Soybean Flour Fortified with Gryllus assimilis Powder to Increase Iron Bioavailability Improves Gut Health and Oxidative Balance In Vivo
Abstract
1. Introduction
2. Materials and Methods
2.1. Obtaining and Preparing Flours
2.2. Chemical Composition of Soy Flour and Cricket Powder
2.3. Iron Determination
2.4. Experimental Animals and Diets
2.5. Hemoglobin and Serum Ferritin and Transferrin Assays
2.6. Iron Bioavailability
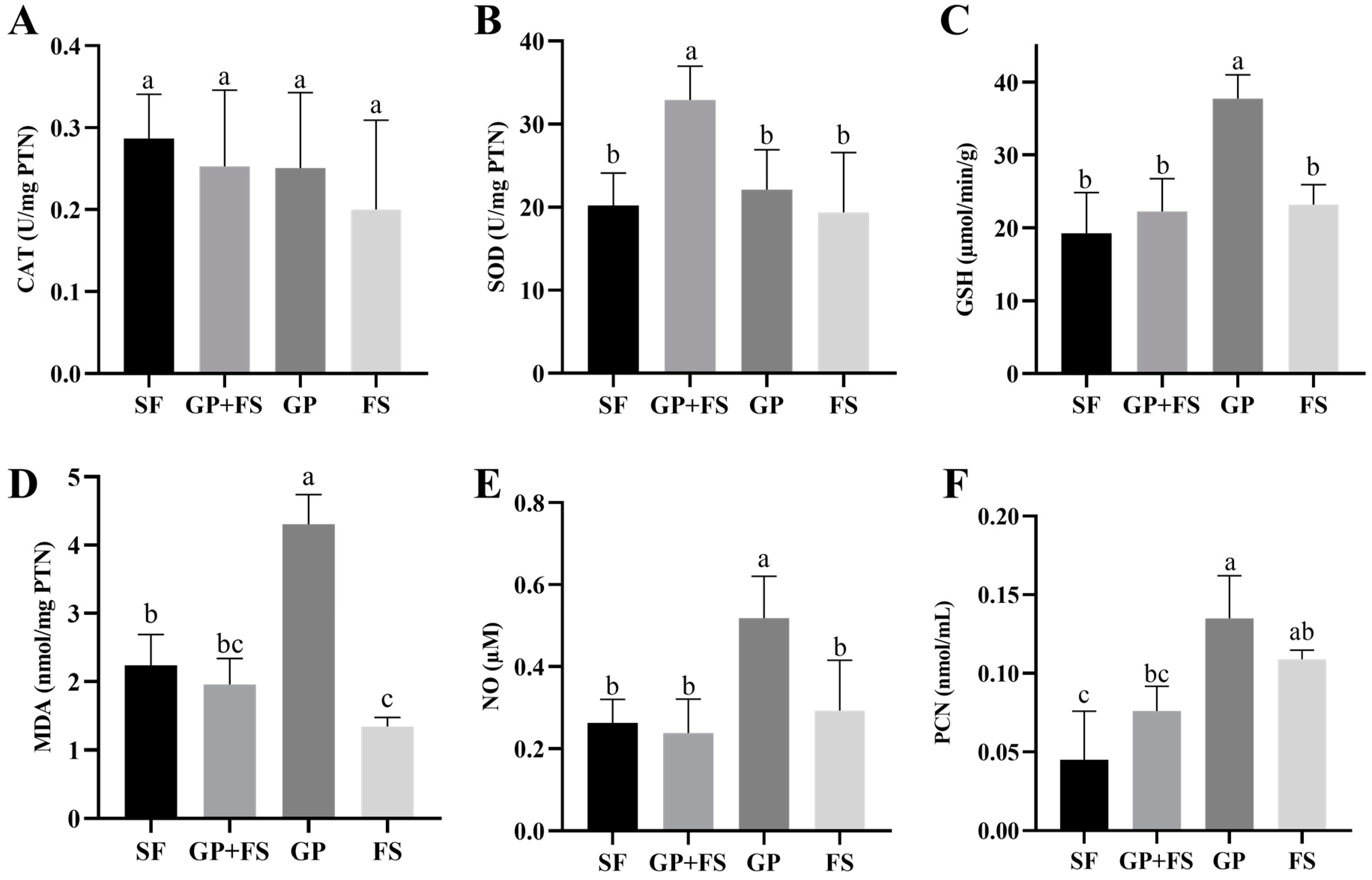
2.7. Oxidative Stress Biomarkers
2.7.1. Tissue Preparation
2.7.2. Catalase Activity
2.7.3. Superoxide Dismutase Activity
2.7.4. Glutathione S-Transferase Activity
2.7.5. Malondialdehyde Concentration
2.7.6. Nitric Oxide Concentration
2.7.7. Carbonylated Protein Concentration
2.7.8. Total Protein Concentration
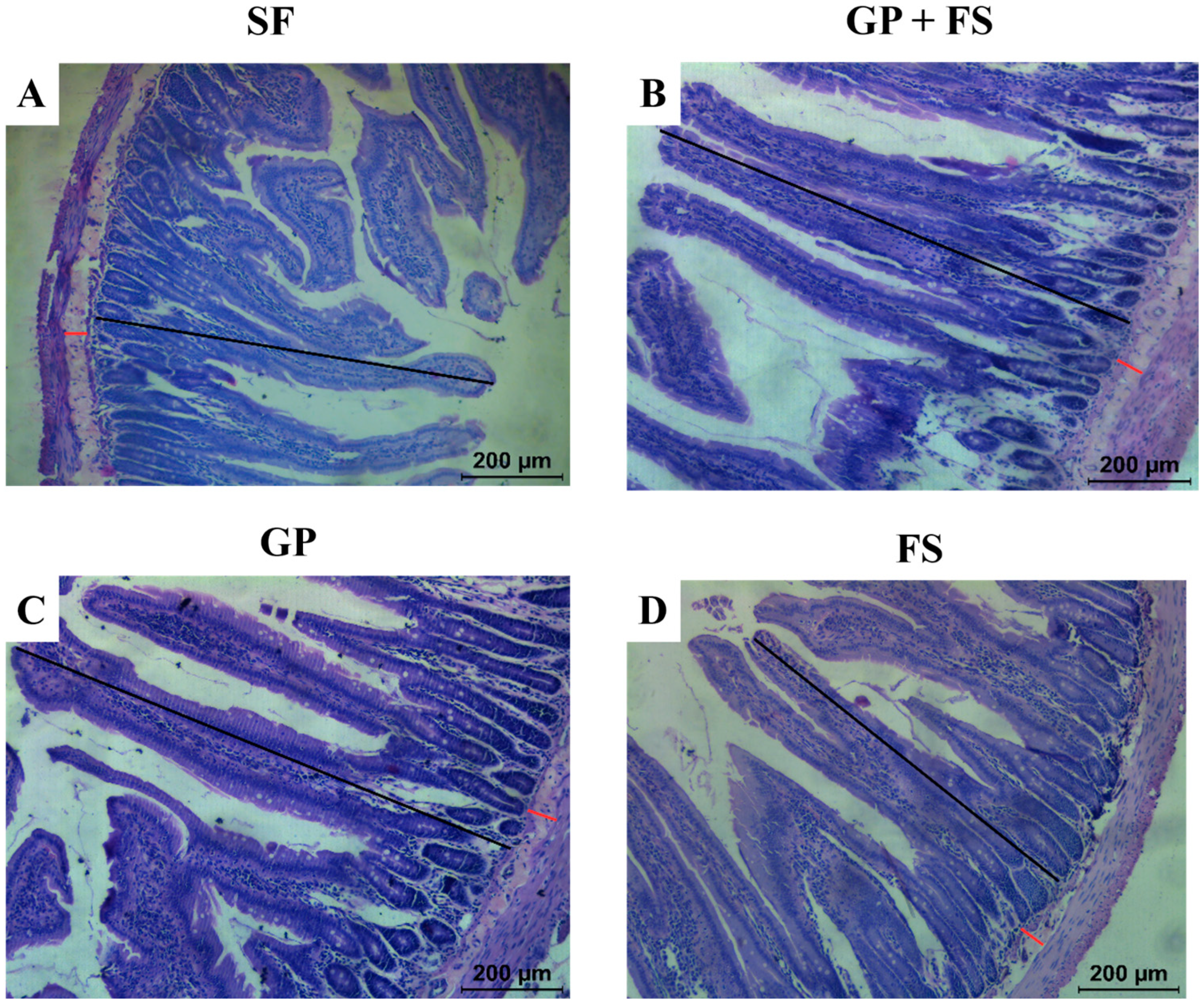
2.8. Analysis of Fecal Content and Duodenal Morphology
2.8.1. Fecal Moisture
2.8.2. Fecal Color and Consistency (Bristol Stool Scale)
2.8.3. Cecal Fecal pH Analysis
2.8.4. Quantification of Short-Chain Fatty Acids (SCFAs)
2.8.5. Histological Analysis of the Duodenum
2.9. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Skalnaya, M.G.; Skalny, A.V. Essential Trace Elements in Human Health: A Physician’s View; Publishing House of Tomsk State University: Tomsk, Russia, 2018; ISBN 978-5-94621-683-8. [Google Scholar]
- Pasricha, S.R.; Tye-Din, J.; Muckenthaler, M.U.; Swinkels, D.W. Iron Deficiency. Lancet 2021, 397, 233–248. [Google Scholar] [CrossRef]
- Yang, J.; Li, Q.; Feng, Y.; Zeng, Y. Iron Deficiency and Iron Deficiency Anemia: Potential Risk Factors in Bone Loss. Int. J. Mol. Sci. 2023, 24, 6891. [Google Scholar] [CrossRef]
- Iglesias Vázquez, L.; Valera, E.; Villalobos, M.; Tous, M.; Arija, V. Prevalence of Anemia in Children from Latin America and the Caribbean and Effectiveness of Nutritional Interventions: Systematic Review and Meta-Analysis. Nutrients 2019, 11, 183. [Google Scholar] [CrossRef] [PubMed]
- Melse-Boonstra, A. Bioavailability of Micronutrients from Nutrient-Dense Whole Foods: Zooming in on Dairy, Vegetables, and Fruits. Front. Nutr. 2020, 7, 101. [Google Scholar] [CrossRef]
- Hallberg, L. Bioavailability of dietary iron in man. Annu. Rev. Nutr. 1981, 1, 123–147. [Google Scholar] [CrossRef] [PubMed]
- Piskin, E.; Cianciosi, D.; Gulec, S.; Tomas, M.; Capanoglu, E. Iron Absorption: Factors, Limitations, and Improvement Methods. ACS Omega 2022, 7, 20441–20456. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Mao, C.; Li, X.; Jiang, L.; Zhang, W.; Li, M.; Hou, X. Edible Insects: A New Sustainable Nutritional Resource Worth Promoting. Foods 2023, 12, 4073. [Google Scholar] [CrossRef] [PubMed]
- Ojha, S.; Bekhit, A.E.D.; Grune, T.; Schlüter, O.K. Bioavailability of Nutrients from Edible Insects. Curr. Opin. Food Sci. 2021, 41, 240–248. [Google Scholar] [CrossRef]
- Tanga, C.M.; Ekesi, S. Dietary and Therapeutic Benefits of Edible Insects: A Global Perspective. Annu. Rev. Entomol. 2024, 69, 303–331. [Google Scholar] [CrossRef] [PubMed]
- Starčević, K.; Gavrilović, A.; Gottstein, Ž.; Mašek, T. Influence of Substitution of Sunflower Oil by Different Oils on the Growth, Survival Rate, and Fatty Acid Composition of Jamaican Field Cricket (Gryllus assimilis). Anim. Feed Sci. Technol. 2017, 228, 66–71. [Google Scholar] [CrossRef]
- Araújo, R.R.S.; dos Santos Benfica, T.A.R.; Ferraz, V.P.; Santos, E.M. Nutritional Composition of Insects Gryllus assimilis and Zophobas morio: Potential Foods Harvested in Brazil. J. Food Compos. Anal. 2019, 76, 22–26. [Google Scholar] [CrossRef]
- Abril, S.; Pinzón, M.; Hernández-Carrión, M.; Sanchez-Camargo, A.D.P. Edible Insects in Latin America: A Sustainable Alternative for Our Food Security. Front. Nutr. 2022, 9, 904812. [Google Scholar] [CrossRef]
- Messina, C.M.; Gaglio, R.; Morghese, M.; Tolone, M.; Arena, R.; Moschetti, G.; Settanni, L. Microbiological Profile and Bioactive Properties of Insect Powders Used in Food and Feed Formulations. Foods 2019, 8, 400. [Google Scholar] [CrossRef] [PubMed]
- Nowakowski, A.C.; Miller, A.C.; Miller, M.E.; Xiao, H.; Wu, X. Potential Health Benefits of Edible Insects. Crit. Rev. Food Sci. Nutr. 2022, 62, 3499–3508. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.A.; Pereira, S.M.S.; Dias, K.A.; da Silva Paes, S.; Grancieri, M.; Jimenez, L.G.S.; Della Lucia, C.M. Nutritional Content, Amino Acid Profile, and Protein Properties of Edible Insects (Tenebrio molitor and Gryllus assimilis) Powders at Different Stages of Development. J. Food Compos. Anal. 2024, 125, 105804. [Google Scholar] [CrossRef]
- Seabrooks, L.; Hu, L. Insects: An Underrepresented Resource for the Discovery of Biologically Active Natural Products. Acta Pharm. Sin. B 2017, 7, 409–426. [Google Scholar] [CrossRef] [PubMed]
- Quinteros, M.F.; Martínez, J.; Barrionuevo, A.; Rojas, M.; Carrillo, W. Functional, Antioxidant, and Anti-Inflammatory Properties of Cricket Protein Concentrate (Gryllus assimilis). Biology 2022, 11, 776. [Google Scholar] [CrossRef]
- Carvalho, A.W.D.; Natal, D.I.G.; Silva, C.O.D.; Dantas, M.I.D.S.; Barros, E.G.D.; Ribeiro, S.M.R.; Martino, H.S.D. Heat-Treatment Reduces Anti-Nutritional Phytochemicals and Maintains Protein Quality in Genetically Improved Hulled Soybean Flour. Food Sci. Technol. 2013, 33, 310–315. [Google Scholar] [CrossRef]
- Raheem, D.; Raposo, A.; Oluwole, O.B.; Nieuwland, M.; Saraiva, A.; Carrascosa, C. Entomophagy: Nutritional, Ecological, Safety, and Legislation Aspects. Food Res. Int. 2019, 126, 108672. [Google Scholar] [CrossRef] [PubMed]
- Oibiokpa, F.I.; Akanya, H.O.; Jigam, A.A.; Saidu, A.N.; Egwim, E.C. Protein Quality of Four Indigenous Edible Insect Species in Nigeria. Food Sci. Hum. Wellness 2018, 7, 175–183. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, H.; Huang, G. Protein hydrolysates as promoters of non-haem iron absorption. Nutrients 2017, 9, 609. [Google Scholar] [CrossRef]
- Latunde-Dada, G.O.; Yang, W.; Vera Aviles, M. In vitro iron availability from insects and sirloin beef. J. Agric. Food Chem. 2016, 64, 8420–8424. [Google Scholar] [CrossRef]
- Mwangi, M.N.; Oonincx, D.G.; Hummel, M.; Utami, D.A.; Gunawan, L.; Veenenbos, M.; Melse-Boonstra, A. Absorption of iron from edible house crickets: A randomized crossover stable-isotope study in humans. Am. J. Clin. Nutr. 2022, 116, 1146–1156. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, K.B.F.; Costa, N.M.B.; Alfenas, R.C.G.; Paula, S.O.; Minim, V.P.R.; Bressan, J. Oxidative stress: Concept, implications and modulating factors. Rev. Nutr. 2010, 23, 629–643. [Google Scholar] [CrossRef]
- Barboza, G.D.; Guizzardi, S.; Moine, L.; de Talamoni, N.T. Oxidative Stress, Antioxidants, and Intestinal Calcium Absorption. World J. Gastroenterol. 2017, 23, 2841. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, L.; Jiang, G.; Lei, A.; Yu, Q.; Xie, J.; Chen, Y. Evaluation of the Protective Effects of Ganoderma atrum Polysaccharide on Acrylamide-Induced Injury in Small Intestine Tissue of Rats. Food Funct. 2019, 10, 5863–5872. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Y.; Zhang, X.; Lu, Y.; Chen, H. New Insights in Intestinal Oxidative Stress Damage and the Health Intervention Effects of Nutrients: A Review. J. Funct. Foods 2020, 75, 104248. [Google Scholar] [CrossRef]
- Yoon, G.A.; Park, S. Antioxidant action of soy isoflavones on oxidative stress and antioxidant enzyme activities in exercised rats. Nutr. Res. Pract. 2014, 8, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Cao, W.; Huang, Y.; Fang, Y.; Cheng, Y.; Pan, S.; Xu, X. Isoflavone biochanin A, a novel nuclear factor erythroid 2-related factor 2 (Nrf2)-antioxidant response element activator, protects against oxidative damage in HepG2 cells. Biofactors 2019, 45, 563–574. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis of AOAC International: Agricultural Chemicals, Contaminants, Drugs; AOAC International: Gaithersburg, MD, USA, 2012; Volume 16. [Google Scholar]
- Fontelles, M.J.; Simões, M.G.; Almeida, J.C.D.; Fontelles, R.G.S. Metodologia da pesquisa: Diretrizes para o cálculo do tamanho da amostra. Rev. Para. Med 2010, 24, 57–64. [Google Scholar]
- AOAC International. Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Rockville, MD, USA, 2000. [Google Scholar]
- López-Alarcón, M.; Hernández, M.; Sousa, V.; Moreno, Á.; Villapando, S. Iron bioavailability and utilization in rats are lower from lime-treated corn flour than from wheat flour when they are fortified with different sources of iron. J. Nutr. 2003, 133, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase In Vitro. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 121–126. [Google Scholar] [CrossRef]
- Marklund, S.L. Superoxide Dismutase Isoenzymes in Tissues and Plasma from New Zealand Black Mice, Nude Mice, and Normal BALB/c Mice. Mutat. Res./Fundam. Mol. Mech. Mutagen. 1985, 148, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-Transferases: The First Enzymatic Step in Mercapturic Acid Formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef] [PubMed]
- Buege, J.A.; Aust, S.D. Microsomal Lipid Peroxidation. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1978; Volume 52, pp. 302–310. [Google Scholar] [CrossRef]
- Green, C.L. Analysis of Nitrate, Nitrite, and [15N] Nitrate in Biological Fluids. Infect. Immun. 1994, 62, 1171. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.L. Carbonyl Assay for Determination of Oxidatively Modified Proteins. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1994; Volume 233, pp. 246–257. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Paez, V.; Barrett, W.B.; Deng, X.; Diaz-Amigo, C.; Fiedler, K.; Fuerer, C.; Coates, S.G. AOAC SMPR® 2016.002. J. AOAC Int. 2016, 99, 1122–1124. [Google Scholar] [CrossRef]
- Silveira Junior, A.O.O. Exame Coprológico E As Funções Digestivas, 1st ed.; Santos: São Paulo, Brazil, 1988. [Google Scholar]
- Canani, R.B.; Cirillo, P.; Terrin, G.; Cesarano, L.; Spagnuolo, M.I.; De Vincenzo, A.; Guarino, A. Probiotics for Treatment of Acute Diarrhoea in Children: Randomised Clinical Trial of Five Different Preparations. BMJ 2007, 335, 340. [Google Scholar] [CrossRef] [PubMed]
- Grancieri, M.; Costa, N.M.B.; Tostes, M.D.G.V.; de Oliveira, D.S.; de Carvalho Nunes, L.; de Nadai Marcon, L.; Viana, M.L. Yacon Flour (Smallanthus sonchifolius) Attenuates Intestinal Morbidity in Rats with Colon Cancer. J. Funct. Foods 2017, 37, 666–675. [Google Scholar] [CrossRef]
- Siegfried, R.; Rückemann, H.; Stumpf, G. An HPLC Method for Determining Organic Acids in Silage. J. Sci. Food Agric. 1984, 35, 1211–1217. [Google Scholar]
- Araújo, R.R.S.; Fagundes, M.M.A.; Viana, A.M.F.; Paulino, A.H.S.; Silva, M.E.; Santos, E.M. Protein Quality Evaluation In Vivo of Cricket Flour (Gryllus assimilis) Reared in Brazil. J. Insects Food Feed 2022, 8, 409–416. [Google Scholar] [CrossRef]
- Bergmans, R.S.; Nikodemova, M.; Stull, V.J.; Rapp, A.; Malecki, K.M. Comparison of Cricket Diet with Peanut-Based and Milk-Based Diets in the Recovery from Protein Malnutrition in Mice and the Impact on Growth, Metabolism, and Immune Function. PLoS ONE 2020, 15, e0234559. [Google Scholar] [CrossRef]
- Deuchi, K.; Kanauchi, O.; Shizukuishi, M.; Kobayashi, E. Continuous and Massive Intake of Chitosan Affects Mineral and Fat-Soluble Vitamin Status in Rats Fed on a High-Fat Diet. Biosci. Biotechnol. Biochem. 1995, 59, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- Mwangi, M.N.; Oonincx, D.G.; Stouten, T.; Veenenbos, M.; Melse-Boonstra, A.; Dicke, M.; Van Loon, J.J. Insects as Sources of Iron and Zinc in Human Nutrition. Nutr. Res. Rev. 2018, 31, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Oonincx, D.; Finke, M.J.J. Nutritional Value of Insects and Ways to Manipulate Their Composition. J. Insects Food Feed 2021, 7, 639–659. [Google Scholar] [CrossRef]
- Costa, B.M.N.; Martino, D.S.H. Biodisponibilidade de Minerais. In Tratado de Alimentação, Nutrição E Dietoterapia; Editora Payá: São Paulo, Brazil, 2016; pp. 107–135. [Google Scholar]
- Grotto, H.Z. Fisiologia e Metabolismo do Ferro. Rev. Bras. Hematol. E Hemoter. 2010, 32, 8–17. [Google Scholar] [CrossRef]
- Frazer, D.M.; Anderson, G.J. The Regulation of Iron Transport. Biofactors 2014, 40, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Waldvogel-Abramowski, S.; Waeber, G.; Gassner, C.; Buser, A.; Frey, B.M.; Favrat, B.; Tissot, J.D. Physiology of Iron Metabolism. Transfus. Med. Hemotherapy 2014, 41, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Moniem, M.M.; Hassan, A.M.; Said, M.M.; Esmat, A.Y. Iron Supplementation Ameliorates Aloin-Induced Iron Deficiency Anemia in Rats. Exp. Mol. Pathol. 2022, 124, 104740. [Google Scholar] [CrossRef] [PubMed]
- Sciacqua, A.; Ventura, E.; Tripepi, G.; Cassano, V.; D’Arrigo, G.; Roumeliotis, S.; Perticone, F. Ferritin Modifies the Relationship Between Inflammation and Arterial Stiffness in Hypertensive Patients with Different Glucose Tolerance. Cardiovasc. Diabetol. 2020, 19, 123. [Google Scholar] [CrossRef] [PubMed]
- Ueda, N.; Takasawa, K. Impact of Inflammation on Ferritin, Hepcidin, and the Management of Iron Deficiency Anemia in Chronic Kidney Disease. Nutrients 2018, 10, 1173. [Google Scholar] [CrossRef] [PubMed]
- Basson, A.R.; Ahmed, S.; Almutairi, R.; Seo, B.; Cominelli, F. Regulation of intestinal inflammation by soybean and soy-derived compounds. Foods 2021, 10(4), 774. [Google Scholar] [CrossRef] [PubMed]
- Al-Nakkash, L.; Kubinski, A. Soy Isoflavones and Gastrointestinal Health. Curr. Nutr. Rep. 2020, 9, 193–201. [Google Scholar] [CrossRef]
- Huang, L.; Zheng, T.; Hui, H.; Xie, G. Soybean Isoflavones Modulate Gut Microbiota to Benefit Health, Weight, and Metabolism. Front. Cell. Infect. Microbiol. 2022, 12, 1004765. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, H.; Zhang, Y.; Zhang, C.; Zhang, J.; Liu, X. Differences in the Gut Microbiota Composition of Rats Fed with Soybean Protein and Their Derived Peptides. J. Food Sci. 2021, 86, 5452–5465. [Google Scholar] [CrossRef] [PubMed]
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Verbeke, K. Short Chain Fatty Acids in Human Gut and Metabolic Health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef] [PubMed]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The Role of Short-Chain Fatty Acids in Microbiota–Gut–Brain Communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef]
- De Vries, J.; Le Bourgot, C.; Calame, W.; Respondek, F. Effects of β-Fructans Fiber on Bowel Function: A Systematic Review and Meta-Analysis. Nutrients 2019, 11, 91. [Google Scholar] [CrossRef] [PubMed]
- Brandl, B.; Lee, Y.M.; Dunkel, A.; Hofmann, T.; Hauner, H.; Skurk, T. Effects of Extrinsic Wheat Fiber Supplementation on Fecal Weight: A Randomized Controlled Trial. Nutrients 2020, 12, 298. [Google Scholar] [CrossRef] [PubMed]
- Remes-Troche, J.M.; Taboada-Liceaga, H.; Gill, S.; Amieva-Balmori, M.; Rossi, M.; Hernández-Ramírez, G.; Whelan, K. Nopal Fiber (Opuntia ficus-indica) Improves Symptoms in Irritable Bowel Syndrome in the Short Term: A Randomized Controlled Trial. Neurogastroenterol. Motil. 2021, 33, e13986. [Google Scholar] [CrossRef]
- Kwon, O.; Han, T.S.; Son, M.Y. Intestinal Morphogenesis in Development, Regeneration, and Disease: The Potential Utility of Intestinal Organoids for Studying Compartmentalization of the Crypt-Villus Structure. Front. Cell Dev. Biol. 2020, 8, 593969. [Google Scholar] [CrossRef]
- Selenius, O.; Korpela, J.; Salminen, S.; Gallego, C.G. Effect of Chitin and Chitooligosaccharide on In Vitro Growth of Lactobacillus rhamnosus GG and Escherichia coli TG. Appl. Food Biotechnol. 2018, 5, 163–172. [Google Scholar] [CrossRef]
- Kim, Y.S.; Ho, S.B. Intestinal Goblet Cells and Mucins in Health and Disease: Recent Insights and Progress. Curr. Gastroenterol. Rep. 2010, 12, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.K.; Choudhury, B. Suitability of Organic Solvent and Cholinium-Based Ionic Liquid Activated Novel Lignolytic Enzymes of H. aswanensis for Enhanced Kalson Lignin Degradation. Int. J. Biol. Macromol. 2020, 165, 107–117. [Google Scholar] [CrossRef]
- Yuan, H.; Xu, F.; Tian, X.; Wei, H.; Zhang, R.; Ge, Y.; Xu, H. Oxidative Stress and Inflammation Caused by 1-Tetradecyl-3-Methylimidazolium Tetrafluoroborate in Rat Livers. Environ. Sci. Pollut. Res. 2022, 29, 86680–86691. [Google Scholar] [CrossRef]
- Xie, D.; Jiang, L.; Lin, Y.; Liu, Z. Antioxidant Activity of Selenium-Enriched Chrysomyia megacephala (Fabricius) Larvae Powder and Its Impact on Intestinal Microflora in D-Galactose-Induced Aging Mice. BMC Complement. Med. Ther. 2020, 20, 264. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, C.; Gleddie, S.; Xiao, C.W. Soybean Bioactive Peptides and Their Functional Properties. Nutrients 2018, 10, 1211. [Google Scholar] [CrossRef] [PubMed]
- Kusumah, J.; de Mejia, E.G. Impact of Soybean Bioactive Compounds as Response to Diet-Induced Chronic Inflammation: A Systematic Review. Food Res. Int. 2022, 162, 111928. [Google Scholar] [CrossRef]
- Ahn, M.Y.; Kim, M.J.; Kwon, R.H.; Hwang, J.S.; Park, K.K. Gene Expression Profiling and Inhibition of Adipose Tissue Accumulation of G. bimaculatus Extract in Rats on High-Fat Diet. Lipids Health Dis. 2015, 14, 116. [Google Scholar] [CrossRef] [PubMed]
- Hwang, B.B.; Chang, M.H.; Lee, J.H.; Heo, W.; Kim, J.K.; Pan, J.H.; Kim, J.H. The Edible Insect Gryllus bimaculatus Protects Against Gut-Derived Inflammatory Responses and Liver Damage in Mice After Acute Alcohol Exposure. Nutrients 2019, 11, 857. [Google Scholar] [CrossRef]
- Cui, X.; Gong, J.; Han, H.; He, L.; Teng, Y.; Tetley, T.; Zhang, J.J. Relationship Between Free and Total Malondialdehyde, a Well-Established Marker of Oxidative Stress, in Various Types of Human Biospecimens. J. Thorac. Dis. 2018, 10, 3088. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 5th ed.; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Forman, H.J.; Zhang, H. Targeting Oxidative Stress in Disease: Promise and Limitations of Antioxidant Therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Hybertson, B.M.; Gao, B.; Bose, S.K.; McCord, J.M. Oxidative Stress in Health and Disease: The Therapeutic Potential of Nrf2 Activation. Mol. Asp. Med. 2011, 32, 234–246. [Google Scholar] [CrossRef]

| Compounds | Gryllus assimilis | Soy Flour |
|---|---|---|
| Moisture (g/100 g) | 3.68 ± 0.01 | 5.92 ± 0.23 |
| Ash (g/100 g) | 4.04 ± 0.16 | 5.02 ± 0.05 |
| Lipids (g/100 g) | 18.28 ± 1.01 | 22.55 ± 0.59 |
| Proteins (g/100 g) | 64.75 ± 0.79 | 43.90 ± 1.63 |
| Total dietary fiber (g/100 g) | 9.04 ± 0.01 | 13.35 ± 0.48 |
| Carbohydrates (g/100 g) | 0.21 ± 0.01 | 9.26 ± 2.75 |
| Iron (mg/100 g) | 23.72 ± 1.31 | 7.94 ± 0.69 |
| Variables | SF | GP + FS | GP | FS |
|---|---|---|---|---|
| Total intake (g) | 338.41 ± 15.98 a | 348.82 ± 17.91 a | 343.61 ± 32.30 a | 342.28 ± 18.17 a |
| Weight gain (g) | 30.42 ± 18.85 a | 32.81 ± 20.41 a | 30.04 ± 19.13 a | 20.33 ± 16.89 a |
| FER | 0.09 ± 0.05 a | 0.10 ± 0.05 a | 0.10 ± 0.04 a | 0.8 ± 0.04 a |
| Iron intake (g) | 7.39 ± 0.35 ab | 8.02 ± 0.41 a | 7.90 ± 0.74 ab | 7.29 ± 0.39 b |
| HRE % | 41.37 ± 10.86 a | 41.13 ± 10.86 a | 36.40 ± 6.26 a | 40.82 ± 8.03 a |
| RBV-HRE | 1.00 ± 0.15 a | 0.99 ± 0.18 a | 0.88 ± 0.12 a | 0.98 ± 0.11 a |
| Iron utilization | 0.90 ± 0.14 ab | 0.94 ± 0.15 a | 0.83 ± 0.11 b | 0.86 ± 0.10 ab |
| Variables | SF | GP + FS | GP | FS |
|---|---|---|---|---|
| Initial hemoglobin (g/dL) | 7.52 ± 1.51 a | 7.51 ± 1.47 a | 7.57 ± 1.47 a | 7.67 ± 1.38 a |
| Final hemoglobin (g/dL) | 14.46 ± 1.99 a | 14.49 ± 1.25 a | 12.05 ± 1.82 b | 14.37 ± 1.01 a |
| Hemoglobin gain (g/dL) | 7.53 ± 2.00 a | 6.98 ± 1.08 a | 4.49 ± 2.00 b | 6.70 ± 1.38 ab |
| Transferrin (mg/dL) | 163.40 ± 8.26 b | 178.03 ± 4.90 a | 185.91 ± 8.19 a | 160.22 ± 7.44 b |
| Ferritin (ng/dL) | 5.18 ± 1.01 ab | 4.56 ± 0.97 b | 6.41 ± 1.10 a | 4.64 ± 0.84 ab |
| Variables | SF | GP + FS | GP | FS |
|---|---|---|---|---|
| Acetic acid (mmol/L) | 7.58 ± 0.92 d | 10.02 ± 0.76 c | 11.59 ± 0.97 b | 13.95 ± 1.80 a |
| Propionic acid (mmol/L) | 3.60 ± 0.53 b | 3.86 ± 0.64 b | 4.27 ± 0.47 ab | 4.78 ± 0.21 a |
| Butyric acid (mmol/L) | 0.99 ± 0.12 a | 1.17 ± 0.32 a | 1.31 ± 0.29 a | 1.15 ± 0.27 a |
| Total SCFA | 12.56 ± 1.84 | 15.87 ± 0.86 | 17.24 ± 1.74 | 19.33 ± 1.63 |
| Fecal moisture (%) | 12.32 ± 1.37 b | 21.29 ± 3.80 a | 16.08 ± 2.53 b | 18.72 ± 1.30 ab |
| Cecal stool pH | 8.25 ± 0.69 a | 7.96 ± 0.31 a | 7.74 ± 0.36 a | 8.03 ± 0.55 a |
| Stool color | 3.00 ± 0.00 a | 2.00 ± 0.00 a | 2.00 ± 0.00 a | 1.00 ± 0.00 a |
| Stool consistency | 2.00 ± 0.00 a | 1.00 ± 0.00 a | 2.00 ± 0.00 a | 1.00 ± 0.00 a |
| Variables | SF | GP + FS | GP | FS |
|---|---|---|---|---|
| Mucosal thickness (µm) | 600.64 ± 78.48 b | 725.26 ± 79.41 ab | 798.81 ± 78.53 a | 796.78 ± 46.29 a |
| Submucosal thickness (µm) | 47.14 ± 7.68 a | 46.24 ± 3.88 a | 48.84 ± 3.20 a | 45.23 ± 10.31 a |
| Goblet cell diameter (µm) | 16.86 ± 0.94 b | 17.95 ± 0.97 b | 19.21 ± 0.92 a | 16.78 ± 0.96 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barnabé, M.L.d.F.; Vicente, L.C.d.O.S.; Martins, K.V.C.; Lacerda, G.F.; Rodrigues, E.; Oliveira, L.A.; Dias, K.A.; Pereira, S.M.S.; José, V.P.B.d.S.; Dias, M.M.d.S.; et al. Soybean Flour Fortified with Gryllus assimilis Powder to Increase Iron Bioavailability Improves Gut Health and Oxidative Balance In Vivo. Nutrients 2025, 17, 437. https://doi.org/10.3390/nu17030437
Barnabé MLdF, Vicente LCdOS, Martins KVC, Lacerda GF, Rodrigues E, Oliveira LA, Dias KA, Pereira SMS, José VPBdS, Dias MMdS, et al. Soybean Flour Fortified with Gryllus assimilis Powder to Increase Iron Bioavailability Improves Gut Health and Oxidative Balance In Vivo. Nutrients. 2025; 17(3):437. https://doi.org/10.3390/nu17030437
Chicago/Turabian StyleBarnabé, Michele Lílian da Fonseca, Laura Célia de Oliveira Souza Vicente, Karina Vitoria Cipriana Martins, Gabrieli Fernandes Lacerda, Elias Rodrigues, Lívya Alves Oliveira, Kelly Aparecida Dias, Stephanie Michelin Santana Pereira, Vinicius Parzanini Brilhante de São José, Manoela Maciel dos Santos Dias, and et al. 2025. "Soybean Flour Fortified with Gryllus assimilis Powder to Increase Iron Bioavailability Improves Gut Health and Oxidative Balance In Vivo" Nutrients 17, no. 3: 437. https://doi.org/10.3390/nu17030437
APA StyleBarnabé, M. L. d. F., Vicente, L. C. d. O. S., Martins, K. V. C., Lacerda, G. F., Rodrigues, E., Oliveira, L. A., Dias, K. A., Pereira, S. M. S., José, V. P. B. d. S., Dias, M. M. d. S., Calhelha, R. C., Leite, L. B., Ribeiro, L., Carvalho, I. M. M. d., Silva, B. P. d., Martino, H. S. D., Gonçalves, R. V., & Mattos Della Lucia, C. (2025). Soybean Flour Fortified with Gryllus assimilis Powder to Increase Iron Bioavailability Improves Gut Health and Oxidative Balance In Vivo. Nutrients, 17(3), 437. https://doi.org/10.3390/nu17030437









