Glycyrrhiza uralensis Polysaccharide Modulates Characteristic Bacteria and Metabolites, Improving the Immune Function of Healthy Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and Structure of GP1
2.2. Antioxidant Capacity of GP1
2.2.1. 1,1-Diphenyl-2-picrylhydrazyl (DPPH) Radical Scavenging
2.2.2. 2,2′-Azino-bis3-ethylbenzothiazoline-6-sulfonic Acid) (ABTS) Radical Scavenging
2.3. Cytotoxicity and Immunomodulatory Activity of GP1
2.3.1. Cytotoxicity Analysis
2.3.2. Phagocytic Activity
2.3.3. Determination of Nitric Oxide (NO) and Cytokines
2.4. Animal Experiment
2.4.1. Animals and Experimental Process
2.4.2. Tissues’ Antioxidant Capacity
2.4.3. Histopathological Analysis
2.4.4. Goblet Cell Number
2.4.5. Immune Cell Number
2.4.6. Immune Factor Content
2.4.7. Gut Microbiota Structure Analysis
2.4.8. Proteomic Analysis
2.4.9. Non-Targeted Metabonomics Analysis
2.5. Statistical Analysis
3. Results
3.1. Antioxidant Activities of GP1
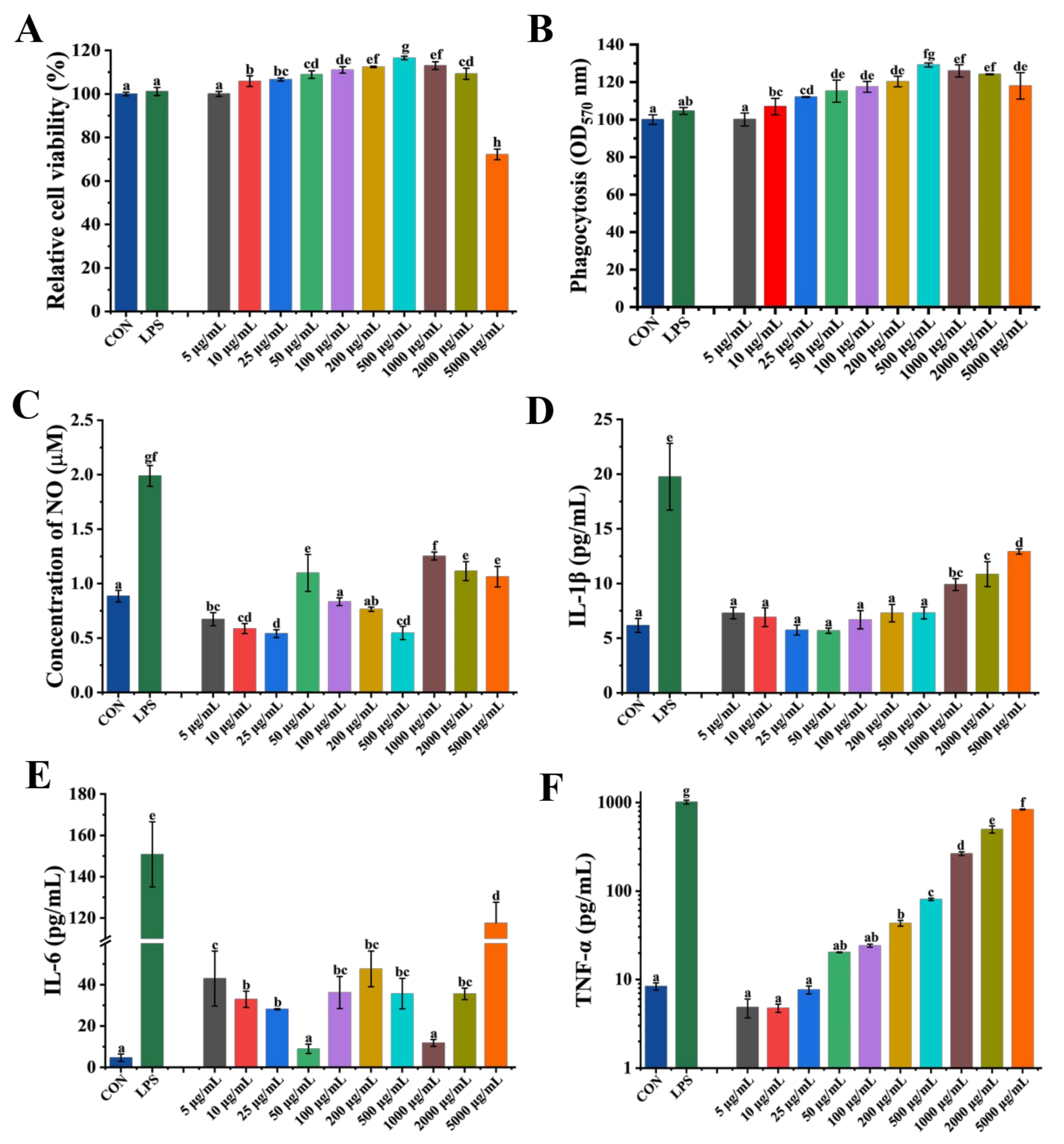
3.2. Cytotoxicity and Immunomodulatory Activity of GP1 in RAW 264.7
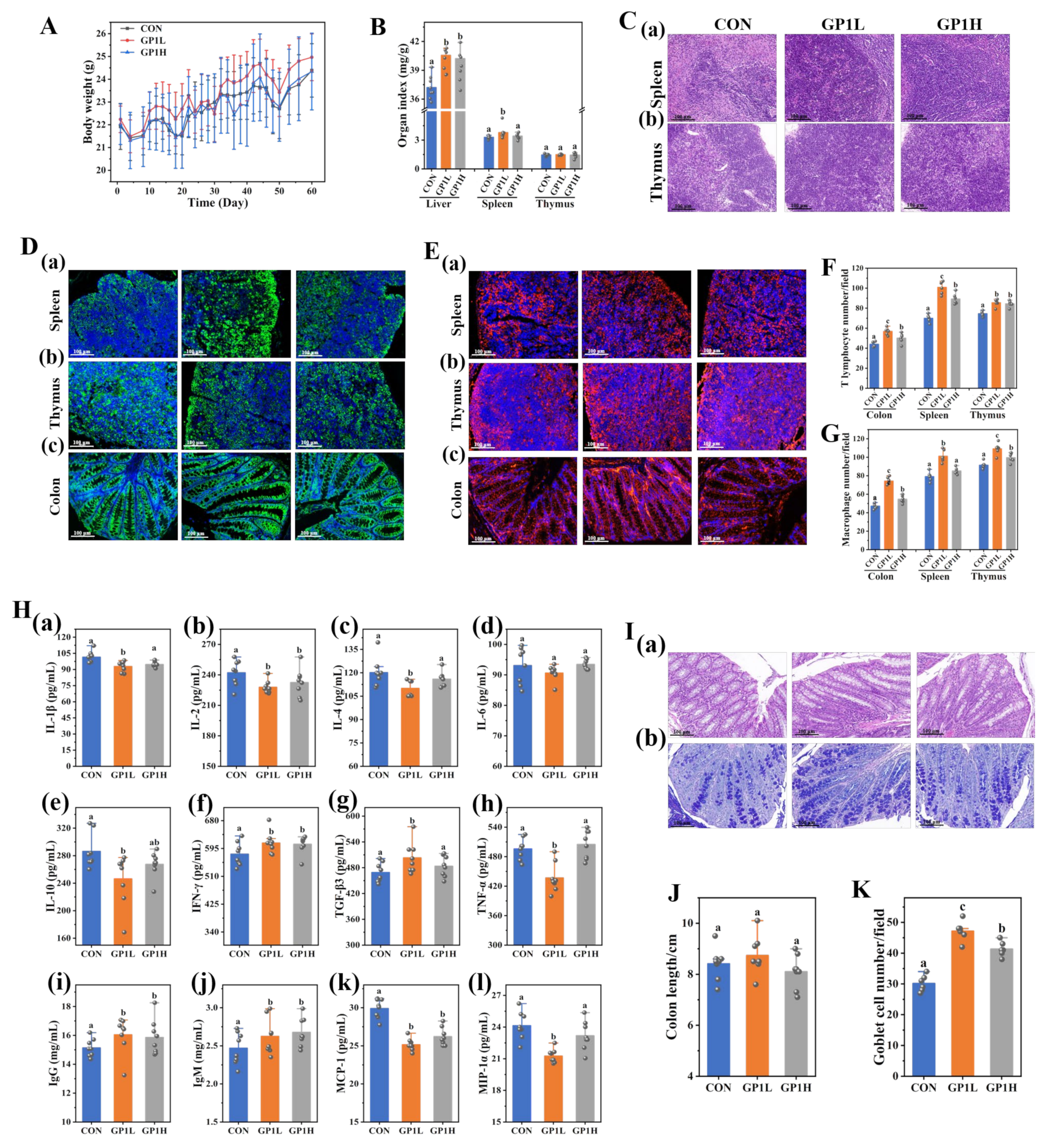
3.3. GP1 Enhances the Immunomodulatory Effect of Healthy Mice
3.4. GP1 Optimizes the Composition Structure of Gut Microbiota
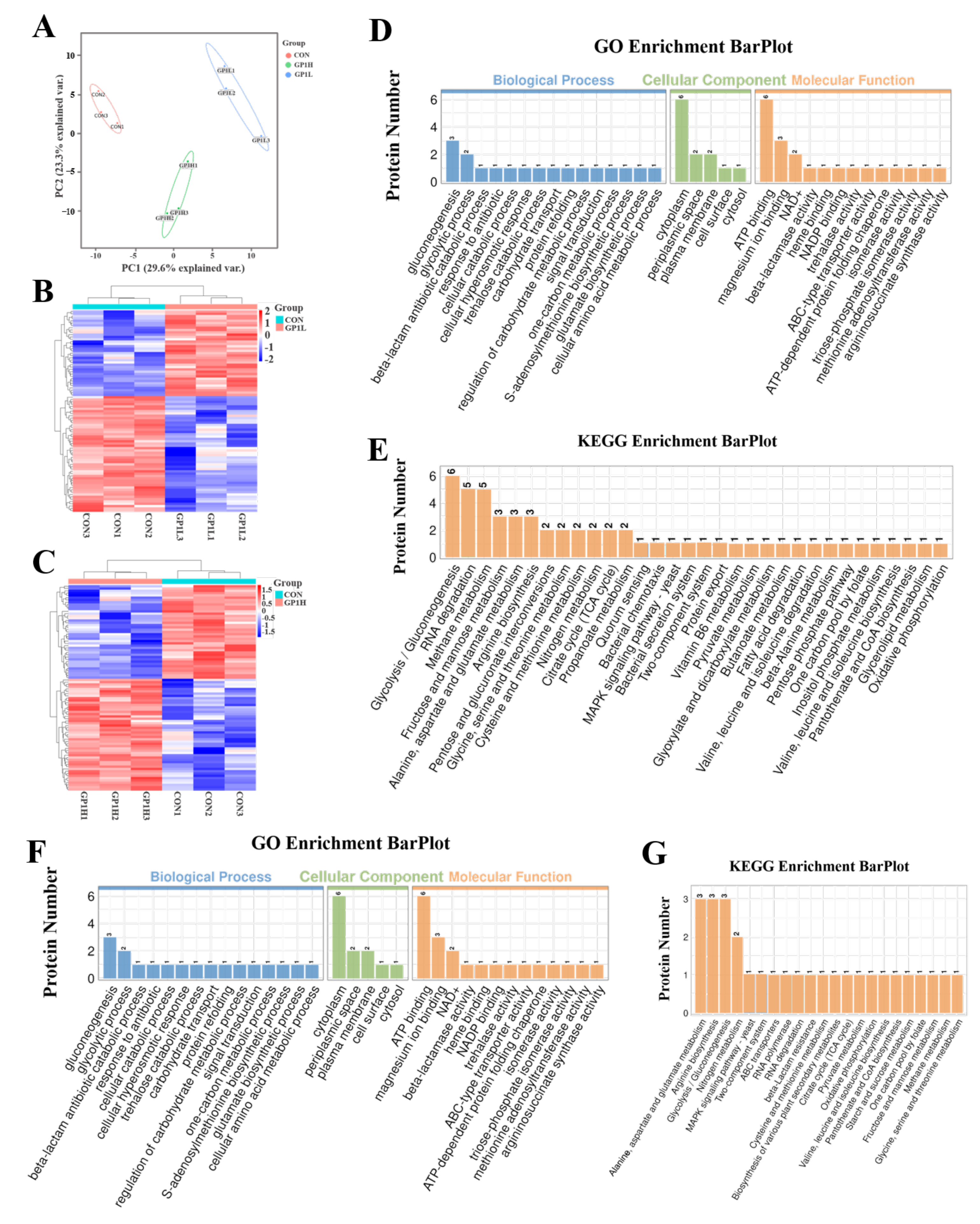
3.5. GP1 Changes Expression of Functional Proteins in Gut Microbiota
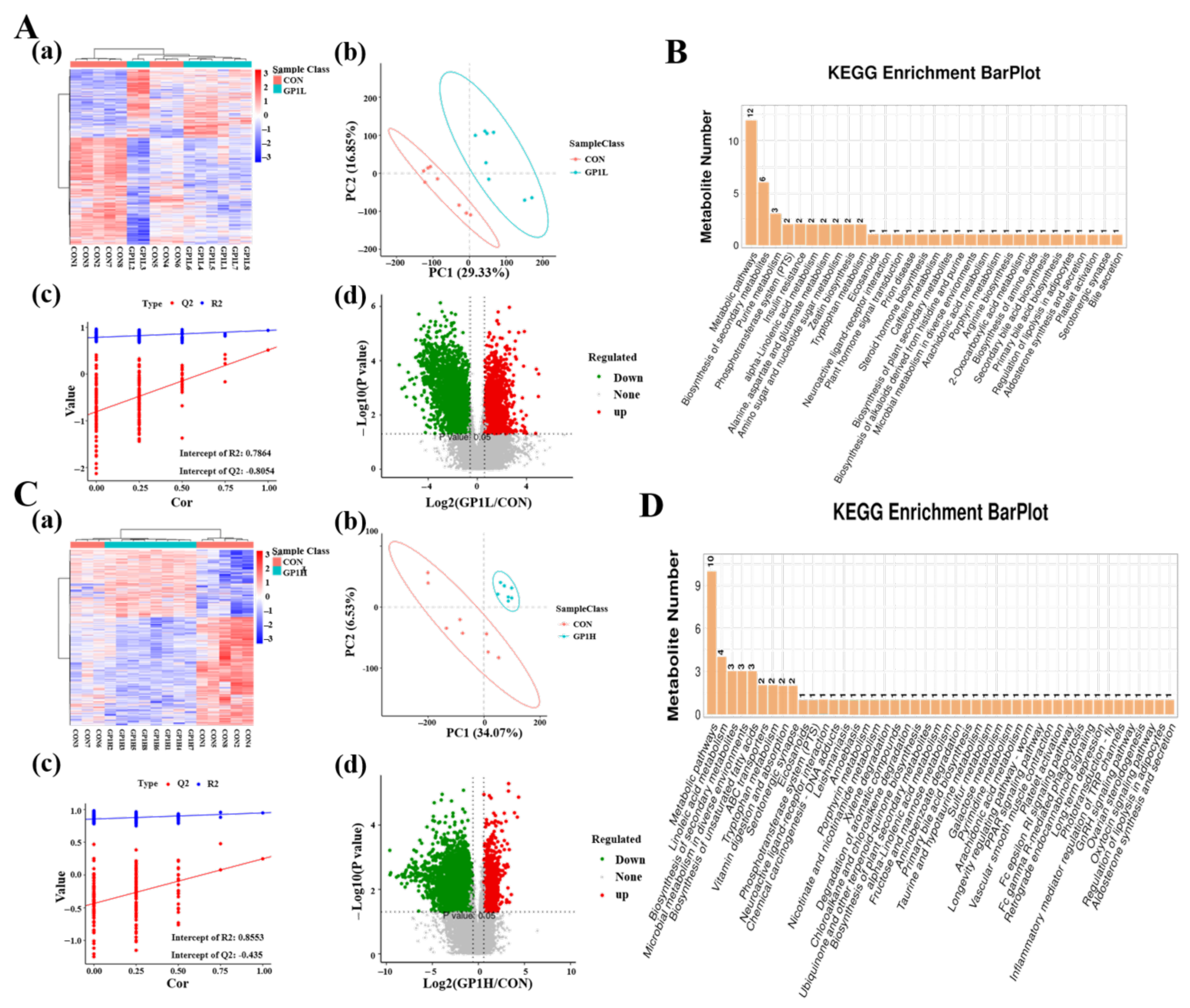
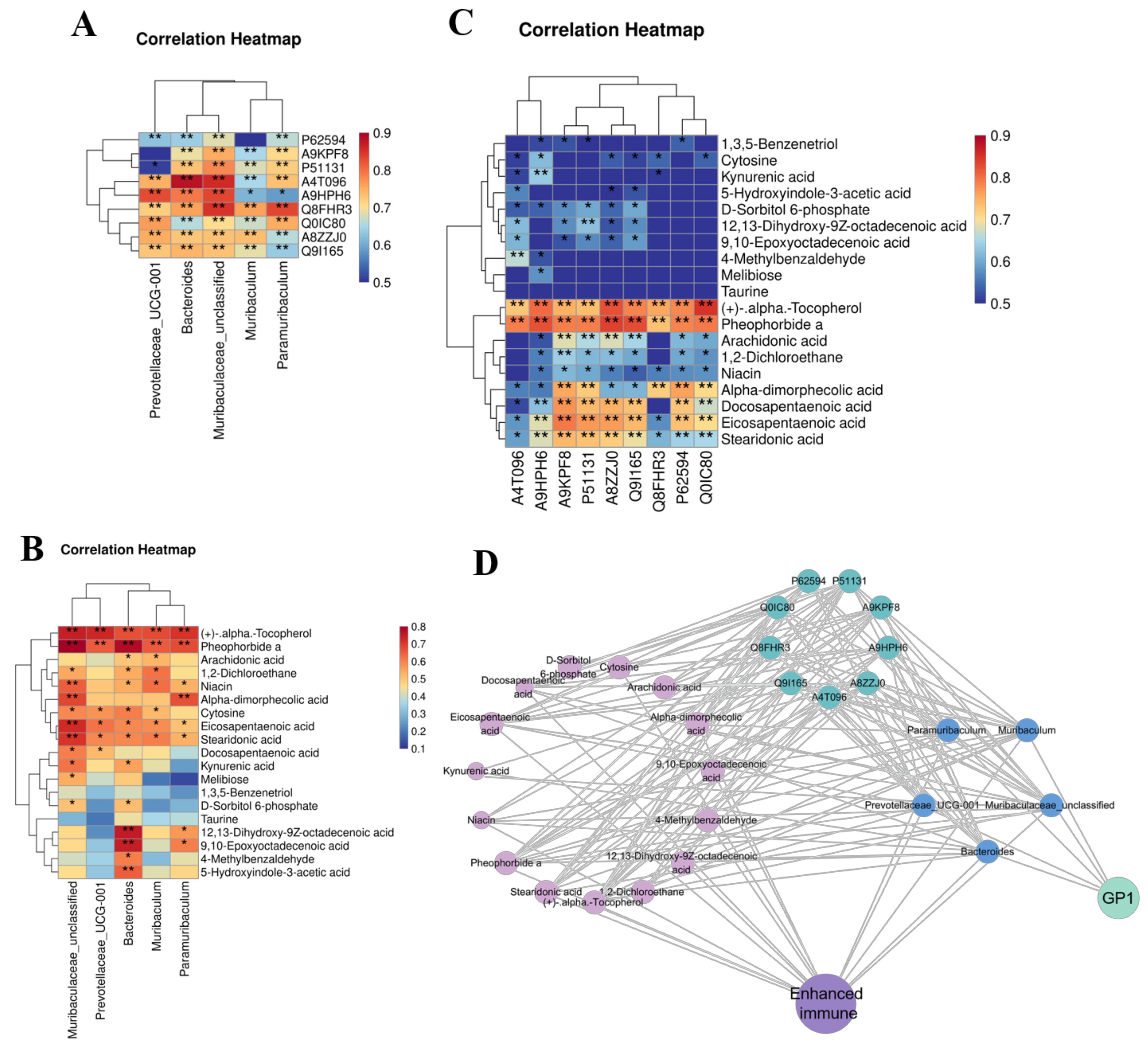
3.6. GP1 Promotes Health by Producing Beneficial Metabolites
3.7. Prebiotic Effect of GP1L
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviations | Full Name |
| GP | Glycyrrhiza polysaccharide |
| T-AOC | Total antioxidant capacity |
| GSH-Px | Glutathione peroxidase |
| SOD | Superoxide dismutase |
| MDA | Malondialdehyde |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| LPS | Lipopolysaccharide |
| PBS | Phosphate-Buffered Saline |
| CCK-8 | Cell Counting Kit-8 |
| NO | Nitric Oxide |
| IL-1β | Interleukin 1β |
| IL-2 | Interleukin 2 |
| IL-4 | Interleukin 4 |
| IL-6 | Interleukin 6 |
| IL-10 | Interleukin 10 |
| IFN-γ | Interferon-gamma |
| TGF-β3 | Transforming growth factor-β3 |
| TNF-α | Tumor necrosis factor α |
| IgG | immunoglobulin G |
| IgM | immunoglobulin M |
| MIP-1α | Macrophage Inflammatory Protein-1 alpha |
| MCP-1 | Monocyte chemoattractant protein-1 |
| KEGG | Kyoto encyclopedia of genes and genomes |
| GO | Gene ontology |
| PLS-DA | Partial Least Squares Discriminant Analysis |
References
- Liu, X.Q.; Su, S.P.; Yao, J.Y.; Zhang, X.Y.; Wu, Z.F.; Jia, L.L.; Liu, L.Y.; Hou, R.Y.; Farag, M.A.; Liu, L.L. Research advance about plant polysaccharide prebiotics, benefit for probiotics on gut homeostasis modulation. Food Biosci. 2024, 59, 103831. [Google Scholar] [CrossRef]
- Fu, Q.W.; Tian, M.Y.; Yang, Y.; Zhu, Y.; Zhou, H.L.; Tan, J.; Wang, J.; Huang, Q.W. Paotianxiong polysaccharides potential prebiotics: Structural analysis and prebiotic properties. Food Chem. 2024, 451, 139499. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.Q.; Wang, Y.K.; Huang, L.X.; Shen, M.Y.; Yu, Y.; Yu, Q.; Chen, Y.; Xie, J.H. Review of the relationships among polysaccharides, gut microbiota, and human health. Food Res. Int. 2020, 140, 109858. [Google Scholar] [CrossRef] [PubMed]
- Do, M.H.; Seo, Y.S.; Park, H.Y. Polysaccharides: Bowel health and gut microbiota. Crit. Rev. Food Sci. 2020, 61, 1212–1224. [Google Scholar] [CrossRef]
- Li, A.Y. Shennong’s Herbal Classics; Democracy and Construction Press: Beijing, China, 2021. (In Chinese) [Google Scholar]
- Li, S.Z. Compendium of Materia Medica: The World’s First Pharmacopoeia; Beijing Science and Technology Press: Beijing, China, 2006. (In Chinese) [Google Scholar]
- Simayi, Z.; Rozi, P.; Yang, X.; Ababaikeri, G.; Maimaitituoheti, W.; Bao, X.; Ma, S.; Askar, G.; Yadikar, N. Isolation, structural characterization, biological activity, and application of Glycyrrhiza polysaccharides: Systematic review. Int. J. Biol. Macromol. 2021, 183, 387–398. [Google Scholar] [CrossRef]
- Mutaillifu, P.; Bobakulov, K.; Abuduwaili, A.; Huojiaaihemaiti, H.; Nuerxiati, R.; Aisa, H.A.; Yili, A. Structural characterization and antioxidant activities of a water-soluble polysaccharide isolated from Glycyrrhiza glabra. Int. J. Biol. Macromol. 2020, 144, 751–759. [Google Scholar] [CrossRef]
- Aipire, A.; Yuan, P.; Aimaier, A.; Cai, S.; Mahabati, M.; Lu, J.; Ying, T.; Zhang, B.; Li, J. Preparation, Characterization, and Immuno-Enhancing Activity of Polysaccharides from Glycyrrhiza uralensis. Biomolecules 2020, 10, 159. [Google Scholar] [CrossRef]
- Song, W.D.; Wang, Y.Y.; Li, G.C.; Xue, S.N.; Zhang, G.L.; Dang, Y.Y.; Wang, B.H. Modulating the gut microbiota is involved in the effect of low-molecular-weight Glycyrrhiza polysaccharide on immune function. Gut Microbes 2023, 15, 2276814. [Google Scholar] [CrossRef]
- Chen, H.; Zeng, J.; Wang, B.; Cheng, Z.; Xu, J.; Gao, W.; Chen, K. Structural characterization and antioxidant activities of Bletilla striata polysaccharide extracted by different methods. Carbohyd. Polym. 2021, 266, 118149. [Google Scholar] [CrossRef]
- Ren, Y.; Zheng, G.; You, L.; Wen, L.; Li, C.; Fu, X.; Zhou, L. Structural characterization and macrophage immunomodulatory activity of a polysaccharide isolated from Gracilaria lemaneiformis. J. Funct. Foods 2017, 33, 286–296. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Groer, M.; Dutra, S.V.O.; Sarkar, A.; McSkimming, D.I. Gut Microbiota and Immune System Interactions. Microorganisms 2020, 8, 2046. [Google Scholar] [CrossRef]
- Fehily, S.R.; Basnayake, C.; Wright, E.K.; Kamm, M.A. The gut microbiota and gut disease. Intern. Med. J. 2021, 51, 1594–1604. [Google Scholar] [CrossRef]
- Hao, J.; Zhu, Y.F.; Zhang, Y.F.; Li, L.Z.; Li, Z.G.; Wang, L.; Qu, Y.D.; Qi, L.L.; Yu, H.L.; Wang, D. Structural characterization and hypolipidemic activity of a hetero-galactan purified from Sanghuangporus vaninii based on modulation of TLR4/NF-κB pathway. Carbohyd. Polym. 2024, 347, 122702. [Google Scholar] [CrossRef]
- Cortes-Perez, N.G.; de LeBlanc, A.D.; Gomez-Gutierrez, J.G.; LeBlanc, J.G.; Bermudez-Humaran, L.G. Probiotics and Trained Immunity. Biomolecules 2021, 11, 1402. [Google Scholar] [CrossRef]
- Wang, X.Y.; Yin, J.Y.; Nie, S.P.; Xie, M.Y. Isolation, purification and physicochemical properties of polysaccharide from fruiting body of Hericium erinaceus and its effect on colonic health of mice. Int. J. Biol. Macromol. 2017, 107, 1310–1319. [Google Scholar] [CrossRef]
- Huang, R.Z.; Zhang, J.; Xu, X.X.; Sun, M.Z.; Xu, L.G.; Kuang, H.; Xu, C.L.; Guo, L.L. The multiple benefits of bioactive polysaccharides: From the gut to overall health. Trends Food Sci. Tech. 2024, 152, 104677. [Google Scholar] [CrossRef]
- Su, Y.; Li, L. Structural characterization and antioxidant activity of polysaccharide from four auriculariales. Carbohyd. Polym. 2020, 229, 115407. [Google Scholar] [CrossRef]
- Teng, C.; Qin, P.; Shi, Z.; Zhang, W.; Yang, X.; Yao, Y.; Ren, G. Structural characterization and antioxidant activity of alkali-extracted polysaccharides from quinoa. Food Hydrocoll. 2021, 113, 106392. [Google Scholar] [CrossRef]
- Chen, F.; Huang, G.L. Extraction and antioxidant activities of cushaw polysaccharide. Int. J. Biol. Macromol. 2018, 120, 1646–1649. [Google Scholar] [CrossRef]
- Fan, J.; Feng, H.B.; Yu, Y.; Sun, M.X.; Liu, Y.R.; Li, T.Z.; Sun, X.; Liu, S.J.; Sun, M.D. Antioxidant activities of the polysaccharides of Chuanminshen violaceum. Carbohyd. Polym. 2017, 157, 629–636. [Google Scholar] [CrossRef]
- Jiang, J.; Kong, F.; Li, N.; Zhang, D.; Yan, C.; Lv, H. Purification, structural characterization and in vitro antioxidant activity of a novel polysaccharide from Boshuzhi. Carbohyd. Polym. 2016, 147, 365–371. [Google Scholar] [CrossRef]
- Zhu, Y.Q.; Chen, B.R.; Zhang, X.Y.; Akbar, M.T.; Wu, T.; Zhang, Y.Y.; Zhi, L.; Shen, Q. Exploration of the Muribaculaceae Family in the Gut Microbiota: Diversity, Metabolism, and Function. Nutrients 2024, 16, 2660. [Google Scholar] [CrossRef] [PubMed]
- Bang, S.; Shin, Y.H.; Ma, X.; Park, S.M.; Graham, D.B.; Xavier, R.J.; Clardy, J.A. Cardiolipin from Muribaculum intestinale Induces Antigen-Specific Cytokine Responses. J. Am. Chem. Soc. 2023, 145, 23422–23426. [Google Scholar] [CrossRef]
- Luo, Y.H.; Zhou, Y.; Huang, P.F.; Zhang, Q.Q.; Luan, F.Y.; Peng, Y.H.; Wei, J.L.; Li, N.N.; Wang, C.Y.; Wang, X.B. Causal relationship between gut Prevotellaceae and risk of sepsis: A two-sample Mendelian randomization and clinical retrospective study in the framework of predictive, preventive, and personalized medicine. EPMA J. 2023, 14, 697–711. [Google Scholar] [CrossRef]
- Usyk, M.; Pandey, A.; Hayes, R.B.; Moran, U.; Pavlick, A.; Osman, I.; Weber, J.S.; Ahn, J. Bacteroides vulgatus and Bacteroides dorei predict immune-related adverse events in immune checkpoint blockade treatment of metastatic melanoma. Genome Med. 2021, 13, 160. [Google Scholar] [CrossRef]
- Zhu, Z.P.; Luo, Y.R.; Lin, L.T.; Gao, T.H.; Yang, Q.S.; Fan, Y.Q.; Wang, S.Y.; Fu, C.M.; Liao, W. Modulating Effects of Turmeric Polysaccharides on Immune Response and Gut Microbiota in Cyclophosphamide-Treated Mice. J. Agric. Food Chem. 2024, 72, 3469–3482. [Google Scholar] [CrossRef]
- Pratap, K.; Majzoub, M.E.; Taki, A.C.; Hernandez, S.M.; Magnusson, M.; Glasson, C.R.K.; de Nys, R.; Thomas, T.; Lopata, A.L.; Kamath, S.D. The Algal Polysaccharide Ulvan and Carotenoid Astaxanthin Both Positively Modulate Gut Microbiota in Mice. Foods 2022, 11, 565. [Google Scholar] [CrossRef]
- Dong, J.; Liang, Q.X.; Niu, Y.; Jiang, S.J.; Zhou, L.; Wang, J.M.; Ma, C.Y.; Kang, W.Y. Effects of Nigella sativa seed polysaccharides on type 2 diabetic mice and gut microbiota. Int. J. Biol. Macromol. 2020, 159, 725–738. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Xing, H.; Bolotnikov, G.; Krämer, M.; Gotzmann, N.; Knippschild, U.; Kissmann, A.K.; Rosenau, F. Enriched Aptamer Libraries in Fluorescence-Based Assays for Rikenella microfusus-Specific Gut Microbiome Analyses. Microorganisms 2023, 11, 2266. [Google Scholar] [CrossRef]
- Bai, Y.J.; Zhou, Y.; Li, X.; Zhang, R.F.; Huang, F.; Fan, B.; Tong, L.T.; Wang, F.Z.; Zhang, M.W. Longan pulp polysaccharides regulate gut microbiota and metabolites to protect intestinal epithelial barrier. Food Chem. 2023, 422, 136225. [Google Scholar] [CrossRef]
- Hao, R.L.; Zhou, X.; Zhao, X.Y.; Lv, X.Q.; Zhu, X.Y.; Gao, N.N.; Jiang, Y.; Wu, M.Y. Flammulina velutipes polysaccharide counteracts cadmium-induced gut injury in mice via modulating gut inflammation, gut microbiota and intestinal barrier. Sci. Total Environ. 2023, 877, 162910. [Google Scholar] [CrossRef]
- Yang, Y.; Ye, H.Q.; Zhao, C.H.; Ren, L.; Wang, C.N.; Georgiev, M.I.; Xiao, J.B.; Zhang, T.H. Value added immunoregulatory polysaccharides of Hericium erinaceus and their effect on the gut microbiota. Carbohyd. Polym. 2021, 262, 117668. [Google Scholar] [CrossRef]
- Liu, G.M.; Liang, L.; Yu, G.Y.; Li, Q.H. Pumpkin polysaccharide modifies the gut microbiota during alleviation of type 2 diabetes in rats. Int. J. Biol. Macromol. 2018, 115, 711–717. [Google Scholar] [CrossRef]
- Kiran, S.; Parvathy, J.; Sukumaran, T.; Varghese, J.; Lakshmi, S.; Kumar, S.S.; Babu, A.; Harikumar, K.B.; Ragupathy, L. Immunomodulatory properties of D-sorbitol/Dmannitol incorporated linear step-growth Copolymers. Int. J. Polym. Mater. 2023, 72, 690–701. [Google Scholar] [CrossRef]
- Laureano, G.; Matos, A.R.; Figueiredo, A. Eicosapentaenoic acid: New insights into an oomycete-driven elicitor to enhance grapevine immunity. Plant Physiol. Bioch. 2024, 213, 108799. [Google Scholar] [CrossRef]
- Qi, H.Q.; Liu, Y.; Jian, F.J.; Xing, X.; Wang, J.H.; Li, C. Effects of dietary arachidonic acid (ARA) on immunity, growth and fatty acids of Apostichopus japonicus. Fish Shellfish Immunol. 2022, 127, 901–909. [Google Scholar] [CrossRef]
- Patel, D.; Goruk, S.; Newell, M.; Chen, G.Q.; Richard, C.; Field, C.J. Feeding a Bioactive Oil Enriched in Stearidonic Acid during Early Life Influences Immune System Maturation in Neonatal Sprague-Dawley Rats. J. Nutr. Nutr. Immunol. 2020, 150, 606–615. [Google Scholar] [CrossRef]
- Kaur, G.; Guo, X.; Sinclair, A.J. Short update on docosapentaenoic acid: A bioactive long-chain n-3 fatty acid. Curr. Opin. Clin. Nutr. 2016, 19, 88–91. [Google Scholar] [CrossRef]
- Tomita, K.; Nagura, T.; Okuhara, Y.; Nakajima-Adachi, H.; Shigematsu, N.; Aritsuka, T.; Kaminogawa, S.; Hachimura, S. Dietary melibiose regulates Th cell response and enhances the induction of oral tolerance. Biosci. Biotech. Bioch. 2007, 71, 2774–2780. [Google Scholar] [CrossRef][Green Version]
- Laureano, G.; Matos, A.R.; Figueiredo, A. Effects of taurine supplementation in low fishmeal diet on growth, immunity and intestinal health of Litopenaeus vannamei. Aquacult. Rep. 2023, 32, 101713. [Google Scholar]
- Feng, J.S.; Wang, L.; Chen, Y.B.; Xiong, Y.X.; Wu, Q.W.; Jiang, Z.Y.; Yi, H.B. Effects of niacin on intestinal immunity, microbial community and intestinal barrier in weaned piglets during starvation. Int. Immunopharmacol. 2021, 95, 107584. [Google Scholar] [CrossRef]
- Lodaya, R.N.; Kanitkar, A.P.; Ashraf, A.; Bamba, D.; Amiji, M.M.; O’Hagan, D.T. A Self-Emulsified Adjuvant System Containing the Immune Potentiator Alpha Tocopherol Induces Higher Neutralizing Antibody Responses than a Squalene-Only Emulsion When Evaluated with a Recombinant Cytomegalovirus (CMV) Pentamer Antigen in Mice. Pharmaceutics 2023, 15, 238. [Google Scholar] [CrossRef] [PubMed]
- Dimitroff, C.J. Leveraging fluorinated glucosamine action to boost antitumor immunity. Curr. Opin. Immunol. 2013, 25, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Kyoung, H.; Lee, J.J.; Cho, J.H.; Choe, J.; Kang, J.; Lee, H.; Liu, Y.H.; Kim, Y.; Kim, H.B.; Song, M. Dietary Glutamic Acid Modulates Immune Responses and Gut Health of Weaned Pigs. Animals 2021, 11, 504. [Google Scholar] [CrossRef]
- Wang, L.L.; Yan, C.H.; Wang, L.L.; Ai, C.Q.; Wang, S.T.; Shen, C.H.; Tong, Y.Q.; Song, S. Ascophyllum nodosum polysaccharide regulates gut microbiota metabolites to protect against colonic inflammation in mice. Food Funct. 2022, 14, 810–821. [Google Scholar] [CrossRef]
- Huang, R.; Zhu, Z.J.; Wu, S.J.; Wang, J.; Chen, M.F.; Liu, W.; Huang, A.H.; Zhang, J.M.; Wu, Q.P.; Ding, Y. Polysaccharides from Cordyceps militaris prevent obesity in association with modulating gut microbiota and metabolites in high-fat diet-fed mice. Food Res. Int. 2022, 157, 111197. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, W.; Zhang, T.; Wang, Y.; Xue, S.; Zhang, Y.; Zhang, G. Glycyrrhiza uralensis Polysaccharide Modulates Characteristic Bacteria and Metabolites, Improving the Immune Function of Healthy Mice. Nutrients 2025, 17, 225. https://doi.org/10.3390/nu17020225
Song W, Zhang T, Wang Y, Xue S, Zhang Y, Zhang G. Glycyrrhiza uralensis Polysaccharide Modulates Characteristic Bacteria and Metabolites, Improving the Immune Function of Healthy Mice. Nutrients. 2025; 17(2):225. https://doi.org/10.3390/nu17020225
Chicago/Turabian StyleSong, Wangdi, Taifeng Zhang, Yunyun Wang, Shengnan Xue, Yan Zhang, and Genlin Zhang. 2025. "Glycyrrhiza uralensis Polysaccharide Modulates Characteristic Bacteria and Metabolites, Improving the Immune Function of Healthy Mice" Nutrients 17, no. 2: 225. https://doi.org/10.3390/nu17020225
APA StyleSong, W., Zhang, T., Wang, Y., Xue, S., Zhang, Y., & Zhang, G. (2025). Glycyrrhiza uralensis Polysaccharide Modulates Characteristic Bacteria and Metabolites, Improving the Immune Function of Healthy Mice. Nutrients, 17(2), 225. https://doi.org/10.3390/nu17020225





