Evaluating Bioactive-Substance-Based Interventions for Adults with MASLD: Results from a Systematic Scoping Review
Abstract
1. Introduction
2. Methods
2.1. Eligibility Criteria
2.2. Search Strategy
2.3. Article Selection and Data Extraction
2.4. Synthesis of Results
3. Results
3.1. Population Characteristics
3.2. Interventions
3.3. Systematic Reviews
3.4. Outcomes Reported in Primary Studies
3.5. Funding
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zeng, X.F.; Varady, K.A.; Wang, X.D.; Targher, G.; Byrne, C.D.; Tayyem, R.; Latella, G.; Bergheim, I.; Valenzuela, R.; George, J.; et al. The role of dietary modification in the prevention and management of metabolic dysfunction-associated fatty liver disease: An international multidisciplinary expert consensus. Metabolism 2024, 161, 156028. [Google Scholar] [CrossRef] [PubMed]
- Yates, A.A.; Dwyer, J.T.; Erdman, J.W.; King, J.C.; Lyle, B.J.; Schneeman, B.O.; Weaver, C.M.; serving as an ad hoc Working Group on a Framework for Developing Recommended Intakes for Dietary Bioactives. Perspective: Framework for Developing Recommended Intakes of Bioactive Dietary Substances. Adv. Nutr. 2021, 12, 1087–1099. [Google Scholar] [CrossRef]
- Lai, J.C.; Ring, M.; Dhruva, A.; Yeh, G.Y. A patient-centered approach to dietary supplements for patients with chronic liver disease. Hepatol. Commun. 2024, 8, e0552. [Google Scholar] [CrossRef] [PubMed]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Levac, D.; Colquhoun, H.; O’Brien, K.K. Scoping studies: Advancing the methodology. Implement. Sci. 2010, 5, 69. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.D.J.; Marnie, C.; Tricco, A.C.; Pollock, D.; Munn, Z.; Alexander, L.; McInerney, P.; Godfrey, C.M.; Khalil, H. Updated methodological guidance for the conduct of scoping reviews. JBI Evid. Synth. 2020, 18, 2119–2126. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Mohr, A.E.; Handu, D.; Senkus, K. Adult Nutrition and Dietary Management Interventions for Metabolic Dysfunction Associated Steatotic Liver Disease: A Systematic Scoping Review. 2023. Available online: https://osf.io/gxfcn/ (accessed on 10 November 2024).
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Abenavoli, L.; Greco, M.; Nazionale, I.; Peta, V.; Milic, N.; Accattato, F.; Foti, D.; Gulletta, E.; Luzza, F. Effects of Mediterranean diet supplemented with silybin-vitamin E-phospholipid complex in overweight patients with non-alcoholic fatty liver disease. Expert Rev. Gastroenterol. Hepatol. 2015, 9, 519–527. [Google Scholar] [CrossRef]
- Aller, R.; Izaola, O.; Gómez, S.; Tafur, C.; González, G.; Berroa, E.; Mora, N.; González, J.M.; de Luis, D.A. Effect of silymarin plus vitamin E in patients with non-alcoholic fatty liver disease. A randomized clinical pilot study. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 3118–3124. [Google Scholar]
- Anushiravani, A.; Haddadi, N.; Pourfarmanbar, M.; Mohammadkarimi, V. Treatment options for nonalcoholic fatty liver disease: A double-blinded randomized placebo-controlled trial. Eur. J. Gastroenterol. Hepatol. 2019, 31, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Asghari, S.; Asghari-Jafarabadi, M.; Somi, M.-H.; Ghavami, S.-M.; Rafraf, M. Comparison of Calorie-Restricted Diet and Resveratrol Supplementation on Anthropometric Indices, Metabolic Parameters, and Serum Sirtuin-1 Levels in Patients With Nonalcoholic Fatty Liver Disease: A Randomized Controlled Clinical Trial. J. Am. Coll. Nutr. 2018, 37, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Asghari, S.; Rafraf, M.; Farzin, L.; Asghari-Jafarabadi, M.; Ghavami, S.M.; Somi, M.H. Effects of pharmacologic dose of resveratrol supplementation on oxidative/antioxidative status biomarkers in nonalcoholic fatty liver disease patients: A randomized, double-blind, placebo- controlled trial. Adv. Pharm. Bull. 2018, 8, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Atarodi, H.; Pazouki, A.; Gholizadeh, B.; Karami, R.; Kabir, A.; Sadri, G.; Kassir, R.; Kermansaravi, M. Effect of silymarin on liver size and nonalcoholic fatty liver disease in morbidly obese patients: A randomized double-blind clinical trial. J. Res. Med. Sci. 2022, 27, 76. [Google Scholar] [CrossRef] [PubMed]
- Chachay, V.S.; Macdonald, G.A.; Martin, J.H.; Whitehead, J.P.; O’Moore-Sullivan, T.M.; Lee, P.; Franklin, M.; Klein, K.; Taylor, P.J.; Ferguson, M.; et al. Resveratrol Does Not Benefit Patients With Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2014, 12, 2092–2103. [Google Scholar] [CrossRef]
- Chashmniam, S.; Mirhafez, S.R.; Dehabeh, M.; Hariri, M.; Azimi Nezhad, M.; Nobakht M. Gh, B.F. A pilot study of the effect of phospholipid curcumin on serum metabolomic profile in patients with non-alcoholic fatty liver disease: A randomized, double-blind, placebo-controlled trial. Eur. J. Clin. Nutr. 2019, 73, 1224–1235. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhao, X.; Ran, L.; Wan, J.; Wang, X.; Qin, Y.; Shu, F.; Gao, Y.; Yuan, L.; Zhang, Q.; et al. Resveratrol improves insulin resistance, glucose and lipid metabolism in patients with non-alcoholic fatty liver disease: A randomized controlled trial. Dig. Liver Dis. 2015, 47, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Chiurazzi, M.; Cacciapuoti, N.; Di Lauro, M.; Nasti, G.; Ceparano, M.; Salomone, E.; Guida, B.; Lonardo, M.S. The Synergic Effect of a Nutraceutical Supplementation Associated to a Mediterranean Hypocaloric Diet in a Population of Overweight/Obese Adults with NAFLD. Nutrients 2022, 14, 4750. [Google Scholar] [CrossRef] [PubMed]
- Dallio, M.; Masarone, M.; Romeo, M.; Tuccillo, C.; Morisco, F.; Persico, M.; Loguercio, C.; Federico, A. PNPLA3, TM6SF2, and MBOAT7 Influence on Nutraceutical Therapy Response for Non-alcoholic Fatty Liver Disease: A Randomized Controlled Trial. Front. Med. 2021, 8, 734847. [Google Scholar] [CrossRef] [PubMed]
- Darand, M.; Darabi, Z.; Yari, Z.; Hedayati, M.; Shahrbaf, M.A.; Khoncheh, A.; Hosseini-Ahangar, B.; Alavian, S.M.; Hekmatdoost, A.; Hosseini-Ahangar, B. The effects of black seed supplementation on cardiovascular risk factors in patients with nonalcoholic fatty liver disease: A randomized, double-blind, placebo-controlled clinical trial. Phytother. Res. 2019, 33, 2369–2377. [Google Scholar] [CrossRef] [PubMed]
- Darand, M.; Darabi, Z.; Yari, Z.; Saadati, S.; Hedayati, M.; Khoncheh, A.; Hosseini-Ahangar, B.; Alavian, S.m.; Hekmatdoost, A. Nigella sativa and inflammatory biomarkers in patients with non-alcoholic fatty liver disease: Results from a randomized, double-blind, placebo-controlled, clinical trial. Complement. Ther. Med. 2019, 44, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Faghihzadeh, F.; Adibi, P.; Rafiei, R.; Hekmatdoost, A. Resveratrol supplementation improves inflammatory biomarkers in patients with nonalcoholic fatty liver disease. Nutr. Res. 2014, 34, 837–843. [Google Scholar] [CrossRef]
- Faghihzadeh, F.; Adibi, P.; Hekmatdoost, A. The effects of resveratrol supplementation on cardiovascular risk factors in patients with non-alcoholic fatty liver disease: A randomised, double-blind, placebo-controlled study. Br. J. Nutr. 2015, 114, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Farzin, L.; Asghari, S.; Rafraf, M.; Asghari-Jafarabadi, M.; Shirmohammadi, M. No beneficial effects of resveratrol supplementation on atherogenic risk factors in patients with nonalcoholic fatty liver disease. Int. J. Vitam. Nutr. Res. 2020, 90, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Federico, A.; Conti, V.; Russomanno, G.; Dallio, M.; Masarone, M.; Stiuso, P.; Tuccillo, C.; Caraglia, M.; Manzo, V.; Persico, M.; et al. A Long-term Treatment with Silybin in Patients with Non-alcoholic Steatohepatitis Stimulates Catalase Activity in Human Endothelial Cells. In Vivo 2017, 31, 609–618. [Google Scholar] [CrossRef]
- Federico, A.; Dallio, M.; Masarone, M.; Gravina, A.G.; Di Sarno, R.; Tuccillo, C.; Cossiga, V.; Lama, S.; Stiuso, P.; Morisco, F.; et al. Evaluation of the Effect Derived from Silybin with Vitamin D and Vitamin E Administration on Clinical, Metabolic, Endothelial Dysfunction, Oxidative Stress Parameters, and Serological Worsening Markers in Nonalcoholic Fatty Liver Disease Patients. Oxidative Med. Cell. Longev. 2019, 2019, 8742075. [Google Scholar] [CrossRef]
- Ferro, Y.; Maurotti, S.; Mazza, E.; Pujia, R.; Sciacqua, A.; Musolino, V.; Mollace, V.; Pujia, A.; Montalcini, T. Citrus Bergamia and Cynara Cardunculus Reduce Serum Uric Acid in Individuals with Non-Alcoholic Fatty Liver Disease. Medicina 2022, 58, 1728. [Google Scholar] [CrossRef] [PubMed]
- Ferro, Y.; Montalcini, T.; Mazza, E.; Foti, D.; Angotti, E.; Gliozzi, M.; Nucera, S.; Paone, S.; Bombardelli, E.; Aversa, I.; et al. Randomized Clinical Trial: Bergamot Citrus and Wild Cardoon Reduce Liver Steatosis and Body Weight in Non-diabetic Individuals Aged Over 50 Years. Front. Endocrinol. 2020, 11, 494. [Google Scholar] [CrossRef]
- Ferro, Y.; Pujia, R.; Mazza, E.; Lascala, L.; Lodari, O.; Maurotti, S.; Pujia, A.; Montalcini, T. A new nutraceutical (Livogen Plus®) improves liver steatosis in adults with non-alcoholic fatty liver disease. J. Transl. Med. 2022, 20, 377. [Google Scholar] [CrossRef] [PubMed]
- Geng, Q.; Zhang, P.; Liu, X.; Xue, L. Effect of berberine and bicyclol on Chinese patients with nonalcoholic fatty liver disease: A retrospective study. Postgrad. Med. 2022, 134, 507–515. [Google Scholar] [CrossRef]
- Ghaffari, A.; Rafraf, M.; Navekar, R.; Asghari-Jafarabadi, M. Effects of turmeric and chicory seed supplementation on antioxidant and inflammatory biomarkers in patients with non-alcoholic fatty liver disease (NAFLD). Adv. Integr. Med. 2018, 5, 89–95. [Google Scholar] [CrossRef]
- Ghaffari, A.; Rafraf, M.; Navekar, R.; Sepehri, B.; Asghari-Jafarabadi, M.; Ghavami, S.-M. Turmeric and chicory seed have beneficial effects on¬†obesity markers and lipid profile in non-alcoholic fatty liver disease (NAFLD). Int. J. Vitam. Nutr. Res. Int. Z. Fur Vitam.-Und Ernahrungsforschung. J. Int. De Vitaminol. Et De Nutr. 2019, 89, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, A.; Rafraf, M.; Navekar, R.; Sepehri, B.; Asghari-Jafarabadi, M.; Ghavami, S.M. Effects of turmeric on homocysteine and Fetuin-A in patients with nonalcoholic fatty liver disease: A randomized Double-Blind placebo-controlled study. Iran. Red Crescent Med. J. 2017, 19, e43193. [Google Scholar] [CrossRef]
- Hariri, M.; Gholami, A.; Mirhafez, S.R.; Bidkhori, M.; Sahebkar, A. A pilot study of the effect of curcumin on epigenetic changes and DNA damage among patients with non-alcoholic fatty liver disease: A randomized, double-blind, placebo-controlled, clinical trial. Complement. Ther. Med. 2020, 51, 102447. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S.J.; Hajiani, E.; Sardabi, E.H. A Placebo-Controlled Trial of Silymarin in Patients with Nonalcoholic Fatty Liver Disease. Hepat. Mon. 2009, 9, 265–270. [Google Scholar]
- Heebøll, S.; Kreuzfeldt, M.; Hamilton-Dutoit, S.; Kjær Poulsen, M.; Stødkilde-Jørgensen, H.; Møller, H.J.; Jessen, N.; Thorsen, K.; Kristina Hellberg, Y.; Bønløkke Pedersen, S.; et al. Placebo-controlled, randomised clinical trial: High-dose resveratrol treatment for non-alcoholic fatty liver disease. Scand. J. Gastroenterol. 2016, 51, 456–464. [Google Scholar] [CrossRef]
- Hosseinabadi, S.; Rafraf, M.; Asghari, S.; Asghari-Jafarabadi, M.; Vojouhi, S. Effect of green coffee extract supplementation on serum adiponectin concentration and lipid profile in patients with non-alcoholic fatty liver disease: A randomized, controlled trial. Complement. Ther. Med. 2020, 49, 102290. [Google Scholar] [CrossRef]
- Hosseinabadi, S.; Rafraf, M.; Asghari-Jafarabadi, M. Effects of green coffee extract supplementation on blood pressure and antioxidants status in patients with non-alcoholic fatty liver disease. Prog. Nutr. 2019, 21, 180–189. [Google Scholar] [CrossRef]
- Hosseinabadi, S.; Rafraf, M.; Mahmoodzadeh, A.; Asghari-Jafarabadi, M.; Asghari, S. Effects of green coffee extract supplementation on glycemic indexes, leptin, and obesity values in patients with non-alcoholic fatty liver disease. J. Herb. Med. 2020, 22, 100340. [Google Scholar] [CrossRef]
- Hsu, C.H.; Tsai, T.H.; Kao, Y.H.; Hwang, K.C.; Tseng, T.Y.; Chou, P. Effect of green tea extract on obese women: A randomized, double-blind, placebo-controlled clinical trial. Clin. Nutr. 2008, 27, 363–370. [Google Scholar] [CrossRef]
- Hussain, M.; Habib Ur, R.; Akhtar, L. Therapeutic benefits of green tea extract on various parameters in non-alcoholic fatty liver disease patients. Pak. J. Med. Sci. 2017, 33, 931–936. [Google Scholar] [CrossRef]
- Jarhahzadeh, M.; Alavinejad, P.; Farsi, F.; Husain, D.; Rezazadeh, A. The effect of turmeric on lipid profile, malondialdehyde, liver echogenicity and enzymes among patients with nonalcoholic fatty liver disease: A randomized double blind clinical trial. Diabetol. Metab. Syndr. 2021, 13, 112. [Google Scholar] [CrossRef] [PubMed]
- Jazayeri-Tehrani, S.A.; Rezayat, S.M.; Mansouri, S.; Qorbani, M.; Alavian, S.M.; Daneshi-Maskooni, M.; Hosseinzadeh-Attar, M.-J. Nano-curcumin improves glucose indices, lipids, inflammation, and Nesfatin in overweight and obese patients with non-alcoholic fatty liver disease (NAFLD): A double-blind randomized placebo-controlled clinical trial. Nutr. Metab. 2019, 16, 8. [Google Scholar] [CrossRef]
- Kavyani, M.; Saleh-Ghadimi, S.; Dehghan, P.; Abbasalizad Farhangi, M.; Khoshbaten, M. Co-supplementation of camelina oil and a prebiotic is more effective for in improving cardiometabolic risk factors and mental health in patients with NAFLD: A randomized clinical trial. Food Funct. 2021, 12, 8594–8604. [Google Scholar] [CrossRef]
- Khonche, A.; Huseini, H.F.; Gholamian, M.; Mohtashami, R.; Nabati, F.; Kianbakht, S. Standardized Nigella sativa seed oil ameliorates hepatic steatosis, aminotransferase and lipid levels in non-alcoholic fatty liver disease: A randomized, double-blind and placebo-controlled clinical trial. J. Ethnopharmacol. 2019, 234, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Mansour, A.; Mohajeri-Tehrani, M.R.; Karimi, S.; Sanginabadi, M.; Poustchi, H.; Enayati, S.; Asgarbeik, S.; Nasrollahzadeh, J.; Hekmatdoost, A. Short term effects of coffee components consumption on gut microbiota in patients with non-alcoholic fatty liver and diabetes: A pilot randomized placebo-controlled, clinical trial. Excli J. 2020, 19, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Mansour, A.; Mohajeri-Tehrani, M.R.; Samadi, M.; Qorbani, M.; Merat, S.; Adibi, H.; Poustchi, H.; Hekmatdoost, A. Effects of supplementation with main coffee components including caffeine and/or chlorogenic acid on hepatic, metabolic, and inflammatory indices in patients with non-alcoholic fatty liver disease and type 2 diabetes: A randomized, double-blind, placebo-controlled, clinical trial. Nutr. J. 2021, 20, 35. [Google Scholar] [CrossRef]
- Memon, I.A.; Akbar, M.; Bhurgri, A.N. Effect of silymarin therapy on liver aminotransferase in non-alcoholic fatty liver disease. Med. Forum Mon. 2015, 26, 46–49. [Google Scholar]
- Mielgo-Ayuso, J.; Barrenechea, L.; Alcorta, P.; Larrarte, E.; Margareto, J.; Labayen, I. Effects of dietary supplementation with epigallocatechin-3-gallate on weight loss, energy homeostasis, cardiometabolic risk factors and liver function in obese women: Randomised, double-blind, placebo-controlled clinical trial. Br. J. Nutr. 2014, 111, 1263–1271. [Google Scholar] [CrossRef]
- Mirhafez, S.R.; Dehabeh, M.; Hariri, M.; Farimani, A.R.; Movahedi, A.; Naderan, R.D.; Jamialahmadi, T.; Simental-Mendía, L.E.; Sahebkar, A. Curcumin and Piperine Combination for the Treatment of Patients with Non-alcoholic Fatty Liver Disease: A Double-Blind Randomized Placebo-Controlled Trial. Adv. Exp. Med. Biol. 2021, 1328, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Mirhafez, S.R.; Farimani, A.R.; Dehhabe, M.; Bidkhori, M.; Hariri, M.; Motlagh Ghouchani, B.F.N.; Abdollahi, F. Effect of phytosomal curcumin on circulating levels of adiponectin and leptin in patients with non-alcoholic fatty liver disease: A randomized, double-blind, placebo-controlled clinical trial. J. Gastrointest. Liver Dis. 2019, 28, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Mirhafez, S.R.; Farimani, A.R.; Gholami, A.; Hooshmand, E.; Tavallaie, S.; Nobakht Gh, B.F.M. The effect of curcumin with piperine supplementation on pro-oxidant and antioxidant balance in patients with non-alcoholic fatty liver disease: A randomized, double-blind, placebo-controlled trial. Drug Metab. Pers. Ther. 2019, 34, 20180040. [Google Scholar] [CrossRef] [PubMed]
- Mirhafez, S.R.; Rezai, A.; Dehabeh, M.; Nobakht, M.G.B.F.; Bidkhori, M.; Sahebkar, A.; Hariri, M. Efficacy of phytosomal curcumin among patients with non-alcoholic fatty liver disease. Int. J. Vitam. Nutr. Res. 2019, 91, 1–9. [Google Scholar] [CrossRef]
- Mirhashemi, S.H.; Hakakzadeh, A.; Yeganeh, F.E.; Oshidari, B.; Rezaee, S.P. Effect of 8 Weeks milk thistle powder (silymarin extract) supplementation on fatty liver disease in patients candidates for bariatric surgery. Metab. Open 2022, 14, 100190. [Google Scholar] [CrossRef] [PubMed]
- Moradi Kelardeh, B.; Rahmati-Ahmadabad, S.; Farzanegi, P.; Helalizadeh, M.; Azarbayjani, M.-A. Effects of non-linear resistance training and curcumin supplementation on the liver biochemical markers levels and structure in older women with non-alcoholic fatty liver disease. J. Bodyw. Mov. Ther. 2020, 24, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Masoodi, M.; Rezadoost, A.; Panahian, M.; Vojdanian, M. Effects of Silymarin on Reducing Liver Aminotransferases in Patients with Nonalcoholic Fatty Liver Diseases. Iran. J. Gastroenterol. Hepatol. 2013, 18, 181–185. [Google Scholar]
- Musazadeh, V.; Dehghan, P.; Khoshbaten, M. Efficacy of omega-3-rich Camelina sativa on the metabolic and clinical markers in nonalcoholic fatty liver disease: A randomized, controlled trial. Eur. J. Gastroenterol. Hepatol. 2022, 34, 537–545. [Google Scholar] [CrossRef]
- Musazadeh, V.; Dehghan, P.; Saleh-Ghadimi, S.; Abbasalizad Farhangi, M. Omega 3-rich Camelina sativa oil in the context of a weight loss program improves glucose homeostasis, inflammation and oxidative stress in patients with NAFLD: A randomised placebo-controlled clinical trial. Int. J. Clin. Pract. 2021, 75, e14744. [Google Scholar] [CrossRef]
- Musolino, V.; Gliozzi, M.; Bombardelli, E.; Nucera, S.; Carresi, C.; Maiuolo, J.; Mollace, R.; Paone, S.; Bosco, F.; Scarano, F.; et al. The synergistic effect of Citrus bergamia and Cynara cardunculus extracts on vascular inflammation and oxidative stress in non-alcoholic fatty liver disease. J. Tradit. Complement. Med. 2020, 10, 268–274. [Google Scholar] [CrossRef]
- Naseri, K.; Saadati, S.; Yari, Z.; Askari, B.; Mafi, D.; Hoseinian, P.; Asbaghi, O.; Hekmatdoost, A.; de Courten, B. Curcumin Offers No Additional Benefit to Lifestyle Intervention on Cardiometabolic Status in Patients with Non-Alcoholic Fatty Liver Disease. Nutrients 2022, 14, 3224. [Google Scholar] [CrossRef]
- Navarro, V.J.; Belle, S.H.; D’Amato, M.; Adfhal, N.; Brunt, E.M.; Fried, M.W.; Reddy, K.R.; Wahed, A.S.; Harrison, S.; on behalf of the Silymarin in NASH and C Hepatitis (SyNCH) Study Group. Silymarin in non-cirrhotics with non-alcoholic steatohepatitis: A randomized, double-blind, placebo controlled trial. PLoS ONE 2019, 14, e0221683. [Google Scholar] [CrossRef]
- Navekar, R.; Rafraf, M.; Ghaffari, A.; Asghari-Jafarabadi, M.; Khoshbaten, M. Turmeric Supplementation Improves Serum Glucose Indices and Leptin Levels in Patients with Nonalcoholic Fatty Liver Diseases. J. Am. Coll. Nutr. 2017, 36, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Nejati, L.; Movahedi, A.; Salari, G.; Moeineddin, R.; Nejati, P. The Effect of Berberine on Lipid Profile, Liver Enzymes, and Fasting Blood Glucose in Patients with Non-alcoholic Fatty Liver Disease (NAFLD): A Randomized Controlled Trial. Med. J. Islam. Repub. Iran 2022, 36, 39. [Google Scholar] [CrossRef]
- Panahi, Y.; Kianpour, P.; Mohtashami, R.; Jafari, R.; Simental-Mendía, L.E.; Sahebkar, A. Curcumin Lowers Serum Lipids and Uric Acid in Subjects With Nonalcoholic Fatty Liver Disease: A Randomized Controlled Trial. J. Cardiovasc. Pharmacol. 2016, 68, 223–229. [Google Scholar] [CrossRef]
- Panahi, Y.; Kianpour, P.; Mohtashami, R.; Jafari, R.; Simental-Mendía, L.E.; Sahebkar, A. Efficacy and Safety of Phytosomal Curcumin in Non-Alcoholic Fatty Liver Disease: A Randomized Controlled Trial. Drug Res. 2017, 67, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Panahi, Y.; Valizadegan, G.; Ahamdi, N.; Ganjali, S.; Majeed, M.; Sahebkar, A. Curcuminoids plus piperine improve nonalcoholic fatty liver disease: A clinical trial. J. Cell. Biochem. 2019, 120, 15989–15996. [Google Scholar] [CrossRef] [PubMed]
- Pezeshki, A.; Safi, S.; Feizi, A.; Askari, G.; Karami, F. The effect of green tea extract supplementation on liver enzymes in patients with nonalcoholic fatty liver disease. Int. J. Prev. Med. 2015, 2015, 173051. [Google Scholar] [CrossRef]
- Poulos, J.E.; Kalogerinis, P.T.; Milanov, V.; Kalogerinis, C.T.; Poulos, E.J. The Effects of Vitamin E, Silymarin and Carnitine on the Metabolic Abnormalities Associated with Nonalcoholic Liver Disease. J. Diet. Suppl. 2022, 19, 287–302. [Google Scholar] [CrossRef]
- Poulsen, M.K.; Nellemann, B.; Bibby, B.M.; Stødkilde-jørgensen, H.; Pedersen, S.B.; Grønbæk, H.; Nielsen, S. No effect of resveratrol on VLDL-TG kinetics and insulin sensitivity in obese men with nonalcoholic fatty liver disease. Diabetes Obes. Metab. 2018, 20, 2504–2509. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, S.; Asgary, S.; Askari, G.; Keshvari, M.; Hatamipour, M.; Feizi, A.; Sahebkar, A. Treatment of Non-alcoholic Fatty Liver Disease with Curcumin: A Randomized Placebo-controlled Trial. Phytother. Res. PTR 2016, 30, 1540–1548. [Google Scholar] [CrossRef]
- Rashidmayvan, M.; Vandyousefi, S.; Barati, M.; Salamat, S.; Ghodrat, S.; Khorasanchi, M.; Jahan-Mihan, A.; Nattagh-Eshtivani, E.; Mohammadshahi, M. The effect of nigella sativa supplementation on cardiometabolic outcomes in patients with non-alcoholic fatty liver: A randomized double-blind, placebo-controlled trial. Complement. Ther. Clin. Pract. 2022, 48, 101598. [Google Scholar] [CrossRef] [PubMed]
- Saadati, S.; Hatami, B.; Yari, Z.; Shahrbaf, M.A.; Eghtesad, S.; Mansour, A.; Poustchi, H.; Hedayati, M.; Aghajanpoor-pasha, M.; Sadeghi, A.; et al. The effects of curcumin supplementation on liver enzymes, lipid profile, glucose homeostasis, and hepatic steatosis and fibrosis in patients with non-alcoholic fatty liver disease. Eur. J. Clin. Nutr. 2019, 73, 441–449. [Google Scholar] [CrossRef]
- Saadati, S.; Hekmatdoost, A.; Hatami, B.; Mansour, A.; Yari, Z.; Hedayati, M.; Sadeghi, A. Comparing different non-invasive methods in assessment of the effects of curcumin on hepatic fibrosis in patients with non-alcoholic fatty liver disease. Gastroenterol. Hepatol. Bed Bench 2018, 11, S8–S13. [Google Scholar] [PubMed]
- Saadati, S.; Sadeghi, A.; Mansour, A.; Yari, Z.; Poustchi, H.; Hedayati, M.; Hatami, B.; Hekmatdoost, A. Curcumin and inflammation in non-alcoholic fatty liver disease: A randomized, placebo controlled clinical trial. BMC Gastroenterol. 2019, 19, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Saberi-Karimian, M.; Keshvari, M.; Ghayour-Mobarhan, M.; Salehizadeh, L.; Rahmani, S.; Behnam, B.; Jamialahmadi, T.; Asgary, S.; Sahebkar, A. Effects of curcuminoids on inflammatory status in patients with non-alcoholic fatty liver disease: A randomized controlled trial. Complement. Ther. Med. 2020, 49, 102322. [Google Scholar] [CrossRef] [PubMed]
- Safari, Z.; Bagherniya, M.; Khoram, Z.; Ebrahimi Varzaneh, A.; Heidari, Z.; Sahebkar, A.; Askari, G. The effect of curcumin on anthropometric indices, blood pressure, lipid profiles, fasting blood glucose, liver enzymes, fibrosis, and steatosis in non-alcoholic fatty livers. Front. Nutr. 2023, 10, 1163950. [Google Scholar] [CrossRef] [PubMed]
- Sakata, R.; Nakamura, T.; Torimura, T.; Ueno, T.; Sata, M. Green tea with high-density catechins improves liver function and fat infiltration in non-alcoholic fatty liver disease (NAFLD) patients: A double-blind placebo-controlled study. Int. J. Mol. Med. 2013, 32, 989–994. [Google Scholar] [CrossRef]
- Sangouni, A.A.; Mohammad Hosseini Azar, M.R.; Alizadeh, M. Effect of garlic powder supplementation on hepatic steatosis, liver enzymes and lipid profile in patients with non-alcoholic fatty liver disease: A double-blind randomised controlled clinical trial. Br. J. Nutr. 2020, 124, 450–456. [Google Scholar] [CrossRef]
- Sangouni, A.A.; Mohammad Hosseini Azar, M.R.; Alizadeh, M. Effects of garlic powder supplementation on insulin resistance, oxidative stress, and body composition in patients with non-alcoholic fatty liver disease: A randomized controlled clinical trial. Complement. Ther. Med. 2020, 51, 102428. [Google Scholar] [CrossRef] [PubMed]
- Sangouni, A.A.; Sangsefidi, Z.S.; Yarhosseini, F.; Hosseinzadeh, M.; Akhondi-Meybodi, M.; Ranjbar, A.; Madadizadeh, F.; Mozaffari-Khosravi, H. Effect of Cornus mas L. fruit extract on lipid accumulation product and cardiovascular indices in patients with non-alcoholic fatty liver disease: A double-blind randomized controlled trial. Clin. Nutr. ESPEN 2022, 47, 51–57. [Google Scholar] [CrossRef]
- Sangsefidi, Z.S.; Yarhosseini, F.; Hosseinzadeh, M.; Ranjbar, A.; Akhondi-Meybodi, M.; Fallahzadeh, H.; Mozaffari-Khosravi, H. The effect of (Cornus mas L.) fruit extract on liver function among patients with nonalcoholic fatty liver: A double-blind randomized clinical trial. Phytother. Res. PTR 2021, 35, 5259–5268. [Google Scholar] [CrossRef] [PubMed]
- Shahmohammadi, H.A.; Hosseini, S.A.; Hajiani, E.; Malehi, A.S.; Alipour, M. Effects of green coffee bean extract supplementation on patients with non-alcoholic fatty liver disease: A randomized clinical trial. Hepat. Mon. 2017, 17, e12299. [Google Scholar] [CrossRef]
- Sharifi, S.; Bagherniya, M.; Khoram, Z.; Ebrahimi Varzaneh, A.; Atkin, S.L.; Jamialahmadi, T.; Sahebkar, A.; Askari, G. Efficacy of curcumin plus piperine co-supplementation in moderate-to-high hepatic steatosis: A double-blind, randomized, placebo-controlled clinical trial. Phytother. Res. PTR 2023, 37, 2217–2229. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, D.; Paknahad, Z.; Rouhani, M.H. Therapeutic Effects of Garlic on Hepatic Steatosis in Nonalcoholic Fatty Liver Disease Patients: A Randomized Clinical Trial. Diabetes Metab. Syndr. Obes. 2020, 13, 2389–2397. [Google Scholar] [CrossRef] [PubMed]
- Solhi, H.; Ghahremani, R.; Kazemifar, A.M.; Yazdi, Z.H. Silymarin in treatment of non-alcoholic steatohepatitis: A randomized clinical trial. Casp. J. Intern. Med. 2014, 5, 9–12. [Google Scholar]
- Sorrentino, G.; Crispino, P.; Coppola, D.; De Stefano, G. Efficacy of Lifestyle Changes in Subjects with Non-Alcoholic Liver Steatosis and Metabolic Syndrome May Be Improved with an Antioxidant Nutraceutical: A Controlled Clinical Study. Drugs R D 2015, 15, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaee, S.M.; Alavian, S.M.; Ghalichi, L.; Miryounesi, S.M.; Mousavizadeh, K.; Jazayeri, S.; Vafa, M.R. Green tea in non-alcoholic fatty liver disease: A double blind randomized clinical trial. Hepat. Mon. 2017, 17, 6. [Google Scholar] [CrossRef]
- Theodotou, M.; Fokianos, K.; Moniatis, D.; Kadlenic, R.; Chrysikou, A.; Aristotelous, A.; Mouzouridou, A.; Diakides, J.; Stavrou, E. Effect of resveratrol on non-alcoholic fatty liver disease. Exp. Ther. Med. 2019, 18, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Wah Kheong, C.; Nik Mustapha, N.R.; Mahadeva, S. A Randomized Trial of Silymarin for the Treatment of Nonalcoholic Steatohepatitis. Clin. Gastroenterol. Hepatol. 2017, 15, 1940. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.-M.; Xia, M.-F.; Wang, Y.; Chang, X.-X.; Yao, X.-Z.; Rao, S.-X.; Zeng, M.-S.; Tu, Y.-F.; Feng, R.; Jia, W.-P.; et al. Efficacy of Berberine in Patients with Non-Alcoholic Fatty Liver Disease. PLoS ONE 2015, 10, e0134172. [Google Scholar] [CrossRef] [PubMed]
- Yarhosseini, F.; Sangouni, A.A.; Sangsefidi, Z.S.; Hosseinzadeh, M.; Akhondi-Meybodi, M.; Ranjbar, A.; Fallahzadeh, H.; Mozaffari-Khosravi, H. Effect of Cornus mas L. fruit extract on blood pressure, anthropometric and body composition indices in patients with non-alcoholic fatty liver disease: A double-blind randomized controlled trial. Clin. Nutr. ESPEN 2023, 56, 18–24. [Google Scholar] [CrossRef]
- Afsharinasab, M.; Mohammad-Sadeghipour, M.; Reza Hajizadeh, M.; Khoshdel, A.; Mirzaiey, V.; Mahmoodi, M. The effect of hydroalcoholic Berberis integerrima fruits extract on the lipid profile, antioxidant parameters and liver and kidney function tests in patients with nonalcoholic fatty liver disease. Saudi J. Biol. Sci. 2020, 27, 2031–2037. [Google Scholar] [CrossRef] [PubMed]
- Cossiga, V.; Lembo, V.; Guarino, M.; Tuccillo, C.; Morando, F.; Pontillo, G.; Fiorentino, A.; Caporaso, N.; Morisco, F. Berberis aristata, Elaeis guineensis and Coffea canephora Extracts Modulate the Insulin Receptor Expression and Improve Hepatic Steatosis in NAFLD Patients: A Pilot Clinical Trial. Nutrients 2019, 11, 3070. [Google Scholar] [CrossRef]
- Afzali, N.; Ebadi, S.S.; Afzali, H.; Sharif, M.R.; Vazirian, M.; Ebadi, S.A.; Shahkarami, V.; Rahimi, H. Effect of Beta vulgaris Extract on Liver Enzymes in Patients with Non-Alcoholic Fatty Liver Disease: A Randomized Clinical Trial. Hepat. Mon. 2020, 20, e102125. [Google Scholar] [CrossRef]
- Akbari, S.; Sohouli, M.H.; Ebrahimzadeh, S.; Ghanaei, F.M.; Hosseini, A.F.; Aryaeian, N. Effect of rosemary leaf powder with weight loss diet on lipid profile, glycemic status, and liver enzymes in patients with nonalcoholic fatty liver disease: A randomized, double-blind clinical trial. Phytother. Res. PTR 2022, 36, 2186–2196. [Google Scholar] [CrossRef] [PubMed]
- Amanat, S.; Eftekhari, M.H.; Fararouei, M.; Bagheri Lankarani, K.; Massoumi, S.J. Genistein supplementation improves insulin resistance and inflammatory state in non-alcoholic fatty liver patients: A randomized, controlled trial. Clin. Nutr. 2018, 37, 1210–1215. [Google Scholar] [CrossRef] [PubMed]
- Amerikanou, C.; Kanoni, S.; Kaliora, A.C.; Barone, A.; Bjelan, M.; D’Auria, G.; Gioxari, A.; Gosalbes, M.a.J.; Mouchti, S.; Stathopoulou, M.G.; et al. Effect of Mastiha supplementation on NAFLD: The MAST4HEALTH Randomised, Controlled Trial. Mol. Nutr. Food Res. 2021, 65, e2001178. [Google Scholar] [CrossRef]
- Askari, F.; Rashidkhani, B.; Hekmatdoost, A. Cinnamon may have therapeutic benefits on lipid profile, liver enzymes, insulin resistance, and high-sensitivity C-reactive protein in nonalcoholic fatty liver disease patients. Nutr. Res. 2014, 34, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Babaei, A.; Taghavi, S.A.; Mohammadi, A.; Mahdiyar, M.A.; Iranpour, P.; Ejtehadi, F.; Mohagheghzadeh, A. Comparison of the efficacy of oral fenugreek seeds hydroalcoholic extract versus placebo in nonalcoholic fatty liver disease; a randomized, triple-blind controlled pilot clinical trial. Indian J. Pharmacol. 2020, 52, 86–93. [Google Scholar] [CrossRef]
- Barghchi, H.; Milkarizi, N.; Belyani, S.; Norouzian Ostad, A.; Askari, V.R.; Rajabzadeh, F.; Goshayeshi, L.; Ghelichi Kheyrabadi, S.Y.; Razavidarmian, M.; Dehnavi, Z.; et al. Pomegranate (Punica granatum L.) peel extract ameliorates metabolic syndrome risk factors in patients with non-alcoholic fatty liver disease: A randomized double-blind clinical trial. Nutr. J. 2023, 22, 40. [Google Scholar] [CrossRef]
- Castellino, G.; Nikolic, D.; Magán-Fernández, A.; Malfa, G.A.; Chianetta, R.; Patti, A.M.; Amato, A.; Montalto, G.; Toth, P.P.; Banach, M.; et al. Altilix® Supplement Containing Chlorogenic Acid and Luteolin Improved Hepatic and Cardiometabolic Parameters in Subjects with Metabolic Syndrome: A 6 Month Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2019, 11, 2580. [Google Scholar] [CrossRef] [PubMed]
- Cerletti, C.; Colucci, M.; Storto, M.; Semeraro, F.; Ammollo, C.T.; Incampo, F.; Costanzo, S.; De Bartolomeo, G.; Portincasa, P.; Barone, M.; et al. Randomized trial of chronic supplementation with a nutraceutical mixture to subjects with Non Alcoholic Fatty Liver Disease (NAFLD). Br. J. Nutr. 2020, 123, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Cheraghpour, M.; Imani, H.; Ommi, S.; Alavian, S.M.; Karimi-Shahrbabak, E.; Hedayati, M.; Yari, Z.; Hekmatdoost, A. Hesperidin improves hepatic steatosis, hepatic enzymes, and metabolic and inflammatory parameters in patients with nonalcoholic fatty liver disease: A randomized, placebo-controlled, double-blind clinical trial. Phytother. Res. PTR 2019, 33, 2118–2125. [Google Scholar] [CrossRef] [PubMed]
- Chiou, Y.-L.; Chyau, C.-C.; Li, T.-J.; Kuo, C.-F.; Kang, Y.-Y.; Chen, C.-C.; Ko, W.-S. Hepatoprotective Effect of Mycelium in Patients with Nonalcoholic Steatohepatitis: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Am. Coll. Nutr. 2021, 40, 349–357. [Google Scholar] [CrossRef]
- Chou, S.C.; Chen, K.W.; Hwang, J.S.; Lu, W.T.; Chu, Y.Y.; Lin, J.D.; Chang, H.J.; See, L.C. The add-on effects of Gynostemma pentaphyllum on nonalcoholic fatty liver disease. Altern. Ther. Health Med. 2006, 12, 34–39. [Google Scholar]
- Daneshi-Maskooni, M.; Keshavarz, S.A.; Qorbani, M.; Mansouri, S.; Alavian, S.M.; Badri-Fariman, M.; Jazayeri-Tehrani, S.A.; Sotoudeh, G. Green cardamom increases Sirtuin-1 and reduces inflammation in overweight or obese patients with non-alcoholic fatty liver disease: A double-blind randomized placebo-controlled clinical trial. Nutr. Metab. 2018, 15, 63. [Google Scholar] [CrossRef] [PubMed]
- Daneshi-Maskooni, M.; Keshavarz, S.A.; Qorbani, M.; Mansouri, S.; Alavian, S.M.; Badri-Fariman, M.; Jazayeri-Tehrani, S.A.; Sotoudeh, G. Green cardamom supplementation improves serum irisin, glucose indices, and lipid profiles in overweight or obese non-alcoholic fatty liver disease patients: A double-blind randomized placebo-controlled clinical trial. BMC Complement. Altern. Med. 2019, 19, 59. [Google Scholar] [CrossRef]
- Darvish Damavandi, R.; Shidfar, F.; Najafi, M.; Janani, L.; Masoodi, M.; Akbari-Fakhrabadi, M.; Dehnad, A. Effect of Portulaca Oleracea (purslane) extract on liver enzymes, lipid profile, and glycemic status in nonalcoholic fatty liver disease: A randomized, double-blind clinical trial. Phytother. Res. PTR 2021, 35, 3145–3156. [Google Scholar] [CrossRef]
- Ebrahimi-Mameghani, M.; Aliashrafi, S.; Javadzadeh, Y.; AsghariJafarabadi, M. The Effect of Chlorella vulgaris Supplementation on Liver Enzymes, Serum Glucose and Lipid Profile in Patients with Non-Alcoholic Fatty Liver Disease. Health Promot. Perspect. 2014, 4, 107–115. [Google Scholar] [CrossRef][Green Version]
- Ebrahimi-Mameghani, M.; Sadeghi, Z.; Abbasalizad Farhangi, M.; Vaghef-Mehrabany, E.; Aliashrafi, S. Glucose homeostasis, insulin resistance and inflammatory biomarkers in patients with non-alcoholic fatty liver disease: Beneficial effects of supplementation with microalgae Chlorella vulgaris: A double-blind placebo-controlled randomized clinical trial. Clin. Nutr. 2017, 36, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Haidari, F.; Hojhabrimanesh, A.; Helli, B.; Seyedian, S.S.; Ahmadi-Angali, K. An energy-restricted high-protein diet supplemented with β-cryptoxanthin alleviated oxidative stress and inflammation in nonalcoholic fatty liver disease: A randomized controlled trial. Nutr. Res. 2020, 73, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Haidari, F.; Hojhabrimanesh, A.; Helli, B.; Seyedian, S.S.; Ahmadi-Angali, K.; Abiri, B. A hypocaloric high-protein diet supplemented with β-cryptoxanthin improves non-alcoholic fatty liver disease: A randomized controlled trial. BMC Gastroenterol. 2020, 20, 349. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Kim, S.M.; Nam, G.E.; Kim, S.H.; Park, S.J.; Park, Y.K.; Baik, H.W. A Randomized, Double-Blind, Placebo-Controlled, Multi-Centered Clinical Study to Evaluate the Efficacy and Safety of Artemisia annua L. Extract for Improvement of Liver Function. Clin. Nutr. Res. 2020, 9, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Hongguang, J.; Xingjie, H.; Mingliang, J.; Yun, L.; Tongjian, L.; Jingmo, Y.; Liang, L.; Lifang, Z. Clinical effect of the extract of TCM Fructus akebiae combined with ursodeoxycholic acid on nonalcoholic fatty liver disease. Pak. J. Pharm. Sci. 2019, 32, 433–437. [Google Scholar]
- Hormoznejad, R.; Mohammad Shahi, M.; Rahim, F.; Helli, B.; Alavinejad, P.; Sharhani, A. Combined cranberry supplementation and weight loss diet in non-alcoholic fatty liver disease: A double-blind placebo-controlled randomized clinical trial. Int. J. Food Sci. Nutr. 2020, 71, 991–1000. [Google Scholar] [CrossRef]
- Illnait, J.; Rodriguez, I.; Mendoza, S.; Fernandez, Y.; Mas, R.; Miranda, M.; Pinera, J.; Fernandez, J.C.; Mesa, M.; Fernandez, L.; et al. Effects of D-002, a mixture of high molecular weight beeswax alcohols, on patients with nonalcoholic fatty liver disease. Korean J. Intern. Med. 2013, 28, 439–448. [Google Scholar] [CrossRef]
- Izadi, F.; Farrokhzad, A.; Tamizifar, B.; Tarrahi, M.J.; Entezari, M.H. Effect of sour tea supplementation on liver enzymes, lipid profile, blood pressure, and antioxidant status in patients with non-alcoholic fatty liver disease: A double-blind randomized controlled clinical trial. Phytother. Res. PTR 2020, 35, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Jazayeri, S.F.; Ghods, R.; Hashem Dabaghian, F.; Shojaii, A.; Moravej, S.A.A.-H.; Khadem, E.; Seyedian, S.S. The Efficacy of Plantago major Seed on Liver Enzymes in Nonalcoholic Fatty Liver Disease: A Randomized Double-Blind Clinical Trial. Evid.-Based Complement. Altern. Med. 2021, 2021, 6693887. [Google Scholar] [CrossRef] [PubMed]
- Jinato, T.; Chayanupatkul, M.; Dissayabutra, T.; Chutaputti, A.; Tangkijvanich, P.; Chuaypen, N. Litchi-Derived Polyphenol Alleviates Liver Steatosis and Gut Dysbiosis in Patients with Non-Alcoholic Fatty Liver Disease: A Randomized Double-Blinded, Placebo-Controlled Study. Nutrients 2022, 14, 2921. [Google Scholar] [CrossRef] [PubMed]
- Kanoni, S.; Kumar, S.; Amerikanou, C.; Kurth, M.J.; Stathopoulou, M.G.; Bourgeois, S.; Masson, C.; Kannt, A.; Cesarini, L.; Kontoe, M.-S.; et al. Nutrigenetic Interactions Might Modulate the Antioxidant and Anti-Inflammatory Status in Mastiha-Supplemented Patients With NAFLD. Front. Immunol. 2021, 12, 683028. [Google Scholar] [CrossRef]
- Kazemi, S.; Shidfar, F.; Ehsani, S.; Adibi, P.; Janani, L.; Eslami, O. The effects of sumac (Rhus coriaria L.) powder supplementation in patients with non-alcoholic fatty liver disease: A randomized controlled trial. Complement. Ther. Clin. Pract. 2020, 41, 101259. [Google Scholar] [CrossRef] [PubMed]
- Khoshbaten, M.; Aliasgarzadeh, A.; Masnadi, K.; Farhang, S.; Tarzamani, M.K.; Babaei, H.; Kiani, J.; Zaare, M.; Najafipoor, F. Grape seed extract to improve liver function in patients with nonalcoholic fatty liver change. Saudi J. Gastroenterol. 2010, 16, 194–197. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Lim, Y.; Kwon, S.W.; Kwon, O. Pinitol consumption improves liver health status by reducing oxidative stress and fatty acid accumulation in subjects with non-alcoholic fatty liver disease: A randomized, double-blind, placebo-controlled trial. J. Nutr. Biochem. 2019, 68, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Majnooni, M.B.; Ataee, M.; Bahrami, G.; Heydarpour, F.; Aneva, I.Y.; Farzaei, M.H.; Ahmadi-Juoibari, T. The effects of co-administration of artichoke leaf extract supplementation with metformin and vitamin E in patients with nonalcoholic fatty liver disease: A randomized clinical trial. Phytother. Res. PTR 2021, 35, 6324–6334. [Google Scholar] [CrossRef] [PubMed]
- Masnadi Shirazi, K.; Shirinpour, E.; Masnadi Shirazi, A.; Nikniaz, Z. Effect of cranberry supplementation on liver enzymes and cardiometabolic risk factors in patients with NAFLD: A randomized clinical trial. BMC Complement. Med. Ther. 2021, 21, 283. [Google Scholar] [CrossRef]
- Namkhah, Z.; Naeini, F.; Mahdi Rezayat, S.; Yaseri, M.; Mansouri, S.; Javad Hosseinzadeh-Attar, M. Does naringenin supplementation improve lipid profile, severity of hepatic steatosis and probability of liver fibrosis in overweight/obese patients with NAFLD? A randomised, double-blind, placebo-controlled, clinical trial. Int. J. Clin. Pract. 2021, 75, 1–11. [Google Scholar] [CrossRef]
- Nomi-Golzar, S.; Mahboob, S.; Tavakkoli, S.; Asghari Jafarabadi, M.; Rezazadeh, K.; Vaghef-Mehrabany, E.; Ebrahimi-Mameghani, M. Effects of hydroxy citric acid on body weight and serum hepcidin level in women with non-alcoholic fatty liver disease. Adv. Integr. Med. 2021, 8, 122–128. [Google Scholar] [CrossRef]
- Panahi, Y.; Kianpour, P.; Mohtashami, R.; Atkin, S.L.; Butler, A.E.; Jafari, R.; Badeli, R.; Sahebkar, A. Efficacy of artichoke leaf extract in non-alcoholic fatty liver disease: A pilot double-blind randomized controlled trial. Phytother. Res. 2018, 32, 1382–1387. [Google Scholar] [CrossRef] [PubMed]
- Pour, F.K.; Aryaeian, N.; Mokhtare, M.; Mirnasrollahi Parsa, R.S.; Jannani, L.; Agah, S.; Fallah, S.; Moradi, N. The effect of saffron supplementation on some inflammatory and oxidative markers, leptin, adiponectin, and body composition in patients with nonalcoholic fatty liver disease: A double-blind randomized clinical trial. Phytother. Res. 2020, 34, 3367–3378. [Google Scholar] [CrossRef]
- Rafie, R.; Hosseini, S.A.; Hajiani, E.; Saki Malehi, A.; Mard, S.A. Effect of Ginger Powder Supplementation in Patients with Non-Alcoholic Fatty Liver Disease: A Randomized Clinical Trial. Clin. Exp. Gastroenterol. 2020, 13, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Rahimlou, M.; Yari, Z.; Hekmatdoost, A.; Alavian, S.M.; Keshavarz, S.A. Ginger Supplementation in Nonalcoholic Fatty Liver Disease: A Randomized, Double-Blind, Placebo-Controlled Pilot Study. Hepat. Mon. 2016, 16, e34897. [Google Scholar] [CrossRef]
- Rostamizadeh, P.; Asl, S.M.K.H.; Far, Z.G.; Ahmadijoo, P.; Mahmudiono, T.; Bokov, D.O.; Alsaikhan, F.; Jannat, B.; Mazloom, Z. Effects of licorice root supplementation on liver enzymes, hepatic steatosis, metabolic and oxidative stress parameters in women with nonalcoholic fatty liver disease: A randomized double-blind clinical trial. Phytother. Res. PTR 2022, 36, 3949–3956. [Google Scholar] [CrossRef] [PubMed]
- Shih, P.H.; Shiue, S.J.; Chen, C.N.; Cheng, S.W.; Lin, H.Y.; Wu, L.W.; Wu, M.S. Fucoidan and Fucoxanthin Attenuate Hepatic Steatosis and Inflammation of NAFLD through Modulation of Leptin/Adiponectin Axis. Mar. Drugs 2021, 19, 148. [Google Scholar] [CrossRef]
- Tutunchi, H.; Arefhosseini, S.; Ebrahimi-Mameghani, M. Clinical effectiveness of α-lipoic acid, myo-inositol and propolis supplementation on metabolic profiles and liver function in obese patients with NAFLD: A randomized controlled clinical trial. Clin. Nutr. ESPEN 2023, 54, 412–420. [Google Scholar] [CrossRef]
- Tutunchi, H.; Arefhosseini, S.; Nomi-Golzar, S.; Ebrahimi-Mameghani, M. Effects of Hydroxycitric Acid Supplementation on Body Composition, Obesity Indices, Appetite, Leptin, and Adiponectin of Women with NAFLD on a Calorie-Restricted Diet. Int. J. Clin. Pract. 2023, 2023, 6492478. [Google Scholar] [CrossRef] [PubMed]
- Yari, Z.; Rahimlou, M.; Eslamparast, T.; Ebrahimi-Daryani, N.; Poustchi, H.; Hekmatdoost, A. Flaxseed supplementation in non-alcoholic fatty liver disease: A pilot randomized, open labeled, controlled study. Int. J. Food Sci. Nutr. 2016, 67, 461–469. [Google Scholar] [CrossRef]
- Yari, Z.; Cheraghpour, M.; Alavian, S.M.; Hedayati, M.; Eini-Zinab, H.; Hekmatdoost, A. The efficacy of flaxseed and hesperidin on non-alcoholic fatty liver disease: An open-labeled randomized controlled trial. Eur. J. Clin. Nutr. 2021, 75, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Zamani, N.; Shams, M.; Nimrouzi, M.; Zarshenas, M.M.; Abolhasani Foroughi, A.; Fallahzadeh Abarghooei, E.; Fattahi, M.R. The effects of Zataria multiflora Boiss. (Shirazi thyme) on nonalcoholic fatty liver disease and insulin resistance: A randomized double-blind placebo-controlled clinical trial. Complement. Ther. Med. 2018, 41, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.W.; Chen, F.X.; Li, D.; Ling, W.H.; Guo, H.H. A CONSORT-compliant, randomized, double-blind, placebo-controlled pilot trial of purified anthocyanin in patients with nonalcoholic fatty liver disease. Medicine 2015, 94, e758. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abunofal, O.; Mohan, C. Salubrious Effects of Green Tea Catechins on Fatty Liver Disease: A Systematic Review. Medicines 2022, 9, 20. [Google Scholar] [CrossRef]
- Asbaghi, O.; Kashkooli, S.; Mardani, M.; Rezaei Kelishadi, M.; Fry, H.; Kazemi, M.; Kaviani, M. Effect of green coffee bean extract supplementation on liver function and inflammatory biomarkers: A meta-analysis of randomized clinical trials. Complement. Ther. Clin. Pract. 2021, 43, 101349. [Google Scholar] [CrossRef] [PubMed]
- Baziar, N.; Parohan, M. The effects of curcumin supplementation on body mass index, body weight, and waist circumference in patients with nonalcoholic fatty liver disease: A systematic review and dose-response meta-analysis of randomized controlled trials. Phytother. Res. 2020, 34, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Ebadi, M.; Ip, S.; Bhanji, R.A.; Montano-Loza, A.J. Effect of Coffee Consumption on Non-Alcoholic Fatty Liver Disease Incidence, Prevalence and Risk of Significant Liver Fibrosis: Systematic Review with Meta-Analysis of Observational Studies. Nutrients 2021, 13, 3042. [Google Scholar] [CrossRef] [PubMed]
- Elgebaly, A.; Radwan, I.A.I.; AboElnas, M.M.; Ibrahim, H.H.; Eltoomy, M.F.M.; Atta, A.A.; Mesalam, H.A.; Sayed, A.A.; Othman, A.A. Resveratrol Supplementation in Patients with Non-Alcoholic Fatty Liver Disease: Systematic Review and Meta-analysis. J. Gastrointest. Liver Dis. JGLD 2017, 26, 59–67. [Google Scholar] [CrossRef]
- Fakhri, M.; Fakheri, H.; Azadbakht, M.; Moosazadeh, M.; Yousefi, S.S. Effect of Medicinal Plants and Natural Products on Liver Enzymes in Non-alcoholic Fatty Liver Patients in Iran: A Systematic Review and Meta-Analysis. Int. J. Prev. Med. 2022, 13, 87. [Google Scholar] [CrossRef]
- Goodarzi, R.; Sabzian, K.; Shishehbor, F.; Mansoori, A. Does turmeric/curcumin supplementation improve serum alanine aminotransferase and aspartate aminotransferase levels in patients with nonalcoholic fatty liver disease? A systematic review and meta-analysis of randomized controlled trials. Phytother. Res. 2019, 33, 561–570. [Google Scholar] [CrossRef]
- Hall, R.; George, E.; Tierney, A.; Reddy, A.J. The effect of dietary intervention, with or without co-interventions, on inflammatory markers in patients with non-alcoholic fatty liver disease: A systematic review and meta-analysis. Proc. Nutr. Soc. 2023, 82, E139. [Google Scholar] [CrossRef]
- Hayat, U.; Siddiqui, A.A.; Okut, H.; Afroz, S.; Tasleem, S.; Haris, A. The effect of coffee consumption on the non-alcoholic fatty liver disease and liver fibrosis: A meta-analysis of 11 epidemiological studies. Ann. Hepatol. 2021, 20, 100254. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Li, Y.; Deng, X.; Xiao, X.; Zeng, J. Integrative evidence construction for resveratrol treatment of nonalcoholic fatty liver disease: Preclinical and clinical meta-analyses. Front. Pharmacol. 2023, 14, 1230783. [Google Scholar] [CrossRef] [PubMed]
- Jafarirad, S.; Mansoori, A.; Adineh, A.; Panahi, Y.; Hadi, A.; Goodarzi, R. Does Turmeric/curcumin Supplementation Change Anthropometric Indices in Patients with Non-alcoholic Fatty Liver Disease? A Systematic Review and Meta-analysis of Randomized Controlled Trials. Clin. Nutr. Res. 2019, 8, 196–208. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Skonieczna-Żydecka, K.; Kałduńska, J.; Stachowska, E.; Gutowska, I.; Janda, K. Effects of Resveratrol Supplementation in Patients with Non-Alcoholic Fatty Liver Disease-A Meta-Analysis. Nutrients 2020, 12, 2435. [Google Scholar] [CrossRef] [PubMed]
- Jalali, M.; Mahmoodi, M.; Mosallanezhad, Z.; Jalali, R.; Imanieh, M.H.; Moosavian, S.P. The effects of curcumin supplementation on liver function, metabolic profile and body composition in patients with non-alcoholic fatty liver disease: A systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Med. 2020, 48, 102283. [Google Scholar] [CrossRef] [PubMed]
- Kalopitas, G.; Antza, C.; Doundoulakis, I.; Siargkas, A.; Kouroumalis, E.; Germanidis, G.; Samara, M.; Chourdakis, M. Impact of Silymarin in individuals with nonalcoholic fatty liver disease: A systematic review and meta-analysis. Nutrition 2021, 83, 111092. [Google Scholar] [CrossRef]
- Kamel, A.M.; Farag, M.A. Therapeutic Potential of Artichoke in the Treatment of Fatty Liver: A Systematic Review and Meta-Analysis. J. Med. Food 2022, 25, 931–942. [Google Scholar] [CrossRef]
- Khalili, L.; Nammi, S. The Effects of Curcumin Supplementation on Metabolic Biomarkers and Body Mass Index in Patients with Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Curr. Pharm. Des. 2022, 28, 1911–1925. [Google Scholar] [CrossRef] [PubMed]
- Kositamongkol, C.; Kanchanasurakit, S.; Auttamalang, C.; Inchai, N.; Kabkaew, T.; Kitpark, S.; Chaiyakunapruk, N.; Duangjai, A.; Saokaew, S.; Phisalprapa, P. Coffee Consumption and Non-alcoholic Fatty Liver Disease: An Umbrella Review and a Systematic Review and Meta-analysis. Front. Pharmacol. 2021, 12, 786596. [Google Scholar] [CrossRef] [PubMed]
- Kosmalski, M.; Frankowski, R.; Deska, K.; Różycka-Kosmalska, M.; Pietras, T. Exploring the Impact of Nutrition on Non-Alcoholic Fatty Liver Disease Management: Unveiling the Roles of Various Foods, Food Components, and Compounds. Nutrients 2023, 15, 2838. [Google Scholar] [CrossRef]
- Liu, Z.L.; Xie, L.Z.; Zhu, J.; Li, G.Q.; Grant, S.J.; Liu, J.P. Herbal medicines for fatty liver diseases. Cochrane Database Syst. Rev. 2013, 8, CD009059. [Google Scholar] [CrossRef]
- Lukkunaprasit, T.; Tansawet, A.; Boonmanunt, S.; Sobhonslidsuk, A.; McKay, G.J.; Attia, J.; Thakkinstian, A. An updated meta-analysis of effects of curcumin on metabolic dysfunction-associated fatty liver disease based on available evidence from Iran and Thailand. Sci. Rep. 2023, 13, 5824. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi, M.; Hosseini, R.; Kazemi, A.; Ofori-Asenso, R.; Mazidi, M.; Mazloomi, S.M.; Ofori-Asenso, R. Effects of green tea or green tea catechin on liver enzymes in healthy individuals and people with nonalcoholic fatty liver disease: A systematic review and meta-analysis of randomized clinical trials. Phytother. Res. 2020, 34, 1587–1598. [Google Scholar] [CrossRef] [PubMed]
- Mansour-Ghanaei, F.; Hadi, A.; Pourmasoumi, M.; Joukar, F.; Golpour, S.; Najafgholizadeh, A.; Mansour-Ghanaei, F. Green tea as a safe alternative approach for nonalcoholic fatty liver treatment: A systematic review and meta-analysis of clinical trials. Phytother. Res. 2018, 32, 1876–1884. [Google Scholar] [CrossRef]
- Marventano, S.; Salomone, F.; Godos, J.; Pluchinotta, F.; Del Rio, D.; Mistretta, A.; Grosso, G. Coffee and tea consumption in relation with non-alcoholic fatty liver and metabolic syndrome: A systematic review and meta-analysis of observational studies. Clin. Nutr. 2016, 35, 1269–1281. [Google Scholar] [CrossRef] [PubMed]
- Moradi, M.; Sohrabi, G.; Golbidi, M.; Yarmohammadi, S.; Hemati, N.; Campbell, M.S.; Moradi, S.; Kermani, M.a.H.; Farzaei, M.H. Effects of artichoke on blood pressure: A systematic review and meta-analysis. Complement. Ther. Med. 2021, 57, 102668. [Google Scholar] [CrossRef] [PubMed]
- Ngu, M.H.; Norhayati, M.N.; Rosnani, Z.; Maryam, M.Z.; Zulkifli, M.M. Curcumin as adjuvant treatment in patients with non-alcoholic fatty liver (NAFLD) disease: A systematic review and meta-analysis. Complement. Ther. Med. 2022, 66, 102843. [Google Scholar] [CrossRef]
- Perna, S.; Rafique, A.; Rondanelli, M.; Allehdan, S.; Riso, P.; Marino, M. Effect of caper fruit (Capparis spinosa L.) consumption on liver enzymes, lipid profile, fasting plasma glucose, and weight loss. A systematic review and a preliminary meta-analysis of randomized controlled trials. Biomed. Pharmacother.=Biomed. Pharmacother. 2023, 168, 115638. [Google Scholar] [CrossRef]
- Pourreza, S.; Azar, P.S.; Sanaie, S.; Noshadi, N.; Jalali, S.; Niazkar, H.R.; Karimi, A.; Vajdi, M. Therapeutic Effects and Mechanisms of Action of Garlic (Allium sativum) on Nonalcoholic Fatty Liver Disease: A Comprehensive Systematic Literature Review. Evid.-Based Complement. Altern. Med. 2022, 2022, 6960211. [Google Scholar] [CrossRef] [PubMed]
- Rafiee, S.; Mohammadi, H.; Ghavami, A.; Sadeghi, E.; Safari, Z.; Askari, G. Efficacy of resveratrol supplementation in patients with nonalcoholic fatty liver disease: A systematic review and meta-analysis of clinical trials. Complement. Ther. Clin. Pract. 2021, 42, 101281. [Google Scholar] [CrossRef]
- Reddy, A.J.; George, E.S.; Roberts, S.K.; Tierney, A.C. Effect of dietary intervention, with or without co-interventions, on inflammatory markers in patients with nonalcoholic fatty liver disease: A systematic literature review. Nutr. Rev. 2019, 77, 765–786. [Google Scholar] [CrossRef]
- Saab, S.; Mallam, D.; Cox, G.A., 2nd; Tong, M.J. Impact of coffee on liver diseases: A systematic review. Liver Int. Off. J. Int. Assoc. Study Liver 2014, 34, 495–504. [Google Scholar] [CrossRef]
- Sewter, R.; Heaney, S.; Patterson, A. Coffee Consumption and the Progression of NAFLD: A Systematic Review. Nutrients 2021, 13, 2381. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.Q.; Fan, Y.C.; Liu, W.Y.; Li, L.F.; Chen, Y.P.; Zheng, M.H. Traditional Chinese medicines benefit to nonalcoholic fatty liver disease: A systematic review and meta-analysis. Mol. Biol. Rep. 2012, 39, 9715–9722. [Google Scholar] [CrossRef]
- Tsompanaki, E.; Thanapirom, K.; Papatheodoridi, M.; Parikh, P.; Chotai de Lima, Y.; Tsochatzis, E.A. Systematic Review and Meta-analysis: The Role of Diet in the Development of Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2023, 21, 1462. [Google Scholar] [CrossRef]
- Wang, L.-L.; Zhang, P.-H.; Yan, H.-H. Functional foods and dietary supplements in the management of non-alcoholic fatty liver disease: A systematic review and meta-analysis. Front. Nutr. 2023, 10, 1014010. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jin, X.; Li, H.; Zhang, X.; Chen, X.; Lu, K.; Chu, C. Effects of various interventions on non-alcoholic fatty liver disease (NAFLD): A systematic review and network meta-analysis. Front. Pharmacol. 2023, 14, 1180016. [Google Scholar] [CrossRef]
- Wei, S.; Yu, X. Efficacy of resveratrol supplementation on liver enzymes in patients with non-alcoholic fatty liver disease: A systematic review and meta-analysis. Complement. Ther. Med. 2021, 57, 102635. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Liu, N.; Tantai, X.; Xing, X.; Xiao, C.; Chen, L.; Wang, J. The effects of curcumin on the metabolic parameters of non-alcoholic fatty liver disease: A meta-analysis of randomized controlled trials. Hepatol. Int. 2019, 13, 302–313. [Google Scholar] [CrossRef]
- White, C.M.; Ji-Young, L.E.E. The impact of turmeric or its curcumin extract on nonalcoholic fatty liver disease: A systematic review of clinical trials. Pharm. Pract. 2019, 17, 1350. [Google Scholar] [CrossRef]
- Wijarnpreecha, K.; Thongprayoon, C.; Ungprasert, P. Coffee consumption and risk of nonalcoholic fatty liver disease: A systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 2017, 29, e8–e12. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Chen, J.; Zhang, T.; Yuan, X.; Ge, A.; Wang, S.; Xu, H.; Zeng, L.; Ge, J. Efficacy and safety of dietary polyphenol supplementation in the treatment of non-alcoholic fatty liver disease: A systematic review and meta-analysis. Front. Immunol. 2022, 13, 949746. [Google Scholar] [CrossRef] [PubMed]
- Zeraattalab-Motlagh, S.; Jayedi, A.; Shab-Bidar, S. effects of resveratrol supplementation in patients with type 2 diabetes, metabolic syndrome, and nonalcoholic fatty liver disease: An umbrella review of meta-analyses of randomized controlled trials. Am. J. Clin. Nutr. 2021, 114, 1675–1685. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yuan, W.; Fang, J.; Wang, W.; He, P.; Lei, J.; Wang, C. Efficacy of Resveratrol Supplementation against Non-Alcoholic Fatty Liver Disease: A Meta-Analysis of Placebo-Controlled Clinical Trials. PLoS ONE 2016, 11, e0161792. [Google Scholar] [CrossRef]
- Zhong, S.; Fan, Y.; Yan, Q.; Fan, X.; Wu, B.; Han, Y.; Zhang, Y.; Chen, Y.; Zhang, H.; Niu, J. The therapeutic effect of silymarin in the treatment of nonalcoholic fatty disease: A meta-analysis (PRISMA) of randomized control trials. Medicine 2017, 96, e9061. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chen, Y.; Yu, J.; Li, T.; Lu, Z.; Chen, Y.; Zhang, X.; Ye, F. The efficacy of novel metabolic targeted agents and natural plant drugs for nonalcoholic fatty liver disease treatment: A PRISMA-compliant network meta-analysis of randomized controlled trials. Medicine 2021, 100, e24884. [Google Scholar] [CrossRef]
- Zhou, Q.; Peng, Y.; Chen, F.; Dai, J. Ginger supplementation for the treatment of non-alcoholic fatty liver disease: A meta-analysis of randomized controlled trials. Afr. Health Sci. 2023, 23, 614–621. [Google Scholar] [CrossRef]
- Mansour-Ghanaei, F.; Pourmasoumi, M.; Hadi, A.; Joukar, F. Efficacy of curcumin/turmeric on liver enzymes in patients with non-alcoholic fatty liver disease: A systematic review of randomized controlled trials. Integr. Med. Res. 2019, 8, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Różański, G.; Tabisz, H.; Zalewska, M.; Niemiro, W.; Kujawski, S.; Newton, J.; Zalewski, P.; Słomko, J. Meta-Analysis of Exploring the Effect of Curcumin Supplementation with or without Other Advice on Biochemical and Anthropometric Parameters in Patients with Metabolic-Associated Fatty Liver Disease (MAFLD). Int J Environ. Res. Public Health 2023, 20, 4266. [Google Scholar] [CrossRef] [PubMed]
- Xiong, P.; Zhu, Y.F. Soy diet for nonalcoholic fatty liver disease: A meta-analysis of randomized controlled trials. Medicine 2021, 100, e25817. [Google Scholar] [CrossRef]
- Zhang, Q.; Jia, Y.; Zhang, Y.; Wang, Y.; Li, X.; Tian, X.; Han, S. The effects of medicinal and food homologous substances on blood lipid and blood glucose levels and liver function in patients with nonalcoholic fatty liver disease: A systematic review of randomized controlled trials. Lipids Health Dis. 2023, 22, 137. [Google Scholar] [CrossRef] [PubMed]
- Guariglia, M.; Saba, F.; Rosso, C.; Bugianesi, E. Molecular Mechanisms of Curcumin in the Pathogenesis of Metabolic Dysfunction Associated Steatotic Liver Disease. Nutrients 2023, 15, 5053. [Google Scholar] [CrossRef]
- Anushiravani, A.; Ghajarieh Sepanlou, S. Burden of Liver Diseases: A Review from Iran. Middle East J. Dig. Dis. 2019, 11, 189–191. [Google Scholar] [CrossRef] [PubMed]
- Tabaeian, S.P.; Rezapour, A.; Azari, S.; Martini, M.; Saran, M.; Behzadifar, M.; Shahabi, S.; Sayyad, A.; Tahernejad, A.; Bragazzi, N.L.; et al. Prevalence of Non-alcoholic Fatty Liver Disease in Iran: A Systematic Review and Meta-analysis. J. Clin. Exp. Hepatol. 2024, 14, 101209. [Google Scholar] [CrossRef]
- Lichtenstein, A.H.; Petersen, K.; Barger, K.; Hansen, K.E.; Anderson, C.A.M.; Baer, D.J.; Lampe, J.W.; Rasmussen, H.; Matthan, N.R. Perspective: Design and Conduct of Human Nutrition Randomized Controlled Trials. Adv. Nutr. 2021, 12, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Sorkin, B.C.; Murch, S.J.; Weaver, C.M.; Jafari, M. Editorial: Plant Foods and Dietary Supplements: Building Solid Foundations for Clinical Trials. Front. Nutr. 2022, 9, 881688. [Google Scholar] [CrossRef] [PubMed]
- Pollock, D.; Evans, C.; Menghao Jia, R.; Alexander, L.; Pieper, D.; Brandao de Moraes, E.; Peters, M.D.J.; Tricco, A.C.; Khalil, H.; Godfrey, C.M.; et al. “How-to”: Scoping review? J. Clin. Epidemiol. 2024, 176, 111572. [Google Scholar] [CrossRef]
- Brown, P. The 2023 ADA Standards of Care: What’s new? Diabetes Prim. Care 2023, 25, 5–6. [Google Scholar]
- Ali Sangouni, A.; Abdollahi, S.; Mozaffari-Khosravi, H. Effect of resveratrol supplementation on hepatic steatosis and cardiovascular indices in overweight subjects with type 2 diabetes: A double-blind, randomized controlled trial. BMC Cardiovasc. Disord. 2022, 22, 212. [Google Scholar] [CrossRef]
- Abhari, K.; Saadati, S.; Yari, Z.; Hosseini, H.; Hedayati, M.; Abhari, S.; Alavian, S.M.; Hekmatdoost, A. The effects of Bacillus coagulans supplementation in patients with non-alcoholic fatty liver disease: A randomized, placebo-controlled, clinical trial. Clin. Nutr. ESPEN 2020, 39, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ma, P.; Wang, Z.; Sun, M.; Hou, B.; Xu, T.; Li, W.; Yang, X.; Du, G.; Ji, T.; et al. Uncovering the effect and mechanism of Panax notoginseng saponins on metabolic syndrome by network pharmacology strategy. J. Ethnopharmacol. 2023, 300, 115680. [Google Scholar] [CrossRef]
- Hall, R.L.; George, E.S.; Tierney, A.C.; Reddy, A.J. Effect of Dietary Intervention, with or without Cointerventions, on Inflammatory Markers in Patients with Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Adv. Nutr. (Bethesda Md.) 2023, 14, 475–499. [Google Scholar] [CrossRef] [PubMed]
- Moradi, F.; Kooshki, F.; Nokhostin, F.; Khoshbaten, M.; Bazyar, H.; Pourghassem Gargari, B. A pilot study of the effects of chromium picolinate supplementation on serum fetuin-A, metabolic and inflammatory factors in patients with nonalcoholic fatty liver disease: A double-blind, placebo-controlled trial. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. (GMS) 2021, 63, 126659. [Google Scholar] [CrossRef]
- Mirhafez, S.R.; Azimi-Nezhad, M.; Dehabeh, M.; Hariri, M.; Naderan, R.D.; Movahedi, A.; Abdalla, M.; Sathyapalan, T.; Sahebkar, A. The Effect of Curcumin Phytosome on the Treatment of Patients with Non-alcoholic Fatty Liver Disease: A Double-Blind, Randomized, Placebo-Controlled Trial. Adv. Exp. Med. Biol. 2021, 1308, 25–35. [Google Scholar] [CrossRef] [PubMed]
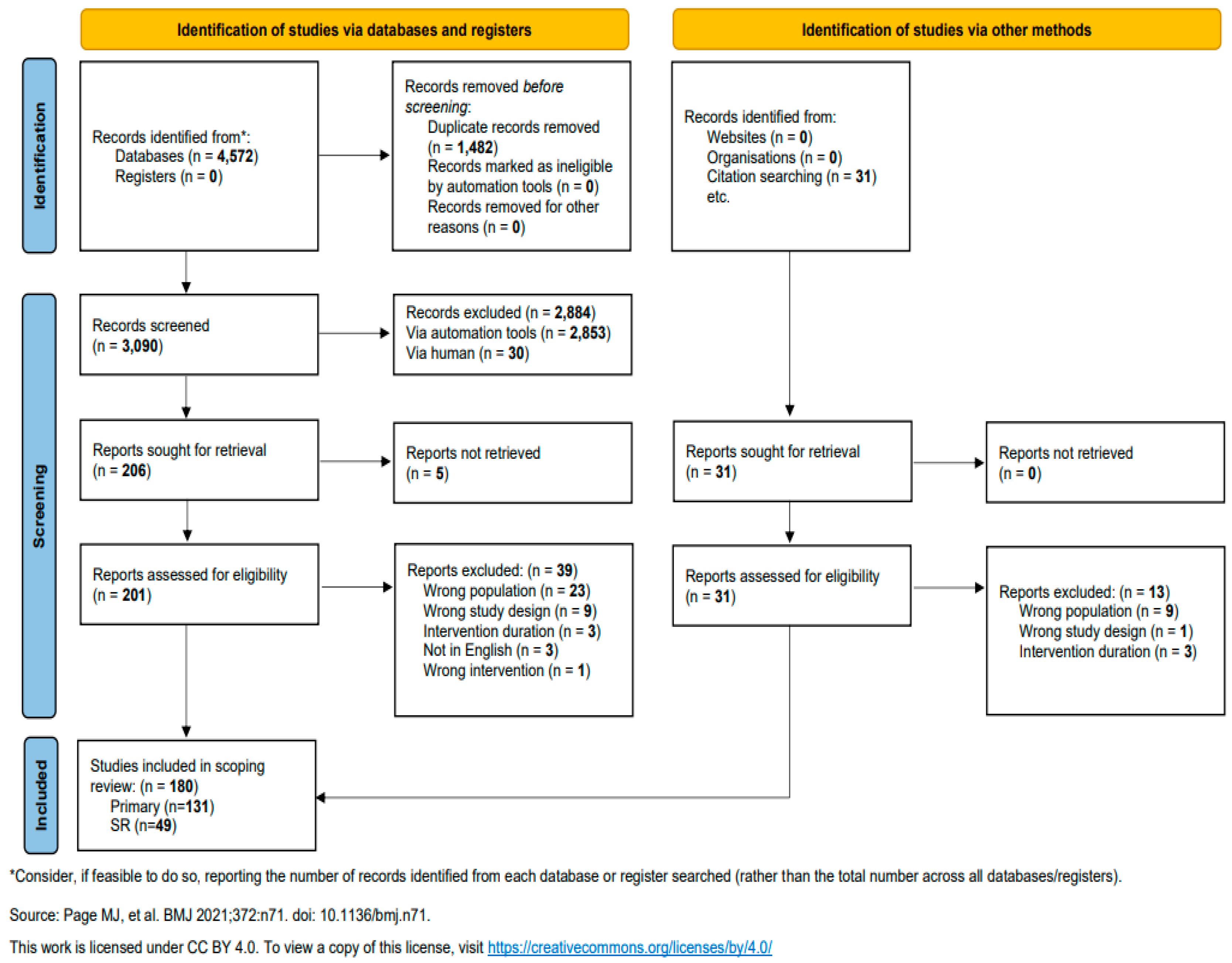

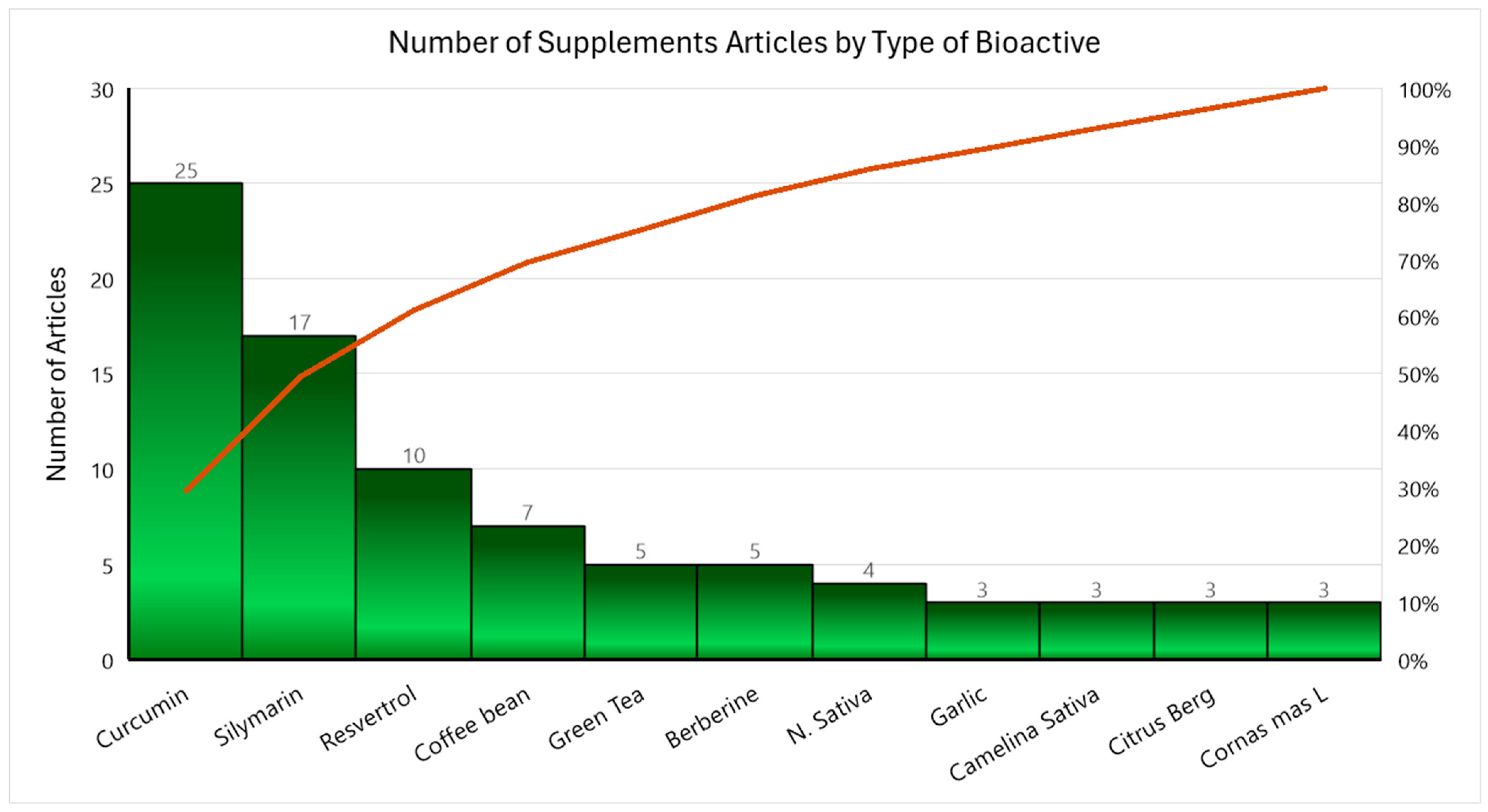

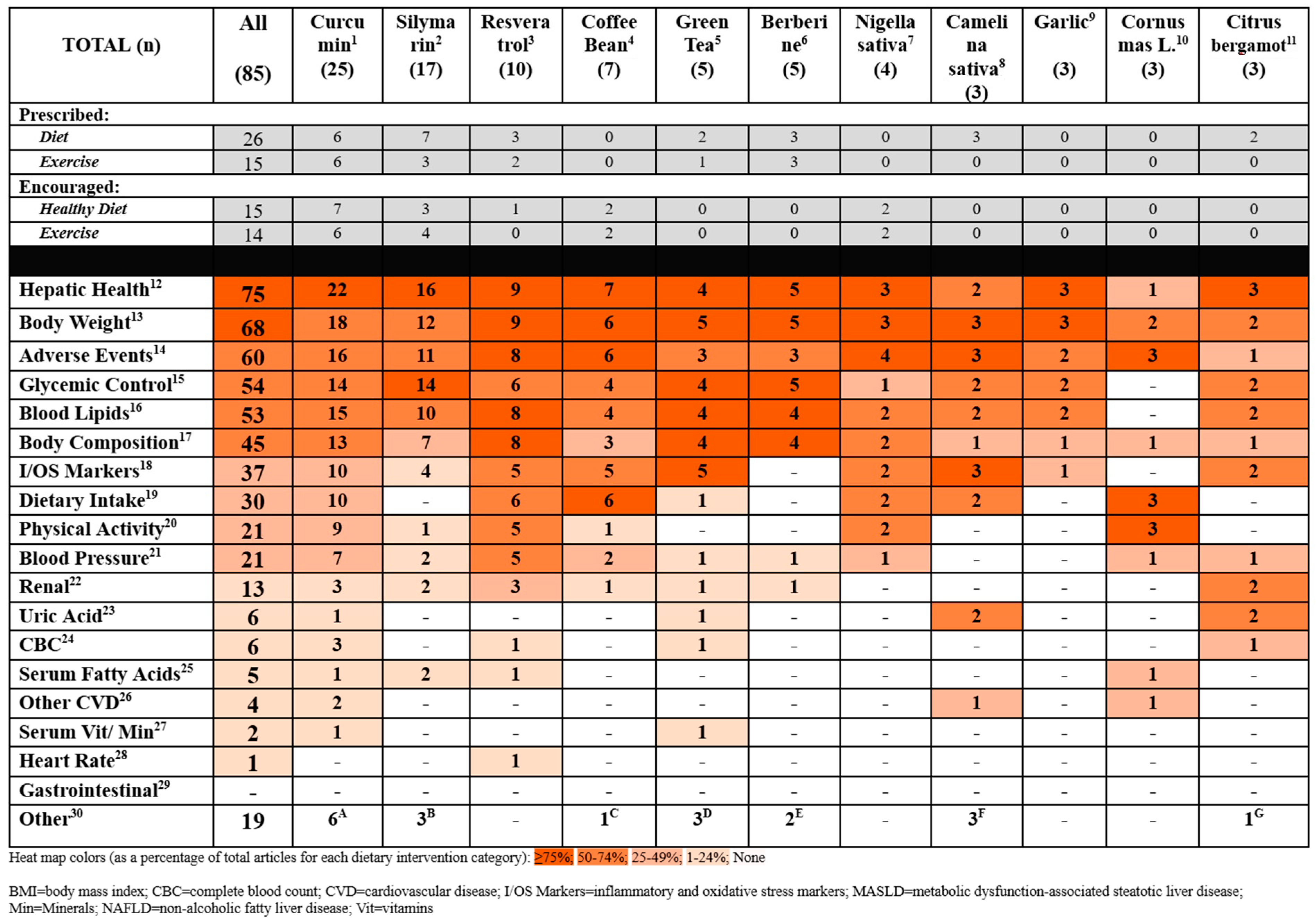
| PICO | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Population | Free-living adults (>18 years old) With metabolic dysfunction-associated steatotic liver disease (MASLD, previously NAFLD) MASLD/NAFLD (absence of significant alcohol use and other causes of hepatic steatosis) with or without overweight or obesity and with or without established disease (T2DM, T1DM, HTN, CVD, hyperlipidemia, or insulin resistance) NASH | Nonadults < 18 years (adolescents, children, and infants) Institutionalized individuals (i.e., inmates and patients of mental disease institutions) Pregnant, postpartum, or lactating women Established or diagnosed alcoholic fatty liver disease Significant alcohol use and other causes of hepatic steatosis (SLD) |
| Intervention | Any interventions (e.g., behavioral counseling, nutrition education, dietary prescription, etc.) that are provided by a healthcare provider (dietitian, nutritionist, physician, or nurses) Carbohydrate- and fructose-restricted diet Hypocaloric diets Fat-restricted diets Med diet DASH diet Intermittent fasting Dietary patterns (vegetarian/vegan) High polyphenol diets (Coffee and Tea) Bioactives MNT Micronutrients (Vitamin E) | Interventions that included fasting (enteral feedings or parenteral nutrition) Intervention length < 4 weeks |
| Comparison | At least one comparator group (e.g., usual diet or another contrasting diet) | No control group or comparator diet |
| Outcomes | Any | Does not report any outcomes of interest |
| Limits | ||
| Study Designs | RCTs and controlled clinical trials Cohort studies Systematic reviews; meta-analyses Guidelines Relevant systematic reviews will be searched for potentially included articles by the database search | Case–control, cross-sectional, before–after studies, ecological studies, single case study, case report, case series, or non-comparative Letters to the editor/commentary, poster session, abstract, or study protocol |
| Age range | Adults > 18 years old | |
| Date range | January 2000 to 12 October 2023 | |
| Language | English only | |
| Databases | MEDLINE, CINAHL, Cochrane Database of Systematic Reviews, Cochrane CENTRAL, Food Science Source, and SportDiscus | |
| At Least 3 Articles per Bioactive (n = 85) | Less Than 3 Articles per Bioactive (n = 46) |
|---|---|
| Berberine (berberis) Camelina sativa Citrus bergamia Coffee Bean Cornus mas L. Fruit Curcumin (Turmeric) Nigella sativa Resveratrol Silymarin (Milk Thistle) Garlic Green Tea | Anthocyanin (from bilberry and black current) Antrodia cinnamomea Mycelium (mushroom) Artemisia anuua I. Artichoke Leaf Beta cryptoxanthin Beta vulgaris (beet) Chlorella vulgaris (algae) Cinnamon Cranberry D-002 (Beeswax Alcohol) Fenugreek Flaxseed Fructus akebiae Fucoidan, Fucoxanthin Genistein Ginger Grape Seed Green Cardamom Gynostemma pentaphyllum Hesperidin Hydroxycitric acid Licorice Root Mastiha Naringenin Nutraceutical mixture Oligonol (Litchi-derived polyphenol) Pinitol (polyol) Plantago major seed Pomegranate Propolis Purslane Rosemary Saffron Shirazi Thyme Sour Tea Sumac |
| ALL | Curcumin | Silymarin | Resveratrol | Coffee/Tea Extracts | Multiple Bioactives A | Garlic | Ginger | Artichoke Leaf | Caper Fruit | Soy/Genestein | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | 49 | 13 | 2 | 6 | 12 | 10 | 1 | 1 | 2 | 1 | 1 |
| Study Designs Included in SRs | |||||||||||
| RCT | 40 | 13 B | 2 | 6 C | 4 | 9 | 1 | 1 | 2 | 1 | 1 C |
| RCT, NRCT | 1 | 1 | |||||||||
| OBS | 8 | 8 D,E | |||||||||
| Quality of SR | |||||||||||
| ≥2 databases | 47 | 13 | 2 | 6 | 11 | 9 | 1 | 1 | 2 | 1 | 1 |
| PROSPERO | 20 | 8 | 1 | 1 | 2 | 5 | 1 | 0 | 1 | 1 | 0 |
| PRISMA | 39 | 11 | 1 | 5 | 8 | 8 | 1 | 1 | 2 | 1 | 1 |
| ROB | 43 | 11 | 2 | 5 | 11 | 9 | 1 | 1 | 1 | 1 | 1 |
| QOE/GRADE | 4 | 2 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Handu, D.; Stote, K.; Piemonte, T. Evaluating Bioactive-Substance-Based Interventions for Adults with MASLD: Results from a Systematic Scoping Review. Nutrients 2025, 17, 453. https://doi.org/10.3390/nu17030453
Handu D, Stote K, Piemonte T. Evaluating Bioactive-Substance-Based Interventions for Adults with MASLD: Results from a Systematic Scoping Review. Nutrients. 2025; 17(3):453. https://doi.org/10.3390/nu17030453
Chicago/Turabian StyleHandu, Deepa, Kim Stote, and Tami Piemonte. 2025. "Evaluating Bioactive-Substance-Based Interventions for Adults with MASLD: Results from a Systematic Scoping Review" Nutrients 17, no. 3: 453. https://doi.org/10.3390/nu17030453
APA StyleHandu, D., Stote, K., & Piemonte, T. (2025). Evaluating Bioactive-Substance-Based Interventions for Adults with MASLD: Results from a Systematic Scoping Review. Nutrients, 17(3), 453. https://doi.org/10.3390/nu17030453





