Brussels Chicory Enhances Exhaustive Aerobic Exercise Performance and Post-Exercise Recovery, Possibly Through Promotion of Lactate Oxidation: A Pilot Randomized, Single-Blind, Placebo-Controlled, Two-Way Crossover Study
Abstract
1. Introduction
2. Material and Methods
2.1. Study Participants
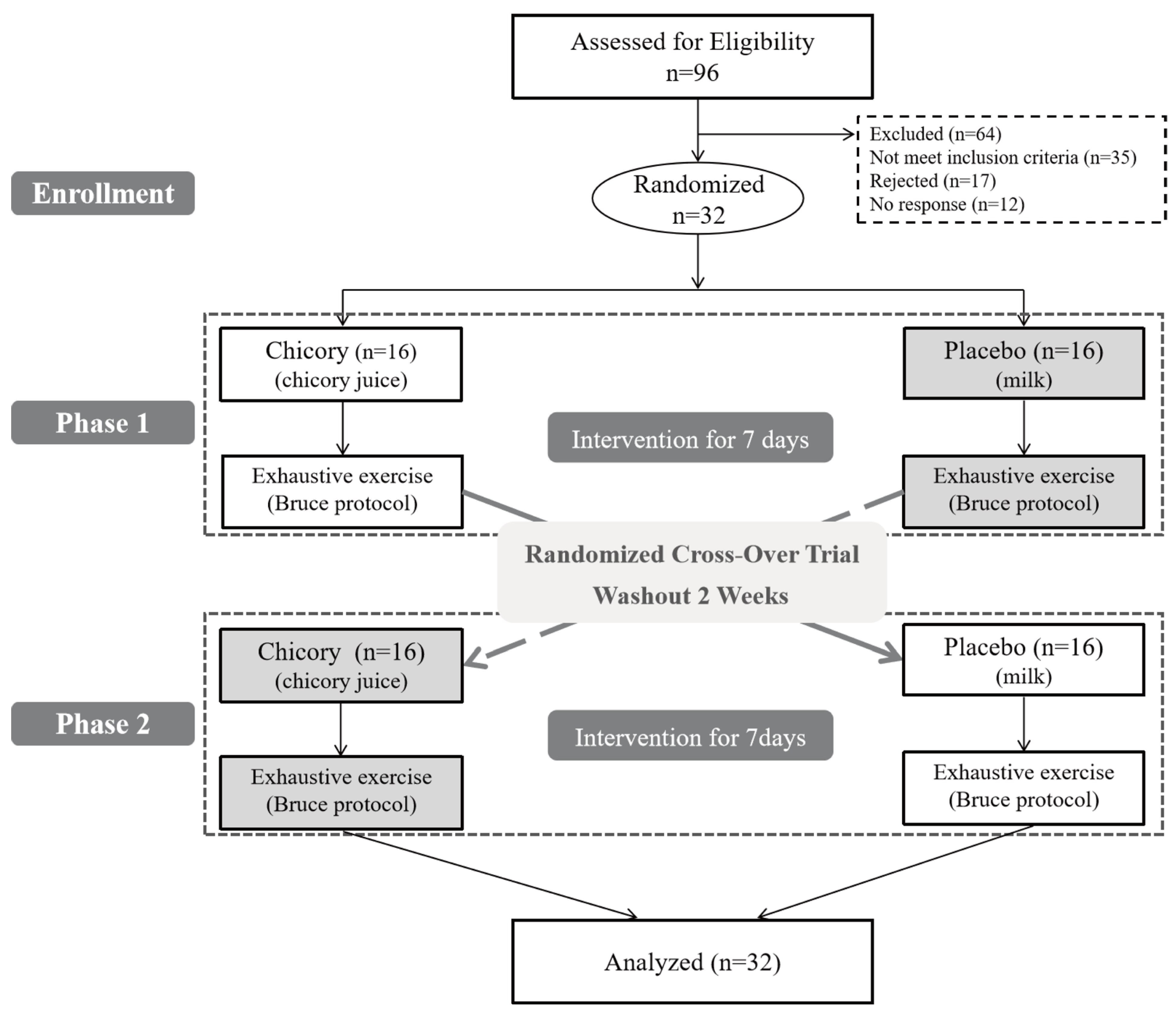
2.2. Randomization and Blinding
2.3. Study Design and Trial Protocol
2.4. Outcome Measurement and Sample Size Calculation
2.5. Sociodemographic Data Collection and Anthropometric Assessments
2.6. High-Intensity Exhaustive Aerobic Exercise from the Bruce Protocol
2.7. Phenolic Acids in Brussels Chicory and Serum Samples
2.8. Cell Culture
2.9. Cell Viability
2.10. Measurements of Intracellular and Conditioned Medium Lactate Concentrations
2.11. Seahorse Oxidative and Glycolytic Metabolic Assay
2.12. Isolation of Mitochondria
2.13. Western Blot
2.14. RNA Preparation and Quantitative Real-Time PCR (qRT-PCR) Analysis
2.15. Small Interfering RNA (siRNA) Strategy
2.16. Lactate Oxidation Assay
2.17. Lactate Dehydrogenase Activity
2.18. Statistical Analyses
3. Results
3.1. Participants’Characteristics
3.2. Brussels Chicory Consumption Increased Time to Exhaustion During Exhaustive Aerobic Exercise in Healthy Participants
3.3. Brussels Chicory Consumption Promoted Post-Exercise Recovery in Healthy Participants
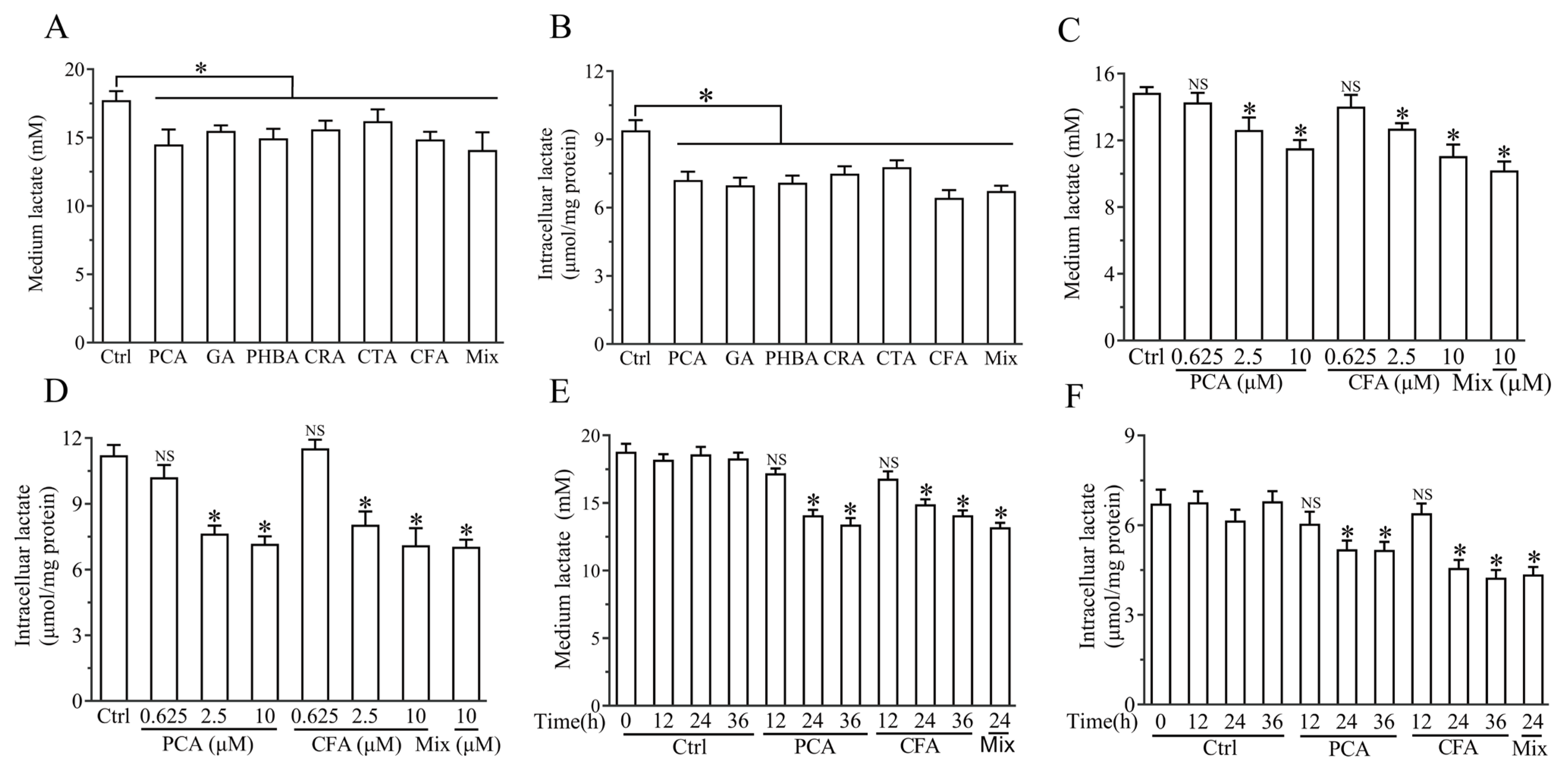
3.4. Brussels Chicory Phenolic Acids Improved Lactate Metabolism in C2C12 Myotubes
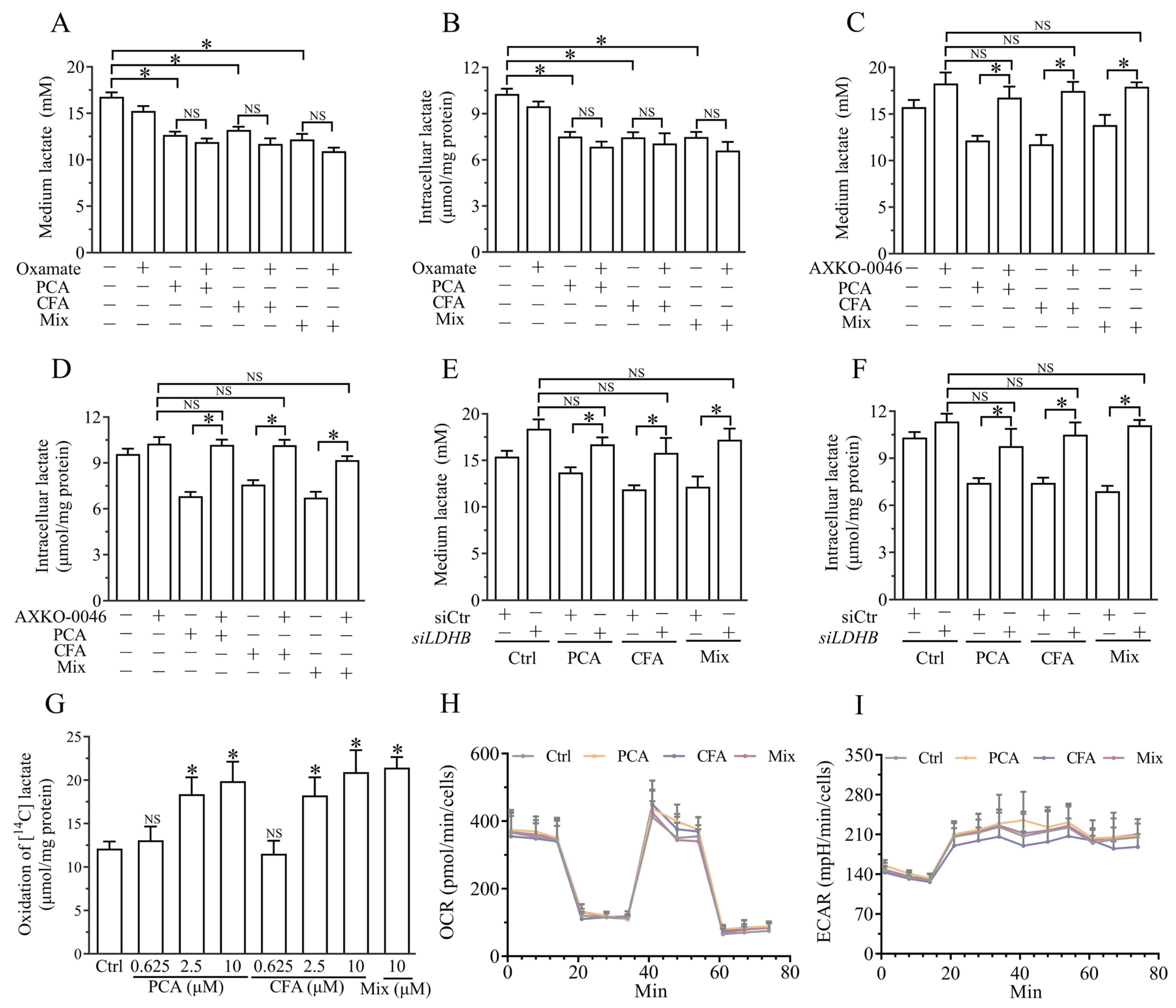
3.5. Brussels Chicory Phenolic Acids Promoted Lactate Clearance by Targeting LDHB-Mediated Lactate Oxidation
3.6. Brussels Chicory Phenolic Acids Increased Mitochondria LDHB Without Affecting Its Expression and Then Promoted Lactate Clearance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. American College of Sports Medicine Joint Position Statement. Nutrition and Athletic Performance. Med. Sci. Sports Exerc. 2016, 48, 543–568. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Kempe, M.; Brink, M.; Lemmink, K. Effectiveness of Recovery Strategies After Training and Competition in Endurance Athletes: An Umbrella Review. Sports Med. Open 2024, 10, 55. [Google Scholar] [CrossRef]
- McCartney, D.; Desbrow, B.; Irwin, C. Post-exercise Ingestion of Carbohydrate, Protein and Water: A Systematic Review and Meta-analysis for Effects on Subsequent Athletic Performance. Sports Med. 2018, 48, 379–408. [Google Scholar] [CrossRef] [PubMed]
- Cairns, S.P. Lactic acid and exercise performance: Culprit or friend? Sports Med. 2006, 36, 279–291. [Google Scholar] [CrossRef]
- Lancha Junior, A.H.; Painelli, V.d.S.; Saunders, B.; Artioli, G.G. Nutritional Strategies to Modulate Intracellular and Extracellular Buffering Capacity During High-Intensity Exercise. Sports Med. 2015, 45 (Suppl. S1), S71–S81. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.G.; Lamb, G.D.; Westerblad, H. Skeletal muscle fatigue: Cellular mechanisms. Physiol. Rev. 2008, 88, 287–332. [Google Scholar] [CrossRef] [PubMed]
- Akinci, B.; Zenginler Yazgan, Y.; Altinoluk, T. The effectiveness of three different recovery methods on blood lactate, acute muscle performance, and delayed-onset muscle soreness: A randomized comparative study. J. Sports Med. Phys. Fit. 2020, 60, 345–354. [Google Scholar] [CrossRef]
- Brooks, G.A. Lactate as a fulcrum of metabolism. Redox Biol. 2020, 35, 101454. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A. Lactate: Link between glycolytic and oxidative metabolism. Sports Med. 2007, 37, 341–343. [Google Scholar] [CrossRef]
- Morris, D. Effects of oral lactate consumption on metabolism and exercise performance. Curr. Sports Med. Rep. 2012, 11, 185–188. [Google Scholar] [CrossRef]
- Morris, D.; Beloni, R.; Wofford, H. Metabolic and exercise performance responses to two different oral doses of calcium lactate. Med. Sci. Sports Exerc. 2016, 48, 250. [Google Scholar] [CrossRef]
- Bordoli, C.; Varley, I.; Sharpe, G.R.; Johnson, M.A.; Hennis, P.J. Effects of Oral Lactate Supplementation on Acid–Base Balance and Prolonged High-Intensity Interval Cycling Performance. J. Funct. Morphol. Kinesiol. 2024, 9, 139. [Google Scholar] [CrossRef]
- Fahey, T.D.; Larsen, J.D.; Brooks, G.A.; Colvin, W.; Henderson, S.; Lary, D. The effects of ingesting polylactate or glucose polymer drinks during prolonged exercise. Int. J. Sport Nutr. 1991, 1, 249–256. [Google Scholar] [CrossRef]
- Morris, D.M.; Shafer, R.S.; Fairbrother, K.R.; Woodall, M.W. Effects of lactate consumption on blood bicarbonate levels and performance during high-intensity exercise. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Russ, A.E.; Schifino, A.G.; Leong, C.-H.J.A.G. Effect of lactate supplementation on VO2peak and onset of blood lactate accumulation: A double-blind, placebo-controlled trial. Acta Gymnica 2019, 49, 51–57. [Google Scholar] [CrossRef]
- Oliveira, L.F.; Painelli, V.d.S.; Nemezio, K.; Gonçalves, L.S.; Yamaguchi, G.; Saunders, B.; Gualano, B.; Artioli, G.G. Chronic lactate supplementation does not improve blood buffering capacity and repeated high-intensity exercise. Scand. J. Med. Sci. Sports 2017, 27, 1231–1239. [Google Scholar] [CrossRef]
- Summermatter, S.; Santos, G.; Pérez-Schindler, J.; Handschin, C. Skeletal muscle PGC-1α controls whole-body lactate homeostasis through estrogen-related receptor α-dependent activation of LDH B and repression of LDH A. Proc. Natl. Acad. Sci. USA 2013, 110, 8738–8743. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Hussien, R.; Brooks, G.A. Colocalization of MCT1, CD147, and LDH in mitochondrial inner membrane of L6 muscle cells: Evidence of a mitochondrial lactate oxidation complex. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E1237–E1244. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Vitaglione, P.; Mennella, I.; Ferracane, R.; Rivellese, A.A.; Giacco, R.; Ercolini, D.; Gibbons, S.M.; La Storia, A.; Gilbert, J.A.; Jonnalagadda, S.; et al. Whole-grain wheat consumption reduces inflammation in a randomized controlled trial on overweight and obese subjects with unhealthy dietary and lifestyle behaviors: Role of polyphenols bound to cereal dietary fiber. Am. J. Clin. Nutr. 2015, 101, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Ortega, D.R.; López, A.M.; Amaya, H.M.; de la Rosa, F.J.B. Tart cherry and pomegranate supplementations enhance recovery from exercise-induced muscle damage: A systematic review. Biol. Sport 2021, 38, 97–111. [Google Scholar] [CrossRef]
- Ostojic, S.M.; Stojanovic, M.D.; Djordjevic, B.; Jourkesh, M.; Vasiljevic, N. The effects of a 4-week coffeeberry supplementation on antioxidant status, endurance, and anaerobic performance in college athletes. Res. Sports Med. 2008, 16, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Lowery, L.M.; Anderson, D.E.; Scanlon, K.F.; Stack, A.; Escalante, G.; Campbell, S.C.; Kerksick, C.M.; Nelson, M.T.; Ziegenfuss, T.N.; VanDusseldorp, T.A.; et al. International society of sports nutrition position stand: Coffee and sports performance. J. Int. Soc. Sports Nutr. 2023, 20, 2237952. [Google Scholar] [CrossRef]
- Bowtell, J.; Kelly, V. Fruit-Derived Polyphenol Supplementation for Athlete Recovery and Performance. Sports Med. 2019, 49, 3–23. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, X.; Sha, M.; Feng, Z.; Liu, Y.J.F.B. Natural bioactive flavonoids as promising agents in alleviating exercise-induced fatigue. Food Biosci. 2023, 51, 102360. [Google Scholar] [CrossRef]
- Perović, J.; Tumbas Šaponjac, V.; Kojić, J.; Krulj, J.; Moreno, D.A.; García-Viguera, C.; Bodroža-Solarov, M.; Ilić, N. Chicory (Cichorium intybus L.) as a food ingredient—Nutritional composition, bioactivity, safety, and health claims: A review. Food Chem. 2021, 336, 127676. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.A.; Weir, T.L. Fresh Take on the Relationship between Diet, Gut Microbiota, and Atherosclerosis: A Food-Based Approach with Brussels Chicory. J. Nutr. 2022, 152, 2181–2183. [Google Scholar] [CrossRef]
- Wang, D.; Wei, X.; Yan, X.; Jin, T.; Ling, W. Protocatechuic acid, a metabolite of anthocyanins, inhibits monocyte adhesion and reduces atherosclerosis in apolipoprotein E-deficient mice. J. Agric. Food Chem. 2010, 58, 12722–12728. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, X.; Du, Y.; Zhang, X.; Xiang, P.; Chen, G.; Ling, W.; Wang, D. Protocatechuic acid boosts continual efferocytosis in macrophages by derepressing KLF4 to transcriptionally activate MerTK. Sci. Signal 2023, 16, eabn1372. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Liu, C.; Yang, H.; Wang, W.; Ling, W.; Wang, D. Chicory, a typical vegetable in Mediterranean diet, exerts a therapeutic role in established atherosclerosis in apolipoprotein E-deficient mice. Mol. Nutr. Food Res. 2015, 59, 1803–1813. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, X.; Du, Y.; Liu, X.; Chen, G.; Xiang, P.; Wu, H.; Liu, C.; Wang, D. Brussels Chicory Stabilizes Unstable Atherosclerotic Plaques and Reshapes the Gut Microbiota in Apoe−/− Mice. J. Nutr. 2022, 152, 2209–2217. [Google Scholar] [CrossRef]
- Sasaki, K.; Iwata, N.; Ferdousi, F.; Isoda, H. Antidepressant-Like Effect of Ferulic Acid via Promotion of Energy Metabolism Activity. Mol. Nutr. Food Res. 2019, 63, e1900327. [Google Scholar] [CrossRef] [PubMed]
- Xiang, P.; Du, Y.; Chen, G.; Mao, Y.; Li, S.; Li, Q.; Yang, Y.; Li, X.; Wang, D. Dietary Achievable Dose of Protocatechuic Acid, a Metabolite of Flavonoids, Inhibits High-Fat Diet-Induced Obesity in Mice. Mol. Nutr. Food Res. 2024, 68, e2300451. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Huang, R.; Wang, Y.; Han, S.; Qin, X.; Li, Z.; Wang, X.; Han, Y.; Wang, T.; Xia, B.J.M.n.; et al. Protocatechuic Acid Ameliorates High Fat Diet-Induced Obesity and Insulin Resistance in Mice. Mol. Nutr. Food Res. 2023, 67, 2200244. [Google Scholar] [CrossRef] [PubMed]
- Matacchione, G.; Gurău, F.; Baldoni, S.; Prattichizzo, F.; Silvestrini, A.; Giuliani, A.; Pugnaloni, A.; Espinosa, E.; Amenta, F.; Bonafè, M.; et al. Pleiotropic effects of polyphenols on glucose and lipid metabolism: Focus on clinical trials. Ageing Res. Rev. 2020, 61, 101074. [Google Scholar] [CrossRef]
- Wang, D.; Xia, M.; Yan, X.; Li, D.; Wang, L.; Xu, Y.; Jin, T.; Ling, W. Gut microbiota metabolism of anthocyanin promotes reverse cholesterol transport in mice via repressing miRNA-10b. Circ. Res. 2012, 111, 967–981. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, G.; He, M.; Pan, X.; Wang, Z.; Yang, J.; Zi, L. China Food Composition List, 6th ed.; Peking University Medical Press: Beijing, China, 2018. [Google Scholar]
- Park, C.H.; Kwak, Y.S.; Seo, H.K.; Kim, H.Y. Assessing the values of blueberries intake on exercise performance, TAS, and inflammatory factors. Iran. J. Public Health 2018, 47, 27. [Google Scholar] [PubMed]
- Hallal, P.C.; Victora, C.G. Reliability and validity of the International Physical Activity Questionnaire (IPAQ). Med. Sci. Sports Exerc. 2004, 36, 556. [Google Scholar] [CrossRef]
- National Institute of Nutrition and Food Safety Center for Disease Control and Prevention. China Food Composition; Peking University Medical Press: Beijing, China, 2002. [Google Scholar]
- Asad, M.R.; Arazı, H.; Poormohammad, N.; Gholızadeh, R. The Effects of Cherry Juice Supplementation on Antioxidant Capacity, Hydrogen Peroxide and Creatine Kinase Following an Exhaustive Aerobic Exercise in Non-Athlete Men. Int. J. Sport Cult. Sci. 2018, 6, 40–47. [Google Scholar] [CrossRef]
- Marshall, M.R.; Coe, D.P.; Pivarnik, J.M. Development of a prediction model to predict VO2peak in adolescent girls using the Bruce protocol to exhaustion. Res. Q. Exerc. Sport 2014, 85, 251–256. [Google Scholar] [CrossRef]
- Zheng, J.; Xiong, H.; Li, Q.; He, L.; Weng, H.; Ling, W.; Wang, D. Protocatechuic acid from chicory is bioavailable and undergoes partial glucuronidation and sulfation in healthy humans. Food Sci. Nutr. 2019, 7, 3071–3080. [Google Scholar] [CrossRef] [PubMed]
- Biswas, G.; Adebanjo, O.A.; Freedman, B.D.; Anandatheerthavarada, H.K.; Vijayasarathy, C.; Zaidi, M.; Kotlikoff, M.; Avadhani, N.G. Retrograde Ca2+ signaling in C2C12 skeletal myocytes in response to mitochondrial genetic and metabolic stress: A novel mode of inter-organelle crosstalk. EMBO J. 1999, 18, 522–533. [Google Scholar] [CrossRef] [PubMed]
- Wensaas, A.J.; Rustan, A.C.; Lövstedt, K.; Kull, B.; Wikström, S.; Drevon, C.A.; Hallén, S. Cell-based multiwell assays for the detection of substrate accumulation and oxidation. J. Lipid Res. 2007, 48, 961–967. [Google Scholar] [CrossRef]
- Presti, N.; Mansouri, T.; Maloney, M.K.; Hostler, D. The Impact Plant-Based Diets Have on Athletic Performance and Body Composition: A Systematic Review. J. Am. Nutr. Assoc. 2024, 43, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Billat, V.; Hamard, L.; Koralsztein, J.P.; Morton, R.H.J.J.o.A.P. Differential modeling of anaerobic and aerobic metabolism in the 800-m and 1,500-m run. J. Appl. Physiol. 2009, 107, 478–487. [Google Scholar] [CrossRef]
- de Matos, C.C.; Marinho, D.A.; Duarte-Mendes, P.; de Souza Castro, F.A. VO2 kinetics and bioenergetic responses to sets performed at 90%, 92.5%, and 95% of 400-m front crawl speed in male swimmers. Sport Sci. Health. 2022, 18, 1321–1329. [Google Scholar] [CrossRef]
- Rodríguez, F.A.; Mader, A. Energy systems in swimming. In World Book of Swimming: From Science to Performance; Nova Science Publishers, Inc: New York, NY, USA, 2011; Volume 225. [Google Scholar]
- Li, Q.; Liu, X.; Zhang, X.; Du, Y.; Chen, G.; Xiang, P.; Ling, W.; Wang, D. Terpene Lactucopicrin Limits Macrophage Foam Cell Formation by a Reduction of Lectin-Like Oxidized Low-Density Lipoprotein Receptor-1 in Lipid Rafts. Mol. Nutr. Food Res. 2022, 66, e2100905. [Google Scholar] [CrossRef]
- Weng, H.; He, L.; Liu, X.; Li, Q.; Du, Y.; Zheng, J.; Wang, D. Natural lactucopicrin alleviates importin-α3-mediated NF-κB activation in inflammated endothelial cells and improves sepsis in mice. Biochem. Pharmacol. 2021, 186, 114501. [Google Scholar] [CrossRef]
- Chang, W.-H.; Hu, S.-P.; Huang, Y.-F.; Yeh, T.-S.; Liu, J.-F. Effect of purple sweet potato leaves consumption on exercise-induced oxidative stress and IL-6 and HSP72 levels. J. Appl. Physiol. 2010, 109, 1710–1715. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Wang, W.; Yang, H.; Wang, D.; Ling, W. Influence of Intestinal Microbiota on the Catabolism of Flavonoids in Mice. J. Food Sci. 2016, 81, H3026–H3034. [Google Scholar] [CrossRef]
- Jaganath, I.B.; Mullen, W.; Lean, M.E.J.; Edwards, C.A.; Crozier, A. In vitro catabolism of rutin by human fecal bacteria and the antioxidant capacity of its catabolites. Free Radic. Biol. Med. 2009, 47, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, G.; Cumming, K.T.; Holden, G.; Hallén, J.; Rønnestad, B.R.; Sveen, O.; Skaug, A.; Paur, I.; Bastani, N.E.; Østgaard, H.N.; et al. Vitamin C and E supplementation hampers cellular adaptation to endurance training in humans: A double-blind, randomised, controlled trial. J. Physiol. 2014, 592, 1887–1901. [Google Scholar] [CrossRef] [PubMed]
- Rogers, D.R.; Lawlor, D.J.; Moeller, J.L. Vitamin C Supplementation and Athletic Performance: A Review. Curr. Sports Med. Rep. 2023, 22, 255–259. [Google Scholar] [CrossRef]
- Nikolaidis, M.G.; Kerksick, C.M.; Lamprecht, M.; McAnulty, S.R. Does vitamin C and E supplementation impair the favorable adaptations of regular exercise? Oxidative Med. Cell. Longev. 2012, 2012, 707941. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, M.; Spriet, L.L. Skeletal muscle energy metabolism during exercise. Nat. Metab. 2020, 2, 817–828. [Google Scholar] [CrossRef]
- Liu, C.; Wang, W.; Lin, W.; Ling, W.; Wang, D. Established atherosclerosis might be a prerequisite for chicory and its constituent protocatechuic acid to promote endothelium-dependent vasodilation in mice. Mol. Nutr. Food Res. 2016, 60, 2141–2150. [Google Scholar] [CrossRef]
- Masodsai, K.; Lin, Y.-Y.; Chaunchaiyakul, R.; Su, C.-T.; Lee, S.-D.; Yang, A.-L. Twelve-Week Protocatechuic Acid Administration Improves Insulin-Induced and Insulin-Like Growth Factor-1-Induced Vasorelaxation and Antioxidant Activities in Aging Spontaneously Hypertensive Rats. Nutrients 2019, 11, 699. [Google Scholar] [CrossRef] [PubMed]
- Dalle, S.; Koppo, K.; Hespel, P. Sodium bicarbonate improves sprint performance in endurance cycling. J. Sci. Med. Sport 2021, 24, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.M.; Pyne, D.B. Bicarbonate loading to enhance training and competitive performance. Int. J. Sports Physiol. Perform. 2007, 2, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Horswill, C.A. Effects of bicarbonate, citrate, and phosphate loading on performance. Int. J. Sport. Nutr. 1995, 5, S111–S119. [Google Scholar] [CrossRef]
- Cook, M.D.; Myers, S.D.; Blacker, S.D.; Willems, M.E.T. New Zealand blackcurrant extract improves cycling performance and fat oxidation in cyclists. Eur. J. Appl. Physiol. 2015, 115, 2357–2365. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.-K.; Shin, Y.-O.; Yoon, J.-H.; Kim, S.H.; Shin, H.-C.; Hwang, H.J. Effect of supplementation with Ecklonia cava polyphenol on endurance performance of college students. Int. J. Sport Nutr. Exerc. Metab. 2010, 20, 72–79. [Google Scholar] [CrossRef]
- Yarahmadi, M.; Askari, G.; Kargarfard, M.; Ghiasvand, R.; Hoseini, M.; Mohamadi, H.; Asadi, A. The effect of anthocyanin supplementation on body composition, exercise performance and muscle damage indices in athletes. Int. J. Prev. Med. 2014, 5, 1594–1600. [Google Scholar] [PubMed]
- Liang, X.; Liu, L.; Fu, T.; Zhou, Q.; Zhou, D.; Xiao, L.; Liu, J.; Kong, Y.; Xie, H.; Yi, F.; et al. Exercise Inducible Lactate Dehydrogenase B Regulates Mitochondrial Function in Skeletal Muscle. J. Biol. Chem. 2016, 291, 25306–25318. [Google Scholar] [CrossRef]
- Sinkovič, L.; Demšar, L.; Žnidarčič, D.; Vidrih, R.; Hribar, J.; Treutter, D. Phenolic profiles in leaves of chicory cultivars (Cichorium intybus L.) as influenced by organic and mineral fertilizers. Food Chem. 2015, 166, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Qadir, O.; Siervo, M.; Seal, C.J.; Brandt, K. Manipulation of Contents of Nitrate, Phenolic Acids, Chlorophylls, and Carotenoids in Lettuce (Lactuca sativa L.) via Contrasting Responses to Nitrogen Fertilizer When Grown in a Controlled Environment. J. Agric. Food Chem. 2017, 65, 10003–10010. [Google Scholar] [CrossRef] [PubMed]
- Billat, V.; Renoux, J.C.; Pinoteau, J.; Petit, B.; Koralsztein, J.P. Reproducibility of running time to exhaustion at VO2max in subelite runners. Med. Sci. Sports Exerc. 1994, 26, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Coggan, A.R.; Costill, D.L. Biological and technological variability of three anaerobic ergometer tests. Int. J. Sports Med. 1984, 5, 142–145. [Google Scholar] [CrossRef]



| Stages | Speed Mph (km/h) | Grade (%) | Duration (min) |
|---|---|---|---|
| Warm-up | 1.7 (2.7) | 0 | 3 |
| Warm-up | 1.7 (2.7) | 5 | 3 |
| 1 | 1.7 (2.7) | 10 | 3 |
| 2 | 2.5 (4.0) | 12 | 3 |
| 3 | 3.4 (5.4) | 14 | 3 |
| 4 | 4.2 (6.7) | 16 | 3 |
| 5 | 5.0 (8.0) | 18 | 3 |
| 6 | 5.5 (8.8) | 20 | 3 |
| 7 | 6.0 (9.6) | 22 | 3 |
| Characteristics | Placebo Then Brussels Chicory (n = 16) | Brussels Chicory Then Placebo (n = 16) | p b |
|---|---|---|---|
| Age (years) | 24.31 ± 3.52 | 22.37 ± 2.22 | 0.097 |
| Gender | 0.432 | ||
| Women [n (%)] | 7 (44.75%) | 7 (44.75%) | |
| Men [n (%)] | 9 (56.25%) | 9 (56.25%) | |
| Weight (kg) | 62.95 ± 6.13 | 65.63 ± 6.85 | 0.056 |
| Body mass index (kg/m2) | 21.60 ± 1.78 | 22.67 ± 1.54 | 0.213 |
| Percentage body fat (%) | 17.23 ± 4.62 | 20.44 ± 5.83 | 0.069 |
| Systolic blood pressure (mmHg) | 114.95 ± 5.51 | 118.05 ± 6.96 | 0.369 |
| Diastolic blood pressure (mmHg) | 72.85 ± 4.97 | 76.72 ± 5.54 | 0.082 |
| Heart rate (beats/min) | 73.15 ± 6.03 | 72.43 ± 8.15 | 0.724 |
| Physical activity level (MET-min/w) | 1802.27 ± 494.45 | 2132.12 ± 505.77 | 0.432 |
| Walking (MET-min/w) | 808.09 (724.10, 995.17) | 802.45 (620.46, 953.57) | 0.386 |
| MPA c (MET-min/w) | 801.07 (526.18, 890.67) | 581.21 (451.82, 702.17) | 0.142 |
| VPA d (MET-min/w) | 458.42 (283.43, 640.83) | 326.90 (217.93, 541.82) | 0.152 |
| Energy (kcal/day) | 1963.32 ± 266.55 | 2047.86 ± 262.91 | 0.102 |
| Carbohydrates (g/day) | 312.67 ± 57.82 | 349.45 ± 56.93 | 0.200 |
| Proteins (g/day) | 64.33 ± 11.58 | 69.28 ± 10.49 | 0.445 |
| Fat (g/day) | 48.64 ± 11.33 | 54.86 ± 12.44 | 0.521 |
| 0 min Post-Exercise | 60 min Post-Exercise | p c | p d | p e | |||
|---|---|---|---|---|---|---|---|
| Placebo | Chicory b | Placebo | Chicory | ||||
| IL-6 pg/mL | 2.84 ± 1.43 | 2.68 ± 1.24 | 3.08 ± 1.36 | 2.82 ± 1.54 | 0.424 | 0.623 | 0.829 |
| IL-8 pg/mL | 3.21 ± 1.23 | 2.91 ± 1.52 | 3.30 ± 1.98 | 3.09 ± 2.11 | 0.459 | 0.684 | 0.878 |
| CRP mg/dL | 0.75 ± 0.43 | 0.63 ± 0.38 | 0.92 ± 0.65 | 0.77 ± 0.45 | 0.389 | 0.054 | 0.852 |
| SOD activity mU/mg prot | 0.43 ± 0.16 | 0.56 ± 0.20 | 0.41 ± 0.19 | 0.49 ± 0.28 | 0.052 | 0.266 | 0.428 |
| GSH-PX activity mU/mg prot | 8.84 ± 3.43 | 9.68 ± 3.24 | 9.08 ± 4.36 | 10.82 ± 4.54 | 0.171 | 0.445 | 0.583 |
| CAT activity mU/mg prot | 44.51 ± 17.08 | 48.48 ± 21.44 | 46.57 ± 21.07 | 53.56 ± 24.06 | 0.170 | 0.525 | 0.715 |
| 0 min Post-Exercise | 60 min Post-Exercise | p c | p d | p e | |||
|---|---|---|---|---|---|---|---|
| Placebo | Chicory b | Placebo | Chicory | ||||
| IL-6 pg/mL | 2.44 ± 1.28 | 1.93 ± 1.23 | 2.01 ± 1.20 | 1.94 ± 1.72 | 0.352 | 0.630 | 0.538 |
| IL-8 pg/mL | 3.10 ± 1.65 | 2.26 ± 1.77 | 3.23 ± 1.67 | 2.55 ± 1.22 | 0.055 | 0.713 | 0.841 |
| CRP mg/dL | 1.04 ± 0.65 | 0.85 ± 0.16 | 1.16 ± 0.70 | 1.18 ± 0.63 | 0.439 | 0.266 | 0.252 |
| SOD activity mU/mg prot | 0.49 ± 0.29 | 0.63 ± 0.33 | 0.44 ± 0.25 | 0.58 ± 0.18 | 0.051 | 0.989 | 0.437 |
| GSH-PX activity mU/mg prot | 10.44 ± 4.28 | 8.93 ± 3.23 | 9.61 ± 2.20 | 9.94 ± 3.72 | 0.270 | 0.941 | 0.187 |
| CAT activity mU/mg prot | 56.47 ± 26.09 | 50.44 ± 19.13 | 52.49 ± 19.05 | 55.51 ± 21.08 | 0.595 | 0.942 | 0.211 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, Y.; Huang, J.; Li, S.; Chen, G.; Du, Y.; Kang, M.; Zhu, S.; Zhang, W.; Xu, Q.; Wang, Y.; et al. Brussels Chicory Enhances Exhaustive Aerobic Exercise Performance and Post-Exercise Recovery, Possibly Through Promotion of Lactate Oxidation: A Pilot Randomized, Single-Blind, Placebo-Controlled, Two-Way Crossover Study. Nutrients 2025, 17, 365. https://doi.org/10.3390/nu17020365
Mao Y, Huang J, Li S, Chen G, Du Y, Kang M, Zhu S, Zhang W, Xu Q, Wang Y, et al. Brussels Chicory Enhances Exhaustive Aerobic Exercise Performance and Post-Exercise Recovery, Possibly Through Promotion of Lactate Oxidation: A Pilot Randomized, Single-Blind, Placebo-Controlled, Two-Way Crossover Study. Nutrients. 2025; 17(2):365. https://doi.org/10.3390/nu17020365
Chicago/Turabian StyleMao, Yihui, Junhao Huang, Shuangshuang Li, Guanyu Chen, Yushi Du, Mengxi Kang, Shasha Zhu, Wenyu Zhang, Qiuhui Xu, Yihan Wang, and et al. 2025. "Brussels Chicory Enhances Exhaustive Aerobic Exercise Performance and Post-Exercise Recovery, Possibly Through Promotion of Lactate Oxidation: A Pilot Randomized, Single-Blind, Placebo-Controlled, Two-Way Crossover Study" Nutrients 17, no. 2: 365. https://doi.org/10.3390/nu17020365
APA StyleMao, Y., Huang, J., Li, S., Chen, G., Du, Y., Kang, M., Zhu, S., Zhang, W., Xu, Q., Wang, Y., Ling, W., Luo, X., & Wang, D. (2025). Brussels Chicory Enhances Exhaustive Aerobic Exercise Performance and Post-Exercise Recovery, Possibly Through Promotion of Lactate Oxidation: A Pilot Randomized, Single-Blind, Placebo-Controlled, Two-Way Crossover Study. Nutrients, 17(2), 365. https://doi.org/10.3390/nu17020365









