The Effects of Essential Amino Acid Supplementation on Hippocampal Neurotrophin, Dopaminergic and Serotonergic Changes in an Overtraining Mouse Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
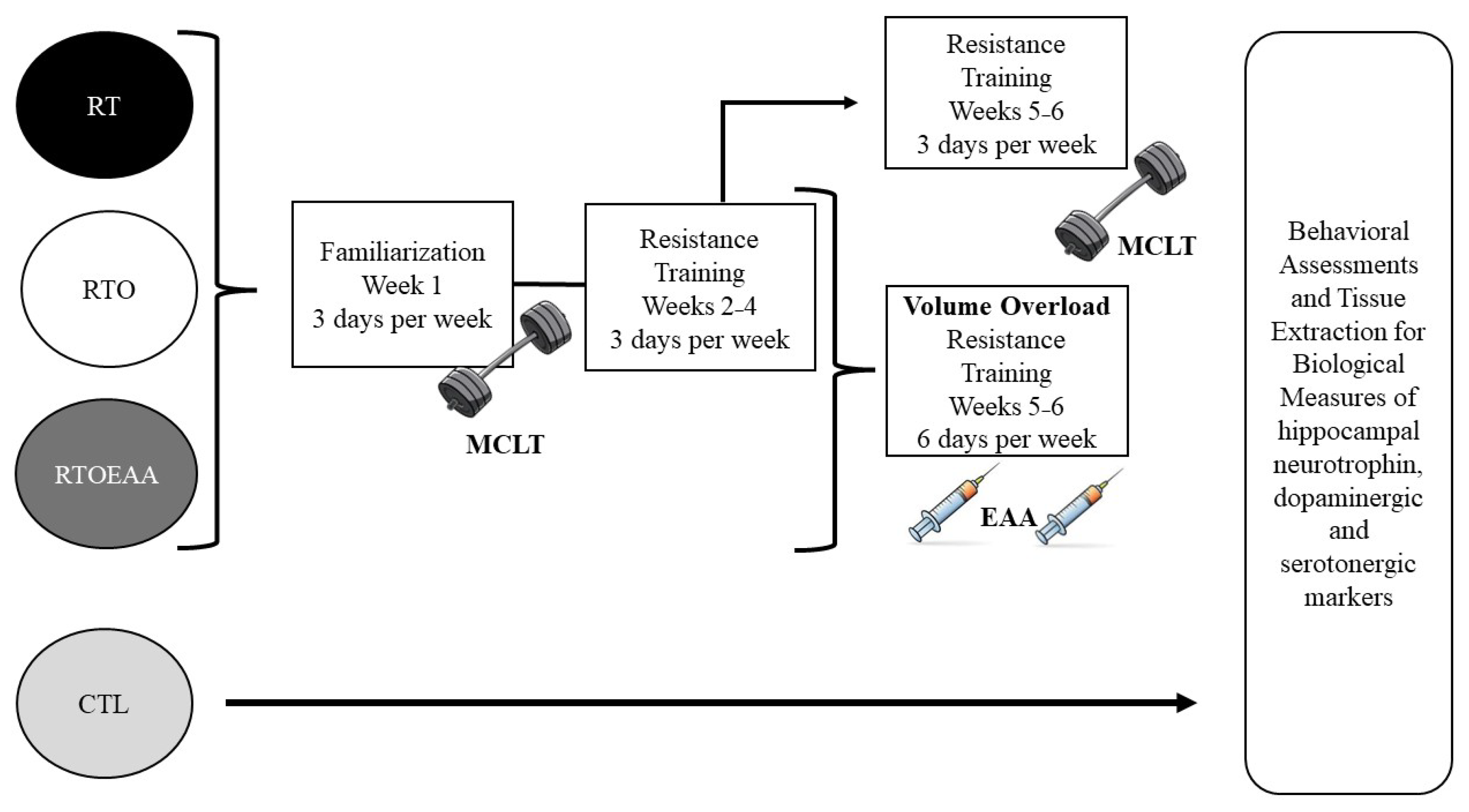
2.2. Experimental Design
2.3. Resistance Training Protocol
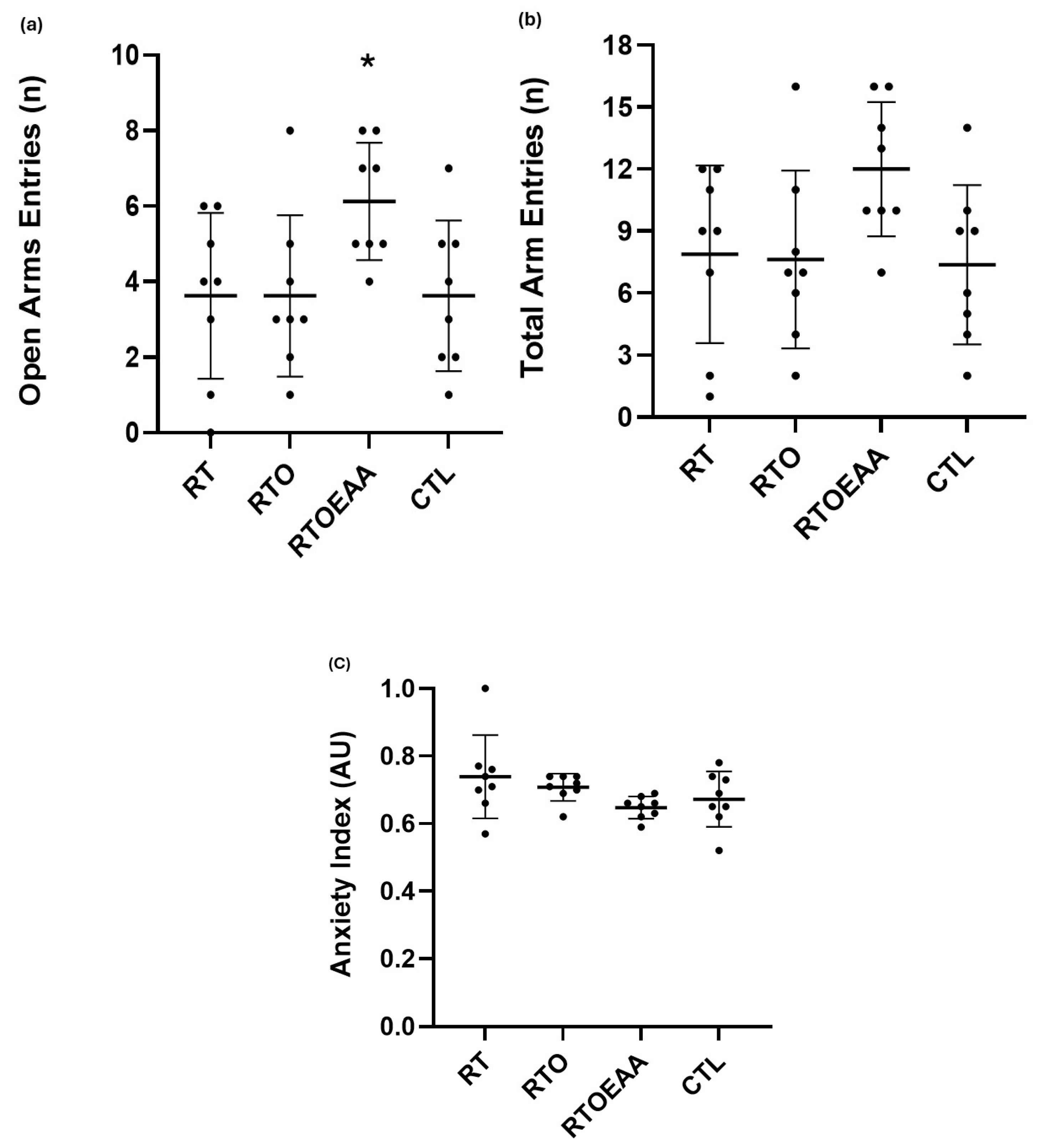
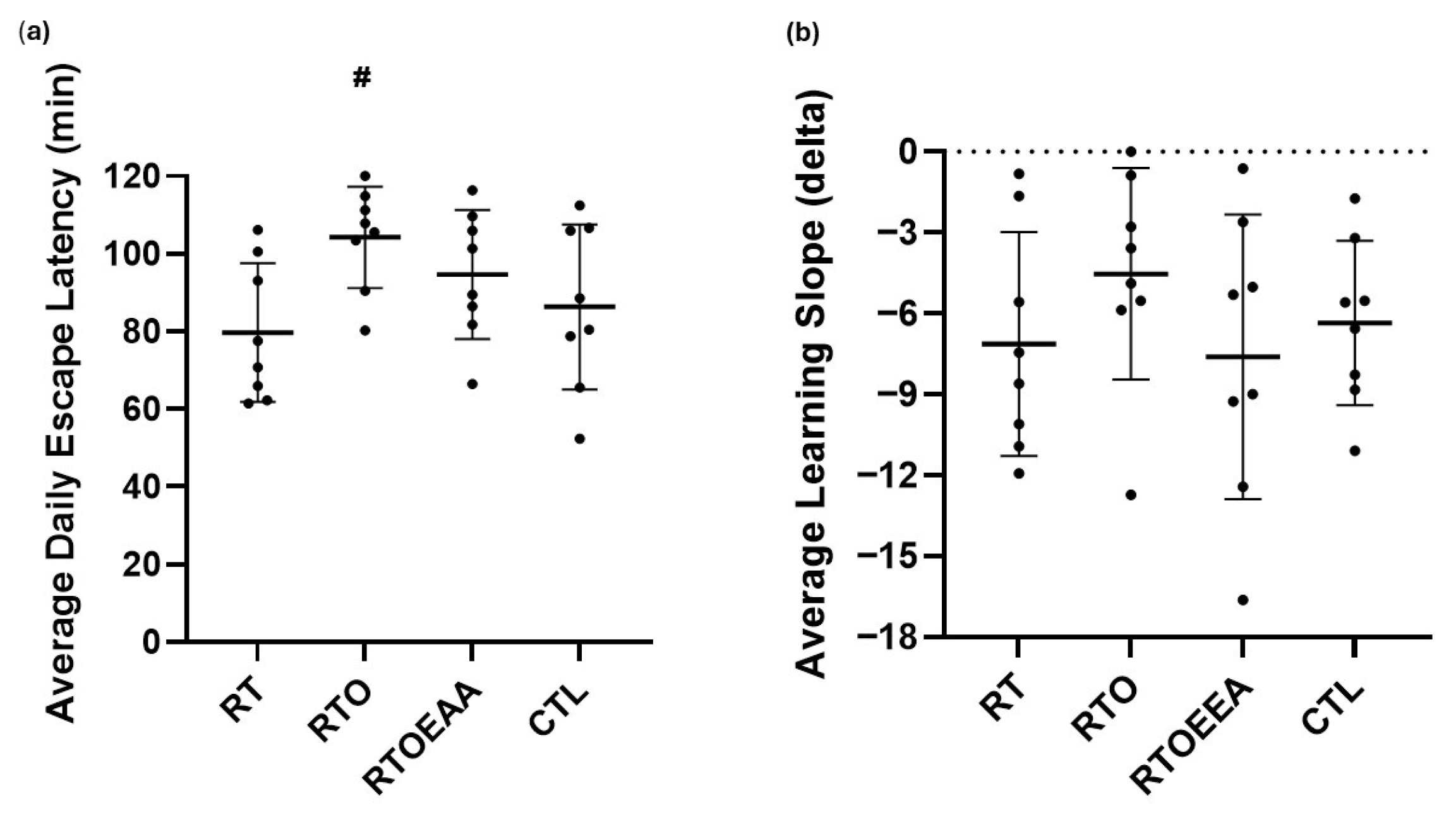
2.4. Behavioral Measures
2.5. Tissue Preparation
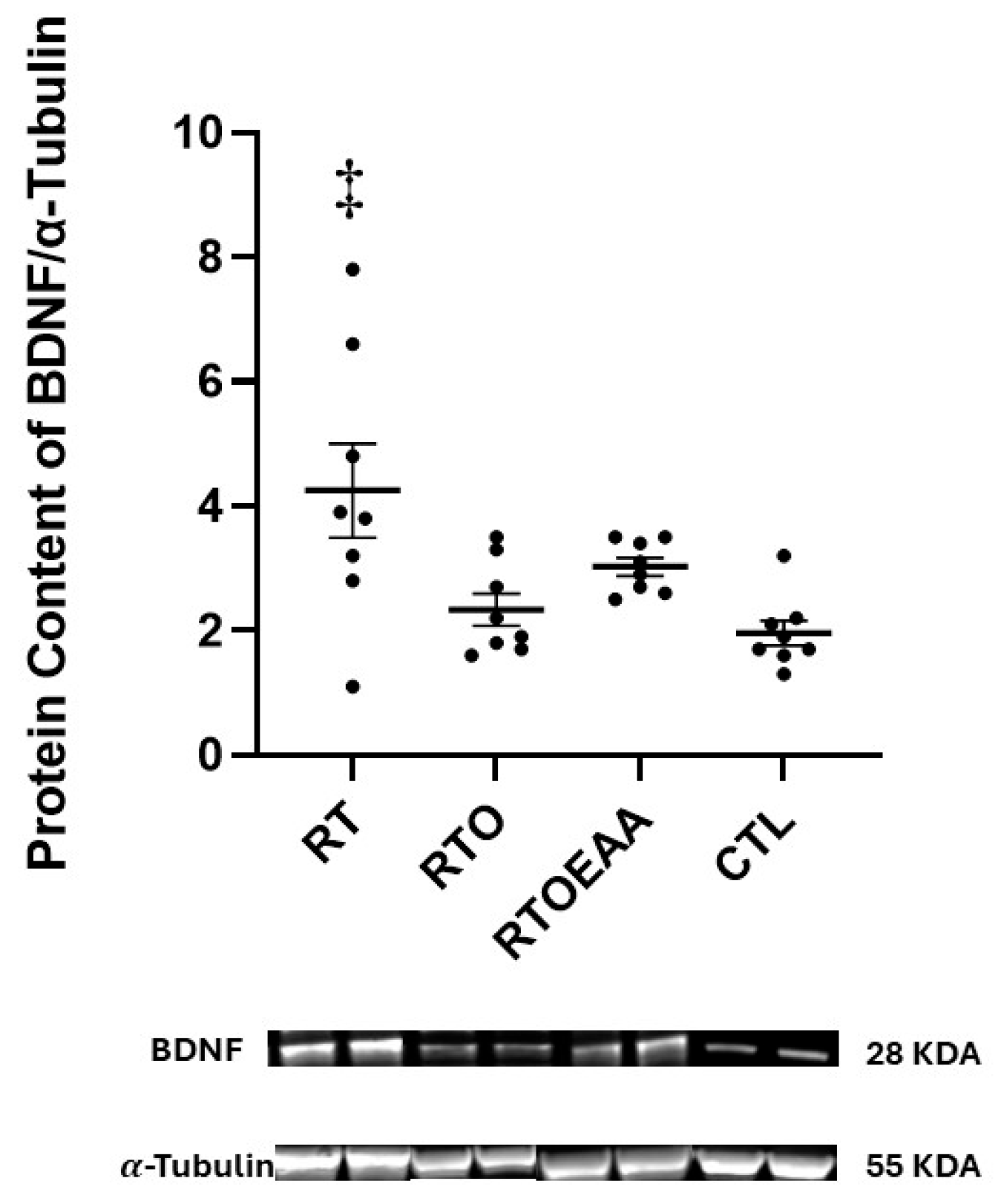
2.6. Western Blot Analysis
2.7. Statistical Analysis
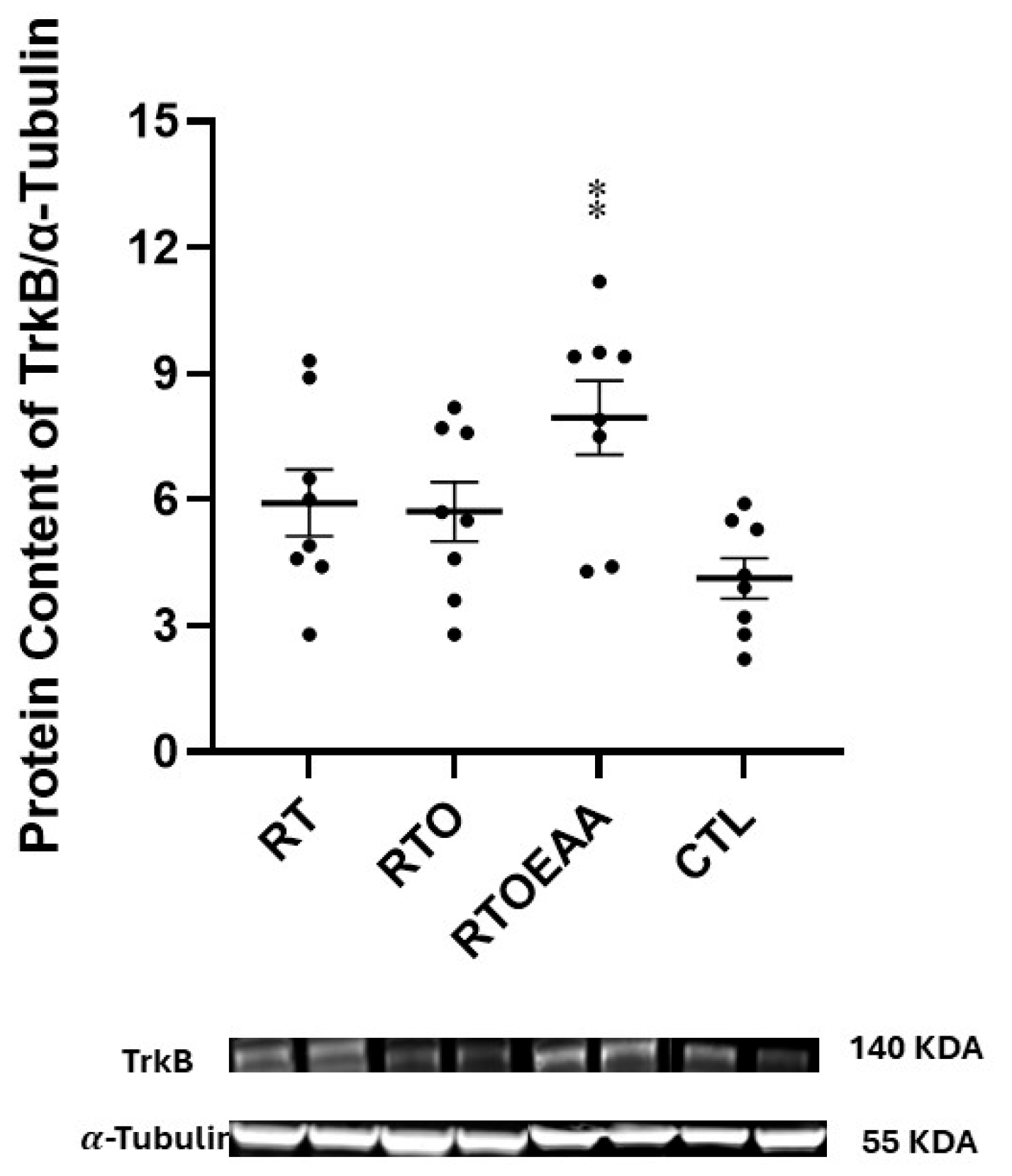
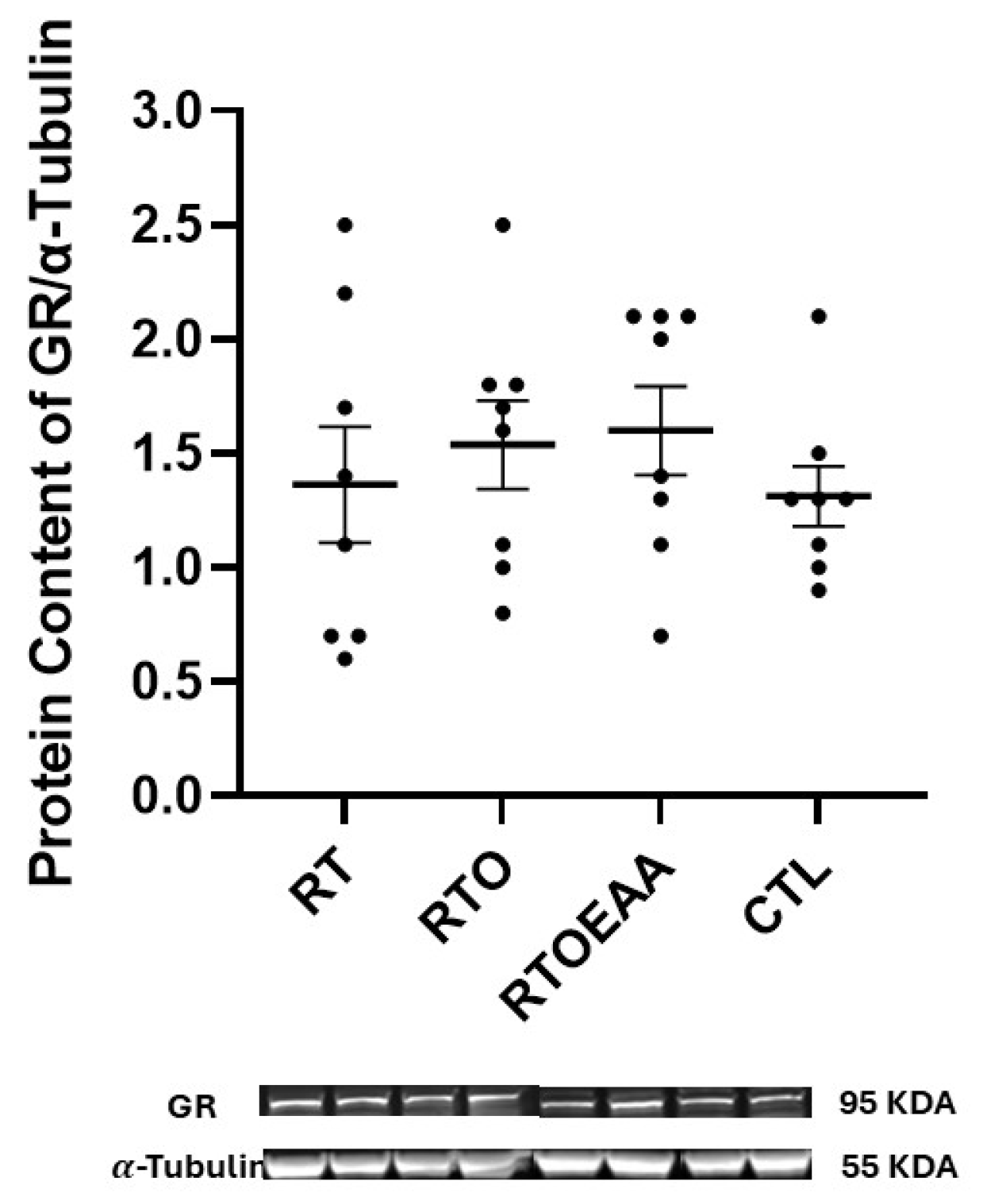
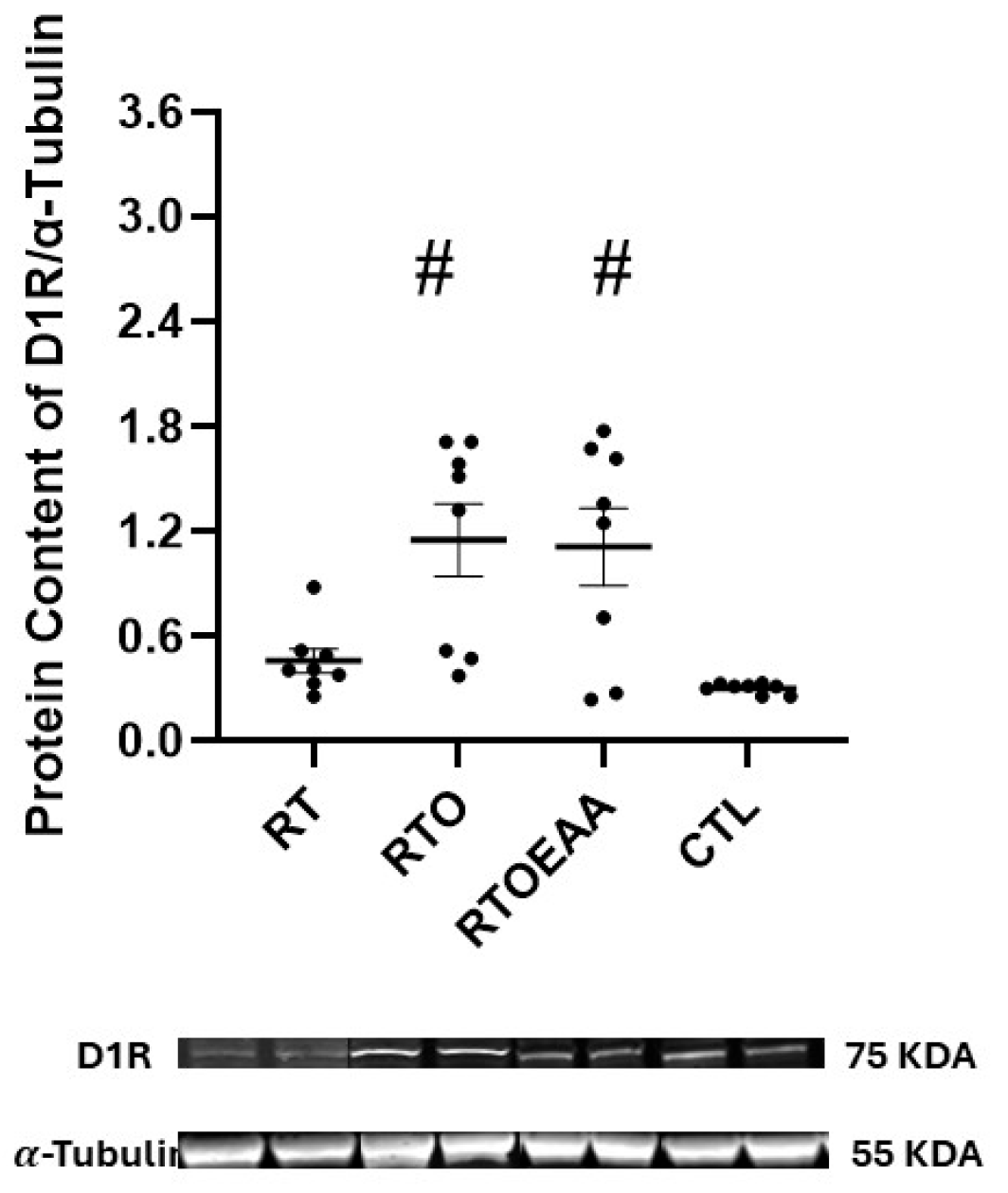
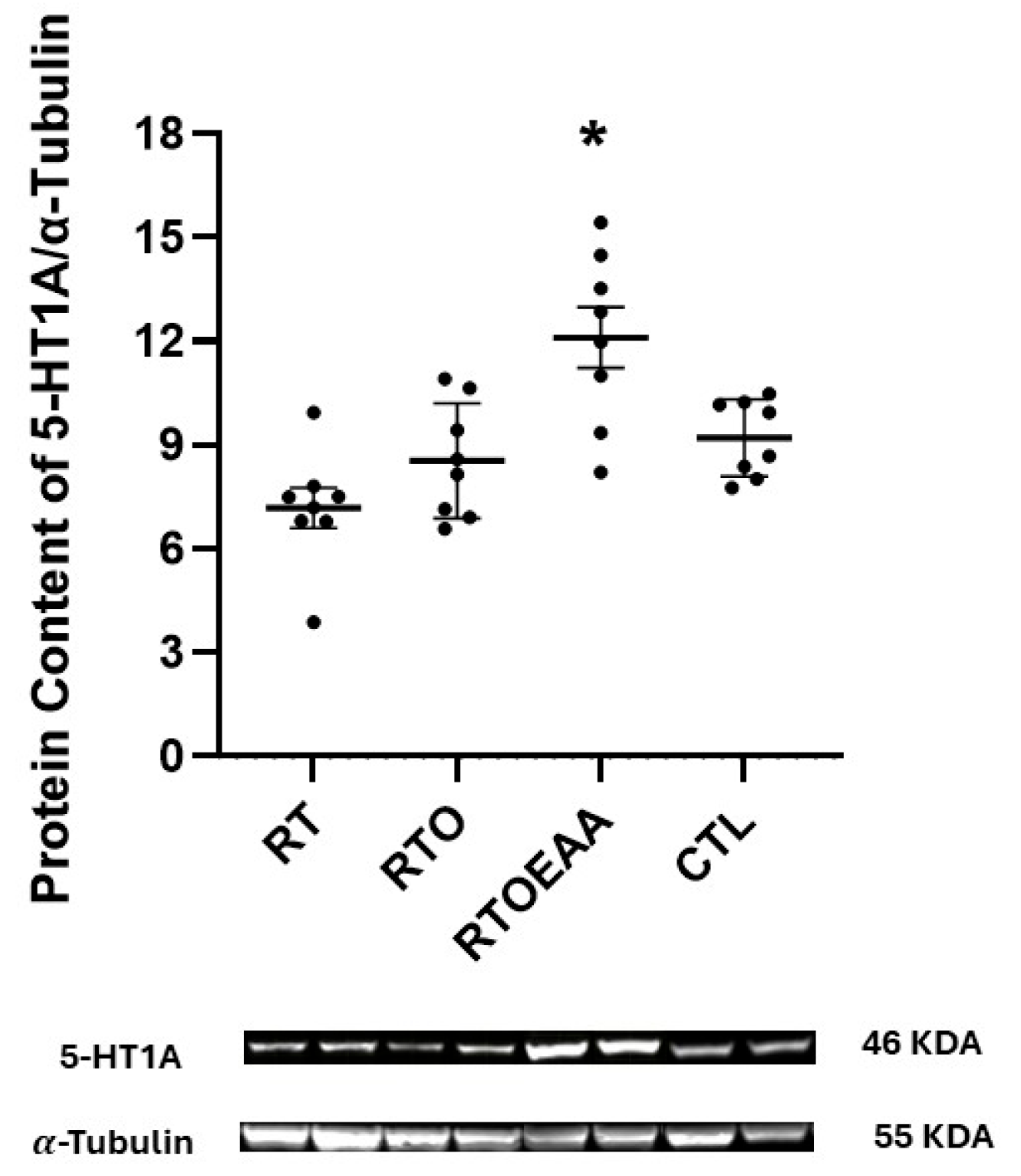
3. Results
3.1. Performance and Body Mass
3.2. Behavior Measures
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kraemer, W.J.; Adams, K.; Cafarelli, E.; Dudley, G.A.; Dooly, C.; Feigenbaum, M.S.; Fleck, S.J.; Franklin, B.; Fry, A.C.; Hoffman, J.R.; et al. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med. Sci. Sports Exerc. 2002, 34, 364–380. [Google Scholar] [CrossRef] [PubMed]
- Schwellnus, M.; Soligard, T.; Alonso, J.M.; Bahr, R.; Clarsen, B.; Dijkstra, H.P.; Gabbett, T.J.; Gleeson, M.; Hägglund, M.; Hutchinson, M.R.; et al. How much is too much? (Part 2) International Olympic Committee consensus statement on load in sport and risk of illness. Br. J. Sports Med. 2016, 50, 1043–1052. [Google Scholar] [CrossRef]
- Lehmann, M.J.; Lormes, W.; Opitz-Gress, A.; Steinacker, J.M.; Netzer, N.; Foster, C.; Gastmann, U. Training and overtraining: An overview and experimental results in endurance sports. J. Sports Med. Phys. Fit. 1997, 37, 7–17. [Google Scholar]
- Hynynen, E.; Uusitalo, A.; Konttinen, N.; Rusko, H. Cardiac autonomic responses to standing up and cognitive task in overtrained athletes. Int. J. Sports Med. 2008, 29, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, L.E.; VanHeest, J.L. The unknown mechanism of the overtraining syndrome: Clues from depression and psychoneuroimmunology. Sports Med. 2002, 32, 185–209. [Google Scholar] [CrossRef] [PubMed]
- Birrer, D.; Lienhard, D.; Williams, C.A.; Röthlin, P.; Morgan, G. Prevalence of non-functional overreaching and the overtraining syndrome in Swiss elite athletes. Schweiz. Z. Sport. Sport. 2013, 61, 23–29. [Google Scholar] [CrossRef]
- Nederhof, E.; Lemmink, K.A.P.M.; Visscher, C.; Meeusen, R.; Mulder, T. Psychomotor speed: Possibly a new marker for overtraining syndrome. Sports Med. 2006, 36, 817–828. [Google Scholar] [CrossRef]
- Cadegiani, F.A.; Kater, C.E. Novel insights of overtraining syndrome discovered from the EROS study. BMJ Open Sport Exerc. Med. 2019, 5, e000542. [Google Scholar] [CrossRef]
- La Torre, M.E.; Monda, A.; Messina, A.; de Stefano, M.I.; Monda, V.; Moscatelli, F.; Tafuri, F.; Saraiello, E.; Latino, F.; Monda, M.; et al. The potential role of nutrition in overtraining syndrome: A narrative review. Nutrients 2023, 15, 4916. [Google Scholar] [CrossRef]
- Angeli, A.; Minetto, M.; Dovio, A.; Paccotti, P. The overtraining syndrome in athletes: A stress-related disorder. J. Endocrinol. Investig. 2004, 27, 603–612. [Google Scholar] [CrossRef]
- Buyse, L.; Decroix, L.; Timmermans, N.; Barbé, K.; Verrelst, R.; Meeusen, R. Improving the diagnosis of nonfunctional overreaching and overtraining syndrome. Med. Sci. Sports Exerc. 2019, 51, 2524–2530. [Google Scholar] [CrossRef]
- Cadegiani, F.A.; Kater, C.E. Hypothalamic-Pituitary-Adrenal (HPA) Axis Functioning in Overtraining Syndrome: Findings from Endocrine and Metabolic Responses on Overtraining Syndrome (EROS)—EROS-HPA Axis. Sports Med. Open 2017, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Yeager, M.P.; Pioli, P.A.; Guyre, P.M. Cortisol exerts bi-phasic regulation of inflammation in humans. Dose-Response 2011, 9, 332–347. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.-J.; Liu, M.-Y.; Li, H.; Liu, X.; Chen, C.; Han, Z.; Wu, H.-Y.; Jing, X.; Zhou, H.-H.; Suh, H.; et al. The different roles of glucocorticoids in the hippocampus and hypothalamus in chronic stress-induced HPA axis hyperactivity. PLoS ONE 2014, 9, e97689. [Google Scholar] [CrossRef]
- Franco, A.J.; Chen, C.; Scullen, T.; Zsombok, A.; Salahudeen, A.A.; Di, S.; Herman, J.P.; Tasker, J.G. Sensitization of the hypothalamic-pituitary-adrenal axis in a male rat chronic stress model. Endocrinology 2016, 157, 2346–2355. [Google Scholar] [CrossRef]
- Algamal, M.; Pearson, A.J.; Hahn-Townsend, C.; Burca, I.; Mullan, M.; Crawford, F.; Ojo, J.O. Repeated unpredictable stress and social isolation induce chronic HPA axis dysfunction and persistent abnormal fear memory. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 104, 110035. [Google Scholar] [CrossRef]
- Karin, O.; Raz, M.; Tendler, A.; Bar, A.; Kohanim, Y.K.; Milo, T.; Alon, U. A new model for the HPA axis explains dysregulation of stress hormones on the timescale of weeks. Mol. Syst. Biol. 2020, 16, e9510. [Google Scholar] [CrossRef]
- Brooks, K.A.; Carter, J.G. Overtraining, Exercise, and Adrenal Insufficiency. J. Nov. Physiother. 2013, 3, 125. [Google Scholar] [CrossRef]
- Baumeister, D.; Lightman, S.L.; Pariante, C.M. The Interface of Stress and the HPA Axis in Behavioural Phenotypes of Mental Illness. Curr. Top. Behav. Neurosci. 2014, 18, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.; Makino, S.; Kvetnansky, R.; Post, R.M. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J. Neurosci. 1995, 15, 1766–1777. [Google Scholar] [CrossRef]
- Numakawa, T.; Odaka, H.; Adachi, N. Actions of Brain-Derived Neurotrophin Factor in the Neurogenesis and Neuronal Function, and Its Involvement in the Pathophysiology of Brain Diseases. Int. J. Mol. Sci. 2018, 19, 3650. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Numakawa, T.; Kajihara, R. Involvement of brain-derived neurotrophic factor signaling in the pathogenesis of stress-related brain diseases. Front. Mol. Neurosci. 2023, 16, 1247422. [Google Scholar] [CrossRef]
- Beyer, D.K.E.; Mattukat, A.; Freund, N. Prefrontal dopamine D1 receptor manipulation influences anxiety behavior and induces neuroinflammation within the hippocampus. Int. J. Bipolar Disord. 2021, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Tyler, J.; Podaras, M.; Richardson, B.; Roeder, N.; Hammond, N.; Hamilton, J.; Blum, K.; Gold, M.; Baron, D.A.; Thanos, P.K. High-intensity interval training exercise increases dopamine D2 levels and modulates brain dopamine signaling. Front. Public Health 2023, 11, 1257629. [Google Scholar] [CrossRef]
- Espadas, I.; Ortiz, O.; García-Sanz, P.; Sanz-Magro, A.; Alberquilla, S.; Solis, O.; Delgado-García, J.M.; Gruart, A.; Moratalla, R. Dopamine D2R is Required for Hippocampal-dependent Memory and Plasticity at the CA3-CA1 Synapse. Cereb Cortex. 2021, 5, 2187–2204. [Google Scholar] [CrossRef] [PubMed]
- López, J.F.; Chalmers, D.T.; Little, K.Y.; Watson, S.J. Regulation of Serotonin1A, Glucocorticoid, and Mineralocorticoid Receptor in Rat and Human Hippocampus: Implications for the Neurobiology of Depression. Biol. Psychiatry 1998, 43, 547–573. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Sakai, R.R.; McEwen, B.S.; Mendelson, S. Stress and antidepressant effects on hippocampal and cortical 5-HT1A and 5-HT2 receptors and transport sites for serotonin. Brain Res. 1993, 615, 87–94. [Google Scholar] [CrossRef]
- Ferrando, A.A.; Wolfe, R.R.; Hirsch, K.R.; Church, D.D.; Kviatkovsky, S.A.; Roberts, M.D.; Stout, J.R.; Gonzalez, D.E.; Sowinski, R.J.; Kreider, R.B.; et al. International Society of Sports Nutrition Position Stand: Effects of essential amino acid supplementation on exercise and performance. J. Int. Soc. Sports Nutr. 2023, 20, 2263409. [Google Scholar] [CrossRef]
- Sato, H.; Tsukamoto-Yasui, M.; Takado, Y.; Kawasaki, N.; Matsunaga, K.; Ueno, S.; Kanda, M.; Nishimura, M.; Karakawa, S.; Isokawa, M.; et al. Protein deficiency-induced behavioral abnormalities and neurotransmitter loss in aged mice are ameliorated by essential amino acids. Front. Nutr. 2020, 7, 23. [Google Scholar] [CrossRef]
- Wilke, J.; Giesche, F.; Klier, K.; Vogt, L.; Herrmann, E.; Banzer, W. Acute Effects of Resistance Exercise on Cognitive Function in Healthy Adults: A Systematic Review with Multilevel Meta-Analysis. Sports Med. 2019, 49, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; So, B.; Choi, M.; Kang, D.; Song, W. Resistance exercise training increases the expression of irisin concomitant with improvement of muscle function in aging mice and humans. Exp. Gerontol. 2015, 70, 11–17. [Google Scholar] [CrossRef]
- Deus, A.P.L.; Bassi, D.; Simões, R.P.; Oliveira, C.R.; Baldissera, V.; Marqueti, R.d.C.; Araujo, H.S.; Arena, R.; Borghi-Silva, A. MMP-2 expression in skeletal muscle after strength training. Int. J. Sports Med. 2012, 33, 137–141. [Google Scholar] [CrossRef]
- Krause Neto, W.; Silva, W.A.; Oliveira, T.V.; Boas, A.E.d.S.V.; Ciena, A.P.; Anaruma, C.A.; Gorzi, A.; Caperuto, É.C.; Gama, E.F. Muscle hypertrophy is correlated with load progression delta, climb volume, and total load volume in rodents undergoing different ladder-based resistance training protocols. Tissue Cell 2022, 75, 101725. [Google Scholar] [CrossRef]
- Cohen, H.; Liu, T.; Kozlovsky, N.; Kaplan, Z.; Zohar, J.; Mathé, A.A. The neuropeptide Y (NPY)-ergic system is associated with behavioral resilience to stress exposure in an animal model of post-traumatic stress disorder. Neuropsychopharmacology 2012, 37, 350–363. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barnes, C.A. Memory deficits associated with senescence: A neurophysiological and behavioral study in the rat. J. Comp. Physiol. Psychol. 1979, 93, 74–104. [Google Scholar] [CrossRef] [PubMed]
- Church, D.D.; Hirsch, K.R.; Kviatkovsky, S.A.; Matthews, J.J.; Henderson, R.A.; Azhar, G.; Wolfe, R.R.; Ferrando, A.A. Effect of 3 Different Daily Protein Intakes in a 2-Meal Eating Pattern on Protein Turnover in Middle Age and Older Adults: A Randomized Controlled Trial. J. Nutr. 2025, 155, 1364–1372. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hoffman, J.R. Overtraining. In Physiological Aspects of Sport Training and Performance; Human Kinetics: Champaign, IL, USA, 2014; pp. 395–411. [Google Scholar]
- Wang, C.; Kang, Y.; Liu, P.; Liu, W.; Chen, W.; Hayashi, T.; Mizuno, K.; Hattori, S.; Fujisaki, H.; Ikejima, T. Combined use of dasatinib and quercetin alleviates overtraining-induced deficits in learning and memory through eliminating senescent cells and reducing apoptotic cells in rat hippocampus. Behav. Brain Res. 2023, 440, 114260. [Google Scholar] [CrossRef] [PubMed]
- Jahangiri, Z.; Gholamnezhad, Z.; Hosseini, M.; Beheshti, F.; Kasraie, N. The effects of moderate exercise and overtraining on learning and memory, hippocampal inflammatory cytokine levels, and brain oxidative stress markers in rats. J. Physiol. Sci. 2019, 69, 993–1004. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dowlati, Y.; Ravindran, A.V.; Segal, Z.V.; Stewart, D.E.; Steiner, M.; Meyer, J.H. Selective dietary supplementation in early postpartum is associated with high resilience against depressed mood. Proc. Natl. Acad. Sci. USA 2017, 114, 3509–3514. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rondanelli, M.; Opizzi, A.; Antoniello, N.; Boschi, F.; Iadarola, P.; Pasini, E.; Aquilani, R.; Dioguardi, F.S. Effect of essential amino acid supplementation on quality of life, amino acid profile and strength in institutionalized elderly patients. Clin. Nutr. 2011, 30, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.D.; Milner, T.A.; McEwen, B.S. Dynamic plasticity: The role of glucocorticoids, brain-derived neurotrophic factor and other trophic factors. Neuroscience 2013, 239, 214–227. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ozawa, T.; Yamada, K.; Ichitani, Y. Hippocampal BDNF treatment facilitates consolidation of spatial memory in spontaneous place recognition in rats. Behav. Brain Res. 2014, 263, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.R.; Ostfeld, I.; Stout, J.R.; Harris, R.C.; Kaplan, Z.; Cohen, H. β-Alanine supplemented diets enhance behavioral resilience to stress exposure in an animal model of PTSD. Amino Acids. 2015, 47, 1247–1257. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hoffman, J.R.; Zuckerman, A.; Ram, O.; Sadot, O.; Stout, J.R.; Ostfeld, I.; Cohen, H. Behavioral and inflammatory response in animals exposed to a low-pressure blast wave and supplemented with β-alanine. Amino Acids. 2017, 49, 871–886. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Church, D.D.; Hoffman, J.R.; Mangine, G.T.; Jajtner, A.R.; Townsend, J.R.; Beyer, K.S.; Wang, R.; La Monica, M.B.; Fukuda, D.H.; Stout, J.R. Comparison of high intensity vs. high-volume resistance training on the BDNF response to exercise. J. Appl. Physiol. 2016, 121, 123–128. [Google Scholar] [CrossRef]
- Suijo, K.; Inoue, S.; Ohya, Y.; Odagiri, Y.; Takamiya, T.; Ishibashi, H.; Itoh, M.; Fujieda, Y.; Shimomitsu, T. Resistance exercise enhances cognitive function and increases hippocampal BDNF and CREB expression in mice. Int. J. Sports Med. 2013, 34, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zheng, Y.L.; Yin, X.; Xu, S.J.; Tian, D.; Zhang, C.Y.; Wang, S.; Ma, J.Z. Excessive treadmill training enhances brain-specific microRNA-34a in the mouse hippocampus. Front. Mol. Neurosci. 2020, 13, 7. [Google Scholar] [CrossRef]
- Parwata, N.M.N. Overtraining physical aerobic exercise against the decline of memory on mice (Rattus norvegicus). J. Profesi Med. 2020, 14, 22–28. [Google Scholar] [CrossRef]
- Dora, K.; Tsukamoto, H.; Suga, T.; Tomoo, K.; Suzuki, A.; Adachi, Y.; Takeshita, M.; Kato, Y.; Kawasaki, M.; Sato, W.; et al. Essential amino acid supplements ingestion has a positive effect on executive function after moderate-intensity aerobic exercise. Sci. Rep. 2023, 13, 22644. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cassilhas, R.; Lee, K.; Fernandes, J.; Oliveira, M.; Tufik, S.; Meeusen, R.; de Mello, M. Spatial memory is improved by aerobic and resistance exercise through divergent molecular mechanisms. Neuroscience 2012, 202, 309–317. [Google Scholar] [CrossRef]
- Nokia, M.S.; Lensu, S.; Ahtiainen, J.P.; Johansson, P.P.; Koch, L.G.; Britton, S.L.; Kainulainen, H. Physical exercise increases adult hippocampal neurogenesis in male rats provided it is aerobic and sustained. J. Physiol. 2016, 594, 1855–1873. [Google Scholar] [CrossRef] [PubMed]
- Orlovsky, M.A.; Dosenko, V.E.; Spiga, F.; Skibo, G.G.; Lightman, S.L. Hippocampus remodeling by chronic stress accompanied by GR, proteasome and caspase-3 overexpression. Brain Res. 2014, 1593, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Kitraki, E.; Kremmyda, O.; Youlatos, D.; Alexis, M.; Kittas, C. Spatial performance and corticosteroid receptor status in the 21-day restraint stress paradigm. Ann. N. Y. Acad. Sci. 2004, 1018, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.D.; Kogan, J.F.; Marrocco, J.; McEwen, B.S. Genomic and epigenomic mechanisms of glucocorticoids in the brain. Nat. Rev. Endocrinol. 2017, 13, 661–673. [Google Scholar] [CrossRef] [PubMed]
- Chaves, T.; Fazekas, C.L.; Horváth, K.; Correia, P.; Szabó, A.; Török, B.; Bánrévi, K.; Zelena, D. Stress Adaptation and the Brainstem with Focus on Corticotropin-Releasing Hormone. Int. J. Mol. Sci. 2021, 22, 9090. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, S.H.; Jung, E.-M. Adverse effects of early-life stress: Focus on the rodent neuroendocrine system. Neural Regen. Res. 2024, 19, 336–341. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, L.; Wang, W.; Li, Y.; Jin, H.; Xiao, B.; Jin, Q. Dopamine and D1 receptor in hippocampal dentate gyrus involved in chronic stress–induced alteration of spatial learning and memory in rats. Neurobiol. Stress 2024, 33, 100685. [Google Scholar] [CrossRef]
- Wang, F.; Wan, P.; Wang, W.; Xiao, B.; Jin, H.; Jin, Q. Dopamine in the hippocampal dentate gyrus modulates spatial learning via D1-like receptors. Brain Res. Bull. 2019, 144, 101–107. [Google Scholar] [CrossRef]
- Godino, A.; Salery, M.; Minier-Toribio, A.M.; Patel, V.; Fullard, J.F.; Kondev, V.; Parise, E.M.; Martinez-Rivera, F.J.; Morel, C.; Roussos, P.; et al. Dopamine D1–D2 signalling in hippocampus arbitrates approach and avoidance. Nature 2025, 643, 448–457. [Google Scholar] [CrossRef]
- Naumenko, V.S.; Kondaurova, E.M.; Bazovkina, D.V.; Tsybko, A.S.; Ilchibaeva, T.V.; Khotskin, N.V.; Semenova, A.A.; Popova, N.K. Effect of GDNF on depressive-like behavior, spatial learning and key genes of the brain dopamine system in genetically predisposed to behavioral disorders mouse strains. Behav. Brain Res. 2014, 274, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bauer, E.E.; Buhr, T.J.; Reed, C.H.; Clark, P.J. Exercise-Induced Adaptations to the Mouse Striatal Adenosine System. Neural Plast. 2020, 2020, 5859098. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vučcković, M.G.; Li, Q.; Fisher, B.; Nacca, A.; Leahy, R.M.; Walsh, J.P.; Mukherjee, J.; Williams, C.; Jakowec, M.W.; Petzinger, G.M. Exercise elevates dopamine D2 receptor in a mouse model of Parkinson’s disease: In vivo imaging with [18F] fallypride. Mov. Disord. 2010, 25, 2777–2784. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fernstrom, J.D. Large neutral amino acids: Dietary effects on brain neurochemistry and function. Amino Acids 2013, 45, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Glikmann-Johnston, Y.; Saling, M.M.; Reutens, D.C.; Stout, J.C. Hippocampal 5-HT1A Receptor and Spatial Learning and Memory. Front. Pharmacol. 2015, 6, 289. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ögren, S.O.; Eriksson, T.M.; Elvander-Tottie, E.; D’aDdario, C.; Ekström, J.C.; Svenningsson, P.; Meister, B.; Kehr, J.; Stiedl, O. The role of 5-HT(1A) receptors in learning and memory. Behav. Brain Res. 2008, 195, 54–77. [Google Scholar] [CrossRef] [PubMed]
- Uusitalo, A.L.; Vanninen, E.; Valkonen-Korhonen, M.; Kuikka, J.T. Brain serotonin reuptake did not change during one year in overtrained athletes. Int. J. Sports Med. 2006, 27, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Uusitalo, A.L.; Valkonen-Korhonen, M.; Helenius, P.; Vanninen, E.; Bergström, K.A.; Kuikka, J.T. Abnormal serotonin reuptake in an overtrained, insomnic and depressed team athlete. Int. J. Sports Med. 2004, 25, 150–153. [Google Scholar] [CrossRef]
- Smriga, M.; Kameishi, M.; Torii, K. Exercise-dependent preference for a mixture of branched-chain amino acids and homeostatic control of brain serotonin in exercising rats. J. Nutr. 2006, 136, 548S–552S. [Google Scholar] [CrossRef] [PubMed]
- Coppola, A.; Wenner, B.R.; Ilkayeva, O.; Stevens, R.D.; Maggioni, M.; Slotkin, T.A.; Levin, E.D.; Newgard, C.B. Branched-chain amino acids alter neurobehavioral function in rats. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E405–E413. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Choi, S.; DiSilvio, B.; Fernstrom, M.H.; Fernstrom, J.D. Oral branched-chain amino acid supplements that reduce brain serotonin during exercise in rats also lower brain catecholamines. Amino Acids 2013, 45, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Knobelman, D.A.; Hen, R.; Blendy, J.A.; Lucki, I. Regional patterns of compensation following genetic deletion of either 5-hydroxytryptamine(1A) or 5-hydroxytryptamine(1B) receptor in the mouse. J. Pharmacol. Exp. Ther. 2001, 298, 1092–1100. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Binman, L.; Ben-Zeev, T.; Harris, A.; Levi, C.; Weissman, I.; Church, D.D.; Ferrando, A.A.; Hoffman, J.R. The Effects of Essential Amino Acid Supplementation on Hippocampal Neurotrophin, Dopaminergic and Serotonergic Changes in an Overtraining Mouse Model. Nutrients 2025, 17, 2957. https://doi.org/10.3390/nu17182957
Binman L, Ben-Zeev T, Harris A, Levi C, Weissman I, Church DD, Ferrando AA, Hoffman JR. The Effects of Essential Amino Acid Supplementation on Hippocampal Neurotrophin, Dopaminergic and Serotonergic Changes in an Overtraining Mouse Model. Nutrients. 2025; 17(18):2957. https://doi.org/10.3390/nu17182957
Chicago/Turabian StyleBinman, Lior, Tavor Ben-Zeev, Asher Harris, Chagai Levi, Inbal Weissman, David D. Church, Arny A. Ferrando, and Jay R. Hoffman. 2025. "The Effects of Essential Amino Acid Supplementation on Hippocampal Neurotrophin, Dopaminergic and Serotonergic Changes in an Overtraining Mouse Model" Nutrients 17, no. 18: 2957. https://doi.org/10.3390/nu17182957
APA StyleBinman, L., Ben-Zeev, T., Harris, A., Levi, C., Weissman, I., Church, D. D., Ferrando, A. A., & Hoffman, J. R. (2025). The Effects of Essential Amino Acid Supplementation on Hippocampal Neurotrophin, Dopaminergic and Serotonergic Changes in an Overtraining Mouse Model. Nutrients, 17(18), 2957. https://doi.org/10.3390/nu17182957





