The Microbiota–Diet–Immunity Axis in Cancer Care: From Prevention to Treatment Modulation and Survivorship
Abstract
1. Introduction
2. Anti- and Pro-Tumorigenic Effects of the Microbiota
2.1. Anti-Tumorigenic Effects of Microbiota
2.1.1. Competition with Pro-Tumorigenic Species
2.1.2. Anti-Tumor Immune Responses
2.1.3. Beneficial Metabolites
2.2. Pro-Tumorigenic Effects of Microbiota
2.2.1. Genotoxicity
2.2.2. Inflammation
3. Diet–Microbiota Interaction in Tumorigenesis
3.1. Mediterranean Diet, Gut Microbiota, and Cancer Prevention
3.2. Plant-Based Diets, Gut Microbiota, and Cancer Prevention
3.3. Ketogenic Diet, Microbiota, and Tumor Immunomodulation
3.4. Western Diet, Dysbiosis, and Tumor-Promoting Microbiota
4. Microbiota and Cancer Therapy
4.1. Microbiota and Chemotherapy
4.2. Microbiota and Immunotherapy
4.3. Microbiota and Radiotherapy
4.4. Fecal Microbiota Transplantation
5. Diet–Microbiota Interaction in Cancer Survivorship
5.1. Microbiota Modulation by Dietary Components and Patterns: Impact on Cancer Recurrence and Survival
5.2. Microbiota Modulation by Diet and Lifestyle: Impact on Comorbidities and Quality of Life in Cancer Survivors
6. Limitations and Future Perspectives
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Park, E.M.; Chelvanambi, M.; Bhutiani, N.; Kroemer, G.; Zitvogel, L.; Wargo, J.A. Targeting the gut and tumor microbiota in cancer. Nat. Med. 2022, 28, 690–703. [Google Scholar] [CrossRef]
- Evans, J.M.; Morris, L.S.; Marchesi, J.R. The gut microbiome: The role of a virtual organ in the endocrinology of the host. J. Endocrinol. 2013, 218, R37–R47. [Google Scholar] [CrossRef] [PubMed]
- Ursell, L.K.; Metcalf, J.L.; Parfrey, L.W.; Knight, R. Defining the human microbiome. Nutr. Rev. 2012, 70 (Suppl. S1), S38–S44. [Google Scholar] [CrossRef]
- Mills, S.; Stanton, C.; Lane, J.A.; Smith, G.J.; Ross, R.P. Precision Nutrition and the Microbiome, Part I: Current State of the Science. Nutrients 2019, 11, 923. [Google Scholar] [CrossRef]
- Sun, J.; Song, S.; Liu, J.; Chen, F.; Li, X.; Wu, G. Gut microbiota as a new target for anticancer therapy: From mechanism to means of regulation. NPJ Biofilms Microbiomes 2025, 11, 43. [Google Scholar] [CrossRef] [PubMed]
- Alum, E.U.; Uti, D.E.; Ugwu, O.P.-C.; Alum, B.N.; Edeh, F.O.; Ainebyoona, C. Unveiling the microbial orchestra: Exploring the role of microbiota in cancer development and treatment. Discov. Oncol. 2025, 16, 646. [Google Scholar] [CrossRef]
- Zitvogel, L.; Galluzzi, L.; Viaud, S.; Vétizou, M.; Daillère, R.; Merad, M.; Kroemer, G. Cancer and the gut microbiota: An unexpected link. Sci. Transl. Med. 2015, 7, 271ps1. [Google Scholar] [CrossRef]
- Beam, A.; Clinger, E.; Hao, L. Effect of Diet and Dietary Components on the Composition of the Gut Microbiota. Nutrients 2021, 13, 2795. [Google Scholar] [CrossRef] [PubMed]
- Adolph, T.E.; Tilg, H. Western diets and chronic diseases. Nat. Med. 2024, 30, 2133–2147. [Google Scholar] [CrossRef]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef]
- Moran-Ramos, S.; López-Contreras, B.E.; Canizales-Quinteros, S. Gut Microbiota in Obesity and Metabolic Abnormalities: A Matter of Composition or Functionality? Arch. Med. Res. 2017, 48, 735–753. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.; Dai, L.; Avesani, C.M.; Kublickiene, K.; Stenvinkel, P. The dietary source of trimethylamine N-oxide and clinical outcomes: An unexpected liaison. Clin. Kidney J. 2023, 16, 1804–1812. [Google Scholar] [CrossRef]
- Newgard, C.B. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 2012, 15, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Hamaya, R.; Mora, S.; Lawler, P.R.; Cook, N.R.; Ridker, P.M.; Buring, J.E.; Lee, I.M.; Manson, J.E.; Tobias, D.K. Association of Plasma Branched-Chain Amino Acid With Biomarkers of Inflammation and Lipid Metabolism in Women. Circ. Genom. Precis. Med. 2021, 14, e003330. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, S.; Loo, T.M.; Atarashi, K.; Kanda, H.; Sato, S.; Oyadomari, S.; Iwakura, Y.; Oshima, K.; Morita, H.; Hattori, M.; et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 2013, 499, 97–101. [Google Scholar] [CrossRef]
- Sulekha Suresh, D.; Jain, T.; Dudeja, V.; Iyer, S.; Dudeja, V. From Microbiome to Malignancy: Unveiling the Gut Microbiome Dynamics in Pancreatic Carcinogenesis. Int. J. Mol. Sci. 2025, 26, 3112. [Google Scholar] [CrossRef]
- La Vecchia, M.; Clavenna, M.G.; Sculco, M.; Sala, G.; Marradi, D.; Barberis, E.; Joseph, S.; Mellai, M.; Pagano, N.; Boldorini, R.; et al. Gut microbiota and metabolome signatures in obese and normal-weight patients with colorectal tumors. iScience 2025, 28, 112221. [Google Scholar] [CrossRef]
- Zhang, W.; Qin, X.; Zhang, K.; Ma, J.; Li, M.; Jin, G.; Liu, X.; Wang, S.; Wang, B.; Wu, J.; et al. Microbial metabolite trimethylamine-N-oxide induces intestinal carcinogenesis through inhibiting farnesoid X receptor signaling. Cell Oncol. 2024, 47, 1183–1199. [Google Scholar] [CrossRef]
- Delzenne, N.M.; Bindels, L.B.; Neyrinck, A.M.; Walter, J. The gut microbiome and dietary fibres: Implications in obesity, cardiometabolic diseases and cancer. Nat. Rev. Microbiol. 2025, 23, 225–238. [Google Scholar] [CrossRef]
- Ross, F.C.; Patangia, D.; Grimaud, G.; Lavelle, A.; Dempsey, E.M.; Ross, R.P.; Stanton, C. The interplay between diet and the gut microbiome: Implications for health and disease. Nat. Rev. Microbiol. 2024, 22, 671–686. [Google Scholar] [CrossRef]
- Morel, S.; Delvin, E.; Marcil, V.; Levy, E. Intestinal Dysbiosis and Development of Cardiometabolic Disorders in Childhood Cancer Survivors: A Critical Review. Antioxid. Redox Signal. 2021, 34, 223–251. [Google Scholar] [CrossRef]
- Clifford, B.K.; Amorim, N.M.L.; Kaakoush, N.O.; Boysen, L.; Tedla, N.; Goldstein, D.; Hardeman, E.C.; Simar, D. Irradiation-Induced Dysbiosis: The Compounding Effect of High-Fat Diet on Metabolic and Immune Functions in Mice. Int. J. Mol. Sci. 2023, 24, 5631. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Cheng, L.; Cheng, X.; Wang, Y.; Lin, X.; Xia, S. The Mediating Role of Gut Microbiota on the Association Between Dietary Quality and Cancer-Related Fatigue Among Breast Cancer Patients: A Cross-Sectional Study. Nutrients 2024, 16, 4371. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.R.; Aggarwal, P.; Costa, R.G.F.; Cole, A.M.; Trinchieri, G. Targeting the gut microbiota for cancer therapy. Nat. Rev. Cancer 2022, 22, 703–722. [Google Scholar] [CrossRef]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Vivarelli, S.; Salemi, R.; Candido, S.; Falzone, L.; Santagati, M.; Stefani, S.; Torino, F.; Banna, G.L.; Tonini, G.; Libra, M. Gut Microbiota and Cancer: From Pathogenesis to Therapy. Cancers 2019, 11, 38. [Google Scholar] [CrossRef]
- Greco, G.; Zeppa, S.D.; Agostini, D.; Attisani, G.; Stefanelli, C.; Ferrini, F.; Sestili, P.; Fimognari, C. The Anti- and Pro-Tumorigenic Role of Microbiota and Its Role in Anticancer Therapeutic Strategies. Cancers 2022, 15, 190. [Google Scholar] [CrossRef]
- Ivleva, E.A.; Grivennikov, S.I. Microbiota-driven mechanisms at different stages of cancer development. Neoplasia 2022, 32, 100829. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Shukla, G. Probiotics Lactobacillus rhamnosus GG, Lactobacillus acidophilus suppresses DMH-induced procarcinogenic fecal enzymes and preneoplastic aberrant crypt foci in early colon carcinogenesis in Sprague Dawley rats. Nutr. Cancer 2013, 65, 84–91. [Google Scholar] [CrossRef]
- Asha, A.; Gayathri, D. Synergistic impact of Lactobacillus fermentum, Lactobacillus plantarum and vincristine on 1,2-dimethylhydrazine-induced colorectal carcinogenesis in mice. Exp. Ther. Med. 2012, 3, 1049–1054. [Google Scholar] [CrossRef]
- Chou, Y.C.; Ho, P.Y.; Chen, W.J.; Wu, S.H.; Pan, M.H. Lactobacillus fermentum V3 ameliorates colitis-associated tumorigenesis by modulating the gut microbiome. Am. J. Cancer Res. 2020, 10, 1170–1181. [Google Scholar]
- Kim, J.; Lee, H.K. Potential Role of the Gut Microbiome In Colorectal Cancer Progression. Front. Immunol. 2022, 12, 807648. [Google Scholar] [CrossRef]
- Konishi, H.; Fujiya, M.; Tanaka, H.; Ueno, N.; Moriichi, K.; Sasajima, J.; Ikuta, K.; Akutsu, H.; Tanabe, H.; Kohgo, Y. Probiotic-derived ferrichrome inhibits colon cancer progression via JNK-mediated apoptosis. Nat. Commun. 2016, 7, 12365. [Google Scholar] [CrossRef]
- Zhu, W.; Miyata, N.; Winter, M.G.; Arenales, A.; Hughes, E.R.; Spiga, L.; Kim, J.; Sifuentes-Dominguez, L.; Starokadomskyy, P.; Gopal, P.; et al. Editing of the gut microbiota reduces carcinogenesis in mouse models of colitis-associated colorectal cancer. J. Exp. Med. 2019, 216, 2378–2393. [Google Scholar] [CrossRef]
- Cai, S.; Kandasamy, M.; Rahmat, J.N.; Tham, S.M.; Bay, B.H.; Lee, Y.K.; Mahendran, R. Lactobacillus rhamnosus GG Activation of Dendritic Cells and Neutrophils Depends on the Dose and Time of Exposure. J. Immunol. Res. 2016, 2016, 7402760. [Google Scholar] [CrossRef]
- Fan, S.; Jiang, Z.; Zhang, Z.; Xing, J.; Wang, D.; Tang, D. Akkermansia muciniphila: A potential booster to improve the effectiveness of cancer immunotherapy. J. Cancer Res. Clin. Oncol. 2023, 149, 13477–13494. [Google Scholar] [CrossRef] [PubMed]
- Al-Ishaq, R.K.; Koklesova, L.; Kubatka, P.; Büsselberg, D. Immunomodulation by Gut Microbiome on Gastrointestinal Cancers: Focusing on Colorectal Cancer. Cancers 2022, 14, 2140. [Google Scholar] [CrossRef]
- Qiu, Q.; Lin, Y.; Ma, Y.; Li, X.; Liang, J.; Chen, Z.; Liu, K.; Huang, Y.; Luo, H.; Huang, R.; et al. Exploring the Emerging Role of the Gut Microbiota and Tumor Microenvironment in Cancer Immunotherapy. Front. Immunol. 2021, 11, 612202. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tian, X.; He, B.; Hoang, T.K.; Taylor, C.M.; Blanchard, E.; Freeborn, J.; Park, S.; Luo, M.; Couturier, J.; et al. Lactobacillus reuteri DSM 17938 feeding of healthy newborn mice regulates immune responses while modulating gut microbiota and boosting beneficial metabolites. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 317, G824–G838. [Google Scholar] [CrossRef] [PubMed]
- Atarashi, K.; Tanoue, T.; Oshima, K.; Suda, W.; Nagano, Y.; Nishikawa, H.; Fukuda, S.; Saito, T.; Narushima, S.; Hase, K.; et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 2013, 500, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Farhadi Rad, H.; Tahmasebi, H.; Javani, S.; Hemati, M.; Zakerhamidi, D.; Hosseini, M.; Alibabaei, F.; Banihashemian, S.Z.; Oksenych, V.; Eslami, M. Microbiota and Cytokine Modulation: Innovations in Enhancing Anticancer Immunity and Personalized Cancer Therapies. Biomedicines 2024, 12, 2776. [Google Scholar] [CrossRef]
- Arshad, T.; Mansur, F.; Palek, R.; Manzoor, S.; Liska, V. A Double Edged Sword Role of Interleukin-22 in Wound Healing and Tissue Regeneration. Front. Immunol. 2020, 11, 2148. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Wang, H.; Deng, L.; Hou, J.; Shi, R.; Yao, M.; Gao, Y.; Yao, A.; Wang, X.; Yu, L.; et al. IL-22 is related to development of human colon cancer by activation of STAT3. BMC Cancer 2013, 13, 59. [Google Scholar] [CrossRef]
- Hernandez, P.; Gronke, K.; Diefenbach, A. A catch-22: Interleukin-22 and cancer. Eur. J. Immunol. 2018, 48, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Gavzy, S.J.; Kensiski, A.; Lee, Z.L.; Mongodin, E.F.; Ma, B.; Bromberg, J.S. Bifidobacterium mechanisms of immune modulation and tolerance. Gut Microbes 2023, 15, 2291164. [Google Scholar] [CrossRef]
- Ma, J.; Huang, L.; Hu, D.; Zeng, S.; Han, Y.; Shen, H. The role of the tumor microbe microenvironment in the tumor immune microenvironment: Bystander, activator, or inhibitor? J. Exp. Clin. Cancer Res. 2021, 40, 327. [Google Scholar] [CrossRef]
- Zhou, Z.; Zheng, J.; Lu, Y.; Mai, Z.; Lin, Y.; Lin, P.; Zheng, Y.; Chen, X.; Xu, R.; Zhao, X.; et al. Optimizing CD8+ T cell-based immunotherapy via metabolic interventions: A comprehensive review of intrinsic and extrinsic modulators. Exp. Hematol. Oncol. 2024, 13, 103. [Google Scholar] [CrossRef]
- Mowat, C.; Dhatt, J.; Bhatti, I.; Hamie, A.; Baker, K. Short chain fatty acids prime colorectal cancer cells to activate antitumor immunity. Front. Immunol. 2023, 14, 1190810. [Google Scholar] [CrossRef]
- Zhu, X.; Li, K.; Liu, G.; Wu, R.; Zhang, Y.; Wang, S.; Xu, M.; Lu, L.; Li, P. Microbial metabolite butyrate promotes anti-PD-1 antitumor efficacy by modulating T cell receptor signaling of cytotoxic CD8 T cell. Gut Microbes 2023, 15, 2249143. [Google Scholar] [CrossRef]
- Zagato, E.; Pozzi, C.; Bertocchi, A.; Schioppa, T.; Saccheri, F.; Guglietta, S.; Fosso, B.; Melocchi, L.; Nizzoli, G.; Troisi, J.; et al. Endogenous murine microbiota member Faecalibaculum rodentium and its human homologue protect from intestinal tumour growth. Nat. Microbiol. 2020, 5, 511–524. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, M.; Greathouse, K.L. Targeting Dietary and Microbial Tryptophan-Indole Metabolism as Therapeutic Approaches to Colon Cancer. Nutrients 2021, 13, 1189. [Google Scholar] [CrossRef]
- Schütz, B.; Krause, F.F.; Taudte, R.V.; Zaiss, M.M.; Luu, M.; Visekruna, A. Modulation of Host Immunity by Microbiome-Derived Indole-3-Propionic Acid and Other Bacterial Metabolites. Eur. J. Immunol. 2025, 55, e202451594. [Google Scholar] [CrossRef]
- Bell, H.N.; Rebernick, R.J.; Goyert, J.; Singhal, R.; Kuljanin, M.; Kerk, S.A.; Huang, W.; Das, N.K.; Andren, A.; Solanki, S.; et al. Reuterin in the healthy gut microbiome suppresses colorectal cancer growth through altering redox balance. Cancer Cell 2022, 40, 185–200.E6. [Google Scholar] [CrossRef]
- Arthur, J.C.; Gharaibeh, R.Z.; Mühlbauer, M.; Perez-Chanona, E.; Uronis, J.M.; McCafferty, J.; Fodor, A.A.; Jobin, C. Microbial genomic analysis reveals the essential role of inflammation in bacteria-induced colorectal cancer. Nat. Commun. 2014, 5, 4724. [Google Scholar] [CrossRef]
- Sadeghi, M.; Mestivier, D.; Sobhani, I. Contribution of pks+ Escherichia coli (E. coli) to Colon Carcinogenesis. Microorganisms 2024, 12, 1111. [Google Scholar] [CrossRef]
- Chung, L.; Orberg, E.T.; Geis, A.L.; Chan, J.L.; Fu, K.; DeStefano Shields, C.E.; Dejea, C.M.; Fathi, P.; Chen, J.; Finard, B.B.; et al. Bacteroides fragilis Toxin Coordinates a Pro-carcinogenic Inflammatory Cascade via Targeting of Colonic Epithelial Cells. Cell Host Microbe 2018, 23, 421. [Google Scholar] [CrossRef]
- Tozzi, M.; Fiore, A.; Travaglione, S.; Marcon, F.; Rainaldi, G.; Germinario, E.A.P.; Laterza, I.; Donati, S.; Macchia, D.; Spada, M.; et al. Coli cytotoxic necrotizing factor-1 promotes colorectal carcinogenesis by causing oxidative stress, DNA damage and intestinal permeability alteration. J. Exp. Clin. Cancer Res. 2025, 44, 29. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef] [PubMed]
- Nishida, A.; Andoh, A. The Role of Inflammation in Cancer: Mechanisms of Tumor Initiation, Progression, and Metastasis. Cells 2025, 14, 488. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, H.; Lee, J.H.; Hwangbo, C. Toll-like receptor 4 (TLR4): New insight immune and aging. Immun. Ageing 2023, 20, 67. [Google Scholar] [CrossRef]
- Duan, T.; Du, Y.; Xing, C.; Wang, H.Y.; Wang, R.F. Toll-Like Receptor Signaling and Its Role in Cell-Mediated Immunity. Front. Immunol. 2022, 13, 812774. [Google Scholar] [CrossRef]
- Bakrim, S.; Fessikh, M.E.; Elhrech, H.; Omari, N.E.; Amanullah, M.; Ming, L.C.; Moshawih, S.; Bouyahya, A. Targeting inflammation in cancer therapy: From mechanistic insights to emerging therapeutic approaches. J. Transl. Med. 2025, 23, 588. [Google Scholar] [CrossRef]
- Andoh, A.; Zhang, Z.; Inatomi, O.; Fujino, S.; Deguchi, Y.; Araki, Y.; Tsujikawa, T.; Kitoh, K.; Kim-Mitsuyama, S.; Takayanagi, A.; et al. Interleukin-22, a member of the IL-10 subfamily, induces inflammatory responses in colonic subepithelial myofibroblasts. Gastroenterology 2005, 129, 969–984. [Google Scholar] [CrossRef] [PubMed]
- Kryczek, I.; Lin, Y.; Nagarsheth, N.; Peng, D.; Zhao, L.; Zhao, E.; Vatan, L.; Szeliga, W.; Dou, Y.; Owens, S.; et al. IL-22(+)CD4(+) T cells promote colorectal cancer stemness via STAT3 transcription factor activation and induction of the methyltransferase DOT1L. Immunity 2014, 40, 772–784. [Google Scholar] [CrossRef]
- Li, J.; Ji, Y.; Chen, N.; Dai, L.; Deng, H. Colitis-associated carcinogenesis: Crosstalk between tumors, immune cells and gut microbiota. Cell Biosci. 2023, 13, 194. [Google Scholar] [CrossRef]
- Guo, J.; Liao, M.; Wang, J. TLR4 signaling in the development of colitis-associated cancer and its possible interplay with microRNA-155. Cell Commun. Signal. 2021, 19, 90. [Google Scholar] [CrossRef]
- Chang, Y.; Liu, Y.; Zou, Y.; Ye, R.D. Recent Advances in Studies of Serum Amyloid A: Implications in Inflammation, Immunity and Tumor Metastasis. Int. J. Mol. Sci. 2025, 26, 987. [Google Scholar] [CrossRef]
- Fourie, C.; Shridas, P.; Davis, T.; de Villiers, W.J.S.; Engelbrecht, A.M. Serum amyloid A and inflammasome activation: A link to breast cancer progression? Cytokine Growth Factor. Rev. 2021, 59, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Sasazuki, S.; Inoue, M.; Sawada, N.; Iwasaki, M.; Shimazu, T.; Yamaji, T.; Tsugane, S.; Japan Public Health Center-Based Prospective Study Group. Plasma levels of C-reactive protein and serum amyloid A and gastric cancer in a nested case-control study: Japan Public Health Center-based prospective study. Carcinogenesis 2010, 31, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Flannigan, K.L.; Denning, T.L. Segmented filamentous bacteria-induced immune responses: A balancing act between host protection and autoimmunity. Immunology 2018, 154, 537–546. [Google Scholar] [CrossRef]
- Holbert, C.E.; Cullen, M.T.; Casero, R.A.; Stewart, T.M. Polyamines in cancer: Integrating organismal metabolism and antitumour immunity. Nat. Rev. Cancer 2022, 22, 467–480. [Google Scholar] [CrossRef]
- Koh, A.; Molinaro, A.; Ståhlman, M.; Khan, M.T.; Schmidt, C.; Mannerås-Holm, L.; Wu, H.; Carreras, A.; Jeong, H.; Olofsson, L.E.; et al. Microbially Produced Imidazole Propionate Impairs Insulin Signaling through mTORC1. Cell 2018, 175, 947–961.e17. [Google Scholar] [CrossRef]
- Ethier, J.L.; Desautels, D.; Templeton, A.; Shah, P.S.; Amir, E. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: A systematic review and meta-analysis. Breast Cancer Res. 2017, 19, 2. [Google Scholar] [CrossRef]
- Liu, Y.; Ning, H.; Li, Y.; Li, Y.; Ma, J. The microbiota in breast cancer: Dysbiosis, microbial metabolites, and therapeutic implications. Am. J. Cancer Res. 2025, 15, 1384–1409. [Google Scholar] [CrossRef]
- Su, Q.; Liu, Q. Factors Affecting Gut Microbiome in Daily Diet. Front. Nutr. 2021, 8, 644138. [Google Scholar] [CrossRef]
- McBurney, M.I.; Davis, C.; Fraser, C.M.; Schneeman, B.O.; Huttenhower, C.; Verbeke, K.; Walter, J.; Latulippe, M.E. Establishing What Constitutes a Healthy Human Gut Microbiome: State of the Science, Regulatory Considerations, and Future Directions. J. Nutr. 2019, 149, 1882–1895. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Bella, F.; Godos, J.; Sciacca, S.; Del Rio, D.; Ray, S.; Galvano, F.; Giovannucci, E.L. Possible role of diet in cancer: Systematic review and multiple meta-analyses of dietary patterns, lifestyle factors, and cancer risk. Nutr. Rev. 2017, 75, 405–419. [Google Scholar] [CrossRef]
- Kang, G.G.; Trevaskis, N.L.; Murphy, A.J.; Febbraio, M.A. Diet-induced gut dysbiosis and inflammation: Key drivers of obesity-driven NASH. iScience 2022, 26, 105905. [Google Scholar] [CrossRef] [PubMed]
- Potrykus, M.; Czaja-Stolc, S.; Stankiewicz, M.; Kaska, Ł.; Małgorzewicz, S. Intestinal Microbiota as a Contributor to Chronic Inflammation and Its Potential Modifications. Nutrients 2021, 13, 3839. [Google Scholar] [CrossRef]
- Perrone, P.; D’Angelo, S. Gut Microbiota Modulation Through Mediterranean Diet Foods: Implications for Human Health. Nutrients 2025, 17, 948. [Google Scholar] [CrossRef] [PubMed]
- Vinelli, V.; Biscotti, P.; Martini, D.; Del Bo’, C.; Marino, M.; Meroño, T.; Nikoloudaki, O.; Calabrese, F.M.; Turroni, S.; Taverniti, V.; et al. Effects of Dietary Fibers on Short-Chain Fatty Acids and Gut Microbiota Composition in Healthy Adults: A Systematic Review. Nutrients 2022, 14, 2559. [Google Scholar] [CrossRef]
- Newsome, R.; Yang, Y.; Jobin, C. Western diet influences on microbiome and carcinogenesis. Semin. Immunol. 2023, 67, 101756. [Google Scholar] [CrossRef]
- Cano, R.; Bermúdez, V.; Galban, N.; Garrido, B.; Santeliz, R.; Gotera, M.P.; Duran, P.; Boscan, A.; Carbonell-Zabaleta, A.K.; Durán-Agüero, S.; et al. Dietary Polyphenols and Gut Microbiota Cross-Talk: Molecular and Therapeutic Perspectives for Cardiometabolic Disease: A Narrative Review. Int. J. Mol. Sci. 2024, 25, 9118. [Google Scholar] [CrossRef] [PubMed]
- Queipo-Ortuño, M.I.; Boto-Ordóñez, M.; Murri, M.; Gomez-Zumaquero, J.M.; Clemente-Postigo, M.; Estruch, R.; Cardona Diaz, F.; Andrés-Lacueva, C.; Tinahones, F.J. Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers. Am. J. Clin. Nutr. 2012, 95, 1323–1334. [Google Scholar] [CrossRef] [PubMed]
- Nash, V.; Ranadheera, C.S.; Georgousopoulou, E.N.; Mellor, D.D.; Panagiotakos, D.B.; McKune, A.J.; Kellett, J.; Naumovski, N. The effects of grape and red wine polyphenols on gut microbiota—A systematic review. Food Res. Int. 2018, 113, 277–287. [Google Scholar] [CrossRef]
- Wang, Q.; Hu, T.; Zhang, Q.; Zhang, Y.; Dong, X.; Jin, Y.; Li, J.; Guo, Y.; Guo, F.; Chen, Z.; et al. Fusobacterium nucleatum promotes colorectal cancer through neogenesis of tumor stem cells. J. Clin. Investig. 2025, 135, e181595. [Google Scholar] [CrossRef]
- Hu, L.; Liu, Y.; Kong, X.; Wu, R.; Peng, Q.; Zhang, Y.; Zhou, L.; Duan, L. Fusobacterium nucleatum Facilitates M2 Macrophage Polarization and Colorectal Carcinoma Progression by Activating TLR4/NF-κB/S100A9 Cascade. Front. Immunol. 2021, 12, 658681. [Google Scholar] [CrossRef]
- Shang, F.; Jiang, X.; Wang, H.; Guo, S.; Kang, S.; Xu, B.; Wang, X.; Chen, S.; Li, N.; Liu, B.; et al. Bifidobacterium longum suppresses colorectal cancer through the modulation of intestinal microbes and immune function. Front. Microbiol. 2024, 15, 1327464. [Google Scholar] [CrossRef]
- Randeni, N.; Bordiga, M.; Xu, B. A Comprehensive Review of the Triangular Relationship among Diet-Gut Microbiota-Inflammation. Int. J. Mol. Sci. 2024, 25, 9366. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Schwedhelm, C.; Galbete, C.; Hoffmann, G. Adherence to Mediterranean Diet and Risk of Cancer: An Updated Systematic Review and Meta-Analysis. Nutrients 2017, 9, 1063. [Google Scholar] [CrossRef] [PubMed]
- Aguilera-Buenosvinos, I.; Morales Berstein, F.; González-Gil, E.M.; Dossus, L.; Gunter, M.J.; Biessy, C.; Masala, G.; Santucci De Magistris, M.; Laouali, N.; Shah, S.; et al. Adherence to the Mediterranean Diet and Obesity-Linked Cancer Risk in EPIC. JAMA Netw. Open 2025, 8, e2461031. [Google Scholar] [CrossRef] [PubMed]
- Abrignani, V.; Salvo, A.; Pacinella, G.; Tuttolomondo, A. The Mediterranean Diet, Its Microbiome Connections, and Cardiovascular Health: A Narrative Review. Int. J. Mol. Sci. 2024, 25, 4942. [Google Scholar] [CrossRef]
- Ghosh, T.S.; Rampelli, S.; Jeffery, I.B.; Santoro, A.; Neto, M.; Capri, M.; Giampieri, E.; Jennings, A.; Candela, M.; Turroni, S.; et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: The NU-AGE 1-year dietary intervention across five European countries. Gut 2020, 69, 1218–1228. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; Nguyen, L.H.; Li, Y.; Yan, Y.; Ma, W.; Rinott, E.; Ivey, K.L.; Shai, I.; Willett, W.C.; Hu, F.B.; et al. The gut microbiome modulates the protective association between a Mediterranean diet and cardiometabolic disease risk. Nat. Med. 2021, 27, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Tu, M.; Wang, W.; Zhang, G.; Hammock, B.D. ω-3 Polyunsaturated Fatty Acids on Colonic Inflammation and Colon Cancer: Roles of Lipid-Metabolizing Enzymes Involved. Nutrients 2020, 12, 3301. [Google Scholar] [CrossRef]
- Mostafavi Abdolmaleky, H.; Zhou, J.R. Gut Microbiota Dysbiosis, Oxidative Stress, Inflammation, and Epigenetic Alterations in Metabolic Diseases. Antioxidants 2024, 13, 985. [Google Scholar] [CrossRef]
- de Moreno de LeBlanc, A.; Matar, C.; Thériault, C.; Perdigón, G. Effects of milk fermented by Lactobacillus helveticus R389 on immune cells associated to mammary glands in normal and a breast cancer model. Immunobiology 2005, 210, 349–358. [Google Scholar] [CrossRef]
- Lakritz, J.R.; Poutahidis, T.; Levkovich, T.; Varian, B.J.; Ibrahim, Y.M.; Chatzigiagkos, A.; Mirabal, S.; Alm, E.J.; Erdman, S.E. Beneficial bacteria stimulate host immune cells to counteract dietary and genetic predisposition to mammary cancer in mice. Int. J. Cancer 2014, 135, 529–540. [Google Scholar] [CrossRef]
- Teng, N.M.Y.; Price, C.A.; McKee, A.M.; Hall, L.J.; Robinson, S.D. Exploring the impact of gut microbiota and diet on breast cancer risk and progression. Int. J. Cancer 2021, 149, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Parida, S.; Sharma, D. The Microbiome-Estrogen Connection and Breast Cancer Risk. Cells 2019, 8, 1642. [Google Scholar] [CrossRef]
- Toledo, E.; Salas-Salvadó, J.; Donat-Vargas, C.; Buil-Cosiales, P.; Estruch, R.; Ros, E.; Corella, D.; Fitó, M.; Hu, F.B.; Arós, F.; et al. Mediterranean Diet and Invasive Breast Cancer Risk Among Women at High Cardiovascular Risk in the PREDIMED Trial: A Randomized Clinical Trial. JAMA Intern. Med. 2015, 175, 1752–1760. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.; Asbaghi, O.; Hooshmand, F.; Aghayan, A.H.; Shariati, A.A.; Kazemi, K.; Amirpour, M.; Davoodi, S.H.; Larijani, B. Adherence to Mediterranean Diet and Breast Cancer Risk: A Meta-Analysis of Prospective Observational Studies. Health Sci. Rep. 2025, 8, e70736. [Google Scholar] [CrossRef]
- Chu, A.H.; Lin, K.; Croker, H.; Kefyalew, S.; Becerra-Tomás, N.; Dossus, L.; González-Gil, E.M.; Ahmadi, N.; Park, Y.; Krebs, J.; et al. Dietary patterns and colorectal cancer risk: Global Cancer Update Programme (CUP Global) systematic literature review. Am. J. Clin. Nutr. 2025, 121, 999–1016. [Google Scholar] [CrossRef] [PubMed]
- Bamia, C.; Lagiou, P.; Buckland, G.; Grioni, S.; Agnoli, C.; Taylor, A.J.; Dahm, C.C.; Overvad, K.; Olsen, A.; Tjønneland, A.; et al. Mediterranean diet and colorectal cancer risk: Results from a European cohort. Eur. J. Epidemiol. 2013, 28, 317–328. [Google Scholar] [CrossRef]
- Mehta, R.S.; Nishihara, R.; Cao, Y.; Song, M.; Mima, K.; Qian, Z.R.; Nowak, J.A.; Kosumi, K.; Hamada, T.; Masugi, Y.; et al. Association of Dietary Patterns With Risk of Colorectal Cancer Subtypes Classified by Fusobacterium nucleatum in Tumor Tissue. JAMA Oncol. 2017, 3, 921–927. [Google Scholar] [CrossRef]
- Chen, H.M.; Yu, Y.N.; Wang, J.L.; Lin, Y.W.; Kong, X.; Yang, C.Q.; Yang, L.; Liu, Z.J.; Yuan, Y.Z.; Liu, F.; et al. Decreased dietary fiber intake and structural alteration of gut microbiota in patients with advanced colorectal adenoma. Am. J. Clin. Nutr. 2013, 97, 1044–1052. [Google Scholar] [CrossRef]
- Adak, A.; Khan, M.R. An insight into gut microbiota and its functionalities. Cell. Mol. Life Sci. 2019, 76, 473–493. [Google Scholar] [CrossRef]
- Gauthier, E.; Milagro, F.I.; Navas-Carretero, S. Effect of low-and non-calorie sweeteners on the gut microbiota: A review of clinical trials and cross-sectional studies. Nutrition 2024, 117, 112237. [Google Scholar] [CrossRef]
- Li, J.; Sung, C.Y.; Lee, N.; Ni, Y.; Pihlajamäki, J.; Panagiotou, G.; El-Nezami, H. Probiotics modulated gut microbiota suppresses hepatocellular carcinoma growth in mice. Proc. Natl. Acad. Sci. USA 2016, 113, E1306–E1315. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Lin, Y.; Cao, C.; Chen, D.; Huang, X.; Li, C.; Xu, H.; Lai, H.; Chen, H.; et al. Roles of the gut microbiota in hepatocellular carcinoma: From the gut dysbiosis to the intratumoral microbiota. Cell Death Discov. 2025, 11, 140. [Google Scholar] [CrossRef]
- Tajiri, K.; Shimizu, Y. Gut bacteria may control development of hepatocellular carcinoma. Hepatobiliary Surg. Nutr. 2017, 6, 417–419. [Google Scholar] [CrossRef]
- Bindels, L.B.; Porporato, P.; Dewulf, E.M.; Verrax, J.; Neyrinck, A.M.; Martin, J.C.; Scott, K.P.; Buc Calderon, P.; Feron, O.; Muccioli, G.G.; et al. Gut microbiota-derived propionate reduces cancer cell proliferation in the liver. Br. J. Cancer 2012, 107, 1337–1344. [Google Scholar] [CrossRef]
- Lu, J.; Dong, X.; Gao, Z.; Yan, H.; Shataer, D.; Wang, L.; Qin, Y.; Zhang, M.; Wang, J.; Cui, J.; et al. Probiotics as a therapeutic strategy for metabolic dysfunction-associated steatotic liver disease: A systematic review and meta-analysis. Curr. Res. Food Sci. 2025, 11, 101138. [Google Scholar] [CrossRef]
- Lau, H.C.; Zhang, X.; Ji, F.; Lin, Y.; Liang, W.; Li, Q.; Chen, D.; Fong, W.; Kang, X.; Liu, W.; et al. Lactobacillus acidophilus suppresses non-alcoholic fatty liver disease-associated hepatocellular carcinoma through producing valeric acid. EBioMedicine 2024, 100, 104952. [Google Scholar] [CrossRef]
- Nguyen, N.A.; Jiang, Y.; McQuade, J.L. Eating away cancer: The potential of diet and the microbiome for shaping immunotherapy outcome. Front. Immunol. 2024, 15, 1409414. [Google Scholar] [CrossRef]
- Fakhri, S.; Moradi, S.Z.; Moradi, S.Y.; Piri, S.; Shiri Varnamkhasti, B.; Piri, S.; Khirehgesh, M.R.; Bishayee, A.; Casarcia, N.; Bishayee, A. Phytochemicals regulate cancer metabolism through modulation of the AMPK/PGC-1α signaling pathway. BMC Cancer 2024, 24, 1079. [Google Scholar] [CrossRef]
- Oliver, A.; Chase, A.B.; Weihe, C.; Orchanian, S.B.; Riedel, S.F.; Hendrickson, C.L.; Lay, M.; Sewall, J.M.; Martiny, J.B.H.; Whiteson, K. High-Fiber, Whole-Food Dietary Intervention Alters the Human Gut Microbiome but Not Fecal Short-Chain Fatty Acids. mSystems 2021, 6, e00115–e00121. [Google Scholar] [CrossRef] [PubMed]
- Yarmand, S.; Rashidkhani, B.; Alimohammadi, A.; Shateri, Z.; Shakeri, M.; Sohrabi, Z.; Nouri, M. A healthful plant-based diet can reduce the risk of developing colorectal cancer: Case-control study. J. Health Popul. Nutr. 2024, 43, 111. [Google Scholar] [CrossRef] [PubMed]
- Prochazkova, M.; Budinska, E.; Kuzma, M.; Pelantova, H.; Hradecky, J.; Heczkova, M.; Daskova, N.; Bratova, M.; Modos, I.; Videnska, P.; et al. Vegan Diet Is Associated With Favorable Effects on the Metabolic Performance of Intestinal Microbiota: A Cross-Sectional Multi-Omics Study. Front. Nutr. 2022, 8, 783302. [Google Scholar] [CrossRef]
- Ionescu, V.A.; Diaconu, C.C.; Gheorghe, G.; Mihai, M.M.; Diaconu, C.C.; Bostan, M.; Bleotu, C. Gut Microbiota and Colorectal Cancer: A Balance Between Risk and Protection. Int. J. Mol. Sci. 2025, 26, 3733. [Google Scholar] [CrossRef]
- Hamamah, S.; Lobiuc, A.; Covasa, M. Antioxidant Role of Probiotics in Inflammation-Induced Colorectal Cancer. Int. J. Mol. Sci. 2024, 25, 9026. [Google Scholar] [CrossRef]
- Rubinstein, M.R.; Wang, X.; Liu, W.; Hao, Y.; Cai, G.; Han, Y.W. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe 2013, 14, 195–206. [Google Scholar] [CrossRef]
- Bai, T.; Peng, J.; Zhu, X.; Wu, C. Vegetarian diets and the risk of gastrointestinal cancers: A meta-analysis of observational studies. Eur. J. Gastroenterol. Hepatol. 2023, 35, 1244–1252. [Google Scholar] [CrossRef]
- Latif, A.; Shehzad, A.; Niazi, S.; Zahid, A.; Ashraf, W.; Iqbal, M.W.; Rehman, A.; Riaz, T.; Aadil, R.M.; Khan, I.M.; et al. Probiotics: Mechanism of action, health benefits and their application in food industries. Front. Microbiol. 2023, 14, 1216674. [Google Scholar] [CrossRef] [PubMed]
- Ho, E.; Wong, C.P.; Bouranis, J.A.; Shannon, J.; Zhang, Z. Cruciferous Vegetables, Bioactive Metabolites, and Microbiome for Breast Cancer Prevention. Annu. Rev. Nutr. 2025, 45, 171–195. [Google Scholar] [CrossRef] [PubMed]
- Cogorno, L.; Formisano, E.; Vignati, A.; Prigione, A.; Tramacere, A.; Borgarelli, C.; Sukkar, S.G.; Pisciotta, L. Non-alcoholic fatty liver disease: Dietary and nutraceutical approaches. Liver Res. 2023, 7, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Orabi, D.; Berger, N.A.; Brown, J.M. Abnormal Metabolism in the Progression of Nonalcoholic Fatty Liver Disease to Hepatocellular Carcinoma: Mechanistic Insights to Chemoprevention. Cancers 2021, 13, 3473. [Google Scholar] [CrossRef]
- Gershuni, V.M.; Yan, S.L.; Medici, V. Nutritional Ketosis for Weight Management and Reversal of Metabolic Syndrome. Curr. Nutr. Rep. 2018, 7, 97–106. [Google Scholar] [CrossRef]
- Zhu, H.; Bi, D.; Zhang, Y.; Kong, C.; Du, J.; Wu, X.; Wei, Q.; Qin, H. Ketogenic diet for human diseases: The underlying mechanisms and potential for clinical implementations. Signal Transduct. Target. Ther. 2022, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Matawali, A.; Yeap, J.W.; Sulaiman, S.F.; Tan, M.L. The effects of ketone bodies and ketogenesis on the PI3K/AKT/mTOR signaling pathway: A systematic review. Nutr. Res. 2025, 139, 16–49. [Google Scholar] [CrossRef]
- Cortez, N.E.; Mackenzie, G.G. Ketogenic Diets in Pancreatic Cancer and Associated Cachexia: Cellular Mechanisms and Clinical Perspectives. Nutrients 2021, 15, 3202. [Google Scholar] [CrossRef]
- Mao, Y.; Xia, Z.; Xia, W.; Jiang, P. Metabolic reprogramming, sensing, and cancer therapy. Cell Rep. 2024, 43, 115064. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.L.; Gong, Y.; Qi, Y.J.; Shao, Z.M.; Jiang, Y.Z. Effects of dietary intervention on human diseases: Molecular mechanisms and therapeutic potential. Signal Transduct. Target. Ther. 2024, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Makuku, R.; Sinaei Far, Z.; Khalili, N.; Moyo, A.; Razi, S.; Keshavarz-Fathi, M.; Mahmoudi, M.; Rezaei, N. The Role of Ketogenic Diet in the Treatment of Neuroblastoma. Integr. Cancer Ther. 2023, 22, 15347354221150787. [Google Scholar] [CrossRef]
- Duraj, T.; Kalamian, M.; Zuccoli, G.; Maroon, J.C.; D’Agostino, D.P.; Scheck, A.C.; Poff, A.; Winter, S.F.; Hu, J.; Klement, R.J.; et al. Clinical research framework proposal for ketogenic metabolic therapy in glioblastoma. BMC Med. 2024, 22, 578. [Google Scholar] [CrossRef] [PubMed]
- Zhenyukh, O.; Civantos, E.; Ruiz-Ortega, M.; Sánchez, M.S.; Vázquez, C.; Peiró, C.; Egido, J.; Mas, S. High concentration of branched-chain amino acids promotes oxidative stress, inflammation and migration of human peripheral blood mononuclear cells via mTORC1 activation. Free Radic. Biol. Med. 2017, 104, 165–177. [Google Scholar] [CrossRef]
- Singh, G.; Akcakanat, A.; Sharma, C.; Luyimbazi, D.; Naff, K.A.; Meric-Bernstam, F. The effect of leucine restriction on Akt/mTOR signaling in breast cancer cell lines in vitro and in vivo. Nutr. Cancer 2011, 63, 264–271. [Google Scholar] [CrossRef]
- Fu, Y.; Zou, T.; Shen, X.; Nelson, P.J.; Li, J.; Wu, C.; Yang, J.; Zheng, Y.; Bruns, C.; Zhao, Y.; et al. Lipid metabolism in cancer progression and therapeutic strategies. MedComm 2020, 2, 27–59. [Google Scholar] [CrossRef]
- Jiang, K.; Zhao, Z.; Yuan, M.; Ji, H.; Zhao, Y.; Ding, H.; Feng, J.; Zhou, Y.; Dai, R. Examining the dietary contributions of lipids to pancreatic cancer burden (1990–2021): Incidence trends and future projections. Lipids Health Dis. 2025, 24, 62, Erratum in: Lipids Health Dis. 2025, 24, 90. https://doi.org/10.1186/s12944-025-02505-w. [Google Scholar] [CrossRef]
- Swierczynski, J.; Hebanowska, A.; Sledzinski, T. Role of abnormal lipid metabolism in development, progression, diagnosis and therapy of pancreatic cancer. World J. Gastroenterol. 2014, 20, 2279–2303. [Google Scholar] [CrossRef]
- Xiang, Y.; Zhang, C.; Wang, J.; Cheng, Y.; Wang, K.; Wang, L.; Tong, Y.; Yan, D. Identification of Metabolic Characteristic-Pancreatic Ductal Adenocarcinoma Associations Using Mendelian Randomization and Metabolomics. J. Gastrointest. Cancer 2025, 56, 48. [Google Scholar] [CrossRef]
- Ferrere, G.; Tidjani Alou, M.; Liu, P.; Goubet, A.G.; Fidelle, M.; Kepp, O.; Durand, S.; Iebba, V.; Fluckiger, A.; Daillère, R.; et al. Ketogenic diet and ketone bodies enhance the anticancer effects of PD-1 blockade. J. Clin. Investig. 2021, 6, e145207. [Google Scholar] [CrossRef]
- Nebeling, L.C.; Miraldi, F.; Shurin, S.B.; Lerner, E. Effects of a ketogenic diet on tumor metabolism and nutritional status in pediatric oncology patients: Two case reports. J. Am. Coll. Nutr. 1995, 14, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Branca, J.J.; Pacini, S.; Ruggiero, M. Effects of Pre-surgical Vitamin D Supplementation and Ketogenic Diet in a Patient with Recurrent Breast Cancer. Anticancer Res. 2015, 35, 5525–5532. [Google Scholar]
- Rew, L.; Harris, M.D.; Goldie, J. The ketogenic diet: Its impact on human gut microbiota and potential consequent health outcomes: A systematic literature review. Gastroenterol. Hepatol. Bed Bench 2022, 15, 326–342. [Google Scholar] [CrossRef]
- Olson, C.A.; Vuong, H.E.; Yano, J.M.; Liang, Q.Y.; Nusbaum, D.J.; Hsiao, E.Y. The Gut Microbiota Mediates the Anti-Seizure Effects of the Ketogenic Diet. Cell 2018, 173, 1728–1741.e13, Erratum in: Cell 2018, 174, 497. https://doi.org/10.1016/j.cell.2018.06.051. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yang, H.; Wu, P.; Yang, S.; Xue, W.; Xu, B.; Zhang, S.; Tang, B.; Xu, D. Akkermansia muciniphila: A promising probiotic against inflammation and metabolic disorders. Virulence 2024, 15, 2375555. [Google Scholar] [CrossRef]
- Alsharairi, N.A. The Therapeutic Role of Short-Chain Fatty Acids Mediated Very Low-Calorie Ketogenic Diet-Gut Microbiota Relationships in Paediatric Inflammatory Bowel Diseases. Nutrients 2022, 14, 4113. [Google Scholar] [CrossRef] [PubMed]
- Ang, Q.Y.; Alexander, M.; Newman, J.C.; Tian, Y.; Cai, J.; Upadhyay, V.; Turnbaugh, J.A.; Verdin, E.; Hall, K.D.; Leibel, R.L.; et al. Ketogenic Diets Alter the Gut Microbiome Resulting in Decreased Intestinal Th17 Cells. Cell 2020, 181, 1263–1275.e16. [Google Scholar] [CrossRef]
- Jeong, S.; Kim, Y.; Park, S.; Lee, D.; Lee, J.; Hlaing, S.P.; Yoo, J.W.; Rhee, S.H.; Im, E. Lactobacillus plantarum Metabolites Elicit Anticancer Effects by Inhibiting Autophagy-Related Responses. Molecules 2023, 28, 1890. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, W.; Zhao, J.; Stanton, C.; Ross, R.P.; Zhang, H.; Chen, W.; Yang, B. Lactobacillus plantarum Ameliorates Colorectal Cancer by Ameliorating the Intestinal Barrier through the CLA-PPAR-γ Axis. J. Agric. Food Chem. 2024, 72, 19766–19785. [Google Scholar] [CrossRef]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.L.; et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef]
- Huang, Y.; Ding, J.; Zhu, Y.; Shi, J.; Liu, R.; Wu, C.; Han, L.; Zhang, M. β-hydroxybutyrate, a ketone body, suppresses tumor growth, stemness, and invasive phenotypes in non-small cell lung cancer. Cancer Biol. Ther. 2025, 26, 2516825. [Google Scholar] [CrossRef]
- Rodrigues, L.M.; Uribe-Lewis, S.; Madhu, B.; Honess, D.J.; Stubbs, M.; Griffiths, J.R. The action of β-hydroxybutyrate on the growth, metabolism and global histone H3 acetylation of spontaneous mouse mammary tumours: Evidence of a β-hydroxybutyrate paradox. Cancer Metab. 2017, 5, 4. [Google Scholar] [CrossRef]
- Sargaço, B.; Oliveira, P.A.; Antunes, M.L.; Moreira, A.C. Effects of the Ketogenic Diet in the Treatment of Gliomas: A Systematic Review. Nutrients 2022, 14, 1007. [Google Scholar] [CrossRef]
- Zou, Y.; Fineberg, S.; Pearlman, A.; Feinman, R.D.; Fine, E.J. The effect of a ketogenic diet and synergy with rapamycin in a mouse model of breast cancer. PLoS ONE 2020, 15, e0233662. [Google Scholar] [CrossRef] [PubMed]
- Jemal, M.; Molla, T.S.; Asmamaw Dejenie, T. Ketogenic Diets and their Therapeutic Potential on Breast Cancer: A Systemic Review. Cancer Manag. Res. 2021, 13, 9147–9155. [Google Scholar] [CrossRef] [PubMed]
- Padilla, J.; Lee, J. A Novel Therapeutic Target, BACH1, Regulates Cancer Metabolism. Cells 2021, 10, 634. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Tonouchi, H.; Sasayama, A.; Ashida, K. A Ketogenic Formula Prevents Tumor Progression and Cancer Cachexia by Attenuating Systemic Inflammation in Colon 26 Tumor-Bearing Mice. Nutrients 2018, 10, 206. [Google Scholar] [CrossRef] [PubMed]
- Gouirand, V.; Gicquel, T.; Lien, E.C.; Jaune-Pons, E.; Da Costa, Q.; Finetti, P.; Metay, E.; Duluc, C.; Mayers, J.R.; Audebert, S.; et al. Ketogenic HMG-CoA lyase and its product β-hydroxybutyrate promote pancreatic cancer progression. EMBO J. 2022, 41, e110466. [Google Scholar] [CrossRef]
- Vaezi, M.A.; Nekoufar, S.; Robati, A.K.; Salimi, V.; Tavakoli-Yaraki, M. Therapeutic potential of β-hydroxybutyrate in the management of pancreatic neoplasms: Exploring novel diagnostic and treatment strategies. Lipids Health Dis. 2024, 23, 376. [Google Scholar] [CrossRef]
- Anastasiou, I.A.; Kounatidis, D.; Vallianou, N.G.; Skourtis, A.; Dimitriou, K.; Tzivaki, I.; Tsioulos, G.; Rigatou, A.; Karampela, I.; Dalamaga, M. Beneath the Surface: The Emerging Role of Ultra-Processed Foods in Obesity-Related Cancer. Curr. Oncol. Rep. 2025, 27, 390–414. [Google Scholar] [CrossRef]
- Lian, Y.; Wang, G.P.; Chen, G.Q.; Chen, H.N.; Zhang, G.Y. Association between ultra-processed foods and risk of cancer: A systematic review and meta-analysis. Front. Nutr. 2023, 10, 1175994. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.H.; Yu, J.C.; Hsu, H.M.; Chu, C.H.; Chang, T.M.; Hong, Z.J.; Feng, A.C.; Fu, C.Y.; Hsu, K.F.; Dai, M.S.; et al. The Risk of Breast Cancer between Western and Mediterranean Dietary Patterns. Nutrients 2023, 15, 2057. [Google Scholar] [CrossRef]
- Zhou, X.; Qiao, K.; Wu, H.; Zhang, Y. The Impact of Food Additives on the Abundance and Composition of Gut Microbiota. Molecules 2023, 28, 631. [Google Scholar] [CrossRef]
- Zheng, J.; Guinter, M.A.; Merchant, A.T.; Wirth, M.D.; Zhang, J.; Stolzenberg-Solomon, R.Z.; Steck, S.E. Dietary patterns and risk of pancreatic cancer: A systematic review. Nutr. Rev. 2017, 75, 883–908. [Google Scholar] [CrossRef] [PubMed]
- Frioux, C.; Ansorge, R.; Özkurt, E.; Ghassemi Nedjad, C.; Fritscher, J.; Quince, C.; Waszak, S.M.; Hildebrand, F. Enterosignatures define common bacterial guilds in the human gut microbiome. Cell Host Microbe 2023, 31, 1111–1125.e6. [Google Scholar] [CrossRef]
- Severino, A.; Tohumcu, E.; Tamai, L.; Dargenio, P.; Porcari, S.; Rondinella, D.; Venturini, I.; Maida, M.; Gasbarrini, A.; Cammarota, G.; et al. The microbiome-driven impact of western diet in the development of noncommunicable chronic disorders. Best. Pract. Res. Clin. Gastroenterol. 2024, 72, 101923. [Google Scholar] [CrossRef]
- Tong, Y.; Gao, H.; Qi, Q.; Liu, X.; Li, J.; Gao, J.; Li, P.; Wang, Y.; Du, L.; Wang, C. High fat diet, gut microbiome and gastrointestinal cancer. Theranostics 2021, 11, 5889–5910. [Google Scholar] [CrossRef]
- Sofi, F.; Dinu, M.; Pagliai, G.; Pierre, F.; Gueraud, F.; Bowman, J.; Gerard, P.; Longo, V.; Giovannelli, L.; Caderni, G.; et al. Fecal microbiome as determinant of the effect of diet on colorectal cancer risk: Comparison of meat-based versus pesco-vegetarian diets (the MeaTIc study). Trials 2019, 20, 688. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Xia, J.; Li, L.; Ke, Y.; Cheng, J.; Xie, Y.; Chu, W.; Cheung, P.; Kim, J.H.; Colditz, G.A.; et al. Associations between dietary patterns and the risk of breast cancer: A systematic review and meta-analysis of observational studies. Breast Cancer Res. 2019, 21, 16. [Google Scholar] [CrossRef]
- Fernández-Murga, M.L.; Gil-Ortiz, F.; Serrano-García, L.; Llombart-Cussac, A. A New Paradigm in the Relationship between Gut Microbiota and Breast Cancer: β-glucuronidase Enzyme Identified as Potential Therapeutic Target. Pathogens 2023, 12, 1086. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, A.M.; Burrington, C.M.; Gillaspie, E.A.; Lynch, D.T.; Horsman, M.J.; Greene, M.W. High-fat Western diet-induced obesity contributes to increased tumor growth in mouse models of human colon cancer. Nutr. Res. 2016, 36, 1325–1334. [Google Scholar] [CrossRef]
- Shen, K.; Shen, D.; Jin, D.; Zheng, Y.; Zhu, Y.; Zhao, X.; Zhang, Z.; Wang, N.; Chen, H.; Yang, L. High-fat diet promotes tumor growth in the patient-derived orthotopic xenograft (PDOX) mouse model of ER positive endometrial cancer. Sci. Rep. 2023, 13, 16537. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Song, N.Y.; Yim, H. Targeting dysregulated lipid metabolism in the tumor microenvironment. Arch. Pharmacal Res. 2023, 46, 855–881. [Google Scholar] [CrossRef]
- Natividad, J.M.; Lamas, B.; Pham, H.P.; Michel, M.L.; Rainteau, D.; Bridonneau, C.; da Costa, G.; van Hylckama Vlieg, J.; Sovran, B.; Chamignon, C.; et al. Bilophila wadsworthia aggravates high fat diet induced metabolic dysfunctions in mice. Nat. Commun. 2018, 9, 2802. [Google Scholar] [CrossRef]
- Wahlström, A.; Brumbaugh, A.; Sjöland, W.; Olsson, L.; Wu, H.; Henricsson, M.; Lundqvist, A.; Makki, K.; Hazen, S.L.; Bergström, G.; et al. Production of deoxycholic acid by low-abundant microbial species is associated with impaired glucose metabolism. Nat. Commun. 2024, 15, 4276. [Google Scholar] [CrossRef]
- Devendran, S.; Shrestha, R.; Alves, J.M.P.; Wolf, P.G.; Ly, L.; Hernandez, A.G.; Méndez-García, C.; Inboden, A.; Wiley, J.; Paul, O.; et al. Clostridium scindens ATCC 35704: Integration of Nutritional Requirements, the Complete Genome Sequence, and Global Transcriptional Responses to Bile Acids. Appl. Environ. Microbiol. 2019, 85, e00052-19. [Google Scholar] [CrossRef]
- Kouhzad, M.; Götz, F.; Navidifar, T.; Taki, E.; Ghamari, M.; Mohammadzadeh, R.; Seyedolmohadesin, M.; Bostanghadiri, N. Carcinogenic and anticancer activities of microbiota-derived secondary bile acids. Front. Oncol. 2025, 15, 1514872. [Google Scholar] [CrossRef]
- Singh, R.K.; Chang, H.W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef]
- Cai, J.; Rimal, B.; Jiang, C.; Chiang, J.Y.L.; Patterson, A.D. Bile acid metabolism and signaling, the microbiota, and metabolic disease. Pharmacol. Ther. 2022, 237, 108238. [Google Scholar] [CrossRef]
- Ohara, Y.; Valenzuela, P.; Hussain, S.P. The interactive role of inflammatory mediators and metabolic reprogramming in pancreatic cancer. Trends Cancer 2022, 8, 556–569. [Google Scholar] [CrossRef]
- Li, S.; Zhu, S.; Yu, J. The role of gut microbiota and metabolites in cancer chemotherapy. J. Adv. Res. 2024, 64, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Iida, N.; Dzutsev, A.; Stewart, C.A.; Smith, L.; Bouladoux, N.; Weingarten, R.A.; Molina, D.A.; Salcedo, R.; Back, T.; Cramer, S.; et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 2013, 342, 967–970. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Zhang, S.; Li, H.; Yang, F.; Mushtaq, N.; Ullah, S.; Shi, Y.; An, C.; Xu, J. The influence of gut microbiota dysbiosis to the efficacy of 5-Fluorouracil treatment on colorectal cancer. Biomed. Pharmacother. 2018, 108, 184–193. [Google Scholar] [CrossRef]
- Viaud, S.; Saccheri, F.; Mignot, G.; Yamazaki, T.; Daillère, R.; Hannani, D.; Enot, D.P.; Pfirschke, C.; Engblom, C.; Pittet, M.J.; et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 2013, 342, 971–976. [Google Scholar] [CrossRef]
- Daillère, R.; Vétizou, M.; Waldschmitt, N.; Yamazaki, T.; Isnard, C.; Poirier-Colame, V.; Duong, C.P.M.; Flament, C.; Lepage, P.; Roberti, M.P.; et al. Enterococcus hirae and Barnesiella intestinihominis Facilitate Cyclophosphamide-Induced Therapeutic Immunomodulatory Effects. Immunity 2016, 45, 931–943. [Google Scholar] [CrossRef]
- Gori, S.; Inno, A.; Belluomini, L.; Bocus, P.; Bisoffi, Z.; Russo, A.; Arcaro, G. Gut microbiota and cancer: How gut microbiota modulates activity, efficacy and toxicity of antitumoral therapy. Crit. Rev. Oncol. Hematol. 2019, 143, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Geller, L.T.; Barzily-Rokni, M.; Danino, T.; Jonas, O.H.; Shental, N.; Nejman, D.; Gavert, N.; Zwang, Y.; Cooper, Z.A.; Shee, K.; et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 2017, 357, 1156–1160. [Google Scholar] [CrossRef]
- Vande Voorde, J.; Sabuncuoğlu, S.; Noppen, S.; Hofer, A.; Ranjbarian, F.; Fieuws, S.; Balzarini, J.; Liekens, S. Nucleoside-catabolizing enzymes in mycoplasma-infected tumor cell cultures compromise the cytostatic activity of the anticancer drug gemcitabine. J. Biol. Chem. 2014, 289, 13054–13065. [Google Scholar] [CrossRef]
- Csendes, D.; Gutlapalli, S.D.; Prakash, K.; Swarnakari, K.M.; Bai, M.; Manoharan, M.P.; Raja, R.; Jamil, A.; Desai, A.; Desai, D.M.; et al. Gastrointestinal Microbiota and Breast Cancer Chemotherapy Interactions: A Systematic Review. Cureus 2022, 14, e31648. [Google Scholar] [CrossRef] [PubMed]
- Bawaneh, A.; Wilson, A.S.; Levi, N.; Howard-McNatt, M.M.; Chiba, A.; Soto-Pantoja, D.R.; Cook, K.L. Intestinal Microbiota Influence Doxorubicin Responsiveness in Triple-Negative Breast Cancer. Cancers 2022, 14, 4849. [Google Scholar] [CrossRef] [PubMed]
- Bronckaers, A.; Balzarini, J.; Liekens, S. The cytostatic activity of pyrimidine nucleosides is strongly modulated by Mycoplasma hyorhinis infection: Implications for cancer therapy. Biochem. Pharmacol. 2008, 76, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Yazdi, M.H.; Mahdavi, M.; Setayesh, N.; Esfandyar, M.; Shahverdi, A.R. Selenium nanoparticle-enriched Lactobacillus brevis causes more efficient immune responses in vivo and reduces the liver metastasis in metastatic form of mouse breast cancer. DARU J. Pharm. Sci. 2013, 21, 33. [Google Scholar] [CrossRef]
- Kurita, A.; Kado, S.; Matsumoto, T.; Asakawa, N.; Kaneda, N.; Kato, I.; Uchida, K.; Onoue, M.; Yokokura, T. Streptomycin alleviates irinotecan-induced delayed-onset diarrhea in rats by a mechanism other than inhibition of β-glucuronidase activity in intestinal lumen. Cancer Chemother. Pharmacol. 2011, 67, 201–213. [Google Scholar] [CrossRef]
- Wallace, B.D.; Wang, H.; Lane, K.T.; Scott, J.E.; Orans, J.; Koo, J.S.; Venkatesh, M.; Jobin, C.; Yeh, L.A.; Mani, S.; et al. Alleviating cancer drug toxicity by inhibiting a bacterial enzyme. Science 2010, 330, 831–835. [Google Scholar] [CrossRef]
- Montassier, E.; Gastinne, T.; Vangay, P.; Al-Ghalith, G.A.; Bruley des Varannes, S.; Massart, S.; Moreau, P.; Potel, G.; de La Cochetière, M.F.; Batard, E.; et al. Chemotherapy-driven dysbiosis in the intestinal microbiome. Aliment. Pharmacol. Ther. 2015, 42, 515–528. [Google Scholar] [CrossRef]
- Fijlstra, M.; Ferdous, M.; Koning, A.M.; Rings, E.H.; Harmsen, H.J.; Tissing, W.J. Substantial decreases in the number and diversity of microbiota during chemotherapy-induced gastrointestinal mucositis in a rat model. Support. Care Cancer 2015, 23, 1513–1522. [Google Scholar] [CrossRef]
- Vétizou, M.; Pitt, J.M.; Daillère, R.; Lepage, P.; Waldschmitt, N.; Flament, C.; Rusakiewicz, S.; Routy, B.; Roberti, M.P.; Duong, C.P.; et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015, 350, 1079–1084. [Google Scholar] [CrossRef]
- Dubin, K.; Callahan, M.K.; Ren, B.; Khanin, R.; Viale, A.; Ling, L.; No, D.; Gobourne, A.; Littmann, E.; Huttenhower, C.; et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat. Commun. 2016, 7, 10391. [Google Scholar] [CrossRef]
- Berman, D.; Parker, S.M.; Siegel, J.; Chasalow, S.D.; Weber, J.; Galbraith, S.; Targan, S.R. Wang HL. Blockade of cytotoxic T-lymphocyte antigen-4 by ipilimumab results in dysregulation of gastrointestinal immunity in patients with advanced melanoma. Cancer Immun. 2010, 10, 11. [Google Scholar]
- Davar, D.; Dzutsev, A.K.; McCulloch, J.A.; Rodrigues, R.R.; Chauvin, J.M.; Morrison, R.M.; Deblasio, R.N.; Menna, C.; Ding, Q.; Pagliano, O.; et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science 2021, 371, 595–602. [Google Scholar] [CrossRef]
- Sánchez-Alcoholado, L.; Laborda-Illanes, A.; Otero, A.; Ordóñez, R.; González-González, A.; Plaza-Andrades, I.; Ramos-Molina, B.; Gómez-Millán, J.; Queipo-Ortuño, M.I. Relationships of Gut Microbiota Composition, Short-Chain Fatty Acids and Polyamines with the Pathological Response to Neoadjuvant Radiochemotherapy in Colorectal Cancer Patients. Int. J. Mol. Sci. 2021, 22, 9549. [Google Scholar] [CrossRef]
- Geng, H.W.; Yin, F.Y.; Zhang, Z.F.; Gong, X.; Yang, Y. Butyrate Suppresses Glucose Metabolism of Colorectal Cancer Cells via GPR109a-AKT Signaling Pathway and Enhances Chemotherapy. Front. Mol. Biosci. 2021, 8, 634874. [Google Scholar] [CrossRef] [PubMed]
- Wastyk, H.C.; Fragiadakis, G.K.; Perelman, D.; Dahan, D.; Merrill, B.D.; Yu, F.B.; Topf, M.; Gonzalez, C.G.; Van Treuren, W.; Han, S.; et al. Gut-microbiota-targeted diets modulate human immune status. Cell 2021, 184, 4137–4153.e14. [Google Scholar] [CrossRef]
- Collins, N.; Han, S.J.; Enamorado, M.; Link, V.M.; Huang, B.; Moseman, E.A.; Kishton, R.J.; Shannon, J.P.; Dixit, D.; Schwab, S.R.; et al. The Bone Marrow Protects and Optimizes Immunological Memory during Dietary Restriction. Cell 2019, 178, 1088–1101.e15. [Google Scholar] [CrossRef] [PubMed]
- Vernieri, C.; Fucà, G.; Ligorio, F.; Huber, V.; Vingiani, A.; Iannelli, F.; Raimondi, A.; Rinchai, D.; Frigè, G.; Belfiore, A.; et al. Fasting-Mimicking Diet Is Safe and Reshapes Metabolism and Antitumor Immunity in Patients with Cancer. Cancer Discov. 2022, 12, 90–107. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, B.; Wei, Y.; Kuang, D.M. Influence of gut and intratumoral microbiota on the immune microenvironment and anti-cancer therapy. Pharmacol. Res. 2021, 174, 105966. [Google Scholar] [CrossRef]
- Lu, Y.; Yuan, X.; Wang, M.; He, Z.; Li, H.; Wang, J.; Li, Q. Gut microbiota influence immunotherapy responses: Mechanisms and therapeutic strategies. J. Hematol. Oncol. 2022, 15, 47. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Xu, C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020, 30, 660–669. [Google Scholar] [CrossRef]
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Matson, V.; Fessler, J.; Bao, R.; Chongsuwat, T.; Zha, Y.; Alegre, M.L.; Luke, J.J.; Gajewski, T.F. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 2018, 359, 104–108. [Google Scholar] [CrossRef]
- Baruch, E.N.; Youngster, I.; Ben-Betzalel, G.; Ortenberg, R.; Lahat, A.; Katz, L.; Adler, K.; Dick-Necula, D.; Raskin, S.; Bloch, N.; et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science 2021, 371, 602–609. [Google Scholar] [CrossRef]
- McCulloch, J.A.; Davar, D.; Rodrigues, R.R.; Badger, J.H.; Fang, J.R.; Cole, A.M.; Balaji, A.K.; Vetizou, M.; Prescott, S.M.; Fernandes, M.R.; et al. Intestinal microbiota signatures of clinical response and immune-related adverse events in melanoma patients treated with anti-PD-1. Nat. Med. 2022, 28, 545–556. [Google Scholar] [CrossRef]
- Luu, M.; Riester, Z.; Baldrich, A.; Reichardt, N.; Yuille, S.; Busetti, A.; Klein, M.; Wempe, A.; Leister, H.; Raifer, H.; et al. Microbial short-chain fatty acids modulate CD8+ T cell responses and improve adoptive immunotherapy for cancer. Nat. Commun. 2021, 12, 4077. [Google Scholar] [CrossRef]
- He, Y.; Fu, L.; Li, Y.; Wang, W.; Gong, M.; Zhang, J.; Dong, X.; Huang, J.; Wang, Q.; Mackay, C.R.; et al. Gut microbial metabolites facilitate anticancer therapy efficacy by modulating cytotoxic CD8+ T cell immunity. Cell Metab. 2021, 33, 988–1000.e7. [Google Scholar] [CrossRef]
- Spencer, C.N.; McQuade, J.L.; Gopalakrishnan, V.; McCulloch, J.A.; Vetizou, M.; Cogdill, A.P.; Khan, M.A.W.; Zhang, X.; White, M.G.; Peterson, C.B.; et al. Dietary fiber and probiotics influence the gut microbiome and melanoma immunotherapy response. Science 2021, 374, 1632–1640. [Google Scholar] [CrossRef]
- Li, Z.; Ke, X.; Zuo, D.; Wang, Z.; Fang, F.; Li, B. New Insights into the Relationship between Gut Microbiota and Radiotherapy for Cancer. Nutrients 2022, 15, 48. [Google Scholar] [CrossRef] [PubMed]
- Shiao, S.L.; Kershaw, K.M.; Limon, J.J.; You, S.; Yoon, J.; Ko, E.Y.; Guarnerio, J.; Potdar, A.A.; McGovern, D.P.B.; Bose, S.; et al. Commensal bacteria and fungi differentially regulate tumor responses to radiation therapy. Cancer Cell 2021, 39, 1202–1213.e6. [Google Scholar] [CrossRef] [PubMed]
- Crawford, P.A.; Gordon, J.I. Microbial regulation of intestinal radiosensitivity. Proc. Natl. Acad. Sci. USA 2005, 102, 13254–13259. [Google Scholar] [CrossRef] [PubMed]
- Delia, P.; Sansotta, G.; Donato, V.; Frosina, P.; Messina, G.; De Renzis, C.; Famularo, G. Use of probiotics for prevention of radiation-induced diarrhea. World J. Gastroenterol. 2007, 13, 912–915. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Yu, T.; Huang, X.; Bilotta, A.J.; Xu, L.; Lu, Y.; Sun, J.; Pan, F.; Zhou, J.; Zhang, W.; et al. Intestinal microbiota-derived short-chain fatty acids regulation of immune cell IL-22 production and gut immunity. Nat. Commun. 2020, 11, 4457. [Google Scholar] [CrossRef]
- Biazzo, M.; Deidda, G. Fecal Microbiota Transplantation as New Therapeutic Avenue for Human Diseases. J. Clin. Med. 2022, 11, 4119. [Google Scholar] [CrossRef]
- Wardill, H.R.; van der Aa, S.A.R.; da Silva Ferreira, A.R.; Havinga, R.; Tissing, W.J.E.; Harmsen, H.J.M. Antibiotic-induced disruption of the microbiome exacerbates chemotherapy-induced diarrhoea and can be mitigated with autologous faecal microbiota transplantation. Eur. J. Cancer 2021, 153, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Santana, A.B.; Souto, B.S.; Santos, N.C.M.; Pereira, J.A.; Tagliati, C.A.; Novaes, R.D.; Corsetti, P.P.; de Almeida, L.A. Murine response to the opportunistic bacterium Pseudomonas aeruginosa infection in gut dysbiosis caused by 5-fluorouracil chemotherapy-induced mucositis. Life Sci. 2022, 307, 120890. [Google Scholar] [CrossRef]
- Taur, Y.; Jenq, R.R.; Ubeda, C.; van den Brink, M.; Pamer, E.G. Role of intestinal microbiota in transplantation outcomes. Best. Pract. Res. Clin. Haematol. 2015, 28, 155–161. [Google Scholar] [CrossRef]
- Peled, J.U.; Gomes, A.L.C.; Devlin, S.M.; Littmann, E.R.; Taur, Y.; Sung, A.D.; Weber, D.; Hashimoto, D.; Slingerland, A.E.; Slingerland, J.B.; et al. Microbiota as Predictor of Mortality in Allogeneic Hematopoietic-Cell Transplantation. N. Engl. J. Med. 2020, 382, 822–834. [Google Scholar] [CrossRef]
- Jenq, R.R.; Taur, Y.; Devlin, S.M.; Ponce, D.M.; Goldberg, J.D.; Ahr, K.F.; Littmann, E.R.; Ling, L.; Gobourne, A.C.; Miller, L.C.; et al. Intestinal Blautia Is Associated with Reduced Death from Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2015, 21, 1373–1383. [Google Scholar] [CrossRef] [PubMed]
- Rolling, T.; Zhai, B.; Gjonbalaj, M.; Tosini, N.; Yasuma-Mitobe, K.; Fontana, E.; Amoretti, L.A.; Wright, R.J.; Ponce, D.M.; Perales, M.A.; et al. Haematopoietic cell transplantation outcomes are linked to intestinal mycobiota dynamics and an expansion of Candida parapsilosis complex species. Nat. Microbiol. 2021, 6, 1505–1515. [Google Scholar] [CrossRef]
- Schluter, J.; Peled, J.U.; Taylor, B.P.; Markey, K.A.; Smith, M.; Taur, Y.; Niehus, R.; Staffas, A.; Dai, A.; Fontana, E.; et al. The gut microbiota is associated with immune cell dynamics in humans. Nature 2020, 588, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Jochems, S.H.J.; Van Osch, F.H.M.; Bryan, R.T.; Wesselius, A.; van Schooten, F.J.; Cheng, K.K.; Zeegers, M.P. Impact of dietary patterns and the main food groups on mortality and recurrence in cancer survivors: A systematic review of current epidemiological literature. BMJ Open 2018, 8, e014530. [Google Scholar] [CrossRef] [PubMed]
- Castro-Espin, C.; Agudo, A. The Role of Diet in Prognosis among Cancer Survivors: A Systematic Review and Meta-Analysis of Dietary Patterns and Diet Interventions. Nutrients 2022, 14, 348. [Google Scholar] [CrossRef]
- Becerra-Tomás, N.; Balducci, K.; Abar, L.; Aune, D.; Cariolou, M.; Greenwood, D.C.; Markozannes, G.; Nanu, N.; Vieira, R.; Giovannucci, E.L.; et al. Postdiagnosis dietary factors, supplement use and breast cancer prognosis: Global Cancer Update Programme (CUP Global) systematic literature review and meta-analysis. Int. J. Cancer 2023, 152, 616–634. [Google Scholar] [CrossRef]
- Lee, E.; Kady, V.; Han, E.; Montan, K.; Normuminova, M.; Rovito, M.J. Healthy Eating and Mortality among Breast Cancer Survivors: A Systematic Review and Meta-Analysis of Cohort Studies. Int. J. Environ. Res. Public Health 2022, 19, 7579. [Google Scholar] [CrossRef]
- Trauchburg, A.; Schwingshackl, L.; Hoffmann, G. Association between Dietary Indices and Dietary Patterns and Mortality and Cancer Recurrence among Cancer Survivors: An Updated Systematic Review and Meta-Analysis of Cohort Studies. Nutrients 2023, 15, 3151. [Google Scholar] [CrossRef]
- Spei, M.E.; Bellos, I.; Samoli, E.; Benetou, V. Post-Diagnosis Dietary Patterns among Cancer Survivors in Relation to All-Cause Mortality and Cancer-Specific Mortality: A Systematic Review and Meta-Analysis of Cohort Studies. Nutrients 2023, 15, 3860. [Google Scholar] [CrossRef]
- Tsilidis, K.K.; Markozannes, G.; Becerra-Tomás, N.; Cariolou, M.; Balducci, K.; Vieira, R.; Kiss, S.; Aune, D.; Greenwood, D.C.; Dossus, L.; et al. Post-diagnosis adiposity, physical activity, sedentary behaviour, dietary factors, supplement use and colorectal cancer prognosis: Global Cancer Update Programme (CUP Global) summary of evidence grading. Int. J. Cancer 2024, 155, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.Y.; Keum, N.; Giovannucci, E.L. Post-diagnosis dietary and lifestyle factors and mortality outcomes among colorectal cancer patients: A meta-analysis. J. Natl. Cancer Inst. 2025, 2, djaf098. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.T.; Kashambwa, R.; Sato, K.; Chiuve, S.E.; Fuchs, C.S.; Wu, K.; Giovannucci, E.; Ogino, S.; Hu, F.B.; Meyerhardt, J.A. Post diagnosis diet quality and colorectal cancer survival in women. PLoS ONE 2014, 9, e115377. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wu, H.; Wang, P.P.; Savas, S.; Woodrow, J.; Wish, T.; Jin, R.; Green, R.; Woods, M.; Roebothan, B.; et al. Dietary patterns and colorectal cancer recurrence and survival: A cohort study. BMJ Open 2013, 3, e002270. [Google Scholar] [CrossRef]
- Van Blarigan, E.L.; Fuchs, C.S.; Niedzwiecki, D.; Zhang, S.; Saltz, L.B.; Mayer, R.J.; Mowat, R.B.; Whittom, R.; Hantel, A.; Benson, A.; et al. Association of Survival With Adherence to the American Cancer Society Nutrition and Physical Activity Guidelines for Cancer Survivors After Colon Cancer Diagnosis: The CALGB 89803/Alliance Trial. JAMA Oncol. 2018, 4, 783–790. [Google Scholar] [CrossRef]
- Guinter, M.A.; McCullough, M.L.; Gapstur, S.M.; Campbell, P.T. Associations of Pre- and Postdiagnosis Diet Quality With Risk of Mortality Among Men and Women With Colorectal Cancer. J. Clin. Oncol. 2018, 36, JCO1800714. [Google Scholar] [CrossRef]
- Park, S.Y.; Kang, M.; Shvetsov, Y.B.; Setiawan, V.W.; Boushey, C.J.; Haiman, C.A.; Wilkens, L.R.; Le Marchand, L. Diet quality and all-cause and cancer-specific mortality in cancer survivors and non-cancer individuals: The Multiethnic Cohort Study. Eur. J. Nutr. 2022, 61, 925–933. [Google Scholar] [CrossRef]
- Rock, C.L.; Thomson, C.A.; Sullivan, K.R.; Howe, C.L.; Kushi, L.H.; Caan, B.J.; Neuhouser, M.L.; Bandera, E.V.; Wang, Y.; Robien, K.; et al. American Cancer Society nutrition and physical activity guideline for cancer survivors. CA Cancer J. Clin. 2022, 72, 230–262. [Google Scholar] [CrossRef]
- Castro-Espin, C.; Bonet, C.; Crous-Bou, M.; Nadal-Zaragoza, N.; Tjønneland, A.; Mellemkjær, L.; Hajji-Louati, M.; Truong, T.; Katzke, V.; Le Cornet, C.; et al. Association of Mediterranean diet with survival after breast cancer diagnosis in women from nine European countries: Results from the EPIC cohort study. BMC Med. 2023, 21, 225. [Google Scholar] [CrossRef]
- World Cancer Research Fund/American Institute for Cancer Research. Diet, Nutrition, Physical Activity and Cancer: A Global Perspective; Continuous Update Project Expert Report: London, UK, 2018. [Google Scholar]
- Kyaw, T.S.; Upadhyay, V.; Tolstykh, I.; Van Loon, K.; Laffan, A.; Stanfield, D.; Gempis, D.; Kenfield, S.A.; Chan, J.M.; Piawah, S.; et al. Variety of Fruit and Vegetables and Alcohol Intake are Associated with Gut Microbial Species and Gene Abundance in Colorectal Cancer Survivors. Am. J. Clin. Nutr. 2023, 118, 518–529. [Google Scholar] [CrossRef]
- Li, Y.; Huang, Y.; Liang, H.; Wang, W.; Li, B.; Liu, T.; Huang, Y.; Zhang, Z.; Qin, Y.; Zhou, X.; et al. The roles and applications of short-chain fatty acids derived from microbial fermentation of dietary fibers in human cancer. Front. Nutr. 2023, 10, 1243390. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Wu, K.; Meyerhardt, J.A.; Ogino, S.; Wang, M.; Fuchs, C.S.; Giovannucci, E.L.; Chan, A.T. Fiber Intake and Survival After Colorectal Cancer Diagnosis. JAMA Oncol. 2018, 4, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Byrd, D.A.; Damerell, V.; Gomez Morales, M.F.; Hogue, S.R.; Lin, T.; Ose, J.; Himbert, C.; Ilozumba, M.N.; Kahlert, C.; Shibata, D.; et al. The gut microbiome is associated with disease-free survival in stage I-III colorectal cancer patients. Int. J. Cancer 2025, 157, 64–73. [Google Scholar] [CrossRef]
- An, J.; Kim, B.S.; Yoon, H.J. Combination of gut microbiota, proinflammatory cytokine, and 18F-FDG PET as potential indicators for predicting breast cancer recurrence. Sci. Rep. 2025, 15, 8313. [Google Scholar] [CrossRef]
- Caputo, M.; Pigni, S.; Antoniotti, V.; Agosti, E.; Caramaschi, A.; Antonioli, A.; Aimaretti, G.; Manfredi, M.; Bona, E.; Prodam, F. Targeting microbiota in dietary obesity management: A systematic review on randomized control trials in adults. Crit. Rev. Food Sci. Nutr. 2023, 63, 11449–11481. [Google Scholar] [CrossRef]
- Cava, E.; Marzullo, P.; Farinelli, D.; Gennari, A.; Saggia, C.; Riso, S.; Prodam, F. Breast Cancer Diet “BCD”: A Review of Healthy Dietary Patterns to Prevent Breast Cancer Recurrence and Reduce Mortality. Nutrients 2022, 14, 476. [Google Scholar] [CrossRef]
- Zarei, I.; Baxter, B.A.; Oppel, R.C.; Borresen, E.C.; Brown, R.J.; Ryan, E.P. Plasma and Urine Metabolite Profiles Impacted by Increased Dietary Navy Bean Intake in Colorectal Cancer Survivors: A Randomized-Controlled Trial. Cancer Prev. Res. 2021, 14, 497–508. [Google Scholar] [CrossRef]
- Baxter, B.A.; Oppel, R.C.; Ryan, E.P. Navy Beans Impact the Stool Metabolome and Metabolic Pathways for Colon Health in Cancer Survivors. Nutrients 2018, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Sheflin, A.M.; Borresen, E.C.; Kirkwood, J.S.; Boot, C.M.; Whitney, A.K.; Lu, S.; Brown, R.J.; Broeckling, C.D.; Ryan, E.P.; Weir, T.L. Dietary supplementation with rice bran or navy bean alters gut bacterial metabolism in colorectal cancer survivors. Mol. Nutr. Food Res. 2017, 61, 1500905. [Google Scholar] [CrossRef]
- Weber, A.M.; Ibrahim, H.; Baxter, B.A.; Kumar, R.; Maurya, A.K.; Kumar, D.; Agarwal, R.; Raina, K.; Ryan, E.P. Integrated Microbiota and Metabolite Changes following Rice Bran Intake during Murine Inflammatory Colitis-Associated Colon Cancer and in Colorectal Cancer Survivors. Cancers 2023, 15, 2231. [Google Scholar] [CrossRef]
- Brown, D.G.; Borresen, E.C.; Brown, R.J.; Ryan, E.P. Heat-stabilised rice bran consumption by colorectal cancer survivors modulates stool metabolite profiles and metabolic networks: A randomised controlled trial. Br. J. Nutr. 2017, 117, 1244–1256. [Google Scholar] [CrossRef] [PubMed]
- Kyrø, C.; Frederiksen, K.; Holm, M.; Nørskov, N.P.; Knudsen, K.E.B.; Overvad, K.; Tjønneland, A.; Olsen, A. Prediagnosis plasma concentrations of enterolactone and survival after colorectal cancer: The Danish Diet, Cancer and Health cohort. Br. J. Nutr. 2019, 122, 552–563. [Google Scholar] [CrossRef]
- Song, M.; Zhang, X.; Meyerhardt, J.A.; Giovannucci, E.L.; Ogino, S.; Fuchs, C.S.; Chan, A.T. Marine ω-3 polyunsaturated fatty acid intake and survival after colorectal cancer diagnosis. Gut 2017, 66, 1790–1796. [Google Scholar] [CrossRef] [PubMed]
- Horigome, A.; Okubo, R.; Hamazaki, K.; Kinoshita, T.; Katsumata, N.; Uezono, Y.; Xiao, J.Z.; Matsuoka, Y.J. Association between blood omega-3 polyunsaturated fatty acids and the gut microbiota among breast cancer survivors. Benef. Microbes 2019, 10, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Mailing, L.J.; Allen, J.M.; Buford, T.W.; Fields, C.J.; Woods, J.A. Exercise and the Gut Microbiome: A Review of the Evidence, Potential Mechanisms, and Implications for Human Health. Exerc. Sport Sci. Rev. 2019, 47, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Aya, V.; Flórez, A.; Perez, L.; Ramírez, J.D. Association between physical activity and changes in intestinal microbiota composition: A systematic review. PLoS ONE 2021, 16, e0247039. [Google Scholar] [CrossRef]
- Donati Zeppa, S.; Natalucci, V.; Agostini, D.; Vallorani, L.; Amatori, S.; Sisti, D.; Rocchi, M.B.L.; Pazienza, V.; Perri, F.; Villani, A.; et al. Changes in gut microbiota composition after 12 weeks of a home-based lifestyle intervention in breast cancer survivors during the COVID-19 lockdown. Front. Oncol. 2023, 13, 1225645. [Google Scholar] [CrossRef]
- Freedland, S.J.; Allen, J.; Jarman, A.; Oyekunle, T.; Armstrong, A.J.; Moul, J.W.; Sandler, H.M.; Posadas, E.; Levin, D.; Wiggins, E.; et al. A Randomized Controlled Trial of a 6-Month Low-Carbohydrate Intervention on Disease Progression in Men with Recurrent Prostate Cancer: Carbohydrate and Prostate Study 2 (CAPS2). Clin. Cancer Res. 2020, 26, 3035–3043. [Google Scholar] [CrossRef]
- Kämmerer, U.; Klement, R.J.; Joos, F.T.; Sütterlin, M.; Reuss-Borst, M. Low carb and ketogenic diets increase quality of life, physical performance, body composition, and metabolic health of women with breast cancer. Nutrients 2021, 13, 1029. [Google Scholar] [CrossRef]
- Gabel, K.; Cares, K.; Varady, K.; Gadi, V.; Tussing-Humphreys, L. Current Evidence and Directions for Intermittent Fasting During Cancer Chemotherapy. Adv. Nutr. 2022, 13, 667–680. [Google Scholar] [CrossRef]
- Yang, H.; Zingaro, V.A.; Lincoff, J.; Tom, H.; Oikawa, S.; Oses-Prieto, J.A.; Edmondson, Q.; Seiple, I.; Shah, H.; Kajimura, S.; et al. Remodelling of the translatome controls diet and its impact on tumorigenesis. Nature 2024, 633, 189–197. [Google Scholar] [CrossRef]
- Ergas, I.J.; Cheng, R.K.; Roh, J.M.; Kresovich, J.K.; Iribarren, C.; Nguyen-Huynh, M.; Rana, J.S.; Rillamas-Sun, E.; Laurent, C.A.; Lee, V.S.; et al. Diet quality and cardiometabolic health in breast cancer survivors: The Pathways Study. Breast Cancer Res. Treat. 2025, 211, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Ergas, I.J.; Cheng, R.K.; Roh, J.M.; Kushi, L.H.; Kresovich, J.K.; Iribarren, C.; Nguyen-Huynh, M.; Rana, J.S.; Rillamas-Sun, E.; Laurent, C.A.; et al. Diet quality and cardiovascular disease risk among breast cancer survivors in the Pathways Study. JNCI Cancer Spectr. 2024, 8, pkae013. [Google Scholar] [CrossRef]
- Bonaccio, M.; Di Castelnuovo, A.; Costanzo, S.; Ruggiero, E.; Esposito, S.; Panzera, T.; Cerletti, C.; Donati, M.B.; de Gaetano, G.; Iacoviello, L. Mediterranean Diet Is Associated With Lower All-Cause and Cardiovascular Mortality Among Long-Term Cancer Survivors. JACC Cardio Oncol. 2024, 6, 602–604. [Google Scholar] [CrossRef]
- Chiavaroli, L.; Viguiliouk, E.; Nishi, S.K.; Blanco Mejia, S.; Rahelić, D.; Kahleová, H.; Salas-Salvadó, J.; Kendall, C.W.; Sievenpiper, J.L. DASH Dietary Pattern and Cardiometabolic Outcomes: An Umbrella Review of Systematic Reviews and Meta-Analyses. Nutrients 2019, 11, 338. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; Gea, A.; Ruiz-Canela, M. The Mediterranean Diet and Cardiovascular Health. Circ. Res. 2019, 124, 779–798. [Google Scholar] [CrossRef]
- Shan, Z.; Li, Y.; Baden, M.Y.; Bhupathiraju, S.N.; Wang, D.D.; Sun, Q.; Rexrode, K.M.; Rimm, E.B.; Qi, L.; Willett, W.C.; et al. Association Between Healthy Eating Patterns and Risk of Cardiovascular Disease. JAMA Intern. Med. 2020, 180, 1090–1100. [Google Scholar] [CrossRef]
- Pellegrini, M.; Ippolito, M.; Monge, T.; Violi, R.; Cappello, P.; Ferrocino, I.; Cocolin, L.S.; De Francesco, A.; Bo, S.; Finocchiaro, C. Gut microbiota composition after diet and probiotics in overweight breast cancer survivors: A randomized open-label pilot intervention trial. Nutrition 2020, 74, 110749. [Google Scholar] [CrossRef]
- Deleemans, J.M.; Chleilat, F.; Reimer, R.A.; Lawal, O.A.; Baydoun, M.; Piedalue, K.A.; Lowry, D.E.; Carlson, L.E. Associations Between Health Behaviors, Gastrointestinal Symptoms, and Gut Microbiota in a Cross-Sectional Sample of Cancer Survivors: Secondary Analysis from the Chemo-Gut Study. Integr. Cancer Ther. 2024, 23, 15347354241240141. [Google Scholar] [CrossRef] [PubMed]
- Inglis, J.E.; Lin, P.J.; Kerns, S.L.; Kleckner, I.R.; Kleckner, A.S.; Castillo, D.A.; Mustian, K.M.; Peppone, L.J. Nutritional Interventions for Treating Cancer-Related Fatigue: A Qualitative Review. Nutr. Cancer 2019, 71, 21–40. [Google Scholar] [CrossRef]
- Ciernikova, S.; Sevcikova, A.; Drgona, L.; Mego, M. Modulating the gut microbiota by probiotics, prebiotics, postbiotics, and fecal microbiota transplantation: An emerging trend in cancer patient care. Biochim. Biophys. Acta Rev. Cancer 2023, 1878, 188990. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Fedirko, V.; Beitler, J.; Bai, J.; Peng, G.; Zhou, C.; Gu, J.; Zhao, H.; Lin, I.H.; Chico, C.E.; et al. The role of the gut microbiome in cancer-related fatigue: Pilot study on epigenetic mechanisms. Support. Care Cancer 2021, 29, 3173–3182. [Google Scholar] [CrossRef]
- Kurz, K.; Fiegl, M.; Holzner, B.; Giesinger, J.; Pircher, M.; Weiss, G.; Denz, H.A.; Fuchs, D. Fatigue in patients with lung cancer is related with accelerated tryptophan breakdown. PLoS ONE 2012, 7, e36956. [Google Scholar] [CrossRef]
- Guillemin, G.J.; Fosså, A.; Smeland, K.H.; Fluge, Ø.; Tronstad, K.J.; Loge, J.H.; Midttun, Ø.; Ueland, P.M.; Kiserud, C.E. Metabolic analysis of amino acids and vitmin B6 pathways in lymphoma survivors with cancer related chronic fatigue. PLoS ONE 2020, 15, e0227384. [Google Scholar]
- Lu, Y.; Yuan, H.; Li, Y.; Liu, Y.; Li, R.; Diao, Y.; Chen, J.; Jia, L.; Dong, X.; Xue, H.; et al. Effects of nutritional interventions on cognitive function in adult cancer survivors: A systematic review. J. Clin. Nurs. 2024, 33, 4227–4253. [Google Scholar] [CrossRef] [PubMed]
- Crowder, S.L.; Gudenkauf, L.M.; Hoogland, A.I.; Han, H.S.; Small, B.J.; Carson, T.L.; Parker, N.H.; Booth-Jones, M.; Jim, H.S.L. Cancer-Related Cognitive Impairment and the Potential of Dietary Interventions for the Prevention and Mitigation of Neurodegeneration. Cancer Res. 2025, 85, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Song, B.C.; Bai, J. Microbiome-gut-brain axis in cancer treatment-related psychoneurological toxicities and symptoms: A systematic review. Support. Care Cancer 2021, 29, 605–617. [Google Scholar] [CrossRef] [PubMed]
- Tümkaya Yılmaz, S.; Malfliet, A.; Elma, Ö.; Deliens, T.; Nijs, J.; Clarys, P.; De Groef, A.; Coppieters, I. Diet/Nutrition: Ready to Transition from a Cancer Recurrence/Prevention Strategy to a Chronic Pain Management Modality for Cancer Survivors? J. Clin. Med. 2022, 11, 653. [Google Scholar] [CrossRef]
- Okubo, R.; Chen, C.; Sekiguchi, M.; Hamazaki, K.; Matsuoka, Y.J. Mechanisms underlying the effects of n-3 polyunsaturated fatty acids on fear memory processing and their hypothetical effects on fear of cancer recurrence in cancer survivors. Prostaglandins Leukot. Essent. Fatty Acids. 2018, 131, 14–23. [Google Scholar] [CrossRef]
- Paulsen, J.A.; Ptacek, T.S.; Carter, S.J.; Liu, N.; Kumar, R.; Hyndman, L.; Lefkowitz, E.J.; Morrow, C.D.; Rogers, L.Q. Gut microbiota composition associated with alterations in cardiorespiratory fitness and psychosocial outcomes among breast cancer survivors. Support. Care Cancer 2017, 25, 1563–1570. [Google Scholar] [CrossRef]
- Marian, M.J. Dietary Supplements Commonly Used by Cancer Survivors: Are There Any Benefits? Nutr. Clin. Pract. 2017, 32, 607–627. [Google Scholar] [CrossRef]
- World Cancer Research Fund. Available online: https://www.wcrf.org/research-policy/evidence-for-our-recommendations/ (accessed on 10 July 2025).
- IARC. Fruit and Vegetables. IARC Handbooks of Cancer Prevention; IARC Publications: Lyon, France, 2003; Volume 8, ISBN 978-92-832-3008-3. [Google Scholar]
- Cava, E.; Spadaccini, D.; Aimaretti, G.; Marzullo, P.; Cavigiolo, B.; Farinelli, D.; Gennari, A.; Saggia, C.; Carbonelli, M.G.; Riso, S.; et al. Weight Management Effectiveness and Predictors of Dropout in Breast Cancer Survivors: A Retrospective Study. Cancers 2023, 15, 4401. [Google Scholar] [CrossRef]
- Eslami, M.; Naderian, R.; Bahar, A.; Babaeizad, A.; Rezanavaz Gheshlagh, S.; Oksenych, V.; Tahmasebi, H. Microbiota as diagnostic biomarkers: Advancing early cancer detection and personalized therapeutic approaches through microbiome profiling. Front. Immunol. 2025, 16, 1559480. [Google Scholar] [CrossRef] [PubMed]
- Murali, S.K.; Mansell, T.J. Next generation probiotics: Engineering live biotherapeutics. Biotechnol. Adv. 2024, 72, 108336. [Google Scholar] [CrossRef] [PubMed]
- Charbonneau, M.R.; Isabella, V.M.; Li, N.; Kurtz, C.B. Developing a new class of engineered live bacterial therapeutics to treat human diseases. Nat. Commun. 2020, 11, 1738. [Google Scholar] [CrossRef] [PubMed]
- Katkowska, M.; Garbacz, K.; Kusiak, A. Probiotics: Should All Patients Take Them? Microorganisms 2021, 9, 2620. [Google Scholar] [CrossRef]
- Gazzaniga, F.S.; Kasper, D.L. The gut microbiome and cancer response to immune checkpoint inhibitors. J. Clin. Investig. 2025, 135, e184321. [Google Scholar] [CrossRef]
- Canale, F.P.; Basso, C.; Antonini, G.; Perotti, M.; Li, N.; Sokolovska, A.; Neumann, J.; James, M.J.; Geiger, S.; Jin, W.; et al. Metabolic modulation of tumours with engineered bacteria for immunotherapy. Nature 2021, 598, 662–666. [Google Scholar] [CrossRef]
- Geiger, R.; Rieckmann, J.C.; Wolf, T.; Basso, C.; Feng, Y.; Fuhrer, T.; Kogadeeva, M.; Picotti, P.; Meissner, F.; Mann, M.; et al. L-Arginine Modulates T Cell Metabolism and Enhances Survival and Anti-tumor Activity. Cell 2016, 167, 829–842.e13. [Google Scholar] [CrossRef]
- Zhu, J.; Ke, Y.; Liu, Q.; Yang, J.; Liu, F.; Xu, R.; Zhou, H.; Chen, A.; Xiao, J.; Meng, F.; et al. Engineered Lactococcus lactis secreting Flt3L and OX40 ligand for in situ vaccination-based cancer immunotherapy. Nat. Commun. 2022, 13, 7466. [Google Scholar] [CrossRef]
- Tanoue, T.; Morita, S.; Plichta, D.R.; Skelly, A.N.; Suda, W.; Sugiura, Y.; Narushima, S.; Vlamakis, H.; Motoo, I.; Sugita, K.; et al. A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature 2019, 565, 600–605. [Google Scholar] [CrossRef]
- Park, J.S.; Gazzaniga, F.S.; Wu, M.; Luthens, A.K.; Gillis, J.; Zheng, W.; LaFleur, M.W.; Johnson, S.B.; Morad, G.; Park, E.M.; et al. Targeting PD-L2-RGMb overcomes microbiome-related immunotherapy resistance. Nature 2023, 617, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Bohm, M.S.; Joseph, S.C.; Sipe, L.M.; Kim, M.; Leathem, C.T.; Mims, T.S.; Willis, N.B.; Tanveer, U.A.; Elasy, J.H.; Grey, E.W.; et al. The gut microbiome enhances breast cancer immunotherapy following bariatric surgery. J. Clin. Investig. 2025, 10, e187683. [Google Scholar] [CrossRef] [PubMed]
- Porcari, S.; Mullish, B.H.; Asnicar, F.; Ng, S.C.; Zhao, L.; Hansen, R.; O’Toole, P.W.; Raes, J.; Hold, G.; Putignani, L.; et al. International consensus statement on microbiome testing in clinical practice. Lancet Gastroenterol. Hepatol. 2025, 10, 154–167. [Google Scholar] [CrossRef] [PubMed]

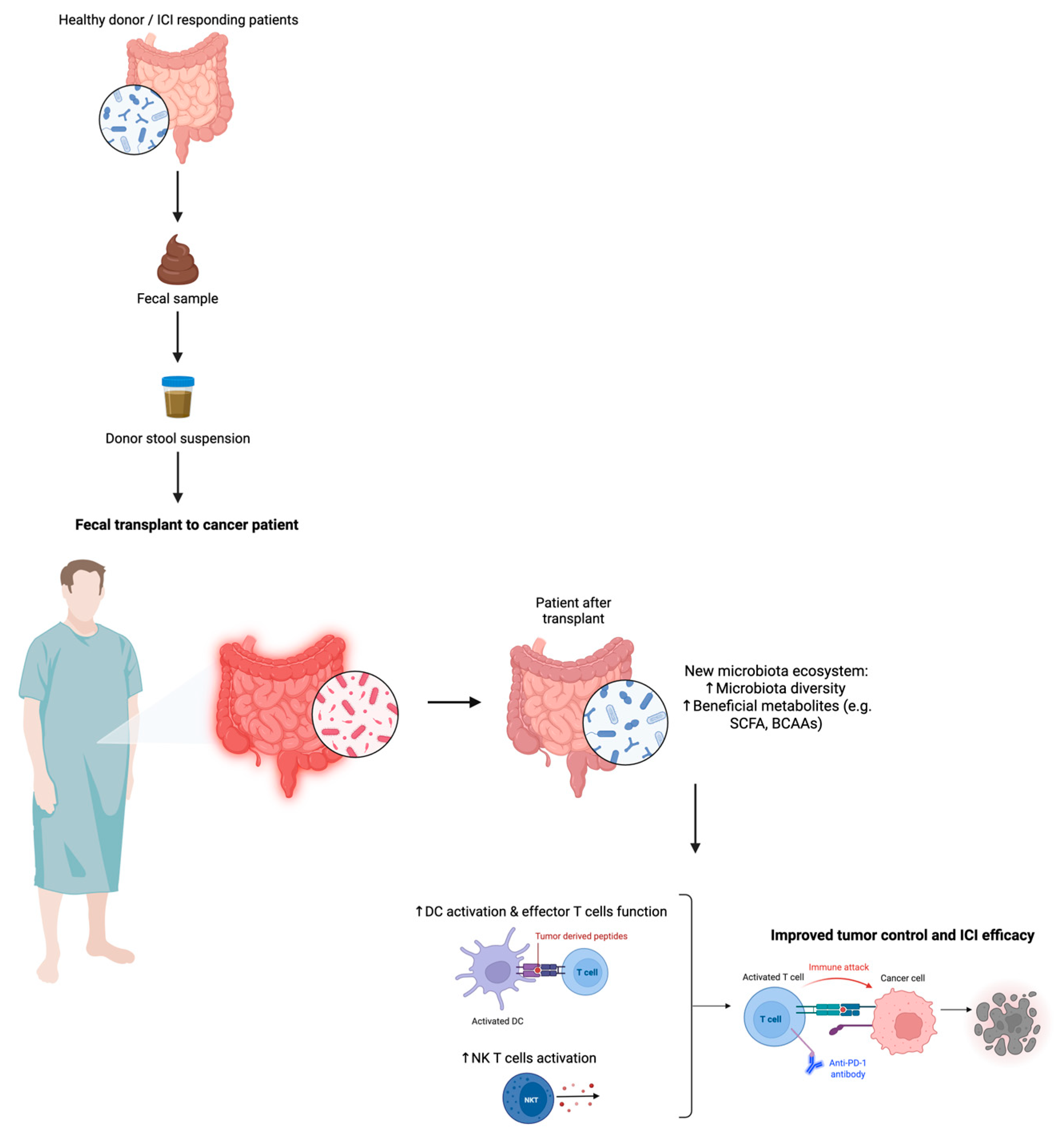

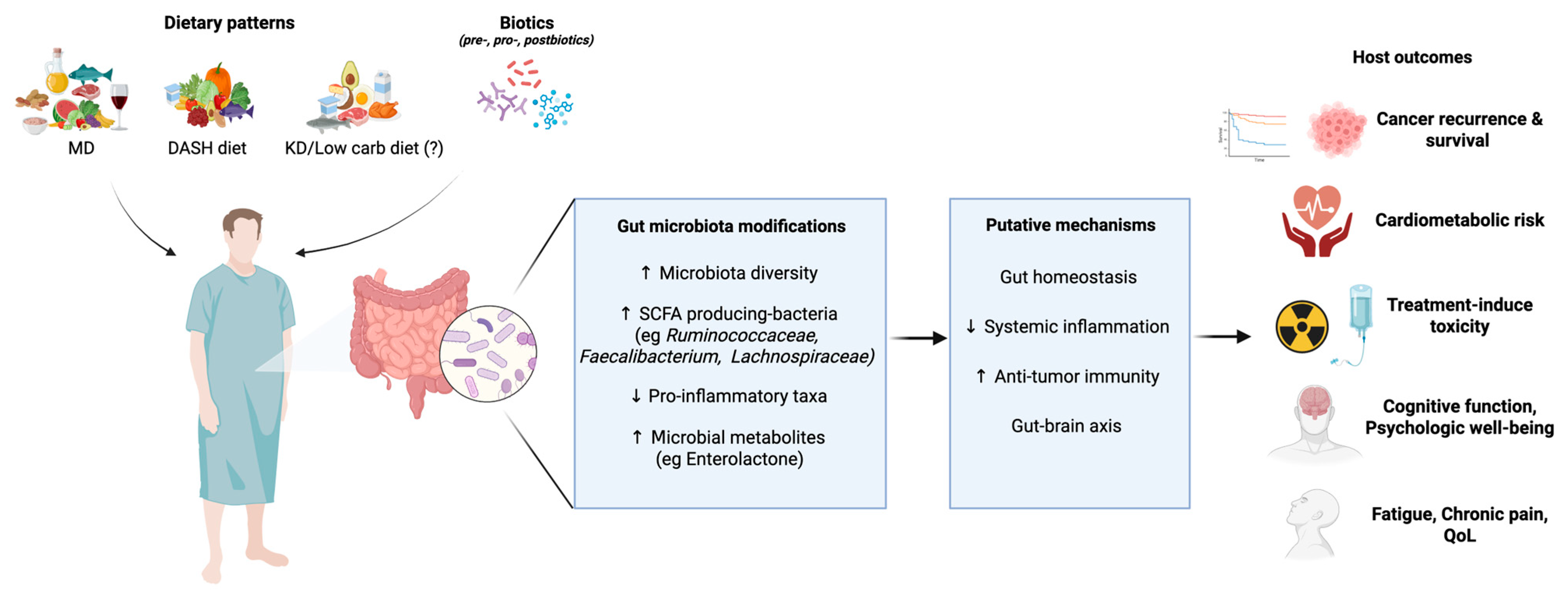
| Therapeutic Molecule | Mechanism | Bacteria | Gut Microbiota Effect on Anticancer Therapy | References |
|---|---|---|---|---|
| Chemotherapies | ||||
| Cyclophosphamide | Alkylating agent | Lactobacillus johnsonii, L. murinus, Enterococcus hirae, Barnesiella intestinihominis | ↑ therapeutic response via gut translocation → secondary lymphoid organs; ↑ Th1 and Th17 induction. | [188,189] |
| Cisplatin/Oxaliplatin | Platinum based agents | Alcaligenes, Lactobacillus, Alistipes | ↑ therapeutic response via TLR4 activation → ↑ ROS production mediated by myeloid cells | [186] |
| Gemcitabine | Nucleoside analogs | Mycoplasma hyorhinis | ↓ therapeutic response via enzymatic drug degradation → ↓ cytostatic activity | [192,195] |
| Paclitaxel | Taxane | Roseburia, Eubacterium, Erysipelotrichaceae | ↓ bacteria diversity and ecological network function; ↓ butyric-producing bacteria | [192,196] |
| CPT-11 (Irinotecan) | DNA topoisomerase I inhibitor | Escherichia Coli | ↑ GI toxicity via SN-38-G conversion into SN-38 induced Bacterial β-glucuronidase; ↓ treatment tolerability | [197,198] |
| Carmustine, Etoposide, Aracytine and Melphalan combination | Conditioning chemotherapy (HSCT) | Firmicutes, Enterococcaceae Actinobacteria, Proteobacteria | ↓ diversity and ecological network function; ↑ drug toxicity; ↑ inflammation (colitis) | [199] |
| Methotrexate | Antimetabolite | Clostridium, Eubacterium, Bifidobacteria, Bacteorides (Anaerobes). Lactobacilli, Streptococci, Enterobacteriaceae (Aerobes) | ↓ microbial diversity ↑ pathogenic taxa → mucositis, inflammation, and GI toxicity. | [200] |
| Immunotherapies | ||||
| CTLA-4 blockade (Ipilimumab) | Anti- CTLA-4 antibody | Bacteroidetes | ↑ treatment response via CD4+ T cell activation effector T cells. ↓ colitis via polyamine/vitamin B modulation | [201,202] |
| Anti-PD-L1 | Checkpoint blockade | Bifidobacterium spp. | ↑ treatment response via tumor-specific T-cell induction; ↑ CD8+ infiltration in tumour microenvironment | [154,201,203] |
| Anti-PD1 | Checkpoint blockade | Lachnospiraceae, Ruminococcaceae, Bifidobacteriaceae, Coriobacteriaceae | ↑ treatment response via CD8+ T cell and innate effectors activation; ↓ suppressive myeloid cells | [204] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tini, S.; Baima, J.; Pigni, S.; Antoniotti, V.; Caputo, M.; De Palma, E.; Cerbone, L.; Grosso, F.; La Vecchia, M.; Bona, E.; et al. The Microbiota–Diet–Immunity Axis in Cancer Care: From Prevention to Treatment Modulation and Survivorship. Nutrients 2025, 17, 2898. https://doi.org/10.3390/nu17172898
Tini S, Baima J, Pigni S, Antoniotti V, Caputo M, De Palma E, Cerbone L, Grosso F, La Vecchia M, Bona E, et al. The Microbiota–Diet–Immunity Axis in Cancer Care: From Prevention to Treatment Modulation and Survivorship. Nutrients. 2025; 17(17):2898. https://doi.org/10.3390/nu17172898
Chicago/Turabian StyleTini, Sabrina, Jessica Baima, Stella Pigni, Valentina Antoniotti, Marina Caputo, Elena De Palma, Luigi Cerbone, Federica Grosso, Marta La Vecchia, Elisa Bona, and et al. 2025. "The Microbiota–Diet–Immunity Axis in Cancer Care: From Prevention to Treatment Modulation and Survivorship" Nutrients 17, no. 17: 2898. https://doi.org/10.3390/nu17172898
APA StyleTini, S., Baima, J., Pigni, S., Antoniotti, V., Caputo, M., De Palma, E., Cerbone, L., Grosso, F., La Vecchia, M., Bona, E., & Prodam, F. (2025). The Microbiota–Diet–Immunity Axis in Cancer Care: From Prevention to Treatment Modulation and Survivorship. Nutrients, 17(17), 2898. https://doi.org/10.3390/nu17172898








