Micronutrient–Antioxidant Therapy and Male Fertility Improvement During ART Cycles
Abstract
:1. Introduction
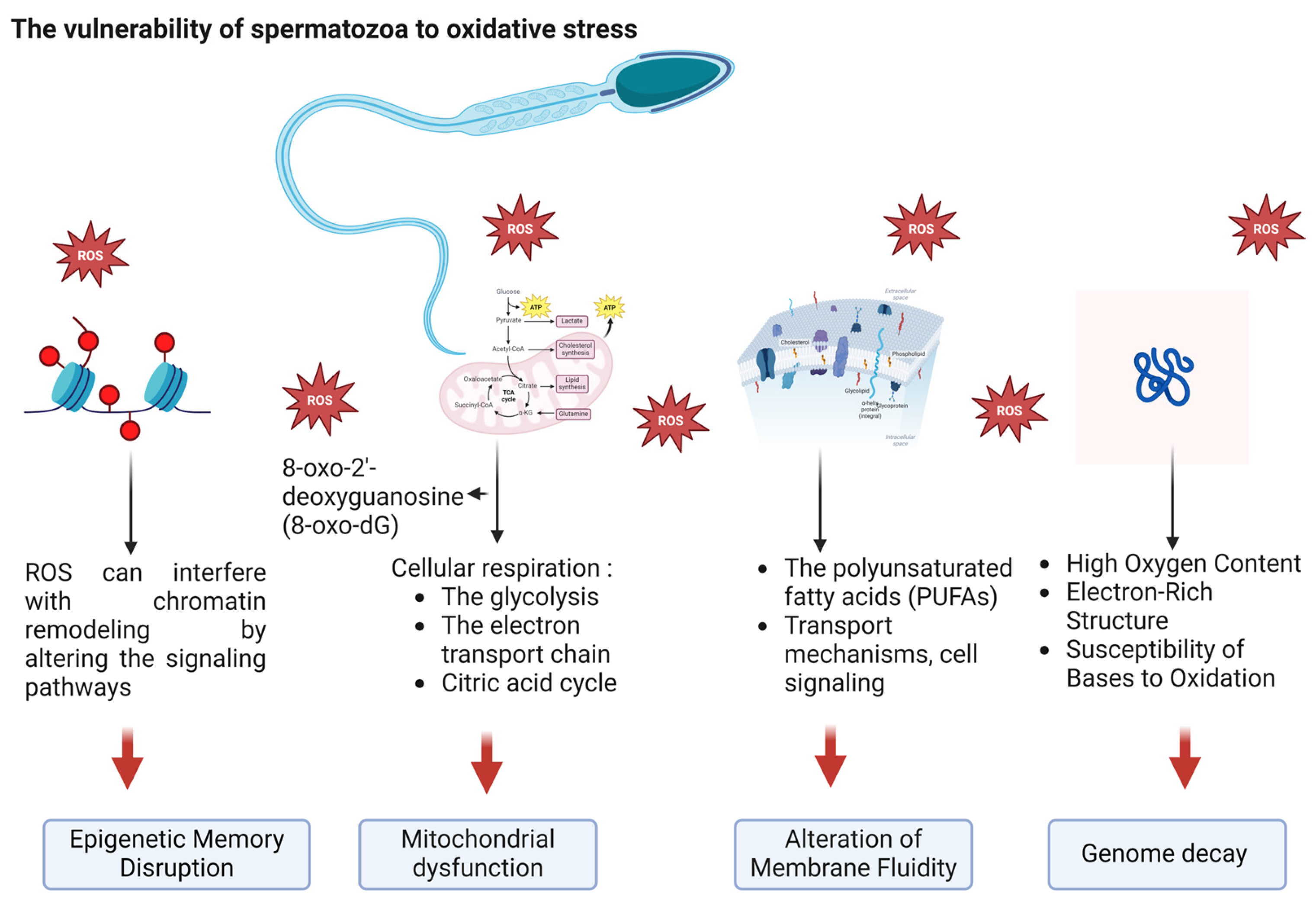
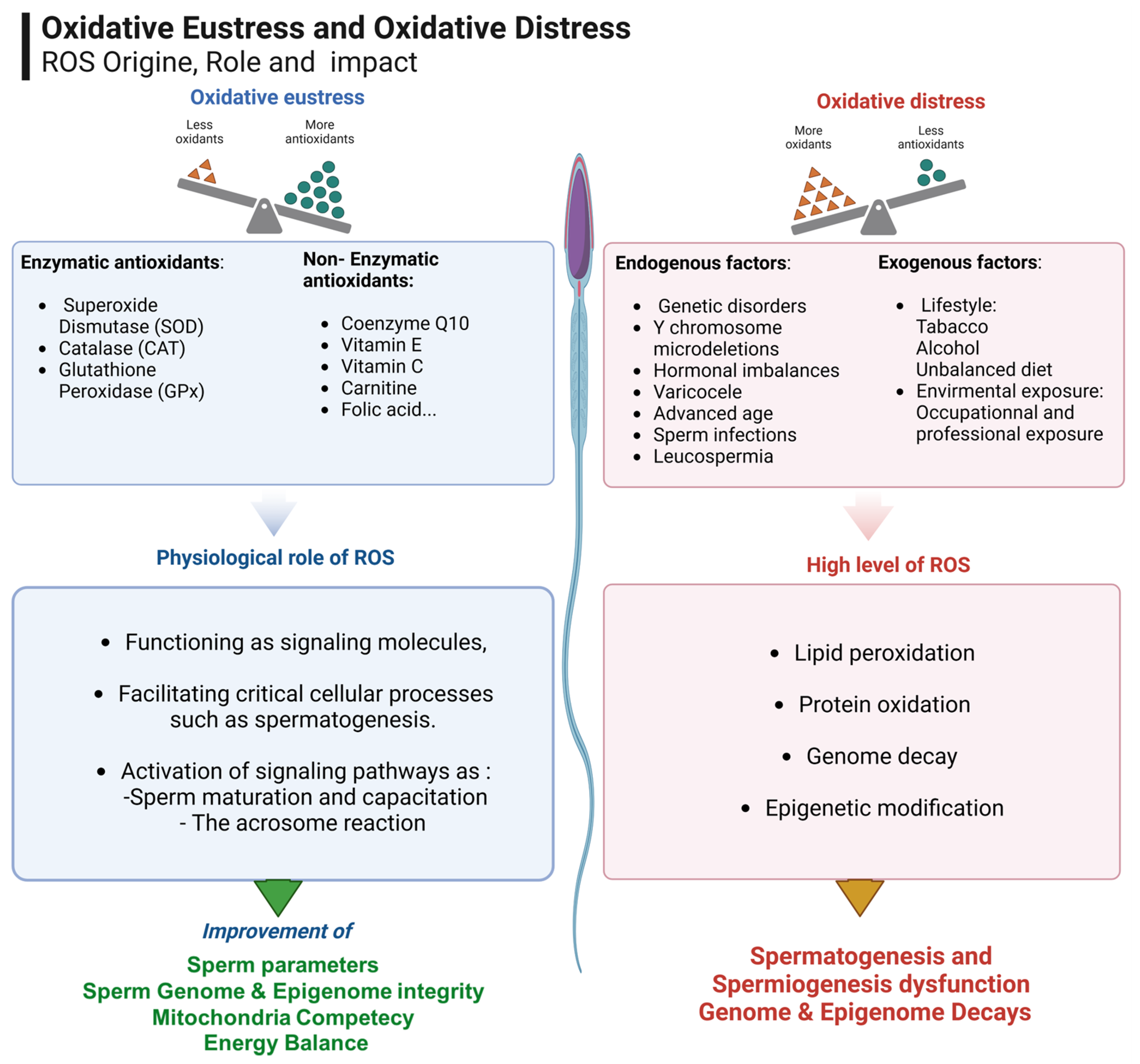
2. Targeting Oxidative Stress in Infertility
3. Micronutrients in Reproductive Medicine: A Path to Improved Fertility
3.1. Semen Quality
3.2. Genome Integrity
3.3. Assisted Reproductive Technology Outcomes
4. What Is Known
5. Conclusions
6. Limitations
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ART | assisted reproductive technology |
| OS | oxidative stress |
| ROS | reactive oxygen species |
| PUFAs | polyunsaturated fatty acids |
| mtDNA | mitochondrial DNA |
| qPCR | quantitative polymerase chain reaction |
| ICSI | intracytoplasmic sperm injection |
| 8-OHdG | 8-hydroxy-2′-deoxyguanosine |
| DNMTs | DNA methyltransferases |
| HDACs | histone deacetylases |
| HMTs | histone methyltransferases |
| LH | luteinizing hormone |
| OAT | Oligo-Astheno-Teratozoospermia |
| ATP | adenosine triphosphate |
| RCTs | randomized controlled trials |
| NAC | N-acetyl-cysteine |
| ALA | alpha-lipoic acid |
| MMP | mitochondrial membrane potential |
| DSBs | double-strand breaks |
| SSBs | single-strand breaks |
| TUNEL | terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling |
| IVF | in vitro fertilization |
| PT | pentoxifylline |
| LC | L-carnitine |
References
- Boitrelle, F.; Shah, R.; Saleh, R.; Henkel, R.; Kandil, H.; Chung, E.; Vogiatzi, P.; Zini, A.; Arafa, M.; Agarwal, A. The Sixth Edition of the WHO Manual for Human Semen Analysis: A Critical Review and SWOT Analysis. Life 2021, 11, 1368. [Google Scholar] [CrossRef] [PubMed]
- Dourou, P.; Gourounti, K.; Lykeridou, A.; Gaitanou, K.; Petrogiannis, N.; Sarantaki, A. Quality of Life among Couples with a Fertility Related Diagnosis. Clin. Pract. 2023, 13, 251–263. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. 1 in 6 People Globally Affected by Infertility. Available online: https://www.who.int/news/item/04-04-2023-1-in-6-people-globally-affected-by-infertility (accessed on 8 January 2025).
- Carlsen, E.; Giwercman, A.; Keiding, N.; Skakkebaek, N.E. Evidence for decreasing quality of semen during past 50 years. BMJ 1992, 305, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Li, B.; Xu, K.; Liu, D.; Hu, J.; Yang, Y.; Nie, H.; Fan, L.; Zhu, W. Decline in semen quality among 30,636 young Chinese men from 2001 to 2015. Fertil. Steril. 2017, 107, 83–88.e2. [Google Scholar] [CrossRef] [PubMed]
- Bahri, H.; Ben Khalifa, M.; Ben Rhouma, M.; Abidi, Z.; Abbassi, E.; Ben Rhouma, K.; Benkhalifa, M. Decline in semen quality of North African men: A retrospective study of 20,958 sperm analyses of men from different North African countries tested in Tunisia over a period of 6 years (2013–2018). Ann. Hum. Biol. 2021, 48, 350–359. [Google Scholar] [CrossRef]
- Mann, U.; Shiff, B.; Patel, P. Reasons for worldwide decline in male fertility. Curr. Opin. Urol. 2020, 30, 296–301. [Google Scholar] [CrossRef]
- Sciorio, R.; Tramontano, L.; Adel, M.; Fleming, S. Decrease in Sperm Parameters in the 21st Century: Obesity, Lifestyle, or Environmental Factors? An Updated Narrative Review. J. Pers. Med. 2024, 14, 198. [Google Scholar] [CrossRef]
- Jørgensen, N.; Lamb, D.J.; Levine, H.; Pastuszak, A.W.; Sigalos, J.T.; Swan, S.H.; Eisenberg, M.L. Are worldwide sperm counts declining? Fertil. Steril. 2021, 116, 1457–1463. [Google Scholar] [CrossRef]
- Lassen, E.; Pacey, A.; Skytte, A.-B.; Montgomerie, R. Recent decline in sperm motility among donor candidates at a sperm bank in Denmark. Hum. Reprod. 2024, 39, 1618–1627. [Google Scholar] [CrossRef]
- Juárez-Rojas, L.; Casillas, F.; López, A.; Betancourt, M.; Ommati, M.M.; Retana-Márquez, S. Physiological role of reactive oxygen species in testis and epididymal spermatozoa. Andrologia 2022, 54, e14367. [Google Scholar] [CrossRef]
- Kalantari, H.; Sabbaghian, M.; Vogiatzi, P.; Rambhatla, A.; Agarwal, A.; Colpi, G.M.; Gilani, M.A.S. Bridging the Gap between AZF Microdeletions and Karyotype: Twelve Years’ Experience of an Infertility Center. World J. Men’s Health 2023, 41, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Cheung, S.; Parrella, A.; Rosenwaks, Z.; Palermo, G.D. Genetic and epigenetic profiling of the infertile male. PLoS ONE. 2019, 14, e0214275. [Google Scholar] [CrossRef] [PubMed]
- Manvelyan, M.; Hunstig, F.; Bhatt, S.; Mrasek, K.; Pellestor, F.; Weise, A.; Simonyan, I.; Aroutiounian, R.; Liehr, T. Chromosome distribution in human sperm—A 3D multicolor banding-study. Mol. Cytogenet. 2008, 1, 25. [Google Scholar] [CrossRef]
- Ilktac, A.; Hamidli, S.; Ersoz, C.; Dogan, B.; Akcay, M. Efficacy of varicocelectomy in primary infertile patients with isolated teratozoospermia. A retrospective analysis. Andrologia 2020, 52, e13875. [Google Scholar] [CrossRef] [PubMed]
- Van der Ven, H.H.; Jeyendran, R.S.; Perez-Pelaez, M.; Al-Hasani, S.; Diedrich, K.; Krebs, D. Leucospermia and the fertilizing capacity of spermatozoa. Eur. J. Obstet. Gynecol. Reprod. Biol. 1987, 24, 49–52. [Google Scholar] [CrossRef] [PubMed]
- du Fossé, N.A.; van der Hoorn, M.-L.P.; van Lith, J.M.M.; le Cessie, S.; Lashley, E.E.L.O. Advanced paternal age is associated with an increased risk of spontaneous miscarriage: A systematic review and meta-analysis. Hum. Reprod. Update 2020, 26, 650–669. [Google Scholar] [CrossRef] [PubMed]
- Lahimer, M.; Montjean, D.; Cabry, R.; Capelle, S.; Lefranc, E.; Bach, V.; Ajina, M.; Ben Ali, H.; Khorsi-Cauet, H.; Benkhalifa, M. Paternal Age Matters: Association with Sperm Criteria’s-Spermatozoa DNA Integrity and Methylation Profile. J. Clin. Med. 2023, 12, 4928. [Google Scholar] [CrossRef] [PubMed]
- Abell, A.; Ernst, E.; Bonde, J.P. Semen quality and sexual hormones in greenhouse workers. Scand. J. Work. Environ. Health 2000, 26, 492–500. [Google Scholar] [CrossRef]
- Auriemma, R.S.; Menafra, D.; de Angelis, C.; Pivonello, C.; Garifalos, F.; Verde, N.; Galdiero, G.; Piscopo, M.; Colao, A.; Pivonello, R. The Role of the Environment in Testicular Dysgenesis Syndrome. In Environmental Endocrinology and Endocrine Disruptors: Endocrine and Endocrine-Targeted Actions and Related Human Diseases; Pivonello, R., Diamanti-Kandarakis, E., Eds.; Springer: Cham, Switzerland, 2020; pp. 1–38. [Google Scholar]
- Lahimer, M.; Diwan, M.A.; Montjean, D.; Cabry, R.; Bach, V.; Ajina, M.; Ben Ali, H.; Benkhalifa, M.; Khorsi-Cauet, H. Endocrine disrupting chemicals and male fertility: From physiological to molecular effects. Front. Public Health 2023, 11, 1232646. Available online: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1232646 (accessed on 10 October 2023). [CrossRef]
- Lahimer, M.; Djekkoun, N.; Tricotteaux-Zarqaoui, S.; Corona, A.; Lafosse, I.; Ben Ali, H.; Ajina, M.; Bach, V.; Benkhalifa, M.; Khorsi-Cauet, H. Impact of Perinatal Coexposure to Chlorpyrifos and a High-Fat Diet on Kisspeptin and GnRHR Presence and Reproductive Organs. Toxics 2023, 11, 789. [Google Scholar] [CrossRef]
- Lahimer, M.; Capelle, S.; Lefranc, E.; Cabry, R.; Montjean, D.; Bach, V.; Ajina, M.; Ben Ali, H.; Benkhalifa, M.; Khorsi-Cauet, H. Effect of pesticide exposure on human sperm characteristics, genome integrity, and methylation profile analysis. Environ. Sci. Pollut. Res. 2023, 30, 77560–77567. [Google Scholar] [CrossRef] [PubMed]
- Aboulmaouahib, S.; Madkour, A.; Kaarouch, I.; Sefrioui, O.; Saadani, B.; Copin, H.; Benkhalifa, M.; Louanjli, N.; Cadi, R. Impact of alcohol and cigarette smoking consumption in male fertility potential: Looks at lipid peroxidation, enzymatic antioxidant activities and sperm DNA damage. Andrologia 2018, 50, e12926. [Google Scholar] [CrossRef] [PubMed]
- Tremellen, K. Oxidative stress and male infertility—A clinical perspective. Hum. Reprod. Update 2008, 14, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J. Reactive oxygen species as mediators of sperm capacitation and pathological damage. Mol. Reprod. Dev. 2017, 84, 1039–1052. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Drevet, J.R.; Moazamian, A.; Gharagozloo, P. Male Infertility and Oxidative Stress: A Focus on the Underlying Mechanisms. Antioxidants 2022, 11, 306. [Google Scholar] [CrossRef]
- Asadi, N.; Bahmani, M.; Kheradmand, A.; Rafieian-Kopaei, M. The Impact of Oxidative Stress on Testicular Function and the Role of Antioxidants in Improving it: A Review. J. Clin. Diagn. Res. 2017, 11, IE01–IE05. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Sulejczak, D.; Kleczkowska, P.; Bukowska-Ośko, I.; Kucia, M.; Popiel, M.; Wietrak, E.; Kramkowski, K.; Wrzosek, K.; Kaczyńska, K. Mitochondrial Oxidative Stress—A Causative Factor and Therapeutic Target in Many Diseases. Int. J. Mol. Sci. 2021, 22, 13384. [Google Scholar] [CrossRef]
- Sawyer, D.E.; Roman, S.D.; Aitken, R.J. Relative susceptibilities of mitochondrial and nuclear DNA to damage induced by hydrogen peroxide in two mouse germ cell lines. Redox Rep. 2001, 6, 182–184. [Google Scholar] [CrossRef]
- Lahimer, M.; Gherissi, O.; Ben Salem, N.; Ben Mustapha, H.; Bach, V.; Khorsi-Cauet, H.; Khairi, H.; Ben Ali, H.; BenKhalifa, M.; Ajina, M. Effect of Micronutrients and L-Carnitine as Antioxidant on Sperm Parameters, Genome Integrity, and ICSI Outcomes: Randomized, Double-Blind, and Placebo-Controlled Clinical Trial. Antioxidants 2023, 12, 1937. [Google Scholar] [CrossRef]
- Lahimer, M.; Mustapha, H.; Bach, V.; Khorsi-Cauet, H.; Benkhalifa, M.; Ajina, M.; Ben Ali, H. Oxidative stress in male infertility and therapeutic approach: A mini-review. Asian Pac. J. Reprod. 2023, 12, 249. [Google Scholar] [CrossRef]
- Ferramosca, A.; Zara, V. Diet and Male Fertility: The Impact of Nutrients and Antioxidants on Sperm Energetic Metabolism. Int. J. Mol. Sci. 2022, 23, 2542. [Google Scholar] [CrossRef] [PubMed]
- Yaris, M.; Akdogan, N.; Öztürk, M.; Bozkurt, A.; Karabakan, M. The effects of two different antioxidant combinations on sperm parameters. Urologia 2022, 89, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Micic, S.; Lalic, N.; Djordjevic, D.; Bojanic, N.; Bogavac-Stanojevic, N.; Busetto, G.M.; Virmani, A.; Agarwal, A. Double-blind, randomised, placebo-controlled trial on the effect of L-carnitine and L-acetylcarnitine on sperm parameters in men with idiopathic oligoasthenozoospermia. Andrologia 2019, 51, e13267. [Google Scholar] [CrossRef] [PubMed]
- Schisterman, E.F.; Sjaarda, L.A.; Clemons, T.; Carrell, D.T.; Perkins, N.J.; Johnstone, E.; Lamb, D.; Chaney, K.; Van Voorhis, B.J.; Ryan, G.; et al. Effect of Folic Acid and Zinc Supplementation in Men on Semen Quality and Live Birth Among Couples Undergoing Infertility Treatment. JAMA 2020, 323, 35–48. [Google Scholar] [CrossRef]
- Agarwal, A.; Leisegang, K.; Majzoub, A.; Henkel, R.; Finelli, R.; Selvam, M.K.P.; Tadros, N.; Parekh, N.; Ko, E.Y.; Cho, C.-L.; et al. Utility of Antioxidants in the Treatment of Male Infertility: Clinical Guidelines Based on a Systematic Review and Analysis of Evidence. World J. Mens Health 2021, 39, 233–290. [Google Scholar] [CrossRef]
- Salas-Huetos, A.; Rosique-Esteban, N.; Becerra-Tomás, N.; Vizmanos, B.; Bulló, M.; Salas-Salvadó, J. The Effect of Nutrients and Dietary Supplements on Sperm Quality Parameters: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Adv. Nutr. 2018, 9, 833–848. [Google Scholar] [CrossRef]
- Cavallini, G.; Cristina Magli, M.; Crippa, A.; Pia Ferraretti, A.; Gianaroli, L. Reduction in sperm aneuploidy levels in severe oligoasthenoteratospermic patients after medical therapy: A preliminary report. Asian J. Androl. 2012, 14, 591–598. [Google Scholar] [CrossRef]
- Pavuluri, H.; Bakhtiary, Z.; Panner Selvam, M.K.; Hellstrom, W.J.G. Oxidative Stress-Associated Male Infertility: Current Diagnostic and Therapeutic Approaches. Medicina 2024, 60, 1008. [Google Scholar] [CrossRef]
- Endale, H.T.; Tesfaye, W.; Mengstie, T.A. ROS induced lipid peroxidation and their role in ferroptosis. Front. Cell Dev. Biol. 2023, 11, 1226044. Available online: https://www.frontiersin.org/journals/cell-and-developmental-biology/articles/10.3389/fcell.2023.1226044/full (accessed on 8 January 2025). [CrossRef]
- Zheng, Y.; Sun, J.; Luo, Z.; Li, Y.; Huang, Y. Emerging mechanisms of lipid peroxidation in regulated cell death and its physiological implications. Cell Death Dis. 2024, 15, 859. [Google Scholar] [CrossRef]
- Su, L.-J.; Zhang, J.-H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.-Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxidative Med. Cell Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef] [PubMed]
- Sinha, K.; Das, J.; Pal, P.B.; Sil, P.C. Oxidative stress: The mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch. Toxicol. 2013, 87, 1157–1180. [Google Scholar] [CrossRef] [PubMed]
- Alahmar, A.T. Role of Oxidative Stress in Male Infertility: An Updated Review. J. Hum. Reprod. Sci. 2019, 12, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Omari Shekaftik, S.; Nasirzadeh, N. 8-Hydroxy-2′-deoxyguanosine (8-OHdG) as a biomarker of oxidative DNA damage induced by occupational exposure to nanomaterials: A systematic review. Nanotoxicology 2021, 15, 850–864. [Google Scholar] [CrossRef]
- Benkhalifa, M.; Ferreira, Y.J.; Chahine, H.; Louanjli, N.; Miron, P.; Merviel, P.; Copin, H. Mitochondria: Participation to infertility as source of energy and cause of senescence. Int. J. Biochem. Cell Biol. 2014, 55, 60–64. [Google Scholar] [CrossRef]
- Sánchez Milán, J.A.; Mulet, M.; Serra, A.; Gallart-Palau, X. Trioxidized cysteine and aging: A molecular binomial that extends far beyond classical proteinopathic paradigms. Aging 2024, 16, 11484–11490. [Google Scholar] [CrossRef]
- Sánchez Milán, J.A.; Fernández-Rhodes, M.; Guo, X.; Mulet, M.; Ngan, S.C.; Iyappan, R.; Katoueezadeh, M.; Sze, S.K.; Serra, A.; Gallart-Palau, X. Trioxidized cysteine in the aging proteome mimics the structural dynamics and interactome of phosphorylated serine. Aging Cell 2024, 23, e14062. [Google Scholar] [CrossRef]
- Balamurli, G.; Liew, A.Q.X.; Tee, W.W.; Pervaiz, S. Interplay between epigenetics, senescence and cellular redox metabolism in cancer and its therapeutic implications. Redox Biol. 2024, 78, 103441. [Google Scholar] [CrossRef]
- Zheng, X.; Sawalha, A.H. The Role of Oxidative Stress in Epigenetic Changes Underlying Autoimmunity. Antioxid. Redox Signal. 2022, 36, 423–440. [Google Scholar] [CrossRef]
- Glorieux, C.; Liu, S.; Trachootham, D.; Huang, P. Targeting ROS in cancer: Rationale and strategies. Nat. Rev. Drug Discov. 2024, 23, 583–606. [Google Scholar] [CrossRef]
- Dimitriadis, F.; Borgmann, H.; Struck, J.P.; Salem, J.; Kuru, T.H. Antioxidant Supplementation on Male Fertility—A Systematic Review. Antioxidants 2023, 12, 836. [Google Scholar] [CrossRef] [PubMed]
- Kaltsas, A. Oxidative Stress and Male Infertility: The Protective Role of Antioxidants. Medicina 2023, 59, 1769. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Bandyopadhyay, A. Antioxidants in mitigating phthalate-induced male reproductive toxicity: A comprehensive review. Chemosphere 2024, 364, 143297. [Google Scholar] [CrossRef]
- Walke, G.; Gaurkar, S.S.; Prasad, R.; Lohakare, T.; Wanjari, M. The Impact of Oxidative Stress on Male Reproductive Function: Exploring the Role of Antioxidant Supplementation. Cureus 2023, 15, e42583. Available online: https://www.cureus.com/articles/169642-the-impact-of-oxidative-stress-on-male-reproductive-function-exploring-the-role-of-antioxidant-supplementation (accessed on 4 February 2024). [CrossRef]
- Wróblewski, M.; Wróblewska, W.; Sobiesiak, M. The Role of Selected Elements in Oxidative Stress Protection: Key to Healthy Fertility and Reproduction. Int. J. Mol. Sci. 2024, 25, 9409. [Google Scholar] [CrossRef]
- João, F.; Duval, C.; Bélanger, M.-C.; Lamoureux, J.; Xiao, C.W.; Ates, S.; Benkhalifa, M.; Miron, P. Reassessing the interpretation of oxidation–reduction potential in male infertility. Reprod. Fertil. 2022, 3, 67–76. [Google Scholar] [CrossRef]
- Office of Dietary Supplements—Carnitine. Available online: https://ods.od.nih.gov/factsheets/Carnitine-HealthProfessional/ (accessed on 2 February 2024).
- Wesselink, E.; Koekkoek, W.A.C.; Grefte, S.; Witkamp, R.F.; Van Zanten, A.R.H. Feeding mitochondria: Potential role of nutritional components to improve critical illness convalescence. Clin. Nutr. 2019, 38, 982–995. [Google Scholar] [CrossRef]
- Ma, L.; Sun, Y. Comparison of L-Carnitine vs. Coq10 and Vitamin E for idiopathic male infertility: A randomized controlled trial. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 4698–4704. [Google Scholar]
- Cheng, J.-B.; Zhu, J.; Ni, F.; Jiang, H. L-carnitine combined with coenzyme Q10 for idiopathic oligoasthenozoospermia: A double-blind randomized controlled trial. Zhonghua Nan Ke Xue Natl. J. Androl. 2018, 24, 33–38. [Google Scholar]
- Sicchieri, F.; Silva, A.B.; Santana, V.P.; Vasconcelos, M.A.C.; Ferriani, R.A.; Vireque, A.A.; dos Reis, R.M. Phosphatidylcholine and L-acetyl-carnitine-based freezing medium can replace egg yolk and preserves human sperm function. Transl. Androl. Urol. 2021, 10, 397–407. [Google Scholar] [CrossRef]
- Alahmar, A.T. Coenzyme Q10 improves sperm motility and antioxidant status in infertile men with idiopathic oligoasthenospermia. Clin. Exp. Reprod. Med. 2022, 49, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.; Bashiri, R.; Ghadiri-Anari, A.; Nadjarzadeh, A. Antioxidant supplements and semen parameters: An evidence based review. Int. J. Reprod. Biomed. 2016, 14, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Croxford, T.P.; McCormick, N.H.; Kelleher, S.L. Moderate Zinc Deficiency Reduces Testicular Zip6 and Zip10 Abundance and Impairs Spermatogenesis in Mice123. J. Nutr. 2011, 141, 359–365. [Google Scholar] [CrossRef]
- Yang, X.; Wang, H.; Huang, C.; He, X.; Xu, W.; Luo, Y.; Huang, K. Zinc enhances the cellular energy supply to improve cell motility and restore impaired energetic metabolism in a toxic environment induced by OTA. Sci. Rep. 2017, 7, 14669. [Google Scholar] [CrossRef]
- Almujaydil, M.S. The Role of Dietary Nutrients in Male Infertility: A Review. Life 2023, 13, 519. [Google Scholar] [CrossRef]
- Abbasihormozi, S.; Kouhkan, A.; Alizadeh, A.; Shahverdi, A.; Nasr-Esfahani, M.; Gilani, M.S.; Yazdi, R.S.; Matinibehzad, A.; Zolfaghari, Z. Association of vitamin D status with semen quality and reproductive hormones in Iranian subfertile men. Andrology 2017, 5, 113–118. [Google Scholar] [CrossRef]
- Hussein, T.M.; Eldabah, N.; Zayed, H.A.; Genedy, R.M. Assessment of serum vitamin D level and seminal vitamin D receptor gene methylation in a sample of Egyptian men with idiopathic infertility. Andrologia 2021, 53, e14172. [Google Scholar] [CrossRef]
- Moslemi, M.K.; Tavanbakhsh, S. Selenium–vitamin E supplementation in infertile men: Effects on semen parameters and pregnancy rate. Int. J. Gen. Med. 2011, 4, 99–104. [Google Scholar] [CrossRef]
- Xu, Z.-J.; Liu, M.; Niu, Q.-J.; Huang, Y.-X.; Zhao, L.; Lei, X.G.; Sun, L.-H. Both selenium deficiency and excess impair male reproductive system via inducing oxidative stress-activated PI3K/AKT-mediated apoptosis and cell proliferation signaling in testis of mice. Free Radic. Biol. Med. 2023, 197, 15–22. [Google Scholar] [CrossRef]
- Li, K.; Yang, X.; Wu, T. The Effect of Antioxidants on Sperm Quality Parameters and Pregnancy Rates for Idiopathic Male Infertility: A Network Meta-Analysis of Randomized Controlled Trials. Front. Endocrinol. 2022, 13, 810242. Available online: https://www.frontiersin.org/articles/10.3389/fendo.2022.810242 (accessed on 27 August 2023). [CrossRef]
- Abad, C.; Amengual, M.J.; Gosálvez, J.; Coward, K.; Hannaoui, N.; Benet, J.; García-Peiró, A.; Prats, J. Effects of oral antioxidant treatment upon the dynamics of human sperm DNA fragmentation and subpopulations of sperm with highly degraded DNA. Andrologia 2013, 45, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Cilio, S.; Rienzo, M.; Villano, G.; Mirto, B.F.; Giampaglia, G.; Capone, F.; Ferretti, G.; Di Zazzo, E.; Crocetto, F. Beneficial Effects of Antioxidants in Male Infertility Management: A Narrative Review. Oxygen 2022, 2, 1–11. [Google Scholar] [CrossRef]
- Jannatifar, R.; Asa, E.; Sahraei, S.S.; Verdi, A.; Piroozmanesh, H. N-acetyl-l-cysteine and alpha lipoic acid are protective supplement on human sperm parameters in cryopreservation of asthenoteratozoospermia patients. Andrologia 2022, 54, e14612. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; De Iuliis, G.N. On the possible origins of DNA damage in human spermatozoa. Mol. Hum. Reprod. 2010, 16, 3–13. [Google Scholar] [CrossRef]
- Hegde, M.L.; Izumi, T.; Mitra, S. Oxidized Base Damage and Single-Strand Break Repair in Mammalian Genomes: Role of Disordered Regions and Posttranslational Modifications in Early Enzymes. Prog. Mol. Biol. Transl. Sci. 2012, 110, 123–153. [Google Scholar]
- Kreuz, S.; Fischle, W. Oxidative Stress Signaling to Chromatin in Health and Disease. Epigenomics 2016, 8, 843–862. [Google Scholar] [CrossRef]
- Fice, H.E.; Robaire, B. Telomere Dynamics Throughout Spermatogenesis. Genes 2019, 10, 525. [Google Scholar] [CrossRef]
- Robinson, L.; Gallos, I.D.; Conner, S.J.; Rajkhowa, M.; Miller, D.; Lewis, S.; Kirkman-Brown, J.; Coomarasamy, A. The effect of sperm DNA fragmentation on miscarriage rates: A systematic review and meta-analysis. Hum. Reprod. 2012, 27, 2908–2917. [Google Scholar] [CrossRef]
- Sengul, M.; Hekim, N.; Asci, R.; Gunes, S. The impact of antioxidants on antioxidant capacity, DNA fragmentation, and chromatin quality in subfertile men: A randomized clinical trial study. Rev. Assoc. Med. Bras. 2024, 70, e20240211. [Google Scholar] [CrossRef]
- Kaltsas, A.; Zikopoulos, A.; Moustakli, E.; Zachariou, A.; Tsirka, G.; Tsiampali, C.; Palapela, N.; Sofikitis, N.; Dimitriadis, F. The Silent Threat to Women’s Fertility: Uncovering the Devastating Effects of Oxidative Stress. Antioxidants 2023, 12, 1490. [Google Scholar] [CrossRef]
- Mauchart, P.; Vass, R.A.; Nagy, B.; Sulyok, E.; Bódis, J.; Kovács, K. Oxidative Stress in Assisted Reproductive Techniques, with a Focus on an Underestimated Risk Factor. Curr. Issues Mol. Biol. 2023, 45, 1272–1286. [Google Scholar] [CrossRef] [PubMed]
- Zejnullahu, V.A.; Zejnullahu, V.A.; Kosumi, E. The role of oxidative stress in patients with recurrent pregnancy loss: A review. Reprod. Health 2021, 18, 207. [Google Scholar] [CrossRef] [PubMed]
- Deluao, J.C.; Winstanley, Y.; Robker, R.L.; Pacella-Ince, L.; Gonzalez, M.B.; McPherson, N.O. Oxidative Stress And Reproductive Function: Reactive Oxygen Species in the Mammalian Pre-Implantation Embryo. 2022. Available online: https://rep.bioscientifica.com/view/journals/rep/164/6/REP-22-0121.xml (accessed on 2 December 2024).
- Truong, T.; Gardner, D.K. Antioxidants improve IVF outcome and subsequent embryo development in the mouse. Hum. Reprod. 2017, 32, 2404–2413. [Google Scholar] [CrossRef] [PubMed]
- Scaruffi, P.; Licata, E.; Maccarini, E.; Massarotti, C.; Bovis, F.; Sozzi, F.; Stigliani, S.; Lago, A.D.; Casciano, I.; Rago, R.; et al. Oral Antioxidant Treatment of Men Significantly Improves the Reproductive Outcome of IVF Cycles. J. Clin. Med. 2021, 10, 3254. [Google Scholar] [CrossRef]
- Imamovic Kumalic, S.; Pinter, B. Review of Clinical Trials on Effects of Oral Antioxidants on Basic Semen and Other Parameters in Idiopathic Oligoasthenoteratozoospermia. BioMed Res. Int. 2014, 2014, e426951. [Google Scholar] [CrossRef]
- Agarwal, A.; Cannarella, R.; Saleh, R.; Harraz, A.M.; Kandil, H.; Salvio, G.; Boitrelle, F.; Kuroda, S.; Farkouh, A.; Rambhatla, A.; et al. Impact of Antioxidant Therapy on Natural Pregnancy Outcomes and Semen Parameters in Infertile Men: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. World J. Mens Health 2023, 41, 14–48. [Google Scholar] [CrossRef]
- Smits, R.M.; Mackenzie-Proctor, R.; Yazdani, A.; Stankiewicz, M.T.; Jordan-Cole, V.; Showell, M. Antioxidants for Male Subfertility: A Systematic Review and Meta-Analysis; Oxford University Press (OUP): Oxford, UK, 2019; Available online: https://researchspace.auckland.ac.nz/handle/2292/48726 (accessed on 4 February 2024).
- Hönscher, C.; Mari, M.; Auffarth, K.; Bohnert, M.; Griffith, J.; Geerts, W.; van der Laan, M.; Cabrera, M.; Reggiori, F.; Ungermann, C. Cellular Metabolism Regulates Contact Sites between Vacuoles and Mitochondria. Dev. Cell 2014, 30, 86–94. [Google Scholar] [CrossRef]
- Hughes, C.E.; Coody, T.K.; Jeong, M.-Y.; Berg, J.A.; Winge, D.R.; Hughes, A.L. Cysteine Toxicity Drives Age-Related Mitochondrial Decline by Altering Iron Homeostasis. Cell 2020, 180, 296–310.e18. [Google Scholar] [CrossRef]
- Helm, M.M.; Alaba, T.; Klimis-Zacas, D.; Izuora, K.; Basu, A. Effect of Dietary Berry Supplementation on Antioxidant Biomarkers in Adults with Cardiometabolic Risks: A Systematic Review of Clinical Trials. Antioxidants 2023, 12, 1182. [Google Scholar] [CrossRef]
- Rago, V.; Di Agostino, S. Novel Insights into the Role of the Antioxidants in Prostate Pathology. Antioxidants 2023, 12, 289. [Google Scholar] [CrossRef]
- Nateghian, Z.; Nasr-Esfahani, M.H.; Talaei-Khozani, T.; Tavalaee, M.; Aliabadi, E. L-Carnitine and Pentoxifylline Supplementation Improves Sperm Viability and Motility at Low Temperature. Int. J. Fertil. Steril. 2023, 17, 61–66. [Google Scholar] [PubMed]
- Vickram, A.; Samad, H.A.; Latheef, S.K.; Chakraborty, S.; Dhama, K.; Sridharan, T.; Sundaram, T.; Gulothungan, G. Human prostasomes an extracellular vesicle—Biomarkers for male infertility and prostrate cancer: The journey from identification to current knowledge. Int. J. Biol. Macromol. 2020, 146, 946–958. [Google Scholar] [CrossRef]
- de Freitas Laiber Pascoal, G.; Geraldi, M.V.; Maróstica, M.R.; Ong, T.P. Effect of Paternal Diet on Spermatogenesis and Offspring Health: Focus on Epigenetics and Interventions with Food Bioactive Compounds. Nutrients 2022, 14, 2150. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhou, H.; Qi, S.; Yang, H.; Hong, X. The association between physical activities combined with dietary habits and cardiovascular risk factors. Heliyon 2024, 10, e28845. [Google Scholar] [CrossRef] [PubMed]
- Scully, T.; Ettela, A.; LeRoith, D.; Gallagher, E.J. Obesity, Type 2 Diabetes, and Cancer Risk. Front. Oncol. 2021, 10, 615375. [Google Scholar] [CrossRef] [PubMed]
- Mai, H.; Ke, J.; Zheng, Z.; Luo, J.; Li, M.; Qu, Y.; Jiang, F.; Cai, S.; Zuo, L. Association of diet and lifestyle factors with semen quality in male partners of Chinese couples preparing for pregnancy. Reprod. Health 2023, 20, 173. [Google Scholar] [CrossRef]
- Tunc, O.; Thompson, J.; Tremellen, K. Improvement in sperm DNA quality using an oral antioxidant therapy. Reprod. BioMedicine Online 2009, 18, 761–768. [Google Scholar] [CrossRef]
- Juanpanich, T.; Suttirojpattana, T.; Parnpai, R.; Vutyavanich, T. The relationship between reactive oxygen species, DNA fragmentation, and sperm parameters in human sperm using simplified sucrose vitrification with or without triple antioxidant supplementation. Clin. Exp. Reprod. Med. 2022, 49, 117–126. [Google Scholar] [CrossRef]
- Kacem, O.; Harzallah, M.; Zedini, C.; Zidi, I.; Meddeb, S.; Fékih, M.; Saidi, H.; Chaib, A.; Boughizane, S.; Ben Ali, H.; et al. Beneficial Effect of an Oral Antioxidant Supplementation (Fertimax2) on IVF-ICSI Outcomes: A Preliminary Clinical Study. Adv. Reprod. Sci. 2014, 2, 47–56. [Google Scholar] [CrossRef]
- Wang, R.C.; Wang, Z. Precision Medicine: Disease Subtyping and Tailored Treatment. Cancers 2023, 15, 3837. [Google Scholar] [CrossRef]
- Sutton, M.Y.; Anachebe, N.F.; Lee, R.; Skanes, H. Racial and Ethnic Disparities in Reproductive Health Services and Outcomes, 2020. Obstet. Gynecol. 2021, 137, 225–233. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lahimer, M.; Capelle, S.; Lefranc, E.; Bosquet, D.; Kazdar, N.; Ledu, A.; Agina, M.; Cabry, R.; BenKhalifa, M. Micronutrient–Antioxidant Therapy and Male Fertility Improvement During ART Cycles. Nutrients 2025, 17, 324. https://doi.org/10.3390/nu17020324
Lahimer M, Capelle S, Lefranc E, Bosquet D, Kazdar N, Ledu A, Agina M, Cabry R, BenKhalifa M. Micronutrient–Antioxidant Therapy and Male Fertility Improvement During ART Cycles. Nutrients. 2025; 17(2):324. https://doi.org/10.3390/nu17020324
Chicago/Turabian StyleLahimer, Marwa, Severine Capelle, Elodie Lefranc, Dorian Bosquet, Nadia Kazdar, Anne Ledu, Mounir Agina, Rosalie Cabry, and Moncef BenKhalifa. 2025. "Micronutrient–Antioxidant Therapy and Male Fertility Improvement During ART Cycles" Nutrients 17, no. 2: 324. https://doi.org/10.3390/nu17020324
APA StyleLahimer, M., Capelle, S., Lefranc, E., Bosquet, D., Kazdar, N., Ledu, A., Agina, M., Cabry, R., & BenKhalifa, M. (2025). Micronutrient–Antioxidant Therapy and Male Fertility Improvement During ART Cycles. Nutrients, 17(2), 324. https://doi.org/10.3390/nu17020324








