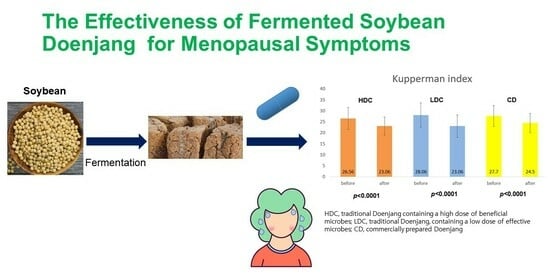
Evaluation of Menopausal Syndrome Relief and Anti-Obesity Efficacy of the Korean Fermented Food Doenjang: A Randomized, Double-Blind Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Safety Assessment
2.4. Indicators of Obesity Assessment
2.5. Inflammation Marker Assessment
2.6. Assessment of Changes in the Gut Microbiome
2.7. Experimental Doenjang Pill Preparation
2.8. Statistical Analysis
3. Results
3.1. Participants
3.2. Anthropometric Parameters
3.3. Safety Assessment
3.4. Effects on Obesity and Inflammation
3.5. Efficacy Evaluation of Kupperman Index Values between the Three Groups
3.6. Microbiome and Short-Chain Fatty Acid Analysis in Feces
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Setchell, K.D. Phytoestrogens: The Biochemistry, Physiology, and Implications for Human Health of Soy Isoflavones. Am. J. Clin. Nutr. 1998, 68, 1333S–1346S. [Google Scholar] [CrossRef] [PubMed]
- Panay, N.; Rees, M. Alternatives to Hormone Replacement Therapy for Management of Menopause Symptoms. Curr. Obstet. Gynaecol. 2005, 15, 259–266. [Google Scholar] [CrossRef]
- Welty, F.K.; Lee, K.S.; Lew, N.S.; Nasca, M.; Zhou, J.R. The association between soy nut consumption and decreased menopausal symptoms. J. Womens Health 2007, 16, 361–369. [Google Scholar] [CrossRef]
- Reed, S.D.; Lampe, J.W.; Qu, C.; Gundersen, G.; Fuller, S.; Copeland, W.K.; Newton, K.M. Self-reported menopausal symptoms in a racially diverse population and soy food consumption. Maturitas 2013, 75, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Park, K.J.; Kim, Y.M.; Lee, B.M.; Lee, B.K. Fungal microflora on Korean home-made meju. Korean J. Mycol. 1997, 5, 7–12. [Google Scholar]
- Namgung, H.J.; Park, H.J.; Cho, I.H.; Choi, H.K.; Kwon, D.Y.; Shim, S.M.; Kim, Y.S. Metabolite profiling of doenjang, fermented soybean paste, during fermentation. J. Sci. Food Agric. 2010, 90, 1926–1935. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.J.; Ryu, M.S.; Yang, H.J.; Wu, X.H.; Jeong, D.Y.; Park, S.M. Bacterial distribution, biogenic amine contents, and functionalities of traditionally made doenjang, a long-term fermented soybean food, from different areas of Korea. Microorganisms 2021, 9, 1348. [Google Scholar] [CrossRef]
- Tao, M.F.; Shao, H.; Li, C.; Teng, Y. Correlation Between the Modified Kupperman Index and the Menopause Rating Scale in Chinese Women. Patient Prefer. Adherence 2013, 7, 223–229. [Google Scholar] [CrossRef]
- Armstrong, T.; Bull, F. Development of the World Health Organization Global Physical Activity Questionnaire (GPAQ). J. Public Health 2006, 14, 66–70. [Google Scholar] [CrossRef]
- Jeon, J.H.; Go, N.Y.; Lee, M.R.; Ndahimana, D.; Kim, E.K. Accuracy of 24-hour diet recalls for estimating energy intake in elderly men using the doubly labeled water method. Korean J. Community Nutr. 2018, 23, 516–524. [Google Scholar] [CrossRef]
- Lobo, R.A. Hormone-replacement therapy: Current thinking. Nat. Rev. Endocrinol. 2017, 13, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Rossouw, J.E.; Anderson, G.L.; Prentice, R.L.; LaCroix, A.Z.; Kooperberg, C.; Stefanick, M.L.; Jackson, R.D.; Beresford, S.A.; Howard, B.V.; Johnson, K.C.; et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results From the Women’s Health Initiative randomized controlled trial. JAMA 2022, 288, 321–333. [Google Scholar] [CrossRef]
- Anderson, G.L.; Limacher, M.; Assaf, A.R.; Bassford, T.; Beresford, S.A.; Black, H.; Bonds, D.; Brunner, R.; Brzyski, R.; Caan, B.; et al. Women’s Health Initiative Steering Committee Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: The Women’s Health Initiative randomized controlled trial. JAMA 2004, 291, 1701–1712. [Google Scholar] [CrossRef]
- Burger, H.G.; MacLennan, A.H.; Huang, K.E.; Castelo-Branco, C. Evidence-based assessment of the impact of the WHI on women’s health. Climacteric 2012, 15, 281–287. [Google Scholar] [CrossRef]
- Simon, J.A. What’s new in hormone replacement therapy: Focus on transdermal estradiol and micronized progesterone. Climacteric 2012, 15 (Suppl. S1), 3–10. [Google Scholar] [CrossRef]
- Manson, J.E.; Aragaki, A.K.; Rossouw, J.E.; Anderson, G.L.; Prentice, R.L.; LaCroix, A.Z.; Chlebowski, R.T.; Howard, B.V.; Thomson, C.A.; Margolis, K.L.; et al. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: The Women’s Health Initiative randomized trials. JAMA 2017, 318, 927–938. [Google Scholar] [CrossRef]
- Russell, L.; Hicks, G.S.; Low, A.K.; Shepherd, J.M.; Brown, C.A. Phytoestrogens: A viable option? Am. J. Med. Sci. 2002, 324, 185–188. [Google Scholar] [CrossRef]
- Cheng, G.; Wilczek, B.; Warner, M.; Gustafsson, J.A.; Landgren, B.M. Isoflavone treatment for acute menopausal symptoms. Menopause 2007, 14, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Omoni, A.O.; Aluko, R.E. Soybean foods and their benefits: Potential mechanisms of action. Nutr. Rev. 2005, 63, 272–283. [Google Scholar] [CrossRef]
- Cancellieri, F.; De Leo, V.; Genazzani, A.D.; Nappi, C.; Parenti, G.L.; Polatti, F.; Ragni, N.; Savoca, S.; Teglio, L.; Finelli, F.; et al. Efficacy on menopausal neurovegetative symptoms and some plasma lipids blood levels of an herbal product containing isoflavones and other plant extracts. Maturitas 2007, 56, 249–256. [Google Scholar] [CrossRef]
- Tice, J.A.; Ettinger, B.; Ensrud, K.; Wallace, R.; Blackwell, T.; Cummings, S.R. Phytoestrogen supplements for the treatment of hot flashes: The Isoflavone Clover Extract (ICE) Study: A randomized controlled trial. JAMA 2003, 290, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.R.; Ko, N.Y.; Chen, K.-H. Isoflavone Supplements for Menopausal Women: A Systematic Review. Nutrients 2019, 11, 2649. [Google Scholar] [CrossRef]
- Kim, J.E.; Choi, K.H. A meta analysis for anti-hyperlipidemia effect of soybeans. J. Korean Data Inf. Sci. Soc. 2010, 21, 651–667. [Google Scholar]
- Cha, Y.S.; Park, Y.; Lee, M.; Chae, S.W.; Park, K.; Kim, Y.; Lee, H.S. Doenjang, a Korean fermented soy food, exerts antiobesity and antioxidative activities in overweight subjects with the PPAR-γ2 C1431T polymorphism: 12-week, double-blind randomized clinical trial. J. Med. Food 2014, 17, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.E.; Kim, K.A.; Han, M.J.; Ki, D.H. Doenjang, a fermented Korean soybean paste, inhibits lipopolysaccharide production of gut microbiota in mice. J. Med. Food 2014, 17, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Wells, C.L.; Barton, R.G.; Wavatne, C.S.; Dunn, D.L.; Cerra, F.B. Intestinal bacterial flora, intestinal pathology, and lipopolysaccharide-induced translocation of intestinal bacteria. Circ. Shock 1992, 37, 117–123. [Google Scholar] [PubMed]
- Kim, K.A.; Jang, S.E.; Jeong, J.J.; Yu, D.H.; Han, M.J.; Kim, D.H. Doenjang, a Korean soybean paste, ameliorates TNBS-induced colitis in mice by suppressing gut microbial lipopolysaccharide production and NF-κB activation. J. Funct. Foods 2014, 11, 417–427. [Google Scholar] [CrossRef]
- Yang, H.J.; Jeong, S.J.; Ryu, M.S.; Ha, G.; Jeong, D.Y.; Park, Y.M.; Lee, H.Y.; Bae, J.S. Protective effect of traditional Korean fermented soybean foods (doenjang) on a dextran sulfate sodium-induced colitis mouse model. Food Funct. 2022, 13, 8616–8626. [Google Scholar] [CrossRef]
- Park, S.; Zhang, T.; Yue, Y.; Jeong, S.J.; Ryu, M.S.; Wu, X.; Yang, H.J.; Jeong, D.Y. Alleviation of metabolic disturbance by substituting kanjang high in Bacillus for salt through modulation of the gut microbiota in estrogen-deficient rats. Foods 2022, 11, 1951. [Google Scholar] [CrossRef]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The influence of probiotics on the firmicutes/bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef]
- Abenavoli, L.; Scarpellini, E.; Colica, C.; Boccuto, L.; Salehi, B.; Sharifi-Rad, J.; Aiello, V.; Romano, B.; De Lorenzo, A.; Izzo, A.A.; et al. Gut microbiota and obesity: A role for probiotics. Nutrients 2019, 11, 2690. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, R.; Xiao, Q.; Liu, C.; Jiang, L.; Deng, F.; Zhou, H. Analysis of bacterial diversity during fermentation of Chinese traditional fermented chopped pepper. Lett. Appl. Microbiol. 2019, 69, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Liu, X.W.; Huang, J.L.; Baloch, S.; Xu, X.; Pei, X.F. Microbial diversity and chemical analysis of Shuidouchi, traditional Chinese fermented soybean. Food Res. Int. 2019, 116, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Conteh, A.R.; Huang, R. Targeting the gut microbiota by Asian and Western dietary constituents: A new avenue for diabetes. Toxicol. Res. 2020, 9, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shimizu, Y.; Kimura, I. Gut microbial metabolite short-chain fatty acids and obesity. Biosci. Microbiota Food Health 2017, 36, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.M.; De Souza, R.; Kendall, C.W.; Emam, A.; Jenkins, D.J. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Lin, S.; Zheng, B.; Cheung, P.C. Short-chain fatty acids in control of energy metabolism. Crit. Rev. Food Sci. Nutr. 2016, 58, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.V.; Frassetto, A.; Kowalik, E.J., Jr.; Nawrocki, A.R.; Lu, M.M.; Kosinski, J.R.; Hubert, J.A.; Szeto, D.; Yao, X.; Forrest, G.; et al. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS ONE 2012, 7, e35240. [Google Scholar] [CrossRef] [PubMed]
- Razavi, A.C.; Potts, K.S.; Kelly, T.N.; Bazzano, L.A. Sex, gut microbiome, and cardiovascular disease risk. Biol. Sex Differ. 2019, 10, 29. [Google Scholar] [CrossRef]
- Den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]




| Ingredient | HDC | LDC | CD | |||
|---|---|---|---|---|---|---|
| Content (g) | Ratio (%) | Content (g) | Ratio (%) | Content (g) | Ratio (%) | |
| Freeze-dried Doenjang powder | 6 | 100 | 6 | 100 | 6 | 100 |
| Total | 6 | 100 | 6 | 100 | 6 | 100 |
| Value | Group | |||
|---|---|---|---|---|
| HDC (n = 18) | LDC (n = 18) | CD (n = 20) | p-Value | |
| Drinking (n) | 18 (100) | 16 (88.9) | 20 (100) | 0.112 |
| Smoking (n) | 18 (100) | 17 (94.4) | 20 (100) | 0.341 |
| Age | 59.61 ± 5.12 | 59.56 ± 5.17 | 60.35 ± 4.08 | 0.848 |
| Weight | 63.68 ± 6.99 | 65.08 ± 7.82 | 64.19 ± 6.26 | 0.833 |
| BMI | 26.29 ± 2.49 | 26.24 ± 3.13 | 26.57 ± 3.07 | 0.935 |
| Value | Group | |||
|---|---|---|---|---|
| HDC (n = 18) | LDC (n = 18) | CD (n = 20) | p-Value | |
| Menarche age | 15.22 ± 1.31 | 14.78 ± 1.35 | 15.8 ± 1.24 | 0.060 |
| Menopause age | 50.94 ± 4.12 | 52.11 ± 2.42 | 52.6 ± 2.23 | 0.237 |
| Menopause period | 105.11 ± 80.96 | 88.67 ± 61.38 | 93.75 ± 57.55 | 0.753 |
| Number of births | 2.28 ± 0.67 | 1.89 ± 0.9 | 2.1 ± 0.72 | 0.321 |
| Value | Group | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| HDC (n = 18) | LDC (n = 18) | CD (n = 20) | |||||||
| Before | After | p-Value | Before | After | p-Value | Before | After | p-Value | |
| WBC | 5.89 ± 1.53 | 5.79 ± 1.25 | 0.647 | 5.94 ± 1.5 | 6.1 ± 1.62 | 0.398 | 6.41 ± 1.65 | 6.46 ± 1.93 | 0.887 |
| RBC | 4.42 ± 0.39 | 4.35 ± 0.37 | 0.165 | 4.26 ± 0.27 | 4.25 ± 0.24 | 0.833 | 4.41 ± 0.28 | 4.41 ± 0.29 | 1.000 |
| Hemoglobin | 13.49 ± 0.87 | 13.2 ± 0.86 | 0.084 | 13.09 ± 0.91 | 12.91 ± 0.86 | 0.312 | 13.59 ± 0.74 | 13.48 ± 0.64 | 0.376 |
| Hematocrit | 39.55 ± 2.51 | 38.78 ± 2.41 | 0.111 | 38.39 ± 2.69 | 38.27 ± 2.24 | 0.789 | 39.84 ± 2.17 | 39.72 ± 1.66 | 0.803 |
| GGT | 24.89 ± 10.78 | 25.06 ± 20.36 | 0.956 | 34.72 ± 26.45 | 32.11 ± 18.96 | 0.504 | 24.8 ± 17.35 | 22.9 ± 12.13 | 0.311 |
| AST | 26.22 ± 7.45 | 26.22 ± 9.93 | 1.000 | 24.67 ± 6.43 | 25.78 ± 10.54 | 0.559 | 28.15 ± 10.44 | 27.5 ± 10.22 | 0.778 |
| ALT | 25.22 ± 13.22 | 25.61 ± 18.36 | 0.819 | 23.56 ± 12.97 | 24.17 ± 15.14 | 0.834 | 25.15 ± 16.44 | 25.45 ± 19.28 | 0.857 |
| BUN | 14.96 ± 3.27 | 13.17 ± 3.04 | 0.040 | 15.79 ± 3.23 | 15.04 ± 4.35 | 0.507 | 13.92 ± 3.59 | 13.06 ± 3.4 | 0.151 |
| Creatinine | 0.68 ± 0.1 | 0.66 ± 0.11 | 0.370 | 0.7 ± 0.1 | 0.69 ± 0.1 | 0.794 | 0.67 ± 0.11 | 0.67 ± 0.08 | 0.954 |
| Uric acid | 4.89 ± 0.88 | 4.46 ± 0.79 | 0.010 | 4.63 ± 1.13 | 4.46 ± 1.22 | 0.157 | 4.62 ± 1.07 | 4.25 ± 0.92 | 0.026 |
| T-protein | 7.29 ± 0.32 | 7.11 ± 0.28 | 0.004 | 7.11 ± 0.42 | 7.01 ± 0.29 | 0.132 | 7.17 ± 0.32 | 7.07 ± 0.32 | 0.222 |
| Albumin | 4.47 ± 0.19 | 4.4 ± 0.13 | 0.111 | 4.52 ± 0.23 | 4.44 ± 0.19 | 0.084 | 4.5 ± 0.18 | 4.47 ± 0.18 | 0.390 |
| T-bilirubin | 0.73 ± 0.29 | 0.74 ± 0.24 | 0.879 | 0.76 ± 0.21 | 0.72 ± 0.22 | 0.269 | 0.78 ± 0.29 | 0.86 ± 0.42 | 0.206 |
| LD | 172.56 ± 45.78 | 179.67 ± 35.85 | 0.513 | 193.94 ± 23.05 | 196.06 ± 27.97 | 0.577 | 211.35 ± 40.1 | 211.05 ± 49.83 | 0.977 |
| ALP | 74.78 ± 22.4 | 74.61 ± 20.11 | 0.939 | 82.72 ± 30.8 | 82.11 ± 25.72 | 0.800 | 79.4 ± 15.4 | 78.55 ± 14.51 | 0.763 |
| CK | 103.17 ± 51.57 | 109.33 ± 70.14 | 0.738 | 130.33 ± 82.46 | 131.11 ± 88.02 | 0.934 | 172.45 ± 239.07 | 130.8 ± 54.94 | 0.384 |
| Value | Group | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| HDC (n = 18) | LDC (n = 18) | CD (n = 20) | |||||||
| Before | After | p-Value | Before | After | p-Value | Before | After | p-Value | |
| SBP | 130.56 ± 9.68 | 132.5 ± 13.04 | 0.545 | 137 ± 12.17 | 136.33 ± 11.81 | 0.783 | 133.55 ± 10.87 | 132.85 ± 13.57 | 0.794 |
| DBP | 77.06 ± 8.15 | 76.78 ± 9.02 | 0.921 | 80 ± 7.98 | 77.33 ± 7.2 | 0.098 | 76 ± 8.72 | 78.6 ± 7.13 | 0.249 |
| Pulse | 74.17 ± 9.97 | 78.28 ± 9.7 | 0.177 | 76 ± 8.13 | 74.06 ± 10.94 | 0.447 | 77.2 ± 9.07 | 78.5 ± 10.52 | 0.454 |
| WC | 87.83 ± 4.09 | 87.54 ± 4.34 | 0.362 | 91.35 ± 6.35 | 90.71 ± 6.82 | 0.282 | 90.52 ± 5.89 | 90.41 ± 5.82 | 0.089 |
| HC | 92.4 ± 16.57 | 95.92 ± 5.79 | 0.303 | 97.12 ± 4.42 | 97.12 ± 4.35 | 1.000 | 98.98 ± 5.75 | 99 ± 5.78 | 0.894 |
| WHR | 0.92 ± 0.04 | 0.92 ± 0.04 | 0.772 | 0.94 ± 0.04 | 0.93 ± 0.04 | 0.381 | 0.92 ± 0.04 | 0.91 ± 0.04 | 0.021 |
| Weight | 63.68 ± 6.99 | 63.89 ± 7.25 | 0.303 | 65.08 ± 7.82 | 65.06 ± 7.49 | 0.933 | 64.19 ± 6.26 | 63.91 ± 6.29 | 0.229 |
| BMI | 26.29 ± 2.49 | 26.36 ± 2.58 | 0.426 | 26.24 ± 3.13 | 26.25 ± 3.1 | 0.942 | 26.57 ± 3.07 | 26.46 ± 3.06 | 0.267 |
| BFM | 24.25 ± 5.21 | 24.43 ± 5.29 | 0.624 | 23.49 ± 4.41 | 23.84 ± 4.46 | 0.065 | 24.02 ± 5.54 | 24.06 ± 5.36 | 0.838 |
| PBF | 37.79 ± 4.15 | 37.87 ± 3.98 | 0.887 | 35.96 ± 3.71 | 36.51 ± 3.64 | 0.0501 | 37.15 ± 6.1 | 37.36 ± 5.67 | 0.444 |
| FFM | 39.43 ± 2.96 | 39.46 ± 2.71 | 0.937 | 41.59 ± 4.91 | 41.22 ± 4.45 | 0.126 | 40.18 ± 3.82 | 39.87 ± 3.54 | 0.156 |
| AFR, Abdominal fat rate | 0.91 ± 0.04 | 0.93 ± 0.05 | 0.020 | 0.90 ± 0.05 | 0.93 ± 0.06 | <0.0001 | 0.91 ± 0.06 | 0.93 ± 0.06 | 0.150 |
| BMR | 1221.56 ± 64.14 | 1222.44 ± 58.32 | 0.907 | 1268.39 ± 106.16 | 1260.33 ± 95.94 | 0.124 | 1237.55 ± 82.39 | 1230.95 ± 76.12 | 0.168 |
| hs-CRP | 1.51 ± 2.02 | 1.27 ± 1.34 | 0.631 | 1.12 ± 1.11 | 1.33 ± 1.76 | 0.645 | 1.19 ± 1.34 | 1.05 ± 1.15 | 0.599 |
| ESR | 8.5 ± 6.53 | 9.39 ± 5.76 | 0.495 | 6.39 ± 5.29 | 8.28 ± 9.56 | 0.314 | 5.45 ± 4.14 | 5.85 ± 3.12 | 0.681 |
| Haptoglobin | 86.28 ± 38.8 | 86.94 ± 39.14 | 0.917 | 106.72 ± 40.81 | 111.94 ± 55.76 | 0.472 | 97.8 ± 48.89 | 99.65 ± 43.98 | 0.809 |
| HDL | 56.06 ± 12.3 | 54.06 ± 14.19 | 0.285 | 52.22 ± 7.76 | 58.72 ± 20.43 | 0.141 | 53.9 ± 10.35 | 54.7 ± 9.52 | 0.583 |
| LDL | 134.28 ± 47.29 | 116.89 ± 42.05 | 0.015 | 123.06 ± 43.1 | 109 ± 38.23 | 0.015 | 117.15 ± 30.17 | 114.3 ± 32.12 | 0.606 |
| TC | 215.89 ± 48.07 | 199.28 ± 43.18 | 0.037 | 203.78 ± 49.23 | 196.33 ± 41.73 | 0.117 | 191.8 ± 34.91 | 192.8 ± 35.15 | 0.860 |
| Glucose | 107.06 ± 9.73 | 106.72 ± 8.99 | 0.823 | 113.72 ± 22.14 | 111.5 ± 24.81 | 0.379 | 117.95 ± 28.83 | 121.95 ± 39.13 | 0.290 |
| Insulin | 6.85 ± 4.28 | 6.87 ± 3.55 | 0.989 | 6.97 ± 4.1 | 8.1 ± 4.88 | 0.087 | 8.23 ± 6.17 | 7.86 ± 5.04 | 0.564 |
| HOMA IR | 33.32 ± 22.52 | 32.82 ± 17.62 | 0.901 | 37.97 ± 31.13 | 44.29 ± 37.67 | 0.191 | 42.72 ± 34.35 | 42.16 ± 27.21 | 0.900 |
| HOMA β | 1.31 ± 0.76 | 1.33 ± 0.67 | 0.879 | 1.22 ± 0.54 | 1.42 ± 0.64 | 0.055 | 1.48 ± 1.03 | 1.38 ± 0.9 | 0.300 |
| QUICKI | 0.36 ± 0.04 | 0.36 ± 0.03 | 0.367 | 0.36 ± 0.04 | 0.36 ± 0.05 | 0.675 | 0.35 ± 0.04 | 0.35 ± 0.05 | 0.808 |
| EQ-5D | 0.92 ± 0.10 | 0.93 ± 0.04 | 0.612 | 0.90 ± 0.07 | 0.92 ± 0.06 | 0.095 | 0.92 ± 0.06 | 0.93 ± 0.04 | 0.909 |
| Value | Group | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| HDC (n = 18) | LDC (n = 18) | CD (n = 20) | |||||||
| Before | After | p-Value | Before | After | p-Value | Before | After | p-Value | |
| Vasomotor | 8.44 ± 3.03 | 8 ± 2.74 | 0.331 | 8.67 ± 3.14 | 7.78 ± 2.56 | 0.104 | 9.60 ± 2.72 | 8.60 ± 2.68 | 0.056 |
| Paresthesia | 3.33 ± 1.94 | 3.33 ± 1.19 | 1.000 | 2.56 ± 1.92 | 2.44 ± 1.10 | 0.772 | 3.20 ± 1.77 | 3.30 ± 1.17 | 0.716 |
| Insomnia | 3.44 ± 2.45 | 3.78 ± 1.35 | 0.507 | 4.00 ± 1.68 | 3.67 ± 1.41 | 0.187 | 4.00 ± 1.72 | 4.20 ± 1.44 | 0.330 |
| Nervousness | 2.00 ± 2.06 | 0.89 ± 1.23 | 0.028 | 2.89 ± 2.08 | 1.83 ± 2.12 | 0.010 | 1.80 ± 2.14 | 0.80 ± 1.20 | 0.004 |
| Melancholia | 0.44 ± 0.70 | 0.11 ± 0.32 | 0.055 | 0.56 ± 0.86 | 0.17 ± 0.38 | 0.049 | 0.55 ± 0.89 | 0.20 ± 0.70 | 0.031 |
| Vertigo | 1.39 ± 0.70 | 1.06 ± 0.54 | 0.029 | 1.44 ± 0.92 | 1.22 ± 0.73 | 0.260 | 1.35 ± 0.88 | 1.20 ± 0.62 | 0.419 |
| Fatigue | 2.22 ± 0.88 | 2.5 ± 0.62 | 0.135 | 2.78 ± 0.43 | 2.72 ± 0.67 | 0.579 | 2.30 ± 0.92 | 2.75 ± 0.44 | 0.083 |
| Headache | 1.22 ± 0.65 | 0.78 ± 0.65 | 0.007 | 1.28 ± 0.83 | 0.83 ± 0.71 | 0.007 | 1.15 ± 0.88 | 0.95 ± 0.83 | 0.297 |
| Arthralgia and myalgia | 2.28 ± 0.96 | 2.22 ± 0.73 | 0.790 | 2.44 ± 0.78 | 2.11 ± 0.58 | 0.111 | 2.30 ± 0.80 | 2.30 ± 0.57 | 1.000 |
| Palpitation | 1.11 ± 1.02 | 0.22 ± 0.55 | 0.001 | 1.06 ± 1.00 | 0.22 ± 0.55 | 0.001 | 1.05 ± 1.10 | 0.20 ± 0.41 | 0.001 |
| Formication | 0.67 ± 1.03 | 0.17 ± 0.51 | 0.015 | 0.39 ± 0.78 | 0.06 ± 0.24 | 0.055 | 0.40 ± 0.68 | 0 ± 0 | 0.017 |
| Total | 26.56 ± 5.03 | 23.06 ± 4.09 | 0.001 | 28.06 ± 5.60 | 23.06 ± 5.15 | 0.000 | 27.7 ± 4.75 | 24.5 ± 4.36 | 0.001 |
| Vaginal dryness | 2.11 ± 1.02 | 1.67 ± 0.84 | 0.002 | 2.00 ± 1.06 | 1.53 ± 0.87 | 0.007 | 2.15 ± 0.93 | 1.6 ± 0.99 | 0.024 |
| Value | HDC | LDC | CD | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Before | After | p-Value | Before | After | p-Value | Before | After | p-Value | |
| Firmicutes (%) | 69.00 ± 13.00 | 63.32 ± 11.11 | 0.140 | 69.37 ± 10.66 | 66.60 ± 13.98 | 0.310 | 70.04 ± 11.51 | 62.98 ± 16.42 | 0.001 |
| Bacteroidetes (%) | 19.14 ± 9.86 | 27.34 ± 13.48 | 0.035 | 20.91 ± 8.83 | 23.22 ± 14.28 | 0.411 | 17.95 ± 10.33 | 27.22 ± 16.96 | 0.001 |
| F/B | 6.41 ± 8.74 | 13.77 ± 46.18 | 0.529 | 5.42 ± 7.08 | 5.20 ± 6.46 | 0.914 | 41.88 ± 110.40 | 19.17 ± 56.62 | 0.398 |
| Beneficial Bacteria | 9.05 ± 7.18 | 27.59 ± 11.52 | <0.0001 | 4.69 ± 2.88 | 21.04 ± 6.88 | <0.0001 | 8.87 ± 8.51 | 26.86 ± 10.20 | <0.0001 |
| Harmful Bacteria | 2.37 ± 1.58 | 2.13 ± 1.57 | 0.658 | 3.36 ± 4.92 | 4.88 ± 9.26 | 0.419 | 4.95 ± 5.61 | 2.52 ± 2.17 | 0.046 |
| Others | 88.58 ± 7.07 | 70.28 ± 12.03 | <0.0001 | 91.94 ± 4.79 | 74.08 ± 9.50 | <0.0001 | 86.18 ± 11.05 | 70.62 ± 10.52 | <0.0001 |
| Value | HDC | LDC | CD | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Before | After | p-Value | Before | After | p-Value | Before | After | p-Value | |
| Acetic acid | 40.32 ± 33.28 | 56.15 ± 50.44 | 0.279 | 48.64 ± 24.71 | 49.2 ± 26.6 | 0.953 | 56.3 ± 38.26 | 41.72 ± 24.9 | 0.148 |
| Propionic acid | 20.21 ± 19.53 | 35.9 ± 39.55 | 0.297 | 22.84 ± 15.42 | 22.15 ± 25.87 | 0.930 | 34.4 ± 27.44 | 14.54 ± 10.16 | 0.020 |
| Butytic acid | 89.68 ± 33.31 | 84.61 ± 30.76 | 0.631 | 91.35 ± 18.32 | 85.89 ± 27.54 | 0.558 | 94.43 ± 27.64 | 74.24 ± 36.75 | 0.040 |
| Total | 143.46 ± 68.85 | 164.68 ± 105.29 | 0.490 | 162.83 ± 48.97 | 157.23 ± 56.05 | 0.791 | 173.71 ± 75.25 | 124.77 ± 62.63 | 0.018 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, A.L.; Ryu, M.S.; Yang, H.-J.; Jeong, D.-Y.; Choi, K.H. Evaluation of Menopausal Syndrome Relief and Anti-Obesity Efficacy of the Korean Fermented Food Doenjang: A Randomized, Double-Blind Clinical Trial. Nutrients 2024, 16, 1194. https://doi.org/10.3390/nu16081194
Han AL, Ryu MS, Yang H-J, Jeong D-Y, Choi KH. Evaluation of Menopausal Syndrome Relief and Anti-Obesity Efficacy of the Korean Fermented Food Doenjang: A Randomized, Double-Blind Clinical Trial. Nutrients. 2024; 16(8):1194. https://doi.org/10.3390/nu16081194
Chicago/Turabian StyleHan, A Lum, Myeong Seon Ryu, Hee-Jong Yang, Do-Youn Jeong, and Keum Ha Choi. 2024. "Evaluation of Menopausal Syndrome Relief and Anti-Obesity Efficacy of the Korean Fermented Food Doenjang: A Randomized, Double-Blind Clinical Trial" Nutrients 16, no. 8: 1194. https://doi.org/10.3390/nu16081194