The Effect of the 14:10-Hour Time-Restricted Feeding (TRF) Regimen on Selected Markers of Glucose Homeostasis in Diet-Induced Prediabetic Male Sprague Dawley Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Housing
2.2. Prediabetes Induction
| Algorithm 1: A priori power analysis for a one-way ANOVA with fixed effects, calculating the required sample size. The analysis is based on the specified input parameters: effect size, significance level, desired power, and number of groups. |
| F tests—ANOVA: Fixed effects, omnibus, one-way Analysis: A priori: Compute required sample size Input: Effect size f = 1.335799 α err prob = 0.05 Power (1 − β err prob) = 0.80 Number of groups = 4 Output: Non-centrality parameter λ = 21.4123076 Critical F = 4.0661806 Nu-merator df = 3 Denominator df = 8 Total sample size = 12 Actual power = 0.8681756 |
2.3. Oral Glucose Tolerance Response
2.4. Intermittent Fasting Protocol

2.5. Blood Collection and Harvesting
2.6. Biochemical Analysis
2.7. Expression of Insulin Signalling Pathway Receptors via Real-Time-PCR
2.8. Glycogen Assay
2.9. Statistical Analysis
3. Results
3.1. Calorie Intake
3.2. Bodyweight Change
3.3. Body Mass Index
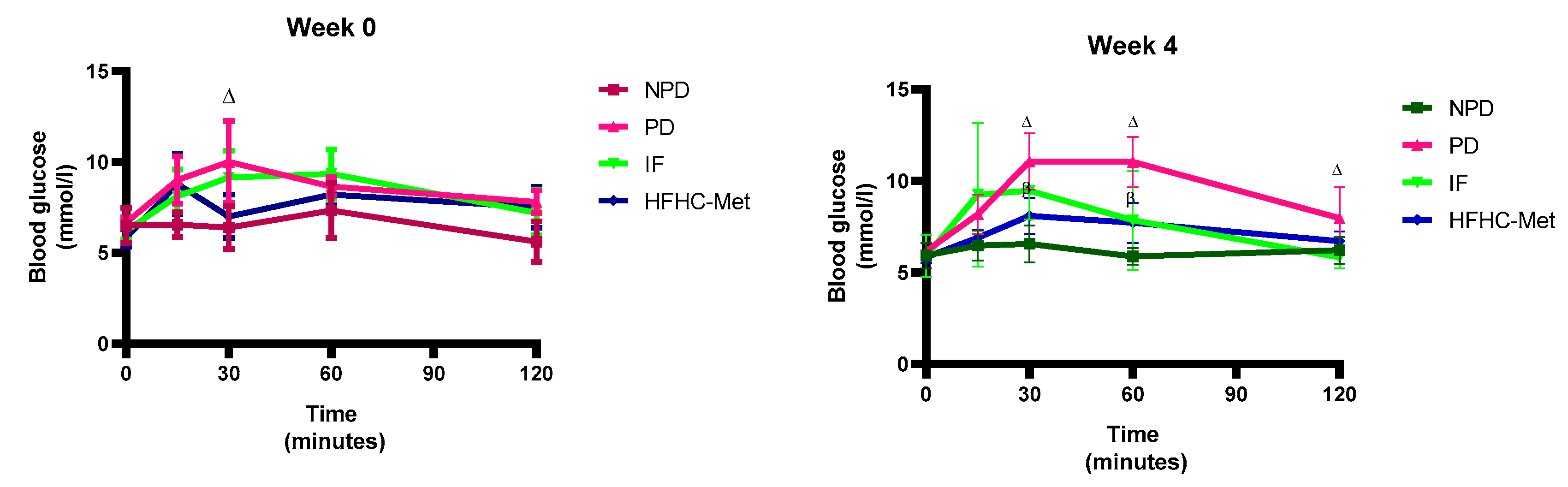
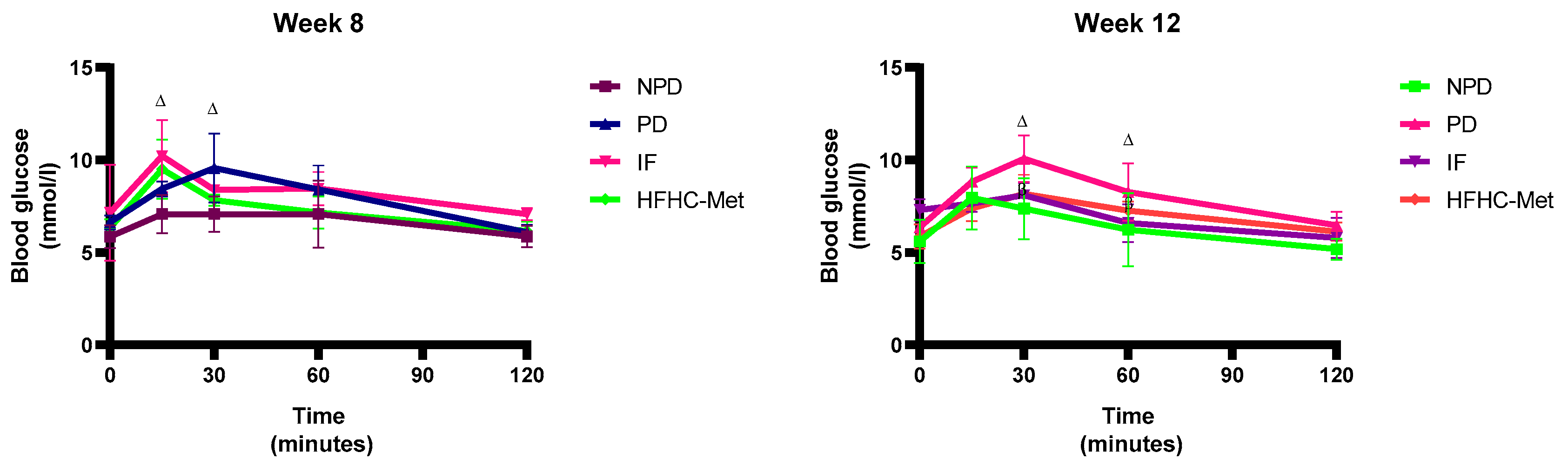
3.4. 2-h Oral Glucose Tolerance Tests
3.5. Fasting Glucose, Plasma Insulin, HOMA-IR and Blood HbA1c
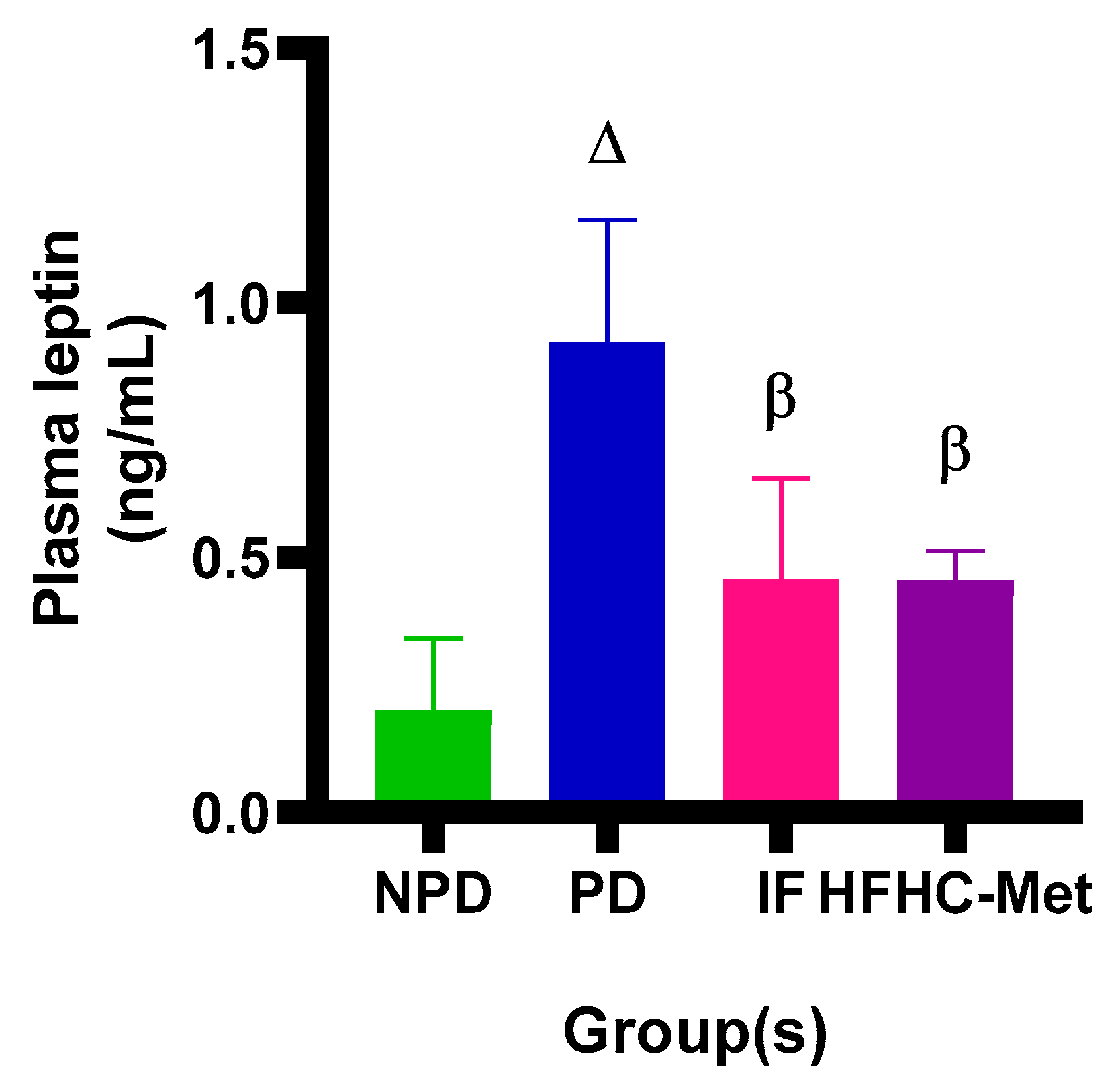
3.6. Plasma Leptin
3.7. Expression of IRS1, IRS2, Akt, PI3K, mTORC1 and GLUT 4
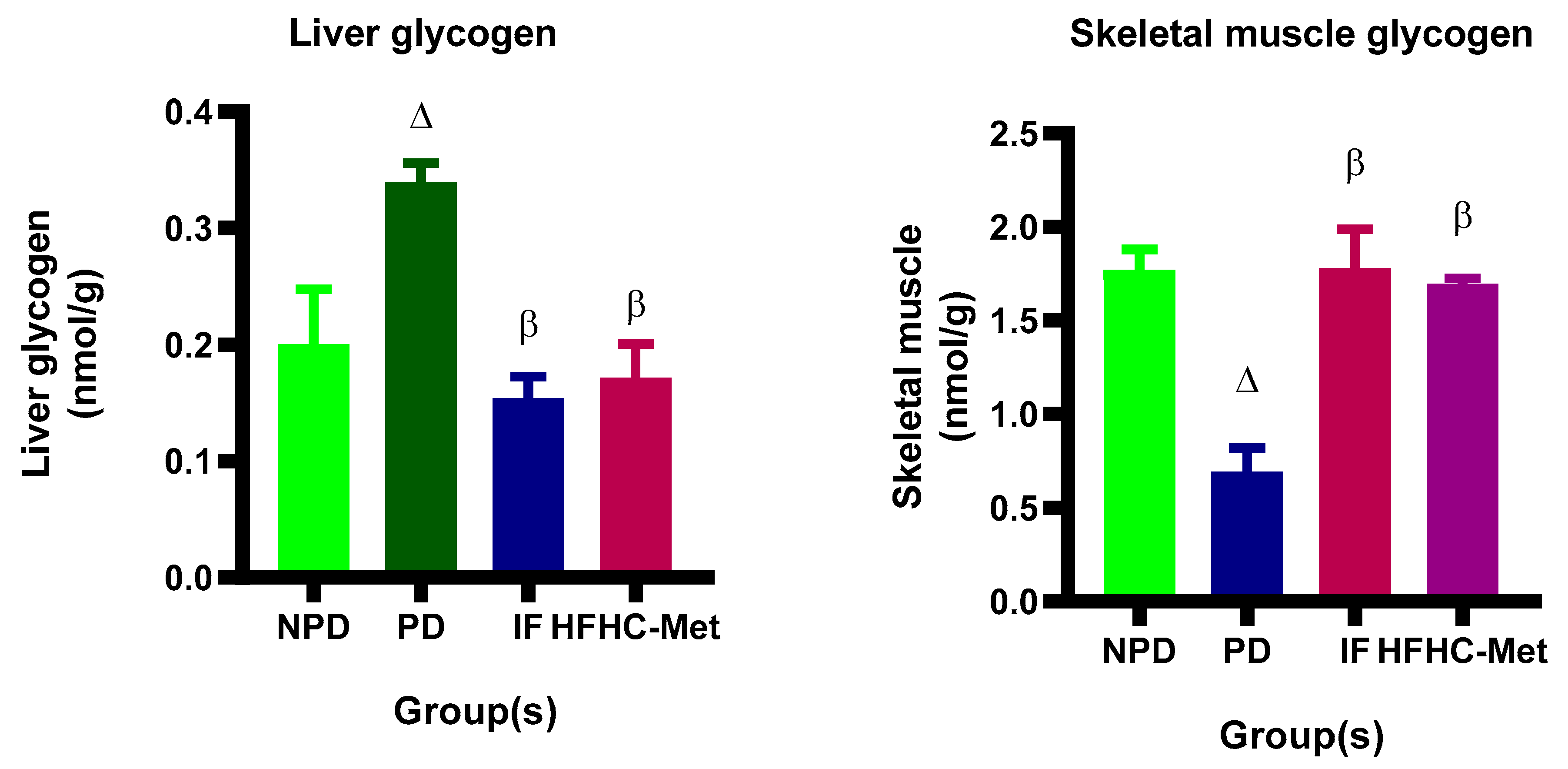
3.8. Glycogen Concentration in Liver and Skeletal Muscle
4. Discussion
5. Limitations and Future Studies
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADA | American Diabetes Association |
| AREC | Animal Research Ethics Committee |
| Akt | Protein kinase B |
| AUC | Area under the curve |
| BRU | Biomedical Research Unit |
| ELISA | Enzyme-linked immunosorbent assay |
| BMI | Body mass index |
| HbA1C | Glycated haemoglobin |
| FG | Fasting glucose |
| IFG | Impaired fasting glucose |
| IGT | Impaired glucose tolerance |
| OGTT | Oral glucose tolerance test |
| GLUT4 | Glucose transporter 4 |
| IRS1 | Insulin receptor substrate 1 |
| IRS2 | Insulin receptor substrate 2 |
| PI3K | Phosphoinositide 3-kinase |
| R | Reverse |
| F | Forward |
| mTORC 1 | Mechanistic target of rapamycin complex 1 |
| HOMA-IR | Homeostasis model assessment of insulin resistance |
| IF | Intermittent fasting |
| HFHC | High-fat, high-carbohydrate |
| TRF | Time-restricted feeding |
| T2DM | Type 2 diabetes mellitus |
| Met | Metformin |
| SEM | Standard error of mean |
| NPD | Non-prediabetic |
| PD | Prediabetes |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| RT-qPCR | Real-time quantitative polymerase chain reaction |
| cDNA | Complementary deoxyribonucleic acid |
| RNA | Ribonucleic acid |
| WHO | World Health Organization |
| GLUT2 | Glucose transporter 2 |
References
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- Tabák, A.G.; Herder, C.; Rathmann, W.; Brunner, E.J.; Kivimäki, M. Prediabetes: A high-risk state for diabetes development. Lancet 2012, 379, 2279–2290. [Google Scholar] [CrossRef] [PubMed]
- Diabetes Canada Clinical Practice Guidelines Expert, C.; Punthakee, Z.; Goldenberg, R.; Katz, P. Definition, Classification and Diagnosis of Diabetes, Prediabetes and Metabolic Syndrome. Can. J. Diabetes 2018, 42 (Suppl. S1), S10–S15. [Google Scholar] [CrossRef]
- Rooney, M.R.; Fang, M.; Ogurtsova, K.; Ozkan, B.; Echouffo-Tcheugui, J.B.; Boyko, E.J.; Magliano, D.J.; Selvin, E. Global Prevalence of Prediabetes. Diabetes Care 2023, 46, 1388–1394. [Google Scholar] [CrossRef]
- Echouffo-Tcheugui, J.B.; Perreault, L.; Ji, L.; Dagogo-Jack, S. Diagnosis and Management of Prediabetes: A Review. JAMA 2023, 329, 1206–1216. [Google Scholar] [CrossRef]
- Cienfuegos, S.; McStay, M.; Gabel, K.; Varady, K.A. Time restricted eating for the prevention of type 2 diabetes. J. Physiol. 2022, 600, 1253–1264. [Google Scholar] [CrossRef]
- Msane, S.; Khathi, A.; Sosibo, A. Therapeutic Potential of Various Intermittent Fasting Regimens in Alleviating Type 2 Diabetes Mellitus and Prediabetes: A Narrative Review. Nutrients 2024, 16, 2692. [Google Scholar] [CrossRef]
- Hatori, M.; Vollmers, C.; Zarrinpar, A.; DiTacchio, L.; Bushong, E.A.; Gill, S.; Leblanc, M.; Chaix, A.; Joens, M.; Fitzpatrick, J.A.; et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012, 15, 848–860. [Google Scholar] [CrossRef]
- Peeke, P.M.; Greenway, F.L.; Billes, S.K.; Zhang, D.; Fujioka, K. Effect of time restricted eating on body weight and fasting glucose in participants with obesity: Results of a randomized, controlled, virtual clinical trial. Nutr. Diabetes 2021, 11, 6. [Google Scholar] [CrossRef]
- Sukkriang, N.; Buranapin, S. Effect of intermittent fasting 16:8 and 14:10 compared with control—Group on weight reduction and metabolic outcomes in obesity with type 2 diabetes patients: A randomized controlled trial. J. Diabetes Investig. 2024, 15, 1297–1305. [Google Scholar] [CrossRef] [PubMed]
- Arnason, T.G.; Bowen, M.W.; Mansell, K.D. Effects of intermittent fasting on health markers in those with type 2 diabetes: A pilot study. World J. Diabetes 2017, 8, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Furmli, S.; Elmasry, R.; Ramos, M.; Fung, J. Therapeutic use of intermittent fasting for people with type 2 diabetes as an alternative to insulin. BMJ Case Rep. 2018, 2018, bcr2017221854. [Google Scholar] [CrossRef] [PubMed]
- Gabel, K.; Hoddy, K.K.; Haggerty, N.; Song, J.; Kroeger, C.M.; Trepanowski, J.F.; Panda, S.; Varady, K.A. Effects of 8-hour time restricted feeding on body weight and metabolic disease risk factors in obese adults: A pilot study. Nutr. Healthy Aging 2018, 4, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Gabel, K.; Kroeger, C.M.; Trepanowski, J.F.; Hoddy, K.K.; Cienfuegos, S.; Kalam, F.; Varady, K.A. Differential Effects of Alternate-Day Fasting Versus Daily Calorie Restriction on Insulin Resistance. Obesity 2019, 27, 1443–1450. [Google Scholar] [CrossRef]
- Gao, Y.; Tsintzas, K.; Macdonald, I.A.; Cordon, S.M.; Taylor, M.A. Effects of intermittent (5:2) or continuous energy restriction on basal and postprandial metabolism: A randomised study in normal-weight, young participants. Eur. J. Clin. Nutr. 2022, 76, 65–73. [Google Scholar] [CrossRef]
- Cefalu, W.T. Animal models of type 2 diabetes: Clinical presentation and pathophysiological relevance to the human condition. ILAR J. 2006, 47, 186–198. [Google Scholar] [CrossRef]
- Srinivasan, K.; Ramarao, P. Animal models in type 2 diabetes research: An overview. Indian. J. Med. Res. 2007, 125, 451–472. [Google Scholar]
- Khathi, A.; Luvuno, M.; Mabandla, M. Voluntary Ingestion of a High-Fat High-Carbohydrate Diet: A Model for Prediabetes. PONTE Int. Sci. Res. J. 2018, 74, 119–143. [Google Scholar] [CrossRef]
- Mapanga, R.F.; Tufts, M.; Shode, F.; Musabayane, C. Renal effects of plant-derived oleanolic acid in streptozotocin-induced diabetic rats. Ren. Fail. 2009, 31, 481–491. [Google Scholar] [CrossRef]
- Parasuraman, S.; Raveendran, R.; Kesavan, R. Blood sample collection in small laboratory animals. J. Pharmacol. Pharmacother. 2010, 1, 87. [Google Scholar] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-T.; Zhao, X.; Yaster, M.; Tao, Y.-X. Expression and distribution of mTOR, p70S6K, 4E-BP1, and their phosphorylated counterparts in rat dorsal root ganglion and spinal cord dorsal horn. Brain Res. 2010, 1336, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Musabayane, C.T.; Xozwa, K.; Ojewole, J.A.O. Effects ofHypoxis hemerocallidea (Fisch. & C.A. Mey.) [Hypoxidaceae] Corm (African Potato) Aqueous Extract on Renal Electrolyte and Fluid Handling in the Rat. Ren. Fail. 2005, 27, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Anjana, R.M.; Pradeepa, R.; Deepa, M.; Datta, M.; Sudha, V.; Unnikrishnan, R.; Bhansali, A.; Joshi, S.R.; Joshi, P.P.; Yajnik, C.S.; et al. Prevalence of diabetes and prediabetes (impaired fasting glucose and/or impaired glucose tolerance) in urban and rural India: Phase I results of the Indian Council of Medical Research–INdia DIABetes (ICMR–INDIAB) study. Diabetologia 2011, 54, 3022–3027. [Google Scholar] [CrossRef]
- Antoni, R.; Robertson, T.M.; Robertson, M.D.; Johnston, J.D. A pilot feasibility study exploring the effects of a moderate time-restricted feeding intervention on energy intake, adiposity and metabolic physiology in free-living human subjects. J. Nutr. Sci. 2018, 7, e22. [Google Scholar] [CrossRef]
- Aoun, A.; Ghanem, C.; Hamod, N.; Sawaya, S. The Safety and Efficacy of Intermittent Fasting for Weight Loss. Nutr. Today 2020, 55, 270–277. [Google Scholar] [CrossRef]
- Arciero, P.J.; Poe, M.; Mohr, A.E.; Ives, S.J.; Arciero, A.; Sweazea, K.L.; Gumpricht, E.; Arciero, K.M. Intermittent fasting and protein pacing are superior to caloric restriction for weight and visceral fat loss. Obesity 2023, 31 (Suppl. S1), 139–149. [Google Scholar] [CrossRef]
- MacNeil, M.D.; Berry, D.P.; Clark, S.A.; Crowley, J.J.; Scholtz, M.M. Evaluation of partial body weight for predicting body weight and average daily gain in growing beef cattle. Transl. Anim. Sci. 2021, 5, txab126. [Google Scholar] [CrossRef]
- Wang, L.; Wang, H.; Zhang, B.; Popkin, B.M.; Du, S. Elevated Fat Intake Increases Body Weight and the Risk of Overweight and Obesity among Chinese Adults: 1991–2015 Trends. Nutrients 2020, 12, 3272. [Google Scholar] [CrossRef]
- Gruzdeva, O.; Borodkina, D.; Uchasova, E.; Dyleva, Y.; Barbarash, O. Leptin resistance: Underlying mechanisms and diagnosis. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Mal, K.; Razaq, M.K.; Magsi, M.; Memon, M.K.; Memon, S.; Afroz, M.N.; Siddiqui, H.F.; Rizwan, A. Association of Leptin With Obesity and Insulin Resistance. Cureus 2020, 12, e12178. [Google Scholar] [CrossRef] [PubMed]
- Doehner, W.; Gerstein, H.C.; Ried, J.; Jung, H.; Asbrand, C.; Hess, S.; Anker, S.D. Obesity and weight loss are inversely related to mortality and cardiovascular outcome in prediabetes and type 2 diabetes: Data from the ORIGIN trial. Eur. Heart J. 2020, 41, 2668–2677. [Google Scholar] [CrossRef] [PubMed]
- Obradovic, M.; Sudar-Milovanovic, E.; Soskic, S.; Essack, M.; Arya, S.; Stewart, A.J.; Gojobori, T.; Isenovic, E.R. Leptin and Obesity: Role and Clinical Implication. Front. Endocrinol. 2021, 12, 585887. [Google Scholar] [CrossRef]
- Sundfør, T.M.; Svendsen, M.; Tonstad, S. Effect of intermittent versus continuous energy restriction on weight loss, maintenance and cardiometabolic risk: A randomized 1-year trial. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 698–706. [Google Scholar] [CrossRef]
- Schubel, R.; Nattenmuller, J.; Sookthai, D.; Nonnenmacher, T.; Graf, M.E.; Riedl, L.; Schlett, C.L.; von Stackelberg, O.; Johnson, T.; Nabers, D.; et al. Effects of intermittent and continuous calorie restriction on body weight and metabolism over 50 wk: A randomized controlled trial. Am. J. Clin. Nutr. 2018, 108, 933–945. [Google Scholar] [CrossRef]
- Sutton, E.F.; Beyl, R.; Early, K.S.; Cefalu, W.T.; Ravussin, E.; Peterson, C.M. Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metab. 2018, 27, 1212–1221.e1213. [Google Scholar] [CrossRef]
- Yerevanian, A.; Soukas, A.A. Metformin: Mechanisms in Human Obesity and Weight Loss. Curr. Obes. Rep. 2019, 8, 156–164. [Google Scholar] [CrossRef]
- Bock, G.; Dalla Man, C.; Campioni, M.; Chittilapilly, E.; Basu, R.; Toffolo, G.; Cobelli, C.; Rizza, R. Pathogenesis of Pre-Diabetes. Diabetes 2006, 55, 3536–3549. [Google Scholar] [CrossRef]
- Marr, L.; Biswas, D.; Daly, L.A.; Browning, C.; Vial, S.C.M.; Maskell, D.P.; Hudson, C.; Bertrand, J.A.; Pollard, J.; Ranson, N.A.; et al. Mechanism of glycogen synthase inactivation and interaction with glycogenin. Nat. Commun. 2022, 13, 3372. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, J.; Yang, S.; Gao, M.; Cao, L.; Li, X.; Hong, D.; Tian, S.; Sun, C. Effect of Intermittent Fasting Diet on Glucose and Lipid Metabolism and Insulin Resistance in Patients with Impaired Glucose and Lipid Metabolism: A Systematic Review and Meta-Analysis. Int. J. Endocrinol. 2022, 2022, 6999907. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Hong, X.; He, X.; Lin, J.; Fan, S.; Wu, J.; Liang, Z.; Chen, S.; Yan, L.; Ren, M.; et al. Intermittent Fasting-Improved Glucose Homeostasis Is Not Entirely Dependent on Caloric Restriction in db/db Male Mice. Diabetes 2024, 73, 864–878. [Google Scholar] [CrossRef] [PubMed]
- González-Ortiz, M.; Martínez-Abundis, E.; Robles-Cervantes, J.A.; Ramos-Zavala, M.G.; Barrera-Durán, C.; González-Canudas, J. Effect of Metformin Glycinate on Glycated Hemoglobin A1c Concentration and Insulin Sensitivity in Drug-Naive Adult Patients with Type 2 Diabetes Mellitus. Diabetes Technol. Ther. 2012, 14, 1140–1144. [Google Scholar] [CrossRef]
- Herman, R.; Kravos, N.A.; Jensterle, M.; Janež, A.; Dolžan, V. Metformin and Insulin Resistance: A Review of the Underlying Mechanisms behind Changes in GLUT4-Mediated Glucose Transport. Int. J. Mol. Sci. 2022, 23, 1264. [Google Scholar] [CrossRef]
- Newsholme, P.; Cruzat, V.; Arfuso, F.; Keane, K. Nutrient regulation of insulin secretion and action. J. Endocrinol. 2014, 221, R105–R120. [Google Scholar] [CrossRef]
- Campbell, J.E.; Newgard, C.B. Mechanisms controlling pancreatic islet cell function in insulin secretion. Nat. Rev. Mol. Cell Biol. 2021, 22, 142–158. [Google Scholar] [CrossRef]
- Chakarova, N.; Dimova, R.; Grozeva, G.; Tankova, T. Assessment of glucose variability in subjects with prediabetes. Diabetes Res. Clin. Pr. 2019, 151, 56–64. [Google Scholar] [CrossRef]
- Utzschneider, K.M.; Tripputi, M.T.; Kozedub, A.; Mather, K.J.; Nadeau, K.J.; Edelstein, S.L.; Hannon, T.S.; Arslanian, S.A.; Cree-Green, M.; Buchanan, T.A.; et al. beta-cells in youth with impaired glucose tolerance or early type 2 diabetes secrete more insulin and are more responsive than in adults. Pediatr. Diabetes 2020, 21, 1421–1429. [Google Scholar] [CrossRef]
- Wang, C.H.; Wang, C.C.; Wei, Y.H. Mitochondrial dysfunction in insulin insensitivity: Implication of mitochondrial role in type 2 diabetes. Ann. N. Y. Acad. Sci. 2010, 1201, 157–165. [Google Scholar] [CrossRef]
- Himanshu, D.; Ali, W.; Wamique, M. Type 2 diabetes mellitus: Pathogenesis and genetic diagnosis. J. Diabetes Metab. Disord. 2020, 19, 1959–1966. [Google Scholar] [CrossRef]
- Ghannadzadeh Yazdi, A.; Masoumvand, M.; Philippou, E.; Hatami, A.; Dehnavi, Z.; Barghchi, H.; Ahmadi-Khorram, M.; Jafarzadeh Esfehani, A.; Nematy, M. The effect of time-restricted eating on arterial stiffness indices in men with metabolic syndrome: Study protocol for a randomized controlled trial. Trials 2024, 25, 497. [Google Scholar] [CrossRef] [PubMed]
- Martens, C.R.; Rossman, M.J.; Mazzo, M.R.; Jankowski, L.R.; Nagy, E.E.; Denman, B.A.; Richey, J.J.; Johnson, S.A.; Ziemba, B.P.; Wang, Y.; et al. Short-term time-restricted feeding is safe and feasible in non-obese healthy midlife and older adults. GeroScience 2020, 42, 667–686. [Google Scholar] [CrossRef] [PubMed]
- Riera-Borrull, M.; García-Heredia, A.; Fernández-Arroyo, S.; Hernández-Aguilera, A.; Cabré, N.; Cuyàs, E.; Luciano-Mateo, F.; Camps, J.; Menendez, J.; Joven, J. Metformin Potentiates the Benefits of Dietary Restraint: A Metabolomic Study. Int. J. Mol. Sci. 2017, 18, 2263. [Google Scholar] [CrossRef] [PubMed]
- Konopka, A.R.; Laurin, J.L.; Schoenberg, H.M.; Reid, J.J.; Castor, W.M.; Wolff, C.A.; Musci, R.V.; Safairad, O.D.; Linden, M.A.; Biela, L.M.; et al. Metformin inhibits mitochondrial adaptations to aerobic exercise training in older adults. Aging Cell 2019, 18, e12880. [Google Scholar] [CrossRef]
- Kanto, K.; Ito, H.; Noso, S.; Babaya, N.; Hiromine, Y.; Taketomo, Y.; Toma, J.; Niwano, F.; Yasutake, S.; Kawabata, Y.; et al. Effects of dosage and dosing frequency on the efficacy and safety of high–dose metformin in Japanese patients with type 2 diabetes mellitus. J. Diabetes Investig. 2018, 9, 587–593. [Google Scholar] [CrossRef]
- Saltiel, A.R. Insulin signaling in health and disease. J. Clin. Investig. 2021, 131, e142241. [Google Scholar] [CrossRef]
- Khamzina, L.; Gruppuso, P.A.; Wands, J.R. Insulin signaling through insulin receptor substrate 1 and 2 in normal liver development. Gastroenterology 2003, 125, 572–585. [Google Scholar] [CrossRef]
- Noch, E.K.; Palma, L.N.; Yim, I.; Bullen, N.; Qiu, Y.; Ravichandran, H.; Kim, J.; Rendeiro, A.; Davis, M.B.; Elemento, O.; et al. Insulin feedback is a targetable resistance mechanism of PI3K inhibition in glioblastoma. Neuro Oncol. 2023, 25, 2165–2176. [Google Scholar] [CrossRef]
- Hoxhaj, G.; Manning, B.D. The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat. Rev. Cancer 2020, 20, 74–88. [Google Scholar] [CrossRef]
- Oliveira, L.D.C.; Morais, G.P.; Ropelle, E.R.; De Moura, L.P.; Cintra, D.E.; Pauli, J.R.; De Freitas, E.C.; Rorato, R.; Da Silva, A.S.R. Using Intermittent Fasting as a Non-pharmacological Strategy to Alleviate Obesity-Induced Hypothalamic Molecular Pathway Disruption. Front. Nutr. 2022, 9, 858320. [Google Scholar] [CrossRef]
- Klip, A.; McGraw, T.E.; James, D.E. Thirty sweet years of GLUT4. J. Biol. Chem. 2019, 294, 11369–11381. [Google Scholar] [CrossRef] [PubMed]
- Świderska, E.; Strycharz, J.; Wróblewski, A.; Szemraj, J.; Drzewoski, J.; Śliwińska, A. Role of PI3K/AKT Pathway in Insulin-Mediated Glucose Uptake; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Samuel, V.T.; Shulman, G.I. The pathogenesis of insulin resistance: Integrating signaling pathways and substrate flux. J. Clin. Investig. 2016, 126, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Haider, N.; Lebastchi, J.; Jayavelu, A.K.; Batista, T.M.; Pan, H.; Dreyfuss, J.M.; Carcamo-Orive, I.; Knowles, J.W.; Mann, M.; Kahn, C.R. Signaling defects associated with insulin resistance in nondiabetic and diabetic individuals and modification by sex. J. Clin. Investig. 2021, 131, e151818. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, V.; Saravanan, R. Glucose uptake through translocation and activation of GLUT4 in PI3K/Akt signaling pathway by asiatic acid in diabetic rats. Hum. Exp. Toxicol. 2015, 34, 884–893. [Google Scholar] [CrossRef]
- Rejeki, P.S.; Pranoto, A.; Widiatmaja, D.M.; Utami, D.M.; Izzatunnisa, N.; Sugiharto; Lesmana, R.; Halim, S. Combined Aerobic Exercise with Intermittent Fasting Is Effective for Reducing mTOR and Bcl-2 Levels in Obese Females. Sports 2024, 12, 116. [Google Scholar] [CrossRef]
- Kubota, N.; Kubota, T.; Itoh, S.; Kumagai, H.; Kozono, H.; Takamoto, I.; Mineyama, T.; Ogata, H.; Tokuyama, K.; Ohsugi, M.; et al. Dynamic Functional Relay between Insulin Receptor Substrate 1 and 2 in Hepatic Insulin Signaling during Fasting and Feeding. Cell Metab. 2008, 8, 49–64. [Google Scholar] [CrossRef]
- Soeters, M.R.; Lammers, N.M.; Dubbelhuis, P.F.; Ackermans, M.; Jonkers-Schuitema, C.F.; Fliers, E.; Sauerwein, H.P.; Aerts, J.M.; Serlie, M.J. Intermittent fasting does not affect whole-body glucose, lipid, or protein metabolism. Am. J. Clin. Nutr. 2009, 90, 1244–1251. [Google Scholar] [CrossRef]
- Rice, S.; Pellatt, L.J.; Bryan, S.J.; Whitehead, S.A.; Mason, H.D. Action of metformin on the insulin-signaling pathway and on glucose transport in human granulosa cells. J. Clin. Endocrinol. Metab. 2011, 96, E427–E435. [Google Scholar] [CrossRef]
- Thota, R.N.; Rosato, J.I.; Dias, C.B.; Burrows, T.L.; Martins, R.N.; Garg, M.L. Dietary Supplementation with Curcumin Reduce Circulating Levels of Glycogen Synthase Kinase-3β and Islet Amyloid Polypeptide in Adults with High Risk of Type 2 Diabetes and Alzheimer’s Disease. Nutrients 2020, 12, 1032. [Google Scholar] [CrossRef]
- Jensen, J.; Jebens, E.; Brennesvik, E.O.; Ruzzin, J.; Soos, M.A.; Engebretsen, E.M.; O’Rahilly, S.; Whitehead, J.P. Muscle glycogen inharmoniously regulates glycogen synthase activity, glucose uptake, and proximal insulin signaling. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E154–E162. [Google Scholar] [CrossRef]
- Morrice, N.; Vainio, S.; Mikkola, K.; van Aalten, L.; Gallagher, J.R.; Ashford, M.L.J.; McNeilly, A.D.; McCrimmon, R.J.; Grosfeld, A.; Serradas, P.; et al. Metformin increases the uptake of glucose into the gut from the circulation in high-fat diet-fed male mice, which is enhanced by a reduction in whole-body Slc2a2 expression. Mol. Metab. 2023, 77, 101807. [Google Scholar] [CrossRef] [PubMed]
- Marks, J.; Carvou, N.J.; Debnam, E.S.; Srai, S.K.; Unwin, R.J. Diabetes increases facilitative glucose uptake and GLUT2 expression at the rat proximal tubule brush border membrane. J. Physiol. 2003, 553, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Thorens, B. GLUT2, glucose sensing and glucose homeostasis. Diabetologia 2015, 58, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Lange, M.G.; Coffey, A.A.; Coleman, P.C.; Barber, T.M.; Van Rens, T.; Oyebode, O.; Abbott, S.; Hanson, P. Metabolic changes with intermittent fasting. J. Hum. Nutr. Diet. 2024, 37, 256–269. [Google Scholar] [CrossRef]
- Mishra, S.; Persons, P.A.; Lorenzo, A.M.; Chaliki, S.S.; Bersoux, S. Time-Restricted Eating and Its Metabolic Benefits. J. Clin. Med. 2023, 12, 7007. [Google Scholar] [CrossRef]
- Shulman, G.I. Cellular mechanisms of insulin resistance. J. Clin. Investig. 2000, 106, 171–176. [Google Scholar] [CrossRef]
- He, J.; Kelley, D.E. Muscle glycogen content in type 2 diabetes mellitus. Am. J. Physiol. Endocrinol. Metab. 2004, 287, E1002–E1007. [Google Scholar] [CrossRef]
- Masedunskas, A.; De Ciutiis, I.; Hein, L.; Ge, A.; Kong, Y.; Qi, M.; Mainali, D.; Rogerson-Wood, L.; Kroeger, C.; Aguirre Candia, Y.; et al. Investigating the Impact of Glycogen-Depleting Exercise Combined with Prolonged Fasting on Autophagy and Cellular Health in Humans: A Randomised Controlled Crossover Trial. Nutrients 2024, 16, 4297. [Google Scholar] [CrossRef]
- Bak, A.M.; Vendelbo, M.H.; Christensen, B.; Viggers, R.; Bibby, B.M.; Rungby, J.; Jorgensen, J.O.L.; Moller, N.; Jessen, N. Prolonged fasting-induced metabolic signatures in human skeletal muscle of lean and obese men. PLoS ONE 2018, 13, e0200817. [Google Scholar] [CrossRef]
- Rajpal, A.; Ismail-Beigi, F. Intermittent fasting and ‘metabolic switch’: Effects on metabolic syndrome, prediabetes and type 2 diabetes. Diabetes Obes. Metab. 2020, 22, 1496–1510. [Google Scholar] [CrossRef]

| Sequence Name | Sequence |
|---|---|
| GLUT 4 F | ATCAACGCCCCACAGAAAGT |
| GLUT 4 R | CCTGCCTACCCAGCCAAGT |
| Akt F | ATGGACTTCCGGTCAGGTTCA |
| Akt R | GCCCTTGCCCAGTAGCTTCA |
| MTORC1 F | TGCAGCCTGACCAATGATGTG |
| MTORC1 R | CTTGTGTCCGGCAGCATCATC |
| IRS1 F | GCCAATCTTCATCCAGTTGC |
| IRS1 R | CATCGTGAAGAAGGCATAGG |
| IRS2 F | CTACCCACTGAGCCCAAGAG |
| IRS2R | CCAGGGATGAAGCAGGACTA |
| PI3K F | ACTGAGATGGAGACACGGAAC |
| PI3K R | GCATCCAAGGGTCCAGTTAGTG |
| GAPDH F | AGTGCCAGCCTCGTCTCATA |
| GAPDH R | GATGGTGATGGGTTTCCCGT |
| Groups | Week 0 | Week 4 | Week 8 | Week 12 | |||
|---|---|---|---|---|---|---|---|
| Caloric Intake (kcal/g) | Caloric Intake (kcal/g) | % Change | Caloric Intake (kcal/g) | % Change | Caloric Intake (kcal/g) | % Change | |
| NPD | 55.1 ± 2.8 | 58.2 ± 6.5 | +5.6 | 61.3 ± 5.9 | +5.3 | 65.7 ± 5.9 | +7.2 |
| PD | 98.4 ± 6.9 ∆ | 110 ± 10.6 ∆ | +11.8 | 112.3 ± 10.1 ∆ | +2.1 | 121.8 ± 6.9 ∆ | +8.5 |
| IF | 91.3 ± 3.3 β | 91.4 ± 6.6 β | +0.1 | 93.2 ± 12.5 β | +2.0 | 108.4 ± 12.0 β | +16.3 |
| HFHC-Met | 93.2 ± 12.5 β | 98.9 ± 6.9 β | +6.1 | 102.6 ± 11.7 β | +3.7 | 112.1 ± 6.7 β | +9.3 |
| Groups | Week 0 | Week 4 | Week 8 | Week 12 | |||
|---|---|---|---|---|---|---|---|
| Body Weight (g) | Body Weight (g) | % Change | Body Weight (g) | % Change | Body Weight (g) | % Change | |
| NPD | 401 ± 10.5 | 425 ± 12.6 | +23 | 489 ± 17.0 | +27 | 503 ± 16.7 | +28 |
| PD | 603 ± 47.7 ∆ | 759 ± 82.8 ∆ | +26 | 786 ± 97.0 ∆ | +26 | 831 ± 102.5 ∆ | +28 |
| IF | 594 ± 7.8 ∆ | 613 ± 7.5 β | +25 | 632 ± 7.2 β | +25 | 658 ± 7.3 β | +26 |
| HFHC-Met | 542 ± 10.4 ∆ | 525 ± 21.8 β | +23 | 570 ± 29.4 β | +25 | 608 ± 29.0 β | +27 |
| BMI (g/cm2) | BMI (g/cm2) | BMI (g/cm2) | BMI (g/cm2) | |
|---|---|---|---|---|
| Groups | Week 0 | Week 4 | Week 8 | Week 12 |
| NPD | 4.3 ± 0.2 | 4.4 ± 0.1 | 4.9 ± 0.1 | 5.2 ± 1.5 |
| PD | 4.8 ± 0.1 | 6.8 ± 0.4 ∆ | 7.0 ± 0.4 ∆ | 7.7 ± 0.4 ∆ |
| IF | 4.7 ± 0.3 | 4.8 ± 0.2 β | 5.1 ± 0.2 β | 6.0 ± 0.12 β |
| HFHC-Met | 4.6 ± 0.4 | 4.7 ± 0.1 β | 4.8 ± 0.2 β | 5.3 ± 0.09 β |
| Groups | Week 0 | Week 4 | Week 8 | Week 12 |
|---|---|---|---|---|
| NPD | 789.5 ± 65.0 | 738.8 ± 33.6 | 804.3 ± 66.4 | 763.4 ± 76.7 |
| PD | 1033 ± 47.8 ∆ | 1152 ± 74.6 ∆ | 952.9 ± 55.5 ∆ | 972.8 ± 61.0 ∆ |
| IF | 1011 ± 66.0 ∆ | 923.1 ± 104.7 β | 988.7 ± 43.0 ∆ | 807.2 ± 53.5 β |
| HFHC-Met | 927.3 ± 47.9 ∆ | 877.0 ± 43.8 β | 873.8 ± 37.3 β | 849.8 ± 29.6 β |
| Groups | Fasting Glucose (mmol/L) | Insulin (μU/mL) | HOMA IR | HbA1c (%) |
|---|---|---|---|---|
| NPD | 5.4 ± 0.2 | 1.1 ± 0.2 | 0.3 ± 0.05 | 5.5 ± 0.6 |
| PD | 6.4 ± 0.1 | 1.9 ± 0.5 | 0.5 ± 0.1 | 8.2 ± 1.1 ∆ |
| IF | 6.0 ± 0.3 | 0.8 ± 0.2 β | 0.2 ± 0.05 β | 6.0 ± 0.5 β |
| HFHC-Met | 5.9 ± 0.4 | 1.8 ± 0.5 | 0.5 ± 0.1 | 6.7 ± 0.6 β |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Msane, S.; Khathi, A.; Sosibo, A.M. The Effect of the 14:10-Hour Time-Restricted Feeding (TRF) Regimen on Selected Markers of Glucose Homeostasis in Diet-Induced Prediabetic Male Sprague Dawley Rats. Nutrients 2025, 17, 292. https://doi.org/10.3390/nu17020292
Msane S, Khathi A, Sosibo AM. The Effect of the 14:10-Hour Time-Restricted Feeding (TRF) Regimen on Selected Markers of Glucose Homeostasis in Diet-Induced Prediabetic Male Sprague Dawley Rats. Nutrients. 2025; 17(2):292. https://doi.org/10.3390/nu17020292
Chicago/Turabian StyleMsane, Sthembiso, Andile Khathi, and Aubrey Mbulelo Sosibo. 2025. "The Effect of the 14:10-Hour Time-Restricted Feeding (TRF) Regimen on Selected Markers of Glucose Homeostasis in Diet-Induced Prediabetic Male Sprague Dawley Rats" Nutrients 17, no. 2: 292. https://doi.org/10.3390/nu17020292
APA StyleMsane, S., Khathi, A., & Sosibo, A. M. (2025). The Effect of the 14:10-Hour Time-Restricted Feeding (TRF) Regimen on Selected Markers of Glucose Homeostasis in Diet-Induced Prediabetic Male Sprague Dawley Rats. Nutrients, 17(2), 292. https://doi.org/10.3390/nu17020292






