Effects of Quercetin and Citrulline on Nitric Oxide Metabolites and Antioxidant Biomarkers in Trained Cyclists
Abstract
:1. Introduction
Study Population
2. Materials and Methods
2.1. Visit Descriptions
2.2. 20 km Time Trial Performance
2.3. Supplementation
2.4. Blood Collection
2.5. Biochemical Assessments
2.6. Statistical Analyses
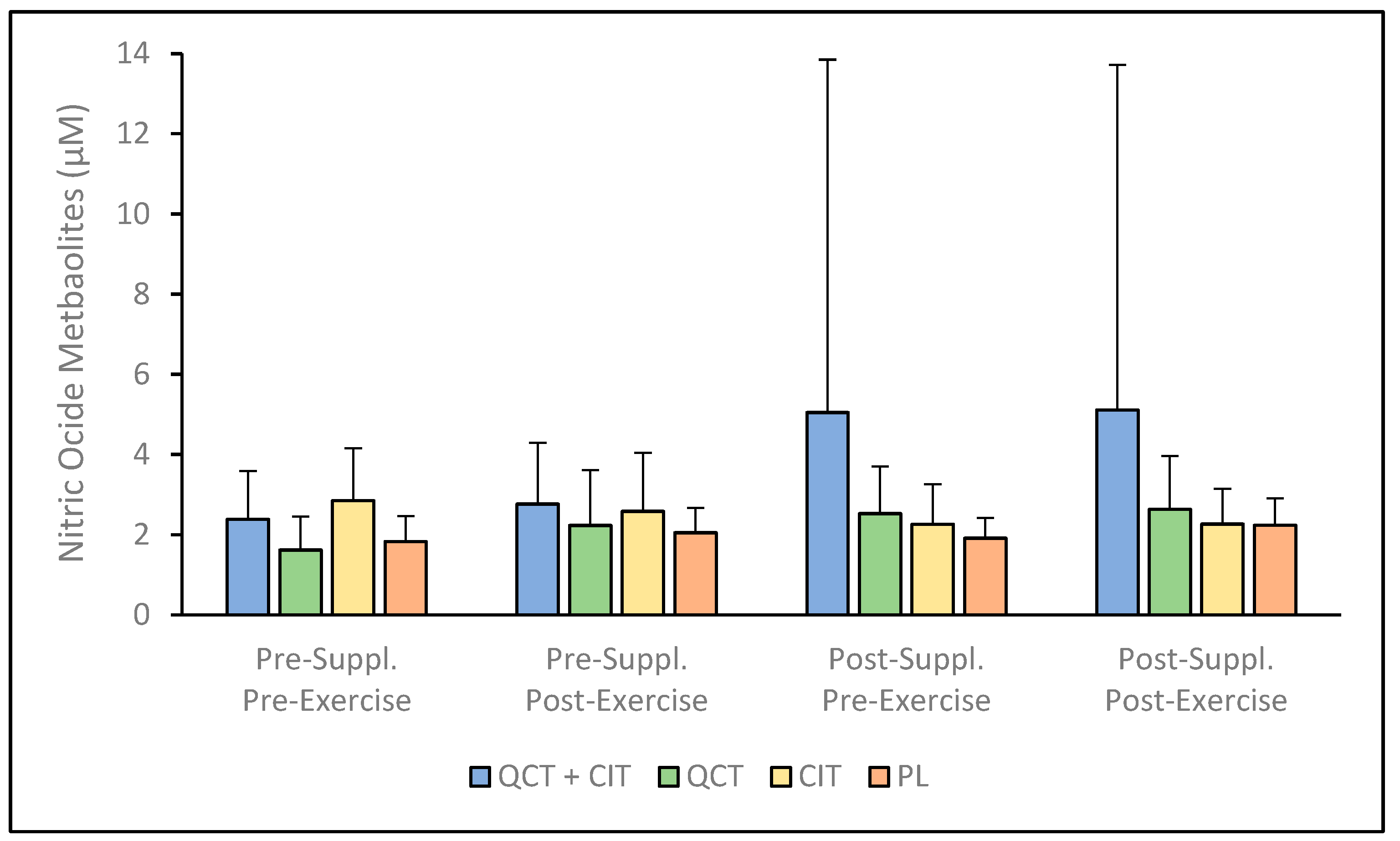
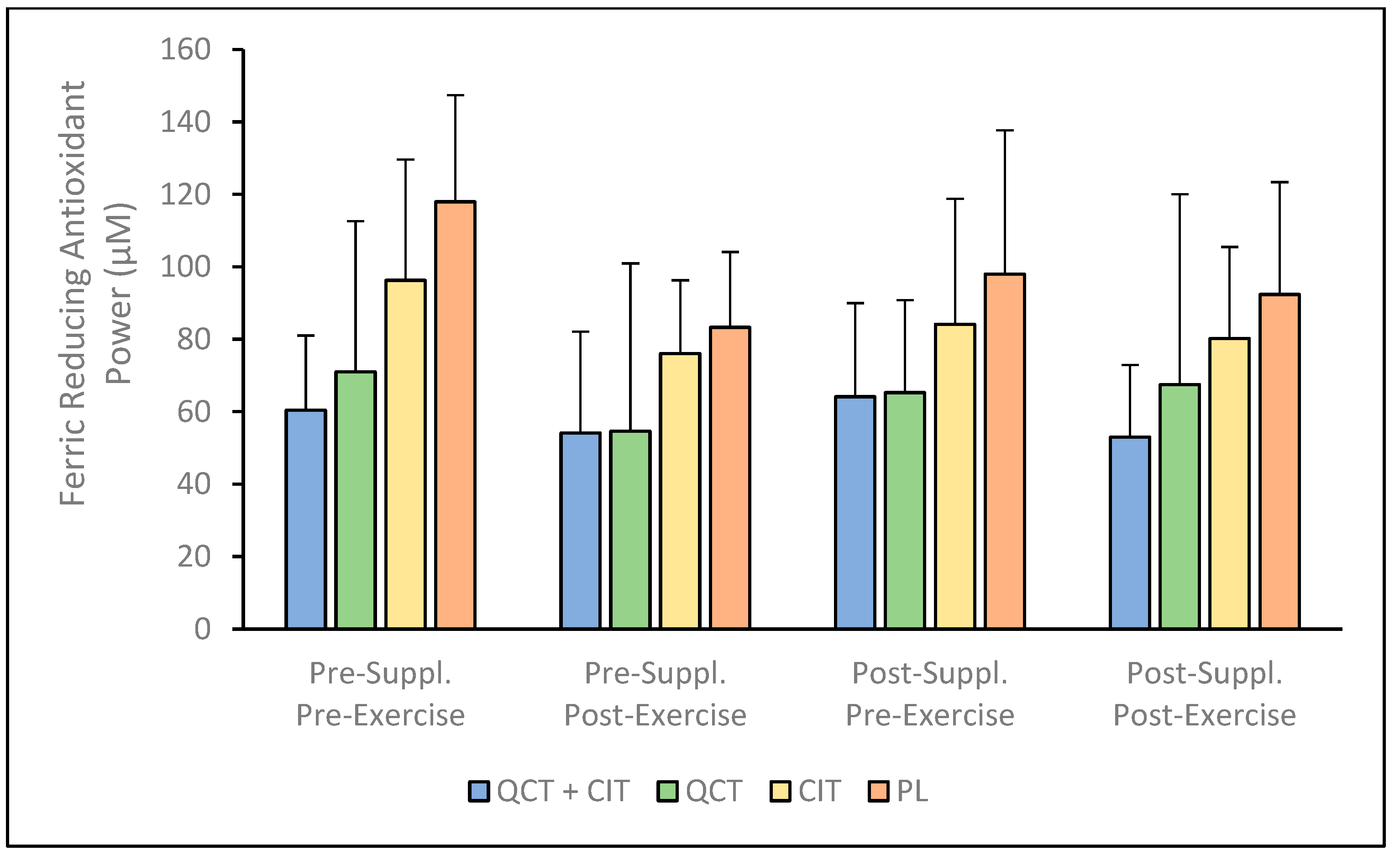
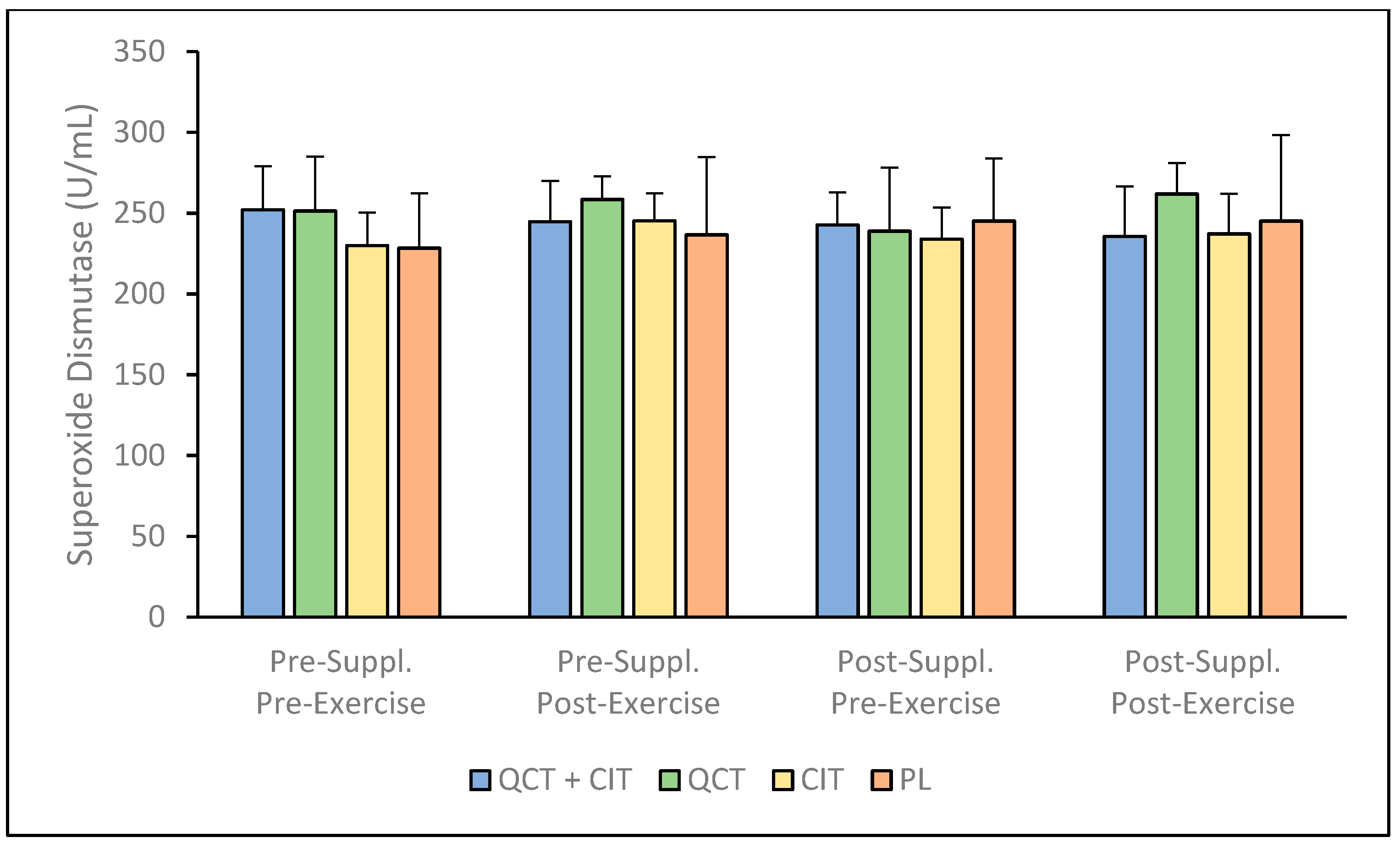
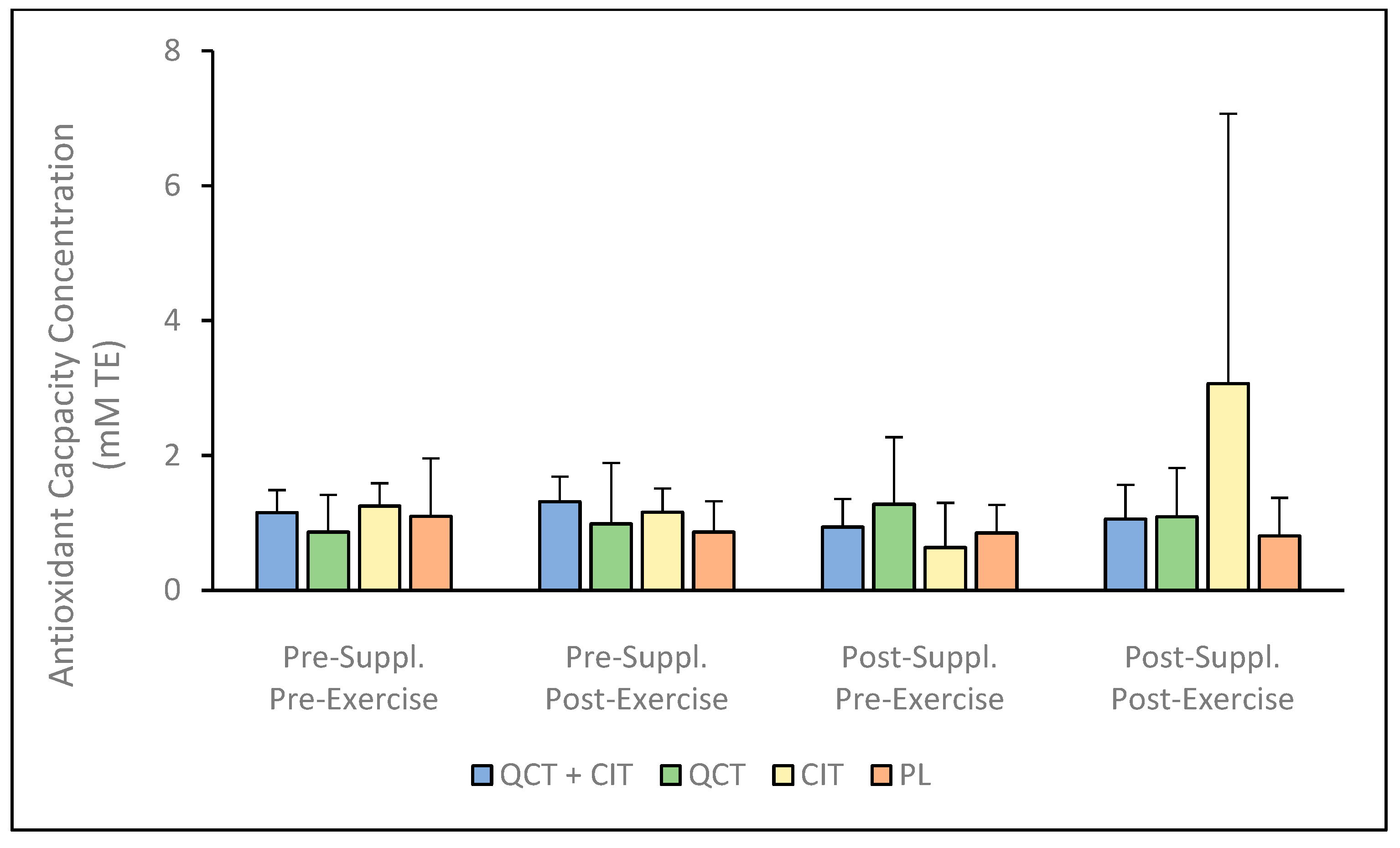
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salehi, B.; Machin, L.; Monzote, L.; Sharifi-Rad, J.; Ezzat, S.M.; Salem, M.A.; Merghany, R.M.; El Mahdy, N.M.; Kılıç, C.S.; Sytar, O. Therapeutic potential of quercetin: New insights and perspectives for human health. Acs Omega 2020, 5, 11849–11872. [Google Scholar] [CrossRef]
- Bentz, A.B. A Review of Quercetin: Chemistry, Antioxident Properties, and Bioavailability. J. Young Investig. 2017. Available online: https://www.jyi.org/2009-april/2017/10/15/a-review-of-quercetin-chemistry-antioxidant-properties-and-bioavailability (accessed on 6 January 2025).
- Aghababaei, F.; Hadidi, M. Recent advances in potential health benefits of quercetin. Pharmaceuticals 2023, 16, 1020. [Google Scholar] [CrossRef]
- Vollmannová, A.; Bojňanská, T.; Musilová, J.; Lidiková, J.; Ňorbová, M. Quercetin as one of the most abundant represented biological valuable plant components with remarkable chemoprotective effects—A review. Heliyon 2024, 10, e33342. [Google Scholar] [CrossRef]
- Yang, D.; Wang, T.; Long, M.; Li, P. Quercetin: Its main pharmacological activity and potential application in clinical medicine. Oxidative Med. Cell Longev. 2020, 2020, 8825387. [Google Scholar] [CrossRef]
- Jantan, I.; Ahmad, W.; Bukhari, S.N.A. Plant-derived immunomodulators: An insight on their preclinical evaluation and clinical trials. Front. Plant Sci. 2015, 6, 655. [Google Scholar] [CrossRef] [PubMed]
- Loke, W.M.; Hodgson, J.M.; Proudfoot, J.M.; McKinley, A.J.; Puddey, I.B.; Croft, K.D. Pure dietary flavonoids quercetin and (−)-epicatechin augment nitric oxide products and reduce endothelin-1 acutely in healthy men. Am. J. Clin. Nutr. 2008, 88, 1018–1025. [Google Scholar] [CrossRef] [PubMed]
- Olabiyi, A.A.; Tope-Eniola, O.S.; Oluwatuyi, A.O.; Alabi, O.; Ademola, O.G.; Oguntimehin, O.M.; AlliSmith, Y.R. Quercetin boosts nitric oxide levels and modulates the activities of arginase, acetylcholinesterase and adenosine deaminase in the corpus cavernosum of cyclosporine-treated rats. Andrologia 2022, 54, e14404. [Google Scholar] [CrossRef]
- Xu, D.; Hu, M.-J.; Wang, Y.-Q.; Cui, Y.-L. Antioxidant activities of quercetin and its complexes for medicinal application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef]
- Tian, C.; Liu, X.; Chang, Y.; Wang, R.; Lv, T.; Cui, C.; Liu, M. Investigation of the anti-inflammatory and antioxidant activities of luteolin, kaempferol, apigenin and quercetin. S. Afr. J. Bot. 2021, 137, 257–264. [Google Scholar] [CrossRef]
- Méndez, I.; Vázquez-Cuevas, F.; Hernández-Muñoz, R.; Valente-Godínez, H.; Vázquez-Martínez, O.; Díaz-Muñoz, M. Redox Reactions in the Physiopathology of the Liver; InTech: London, UK, 2017. [Google Scholar] [CrossRef]
- Collins, J.K.; Wu, G.; Perkins-Veazie, P.; Spears, K.; Claypool, P.L.; Baker, R.A.; Clevidence, B.A. Watermelon consumption increases plasma arginine concentrations in adults. Nutrition 2007, 23, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, A.; Wong, A.; Jaime, S.J.; Gonzales, J.U. Influence of L-citrulline and watermelon supplementation on vascular function and exercise performance. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Uyanga, V.A.; Amevor, F.K.; Liu, M.; Cui, Z.; Zhao, X.; Lin, H. Potential Implications of Citrulline and Quercetin on Gut Functioning of Monogastric Animals and Humans: A Comprehensive Review. Nutrients 2021, 13, 3782. [Google Scholar] [CrossRef] [PubMed]
- Azizi, S.; Ebrahimi-Mameghani, M.; Mobasseri, M.; Karamzad, N.; Mahdavi, R. Oxidative stress and nitrate/nitrite (NOx) status following citrulline supplementation in type 2 diabetes: A randomised, double-blind, placebo-controlled trial. J. Hum. Nutr. Diet. 2021, 34, 64–72. [Google Scholar] [CrossRef]
- Allerton, T.D.; Proctor, D.N.; Stephens, J.M.; Dugas, T.R.; Spielmann, G.; Irving, B.A. l-Citrulline supplementation: Impact on cardiometabolic health. Nutrients 2018, 10, 921. [Google Scholar] [CrossRef]
- Forstermann, U.; Münzel, T. Endothelial nitric oxide synthase in vascular disease: From marvel to menace. Circulation 2006, 113, 1708–1714. [Google Scholar] [CrossRef] [PubMed]
- Villareal, M.O.; Matsukawa, T.; Isoda, H. l-Citrulline Supplementation-Increased Skeletal Muscle PGC-1α Expression Is Associated with Exercise Performance and Increased Skeletal Muscle Weight. Mol. Nutr. Food Res. 2018, 62, 1701043. [Google Scholar] [CrossRef] [PubMed]
- Schwedhelm, E.; Maas, R.; Freese, R.; Jung, D.; Lukacs, Z.; Jambrecina, A.; Spickler, W.; Schulze, F.; Böger, R.H. Pharmacokinetic and pharmacodynamic properties of oral L-citrulline and L-arginine: Impact on nitric oxide metabolism. Br. J. Clin. Pharmacol. 2008, 65, 51–59. [Google Scholar] [CrossRef]
- Kiani, A.K.; Bonetti, G.; Medori, M.C.; Caruso, P.; Manganotti, P.; Fioretti, F.; Nodari, S.; Connelly, S.T.; Bertelli, M. Dietary supplements for improving nitric-oxide synthesis. J. Prev. Med. Hyg. 2022, 63, E239. [Google Scholar]
- Kurtz, J.A.; Grazer, J.; Wilson, K.; Feresin, R.G.; Doyle, J.A.; Middleton, R.; Devis, E.; VanDusseldorp, T.A.; Fasczewski, K.; Otis, J. The effect of quercetin and citrulline on cycling time trial performance. J. Int. Soc. Sports Nutr. 2024, 21, 2416909. [Google Scholar] [CrossRef] [PubMed]
- McKay, T.; Lyon, D.; Sarker-Nag, A.; Priyadarsini, S.; Asara, J.; Karamichos, D. Quercetin attenuates lactate production and extracellular matrix secretion in keratoconus. Sci. Rep. 2015, 5, 9003. [Google Scholar] [CrossRef] [PubMed]
- McKay, A.K.; Stellingwerff, T.; Smith, E.S.; Martin, D.T.; Mujika, I.; Goosey-Tolfrey, V.L.; Sheppard, J.; Burke, L.M. Defining Training and Performance Caliber: A Participant Classification Framework. Int. J. Sports Physiol. Perform. 2022, 17, 317–331. [Google Scholar] [CrossRef]
- Klitzke Borszcz, F. Is the functional threshold power interchangeable. IJSPP 2019, 2018, 0572. [Google Scholar]
- Zavorsky, G.; Murias, J.; Gow, J.; Kim, D.; Poulin-Harnois, C.; Kubow, S.; Lands, L. Laboratory 20-km cycle time trial reproducibility. Int. J. Sports Med. 2007, 28, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Hawley, J.A.; Noakes, T.D. Peak power output predicts maximal oxygen uptake and performance time in trained cyclists. Eur. J. Appl. Physiol. Occup. Physiol. 1992, 65, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Bull, J.R.; Rowland, S.P.; Scherwitzl, E.B.; Scherwitzl, R.; Danielsson, K.G.; Harper, J. Real-world menstrual cycle characteristics of more than 600,000 menstrual cycles. NPJ Digit. Med. 2019, 2, 83. [Google Scholar] [CrossRef]
- Carmichael, M.A.; Thomson, R.L.; Moran, L.J.; Wycherley, T.P. The Impact of Menstrual Cycle Phase on Athletes’ Performance: A Narrative Review. Int. J. Environ. Res. Public Health 2021, 18, 1667. [Google Scholar] [CrossRef]
- Bondarev, D.; Finni, T.; Kokko, K.; Kujala, U.M.; Aukee, P.; Kovanen, V.; Laakkonen, E.K.; Sipilä, S. Physical performance during the menopausal transition and the role of physical activity. J. Gerontol. Ser. A 2021, 76, 1587–1590. [Google Scholar] [CrossRef] [PubMed]
- Burrows, M.; Peters, C.E. The influence of oral contraceptives on athletic performance in female athletes. Sports Med. 2007, 37, 557–574. [Google Scholar] [CrossRef] [PubMed]
- Tramontin, A.F.; Borszcz, F.K.; Costa, V. Functional Threshold Power Estimated from a 20-min Time-trial Test is Warm-up-dependent. Int. J. Sports Med. 2022, 43, 411–417. [Google Scholar] [CrossRef]
- MacInnis, M.J.; Thomas, A.C.; Phillips, S.M. The reliability of 4-min and 20-min time trials and their relationships to functional threshold power in trained cyclists. Int. J. Sports Physiol. Perform. 2019, 14, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Schabort, E.J.; Hawley, J.A.; Hopkins, W.G.; Mujika, I.; Noakes, T.D. A new reliable laboratory test of endurance performance for road cyclists. Med. Sci. Sports Exerc. 1998, 30, 1744–1750. [Google Scholar] [CrossRef] [PubMed]
- Bortolotti, H.; Altimari, L.R.; Vitor-Costa, M.; Cyrino, E.S. Performance during a 20-km cycling time-trial after caffeine ingestion. J. Int. Soc. Sports Nutr. 2014, 11, 45. [Google Scholar] [CrossRef] [PubMed]
- Micklewright, D.; Papadopoulou, E.; Swart, J.; Noakes, T. Previous experience influences pacing during 20 km time trial cycling. Br. J. Sports Med. 2010, 44, 952–960. [Google Scholar] [CrossRef]
- Borszcz, F.K.; Tramontin, A.F.; Costa, V.P. Is the functional threshold power interchangeable with the maximal lactate steady state in trained cyclists? Int. J. Sports Physiol. Perform. 2019, 14, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Borszcz, F.K.; Tramontin, A.F.; Bossi, A.H.; Carminatti, L.J.; Costa, V.P. Functional threshold power in cyclists: Validity of the concept and physiological responses. Int. J. Sports Med. 2018, 39, 737–742. [Google Scholar]
- Lillo-Beviá, J.R.; Courel-Ibáñez, J.; Cerezuela-Espejo, V.; Morán-Navarro, R.; Martínez-Cava, A.; Pallarés, J.G. Is the functional threshold power a valid metric to estimate the maximal lactate steady state in cyclists? J. Strength Cond. Res. 2022, 36, 167–173. [Google Scholar] [CrossRef]
- Nieman, D.C.; Henson, D.A.; Maxwell, K.R.; Williams, A.S.; McAnulty, S.R.; Jin, F.; Shanely, R.A.; Lines, T.C. Effects of quercetin and EGCG on mitochondrial biogenesis and immunity. Med. Sci. Sports Exerc. 2009, 41, 1467–1475. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Henson, D.A.; Davis, J.M.; Dumke, C.L.; Gross, S.J.; Jenkins, D.P.; Murphy, E.A.; Carmichael, M.D.; Quindry, J.C.; McAnulty, S.R. Quercetin ingestion does not alter cytokine changes in athletes competing in the Western States Endurance Run. J. Interferon Cytokine Res. 2007, 27, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- McAnulty, S.R.; McAnulty, L.S.; Nieman, D.C.; Quindry, J.C.; Hosick, P.A.; Hudson, M.H.; Still, L.; Henson, D.A.; Milne, G.L.; Morrow, J.D. Chronic quercetin ingestion and exercise-induced oxidative damage and inflammation. Appl. Physiol. Nutr. Metab. 2008, 33, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Cureton, K.J.; Tomporowski, P.D.; Singhal, A.; Pasley, J.D.; Bigelman, K.A.; Lambourne, K.; Trilk, J.L.; McCully, K.K.; Arnaud, M.J.; Zhao, Q. Dietary quercetin supplementation is not ergogenic in untrained men. J. Appl. Physiol. 2009, 107, 1095–1104. [Google Scholar] [CrossRef]
- MacRae, H.S.; Mefferd, K.M. Dietary antioxidant supplementation combined with quercetin improves cycling time trial performance. Int. J. Sport Nutr. Exerc. Metab. 2006, 16, 405–419. [Google Scholar] [CrossRef]
- Bailey, S.J.; Blackwell, J.R.; Lord, T.; Vanhatalo, A.; Winyard, P.G.; Jones, A.M. l-Citrulline supplementation improves O2 uptake kinetics and high-intensity exercise performance in humans. J. Appl. Physiol. 2015, 119, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Stanelle, S.T.; McLaughlin, K.L.; Crouse, S.F. One week of L-citrulline supplementation improves performance in trained cyclists. J. Strength Cond. Res. 2020, 34, 647–652. [Google Scholar] [CrossRef]
- Davis, J.M.; Carlstedt, C.J.; Chen, S.; Carmichael, M.D.; Murphy, E.A. The dietary flavonoid quercetin increases VO2max and endurance capacity. Int. J. Sport Nutr. Exerc. Metab. 2010, 20, 56–62. [Google Scholar] [CrossRef]
- Daneshvar, P.; Hariri, M.; Ghiasvand, R.; Askari, G.; Darvishi, L.; Mashhadi, N.S.; Khosravi-Boroujeni, H. Effect of eight weeks of quercetin supplementation on exercise performance, muscle damage and body muscle in male badminton players. Int. J. Prev. Med. 2013, 4, S53. [Google Scholar]
- Dabeek, W.M.; Marra, M.V. Dietary quercetin and kaempferol: Bioavailability and potential cardiovascular-related bioactivity in humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, inflammation and immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Qi, W.; Qi, W.; Xiong, D.; Long, M. Quercetin: Its antioxidant mechanism, antibacterial properties and potential application in prevention and control of toxipathy. Molecules 2022, 27, 6545. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D. Review Methods of quantitative analysis of the nitric oxide metabolites nitrite and nitrate in human biological fluids. Free Radic. Res. 2005, 39, 797–815. [Google Scholar] [CrossRef]
- Stevens, C.J.; Dascombe, B.J. The reliability and validity of protocols for the assessment of endurance sports performance: An updated review. Meas. Phys. Educ. Exerc. Sci. 2015, 19, 177–185. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J. [2] Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar]
- Rosa, A.C.; Corsi, D.; Cavi, N.; Bruni, N.; Dosio, F. Superoxide dismutase administration: A review of proposed human uses. Molecules 2021, 26, 1844. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavior Science: Lawrence Eribaum Association; Lawrence Erlbaum Associates: Mahwah, NJ, USA, 1988. [Google Scholar]
- Reckelhoff, J.F.; Hennington, B.S.; Moore, A.G.; Blanchard, E.J.; Cameron, J. Gender differences in the renal nitric oxide (NO) system. Am. J. Hypertens. 1998, 11, 97–104. [Google Scholar] [CrossRef]
- Bescós García, R. The effect of nitric oxide donors on human performance. Sports Med. 2012, 42, 99–117. [Google Scholar] [CrossRef] [PubMed]
- Sandbakk, S.B.; Sandbakk, Ø.; Peacock, O.; James, P.; Welde, B.; Stokes, K.; Böhlke, N.; Tjønna, A.E. Effects of acute supplementation of L-arginine and nitrate on endurance and sprint performance in elite athletes. Nitric Oxide 2015, 48, 10–15. [Google Scholar] [CrossRef]
- Cook, G. The Effects of Chronic Arginine Supplementation on Muscle Strength and Hypertrophy Following Resistance Training. Ph.D. Thesis, Ohio Dominican University, Columbus, OH, USA, 2015. [Google Scholar]
- Wu, G.; Bazer, F.W.; Davis, T.A.; Kim, S.W.; Li, P.; Marc Rhoads, J.; Carey Satterfield, M.; Smith, S.B.; Spencer, T.E.; Yin, Y. Arginine metabolism and nutrition in growth, health and disease. Amino Acids 2009, 37, 153–168. [Google Scholar] [CrossRef]
- Di Meo, S.; Venditti, P. Mitochondria in exercise-induced oxidative stress. Neurosignals 2001, 10, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Tsao, J.-P.; Bernard, J.R.; Hsu, H.-C.; Hsu, C.-L.; Liao, S.-F.; Cheng, I.-S. Short-term oral quercetin supplementation improves post-exercise insulin sensitivity, antioxidant capacity and enhances subsequent cycling time to exhaustion in healthy adults: A pilot study. Front. Nutr. 2022, 9, 875319. [Google Scholar] [CrossRef] [PubMed]
- Boots, A.W.; Haenen, G.R.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef]
- Bendahan, D.; Mattei, J.P.; Ghattas, B.; Confort-Gouny, S.; Le Guern, M.-E.; Cozzone, P.J. Citrulline/malate promotes aerobic energy production in human exercising muscle. Br. J. Sports Med. 2002, 36, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Morita, M.; Kobayashi, Y.; Kamimura, A. Oral L-citrulline supplementation enhances cycling time trial performance in healthy trained men: Double-blind randomized placebo-controlled 2-way crossover study. J. Int. Soc. Sports Nutr. 2016, 13, 6. [Google Scholar] [CrossRef] [PubMed]
- Hämäläinen, M.; Nieminen, R.; Vuorela, P.; Heinonen, M.; Moilanen, E. Anti-inflammatory effects of flavonoids: Genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-κB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-κB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediat. Inflamm. 2007, 2007, 045673. [Google Scholar]
- Granado-Serrano, A.B.; Martín, M.A.; Bravo, L.; Goya, L.; Ramos, S. Quercetin modulates Nrf2 and glutathione-related defenses in HepG2 cells: Involvement of p38. Chem.-Biol. Interact. 2012, 195, 154–164. [Google Scholar] [CrossRef]
- Valaei, K.; Mehrabani, J.; Wong, A. Effects of L-citrulline supplementation on nitric oxide and antioxidant markers after high-intensity interval exercise in young men: A randomized controlled trial. Br. J. Nutr. 2022, 127, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Puga, G.M.; Novais, I.d.P.; Katsanos, C.S.; Zanesco, A. Combined effects of aerobic exercise and l-arginine ingestion on blood pressure in normotensive postmenopausal women: A crossover study. Life Sci. 2016, 151, 323–329. [Google Scholar] [CrossRef]
- Michailidis, Y.; Jamurtas, A.Z.; Nikolaidis, M.G.; Fatouros, I.G.; Koutedakis, Y.; Papassotiriou, I.; Kouretas, D. Sampling time is crucial for measurement of aerobic exercise-induced oxidative stress. Med. Sci. Sports Exerc. 2007, 39, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Cabrera, M.C.; Ristow, M.; Viña, J. Antioxidant supplements in exercise: Worse than useless? Am. J. Physiol.-Endocrinol. Metab. 2012, 302, E476–E477. [Google Scholar] [CrossRef]
- Mason, S.A.; Trewin, A.J.; Parker, L.; Wadley, G.D. Antioxidant supplements and endurance exercise: Current evidence and mechanistic insights. Redox Biol. 2020, 35, 101471. [Google Scholar] [CrossRef]
- Russo, M.; Spagnuolo, C.; Tedesco, I.; Bilotto, S.; Russo, G.L. The flavonoid quercetin in disease prevention and therapy: Facts and fancies. Biochem. Pharmacol. 2012, 83, 6–15. [Google Scholar] [CrossRef]
- Bazzucchi, I.; Patrizio, F.; Ceci, R.; Duranti, G.; Sabatini, S.; Sgrò, P.; Di Luigi, L.; Sacchetti, M. Quercetin Supplementation Improves Neuromuscular Function Recovery from Muscle Damage. Nutrients 2020, 12, 2850. [Google Scholar] [CrossRef] [PubMed]
- Bazzucchi, I.; Patrizio, F.; Ceci, R.; Duranti, G.; Sgrò, P.; Sabatini, S.; Di Luigi, L.; Sacchetti, M.; Felici, F. The effects of quercetin supplementation on eccentric exercise-induced muscle damage. Nutrients 2019, 11, 205. [Google Scholar] [CrossRef] [PubMed]
- Egert, S.; Wolffram, S.; Bosy-Westphal, A.; Boesch-Saadatmandi, C.; Wagner, A.E.; Frank, J.; Rimbach, G.; Mueller, M.J. Daily quercetin supplementation dose-dependently increases plasma quercetin concentrations in healthy humans. J. Nutr. 2008, 138, 1615–1621. [Google Scholar] [CrossRef] [PubMed]
- Rashid, J.; Kumar, S.S.; Job, K.M.; Liu, X.; Fike, C.D.; Sherwin, C.M. Therapeutic potential of citrulline as an arginine supplement: A clinical pharmacology review. Pediatr. Drugs 2020, 22, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Mazur, A.; Scalbert, A. Polyphenols and prevention of cardiovascular diseases. Curr. Opin. Lipidol. 2005, 16, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.J.; Wang, L.; DiCenzo, R.; Morris, M.E. Quercetin pharmacokinetics in humans. Biopharm. Drug Dispos. 2008, 29, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Konrad, M.; Nieman, D.C. Evaluation of quercetin as a countermeasure to exercise-induced physiological stress. In Antioxidants in Sports Nutrition; CRC Press-Taylor & Francis: Boca Raton, FL, USA, 2015; pp. 155–170. [Google Scholar]
- Filipa Almeida, A.; Borge, G.I.A.; Piskula, M.; Tudose, A.; Tudoreanu, L.; Valentova, K.; Williamson, G.; Santos, C.N. Bioavailability of quercetin in humans with a focus on interindividual variation. Compr. Rev. Food Sci. Food Saf. 2018, 17, 714–731. [Google Scholar] [CrossRef] [PubMed]
- Mueller, B.J.; Roberts, M.D.; Mobley, C.B.; Judd, R.L.; Kavazis, A.N. Nitric oxide in exercise physiology: Past and present perspectives. Front. Physiol. 2024, 15, 1504978. [Google Scholar]
- Rodriguez-Lopez, P.; Rueda-Robles, A.; Sánchez-Rodríguez, L.; Blanca-Herrera, R.M.; Quirantes-Piné, R.M.; Borrás-Linares, I.; Segura-Carretero, A.; Lozano-Sánchez, J. Analysis and screening of commercialized protein supplements for sports practice. Foods 2022, 11, 3500. [Google Scholar] [CrossRef] [PubMed]
- Michalczyk, M.; Czuba, M.; Zydek, G.; Zając, A.; Langfort, J. Dietary recommendations for cyclists during altitude training. Nutrients 2016, 8, 377. [Google Scholar] [CrossRef] [PubMed]
- Bardis, C.N.; Kavouras, S.A.; Arnaoutis, G.; Panagiotakos, D.B.; Sidossis, L.S. Mild dehydration and cycling performance during 5-kilometer hill climbing. J. Athl. Train. 2013, 48, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Ferré, M.; Bhupathiraju, S.N.; Hu, F.B. Use of metabolomics in improving assessment of dietary intake. Clin. Chem. 2018, 64, 82–98. [Google Scholar] [CrossRef]
- McAllister, R.M.; Laughlin, M.H. Vascular nitric oxide: Effects of physical activity,-importance for health. Essays Biochem. 2006, 42, 119–131. [Google Scholar] [PubMed]
- Jones, A.M. Dietary nitrate supplementation and exercise performance. Sports Med. 2014, 44, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Deruisseau, K.C.; Quindry, J.; Hamilton, K.L. Dietary antioxidants and exercise. J. Sports Sci. 2004, 22, 81–94. [Google Scholar] [CrossRef] [PubMed]
| QCT + CIT (n = 12) | QCT (n = 13) | CIT (n = 12) | PL (n = 13) | |
|---|---|---|---|---|
| Age (yr) | 33 ± 1 | 35 ± 1 | 37 ± 1 | 37 ± 1 |
| Height (cm) | 176 ± 2 | 173 ± 1 | 178 ± 1 | 177 ± 1 |
| Body Mass (kg) | 78.2 ± 1.8 | 74.6 ± 2.0 | 79.8 ± 1.2 | 77.4 ± 1.1 |
| Lean Tissue (kg or %) | 59.4 ± 1.1 | 55.8 ± 1.5 | 59.0 ± 1.1 | 59.0 ± 0.9 |
| Body Fat (%) | 20.9 ± 0.8 | 22.9 ± 0.9 | 23.3 ± 1.0 | 20.9 ± 1.0 |
| Total Weekly Cycling Volume (AU) | 1416 ± 54 | 1447 ± 62 | 1347 ± 120 | 1619 ± 87 |
| Sex, n (%) | ||||
| Females | 1 (2) | 4 (8) | 1 (2) | 2 (4) |
| Males | 11 (22) | 9 (18) | 11 (22) | 11 (22) |
| Average VO2 (mL/kg/min) | 40.01 ± 6.72 | 40.50 ± 7.18 * | 38.56 ± 5.66 * | 40.56 ± 7.43 |
| Time Trial Performance (minutes) | 30.27 ± 2.35 | 29.96 ± 2.36 | 30.93 ± 2.69 | 30.82 ± 3.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurtz, J.A.; Feresin, R.G.; Grazer, J.; Otis, J.; Wilson, K.E.; Doyle, J.A.; Zwetsloot, K.A. Effects of Quercetin and Citrulline on Nitric Oxide Metabolites and Antioxidant Biomarkers in Trained Cyclists. Nutrients 2025, 17, 224. https://doi.org/10.3390/nu17020224
Kurtz JA, Feresin RG, Grazer J, Otis J, Wilson KE, Doyle JA, Zwetsloot KA. Effects of Quercetin and Citrulline on Nitric Oxide Metabolites and Antioxidant Biomarkers in Trained Cyclists. Nutrients. 2025; 17(2):224. https://doi.org/10.3390/nu17020224
Chicago/Turabian StyleKurtz, Jennifer A., Rafaela G. Feresin, Jacob Grazer, Jeff Otis, Kathryn E. Wilson, J. Andrew Doyle, and Kevin A. Zwetsloot. 2025. "Effects of Quercetin and Citrulline on Nitric Oxide Metabolites and Antioxidant Biomarkers in Trained Cyclists" Nutrients 17, no. 2: 224. https://doi.org/10.3390/nu17020224
APA StyleKurtz, J. A., Feresin, R. G., Grazer, J., Otis, J., Wilson, K. E., Doyle, J. A., & Zwetsloot, K. A. (2025). Effects of Quercetin and Citrulline on Nitric Oxide Metabolites and Antioxidant Biomarkers in Trained Cyclists. Nutrients, 17(2), 224. https://doi.org/10.3390/nu17020224






