Abstract
Type 2 diabetes mellitus (T2DM), a serious metabolic disorder, is a worldwide health problem due to the alarming rise in prevalence and elevated morbidity and mortality. Chronic hyperglycemia, insulin resistance, and ineffective insulin effect and secretion are hallmarks of T2DM, leading to many serious secondary complications. These include, in particular, cardiovascular disorders, diabetic neuropathy, nephropathy and retinopathy, diabetic foot, osteoporosis, liver damage, susceptibility to infections and some cancers. Polyphenols such as flavonoids, phenolic acids, stilbenes, tannins, and lignans constitute an extensive and heterogeneous group of phytochemicals in fresh fruits, vegetables and their products. Various in vitro studies, animal model studies and available clinical trials revealed that flavonoids (e.g., quercetin, kaempferol, rutin, epicatechin, genistein, daidzein, anthocyanins), phenolic acids (e.g., chlorogenic, caffeic, ellagic, gallic acids, curcumin), stilbenes (e.g., resveratrol), tannins (e.g., procyanidin B2, seaweed phlorotannins), lignans (e.g., pinoresinol) have the ability to lower hyperglycemia, enhance insulin sensitivity and improve insulin secretion, scavenge reactive oxygen species, reduce chronic inflammation, modulate gut microbiota, and alleviate secondary complications of T2DM. The interaction between polyphenols and conventional antidiabetic drugs offers a promising strategy in the management and treatment of T2DM, especially in advanced disease stages. Synergistic effects of polyphenols with antidiabetic drugs have been documented, but also antagonistic interactions that may impair drug efficacy. Therefore, additional research is required to clarify mutual interactions in order to use the knowledge in clinical applications. Nevertheless, dietary polyphenols can be successfully applied as part of supportive treatment for T2DM, as they reduce both obvious clinical symptoms and secondary complications.
1. Introduction
Diabetes mellitus (DM), a serious non-communicable disease, represents a global health problem. It is regarded as a chronic metabolic disturbance that is unfortunately on the rise and is distinguished by hyperglycemia due to inadequate insulin production, altered insulin action, or a combination thereof [1,2]. Globally, 537 million people had DM in 2021. Type 2 DM (T2DM), also referred to as non-insulin-dependent diabetes, is the most prevalent type of the disease, accounting for approximately 90% of all cases. Glycated hemoglobin (HbA1c) criteria and fasting or 2-h plasma glucose criteria are typically used to diagnose T2DM. It is anticipated that 592 million people worldwide will have T2DM by 2035, with up to 374 million subjects at increased risk of developing this disease [3,4]. Since about 80% of people with T2DM reside in states with low and middle incomes, the prevalence of this disease is linked to economic disparities [5]. It is widely recognized that obesity, poor diet, physical inactivity, damaged mental health, and genetic predispositions are responsible for T2DM progression [4,6]. The main biological hallmark of T2DM is insulin resistance, which is related to elevated levels of reactive oxygen species (ROS), resulting in chronic oxidative stress and inflammatory process. Superoxide anion radical, hydroxyl radical, singlet oxygen, and hydrogen peroxide are among the most common ROS [7]. Furthermore, oxidized low-density lipoprotein (LDL) cholesterol, which is linked to the development of atherosclerotic plaque, can also be formed by an excess of ROS. Common comorbidities in T2DM include atherosclerosis and associated cardiovascular disorders [4,5]. Diabetic neuropathy, nephropathy and retinopathy, diabetic foot, osteoporosis (also termed as diabetic bone disease), liver damage, susceptibility to infections and some cancers (e.g., pancreas, liver, colon, endometrium, breast, bladder) are additional severe long-term complications of T2DM [8,9,10]. Increased oxidative stress, which results from an imbalance between ROS production and the antioxidant defense system (ADS), is also the cause of all of the secondary complications mentioned above [11]. The ADS is generally essential for preserving cellular redox homeostasis and scavenging ROS. Among its essential components are glutathione peroxidase (GPx), catalase (CAT), and superoxide dismutase (SOD), as well as sirtuins and peroxisome proliferator-activated receptor-gamma (PPAR-γ) [12].
T2DM manifests itself through two main pathological defects at the molecular level, i.e., disturbed insulin secretion due to pancreatic β-cell dysfunction and defective effect of insulin due to insulin receptor abnormalities [13]. Dysfunction of pancreatic β-cells is affected by endoplasmic reticulum stress and mitochondrial dysfunction. In the diabetic stage, there is a disorder of hepatic glucose production. In the liver and peripheral tissues, the effects of insulin are lessened, as evidenced by increased hepatic gluconeogenesis and glycogenolysis, decreased peripheral tissue uptake of glucose, and elevated blood glucose levels [10,14]. Insulin receptors belong to a family of receptors with tyrosine kinase activity. Upon binding of insulin to its receptor, conformational alterations of the receptor and autophosphorylation occur, along with the initiation of its tyrosine kinase activity. These changes are associated with tyrosine phosphorylation of insulin receptor substrate (IRS) proteins, which trigger intracellular signalling cascades [15,16]. As a result of the activation of the phosphatidylinositol-3-kinase (PI3K)/protein kinase B (PKB or Akt) pathway, insulin or insulin-like growth factor (IGF)-1 are activated. Gene expression and mitogenic effects related to insulin are regulated through the mitogen-activated protein kinase/Ras pathway [16]. Insulin is also able to enhance glucose uptake into the target (e.g., skeletal muscle, liver, adipose) tissues by activating the PI3K/Akt signalling pathway, regulating the transport of glucose transporter type 4 (GLUT4) from intracellular compartments to the surface of the cell membrane [5,15]. GLUT4 then imports glucose into the cell [17].
By modifying intracellular signalling pathways, enhancing the ADS, and neutralizing ROS, nutritional antioxidants found in a range of food sources have demonstrated encouraging potential in lowering oxidative stress and enhancing glycemic control [18,19]. In foods of plant origin, polyphenols constitute an extensive and heterogeneous group of phytochemicals. They are concentrated in fresh fruits, vegetables, seeds, spices, and beverages (e.g., tea, wine, coffee). Additionally, they exhibit powerful antioxidant and anti-inflammatory characteristics, and the consumption of foods rich in polyphenols is linked to a low prevalence of metabolic conditions including obesity, T2DM, and hypertension [10,20,21]. The classification commonly used in the medical literature divides polyphenols into non-flavonoid polyphenols and flavonoids [22]. On the basis of their carbon skeleton, non-flavonoid polyphenols can be classified into different subgroups. Phenolic acids, stilbenes, tannins, and lignans are among the most famous. The flavonoids include primarily flavanones, flavones, dihydroflavonols, flavonols, flavan-3-ols, isoflavones, anthocyanidins, and chalcones [23].
Summarizing the current understanding of dietary polyphenols, emphasizing their protective function in the management and treatment of T2DM, and outlining potential mechanisms of action were the primary goals of this review. In this context, detailed characterizations of flavonoids, phenolic acids, stilbenes, tannins, and lignans were conducted. Biosynthesis, metabolism, and the key conclusions from in vitro and in vivo studies suggesting their antidiabetic potential were discussed in each of the previously mentioned groups. Our primary focus was on available clinical trials. To make this review even more comprehensive, interactions between plant-derived polyphenols and antidiabetic drugs were also included.
2. Polyphenols as Antidiabetic Agents
Recent studies suggest that dietary polyphenols have a significant role in the management of T2DM through both insulin-dependent and insulin-independent mechanisms. Insulin-dependent approaches include protecting pancreatic β-cells and promoting their proliferation, reducing pancreatic β-cell apoptosis, alleviating oxidative stress, activating insulin signalling, and stimulating insulin secretion. Mechanisms independent of insulin involve inhibition of glucose absorption, digestive enzymes and advanced glycation end products (AGEs) formation, modification of the inflammatory response, and regulation of gut microbiota. In addition, dietary polyphenols are able to mitigate several serious secondary complications of T2DM [24,25].
More specifically, polyphenols moderate insulin resistance through several mechanisms. They can serve as inhibitors of IRS protein phosphorylation, promoters of GLUT4 translocation, enhancers of Akt phosphorylation, and efficient ROS scavengers [17]. Polyphenols have been shown to improve GLUT4 translocation by the activation of Rab proteins (GTPases and regulators of vesicular transport [26]) and insulin, PI3K, and adenosine monophosphate-activated protein kinase (AMPK) pathways [27,28]. The antioxidant activity of polyphenols may involve direct scavenging of free radicals by hydrogen atom or single electron transfer, or may act through a transition metal chelation mechanism [29], which activates also the action of antioxidant enzymes [1]. Polyphenols are able to protect pancreatic β-cells from oxidative stress both through antioxidant effects and by activating anti-apoptosis signalling [30]. Considering the inflammatory response, polyphenols suppress the production of pro-inflammatory cytokines, including interleukin (IL)-6, IL-8, tumour necrosis factor-alpha (TNF-α) via the inhibited activation of mitogen-activated protein kinases (MAPKs) and nuclear factor kappa B (NF-κB) pathways, and subsequently alleviate inflammatory processes [5]. They stimulate insulin secretion through upregulation of AMPK and IRS pathways and lower oxidative damage to pancreatic β-cells, thus preserving β-cell integrity [31]. Stimulation of insulin release probably occurs through transient inhibition of ATP-sensitive K+ channels and stimulation of whole-cell Ca2+ [17]. Polyphenols also modulate hepatic glucose production by upregulating carnitine palmitoyltransferase 1-β and acyl-CoA oxidase 1 and downregulating phosphoenolpyruvate carboxylase and glucose-6 phosphatase [32]. They further serve as inhibitors of carbohydrate-digesting enzymes and glucose absorption by interacting with α-amylase, α-glucosidase, and sodium-dependent glucose transporter 1 (SLGT1) [33]. Moreover, they activate glucose uptake receptors in insulin-sensitive tissues [34].
Mounting evidence suggests that gut dysbiosis, characterized by a disrupted intestinal balance and an increased prevalence of unfavourable gut microorganisms, plays a significant role in T2DM [35]. Higher levels of Escherichia and Prevotella have been detected in T2DM patients, whereas beneficial bacteria such as Bifidobacterium and Roseburia are more abundant in healthy individuals [36]. At the phylum level, the Firmicutes to Bacteroidetes ratio is elevated in patients compared to non-diabetic controls [37]. It is widely recognized that short-chain fatty acids (SCFAs), the major end products of bacterial fermentation in the gut, may also play a key role in the etiology of T2DM [38,39]. Data indicate that butyrate-producing bacterial taxa are significantly reduced in T2DM subjects [40]. Butyrate, a SCFA produced by gut bacteria in the colon, can prevent the development of insulin resistance [41,42,43,44]. Therefore, gut microbiota modulation is crucial for restoring intestinal balance, improving insulin sensitivity and glucose metabolism, and reducing the risk of DM-associated complications.
Polyphenols and their microbiota-derived metabolites can effectively modulate gut microbiota balance and improve glucose metabolism by promoting the growth of beneficial bacteria, including Bifidobacteria, Akkermansia, and Faecalibacterium prausnitzii [45]. Additionally, polyphenols exert antidiabetic, antihypertensive, and anti-inflammatory activities [46]. Supplementation with prebiotics such as inulin, galactooligosaccharides, fructooligosaccharides, pectic oligosaccharides, starch, polyphenols, and β-glucan, combined with Dendrobium officinale, has been shown to promote glycemic control in T2DM [47]. An in vitro study demonstrated that oregano polyphenols might be used as hypoglycemic and hypolipidemic agents [48]. Numerous animal studies on T2DM have confirmed the favourable impacts of polyphenols in remodelling gut microbiota, which may contribute to improvements in this chronic condition. Tea polyphenols, for instance, counteracted gut dysbiosis and reduced both central and peripheral inflammation in a T2DM rat model. Furthermore, tea polyphenols alleviated memory impairments, potentially through gut microbiota-mediated attenuation of neuroinflammation via inhibition of the TLR4/NF-κB pathway [49]. A review of 11,400 participants with T2DM documented that tea consumption (≥4 cups/day) might play a role in the prevention of T2DM and reduce the risk of disease [50].
Oral gavage of rambutan peel polyphenols improved glucolipid metabolism and altered gut microbiota, particularly the levels of Lactobacillus, Tuzzerella, Odoribacter, Turicibacter, Erysipelatoclostridium, and Lachnospiraceae NK4A136 group in a murine model of T2DM [51]. The extract of Pueraria thomsonii Radix containing nine natural polyphenols reduced pancreatic tissue damage and decreased the Firmicutes/Bacteroidetes ratio in the T2DM murine model [52]. Polyphenols in vinegar extract restored gut composition and upregulated levels of Lactobacillus, Bifidobacterium, Bacteroidetes, and Bacteroides in a diabetic mouse model. Moreover, the intervention decreased blood glucose and inflammation [53]. Punicalagin, a phenolic compound from pomegranates, improved diabetic renal injury, restored gut composition, and increased cecal SCFA levels in mice [54]. Recently, Li et al. [55] investigated that mulberry polyphenols elevated the abundance of Bacteroidetes and increased butyrate and propionate production in diabetic mice. Moreover, this supplementation enhanced glucose homeostasis via increased glucose utilization, reduced pancreatic damage, and elevated antioxidant capacity. Blueberry juice enriched in polyphenols improved glucose tolerance in a prediabetic mouse model [56]. Zuo et al. [57] assessed the effect of metallothionein-kidney bean polyphenol complex on gut microbiota and glucose levels in diabetic rats. The extraction of polyphenol from kidney beans reversed gut dysbiosis and elevated SCFA levels. According to Sun et al. [24], polyphenols from guava tea, coffee, cocoa, olive oil, propolis, red wine, chocolate, blueberries, and grape seeds have demonstrated antidiabetic impacts in T2DM patients by enhancing glucose metabolism, lowering insulin resistance, HbA1c, and ameliorating vascular function. The impact of flavonoids, phenolic acids, stilbenes, tannins, and lignans in relation to T2DM will be presented in the following chapters.
2.1. Flavonoids and T2DM
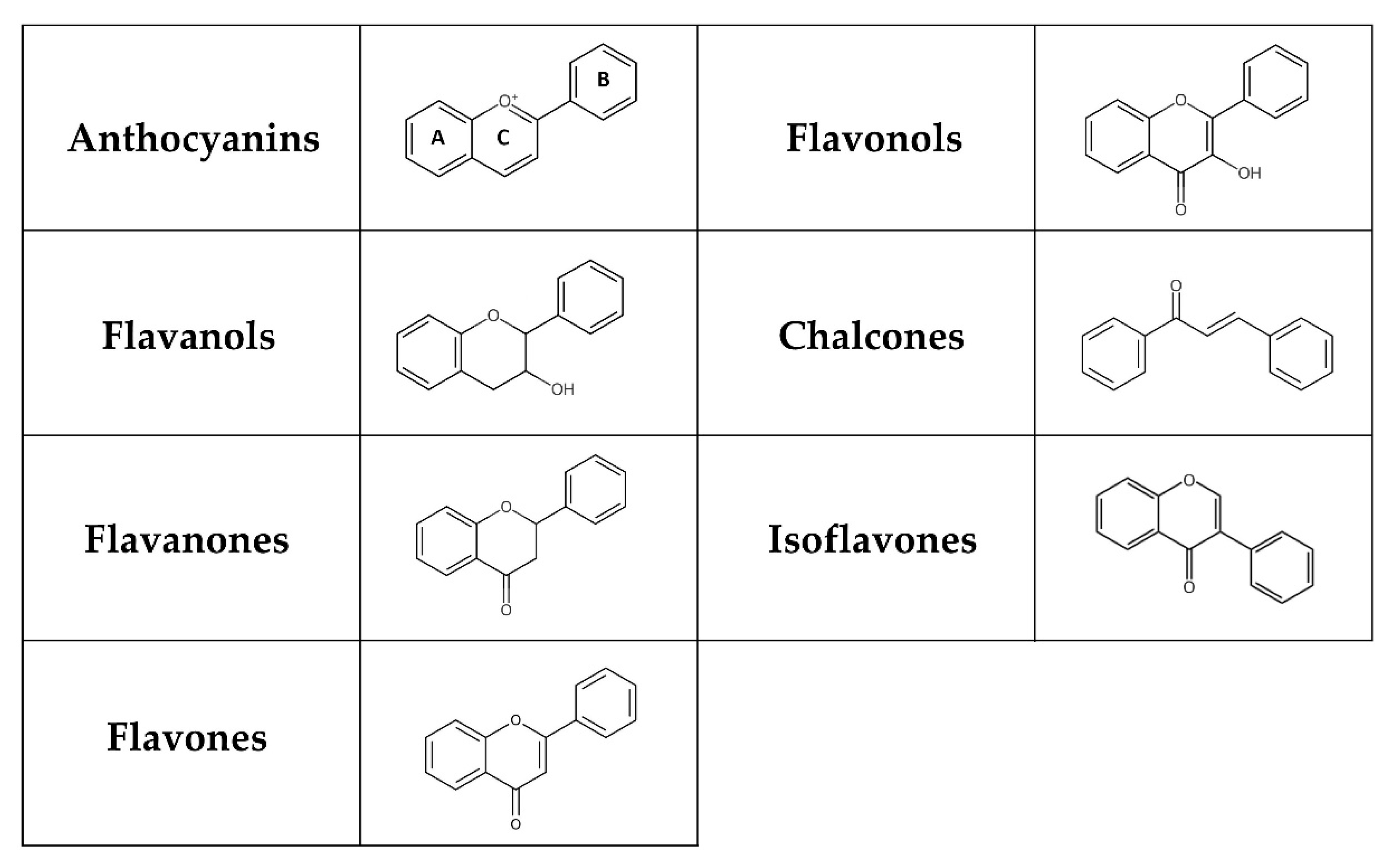
Flavonoids represent a group of polyphenols derived from the benzo-γ-pyrone structure [58]. They are produced through the phenylpropanoid pathway in plants as secondary metabolites, playing a significant role in plant defence mechanisms and the colouration of fruits and flowers [59,60,61]. Flavonoids can be classified into flavones, flavanones, isoflavones, flavonols, chalcones, flavanols, and anthocyanins depending on their chemical structure, degree of hydroxylation and polymerization, substitutions and conjugations (Figure 1) [62,63]. Multiple hydroxyl groups in the flavonoid skeleton offer targets for glycosylation, and thus, dietary flavonoids are mostly present in a chemical structure containing O-glycosides or C-glycosides, including mainly glucose, galactose, arabinose, and rhamnose [63]. The flavonoid glycosides exert a low bioavailability that varies with the location and structure of the glycoside. Furthermore, absorption depends on many factors, such as dosage, diet, sex, and the microbial population in the large intestine. While some flavonoids, like anthocyanins, can be absorbed in the stomach, most anthocyanins primarily reach the colon, where they interact with various bacterial communities [64].
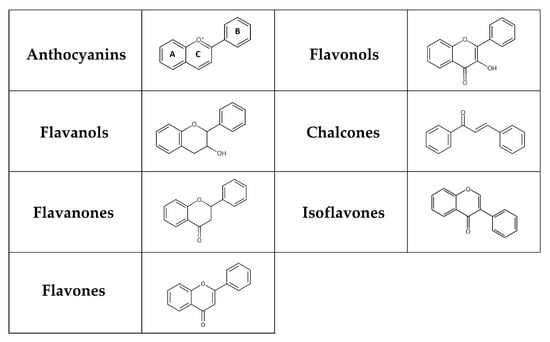
Figure 1.
Chemical structure of flavonoids. Flavonoids possess a basic 15-carbon flavone skeleton, with two benzene rings (A and B) linked by a three-carbon pyran ring (C). Flavonols differ from flavones by the presence of a hydroxyl group at the C-3 position, whereas they differ from flavanones by the presence of a hydroxyl group at the C-3 position and a double bond between C-2 and C-3. Flavanols (flavan-3-ols) are 3-hydroxy derivatives of flavanones lacking a double bond between C-2 and C-3. A charged oxygen atom in ring C and the open form of ring C is found in anthocyanins and chalcones, respectively. In isoflavones, the hydrogen at the C-3 position instead of C-2 is replaced by a phenyl group.
Flavonoid glucosides, including quercetin 3-O-glucoside and quercetin 4′-O-glucoside, are able to be absorbed unaltered in the small intestine through sodium-glucose linked transporter 1 (SGLT1) [65]. An additional method to increase flavonoid glucosides’ bioavailability is their hydrolysis to aglycones by α-glucosidase [66]. Another absorption mechanism in the small intestine is represented by lactase phlorizin hydrolase, which is involved in the hydrolysis of flavonoid glycosides [67]. Finally, unabsorbed glycosides enter the large intestine where they are modified by the gut microbiota into corresponding aglycones and absorbed by epithelial cells into the bloodstream [68]. The pyrone ring in the flavonoid is degraded by gut microbiota, resulting in the formation of phenylacetic acid, phenylpropionic acid and inert by-products. Latterly, flavonoids undergo methylation, hydroxylation, O-methylation, sulfation, and glucuronidation, leading to their excretion in urine and faeces [69].
Tea and wine (mainly red wine) are the main dietary sources of flavonoids in Eastern and Western societies. In addition, vegetables (e.g., lettuce, kale, broccoli, onions, celery, parsley, tomatoes), citrus fruits (e.g., lemons, oranges, grapes, grapefruit), apples, bananas, cherries, peaches, berries (e.g., blueberries, strawberries), soybeans, medicinal plants (e.g., Ginkgo biloba, chamomile, mint), cocoa, dark chocolate are considered important sources of dietary flavonoids [70,71,72].
Numerous flavonoids and extracts rich in flavonoids have been studied as potential antidiabetic agents in clinical, animal, and in vitro studies. Considering total flavonoid consumption, a meta-analysis of prospective cohort studies by Liu et al. [73] demonstrated an association between higher total flavonoid intake and reduced risk of T2DM in a dose-dependent manner. In an observational cohort study of 3430 nondiabetic participants, a 33% reduction in new-onset diabetes was observed in the highest versus the lowest tertile of total flavonoid intake and inverse association with T2DM risk was also found for dihydroflavonols and flavanones after 5.51 years of follow-up [74]. Regarding the effect of specific flavonoids on T2DM, a single oral dose (400 mg) of quercetin, one of the most widespread flavonoids, significantly lowered postprandial blood glucose levels in T2DM patients loaded with maltose (2 g/kg), which could be explained by the inhibition of α-glucosidase [75]. Unfortunately, this study included only 12 individuals per group. However, a longer study conducted by Lee et al. [76] that lasted 10 weeks and included 92 healthy male smokers showed improvements in blood glucose levels, lipid profiles, and blood pressure in subjects who received 100 mg of quercetin each day. Many animal experiments have confirmed the hypoglycemic properties of quercetin, where doses of 15–100 mg/kg for 14–70 days resulted in significant reductions in blood glucose levels, particularly by regeneration of pancreatic islets, improving insulin resistance, affecting glucose metabolism, and promoting insulin release [25,77]. According to the findings, 10 weeks of quercetin supplementation lowered body weight and decreased serum insulin level in diabetic mouse model. Data showed that quercetin reduced the amount of Bacteroides, Proteobacteria, Escherichia coli, and Escherichia-Shigella and decreased the levels of metabolites, including 3-methoxytyramine, L-aspartic acid, L-glutamic acid, and androstenedione [78]. Quercetin is distinguished also by its anti-inflammatory and antioxidant activity. At the molecular level, quercetin increased the expression of insulin-signalling molecules, such as PI3K, and IRS-1. It also activated AMPK and suppressed NF-κB and Jun N-terminal kinase (JNK) pathways [79,80]. In vitro analyses, in silico models and molecular docking studies revealed that quercetin efficiently inhibits α-glucosidase and α-amylase by creating hydrogen bonds with particular amino acid residues [81]. Similarly, kaempferol is considered as an α-glucosidase and α-amylase inhibitor [82]. Furthermore, oral administration of kaempferol improved fasting hyperglycemia, glucose intolerance, and insulin resistance in diet-induced obese mice by inhibiting hepatic gluconeogenesis (reduced pyruvate carboxylase and glucose-6 phosphatase activity) and improving hepatic glucose metabolism (increased Akt and glucose activity) [83]. Bai et al. [84] evaluated the impact of mulberry leaf flavonoids including kaempferol, quercetin, rhamnocitrin, tetramethoxyluteolin, and norartocarpetin on T2DM. They found that kaempferol is capable of binding to protein tyrosine phosphatase 1B (PTP1B) and could be involved in the inhibition of PTP1B.
A randomized clinical trial with a placebo control including 50 participants with T2DM demonstrated that taking 1 g supplement tablet containing 500 mg of rutin daily for 3 months significantly increased antioxidant enzyme levels (SOD, CAT, and GPx) and improved blood pressure markers [85]. In addition, the same dose of rutin reduced levels of fasting blood glucose, insulin, HbA1c, triglycerides, total cholesterol, IL-6, and malondialdehyde. Rutin intake was also associated with increased brain-derived neurotrophic factor (BDNF), which is essential for neurogenesis, synaptic plasticity, and overall brain health [86]. Although there are several contradictory findings, various studies have found lower serum levels of BDNF in T2DM patients, which appears to be linked to insulin resistance and the duration of diabetes. Thus, BDNF might be involved in metabolic control and could play a role in the regulation of glucose metabolism and insulin sensitivity [87]. In a study by Sattanathan et al. [88], T2DM patients received 500 mg of rutin for 60 days. This supplementation lowered fasting blood glucose levels, and systolic and diastolic blood pressures, while increasing high-density lipoprotein (HDL) cholesterol levels. In general, catechins have been shown to inhibit glycogen synthesis, lipogenesis, and glucose oxidation in the liver and weaken glucose transporters in the intestine [25]. The intragastric administration of rutin from Tartary buckwheat reversed gut dysbiosis with increased favourable Akkermansia, Roseburia, and Alisitipes and reduced amount of Escherichia and Mucispirillum in diabetic mice. In addition, rutin decreased glucose and improved serum cholesterol, insulin, triglyceride concentrations, TNF-α, and IL-6 [89].
Epicatechin supplementation improved insulin resistance and fasting serum insulin levels in a clinical study but had no effect on fasting blood glucose levels [90]. On the other hand, epicatechin lowered hyperglycemia and improved insulin response to a glucose load, possibly by modulating pancreatic insulin production and secretion in rats [91]. Similarly, a decreased rate of weight gain, hyperglycemia, and hypertriglyceridemia was found after epicatechin administration in a rat model of high-fat diet (HFD)-induced obesity [92]. In addition, epicatechin alleviated the altered glucose uptake in HepG2 and NRK-52E cell lines [93,94], and demonstrated hydrolyzing enzyme inhibitor properties [82]. Kobayashi et al. [95] reported that epicatechin gallate is able to inhibit SGLT1. In HFD- and streptozotocin (STZ)-induced diabetic mice, epigallocatechin gallate improved glucose homeostasis and inhibited gluconeogenesis and lipogenesis in the liver [96]. Epicatechin, a dietary polyphenol, exerted a beneficial effect on gut microbiota with reduced lipopolysaccharide-producing bacteria, effectively protected glucose homeostasis, and elevated serum level of insulin in T2DM Goto-Kakizaki rats [97]. In a study by Li et al. [98], epigallocatechin gallate (50 and 100 mg/kg for 20 weeks) enhanced lipid profile, suppressed the expression of genes related to fatty acid synthesis, and increased the expression of genes associated with lipolysis and lipid oxidation in white adipose tissue of HFD mice. In addition, galloylated catechins showed higher efficiency than non-galloylated catechins in α-glucosidase and α-amylase inhibition [25]. Within the isoflavones group, a randomized placebo-controlled study by Villa et al. [99], including 50 postmenopausal women, revealed reduced fasting blood glucose and enhanced glucose tolerance and insulin sensitivity after genistein administration (54 mg/day for 24 weeks). In murine models, genistein (600 mg/kg for 4 weeks) significantly lowered levels of triglycerides and glucose [100]. Even a lower dose of genistein (250 mg/kg for 4 weeks) ameliorated hyperglycemia, glucose tolerance, and insulin levels in STZ-induced diabetic mice, which was accompanied by improved β-cell proliferation, survival, and mass [101]. In addition, genistein induced cyclic adenosine monophosphate/protein kinase A (cAMP/PKA) signalling and phosphorylation of extracellular signal-regulated kinase (ERK)1/2 in both INS1 cells and human islets [102]. Numerous other animal and in vitro studies have revealed beneficial effects of genistein on β-cell function [103]. Moreover, genistein is associated with gut microbiota changes and enhanced insulin resistance. A study on obese patients demonstrated that the consumption of genistein decreased insulin resistance and altered gut microbiota with increased Verrucomicrobia phylum [104]. In vitro and in vivo studies documented the preventive effect of daidzein against T2DM [105]. A case–control study by Nguyen et al. [106] including adult humans showed that dietary intake of daidzein correlated with a reduced risk of T2DM. Using non-obese diabetic mice, Choi et al. [107] demonstrated that daidzein supplementation (0.2 g/kg diet for 9 weeks) reduced fasting blood glucose and plasma insulin levels. Moreover, daidzein-treated mice have higher hepatic glucokinase and lower glucose-6-phosphatase and phosphoenolpyruvate carboxykinase activities, indicating that it has a positive impact on lowering neoglucogenesis and glycogenolysis. Other animal studies have confirmed improvements in glucose and lipid metabolism after daidzein administration (0.2 g/kg for 6 weeks; 0.1% for 4 weeks) [105,108,109]. Gut bacteria can convert daidzein into its metabolite equol [110]. In vivo experiments with Zucker diabetic fatty rats revealed that equol supplementation improved insulin secretion failure [111].
The meta-analysis by Guo et al. [112] investigated the association between dietary anthocyanins intake and the development of T2DM in 12,611 participants. According to their results, dietary anthocyanin consumption was linked to a 15% lower risk of T2DM. A double-blind, randomized, placebo-controlled study found that anthocyanin supplementation (320 mg/day for 24 weeks) along with standard treatment significantly lowered levels of triglycerides, LDL cholesterol, fasting plasma glucose, and oxidative stress markers while enhancing antioxidant capacity [113]. Another randomized controlled trial showed that administration of purified anthocyanins (320 mg/day for 12 weeks) positively affected glycemic control and lipid profiles in adults with prediabetes or early untreated diabetes. This study included 138 participants, and significantly decreased levels of HbA1c, LDL cholesterol, apolipoproteins A-1 (apo A1), and B (ApoB) were reported in the anthocyanin group [114]. Pomegranate is a potent antioxidant rich in flavonoids such as anthocyanins; its consumption improves glucose profile in adults [115,116]. In a mouse model, the supplementation with blueberry anthocyanins improved insulin sensitivity via modulated gut microbiota [117]. Combined treatment of metformin (MET) and anthocyanin increased beneficial bacteria with SCFA production and decreased the expression of PTP1B in diabetic mice [118]. PTP1B is a phosphatase regulating diabetes, obesity, and cancer-associated pathways [119,120]. Based on the results, anthocyanin may enhance the effectiveness of MET in the treatment of T2DM [118].
In an animal study, supplementation with scutellarein (50 mg/kg for 16 weeks) resulted in attenuation of obesity, insulin resistance, and oxidative stress [121]. In addition, the positive effect of tangeretin in relation to T2DM indicators was presented by Guo et al. [122] using primary hepatocytes and diabetic mice. Tangeretin (25 and 50 mg/kg for 30 days) ameliorated insulin sensitivity and improved glucose homeostasis in animal models. Moreover, in primary hepatocytes, tangeretin (10 and 20 μM for 48 h) suppressed the mitogen-activated protein kinase (MEK)-ERK1/2 pathway, which is known to regulate insulin sensitivity, and its inhibition improves insulin resistance. Additionally, the treatment of these cells led to the upregulation of insulin-stimulated Akt and glycogen synthase kinase 3 β (GSK3β) phosphorylation, increased glycogen content, and reduced glucose output. Tangeretin administration significantly changed gut microbiota with increased Bacteroides and Lactobacillus and improved insulin resistance and glucose intolerance in HFD mice [123].
Some flavonoids, like diosmetin, formononetin, and luteolin, demonstrated the ability to improve diabetic nephropathy. In STZ-induced diabetic mice, diosmetin administration (50 mg/kg for 5 days) significantly decreased fasting blood glucose level and ameliorated altered levels of oxidative stress parameters and inflammatory cytokines. Furthermore, diosmetin declined the expression of Akt and NF-κB [124]. The administration of diosmetin led to elevated serum insulin level while decreasing blood glucose concentration in diabetic mice. Moreover, diosmetin changed dysbiotic microbiota and elevated the level of Corynebacterium glutamicum [125]. The application of formononetin (10, 20 and 40 mg/kg for 16 weeks) showed a positive effect on hyperglycemia and insulin resistance in diabetic rats. Reduced levels of triglycerides and cholesterol were also noted [126]. The Qijian mixture, rich in formononetin, calycosin, and puerarin, changed gut microbiota and increased genera Bacteroides, Senegalimassilia, and Clostridium sensu stricto 1 in diabetic mice. Gao et al. [127] confirmed the hypoglycemic effect of this traditional Chinese medicine in alleviating T2DM. Luteolin administration (10 and 20 mg/kg for 4 weeks) restored insulin resistance, dyslipidemia, hyperuricemia, and renal inflammatory cell infiltration in STZ-induced diabetic mice [128]. Developed porous starch microspheres loaded with luteolin alleviated injury in liver tissue caused by T2DM. The intervention altered gut microbiota with decreased unfavourable Acetatifactor, Candidatus Arthromitus, and Turicibacter. Further studies might be focused on the hypoglycemic potential of this natural flavonoid in T2DM [129]. The list of studies that investigated the relationships between flavonoids mentioned in this review and T2DM without gut microbiota composition is summarized in Table 1, and those that also looked at gut microbiota composition are listed in Table 2.

Table 1.
List of flavonoids in relation to T2DM without investigating the composition of gut microbiota.

Table 2.
List of studies characterizing gut microbiota alterations after flavonoid intervention in T2DM.
2.2. Phenolic Acids and T2DM
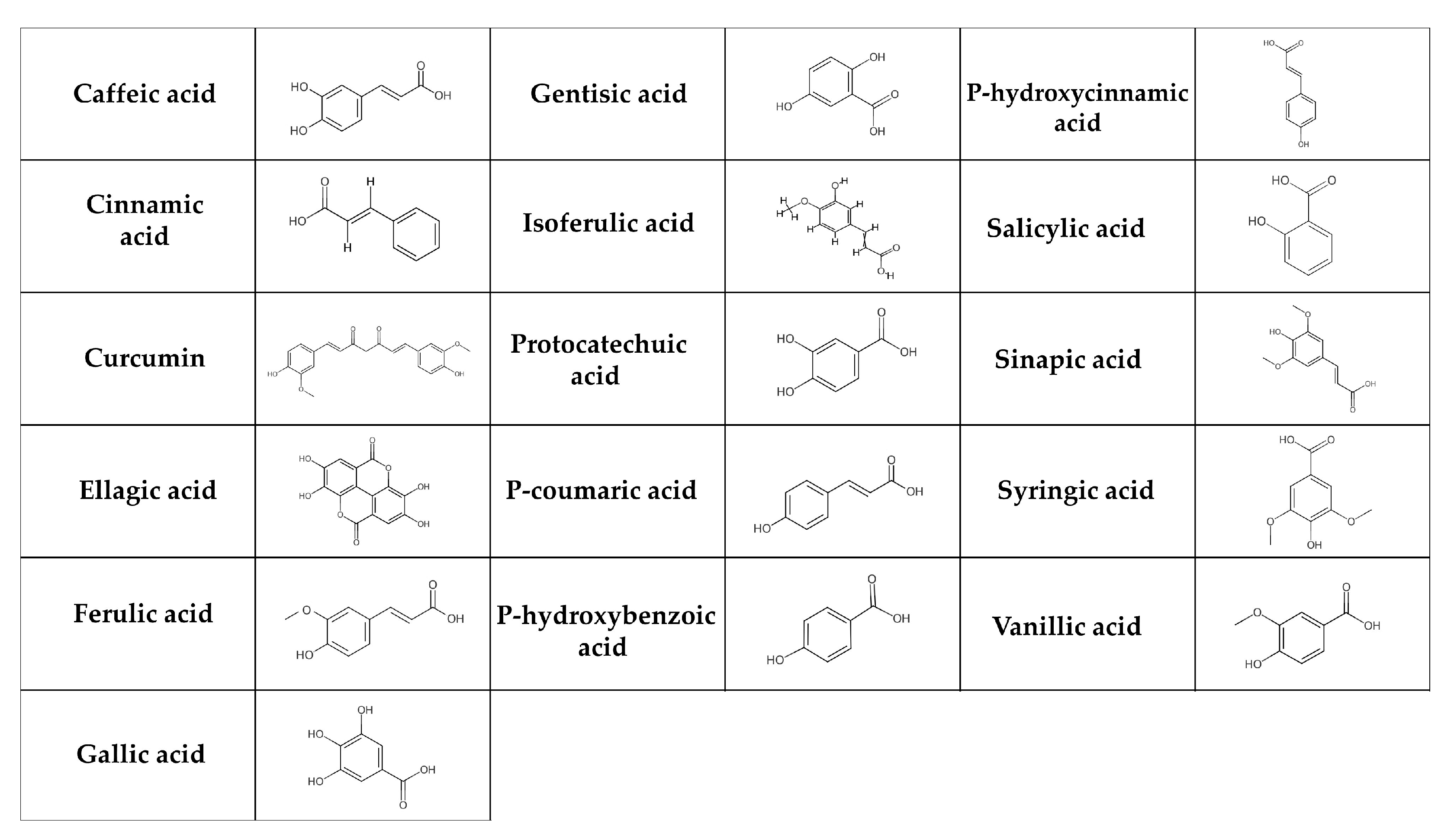
Phenolic acids are the most basic polyphenols in terms of their chemical structure [130]. These are carboxylic acids, divided into hydroxybenzoic acids (e.g., gallic, p-hydroxybenzoic, salicylic, ellagic, gentisic, protocatechuic, syringic and vanillic acids) and hydroxycinnamic acids (e.g., cinnamic, p-coumaric, caffeic, ferulic, isoferulic, sinapic, and chlorogenic acids, curcumin) (Figure 2) [131,132]. The salicylic and gallic acids are synthesized from aromatic amino acids produced through the shikimate pathway [131] in chloroplast [133]. Other hydroxybenzoic and hydroxycinnamic acids are synthesized in the cytosol [134]. In general, phenolic acids can be found in cereals (e.g., barley, oats, rice, wheat), seeds (e.g., quinoa, chia seeds, flax seeds), fruits (e.g., grapes, pears), berries (e.g., blackberries, strawberries), vegetables (e.g., carrots, cabbage, spinach), nuts (e.g., walnuts), spices (e.g., cinnamon, vanilla pods), medicinal herbs (e.g., chicory), tea, cocoa, coffee, algae, and fungi [135,136,137]. They are broken down in multiple tissues and by the intestinal microbiota in the digestive tract. Since only about 10% of degraded phenolic acids is available in the small intestine, the majority is transported to the large intestine [138].
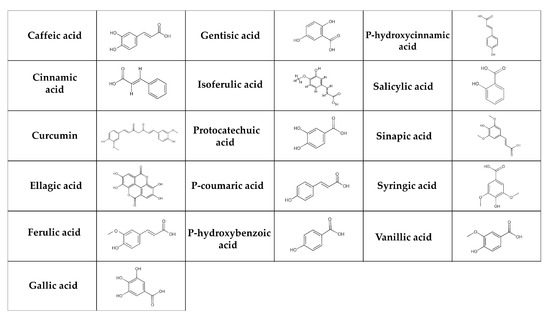
Figure 2.
Chemical structure of phenolic acids. The simple phenolic acids can be classified as hydroxybenzoic (gentisic, salicylic, gallic, protocatechuic, ellagic, syringic, p-hydroxybenzoic, and vanillic acids) and hydroxycinnamic acids (caffeic, chlorogenic, cinnamic, isoferulic, p-coumaric, ferulic, and sinapic acids, curcumin).
Hydroxybenzoic acid breaks down into hippuric acid and glucuronide. This process is highly saturating and affected by the availability of glycine. At high doses, it leads to the urea cycle, gluconeogenesis, fatty acid metabolism, and tricarboxylic acid cycle confiscating coenzyme A prior to its conjugation with glycine [139]. Ellagic acid is absorbed within one hour after consumption [140]. Its metabolite, urolithin B, is conjugated with glucuronic acid and excreted in the urine [141]. Most of the gallic acid is absorbed through the upper digestive tract and the rest through the stomach [142]. Additionally, following ingestion, salicylic acid is found in the blood and urine [143]. Hydroxycinnamic acids are degraded directly in the large intestine, absorbed through the barrier of the gastrointestinal tract, and subsequently enter the peripheral bloodstream [144]. According to El-Seedi et al. [145], cinnamic acid is found in plasma immediately after consumption. Some other phenolic acids, such as caffeic, p-coumaric, and ferulic acids are absorbed through the monocarboxylic acid transporters, across the intestinal epithelial cells [146]. P-coumaric acid is subsequently metabolized in the liver [145].
Although the biological effects of phenolic acids are still controversial, their positive impact on T2DM is undeniable [147]. Phenolic acids can improve the symptoms of T2DM by regulating hepatic glucose homeostasis and influencing carbohydrate metabolism pathways (by inhibiting α-glucosidase and α-amylase) [148,149]. Chlorogenic acid might increase insulin sensitivity and decrease inflammation [5]. A meta-analysis of 10 clinical studies investigated the effects of green coffee bean extract (GCE) supplementation, focusing on chlorogenic acid. A total of 363 participants were included with GCE dosages ranging from 100 to 1000 mg/day for 2 to 16 weeks. The summary from this meta-analysis revealed a significant reduction in fasting blood glucose levels (especially at doses of ≥400 mg/day), but insulin levels were not altered. Some subgroup analyses also highlighted significant improvements in insulin levels after GCE supplementation [150]. To track the effects of chlorogenic acid at a dosage of 1200 mg/day for 12 weeks, 30 subjects with impaired glucose tolerance (IGT) participated in another randomized, double-blind, placebo-controlled clinical trial. They were not taking any other drugs or medications during the trial. Chlorogenic acid administration significantly lowered levels of fasting blood glucose, LDL cholesterol, and triglycerides, and there was an increase in the Matsuda index, indicating improved insulin sensitivity [151]. In diabetic mice, chlorogenic acid administration elevated Lactobacillus, Blautia, and Enterococcus within the gut microbiota. The beneficial effect of this phenolic acid contributed to improved glucose tolerance [152].
There is insufficient evidence from clinical trials specifically monitoring the administration of extracted caffeic acid to patients with diabetes-related symptoms. However, numerous studies have demonstrated the effects of plant and animal products on T2DM, with caffeic acid often cited as a component. The review by Ganguly et al. [153] explored the impacts of caffeic acid on DM-related complications. It highlighted three clinical trials involving propolis as a source of caffeic acid and caffeic acid phenethyl ester. These studies showed that giving propolis to DM patients at doses of 300 and 400 mg for 2, 3, or 6 months increased the Matsuda index, 2-Deoxy-D-glucose (2-DG) uptake, and GPx levels while significantly lowering fasting blood glucose and HbA1c levels. The water extract derived from the leaves of Lycium barbarum L., rich in neochlorogenic acid, chlorogenic acid, caffeic acid, and rutin improved gut dysbiosis and reduced insulin resistance in diabetic rats [154]. Ellagic acid has been shown to exert hypoglycemic effects and is able to improve glycemic control. In a clinical trial, consumption of ellagic acid (180 mg/day for 8 weeks) significantly decreased levels of glucose, insulin, HbA1c, total cholesterol, triglycerides, LDL cholesterol, malondialdehyde, C-reactive protein, TNF-α, and IL-6. However, the values of total antioxidant capacity and activities of SOD and GPx were elevated [155]. In the research by Hosseini et al. [156], 24 overweight and obese adults received 500 mg of pomegranate extract with 40% ellagic acid twice a day for 1 month, and a substantial decrease in homeostatic model assessment for insulin resistance (HOMA-IR) was recorded. Kang et al. [157] documented numerous beneficial effects of ellagic acid against obesity-associated health problems by altering gut microbiota.
An umbrella meta-analysis included 22 studies with a total of 1600 participants and examined the impacts of curcumin supplementation on glycemic indices. The doses of curcumin ranged from 0.08 to 2 g/day, and the duration of the trials varied from 4 to 30 weeks. Curcumin significantly reduced levels of blood glucose, HbA1c, and insulin. Participants involved in this analysis had a variety of health problems, including T2DM, polycystic ovary syndrome, non-alcoholic fatty liver disease, and metabolic syndrome. Notably, many of the included studies focused on populations with insulin resistance, a condition associated with DM [158]. When compared to conventional medication, curcumin supplementation dramatically lowered fasting blood glucose and HbA1c levels in T2DM patients, according to another systematic review and meta-analysis. The meta-analysis included five trials with 349 participants and curcumin doses ranging from 80 to 2100 mg/day [159]. A controlled clinical trial by Hodaei et al. [160] included 53 participants with T2DM and discovered that receiving 1500 mg of curcumin daily for ten weeks considerably lowered fasting blood glucose levels. In animal and in vitro studies, the effect of other phenolic acids on T2DM has been examined. Huang et al. [161] documented that curcumin restored gut microbiota balance by decreasing Enterobacterales and Firmicutes and improved insulin resistance in diabetic rats.
Natural chicory extract (Cichorium intybus) rich in chicoric, chlorogenic, and caffeoylquinic acids enhanced glucose tolerance and reduced basal hyperglycemia in STZ-induced diabetic rats [162]. In HFD mice, chicoric acid also changed the gut microbiota by raising the ratio of Firmicutes to Bacteroidetes [163]. According to Peng et al. [164], chicoric acid enhanced glucose uptake in cell and murine models and increased Akt phosphorylation. HFD mice given 0.5% ferulic acid for 7 weeks showed significantly lower levels of glucose, glucose-6-phosphatase, and phosphoenolpyruvate carboxykinase activities, as well as reduced hyperlipidemia and oxidative stress. They also exhibited elevated insulin and glycogen concentrations [165,166]. Ferulic acid (10 mg/kg for 15 days) demonstrated antioxidant as well as antidiabetic effects and ameliorated liver, kidney, and pancreas damage in mice with alloxan-induced diabetes, presumably through NF-κB inhibition [167]. Ferulic acid (10 and 40 mg/kg for 3 weeks) was found to significantly lower blood glucose, urea, creatinine, glutamic pyruvic transaminases, glutamic oxaloacetate transaminases, and improve the lipid profile in STZ-induced diabetic rats [168]. According to Narasimhan et al. [169], ferulic acid has the ability to regulate the expression of the GLUT2 gene in the liver in a rat model of T2DM. Song et al. [170] determined the protective effect of ferulic acid against diabetic syndrome via modulated gut microbiota with reduced Lactobacillus, Ruminococcus, and promoted Bacteroides, Blautia, Faecalibacterium, Parabacteroides and Phascolarctobacterium.
Rats with alloxan-induced diabetes who were given syringic acid (50 mg/kg for 30 days) showed an increase in insulin and C-peptide levels and a decrease in plasma glucose levels [171]. In the study by Srinivasan et al. [172], syringic acid was administered intragastrically to diabetic rats (25, 50, and 100 mg/kg for 30 days). According to the findings, it significantly improved the levels of plasma glucose, HbA1c, insulin, and glycogen as well as the functional markers of the liver and kidneys. It also restored the activities of important enzymes involved in the carbohydrate metabolism. Yoon et al. [173] demonstrated that L6 skeletal muscle cells treated with 0, 12.5, 25, 50, and 100 μM of p-coumaric acid for 48 h were capable of modulating glucose and lipid metabolism through AMPK activation. Sinapic acid (5, 10, and 25 mg/kg for 3 days) exerted a hypoglycemic effect in STZ-induced diabetic rats [174]. Derebasi et al. [175] revealed a positive impact of p-coumaric acid on probiotic Lactobacillus spp. in vitro.
Cinnamic acid demonstrated antidiabetic activity by enhancing glucose tolerance in rats with STZ-induced diabetes (5 and 10 mg/kg for 14 h) and inducing insulin secretion in isolated islets. Cinnamic acid (10 mg/kg) produced an improvement that was similar to that of glibenclamide (5 mg/kg), a standard drug [176].
The antihyperglycemic, antioxidant, and antilipid peroxidative impacts of gallic acid (10 and 20 mg/kg for 21 days) were demonstrated by Punithavathi et al. [177], which protected the pancreas from diabetes induced by STZ in rats. The elevated insulin secretion after gallic acid treatment positively reversed the impaired carbohydrate metabolism by lowering gluconeogenesis and enhancing glycolysis, ultimately leading to a reduction in hyperglycemia. In another study, gallic acid (20 mg/kg for 30 days) decreased body weight gain, fasting blood glucose and insulin levels in diabetic rats. It also significantly improved the level of PPAR-γ expression, and enhanced glucose uptake through translocation and activation of GLUT4 in the PI3K/Akt signalling pathway [178]. Gallic acid isolated from Terminalia bellerica Roxb. (5, 10, 20 mg/kg for 28 days) reduced levels of plasma glucose, triglyceride, total cholesterol, LDL cholesterol, creatinine, and uric acid in STZ-induced diabetic rats. Conversely, it considerably raised the concentrations of insulin, total protein, C-peptide, glycogen, and albumin [179]. A lower level of gallic acid has been determined in patients with adult-onset type 1 diabetes. The authors observed that a decreased abundance of SCFA-producing bacteria correlated with reduced gallic acid levels [180]. Table 3 shows the list of phenolic acids included in this review in connection with T2DM.

Table 3.
List of phenolic acids in connection with T2DM.
2.3. Stilbenes and T2DM
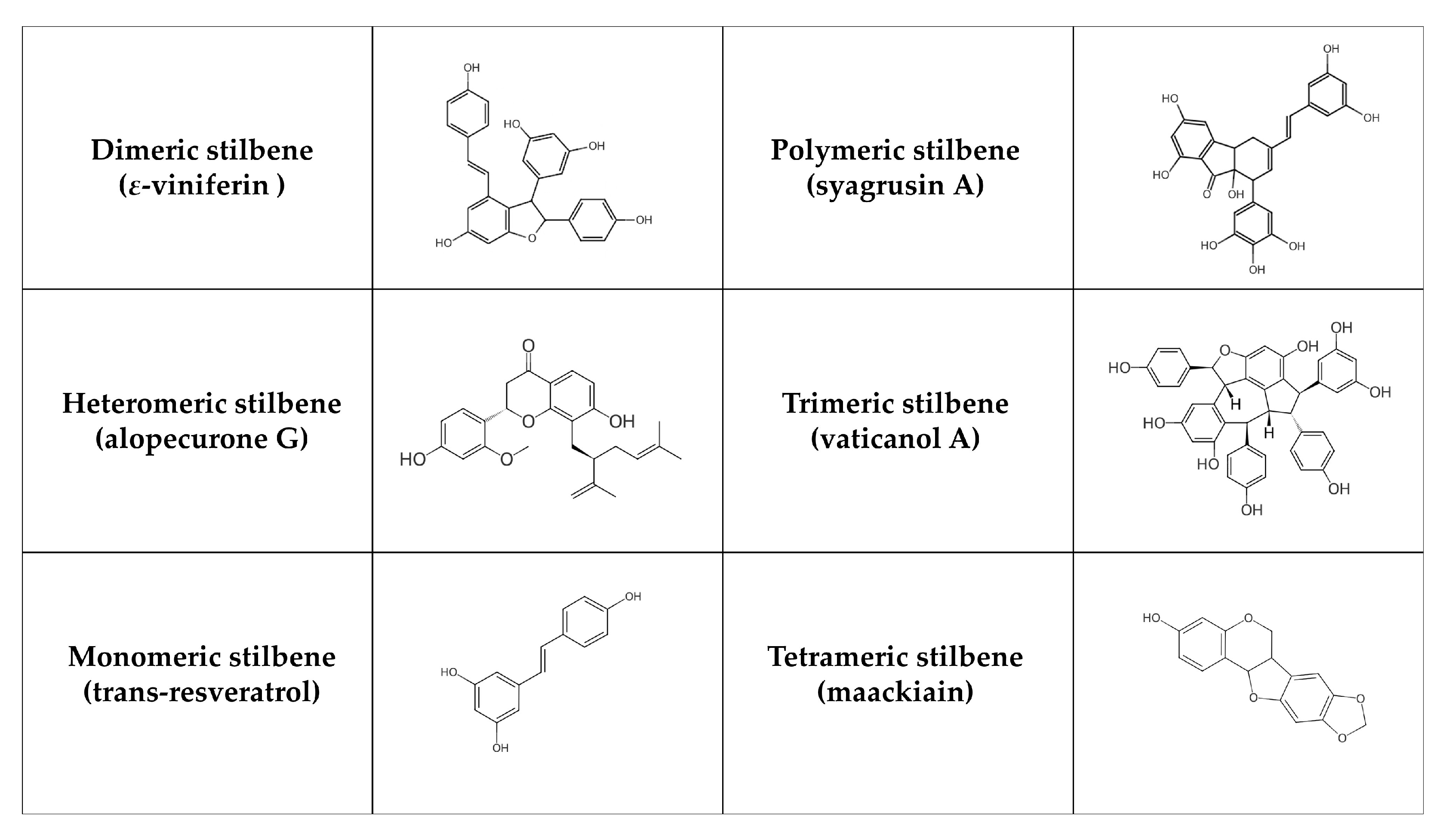
Stilbenes are formed in plants (e.g., Vitaceae, Leguminaceae, Gnetaceae, and Dipterocarpaceae families) as protective agents that protect against external stressors like UV radiation, infection, and pathogen attack [181,182]. Stilbenes can often be identified in grapes as well as in other plants and fruits such as rhubarb, banana, guava, pineapple, apple, peach, passion fruit, pears, and peanuts [183]. Structurally, their main characteristic is the presence of a 1,2-diphenylethylene nucleus [184]. According to their polymerization, monomeric and oligomeric stilbenes can be identified, where oligomeric stilbenes are formed of homogeneous or heterogeneous monomers of stilbene. In general, stilbenes can be divided into six groups [185]: monomeric (styrastilbene A; 13-hydroxykompasinol A; resveratrol; cajanonic acid A; hydrangeic acid; hypargystilbene B, D, E; lonchocarpene; rumexoid), dimeric (multiflorumiside J, K; polygonumnolide D; scirpusin C; ε-viniferin), trimeric (α-viniferin; passiflorinol B; vaticanol A, E, G), tetrameric (maackiain; paeonilactiflorol), polymeric (syagrusin A, B) and heteromeric (polyflavanolstilbene A) (Figure 3). Stilbenes exist in trans and cis isomeric forms, with trans-stilbene being the most common [182]. The metabolism of stilbenes is very rapid. Although 70% of resveratrol taken orally is absorbed, only 5 ng/mL is ultimately accessible, and its half-life in plasma is 9 h [186]. Subsequently, transformed compounds (in the large intestine) are further degraded or excreted by the liver [187]. These compounds are metabolized by uridine 5′-diphosphate glucuronide transferase, sulfotransferase, and catechol O-methyltransferase enzymes. In this phase, metabolism produces water-soluble inactive compounds, which can be easily eliminated [188]. The chemical structure of stilbenes affects the speed of their metabolism. For instance, the presence of a hydroxyl group in oxyresveratrol increases its susceptibility to glucuronidation and sulfation reactions, resulting in a poorer pharmacokinetic profile [189]. On the other hand, pinostilbene contains the dimethoxy group in cycle A instead of the hydroxyl group. Therefore, it is more resistant to metabolism [190]. Overall, the replacement of the hydroxyl group leads to an improvement in stilbene properties. Additionally, acetyl-trans-resveratrol, which contains acetyloxy groups, is more capable of penetrating the cell membrane. Trans-2,3,5,4′-tetrahydroxystilbene-2-O-glucoside, including hydrophilic groups, is more stable and soluble in water than resveratrol [191]. Higher oligomerization and intraperitoneal doses also decrease bioavailability. For example, ε-viniferin has a biological activity of more than 90% after intraperitoneal administration compared to 0.77% after oral administration [192].
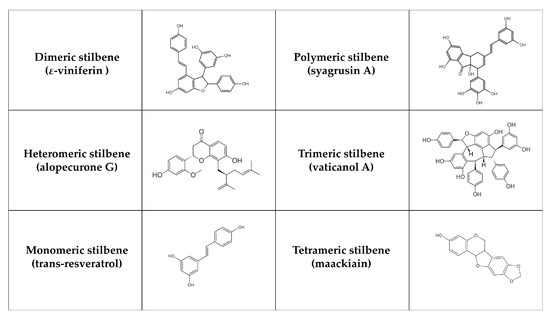
Figure 3.
Chemical structure of selected stilbenes.
Stilbenes may influence T2DM and its comorbidities. After six years of follow-up, an observational cohort study of 3430 non-diabetic participants showed that the highest tertile of stilbene intake reduced the risk of developing new-onset diabetes by 43% [74]. Considering individual stilbenes, various animal studies suggest that resveratrol can increase sirtuin 1 (SIRT1) expression, which stimulates PPAR-γ coactivator 1-α activity. The subsequent upregulation of GLUT4 and AMPK expression is linked to improved insulin sensitivity in peripheral tissues [193,194]. According to Peng Goh et al. [195], 10 patients with T2DM were treated with 3 g/day of resveratrol for 12 weeks. Resveratrol treatment regulated energy expenditure by upregulating AMPK and SIRT1 expression in skeletal muscle, suggesting that it may have favorable exercise-mimetic effects in T2DM patients. In another study, 19 individuals suffering from T2DM received resveratrol (10 mg/day for 4 weeks). This supplementation ameliorated insulin sensitivity, possibly due to less oxidative stress, which in turn led to more efficient insulin signalling through the Akt pathway [196]. The intervention with resveratrol increased the abundance of Erysipelotrichaceae and Ileibacterium in the gut microbiota, while also enhancing insulin sensitivity and glucose tolerance in T2DM mice. The authors [197] noted that, following resveratrol treatment, the microbiota composition closely resembled that of healthy control mice. In a clinical study by Bhatt et al. [198], resveratrol (250 mg/day for 3 months) significantly improved systolic blood pressure, HbA1c, total cholesterol, and total protein levels in 62 participants. However, no significant changes were observed in body weight, LDL and HDL cholesterols. Movahed et al. [199] examined the antihyperglycemic effect of resveratrol (40 and 500 mg/day for 6 months) in 192 patients with T2DM. According to their findings, these patients benefited greatly from resveratrol supplementation in conjunction with conventional antidiabetic treatment, including significant decreases in blood glucose, HbA1c, and insulin levels, mitigating insulin resistance, and HDL cholesterol levels. An important role of PTP1B and α-glucosidase in the pathophysiology and complications of T2DM has been revealed in vivo and in vitro [200]. It has been demonstrated that stilbenes serve as their potent inhibitors (e.g., styrastilbene A, paeonilactiflorol, 13-hydroxykompasinol A, scirpusin C, ε-viniferin, passiflorinol B, polyflavanostilbene A, polygonumnolide D, rumexoid, syagrusin A, B, vaticanol A, E, G), and therefore can have antidiabetic effects [201,202,203,204,205,206,207,208,209,210,211,212]. Cajanonic acid A has been reported to exert inhibitory activity on PPAR-γ and PTP1B. Its hypoglycemic properties in rats (15, 30 and 60 mg/kg for 24 h) were comparable to that of the approved antidiabetic drug rosiglitazone [213]. The relationship of hydrangeic acid to DM was observed by Zhang et al. [214]. Hydrangeic acid markedly elevated the amount of adiponectin in 3T3-L1 cells, 2-deoxyglucose uptake into cells, and GLUT4 translocation. Higher mRNA levels of adiponectin, GLUT4, and PPAR-γ were documented, while TNF-α mRNA expression was reduced. Furthermore, this acid (200 mg/kg/day for 2 weeks) significantly lowered levels of blood glucose, triglycerides, and free fatty acids in a mice model. The effect of two stilbenes, lonchocarpene and 3,5-dimethoxy-4′-O-prenyl-trans-stilbene (DPS), on α-glucosidase activity and postprandial hyperglycemia was investigated by Pereira et al. [205]. Both stilbenes inhibited α-glucosidase activity in vitro. Furthermore, DPS caused a significant reduction in hyperglycemia in a mice model, whereas lonchocarpene did not. Recently, the associations between cajanonic acid A, hydrangeic acid, lonchocarpene, and 3,5-dimethoxy-4′-O-prenyl-trans-stilbene with gut microbiota in T2DM or metabolic diseases have not been fully investigated. Table 4 provides the list of aforementioned stilbenes in relation to T2DM.

Table 4.
List of stilbenes in relation to T2DM.
2.4. Tannins and T2DM
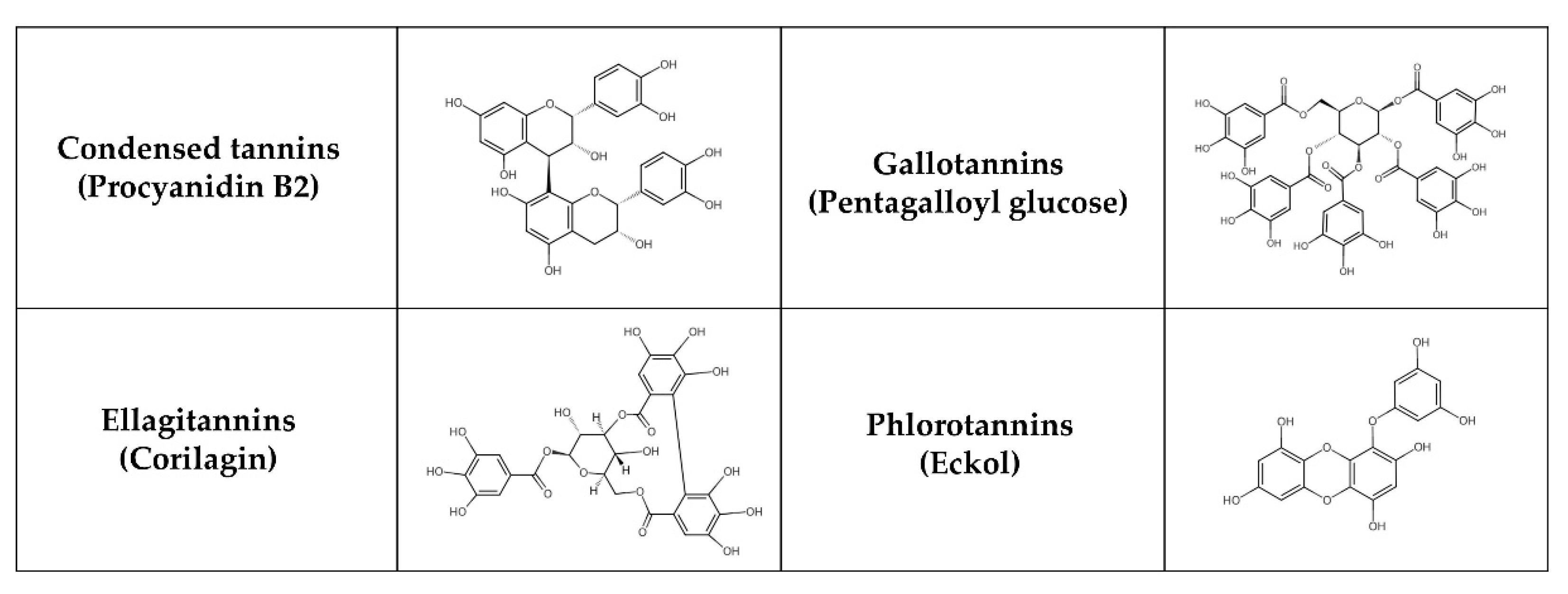
Tannins, natural phenolic compounds, are found in plants and can combine with a variety of minerals and macromolecules, including proteins, cellulose, and starch, to form a strong complex [215,216]. Different groups of tannins can be distinguished based on their chemical structure (Figure 4): hydrolysable tannins—HTs (e.g., gallotannins—GTs and ellagitannins—ETs), condensed tannins—CTs (syn. proanthocyanidins, non-hydrolysable tannins, e.g., procyanidins) and phlorotannins [217]. HTs with smaller molecules consist of sugar esters of polyphenolic carboxylic acids, e.g., gallic or ellagic acids. CTs may be derived from flavanols, such as catechin, flavan-3,4-diols or stilbenes. Polymerization results in more complicated structures [218]. Phlorotannins are present in brown seaweeds and are formed by polymerization of phloroglucinol units. Some classifications also recognize complex tannins (e.g., acutissimins A and B, camelliatannin A), composed of flavane-3-ol, the unit of CTs and HTs, which are linked by carbon-carbon bonds [219,220].
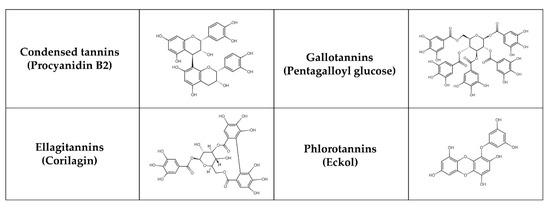
Figure 4.
Chemical structure of selected tannins.
Tannins (except for phlorotannins) are most abundant in cocoa beans, tea, wines (especially red wine), fruits (e.g., apple, berries, plum, pomegranate), juices, nuts (e.g., cashew nuts, peanuts, walnuts), chocolate, legumes (e.g., chickpeas, cowpeas, lentils) and cereal grains (e.g., barley, sorghum) [221]. They can be metabolized in two phases. In the first metabolic phase (non-microbiota mediated), the biological effects of tannins are significantly influenced by their rate of decomposition and absorption. HTs are cleaved in the stomach or small intestine into monomers such as gallic or ellagic acids. Gallic acid from tea is rapidly absorbed and eliminated [222], while its derivatives are absorbed more slowly [142]. For ETs, the duration of intestinal absorption is between 0.5–3 h after oral administration in healthy subjects [140]. After absorption, free ellagic acid undergoes additional conjugation reactions with methyl, glucuronyl or sulfate groups, and these conjugates are detectable in urine and plasma [223]. CTs are absorbed intact from the small intestine, the rate of absorption being influenced by their stereochemistry and chemical structure [224]. After absorption, the aglycone forms of CTs undergo metabolism in the small intestine and later in the liver by glucuronide formation in the endoplasmic reticulum or by sulfonation and methylation in the cytosol [225]. The larger the CTs molecule and the more hydrophilic hydroxyl groups it contains, the lower the absorption rate [226]. In the second metabolic phase (microbiota-mediated), HTs are metabolized by various bacterial enzymes into gallic acid, pyrogallol, phlorogluciol and sometimes also into acetate and butyrate. GTs are later hydrolyzed and degraded by tannase to the hexahyroydiphenoyl. On the contrary, bacterial hydrolysis of ETs undergoes lactonization to produce ellagic acid [225]. Further, ellagic acid is converted into urolithin A and urolithin B, which are able to be easily absorbed [227]. These derivatives have been detected in plasma and urine [228]. CTs in their intact form as dimers, trimers and tetramers are metabolized during phase II in the intestine and liver [229]. Most CTs reach the large intestine intact, where the gut microbiota breaks them down into phenolic acids and phenylvalerolactones [230]. Dietary CTs shifted the gut microbiota in rats toward tannin-resistant bacterial taxa such as Enterobacteriaceae and Bacteroides [231]. Both HTs and CTs can support the growth and function of beneficial bacteria [232]. An extract of red maple leaves enriched with glucitol-core containing GTs modulated the gut microbiota and elevated the levels of Prevotella and Eubacterium in HFD mice. This supplementation led to decreased fat mass and body weight, improved insulin resistance, and reduced inflammation [233]. In a pilot clinical trial, the administration of mango increased GTs and SCFA production while altering bacterial taxa in lean participants, but not in obese individuals [234]. Additionally, the probiotic Lactobacillus plantarum in the gut microbiota can degrade GTs into gallic acid, facilitating its absorption [235].
Tannins have shown promising benefits for T2DM management in several animal and in vitro studies [236]. In rats with STZ-induced diabetes, the administration of tannins (100 and 200 mg/kg of tannin fraction for 30 days) effectively reduced elevated levels of blood glucose, total cholesterol, triglycerides, and LDL cholesterol and restored insulin and HDL cholesterol. Moreover, tannins significantly renewed the activity of antioxidant enzymes (SOD, CAT) and reduced GPx, thus renewing the organs’ antioxidant status to almost normal levels [237]. According to Shahidi and Danielski [5], favorable effects of GTs (e.g., monogalloyl hexoside) and ETs (e.g., ellagic acid hexoside) on reducing oxidative stress, insulin resistance, dyslipidemia, and inflammatory state have been demonstrated. ETs (e.g., lagerstroemin, flosin B) demonstrated strong effects in promoting insulin-like glucose uptake and reducing adipocyte differentiation in 3T3-L1 adipocytes [238]. According to Pinent et al. [239], grape seed procyanidins extracts (GSPE) substantially reduced hyperglycemia in STZ-induced diabetic rats and stimulated glucose uptake in insulin-sensitive cell lines, suggesting that they can exhibit insulinomimetic properties. Additionally, Montagun et al. [240] revealed that procyanidins phosphorylate p38 MAPK and p44/p42 much more than insulin. In insulin-sensitive tissues, they are able to modulate lipogenesis and glucose uptake, as well as ameliorate their oxidative/inflammatory status. Procyanidins also have the ability to modulate the level of active glucagon-like peptide-1 (GLP-1) [241]. Procyanidin B2 (PB2) has become more important due to its inhibitory activity on the formation of AGEs [242] and is one of the major components of GSPE. According to Yin et al. [243], PB2 from grape seeds (30 mg/kg for 10 weeks) alleviated the disturbances of hepatic lipid metabolism in diabetic mice. Body weights, triglycerides, total cholesterol, and free fatty acid levels were all significantly lower, but fasting blood glucose levels were not. In an in vitro study, grape seed PB2 (150 µg/mL for 48 h) reduced adipogenesis of 3T3-L1 cells by targeting PPAR-γ with a mechanism involving miR-483-5p [244]. By enhancing the abundance of Lachnospiraceae NK4A136 group, Alloprevotella, Akkermansia, and Faecalibaculum, peanut skin procyanidins modulate the gut microbiota in T2DM mice. The treatment also elevated levels of IL-10 while reducing lipopolysaccharide, IL-6, and myeloperoxidase. Additionally, this supplementation enhanced gut integrity by increasing the expression of tight junction proteins in the murine colon [245]. Phlorotannins have been shown to exert antidiabetic properties due to their acarbose-like activity, stimulation of glucose uptake, and protection of pancreatic β cells from oxidative stress [246,247]. A small double-blind, randomized, placebo-controlled study found that a commercially available blend of brown seaweed (Ascophyllum nodosum and Fucus vesiculosus) with known inhibitory action on α-amylase and α-glucosidase activities (InSea2TM) was associated with reduced insulin incremental area under the curve and enhanced insulin sensitivity [248]. In a rat model, the same product lowered postprandial blood glucose level and peak insulin secretion [249]. Phlorotannins from Cystoseira compressa demonstrated anti-diabetic effects by reducing glucose, total triglycerides, total cholesterol levels, while increasing antioxidant capacity and insulin concentration in STZ-induced diabetic rats [250]. Similarly, postprandial blood glucose levels were decreased after an administration of ezoishige (Pelvetia babingtonii de Toni) extract in rats [251]. The list of tannins included in this review in connection with T2DM is summarized in Table 5.

Table 5.
List of tannins in connection with T2DM.
2.5. Lignans and T2DM
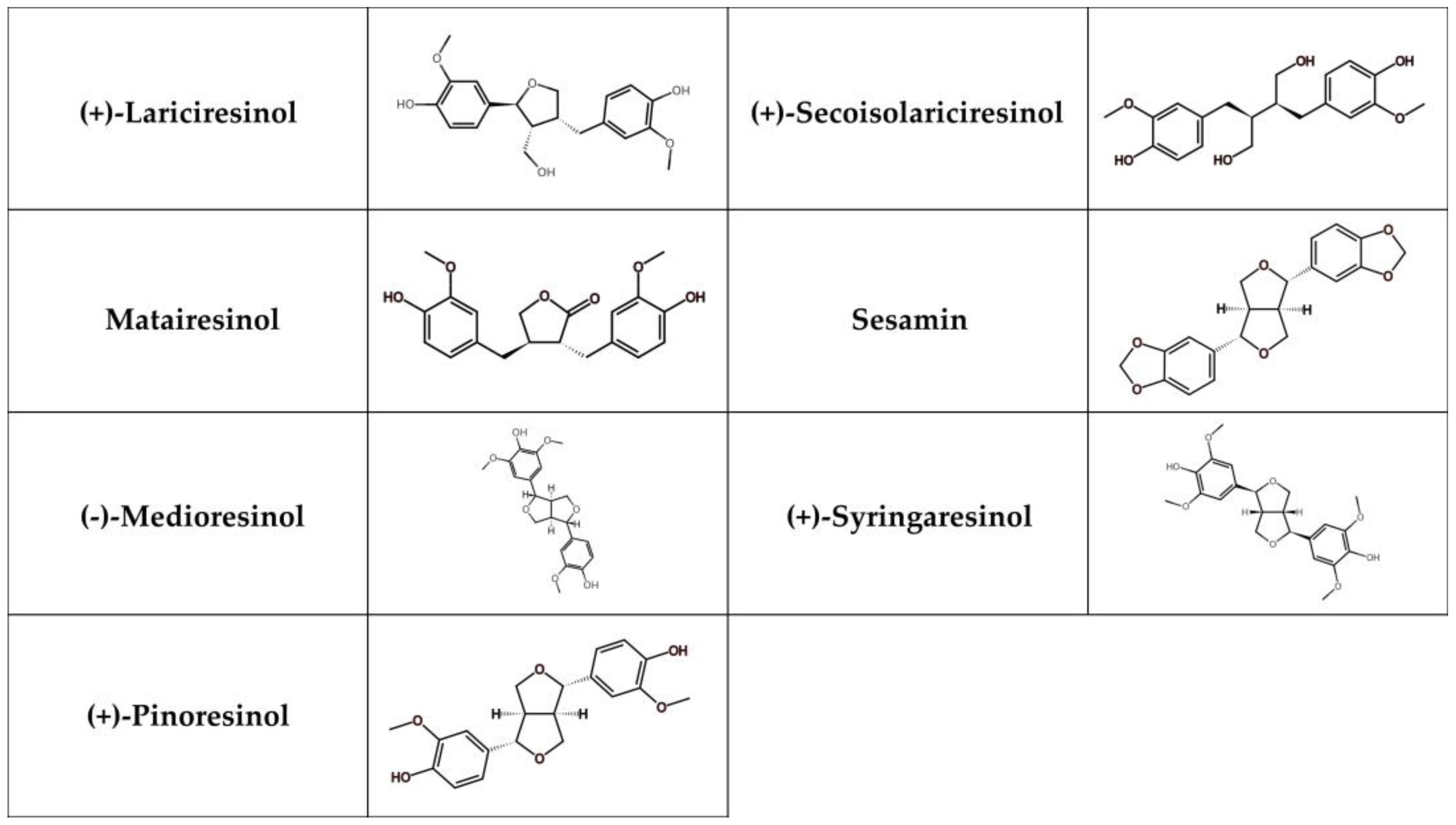
Lignans represent phenolic compounds derived from the biosynthesis of shikimic acid and are found in large quantities in plants [252]. Structurally, they form a part of a diverse family that includes lignans, neolignans, and oxyneolignans [253]. Lignans usually exhibit dimeric structures formed by a β, β’-linkage between two phenylpropane units. Neolignans, on the other hand, are compounds with an alternative linkage. Due to their chemical differences, lignans can be classified into several groups, such as secoisolariciresionol, pinoresinol, matairesinol, medioresinol, sesamin, syringaresinol, and lariciresinol (Figure 5) [254].
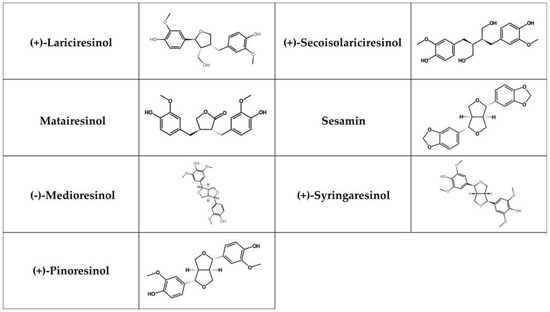
Figure 5.
Chemical structure of selected lignans.
Lignans can be extracted from seeds (e.g., sesame, flaxseed), cereals (e.g., rye grains, buckwheat), vegetables (e.g., broccoli, cucumber), fruits (e.g., grapefruit, pear), beverages (e.g., red wine, coffee) and oils (e.g., olive oil, sesame seed oil) [255]. Numerous bioactivities of lignans result from the gut microbiota’s chemical transformation. Lignan glycosides are hydrolyzed to lignan aglycones and subsequently converted into enterolignans. These compounds have positive health effects and are more bioavailable than their precursors [256]. Sun et al. [257] proposed that enterolactone is linked to a decreased risk of T2DM in women. Lignan intake was related to higher serum enterolactone concentrations. The presence of Coprococcus sp. ART55/1, Butyrivibrio crossotus, Faecalibacterium prausnitzii, Methanobrevibacter smithii, and Alistipes shahii in the gut microbiota was associated with plasma enterolactone concentrations in participating men [258]. Furthermore, supplementation with a lignan-rich extract from Cinnamomum camphora leaves improved glucose and insulin tolerance while increasing the abundance of Lactobacillus in diabetic mice [259].
Several studies suggest that lignans intake may be associated with a lower risk of T2DM. A population-based prospective study conducted on 2882 men and 3665 women revealed that daily consumption of lignans was connected to decreased T2DM risk [260]. A cohort study by Wang et al. [261], involving 201,111 men and women from 3 large studies discovered that a lower risk of T2DM was associated with higher intakes of both total and individual lignans, with the exception of lariciresinol. On the other hand, in an observational cohort study of nondiabetic participants (n = 3430), no significant effect of consuming a lignan-rich diet on the development of T2DM was determined after 6.1 years of follow-up [74]. Several lignans have shown effects on various mechanisms related to T2DM. Pinoresinol was able to inhibit α-glucosidase [262], while syringaresinol reduced the levels of cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) in microglia cells as well as the expression of inflammatory cytokines (TNF-α, IL-1β) [263]. Rats (carrageenan-induced model of inflammation) supplemented with sesamin (50 and 100 mg/kg for 2 days) showed lower levels of TNF-α, IL-1β, and IL-8 [264]. The treatment with secoisolariciresinol diglucoside, the glycosylated form of secoisolariciresionol, also decreased levels of IL-1β, IL-18, TNF-α, and NLR family pyrin domain containing 1 (NLRP1) in mice [265]. The aforementioned findings indicate that lignans may serve as potential therapeutic agents in inflammatory conditions, which are part of T2DM. Table 6 presents the list of lignans involved in this review related to T2DM.

Table 6.
List of lignans related to T2DM.
3. The Interactions Between Plant-Derived Polyphenols and Antidiabetic Drugs
Since T2DM is a complex and complicated disease, treatment must be comprehensive. This complexity makes it impossible to control the disease and its risk factors with a single management approach [266]. In general, biguanides (e.g., MET), thiazolidinediones (e.g., rosiglitazone), insulin secretagogues (sulphonylureas such as glimepiride, meglitinides such as repaglinide), α-glucosidase inhibitors (e.g., acarbose), incretin-based therapies (GLP-1 agonists such as dulaglutide, dipeptidyl peptidase 4 (DPP4) inhibitors such as sitagliptin), and SGLT2 inhibitors (e.g., canagliflozin) belong to the common oral antidiabetic medications for T2DM [2,10]. However, the long-term application of these drugs can lead to adverse side effects, which emphasizes the need for new combination therapies aimed at the complex nature of the disease while minimizing toxicity [266]. For centuries, people around the world have been using traditional plant-based medicines [267]. Many T2DM patients supplement their conventional treatments with herbal medicines, which may influence the effectiveness of the treatment. Plant-drug interactions allow us to reveal how bioactive compounds in plants affect the pharmacokinetics (PK) and pharmacodynamics (PD) of synthetic drugs. PK involves drug absorption, distribution, metabolism, and excretion, primarily through cytochrome P450 (CYP450) enzymes [268,269]. Overall, three types of PD interactions/effects can be distinguished: synergistic, antagonistic, and additive [266,270]. Polyphenols in herbal medicines can interact and influence their bioactive properties. For example, quercetin and rutin from Hippophae rhamnoides L. may exhibit different PK interactions. In the polyherbal formulation, the bioavailability of quercetin was reduced, while that of rutin was increased, compared to co-administration [271]. However, studying these interactions is challenging because existing methods often oversimplify mixtures by focusing on individual compounds rather than the synergistic effects of whole plant extracts [272].
MET is the most common antidiabetic drug used in the treatment of T2DM patients. Multiple in vivo and in vitro studies have documented that the combination of different plant products (e.g., ferulic, p-coumaric, chlorogenic, caffeic, gallic acids, malvidin, cyanidin-3-arabinoside, anthocyanins, quercetin, kaempferol, apigenin, epigallocatechin gallate, avicularin) with MET has synergistic effects (Table 7) [118,168,273,274,275,276]. However, interactions between MET and certain herbal products can reduce its effectiveness by affecting drug transport mechanisms [277,278]. MET is taken up by organic cation transporter 1 (OCT1) for delivery to the liver and eliminated by multidrug and toxin extrusion protein 1 (MATE1) for renal excretion [279]. While combining MET with herbal products may enhance the benefits of treatment, it is essential to carefully assess potential negative interactions to optimize therapeutic outcomes. In this context, Knop et al. [277] revealed inhibitory effects of green tea and epigallocatechin gallate (EGCG) on multiple drug transporters in vitro. As a result, MET uptake mediated by OCT1 and MATE1 was significantly reduced in the presence of green tea and EGCG, suggesting an antagonistic interaction. However, in vivo combination therapy with MET and EGCG ultimately reduced fasting blood glucose in diabetic rats [280]. According to Haque et al. [278], Abelmoschus esculentus (L.) extract showed an agonist effect against the synergistic antidiabetic activity of MET and acarbose (ACR) when co-administered in glucose-induced hyperglycemic mice. Several studies indicate a synergistic effect of thiazolidinediones (TZDs) or PPAR-γ agonists, in combination with plant polyphenols. For example, TZDs combined with hydroxycinnamic derivatives significantly increased 2-DG levels [276], and TZDs combined with ferulic acid improved blood glucose and lipid profiles [168]. Pioglitazone with resveratrol also reduced fasting blood glucose levels and inflammation in diabetic rats [281]. However, naringenin, a citrus flavonoid, attenuated the hypoglycemic impacts of pioglitazone in obese diabetic mice, indicating weak partial antagonistic activity [282]. Several studies have demonstrated that sulfonylureas’ antidiabetic effects are lessened when combined with herbal products [283]. Co-administration of Azadirachta indica extract with glibenclamide or glimepiridine induced an antagonistic interaction, which was manifested by raised blood glucose levels [284]. Similarly, Annona cherimola extract did not show a decrease in blood glucose levels after co-administration with glibenclamide in diabetic mice, suggesting a possible antagonistic impact [285]. Gallic acid potentiated the impacts of ACR, an α-glucosidase inhibitor, in diabetic rats [275]. Rutin from Annona cherimola also showed positive effects when combined with ACR in diabetic mice [285]. Synergistic inhibition of α-glucosidase activity was observed when quercetin, baicalein, or luteolin were combined with ACR, while antagonistic inhibition was determined when (+)-catechin was combined with ACR [286]. Resveratrol improved the PK of DPP4 inhibitors (alogliptin and saxagliptin) in rats [287]. Eugenia jambolana extract combined with sitagliptin lowered fasting blood glucose levels and ameliorated lipid profile in diabetic rats [288]. The study by Valdes et al. [285] revealed that Cherimoya annona extract and rutin with canagliflozin (an SGLT2 inhibitor) significantly reduced blood glucose levels and improved lipid indicators in diabetic mice. The interactions of antidiabetic drugs with various plants and polyphenols obtained from them are summarized in Table 7.

Table 7.
The interactions of antidiabetic drugs with various plants and polyphenols obtained from them.
4. Conclusions
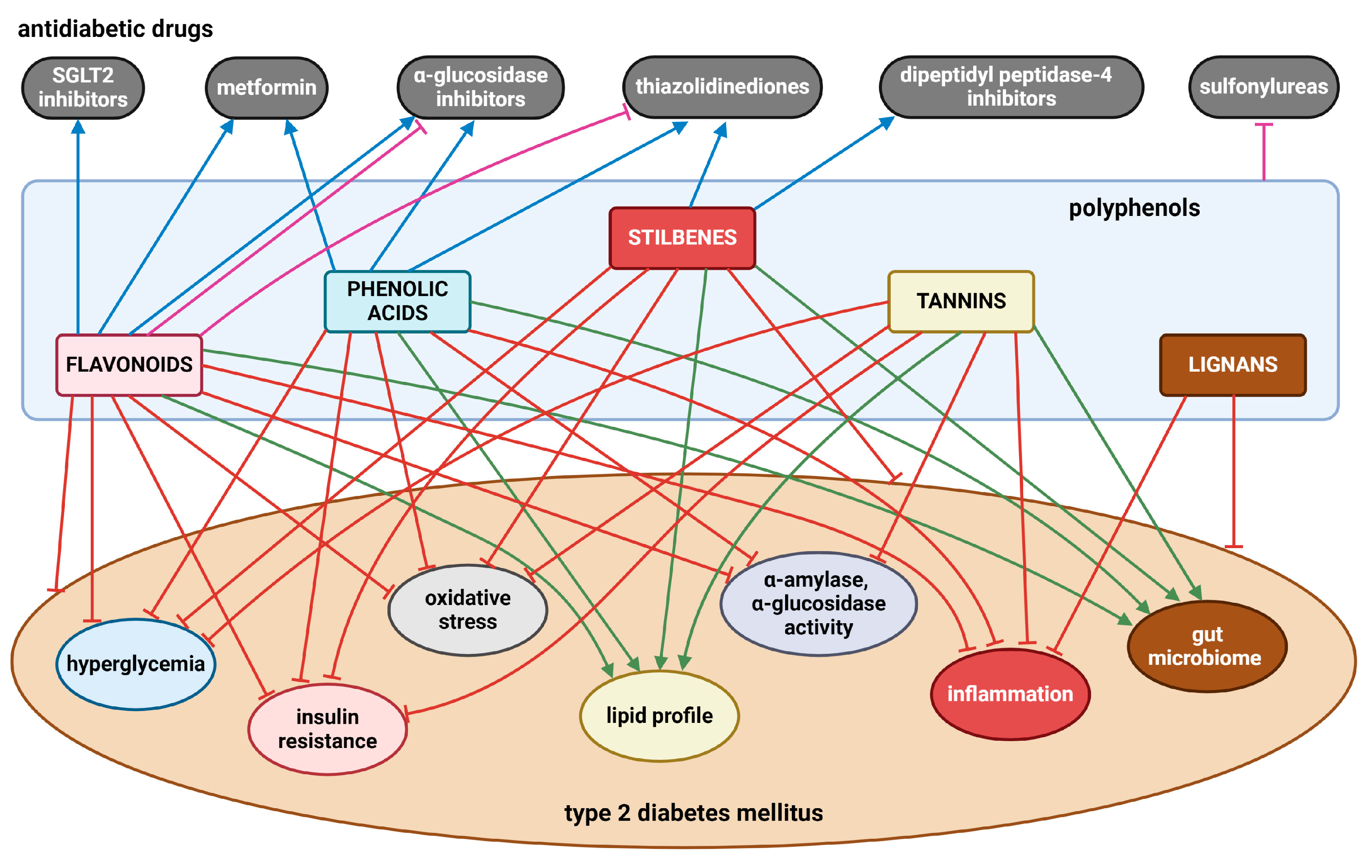
Numerous in vivo and in vitro investigations show that a diet high in polyphenols is linked to multiple health advantages. In T2DM, various flavonoids (e.g., quercetin, kaempferol, rutin, epicatechin, genistein, daidzein, anthocyanins), phenolic acids (e.g., chlorogenic, caffeic, ellagic, gallic acids, curcumin), stilbenes (e.g., resveratrol), tannins (e.g., procyanidin B2, seaweed phlorotannins), and lignans (e.g., pinoresinol) have been demonstrated to lower hyperglycemia, enhance insulin sensitivity and improve insulin secretion, scavenge ROS, and reduce chronic inflammation. In addition, mounting evidence highlights the role of polyphenols in modulating gut microbiota, leading to the abundance of beneficial microbial populations such as Bifidobacteria and Lactobacillus. However, findings remain inconsistent, with some studies reporting conflicting results. Several limitations hinder definitive conclusions, including variability in study design, differences in polyphenol bioavailability, structural variations among polyphenols that affect their metabolism, and significant individual heterogeneity in gut microbiota composition. Figure 6 presents the most significant findings related to this issue.
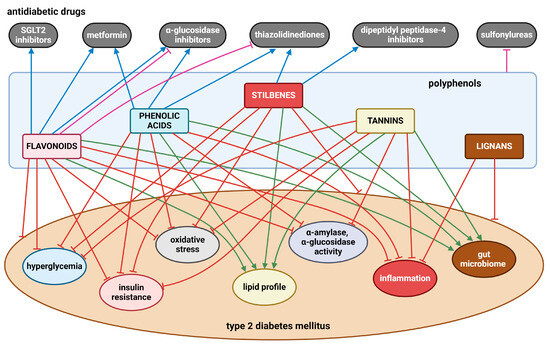
Figure 6.
The effects of polyphenols on T2DM and its obvious clinical symptoms, as well as the interactions between polyphenols and antidiabetic drugs. Blunt red arrows indicate an inhibitory effect; sharp green arrows designate a stimulatory effect or improvement. Blue arrows show a synergistic effect, while blunt purple arrows indicate an antagonistic effect. Created with BioRender.com (accessed on 4 December 2024).
Importantly, dietary polyphenols also have the ability to mitigate serious secondary complications of T2DM, including cardiovascular disorders, diabetic neuropathy, nephropathy and retinopathy, diabetic foot, osteoporosis, liver damage, susceptibility to infections and some cancers. Since food generally contains several types of polyphenols in varying amounts, it is necessary to point out their mutual interactions. Polyphenols from guava tea, coffee, cocoa, olive oil, propolis, red wine, dark chocolate, blueberries, and grape seeds have been found to exert antidiabetic effects in patients with T2DM due to enhanced glucose metabolism, reduced insulin resistance, HbA1c, and improved vascular function. Based on available clinical studies, however, it seems that several individual polyphenols, such as quercetin, kaempferol, epicatechin, anthocyanins, curcumin, and resveratrol, have also demonstrated antidiabetic activity by reducing hyperglycemia, dyslipidemia, and alleviating insulin resistance.
The interactions between polyphenols and conventional antidiabetic drugs offer a promising strategy in the management and treatment of T2DM, especially when the disease is in an advanced stage, by lowering hyperglycemia, improving lipid profiles, and reducing insulin resistance. Although there is generally a promising potential for synergistic effects of polyphenols with antidiabetic drugs, the risk of antagonistic interactions that may impair drug efficacy (Figure 6) underlines the need for careful evaluation. In this direction, more research is required to clarify these mutual interactions with the aim of exploiting the knowledge in clinical applications. Several animal model studies and clinical trials published to date are often limited by small sample sizes, short experimental durations, and the diversity of parameters analyzed. An important limitation of studies analyzing different polyphenolic extracts is insufficient characterization of the experimental material or inadequate phytochemical analysis of the extracts as recommended by the best practice guidelines. Future research should therefore aim to address these shortcomings and include large, controlled clinical studies with diverse populations, taking into account individual variability and confounding factors such as diet and lifestyle.
However, based on the published data, it can be concluded that dietary polyphenol intake can alleviate the overt clinical symptoms of T2DM, as well as its secondary complications. This suggests that they undoubtedly play a protective role in the management and treatment of T2DM.
Author Contributions
Conceptualization, M.M.; methodology, M.M., A.S. (Anna Sarocka) and R.O.; formal analysis, R.B., V.K. and V.M.; writing—original draft preparation, M.M., A.S. (Anna Sarocka), N.P., A.S. (Aneta Sevcikova), S.C. and R.O.; writing—review and editing, M.M., S.C. and R.O.; visualization, A.S. (Anna Sarocka), N.P., R.B. and R.O.; supervision, M.M., S.C. and R.O.; funding acquisition, M.M. and R.O. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the EU NextGenerationEU through the Recovery and Resilience Plan for Slovakia under the project No. 09I03-03-V04-00607.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Barik, S.K.; Sengupta, S.; Arya, R.; Kumar, S.; Kim, J.J.; Chaurasia, R. Dietary Polyphenols as Potential Therapeutic Agents in Type 2 Diabetes Management: Advances and Opportunities. Adv. Nutr. 2024, 16, 100346. [Google Scholar] [CrossRef] [PubMed]
- Martiniakova, M.; Kovacova, V.; Mondockova, V.; Svik, K.; Londzin, P.; Folwarczna, J.; Soltesova Prnova, M.; Stefek, M.; Omelka, R. The Effects of Prolonged Treatment with Cemtirestat on Bone Parameters Reflecting Bone Quality in Non-Diabetic and Streptozotocin-Induced Diabetic Rats. Pharmaceuticals 2023, 16, 628. [Google Scholar] [CrossRef] [PubMed]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes-2023. Diabetes Care 2023, 46, S19–S40. [Google Scholar] [CrossRef] [PubMed]
- Martiniakova, M.; Biro, R.; Penzes, N.; Sarocka, A.; Kovacova, V.; Mondockova, V.; Omelka, R. Links Among Obesity, Type 2 Diabetes Mellitus, and Osteoporosis: Bone as a Target. Int. J. Mol. Sci. 2024, 25, 4827. [Google Scholar] [CrossRef]
- Shahidi, F.; Danielski, R. Review on the Role of Polyphenols in Preventing and Treating Type 2 Diabetes: Evidence from In Vitro and In Vivo Studies. Nutrients 2024, 16, 3159. [Google Scholar] [CrossRef]
- Ahmad, E.; Lim, S.; Lamptey, R.; Webb, D.R.; Davies, M.J. Type 2 Diabetes. Lancet 2022, 400, 1803–1820. [Google Scholar] [CrossRef]
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining Roles of Specific Reactive Oxygen Species (ROS) in Cell Biology and Physiology. Nat. Rev. Mol. Cell Biol. 2022, 23, 499–515. [Google Scholar] [CrossRef]
- Farmaki, P.; Damaskos, C.; Garmpis, N.; Garmpi, A.; Savvanis, S.; Diamantis, E. Complications of the Type 2 Diabetes Mellitus. Curr. Cardiol. Rev. 2020, 16, 249–251. [Google Scholar] [CrossRef]
- Giovannucci, E.; Harlan, D.M.; Archer, M.C.; Bergenstal, R.M.; Gapstur, S.M.; Habel, L.A.; Pollak, M.; Regensteiner, J.G.; Yee, D. Diabetes and Cancer: A Consensus Report. Diabetes Care 2010, 33, 1674–1685. [Google Scholar] [CrossRef]
- Blahova, J.; Martiniakova, M.; Babikova, M.; Kovacova, V.; Mondockova, V.; Omelka, R. Pharmaceutical Drugs and Natural Therapeutic Products for the Treatment of Type 2 Diabetes Mellitus. Pharmaceuticals 2021, 14, 806. [Google Scholar] [CrossRef]
- Aikaeli, F.; Njim, T.; Gissing, S.; Moyo, F.; Alam, U.; Mfinanga, S.G.; Okebe, J.; Ramaiya, K.; Webb, E.L.; Jaffar, S.; et al. Prevalence of Microvascular and Macrovascular Complications of Diabetes in Newly Diagnosed Type 2 Diabetes in Low-and-Middle-Income Countries: A Systematic Review and Meta-Analysis. PLOS Glob. Public Health 2022, 2, e0000599. [Google Scholar] [CrossRef] [PubMed]
- Hassan, W.; Noreen, H.; Rehman, S.; Gul, S.; Kamal, M.A.; Kamdem, J.P.; Zaman, B.; da Rocha, J.B.T. Oxidative Stress and Antioxidant Potential of One Hundred Medicinal Plants. Curr. Top. Med. Chem. 2017, 17, 1336–1370. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2010, 33 (Suppl. S1), S62–S69. [Google Scholar] [CrossRef] [PubMed]
- Rines, A.K.; Sharabi, K.; Tavares, C.D.J.; Puigserver, P. Targeting Hepatic Glucose Metabolism in the Treatment of Type 2 Diabetes. Nat. Rev. Drug Discov. 2016, 15, 786–804. [Google Scholar] [CrossRef]
- van Gerwen, J.; Shun-Shion, A.S.; Fazakerley, D.J. Insulin Signalling and GLUT4 Trafficking in Insulin Resistance. Biochem. Soc. Trans. 2023, 51, 1057–1069. [Google Scholar] [CrossRef]
- Gutiérrez-Rodelo, C.; Roura-Guiberna, A.; Olivares-Reyes, J.A. Molecular Mechanisms of Insulin Resistance: An Update. Gac. Med. Mex. 2017, 153, 214–228. [Google Scholar]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Tudoreanu, L.; Ștefan, G. Plant Polyphenols Mechanisms of Action on Insulin Resistance and Against the Loss of Pancreatic Beta Cells. Crit. Rev. Food Sci. Nutr. 2022, 62, 325–352. [Google Scholar] [CrossRef]
- de Paulo Farias, D.; de Araújo, F.F.; Neri-Numa, I.A.; Pastore, G.M. Antidiabetic Potential of Dietary Polyphenols: A Mechanistic Review. Food Res. Int. 2021, 145, 110383. [Google Scholar] [CrossRef]
- Chen, L.; Gnanaraj, C.; Arulselvan, P.; El-Seedi, H.; Teng, H. A Review on Advanced Microencapsulation Technology to Enhance Bioavailability of Phenolic Compounds: Based on Its Activity in the Treatment of Type 2 Diabetes. Trends Food Sci. Technol. 2019, 85, 149–162. [Google Scholar] [CrossRef]
- Aryaeian, N.; Sedehi, S.K.; Arablou, T. Polyphenols and Their Effects on Diabetes Management: A Review. Med. J. Islam. Repub. Iran. 2017, 31, 134. [Google Scholar] [CrossRef]
- Da Porto, A.; Cavarape, A.; Colussi, G.; Casarsa, V.; Catena, C.; Sechi, L.A. Polyphenols Rich Diets and Risk of Type 2 Diabetes. Nutrients 2021, 13, 1445. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A Concise Overview on the Chemistry, Occurrence, and Human Health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef] [PubMed]
- Khadem, S.; Marles, R.J. Monocyclic Phenolic Acids; Hydroxy- and Polyhydroxybenzoic Acids: Occurrence and Recent Bioactivity Studies. Molecules 2010, 15, 7985–8005. [Google Scholar] [CrossRef]
- Sun, C.; Zhao, C.; Guven, E.C.; Paoli, P.; Simal-Gandara, J.; Ramkumar, K.M.; Wang, S.; Buleu, F.; Pah, A.; Turi, V.; et al. Dietary Polyphenols as Antidiabetic Agents: Advances and Opportunities. Food Front. 2020, 1, 18–44. [Google Scholar] [CrossRef]
- Naz, R.; Saqib, F.; Awadallah, S.; Wahid, M.; Latif, M.F.; Iqbal, I.; Mubarak, M.S. Food Polyphenols and Type II Diabetes Mellitus: Pharmacology and Mechanisms. Molecules 2023, 28, 3996. [Google Scholar] [CrossRef] [PubMed]
- Giacometti, J.; Muhvić, D.; Grubić-Kezele, T.; Nikolić, M.; Šoić-Vranić, T.; Bajek, S. Olive Leaf Polyphenols (OLPs) Stimulate GLUT4 Expression and Translocation in the Skeletal Muscle of Diabetic Rats. Int. J. Mol. Sci. 2020, 21, 8981. [Google Scholar] [CrossRef]
- Yamashita, Y.; Wang, L.; Nanba, F.; Ito, C.; Toda, T.; Ashida, H. Procyanidin Promotes Translocation of Glucose Transporter 4 in Muscle of Mice Through Activation of Insulin and AMPK Signaling Pathways. PLoS ONE 2016, 11, e0161704. [Google Scholar] [CrossRef]
- Nagano, T.; Hayashibara, K.; Ueda-Wakagi, M.; Yamashita, Y.; Ashida, H. Black Tea Polyphenols Promotes GLUT4 Translocation through Both PI3K-and AMPK-Dependent Pathways in Skeletal Muscle Cells. Food Sci. Technol. Res. 2015, 21, 489–494. [Google Scholar] [CrossRef]
- Bhuyan, U.; Handique, J.G. Chapter 6—Plant Polyphenols as Potent Antioxidants: Highlighting the Mechanism of Antioxidant Activity and Synthesis/Development of Some Polyphenol Conjugates. In Bioactive Natural Products; Atta-ur-Rahman, Ed.; Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2022; Volume 75, pp. 243–266. [Google Scholar]
- Dall’Asta, M.; Bayle, M.; Neasta, J.; Scazzina, F.; Bruni, R.; Cros, G.; Del Rio, D.; Oiry, C. Protection of Pancreatic β-Cell Function by Dietary Polyphenols. Phytochem. Rev. 2015, 14, 933–959. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Merino, J.; Sun, Q.; Fitó, M.; Salas-Salvadó, J. Dietary Polyphenols, Mediterranean Diet, Prediabetes, and Type 2 Diabetes: A Narrative Review of the Evidence. Oxid. Med. Cell Longev. 2017, 2017, 6723931. [Google Scholar] [CrossRef]
- Viollet, B.; Guigas, B.; Leclerc, J.; Hébrard, S.; Lantier, L.; Mounier, R.; Andreelli, F.; Foretz, M. AMP-Activated Protein Kinase in the Regulation of Hepatic Energy Metabolism: From Physiology to Therapeutic Perspectives. Acta Physiol. 2009, 196, 81–98. [Google Scholar] [CrossRef] [PubMed]
- Kashtoh, H.; Baek, K.-H. Recent Updates on Phytoconstituent Alpha-Glucosidase Inhibitors: An Approach towards the Treatment of Type Two Diabetes. Plants 2022, 11, 2722. [Google Scholar] [CrossRef] [PubMed]
- Satoh, T. Molecular Mechanisms for the Regulation of Insulin-Stimulated Glucose Uptake by Small Guanosine Triphosphatases in Skeletal Muscle and Adipocytes. Int. J. Mol. Sci. 2014, 15, 18677–18692. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Tripathi, P. Gut Microbiome and Type 2 Diabetes: Where We Are and Where to Go? J. Nutr. Biochem. 2019, 63, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Ejtahed, H.-S.; Hoseini-Tavassol, Z.; Khatami, S.; Zangeneh, M.; Behrouzi, A.; Ahmadi Badi, S.; Moshiri, A.; Hasani-Ranjbar, S.; Soroush, A.-R.; Vaziri, F.; et al. Main Gut Bacterial Composition Differs between Patients with Type 1 and Type 2 Diabetes and Non-Diabetic Adults. J. Diabetes Metab. Disord. 2020, 19, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Craciun, C.-I.; Neag, M.-A.; Catinean, A.; Mitre, A.-O.; Rusu, A.; Bala, C.; Roman, G.; Buzoianu, A.-D.; Muntean, D.-M.; Craciun, A.-E. The Relationships Between Gut Microbiota and Diabetes Mellitus, and Treatments for Diabetes Mellitus. Biomedicines 2022, 10, 308. [Google Scholar] [CrossRef]
- Xiong, R.-G.; Zhou, D.-D.; Wu, S.-X.; Huang, S.-Y.; Saimaiti, A.; Yang, Z.-J.; Shang, A.; Zhao, C.-N.; Gan, R.-Y.; Li, H.-B. Health Benefits and Side Effects of Short-Chain Fatty Acids. Foods 2022, 11, 2863. [Google Scholar] [CrossRef]
- Zemanova, N.; Omelka, R.; Mondockova, V.; Kovacova, V.; Martiniakova, M. Roles of Gut Microbiome in Bone Homeostasis and Its Relationship with Bone-Related Diseases. Biology 2022, 11, 1402. [Google Scholar] [CrossRef]
- Cui, J.; Ramesh, G.; Wu, M.; Jensen, E.T.; Crago, O.; Bertoni, A.G.; Gao, C.; Hoffman, K.L.; Sheridan, P.A.; Wong, K.E.; et al. Butyrate-Producing Bacteria and Insulin Homeostasis: The Microbiome and Insulin Longitudinal Evaluation Study (MILES). Diabetes 2022, 71, 2438–2446. [Google Scholar] [CrossRef]
- Mayorga-Ramos, A.; Barba-Ostria, C.; Simancas-Racines, D.; Guamán, L.P. Protective Role of Butyrate in Obesity and Diabetes: New Insights. Front. Nutr. 2022, 9, 1067647. [Google Scholar] [CrossRef]
- Huang, S.; Chen, J.; Cui, Z.; Ma, K.; Wu, D.; Luo, J.; Li, F.; Xiong, W.; Rao, S.; Xiang, Q.; et al. Lachnospiraceae-Derived Butyrate Mediates Protection of High Fermentable Fiber Against Placental Inflammation in Gestational Diabetes Mellitus. Sci. Adv. 2023, 9, eadi7337. [Google Scholar] [CrossRef] [PubMed]
- Coppola, S.; Nocerino, R.; Paparo, L.; Bedogni, G.; Calignano, A.; Di Scala, C.; de Giovanni di Santa Severina, A.F.; De Filippis, F.; Ercolini, D.; Berni Canani, R. Therapeutic Effects of Butyrate on Pediatric Obesity: A Randomized Clinical Trial. JAMA Netw. Open 2022, 5, e2244912. [Google Scholar] [CrossRef] [PubMed]
- Roshanravan, N.; Mahdavi, R.; Alizadeh, E.; Jafarabadi, M.A.; Hedayati, M.; Ghavami, A.; Alipour, S.; Alamdari, N.M.; Barati, M.; Ostadrahimi, A. Effect of Butyrate and Inulin Supplementation on Glycemic Status, Lipid Profile and Glucagon-Like Peptide 1 Level in Patients with Type 2 Diabetes: A Randomized Double-Blind, Placebo-Controlled Trial. Horm. Metab. Res. 2017, 49, 886–891. [Google Scholar] [CrossRef]
- Liu, J.; Tan, Y.; Cheng, H.; Zhang, D.; Feng, W.; Peng, C. Functions of Gut Microbiota Metabolites, Current Status and Future Perspectives. Aging Dis. 2022, 13, 1106–1126. [Google Scholar] [CrossRef] [PubMed]
- Cherniack, E.P. Polyphenols: Planting the Seeds of Treatment for the Metabolic Syndrome. Nutrition 2011, 27, 617–623. [Google Scholar] [CrossRef]
- Iatcu, O.C.; Hamamah, S.; Covasa, M. Harnessing Prebiotics to Improve Type 2 Diabetes Outcomes. Nutrients 2024, 16, 3447. [Google Scholar] [CrossRef]
- Gutiérrez-Grijalva, E.P.; Antunes-Ricardo, M.; Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Basilio Heredia, J. Cellular Antioxidant Activity and In Vitro Inhibition of α-Glucosidase, α-Amylase and Pancreatic Lipase of Oregano Polyphenols Under Simulated Gastrointestinal Digestion. Food Res. Int. 2019, 116, 676–686. [Google Scholar] [CrossRef]
- Lv, C.; Cheng, L.; Feng, W.; Xie, H.; Kou, J.; Wang, L.; Shi, M.; Song, X.; Wang, X.; Chen, S.; et al. Targeting Microbiota-Immune-Synaptic Plasticity to Explore the Effect of Tea Polyphenols on Improving Memory in the Aged Type 2 Diabetic Rat Model. Nutr. Neurosci. 2024, 27, 1422–1438. [Google Scholar] [CrossRef]
- Jing, Y.; Han, G.; Hu, Y.; Bi, Y.; Li, L.; Zhu, D. Tea Consumption and Risk of Type 2 Diabetes: A Meta-Analysis of Cohort Studies. J. Gen. Intern. Med. 2009, 24, 557–562. [Google Scholar] [CrossRef]
- Liu, Q.; Ma, Q.; Li, J.; Sun, L.; Zhuang, Y. Hypoglycemic Effect of Rambutan (Nephelium Lappaceum L.) Peel Polyphenols on Type 2 Diabetes Mice by Modulating Gut Microbiota and Metabolites. Mol. Nutr. Food Res. 2024, 68, e2400555. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Ouyang, H.; Xu, W.; Sun, Y.; Zhong, Y.; Wang, L.; Huang, J.; Chen, J.; Li, M.; et al. Pueraria Thomsonii Radix Water Extract Alleviate Type 2 Diabetes Mellitus in Db/Db Mice Through Comprehensive Regulation of Metabolism and Gut Microbiota. Molecules 2023, 28, 7471. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Zhang, Z.; Zhao, Y.; Kang, C.; Zhang, X.; Tian, Y.; Yu, J.; Cao, H.; Wang, M. The Anti-Diabetic Activity of Polyphenols-Rich Vinegar Extract in Mice via Regulating Gut Microbiota and Liver Inflammation. Food Chem. 2022, 393, 133443. [Google Scholar] [CrossRef] [PubMed]
- Hua, Q.; Han, Y.; Zhao, H.; Zhang, H.; Yan, B.; Pei, S.; He, X.; Li, Y.; Meng, X.; Chen, L.; et al. Punicalagin Alleviates Renal Injury via the Gut-Kidney Axis in High-Fat Diet-Induced Diabetic Mice. Food Funct. 2022, 13, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Ming, J. Mulberry Polyphenols Restored Both Small and Large Intestinal Microflora in Db/Db Mice, Potentially Alleviating Type 2 Diabetes. Food Funct. 2024, 15, 8521–8543. [Google Scholar] [CrossRef]
- Ferreira, G.; Vieira, P.; Alves, A.; Nunes, S.; Preguiça, I.; Martins-Marques, T.; Ribeiro, T.; Girão, H.; Figueirinha, A.; Salgueiro, L.; et al. Effect of Blueberry Supplementation on a Diet-Induced Rat Model of Prediabetes-Focus on Hepatic Lipid Deposition, Endoplasmic Stress Response and Autophagy. Nutrients 2024, 16, 513. [Google Scholar] [CrossRef]
- Zuo, Z.; Pang, W.; Sun, W.; Lu, B.; Zou, L.; Zhang, D.; Wang, Y. Metallothionein-Kidney Bean Polyphenol Complexes Showed Antidiabetic Activity in Type 2 Diabetic Rats by Improving Insulin Resistance and Regulating Gut Microbiota. Foods 2023, 12, 3139. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An Overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Stavenga, D.G.; Leertouwer, H.L.; Dudek, B.; van der Kooi, C.J. Coloration of Flowers by Flavonoids and Consequences of pH Dependent Absorption. Front. Plant Sci. 2021, 11, 600124. [Google Scholar] [CrossRef]
- Chu, L.; Zheng, W.; Wang, J.; Wang, Z.; Zhao, W.; Zhao, B.; Xu, G.; Xiao, M.; Lou, X.; Pan, F.; et al. Comparative Analysis of the Difference in Flavonoid Metabolic Pathway during Coloring Between Red-Yellow and Red Sweet Cherry (Prunus avium L.). Gene 2023, 880, 147602. [Google Scholar] [CrossRef]
- Chen, S.; Wang, X.; Cheng, Y.; Gao, H.; Chen, X. A Review of Classification, Biosynthesis, Biological Activities and Potential Applications of Flavonoids. Molecules 2023, 28, 4982. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant Flavonoids: Classification, Distribution, Biosynthesis, and Antioxidant Activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef] [PubMed]
- Speciale, A.; Bashllari, R.; Muscarà, C.; Molonia, M.S.; Saija, A.; Saha, S.; Wilde, P.J.; Cimino, F. Anti-Inflammatory Activity of an In Vitro Digested Anthocyanin-Rich Extract on Intestinal Epithelial Cells Exposed to TNF-α. Molecules 2022, 27, 5368. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, J.; Li, Z.; Wang, L.; Gao, W.; Zhang, Z.; Guo, C. Sodium-Dependent Glucose Transporter 1 and Glucose Transporter 2 Mediate Intestinal Transport of Quercetrin in Caco-2 Cells. Food Nutr. Res. 2020, 64, 3745–3753. [Google Scholar] [CrossRef]
- Kotik, M.; Kulik, N.; Valentová, K. Flavonoids as Aglycones in Retaining Glycosidase-Catalyzed Reactions: Prospects for Green Chemistry. J. Agric. Food Chem. 2023, 71, 14890–14910. [Google Scholar] [CrossRef]
- Chen, Z.; Zheng, S.; Li, L.; Jiang, H. Metabolism of Flavonoids in Human: A Comprehensive Review. Curr. Drug Metab. 2014, 15, 48–61. [Google Scholar] [CrossRef]
- Yang, B.; Liu, H.; Yang, J.; Gupta, V.K.; Jiang, Y. New Insights on Bioactivities and Biosynthesis of Flavonoid Glycosides. Trends Food Sci. Technol. 2018, 79, 116–124. [Google Scholar] [CrossRef]
- Zeb, A. Metabolism of Phenolic AntioxidantsAntioxidants. In Phenolic Antioxidants in Foods: Chemistry, Biochemistry and Analysis; Zeb, A., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 333–383. ISBN 978-3-030-74768-8. [Google Scholar]
- Waheed Janabi, A.H.; Kamboh, A.A.; Saeed, M.; Xiaoyu, L.; BiBi, J.; Majeed, F.; Naveed, M.; Mughal, M.J.; Korejo, N.A.; Kamboh, R.; et al. Flavonoid-Rich Foods (FRF): A Promising Nutraceutical Approach Against Lifespan-Shortening Diseases. Iran. J. Basic. Med. Sci. 2020, 23, 140–153. [Google Scholar] [CrossRef]
- Safe, S.; Jayaraman, A.; Chapkin, R.S.; Howard, M.; Mohankumar, K.; Shrestha, R. Flavonoids: Structure-Function and Mechanisms of Action and Opportunities for Drug Development. Toxicol. Res. 2021, 37, 147–162. [Google Scholar] [CrossRef]
- Martiniakova, M.; Babikova, M.; Mondockova, V.; Blahova, J.; Kovacova, V.; Omelka, R. The Role of Macronutrients, Micronutrients and Flavonoid Polyphenols in the Prevention and Treatment of Osteoporosis. Nutrients 2022, 14, 523. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Zhan, J.; Liu, X.-L.; Wang, Y.; Ji, J.; He, Q.-Q. Dietary Flavonoids Intake and Risk of Type 2 Diabetes: A Meta-Analysis of Prospective Cohort Studies. Clin. Nutr. 2014, 33, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Tresserra-Rimbau, A.; Guasch-Ferré, M.; Salas-Salvadó, J.; Toledo, E.; Corella, D.; Castañer, O.; Guo, X.; Gómez-Gracia, E.; Lapetra, J.; Arós, F.; et al. Intake of Total Polyphenols and Some Classes of Polyphenols Is Inversely Associated with Diabetes in Elderly People at High Cardiovascular Disease Risk123. J. Nutr. 2016, 146, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.A.; Ahmed, Z.A.; Mahwi, T.O.; Aziz, T.A. Quercetin Dampens Postprandial Hyperglycemia in Type 2 Diabetic Patients Challenged with Carbohydrates Load. Int. J. Diabetes Res. 2012, 1, 32–35. [Google Scholar]
- Lee, K.-H.; Park, E.; Lee, H.-J.; Kim, M.-O.; Cha, Y.-J.; Kim, J.-M.; Lee, H.; Shin, M.-J. Effects of Daily Quercetin-Rich Supplementation on Cardiometabolic Risks in Male Smokers. Nutr. Res. Pract. 2011, 5, 28–33. [Google Scholar] [CrossRef]
- Shi, G.-J.; Li, Y.; Cao, Q.-H.; Wu, H.-X.; Tang, X.-Y.; Gao, X.-H.; Yu, J.-Q.; Chen, Z.; Yang, Y. In Vitro and In Vivo Evidence That Quercetin Protects Against Diabetes and Its Complications: A Systematic Review of the Literature. Biomed. Pharmacother. 2019, 109, 1085–1099. [Google Scholar] [CrossRef]
- Yuan, M.; Sun, T.; Zhang, Y.; Guo, C.; Wang, F.; Yao, Z.; Yu, L. Quercetin Alleviates Insulin Resistance and Repairs Intestinal Barrier in Db/Db Mice by Modulating Gut Microbiota. Nutrients 2024, 16, 1870. [Google Scholar] [CrossRef]
- Tsalamandris, S.; Antonopoulos, A.S.; Oikonomou, E.; Papamikroulis, G.-A.; Vogiatzi, G.; Papaioannou, S.; Deftereos, S.; Tousoulis, D. The Role of Inflammation in Diabetes: Current Concepts and Future Perspectives. Eur. Cardiol. 2019, 14, 50–59. [Google Scholar] [CrossRef]
- Jiang, H.; Yamashita, Y.; Nakamura, A.; Croft, K.; Ashida, H. Quercetin and Its Metabolite Isorhamnetin Promote Glucose Uptake Through Different Signalling Pathways in Myotubes. Sci. Rep. 2019, 9, 2690. [Google Scholar] [CrossRef]
- Günal-Köroğlu, D.; Catalkaya, G.; Yusufoğlu, B.; Kezer, G.; Esatbeyoglu, T.; Abd El-Aty, A.M.; Capanoglu, E. Quercetin: Potential Antidiabetic Effects through Enzyme Inhibition and Starch Digestibility. Food Saf. Health 2024, 1–14. [Google Scholar] [CrossRef]
- Gong, L.; Feng, D.; Wang, T.; Ren, Y.; Liu, Y.; Wang, J. Inhibitors of α-Amylase and α-Glucosidase: Potential Linkage for Whole Cereal Foods on Prevention of Hyperglycemia. Food Sci. Nutr. 2020, 8, 6320–6337. [Google Scholar] [CrossRef]
- Alkhalidy, H.; Moore, W.; Wang, A.; Luo, J.; McMillan, R.P.; Wang, Y.; Zhen, W.; Hulver, M.W.; Liu, D. Kaempferol Ameliorates Hyperglycemia through Suppressing Hepatic Gluconeogenesis and Enhancing Hepatic Insulin Sensitivity in Diet-Induced Obese Mice. J. Nutr. Biochem. 2018, 58, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Zhao, X.; Liu, K.; Yang, X.; He, Q.; Gao, Y.; Li, W.; Han, W. Mulberry Leaf Compounds and Gut Microbiota in Alzheimer’s Disease and Diabetes: A Study Using Network Pharmacology, Molecular Dynamics Simulation, and Cellular Assays. Int. J. Mol. Sci. 2024, 25, 4062. [Google Scholar] [CrossRef] [PubMed]
- Bazyar, H.; Zare Javid, A.; Ahangarpour, A.; Zaman, F.; Hosseini, S.A.; Zohoori, V.; Aghamohammadi, V.; Yazdanfar, S.; Ghasemi Deh Cheshmeh, M. The Effects of Rutin Supplement on Blood Pressure Markers, Some Serum Antioxidant Enzymes, and Quality of Life in Patients with Type 2 Diabetes Mellitus Compared with Placebo. Front. Nutr. 2023, 10, 1214420. [Google Scholar] [CrossRef]
- Bazyar, H.; Moradi, L.; Zaman, F.; Zare Javid, A. The Effects of Rutin Flavonoid Supplement on Glycemic Status, Lipid Profile, Atherogenic Index of Plasma, Brain-Derived Neurotrophic Factor (BDNF), Some Serum Inflammatory, and Oxidative Stress Factors in Patients with Type 2 Diabetes Mellitus: A Double-Blind, Placebo-Controlled Trial. Phytother. Res. 2023, 37, 271–284. [Google Scholar] [CrossRef]
- Rozanska, O.; Uruska, A.; Zozulinska-Ziolkiewicz, D. Brain-Derived Neurotrophic Factor and Diabetes. Int. J. Mol. Sci. 2020, 21, 841. [Google Scholar] [CrossRef]
- Sattanathan, K.; Dhanapal, C.K.; Umarani, R.; Manavalan, R. Beneficial Health Effects of Rutin Supplementation in Patients with Diabetes Mellitus. J. Appl. Pharm. Sci. 2011, 1, 227–231. [Google Scholar]
- Cai, C.; Cheng, W.; Shi, T.; Liao, Y.; Zhou, M.; Liao, Z. Rutin Alleviates Colon Lesions and Regulates Gut Microbiota in Diabetic Mice. Sci. Rep. 2023, 13, 4897. [Google Scholar] [CrossRef]
- Dower, J.I.; Geleijnse, J.M.; Gijsbers, L.; Zock, P.L.; Kromhout, D.; Hollman, P.C.H. Effects of the Pure Flavonoids Epicatechin and Quercetin on Vascular Function and Cardiometabolic Health: A Randomized, Double-Blind, Placebo-Controlled, Crossover Trial. Am. J. Clin. Nutr. 2015, 101, 914–921. [Google Scholar] [CrossRef]
- Chun, J.H.; Henckel, M.M.; Knaub, L.A.; Hull, S.E.; Pott, G.B.; Ramirez, D.G.; Reusch, J.E.-B.; Keller, A.C. (−)-Epicatechin Reverses Glucose Intolerance in Rats Housed at Thermoneutrality. Planta Med. 2022, 88, 735–744. [Google Scholar] [CrossRef]
- Gutiérrez-Salmeán, G.; Ortiz-Vilchis, P.; Vacaseydel, C.M.; Garduño-Siciliano, L.; Chamorro-Cevallos, G.; Meaney, E.; Villafaña, S.; Villarreal, F.; Ceballos, G.; Ramírez-Sánchez, I. Effects of (−)-Epicatechin on a Diet-Induced Rat Model of Cardiometabolic Risk Factors. Eur. J. Pharmacol. 2014, 728, 24–30. [Google Scholar] [CrossRef]
- Cordero-Herrera, I.; Martín, M.Á.; Goya, L.; Ramos, S. Cocoa Flavonoids Attenuate High Glucose-Induced Insulin Signalling Blockade and Modulate Glucose Uptake and Production in Human HepG2 Cells. Food Chem. Toxicol. 2014, 64, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Cilleros, D.; Martín, M.Á.; Ramos, S. Protective Effects of (−)-Epicatechin and the Colonic Metabolite 3,4-Dihydroxyphenylacetic Acid against Glucotoxicity-Induced Insulin Signalling Blockade and Altered Glucose Uptake and Production in Renal Tubular NRK-52E Cells. Food Chem. Toxicol. 2018, 120, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Suzuki, M.; Satsu, H.; Arai, S.; Hara, Y.; Suzuki, K.; Miyamoto, Y.; Shimizu, M. Green Tea Polyphenols Inhibit the Sodium-Dependent Glucose Transporter of Intestinal Epithelial Cells by a Competitive Mechanism. J. Agric. Food Chem. 2000, 48, 5618–5623. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, S.; Chen, M.; Wang, J.; Xie, B.; Sun, Z. (−)-Epigallocatechin-3-Gallate (EGCG) Inhibits Starch Digestion and Improves Glucose Homeostasis through Direct or Indirect Activation of PXR/CAR-Mediated Phase II Metabolism in Diabetic Mice. Food Funct. 2018, 9, 4651–4663. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Liu, C.; Wan, L.; Peng, L.; Wen, S.; Fang, W.; Chen, H.; Wang, K.; Yang, X.; Huang, J.; et al. (−)-Epicatechin Ameliorates Type 2 Diabetes Mellitus by Reshaping the Gut Microbiota and Gut-Liver Axis in GK Rats. Food Chem. 2024, 447, 138916. [Google Scholar] [CrossRef]
- Li, F.; Gao, C.; Yan, P.; Zhang, M.; Wang, Y.; Hu, Y.; Wu, X.; Wang, X.; Sheng, J. EGCG Reduces Obesity and White Adipose Tissue Gain Partly Through AMPK Activation in Mice. Front. Pharmacol. 2018, 9, 1366. [Google Scholar] [CrossRef]
- Villa, P.; Costantini, B.; Suriano, R.; Perri, C.; Macrì, F.; Ricciardi, L.; Panunzi, S.; Lanzone, A. The Differential Effect of the Phytoestrogen Genistein on Cardiovascular Risk Factors in Postmenopausal Women: Relationship with the Metabolic Status. J. Clin. Endocrinol. Metab. 2009, 94, 552–558. [Google Scholar] [CrossRef]
- Rockwood, S.; Mason, D.; Lord, R.; Lamar, P.; Prozialeck, W.; Al-Nakkash, L. Genistein Diet Improves Body Weight, Serum Glucose and Triglyceride Levels in Both Male and Female Ob/Ob Mice. Diabetes Metab. Syndr. Obes. 2019, 12, 2011–2021. [Google Scholar] [CrossRef]
- Fu, Z.; Gilbert, E.R.; Pfeiffer, L.; Zhang, Y.; Fu, Y.; Liu, D. Genistein Ameliorates Hyperglycemia in a Mouse Model of Nongenetic Type 2 Diabetes. Appl. Physiol. Nutr. Metab. 2012, 37, 480–488. [Google Scholar] [CrossRef]
- Fu, Z.; Zhang, W.; Zhen, W.; Lum, H.; Nadler, J.; Bassaganya-Riera, J.; Jia, Z.; Wang, Y.; Misra, H.; Liu, D. Genistein Induces Pancreatic Beta-Cell Proliferation through Activation of Multiple Signaling Pathways and Prevents Insulin-Deficient Diabetes in Mice. Endocrinology 2010, 151, 3026–3037. [Google Scholar] [CrossRef]
- Gilbert, E.R.; Liu, D. Anti-Diabetic Functions of Soy Isoflavone Genistein: Mechanisms Underlying Its Effects on Pancreatic β-Cell Function. Food Funct. 2013, 4, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Guevara-Cruz, M.; Godinez-Salas, E.T.; Sanchez-Tapia, M.; Torres-Villalobos, G.; Pichardo-Ontiveros, E.; Guizar-Heredia, R.; Arteaga-Sanchez, L.; Gamba, G.; Mojica-Espinosa, R.; Schcolnik-Cabrera, A.; et al. Genistein Stimulates Insulin Sensitivity through Gut Microbiota Reshaping and Skeletal Muscle AMPK Activation in Obese Subjects. BMJ Open Diabetes Res. Care 2020, 8, e000948. [Google Scholar] [CrossRef] [PubMed]
- Cheong, S.H.; Furuhashi, K.; Ito, K.; Nagaoka, M.; Yonezawa, T.; Miura, Y.; Yagasaki, K. Daidzein Promotes Glucose Uptake through Glucose Transporter 4 Translocation to Plasma Membrane in L6 Myocytes and Improves Glucose Homeostasis in Type 2 Diabetic Model Mice. J. Nutr. Biochem. 2014, 25, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.T.; Pham, N.M.; Do, V.V.; Binns, C.W.; Hoang, V.M.; Dang, D.A.; Lee, A.H. Soyfood and Isoflavone Intake and Risk of Type 2 Diabetes in Vietnamese Adults. Eur. J. Clin. Nutr. 2017, 71, 1186–1192. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.S.; Jung, U.J.; Yeo, J.; Kim, M.J.; Lee, M.K. Genistein and Daidzein Prevent Diabetes Onset by Elevating Insulin Level and Altering Hepatic Gluconeogenic and Lipogenic Enzyme Activities in Non-Obese Diabetic (NOD) Mice. Diabetes Metab. Res. Rev. 2008, 24, 74–81. [Google Scholar] [CrossRef]
- Ae Park, S.; Choi, M.-S.; Cho, S.-Y.; Seo, J.-S.; Jung, U.J.; Kim, M.-J.; Sung, M.-K.; Park, Y.B.; Lee, M.-K. Genistein and Daidzein Modulate Hepatic Glucose and Lipid Regulating Enzyme Activities in C57BL/KsJ-Db/Db Mice. Life Sci. 2006, 79, 1207–1213. [Google Scholar] [CrossRef]
- Das, D.; Sarkar, S.; Bordoloi, J.; Wann, S.B.; Kalita, J.; Manna, P. Daidzein, Its Effects on Impaired Glucose and Lipid Metabolism and Vascular Inflammation Associated with Type 2 Diabetes. Biofactors 2018, 44, 407–417. [Google Scholar] [CrossRef]
- Lv, J.; Jin, S.; Zhang, Y.; Zhou, Y.; Li, M.; Feng, N. Equol: A Metabolite of Gut Microbiota with Potential Antitumor Effects. Gut Pathog. 2024, 16, 35. [Google Scholar] [CrossRef]
- Chen, K.; Lang, H.; Wang, L.; Liu, K.; Zhou, Y.; Mi, M. S-Equol Ameliorates Insulin Secretion Failure through Chrebp/Txnip Signaling via Modulating PKA/PP2A Activities. Nutr. Metab. 2020, 17, 7. [Google Scholar] [CrossRef]
- Guo, X.; Yang, B.; Tan, J.; Jiang, J.; Li, D. Associations of Dietary Intakes of Anthocyanins and Berry Fruits with Risk of Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Eur. J. Clin. Nutr. 2016, 70, 1360–1367. [Google Scholar] [CrossRef]
- Li, D.; Zhang, Y.; Liu, Y.; Sun, R.; Xia, M. Purified Anthocyanin Supplementation Reduces Dyslipidemia, Enhances Antioxidant Capacity, and Prevents Insulin Resistance in Diabetic Patients 1, 2, 3. J. Nutr. 2015, 145, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Ling, W.; Yang, Y.; Chen, Y.; Tian, Z.; Du, Z.; Chen, J.; Xie, Y.; Liu, Z.; Yang, L. Role of Purified Anthocyanins in Improving Cardiometabolic Risk Factors in Chinese Men and Women with Prediabetes or Early Untreated Diabetes—A Randomized Controlled Trial. Nutrients 2017, 9, 1104. [Google Scholar] [CrossRef] [PubMed]
- Bahari, H.; Ashtary-Larky, D.; Goudarzi, K.; Mirmohammadali, S.N.; Asbaghi, O.; Hosseini Kolbadi, K.S.; Naderian, M.; Hosseini, A. The Effects of Pomegranate Consumption on Glycemic Indices in Adults: A Systematic Review and Meta-Analysis. Diabetes Metab. Syndr. 2024, 18, 102940. [Google Scholar] [CrossRef] [PubMed]
- Zarfeshany, A.; Asgary, S.; Javanmard, S.H. Potent Health Effects of Pomegranate. Adv. Biomed. Res. 2014, 3, 100. [Google Scholar] [CrossRef]
- Morissette, A.; Kropp, C.; Songpadith, J.-P.; Junges Moreira, R.; Costa, J.; Mariné-Casadó, R.; Pilon, G.; Varin, T.V.; Dudonné, S.; Boutekrabt, L.; et al. Blueberry Proanthocyanidins and Anthocyanins Improve Metabolic Health Through a Gut Microbiota-Dependent Mechanism in Diet-Induced Obese Mice. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E965–E980. [Google Scholar] [CrossRef]
- Tian, J.-L.; Si, X.; Shu, C.; Wang, Y.-H.; Tan, H.; Zang, Z.-H.; Zhang, W.-J.; Xie, X.; Chen, Y.; Li, B. Synergistic Effects of Combined Anthocyanin and Metformin Treatment for Hyperglycemia In Vitro and In Vivo. J. Agric. Food Chem. 2022, 70, 1182–1195. [Google Scholar] [CrossRef]
- Yip, S.-C.; Saha, S.; Chernoff, J. PTP1B: A Double Agent in Metabolism and Oncogenesis. Trends Biochem. Sci. 2010, 35, 442–449. [Google Scholar] [CrossRef]
- Stuible, M.; Doody, K.M.; Tremblay, M.L. PTP1B and TC-PTP: Regulators of Transformation and Tumorigenesis. Cancer Metastasis Rev. 2008, 27, 215–230. [Google Scholar] [CrossRef]
- Gao, L.; Tang, H.; Zeng, Q.; Tang, T.; Chen, M.; Pu, P. The Anti-Insulin Resistance Effect of Scutellarin May Be Related to Antioxidant Stress and AMPKα Activation in Diabetic Mice. Obes. Res. Clin. Pract. 2020, 14, 368–374. [Google Scholar] [CrossRef]
- Guo, J.; Chen, J.; Ren, W.; Zhu, Y.; Zhao, Q.; Zhang, K.; Su, D.; Qiu, C.; Zhang, W.; Li, K. Citrus Flavone Tangeretin Is a Potential Insulin Sensitizer Targeting Hepatocytes Through Suppressing MEK-ERK1/2 Pathway. Biochem. Biophys. Res. Commun. 2020, 529, 277–282. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, D.; Gu, Y.; Jiang, Z.; Zhou, Z. Tangeretin Prevents Obesity by Modulating Systemic Inflammation, Fat Browning, and Gut Microbiota in High-Fat Diet-Induced Obese C57BL/6 Mice. J. Nutr. Biochem. 2022, 101, 108943. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liu, J.; Zhou, Z.; Liu, K.; Liu, C. Diosmetin Attenuates Akt Signaling Pathway by Modulating Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells (NF-κB)/Inducible Nitric Oxide Synthase (iNOS) in Streptozotocin (STZ)-Induced Diabetic Nephropathy Mice. Med. Sci. Monit. 2018, 24, 7007–7014. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Xiong, L.; Bi, C.; Zhang, B. Diosmetin Ameliorate Type 2 Diabetic Mellitus by Up-Regulating Corynebacterium Glutamicum to Regulate IRS/PI3K/AKT-Mediated Glucose Metabolism Disorder in KK-Ay Mice. Phytomedicine 2021, 87, 153582. [Google Scholar] [CrossRef] [PubMed]
- Oza, M.J.; Kulkarni, Y.A. Formononetin Attenuates Kidney Damage in Type 2 Diabetic Rats. Life Sci. 2019, 219, 109–121. [Google Scholar] [CrossRef]
- Gao, K.; Yang, R.; Zhang, J.; Wang, Z.; Jia, C.; Zhang, F.; Li, S.; Wang, J.; Murtaza, G.; Xie, H.; et al. Effects of Qijian Mixture on Type 2 Diabetes Assessed by Metabonomics, Gut Microbiota and Network Pharmacology. Pharmacol. Res. 2018, 130, 93–109. [Google Scholar] [CrossRef]
- Chen, L.; Tian, G.; Tang, W.; Luo, W.; Liu, P.; Ma, Z. Protective Effect of Luteolin on Streptozotocin-Induced Diabetic Renal Damage in Mice via the Regulation of RIP140/NF-κB Pathway and Insulin Signalling Pathway. J. Funct. Foods 2016, 22, 93–100. [Google Scholar] [CrossRef]
- Ge, X.; Liu, T.; Wang, Y.; Wen, H.; Huang, Z.; Chen, L.; Xu, J.; Zhou, H.; Wu, Q.; Zhao, C.; et al. Porous Starch Microspheres Loaded with Luteolin Exhibit Hypoglycemic Activities and Alter Gut Microbial Communities in Type 2 Diabetes Mellitus Mice. Food. Funct. 2025, 16, 54–70. [Google Scholar] [CrossRef]
- Neilson, A.P.; Ferruzzi, M.G. Chapter 22—Bioavailability and Metabolism of Bioactive Compounds from Foods. In Nutrition in the Prevention and Treatment of Disease, 3rd ed.; Coulston, A.M., Boushey, C.J., Ferruzzi, M.G., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 407–423. ISBN 978-0-12-391884-0. [Google Scholar]
- Mamari, H.H.A. Phenolic Compounds: Classification, Chemistry, and Updated Techniques of Analysis and Synthesis. In Phenolic Compounds—Chemistry, Synthesis, Diversity, Non-Conventional Industrial, Pharmaceutical and Therapeutic Applications; IntechOpen: London, UK, 2021; ISBN 978-1-83969-347-2. [Google Scholar]
- Muronetz, V.I.; Barinova, K.; Kudryavtseva, S.; Medvedeva, M.; Melnikova, A.; Sevostyanova, I.; Semenyuk, P.; Stroylova, Y.; Sova, M. Natural and Synthetic Derivatives of Hydroxycinnamic Acid Modulating the Pathological Transformation of Amyloidogenic Proteins. Molecules 2020, 25, 4647. [Google Scholar] [CrossRef]
- Santos-Sánchez, N.F.; Salas-Coronado, R.; Hernández-Carlos, B.; Villanueva-Cañongo, C.; Santos-Sánchez, N.F.; Salas-Coronado, R.; Hernández-Carlos, B.; Villanueva-Cañongo, C. Shikimic Acid Pathway in Biosynthesis of Phenolic Compounds. In Plant Physiological Aspects of Phenolic Compounds; IntechOpen: London, UK, 2019; ISBN 978-1-78984-034-6. [Google Scholar]
- Widhalm, J.R.; Dudareva, N. A Familiar Ring to It: Biosynthesis of Plant Benzoic Acids. Mol. Plant 2015, 8, 83–97. [Google Scholar] [CrossRef]
- Khan, J.; Khan, M.Z.; Ma, Y.; Meng, Y.; Mushtaq, A.; Shen, Q.; Xue, Y. Overview of the Composition of Whole Grains’ Phenolic Acids and Dietary Fibre and Their Effect on Chronic Non-Communicable Diseases. Int. J. Environ. Res. Public Health 2022, 19, 3042. [Google Scholar] [CrossRef]
- Jeelani, P.G.; Sinclair, B.J.; Perinbarajan, G.K.; Ganesan, H.; Ojha, N.; Ramalingam, C.; Muthuramalingam, P.; Mossa, A.-T. The Therapeutic Potential of Chia Seeds as Medicinal Food: A Review. Nutrire 2023, 48, 39. [Google Scholar] [CrossRef]
- Rashmi, H.B.; Negi, P.S. Phenolic Acids from Vegetables: A Review on Processing Stability and Health Benefits. Food Res. Int. 2020, 136, 109298. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C.H. Absorption, Bioavailability, and Metabolism of Flavonoids. Pharm. Biol. 2004, 42, 74–83. [Google Scholar] [CrossRef]
- Irwin, C.; van Reenen, M.; Mason, S.; Mienie, L.J.; Westerhuis, J.A.; Reinecke, C.J. Contribution Towards a Metabolite Profile of the Detoxification of Benzoic Acid through Glycine Conjugation: An Intervention Study. PLoS ONE 2016, 11, e0167309. [Google Scholar] [CrossRef]
- Seeram, N.P.; Lee, R.; Heber, D. Bioavailability of Ellagic Acid in Human Plasma After Consumption of Ellagitannins from Pomegranate (Punica granatum L.) Juice. Clin. Chim. Acta 2004, 348, 63–68. [Google Scholar] [CrossRef]
- Cerdá, B.; Periago, P.; Espín, J.C.; Tomás-Barberán, F.A. Identification of Urolithin A as a Metabolite Produced by Human Colon Microflora from Ellagic Acid and Related Compounds. J. Agric. Food Chem. 2005, 53, 5571–5576. [Google Scholar] [CrossRef]
- Konishi, Y.; Zhao, Z.; Shimizu, M. Phenolic Acids Are Absorbed from the Rat Stomach with Different Absorption Rates. J. Agric. Food Chem. 2006, 54, 7539–7543. [Google Scholar] [CrossRef]
- Paterson, J.; Baxter, G.; Lawrence, J.; Duthie, G. Is There a Role for Dietary Salicylates in Health? Proc. Nutr. Soc. 2006, 65, 93–96. [Google Scholar] [CrossRef]
- Andreasen, M.F.; Kroon, P.A.; Williamson, G.; Garcia-Conesa, M.-T. Intestinal Release and Uptake of Phenolic Antioxidant Diferulic Acids. Free. Radic. Biol. Med. 2001, 31, 304–314. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; El-Said, A.M.A.; Khalifa, S.A.M.; Göransson, U.; Bohlin, L.; Borg-Karlson, A.-K.; Verpoorte, R. Biosynthesis, Natural Sources, Dietary Intake, Pharmacokinetic Properties, and Biological Activities of Hydroxycinnamic Acids. J. Agric. Food Chem. 2012, 60, 10877–10895. [Google Scholar] [CrossRef]
- Alam, M.; Ahmed, S.; Elasbali, A.M.; Adnan, M.; Alam, S.; Hassan, M.I.; Pasupuleti, V.R. Therapeutic Implications of Caffeic Acid in Cancer and Neurological Diseases. Front. Oncol. 2022, 12, 860508. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxidative Med. Cell. Longev. 2009, 2, 897484. [Google Scholar] [CrossRef] [PubMed]
- Manzanaro, S.; Salvá, J.; de la Fuente, J.Á. Phenolic Marine Natural Products as Aldose Reductase Inhibitors. J. Nat. Prod. 2006, 69, 1485–1487. [Google Scholar] [CrossRef] [PubMed]
- Hanhineva, K.; Törrönen, R.; Bondia-Pons, I.; Pekkinen, J.; Kolehmainen, M.; Mykkänen, H.; Poutanen, K. Impact of Dietary Polyphenols on Carbohydrate Metabolism. Int. J. Mol. Sci. 2010, 11, 1365–1402. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, Y.; Wang, Y.; Nazary-Vannani, A.; Clark, C.C.T.; Sedanur Macit, M.; Khani, V.; Zhang, Y. The Influence of Green Coffee Bean Extract Supplementation on Blood Glucose Levels: A Systematic Review and Dose–Response Meta-Analysis of Randomized Controlled Trials. Phytother. Res. 2020, 34, 2159–2169. [Google Scholar] [CrossRef]
- Zuñiga, L.Y.; Aceves-de la Mora, M.C.A.; González-Ortiz, M.; Ramos-Núñez, J.L.; Martínez-Abundis, E. Effect of Chlorogenic Acid Administration on Glycemic Control, Insulin Secretion, and Insulin Sensitivity in Patients with Impaired Glucose Tolerance. J. Med. Food 2018, 21, 469–473. [Google Scholar] [CrossRef]
- Yan, Y.; Li, Q.; Shen, L.; Guo, K.; Zhou, X. Chlorogenic Acid Improves Glucose Tolerance, Lipid Metabolism, Inflammation and Microbiota Composition in Diabetic Db/Db Mice. Front. Endocrinol. 2022, 13, 1042044. [Google Scholar] [CrossRef]
- Ganguly, R.; Singh, S.V.; Jaiswal, K.; Kumar, R.; Pandey, A.K. Modulatory Effect of Caffeic Acid in Alleviating Diabetes and Associated Complications. World J. Diabetes 2023, 14, 62–75. [Google Scholar] [CrossRef]
- Zhao, X.-Q.; Guo, S.; Lu, Y.-Y.; Hua, Y.; Zhang, F.; Yan, H.; Shang, E.-X.; Wang, H.-Q.; Zhang, W.-H.; Duan, J.-A. Lycium barbarum L. Leaves Ameliorate Type 2 Diabetes in Rats by Modulating Metabolic Profiles and Gut Microbiota Composition. Biomed. Pharmacother. 2020, 121, 109559. [Google Scholar] [CrossRef]
- Ghadimi, M.; Foroughi, F.; Hashemipour, S.; Rashidi Nooshabadi, M.; Ahmadi, M.H.; Ahadi Nezhad, B.; Khadem Haghighian, H. Randomized Double-Blind Clinical Trial Examining the Ellagic Acid Effects on Glycemic Status, Insulin Resistance, Antioxidant, and Inflammatory Factors in Patients with Type 2 Diabetes. Phytother. Res. 2021, 35, 1023–1032. [Google Scholar] [CrossRef]
- Hosseini, B.; Saedisomeolia, A.; Wood, L.G.; Yaseri, M.; Tavasoli, S. Effects of Pomegranate Extract Supplementation on Inflammation in Overweight and Obese Individuals: A Randomized Controlled Clinical Trial. Complement. Ther. Clin. Pract. 2016, 22, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Kang, I.; Buckner, T.; Shay, N.F.; Gu, L.; Chung, S. Improvements in Metabolic Health with Consumption of Ellagic Acid and Subsequent Conversion into Urolithins: Evidence and Mechanisms. Adv. Nutr. 2016, 7, 961–972. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhu, J.; Haedi, A.R.; Zhou, M. The Effect of Curcumin Supplementation on Glycemic Indices in Adults: A Meta-Analysis of Meta-Analyses. Prostaglandins Other Lipid Mediat. 2024, 175, 106908. [Google Scholar] [CrossRef] [PubMed]
- Innocent, D.C.; Innocent, R.C.; Kumar, R.; Rabaan, A.A.; Emerole, C.O.; Ayando, O.D.; Dike, I.C.; Duruji, C.O. A Systematic Review and Meta-Analysis on the Effect of Curcumin (Turmeric) on Fasting Blood Glucose and Glycated Haemoglobin in Patients with Type 2 Diabetes Mellitus. medRxiv 2023, 10, 1–31. [Google Scholar] [CrossRef]
- Hodaei, H.; Adibian, M.; Nikpayam, O.; Hedayati, M.; Sohrab, G. The Effect of Curcumin Supplementation on Anthropometric Indices, Insulin Resistance and Oxidative Stress in Patients with Type 2 Diabetes: A Randomized, Double-Blind Clinical Trial. Diabetol. Metab. Syndr. 2019, 11, 41. [Google Scholar] [CrossRef]
- Huang, J.; Guan, B.; Lin, L.; Wang, Y. Improvement of Intestinal Barrier Function, Gut Microbiota, and Metabolic Endotoxemia in Type 2 Diabetes Rats by Curcumin. Bioengineered 2021, 12, 11947–11958. [Google Scholar] [CrossRef]
- Ferrare, K.; Bidel, L.P.R.; Awwad, A.; Poucheret, P.; Cazals, G.; Lazennec, F.; Azay-Milhau, J.; Tournier, M.; Lajoix, A.-D.; Tousch, D. Increase in Insulin Sensitivity by the Association of Chicoric Acid and Chlorogenic Acid Contained in a Natural Chicoric Acid Extract (NCRAE) of Chicory (Cichorium intybus L.) for an Antidiabetic Effect. J. Ethnopharmacol. 2018, 215, 241–248. [Google Scholar] [CrossRef]
- Ding, X.; Jian, T.; Li, J.; Lv, H.; Tong, B.; Li, J.; Meng, X.; Ren, B.; Chen, J. Chicoric Acid Ameliorates Nonalcoholic Fatty Liver Disease via the AMPK/Nrf2/NFκB Signaling Pathway and Restores Gut Microbiota in High-Fat-Diet-Fed Mice. Oxidative Med. Cell. Longev. 2020, 2020, 9734560. [Google Scholar] [CrossRef]
- Peng, Y.; Sun, Q.; Park, Y. Chicoric Acid Promotes Glucose Uptake and Akt Phosphorylation via AMP-Activated Protein Kinase α-Dependent Pathway. J. Funct. Foods 2019, 59, 8–15. [Google Scholar] [CrossRef]
- Son, M.J.; Rico, C.W.; Nam, S.H.; Kang, M.Y. Effect of Oryzanol and Ferulic Acid on the Glucose Metabolism of Mice Fed with a High-Fat Diet. J. Food Sci. 2011, 76, H7–H10. [Google Scholar] [CrossRef]
- Jin Son, M.; W Rico, C.; Hyun Nam, S.; Young Kang, M. Influence of Oryzanol and Ferulic Acid on the Lipid Metabolism and Antioxidative Status in High Fat-Fed Mice. J. Clin. Biochem. Nutr. 2010, 46, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Ramar, M.; Manikandan, B.; Raman, T.; Priyadarsini, A.; Palanisamy, S.; Velayudam, M.; Munusamy, A.; Marimuthu Prabhu, N.; Vaseeharan, B. Protective Effect of Ferulic Acid and Resveratrol Against Alloxan-Induced Diabetes in Mice. Eur. J. Pharmacol. 2012, 690, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, P.K.; Prasad, R.; Ali, S.; Doble, M. Synergistic Interaction of Ferulic Acid with Commercial Hypoglycemic Drugs in Streptozotocin Induced Diabetic Rats. Phytomedicine 2013, 20, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, A.; Chinnaiyan, M.; Karundevi, B. Ferulic Acid Regulates Hepatic GLUT2 Gene Expression in High Fat and Fructose-Induced Type-2 Diabetic Adult Male Rat. Eur. J. Pharmacol. 2015, 761, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wu, M.-S.; Tao, G.; Lu, M.-W.; Lin, J.; Huang, J.-Q. Feruloylated Oligosaccharides and Ferulic Acid Alter Gut Microbiome to Alleviate Diabetic Syndrome. Food Res. Int. 2020, 137, 109410. [Google Scholar] [CrossRef]
- Muthukumaran, J.; Srinivasan, S.; Venkatesan, R.S.; Ramachandran, V.; Muruganathan, U. Syringic Acid, a Novel Natural Phenolic Acid, Normalizes Hyperglycemia with Special Reference to Glycoprotein Components in Experimental Diabetic Rats. J. Acute Dis. 2013, 2, 304–309. [Google Scholar] [CrossRef]
- Srinivasan, S.; Muthukumaran, J.; Muruganathan, U.; Venkatesan, R.S.; Jalaludeen, A.M. Antihyperglycemic Effect of Syringic Acid on Attenuating the Key Enzymes of Carbohydrate Metabolism in Experimental Diabetic Rats. Biomed. Prev. Nutr. 2014, 4, 595–602. [Google Scholar] [CrossRef]
- Yoon, S.-A.; Kang, S.-I.; Shin, H.-S.; Kang, S.-W.; Kim, J.-H.; Ko, H.-C.; Kim, S.-J. P-Coumaric Acid Modulates Glucose and Lipid Metabolism via AMP-Activated Protein Kinase in L6 Skeletal Muscle Cells. Biochem. Biophys. Res. Commun. 2013, 432, 553–557. [Google Scholar] [CrossRef]
- Cherng, Y.-G.; Tsai, C.-C.; Chung, H.-H.; Lai, Y.-W.; Kuo, S.-C.; Cheng, J.-T. Antihyperglycemic Action of Sinapic Acid in Diabetic Rats. J. Agric. Food Chem. 2013, 61, 12053–12059. [Google Scholar] [CrossRef]
- Derebasi, B.N.; Davran Bulut, S.; Aksoy Erden, B.; Sadeghian, N.; Taslimi, P.; Celebioglu, H.U. Effects of P-Coumaric Acid on Probiotic Properties of Lactobacillus acidophilus LA-5 and Lacticaseibacillus rhamnosus GG. Arch. Microbiol. 2024, 206, 223. [Google Scholar] [CrossRef]
- Hafizur, R.M.; Hameed, A.; Shukrana, M.; Raza, S.A.; Chishti, S.; Kabir, N.; Siddiqui, R.A. Cinnamic Acid Exerts Anti-Diabetic Activity by Improving Glucose Tolerance In Vivo and by Stimulating Insulin Secretion In Vitro. Phytomedicine 2015, 22, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Punithavathi, V.R.; Prince, P.S.M.; Kumar, R.; Selvakumari, J. Antihyperglycaemic, Antilipid Peroxidative and Antioxidant Effects of Gallic Acid on Streptozotocin Induced Diabetic Wistar Rats. Eur. J. Pharmacol. 2011, 650, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, G.R.; Jothi, G.; Antony, P.J.; Balakrishna, K.; Paulraj, M.G.; Ignacimuthu, S.; Stalin, A.; Al-Dhabi, N.A. Gallic Acid Attenuates High-Fat Diet Fed-Streptozotocin-Induced Insulin Resistance via Partial Agonism of PPARγ in Experimental Type 2 Diabetic Rats and Enhances Glucose Uptake Through Translocation and Activation of GLUT4 in PI3K/p-Akt Signaling Pathway. Eur. J. Pharmacol. 2014, 745, 201–216. [Google Scholar] [CrossRef]
- Latha, R.C.R.; Daisy, P. Therapeutic Potential of Octyl Gallate Isolated from Fruits of Terminalia Bellerica in Streptozotocin-Induced Diabetic Rats. Pharm. Biol. 2013, 51, 798–805. [Google Scholar] [CrossRef]
- Hu, J.; Ding, J.; Li, X.; Li, J.; Zheng, T.; Xie, L.; Li, C.; Tang, Y.; Guo, K.; Huang, J.; et al. Distinct Signatures of Gut Microbiota and Metabolites in Different Types of Diabetes: A Population-Based Cross-Sectional Study. EClinicalMedicine 2023, 62, 102132. [Google Scholar] [CrossRef]
- Hapeshi, A.; Benarroch, J.M.; Clarke, D.J.; Waterfield, N.R. Iso-Propyl Stilbene: A Life Cycle Signal? Microbiology 2019, 165, 516–526. [Google Scholar] [CrossRef]
- Teka, T.; Zhang, L.; Ge, X.; Li, Y.; Han, L.; Yan, X. Stilbenes: Source Plants, Chemistry, Biosynthesis, Pharmacology, Application and Problems Related to Their Clinical Application—A Comprehensive Review. Phytochemistry 2022, 197, 113128. [Google Scholar] [CrossRef]
- El Khawand, T.; Courtois, A.; Valls, J.; Richard, T.; Krisa, S. A Review of Dietary Stilbenes: Sources and Bioavailability. Phytochem. Rev. 2018, 17, 1007–1029. [Google Scholar] [CrossRef]
- Nguyen, T.-T.A.; Ha, M.T.; Park, S.-E.; Choi, J.S.; Min, B.S.; Kim, J.A. Stilbenes with Potent Protein Tyrosine Phosphatase-1B Inhibitory Activity from the Roots of Polygonum Multiflorum. J. Nat. Prod. 2020, 83, 323–332. [Google Scholar] [CrossRef]
- Su, X.; Zhou, D.; Li, N. Chapter 8—Bioactive Stilbenes from Plants. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 73, pp. 265–403. [Google Scholar]
- Cottart, C.-H.; Nivet-Antoine, V.; Laguillier-Morizot, C.; Beaudeux, J.-L. Resveratrol Bioavailability and Toxicity in Humans. Mol. Nutr. Food Res. 2010, 54, 7–16. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (Poly)Phenolics in Human Health: Structures, Bioavailability, and Evidence of Protective Effects Against Chronic Diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [PubMed]
- Hornedo-Ortega, R.; Jourdes, M.; Da Costa, G.; Courtois, A.; Gabaston, J.; Teissedre, P.-L.; Richard, T.; Krisa, S. Oxyresveratrol and Gnetol Glucuronide Metabolites: Chemical Production, Structural Identification, Metabolism by Human and Rat Liver Fractions, and In Vitro Anti-Inflammatory Properties. J. Agric. Food Chem. 2022, 70, 13082–13092. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Wei, H.; Jia, T.; Tian, H. An Improved Highly Sensitive Method to Determine Low Oxyresveratrol Concentrations in Rat Plasma and Its Pharmacokinetic Application. Biomed. Chromatogr. 2014, 28, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.-R.; Kim, S.-E.; Baek, H.-S.; Kim, B.-J.; Kim, C.-H.; Chung, I.-K.; Park, B.-S.; Shin, S.-H. The Role of Kaempferol-Induced Autophagy on Differentiation and Mineralization of Osteoblastic MC3T3-E1 Cells. BMC Complement. Altern. Med. 2016, 16, 333. [Google Scholar] [CrossRef]
- Han, M.; Zhang, T.; Gu, W.; Yang, X.; Zhao, R.; Yu, J. 2,3,5,4’-tetrahydroxy-stilbene-2-O-β-D-glucoside Attenuates Methionine and Choline-deficient Diet-induced Non-alcoholic Fatty Liver Disease. Exp. Ther. Med. 2018, 16, 1087–1094. [Google Scholar] [CrossRef]
- Kim, J.; Min, J.S.; Kim, D.; Zheng, Y.F.; Mailar, K.; Choi, W.J.; Lee, C.; Bae, S.K. A Simple and Sensitive Liquid Chromatography–Tandem Mass Spectrometry Method for Trans-ε-Viniferin Quantification in Mouse Plasma and Its Application to a Pharmacokinetic Study in Mice. J. Pharm. Biomed. Anal. 2017, 134, 116–121. [Google Scholar] [CrossRef]
- Baur, J.A.; Sinclair, D.A. Therapeutic Potential of Resveratrol: The In Vivo Evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef]
- Jäger, S.; Handschin, C.; St-Pierre, J.; Spiegelman, B.M. AMP-Activated Protein Kinase (AMPK) Action in Skeletal Muscle via Direct Phosphorylation of PGC-1alpha. Proc. Natl. Acad. Sci. USA 2007, 104, 12017–12022. [Google Scholar] [CrossRef]
- Goh, K.P.; Lee, H.Y.; Lau, D.P.; Supaat, W.; Chan, Y.H.; Koh, A.F.Y. Effects of Resveratrol in Patients with Type 2 Diabetes Mellitus on Skeletal Muscle SIRT1 Expression and Energy Expenditure. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 2–13. [Google Scholar] [CrossRef]
- Brasnyó, P.; Molnár, G.A.; Mohás, M.; Markó, L.; Laczy, B.; Cseh, J.; Mikolás, E.; Szijártó, I.A.; Mérei, A.; Halmai, R.; et al. Resveratrol Improves Insulin Sensitivity, Reduces Oxidative Stress and Activates the Akt Pathway in Type 2 Diabetic Patients. Br. J. Nutr. 2011, 106, 383–389. [Google Scholar] [CrossRef]
- Liu, H.; Wang, S.; Yu, X.; Sun, S.; Su, Y. Effect of Resveratrol on the Intestinal Microbiota in Type2 Diabetes Mellitus Mice. Braz. J. Microbiol. 2024, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, J.K.; Thomas, S.; Nanjan, M.J. Resveratrol Supplementation Improves Glycemic Control in Type 2 Diabetes Mellitus. Nutr. Res. 2012, 32, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Movahed, A.; Nabipour, I.; Lieben Louis, X.; Thandapilly, S.J.; Yu, L.; Kalantarhormozi, M.; Rekabpour, S.J.; Netticadan, T. Antihyperglycemic Effects of Short Term Resveratrol Supplementation in Type 2 Diabetic Patients. Evid.-Based Complement. Altern. Med. 2013, 2013, 851267. [Google Scholar] [CrossRef] [PubMed]
- Teimouri, M.; Hosseini, H.; ArabSadeghabadi, Z.; Babaei-Khorzoughi, R.; Gorgani-Firuzjaee, S.; Meshkani, R. The Role of Protein Tyrosine Phosphatase 1B (PTP1B) in the Pathogenesis of Type 2 Diabetes Mellitus and Its Complications. J. Physiol. Biochem. 2022, 78, 307–322. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Pu, Z.; Yi, W.; Ma, Q.; Lin, Q.; Zhong, G.; Yao, P.; Ren, G. Unusual Prenylated Stilbene Derivatives with PTP1B Inhibitory Activity from Artocarpus styracifolius. Planta Med. 2019, 85, 1263–1274. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.-H.; Chen, J.-M.; Kang, C.-J.; Chen, C.-H.; Lee, S.-S. α-Glucosidase Inhibitors from the Seeds of Syagrus romanzoffiana. Phytochemistry 2008, 69, 1173–1178. [Google Scholar] [CrossRef]
- Jin, Q.; Han, X.H.; Hong, S.S.; Lee, C.; Choe, S.; Lee, D.; Kim, Y.; Hong, J.T.; Lee, M.K.; Hwang, B.Y. Antioxidative Oligostilbenes from Caragana sinica. Bioorganic Med. Chem. Lett. 2012, 22, 973–976. [Google Scholar] [CrossRef]
- Zhang, P.-Z.; Gu, J.; Zhang, G.-L. Novel Stilbenes from Artocarpus nanchuanensis. J. Asian Nat. Prod. Res. 2015, 17, 217–223. [Google Scholar] [CrossRef]
- Pereira, A.C.; Arruda, M.S.; da Silva, E.A.; da Silva, M.N.; Lemos, V.S.; Cortes, S.F. Inhibition of α-Glucosidase and Hypoglycemic Effect of Stilbenes from the Amazonian Plant Deguelia rufescens var. urucu (Ducke) AMG Azevedo (Leguminosae). Planta Medica 2011, 78, 36–38. [Google Scholar] [CrossRef]
- Zhang, C.-C.; Geng, C.-A.; Huang, X.-Y.; Zhang, X.-M.; Chen, J.-J. Antidiabetic Stilbenes from Peony Seeds with PTP1B, α-Glucosidase, and DPPIV Inhibitory Activities. J. Agric. Food Chem. 2019, 67, 6765–6772. [Google Scholar] [CrossRef]
- Pan, Z.-H.; Ning, D.-S.; Fu, Y.-X.; Li, D.-P.; Zou, Z.-Q.; Xie, Y.-C.; Yu, L.-L.; Li, L.-C. Preparative Isolation of Piceatannol Derivatives from Passion Fruit (Passiflora edulis) Seeds by High-Speed Countercurrent Chromatography Combined with High-Performance Liquid Chromatography and Screening for α-Glucosidase Inhibitory Activities. J. Agric. Food Chem. 2020, 68, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhan, Z.; Liu, F.; Yang, Y.; Li, L.; Feng, Z.; Jiang, J.; Zhang, P. Polyflavanostilbene A, a New Flavanol-Fused Stilbene Glycoside from Polygonum cuspidatum. Org. Lett. 2013, 15, 674–677. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-B.; Tian, J.-Y.; Dai, Z.; Ye, F.; Ma, S.-C.; Wang, A.-G. A-Glucosidase Inhibitors Extracted from the Roots of Polygonum multiflorum Thunb. Fitoterapia 2017, 117, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Kerem, Z.; Bilkis, I.; Flaishman, M.A.; Sivan, L. Antioxidant Activity and Inhibition of Alpha-Glucosidase by Trans-Resveratrol, Piceid, and a Novel Trans-Stilbene from the Roots of Israeli Rumex bucephalophorus L. J. Agric. Food Chem. 2006, 54, 1243–1247. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.-H.; Lee, S.-S. Unusual Stilbenoids and a Stilbenolignan from Seeds of Syagrus romanzoffiana. Phytochemistry 2010, 71, 792–797. [Google Scholar] [CrossRef]
- Matsuda, H.; Asao, Y.; Nakamura, S.; Hamao, M.; Sugimoto, S.; Hongo, M.; Pongpiriyadacha, Y.; Yoshikawa, M. Antidiabetogenic Constituents from the Thai Traditional Medicine Cotylelobium melanoxylon. Chem. Pharm. Bull. 2009, 57, 487–494. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, R.; Ban, Y.; Cai, J.; Peng, J.; Huang, J.; Wang, J.; Chen, W.; Gao, X.; Zhou, X.; et al. Pharmacokinetics, Tissue Distribution, and Excretion Study of Cajanonic Acid A in Rats by UPLC-MS/MS. Planta Med. 2020, 86, 312–318. [Google Scholar] [CrossRef]
- Zhang, H.; Matsuda, H.; Yamashita, C.; Nakamura, S.; Yoshikawa, M. Hydrangeic Acid from the Processed Leaves of Hydrangea Macrophylla Var. Thunbergii as a New Type of Anti-Diabetic Compound. Eur. J. Pharmacol. 2009, 606, 255–261. [Google Scholar] [CrossRef]
- Pizzi, A. Tannins Medical/Pharmacological and Related Applications: A Critical Review. Sustain. Chem. Pharm. 2021, 22, 100481. [Google Scholar] [CrossRef]
- Govindarajan, R.K.; Revathi, S.; Rameshkumar, N.; Krishnan, M.; Kayalvizhi, N. Microbial Tannase: Current Perspectives and Biotechnological Advances. Biocatal. Agric. Biotechnol. 2016, 6, 168–175. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, X.; Zhao, G.; Hu, T.; Wang, Y. Potential and Challenges of Tannins as an Alternative to In-Feed Antibiotics for Farm Animal Production. Anim. Nutr. 2018, 4, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Moldoveanu, S.C. 7—Analytical Pyrolysis of Polymeric Tannins. In Analytical Pyrolysis of Natural Organic Polymers, 2nd ed.; Moldoveanu, S.C., Ed.; Techniques and Instrumentation in Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2021; Volume 20, pp. 311–313. [Google Scholar]
- Fraga-Corral, M.; Otero, P.; Cassani, L.; Echave, J.; Garcia-Oliveira, P.; Carpena, M.; Chamorro, F.; Lourenço-Lopes, C.; Prieto, M.A.; Simal-Gandara, J. Traditional Applications of Tannin Rich Extracts Supported by Scientific Data: Chemical Composition, Bioavailability and Bioaccessibility. Foods 2021, 10, 251. [Google Scholar] [CrossRef] [PubMed]
- Proença, C.S.; Serrano, B.; Correia, J.; Araújo, M.E.M. Evaluation of Tannins as Potential Green Corrosion Inhibitors of Aluminium Alloy Used in Aeronautical Industry. Metals 2022, 12, 508. [Google Scholar] [CrossRef]
- Serrano, J.; Puupponen-Pimiä, R.; Dauer, A.; Aura, A.-M.; Saura-Calixto, F. Tannins: Current Knowledge of Food Sources, Intake, Bioavailability and Biological Effects. Mol. Nutr. Food Res. 2009, 53 (Suppl. S2), S310–S329. [Google Scholar] [CrossRef]
- Xiang, Z.; Guan, H.; Zhao, X.; Xie, Q.; Xie, Z.; Cai, F.; Dang, R.; Li, M.; Wang, C. Dietary Gallic Acid as an Antioxidant: A Review of Its Food Industry Applications, Health Benefits, Bioavailability, Nano-Delivery Systems, and Drug Interactions. Food Res. Int. 2024, 180, 114068. [Google Scholar] [CrossRef]
- Cerdá, B.; Tomás-Barberán, F.A.; Espín, J.C. Metabolism of Antioxidant and Chemopreventive Ellagitannins from Strawberries, Raspberries, Walnuts, and Oak-Aged Wine in Humans: Identification of Biomarkers and Individual Variability. J. Agric. Food Chem. 2005, 53, 227–235. [Google Scholar] [CrossRef]
- Ottaviani, J.I.; Momma, T.Y.; Heiss, C.; Kwik-Uribe, C.; Schroeter, H.; Keen, C.L. The Stereochemical Configuration of Flavanols Influences the Level and Metabolism of Flavanols in Humans and Their Biological Activity in Vivo. Free. Radic. Biol. Med. 2011, 50, 237–244. [Google Scholar] [CrossRef]
- Requena, T.; Monagas, M.; Pozo-Bayón, M.A.; Martín-Álvarez, P.J.; Bartolomé, B.; del Campo, R.; Ávila, M.; Martínez-Cuesta, M.C.; Peláez, C.; Moreno-Arribas, M.V. Perspectives of the Potential Implications of Wine Polyphenols on Human Oral and Gut Microbiota. Trends Food Sci. Technol. 2010, 21, 332–344. [Google Scholar] [CrossRef]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Proanthocyanidins and Hydrolysable Tannins: Occurrence, Dietary Intake and Pharmacological Effects. Br. J. Pharmacol. 2017, 174, 1244–1262. [Google Scholar] [CrossRef]
- Espín, J.C.; Larrosa, M.; García-Conesa, M.T.; Tomás-Barberán, F. Biological Significance of Urolithins, the Gut Microbial Ellagic Acid-Derived Metabolites: The Evidence so Far. Evid.-Based Complement. Altern. Med. 2013, 2013, 270418. [Google Scholar] [CrossRef]
- Nuñez-Sánchez, M.A.; García-Villalba, R.; Monedero-Saiz, T.; García-Talavera, N.V.; Gómez-Sánchez, M.B.; Sánchez-Álvarez, C.; García-Albert, A.M.; Rodríguez-Gil, F.J.; Ruiz-Marín, M.; Pastor-Quirante, F.A.; et al. Targeted Metabolic Profiling of Pomegranate Polyphenols and Urolithins in Plasma, Urine and Colon Tissues from Colorectal Cancer Patients. Mol. Nutr. Food Res. 2014, 58, 1199–1211. [Google Scholar] [CrossRef] [PubMed]
- Monagas, M.; Urpi-Sarda, M.; Sánchez-Patán, F.; Llorach, R.; Garrido, I.; Gómez-Cordovés, C.; Andres-Lacueva, C.; Bartolomé, B. Insights into the Metabolism and Microbial Biotransformation of Dietary Flavan-3-Ols and the Bioactivity of Their Metabolites. Food Funct. 2010, 1, 233–253. [Google Scholar] [CrossRef] [PubMed]
- Ou, K.; Gu, L. Absorption and Metabolism of Proanthocyanidins. J. Funct. Foods 2014, 7, 43–53. [Google Scholar] [CrossRef]
- Smith, A.H.; Mackie, R.I. Effect of Condensed Tannins on Bacterial Diversity and Metabolic Activity in the Rat Gastrointestinal Tract. Appl. Environ. Microbiol. 2004, 70, 1104–1115. [Google Scholar] [CrossRef]
- Molino, S.; Lerma-Aguilera, A.; Jiménez-Hernández, N.; Gosalbes, M.J.; Rufián-Henares, J.Á.; Francino, M.P. Enrichment of Food with Tannin Extracts Promotes Healthy Changes in the Human Gut Microbiota. Front. Microbiol. 2021, 12, 625782. [Google Scholar] [CrossRef]
- Li, L.; Ma, H.; Liu, T.; Ding, Z.; Liu, W.; Gu, Q.; Mu, Y.; Xu, J.; Seeram, N.P.; Huang, X.; et al. Glucitol-Core Containing Gallotannins-Enriched Red Maple (Acer rubrum) Leaves Extract Alleviated Obesity via Modulating Short-Chain Fatty Acid Production in High-Fat Diet-Fed Mice. J. Funct. Foods 2020, 70, 103970. [Google Scholar] [CrossRef]
- Barnes, R.C.; Kim, H.; Fang, C.; Bennett, W.; Nemec, M.; Sirven, M.A.; Suchodolski, J.S.; Deutz, N.; Britton, R.A.; Mertens-Talcott, S.U.; et al. Body Mass Index as a Determinant of Systemic Exposure to Gallotannin Metabolites During 6-Week Consumption of Mango (Mangifera indica L.) and Modulation of Intestinal Microbiota in Lean and Obese Individuals. Mol. Nutr. Food Res. 2019, 63, e1800512. [Google Scholar] [CrossRef]
- Fang, C.; Kim, H.; Yanagisawa, L.; Bennett, W.; Sirven, M.A.; Alaniz, R.C.; Talcott, S.T.; Mertens-Talcott, S.U. Gallotannins and Lactobacillus plantarum WCFS1 Mitigate High-Fat Diet-Induced Inflammation and Induce Biomarkers for Thermogenesis in Adipose Tissue in Gnotobiotic Mice. Mol. Nutr. Food Res. 2019, 63, e1800937. [Google Scholar] [CrossRef]
- Kwok, A.L.X.; Balasooriya, H.; Ng, K. Efficacy of Ellagic Acid and Ellagitannins on Diabetes Mellitus: A Meta-Analysis of Preclinical and Clinical Trials. Food Biosci. 2023, 53, 102573. [Google Scholar] [CrossRef]
- Velayutham, R.; Sankaradoss, N.; Ahamed, K.N. Protective Effect of Tannins from Ficus racemosa in Hypercholesterolemia and Diabetes Induced Vascular Tissue Damage in Rats. Asian Pac. J. Trop. Med. 2012, 5, 367–373. [Google Scholar] [CrossRef]
- Bai, N.; He, K.; Roller, M.; Zheng, B.; Chen, X.; Shao, Z.; Peng, T.; Zheng, Q. Active Compounds from Lagerstroemia Speciosa, Insulin-like Glucose Uptake-Stimulatory/Inhibitory and Adipocyte Differentiation-Inhibitory Activities in 3T3-L1 Cells. J. Agric. Food Chem. 2008, 56, 11668–11674. [Google Scholar] [CrossRef] [PubMed]
- Pinent, M.; Blay, M.; Bladé, M.C.; Salvadó, M.J.; Arola, L.; Ardévol, A. Grape Seed-Derived Procyanidins Have an Antihyperglycemic Effect in Streptozotocin-Induced Diabetic Rats and Insulinomimetic Activity in Insulin-Sensitive Cell Lines. Endocrinology 2004, 145, 4985–4990. [Google Scholar] [CrossRef] [PubMed]
- Montagut, G.; Onnockx, S.; Vaqué, M.; Bladé, C.; Blay, M.; Fernández-Larrea, J.; Pujadas, G.; Salvadó, M.J.; Arola, L.; Pirson, I.; et al. Oligomers of Grape-Seed Procyanidin Extract Activate the Insulin Receptor and Key Targets of the Insulin Signaling Pathway Differently from Insulin. J. Nutr. Biochem. 2010, 21, 476–481. [Google Scholar] [CrossRef]
- Gonzalez-Abuin, N.; Pinent, M.; Casanova-Marti, A.; Arola, L.; Blay, M.; Ardevol, A. Procyanidins and Their Healthy Protective Effects Against Type 2 Diabetes. Curr. Med. Chem. 2015, 22, 39–50. [Google Scholar] [CrossRef]
- Laddha, A.P.; Kulkarni, Y.A. Tannins and Vascular Complications of Diabetes: An Update. Phytomedicine 2019, 56, 229–245. [Google Scholar] [CrossRef]
- Yin, M.; Zhang, P.; Yu, F.; Zhang, Z.; Cai, Q.; Lu, W.; Li, B.; Qin, W.; Cheng, M.; Wang, H.; et al. Grape Seed Procyanidin B2 Ameliorates Hepatic Lipid Metabolism Disorders in Db/Db Mice. Mol. Med. Rep. 2017, 16, 2844–2850. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Y.; Shao, H.; Bi, Q.; Chen, J.; Ye, Z. Grape Seed Procyanidin B2 Inhibits Adipogenesis of 3T3-L1 Cells by Targeting Peroxisome Proliferator-Activated Receptor γ with miR-483-5p Involved Mechanism. Biomed. Pharmacother. 2017, 86, 292–296. [Google Scholar] [CrossRef]
- Liu, M.; Huang, B.; Wang, L.; Lu, Q.; Liu, R. Peanut Skin Procyanidins Ameliorate Insulin Resistance via Modulation of Gut Microbiota and Gut Barrier in Type 2 Diabetic Mice. J. Sci. Food Agric. 2022, 102, 5935–5947. [Google Scholar] [CrossRef]
- Catarino, M.D.; Silva, A.M.S.; Cardoso, S.M. Fucaceae: A Source of Bioactive Phlorotannins. Int. J. Mol. Sci. 2017, 18, 1327. [Google Scholar] [CrossRef]
- Catarino, M.D.; Silva, A.M.S.; Cardoso, S.M. Phycochemical Constituents and Biological Activities of Fucus spp. Mar. Drugs 2018, 16, 249. [Google Scholar] [CrossRef]
- Paradis, M.-E.; Couture, P.; Lamarche, B. A Randomised Crossover Placebo-Controlled Trial Investigating the Effect of Brown Seaweed (Ascophyllum nodosum and Fucus vesiculosus) on Postchallenge Plasma Glucose and Insulin Levels in Men and Women. Appl. Physiol. Nutr. Metab. 2011, 36, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.-C.; Anguenot, R.; Fillion, C.; Beaulieu, M.; Bérubé, J.; Richard, D. Effect of a Commercially-Available Algal Phlorotannins Extract on Digestive Enzymes and Carbohydrate Absorption In Vivo. Food Res. Int. 2011, 44, 3026–3029. [Google Scholar] [CrossRef]
- Gheda, S.; Naby, M.A.; Mohamed, T.; Pereira, L.; Khamis, A. Antidiabetic and Antioxidant Activity of Phlorotannins Extracted from the Brown Seaweed Cystoseira compressa in Streptozotocin-Induced Diabetic Rats. Environ. Sci. Pollut. Res. Int. 2021, 28, 22886–22901. [Google Scholar] [CrossRef] [PubMed]
- Ohta, T.; Sasaki, S.; Oohori, T.; Yoshikawa, S.; Kurihara, H. Alpha-Glucosidase Inhibitory Activity of a 70% Methanol Extract from Ezoishige (Pelvetia Babingtonii de Toni) and Its Effect on the Elevation of Blood Glucose Level in Rats. Biosci. Biotechnol. Biochem. 2002, 66, 1552–1554. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, İ. Antioxidants and Antioxidant Methods: An Updated Overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Li, Y.; Xie, S.; Ying, J.; Wei, W.; Gao, K. Chemical Structures of Lignans and Neolignans Isolated from Lauraceae. Molecules 2018, 23, 3164. [Google Scholar] [CrossRef]
- Jang, W.Y.; Kim, M.-Y.; Cho, J.Y. Antioxidant, Anti-Inflammatory, Anti-Menopausal, and Anti-Cancer Effects of Lignans and Their Metabolites. Int. J. Mol. Sci. 2022, 23, 15482. [Google Scholar] [CrossRef]
- Berenshtein, L.; Okun, Z.; Shpigelman, A. Stability and Bioaccessibility of Lignans in Food Products. ACS Omega 2024, 9, 2022–2031. [Google Scholar] [CrossRef]
- Baldi, S.; Tristán Asensi, M.; Pallecchi, M.; Sofi, F.; Bartolucci, G.; Amedei, A. Interplay between Lignans and Gut Microbiota: Nutritional, Functional and Methodological Aspects. Molecules 2023, 28, 343. [Google Scholar] [CrossRef]
- Sun, Q.; Wedick, N.M.; Pan, A.; Townsend, M.K.; Cassidy, A.; Franke, A.A.; Rimm, E.B.; Hu, F.B.; van Dam, R.M. Gut Microbiota Metabolites of Dietary Lignans and Risk of Type 2 Diabetes: A Prospective Investigation in Two Cohorts of U.S. Women. Diabetes Care 2014, 37, 1287–1295. [Google Scholar] [CrossRef]
- Li, Y.; Wang, F.; Li, J.; Ivey, K.L.; Wilkinson, J.E.; Wang, D.D.; Li, R.; Liu, G.; Eliassen, H.A.; Chan, A.T.; et al. Dietary Lignans, Plasma Enterolactone Levels, and Metabolic Risk in Men: Exploring the Role of the Gut Microbiome. BMC Microbiol. 2022, 22, 82. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Huang, G.-P.; Zhang, L.-X.; da Yu, E.; Yang, W.-K.; Ye, M.; Zou, S.-Q.; Ni, L.; He, H.-Q. Lignan-Rich Extract from Cinnamomum Camphora Leaf Attenuates Metabolic Syndrome by Modulating Glycolipid Metabolism and Gut Microbiota in T2DM Mice. Phytomedicine 2024, 135, 156118. [Google Scholar] [CrossRef] [PubMed]
- Esfandiar, Z.; Hosseini-Esfahani, F.; Mirmiran, P.; Yuzbashian, E.; Azizi, F. The Association of Dietary Polyphenol Intake with the Risk of Type 2 Diabetes: Tehran Lipid and Glucose Study. Diabetes Metab. Syndr. Obes. 2020, 13, 1643–1652. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Hu, Y.; Liu, B.; Li, Y.; Wang, M.; Sun, Q. Lignan Intake and Type 2 Diabetes Incidence Among US Men and Women. JAMA Netw. Open 2024, 7, e2426367. [Google Scholar] [CrossRef]
- Rasouli, H.; Hosseini-Ghazvini, S.M.-B.; Adibi, H.; Khodarahmi, R. Differential α-Amylase/α-Glucosidase Inhibitory Activities of Plant-Derived Phenolic Compounds: A Virtual Screening Perspective for the Treatment of Obesity and Diabetes. Food Funct. 2017, 8, 1942–1954. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, X.; Zhang, J.; Gao, H.; Yang, L.; Li, D.; Zhang, Q.; Wang, B.; Cui, L.; Wang, X. (−)-Syringaresinol Suppressed LPS-Induced Microglia Activation via Downregulation of NF-κB P65 Signaling and Interaction with ERβ. Int. Immunopharmacol. 2021, 99, 107986. [Google Scholar] [CrossRef]
- Ye, H.; Sun, L.; Li, J.; Wang, Y.; Bai, J.; Wu, L.; Han, Q.; Yang, Z.; Li, L. Sesamin Attenuates Carrageenan-Induced Lung Inflammation Through Upregulation of A20 and TAX1BP1 in Rats. Int. Immunopharmacol. 2020, 88, 107009. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, T.; Yang, C.; Bao, T.; Yang, X.; He, F.; Zhang, Y.; Zhu, L.; Chen, H.; Rong, S.; et al. Secoisolariciresinol Diglucoside Suppresses Dextran Sulfate Sodium Salt-Induced Colitis through Inhibiting NLRP1 Inflammasome. Int. Immunopharmacol. 2020, 78, 105931. [Google Scholar] [CrossRef]
- Hoda, M.; Hemaiswarya, S.; Doble, M. Synergistic Behavior of Phytophenolics with Antidiabetic Drugs. In Role of Phenolic Phytochemicals in Diabetes Management: Phenolic Phytochemicals and Diabetes; Hoda, M., Hemaiswarya, S., Doble, M., Eds.; Springer: Singapore, 2019; pp. 123–143. ISBN 9789811389979. [Google Scholar]
- Chaachouay, N.; Zidane, L. Plant-Derived Natural Products: A Source for Drug Discovery and Development. Drugs Drug Candidates 2024, 3, 184–207. [Google Scholar] [CrossRef]
- Gilani, B.; Cassagnol, M. Biochemistry, Cytochrome P450. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Meng, Q.; Liu, K. Pharmacokinetic Interactions Between Herbal Medicines and Prescribed Drugs: Focus on Drug Metabolic Enzymes and Transporters. Curr. Drug Metab. 2014, 15, 791–807. [Google Scholar] [CrossRef]
- Gupta, R.C.; Chang, D.; Nammi, S.; Bensoussan, A.; Bilinski, K.; Roufogalis, B.D. Interactions Between Antidiabetic Drugs and Herbs: An Overview of Mechanisms of Action and Clinical Implications. Diabetol. Metab. Syndr. 2017, 9, 59. [Google Scholar] [CrossRef]
- Kammalla, A.K.; Ramasamy, M.K.; Chintala, J.; Dubey, G.P.; Agrawal, A.; Kaliappan, I. Comparative Pharmacokinetic Interactions of Quercetin and Rutin in Rats After Oral Administration of European Patented Formulation Containing Hipphophae Rhamnoides and Co-Administration of Quercetin and Rutin. Eur. J. Drug Metab. Pharmacokinet. 2015, 40, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsakris, Z.; Rozos, G.; Tsigalou, C.; Bezirtzoglou, E. Interactions Between Medical Plant-Derived Bioactive Compounds: Focus on Antimicrobial Combination Effects. Antibiotics 2022, 11, 1014. [Google Scholar] [CrossRef] [PubMed]
- Ramon, P.; Bergmann, D.; Abdulla, H.; Sparks, J.; Omoruyi, F. Bioactive Ingredients in K. pinnata Extract and Synergistic Effects of Combined K. pinnata and Metformin Preparations on Antioxidant Activities in Diabetic and Non-Diabetic Skeletal Muscle Cells. Int. J. Mol. Sci. 2023, 24, 6211. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Zhang, C.; Gu, X.; Li, X.; Zhu, H. Metformin in Combination with Malvidin Prevents Progression of Non-Alcoholic Fatty Liver Disease via Improving Lipid and Glucose Metabolisms, and Inhibiting Inflammation in Type 2 Diabetes Rats. Drug Des. Devel Ther. 2021, 15, 2565–2576. [Google Scholar] [CrossRef] [PubMed]
- Oboh, G.; Ogunbadejo, M.D.; Ogunsuyi, O.B.; Oyeleye, S.I. Can Gallic Acid Potentiate the Antihyperglycemic Effect of Acarbose and Metformin? Evidence from Streptozotocin-Induced Diabetic Rat Model. Arch. Physiol. Biochem. 2022, 128, 619–627. [Google Scholar] [CrossRef]
- Prabhakar, P.K.; Doble, M. Effect of Natural Products on Commercial Oral Antidiabetic Drugs in Enhancing 2-Deoxyglucose Uptake by 3T3-L1 Adipocytes. Ther. Adv. Endocrinol. 2011, 2, 103–114. [Google Scholar] [CrossRef]
- Knop, J.; Misaka, S.; Singer, K.; Hoier, E.; Müller, F.; Glaeser, H.; König, J.; Fromm, M.F. Inhibitory Effects of Green Tea and (−)-Epigallocatechin Gallate on Transport by OATP1B1, OATP1B3, OCT1, OCT2, MATE1, MATE2-K and P-Glycoprotein. PLoS ONE 2015, 10, e0139370. [Google Scholar] [CrossRef]
- Haque, M.A.; Hossain, M.S.; Sayed, N.M.A.; Islam, M.T.; Khan, M.R.; Ahmmed, F.; Zohora, F.T.; Ağagündüz, D.; Ming, L.C.; Capasso, R. Abelmoschus esculentus (L.) Moench Pod Extract Revealed Antagonistic Effect Against the Synergistic Antidiabetic Activity of Metformin and Acarbose upon Concomitant Administration in Glucose-Induced Hyperglycemic Mice. Biologics 2022, 2, 128–138. [Google Scholar] [CrossRef]
- Cheng, M.; Ren, L.; Jia, X.; Wang, J.; Cong, B. Understanding the Action Mechanisms of Metformin in the Gastrointestinal Tract. Front. Pharmacol. 2024, 15, 1347047. [Google Scholar] [CrossRef]
- Atsarina, D.M.; Widyastiti, N.S.; Muniroh, M.; Susilaningsih, N.; Maharani, N. Combination of Metformin and Epigallocatechin-3-Gallate Lowers Cortisol, 11β-Hydroxysteroid Dehydrogenase Type 1, and Blood Glucose Levels in Sprague Dawley Rats with Obesity and Diabetes. J. Obes. Metab. Syndr. 2024, 33, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Tamimi, L.N.; Zakaraya, Z.; Hailat, M.; Abu Dayyih, W.; Daoud, E.; Abed, A.; Saadh, M.J.; Majeed, B.; Abumansour, H.; Aburumman, A.; et al. Anti-Diabetic Effect of Cotreatment with Resveratrol and Pioglitazone in Diabetic Rats. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Tsuhako, R.; Atsumi, T.; Narumi, K.; Watanabe, W.; Sugita, C.; Kurokawa, M. Naringenin Interferes with the Anti-Diabetic Actions of Pioglitazone via Pharmacodynamic Interactions. J. Nat. Med. 2017, 71, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Maideen, N.M.P.; Balasubramaniam, R. Pharmacologically Relevant Drug Interactions of Sulfonylurea Antidiabetics with Common Herbs. J. Herbmed Pharmacol. 2018, 7, 200–210. [Google Scholar] [CrossRef]
- Nduka, S.O.; Daniel, A.L.; Ilodigwe, E.E.; Adimorah, U.; Mbagwu, S.I. Pharmacodynamic Herb-Drug Interactions: The Effects of Azadirachta Indica Leaf Extracts on Two Commonly Used Second Generation Sulfonylureas. World J. Pharm. Pharm. Sci. 2015, 4, 1702–1711. [Google Scholar]
- Valdes, M.; Calzada, F.; Martínez-Solís, J.; Martínez-Rodríguez, J. Antihyperglycemic Effects of Annona Cherimola Miller and the Flavonoid Rutin in Combination with Oral Antidiabetic Drugs on Streptozocin-Induced Diabetic Mice. Pharmaceuticals 2023, 16, 112. [Google Scholar] [CrossRef]
- Zhang, B.; Li, X.; Sun, W.; Xing, Y.; Xiu, Z.; Zhuang, C.; Dong, Y. Dietary Flavonoids and Acarbose Synergistically Inhibit α-Glucosidase and Lower Postprandial Blood Glucose. J. Agric. Food Chem. 2017, 65, 8319–8330. [Google Scholar] [CrossRef]
- Surendran, S.; Sapkal, R.; Paul, D.; Nanjappan, S. Effect of Resveratrol on Dipeptidyl Peptidase-4 Inhibitors Pharmacokinetics: An In Vitro and In Vivo Approach. Chem. Biol. Interact. 2020, 315, 108909. [Google Scholar] [CrossRef]
- Vora, A.; Varghese, A.; Kachwala, Y.; Bhaskar, M.; Laddha, A.; Jamal, A.; Yadav, P. Eugenia jambolana Extract Reduces the Systemic Exposure of Sitagliptin and Improves Conditions Associated with Diabetes: A Pharmacokinetic and a Pharmacodynamic Herb-Drug Interaction Study. J. Tradit. Complement. Med. 2019, 9, 364–371. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).