Assessing the Roles of Retinol, Vitamin K2, Carnitine, and Creatine in Plant-Based Diets: A Narrative Review of Nutritional Adequacy and Health Implications
Highlights
- Plant-based diets can provide sufficient levels of retinol through provitamin A carotenoids, even in individuals with reduced conversion efficiency.
- The endogenous synthesis of vitamin K2 meets physiological needs. Supplementation, but not animal-based food consumption, reliably increases serum levels, which should inform clinical practice recommendations and consumer decisions.
- Carnitine and creatine levels differ between omnivorous and plant-based diets, but these differences do not compromise muscle function, cognitive health, or metabolic outcomes.
- Current evidence does not indicate that the absence of these non-essential nutrients in plant-based diets leads to adverse health effects compared to omnivorous diets.
- The absence of retinol, vitamin K2, carnitine, and creatine from plant foods has not been shown to diminish the quality of plant-based diets, which are closely aligned with chronic disease-prevention strategies.
Abstract
:1. Introduction
2. Nonessential Nutrients with Potential Health Implications for Plant-Based Diets
2.1. Retinol
2.2. Vitamin K2
2.3. Carnitine
2.4. Creatine
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADP | Adenosine diphosphate |
| ATP | Adenosine triphosphate |
| BCMO1 | β-carotene 15,15′-monoxygenase |
| BMI | Body mass index |
| CVD | Cardiovascular diseases |
| CI | Confidence interval |
| MK-4 | Menaquinone-4 |
| MK-7 | Menaquinone-7 |
| RR | Risk ratio |
| SD | Standard deviation |
| UBIAD1 | UbiA prenyltransferase domain containing 1 |
| WMD | Weighted mean difference |
References
- Miki, A.J.; Livingston, K.A.; Karlsen, M.C.; Folta, S.C.; McKeown, N.M. Using evidence mapping to examine motivations for following plant-based diets. Curr. Dev. Nutr. 2020, 4, nzaa013. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, B.; Han, H.; Hu, Y.; Zhu, L.; Rimm, E.B.; Hu, F.B.; Sun, Q. Associations between plant-based dietary patterns and risks of type 2 diabetes, cardiovascular disease, cancer, and mortality—A systematic review and meta-analysis. Nutr. J. 2023, 22, 46. [Google Scholar] [CrossRef]
- Cara, K.C.; Goldman, D.M.; Kollman, B.K.; Amato, S.S.; Tull, M.D.; Karlsen, M.C. Commonalities among dietary recommendations from 2010 to 2021 clinical practice guidelines: A meta-epidemiological study from the American College of Lifestyle Medicine. Adv. Nutr. 2023, 14, 500–515. [Google Scholar] [CrossRef] [PubMed]
- Melina, V.; Craig, W.; Levin, S. Position of the Academy of Nutrition and Dietetics: Vegetarian diets. J. Acad. Nutr. Diet 2016, 116, 1970–1980. [Google Scholar] [CrossRef]
- Tang, G. Bioconversion of dietary provitamin A carotenoids to vitamin A in humans. Am. J. Clin. Nutr. 2010, 91, 1468s–1473s. [Google Scholar] [CrossRef] [PubMed]
- Avgerinos, K.I.; Spyrou, N.; Bougioukas, K.I.; Kapogiannis, D. Effects of creatine supplementation on cognitive function of healthy individuals: A systematic review of randomized controlled trials. Exp. Gerontol. 2018, 108, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Virmani, M.A.; Cirulli, M. The role of L-carnitine in mitochondria, prevention of metabolic inflexibility and disease initiation. Int. J. Mol. Sci. 2022, 23, 2717. [Google Scholar] [CrossRef]
- Mladěnka, P.; Macáková, K.; Kujovská Krčmová, L.; Javorská, L.; Mrštná, K.; Carazo, A.; Protti, M.; Remião, F.; Nováková, L. Vitamin K—Sources, physiological role, kinetics, deficiency, detection, therapeutic use, and toxicity. Nutr. Rev. 2022, 80, 677–698. [Google Scholar] [CrossRef] [PubMed]
- Tanumihardjo, S.A.; Palacios, N.; Pixley, K.V. Provitamin a carotenoid bioavailability: What really matters? Int. J. Vitam Nutr. Res. 2010, 80, 336–350. [Google Scholar] [CrossRef]
- Leroy, F.; Barnard, N.D. Children and adults should avoid consuming animal products to reduce risk for chronic disease: NO. Am. J. Clin. Nutr. 2020, 112, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Koeder, C.; Perez-Cueto, F.J.A. Vegan nutrition: A preliminary guide for health professionals. Crit. Rev. Food Sci. Nutr. 2024, 64, 670–707. [Google Scholar] [CrossRef] [PubMed]
- Buxton, J. The Great Plant-Based Con: Why Eating a Plants-Only Diet Won’t Improve Your Health or Save the Planet; Little, Brown Book Group: London, UK, 2022. [Google Scholar]
- Hodge, C.; Taylor, C. Vitamin A Deficiency; StatPearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Kusin, J.A.; Reddy, V.; Sivakumar, B. Vitamin E supplements and the absorption of a massive dose of vitamin A. Am. J. Clin. Nutr. 1974, 27, 774–776. [Google Scholar] [CrossRef] [PubMed]
- Christian, P.; West, K.P., Jr. Interactions between zinc and vitamin A: An update. Am. J. Clin. Nutr. 1998, 68, 435s–441s. [Google Scholar] [CrossRef] [PubMed]
- Chungchunlam, S.M.S.; Moughan, P.J. Comparative bioavailability of vitamins in human foods sourced from animals and plants. Crit. Rev. Food Sci. Nutr. 2024, 64, 11590–11625. [Google Scholar] [CrossRef] [PubMed]
- Tourniaire, F.; Gouranton, E.; von Lintig, J.; Keijer, J.; Bonet, M.L.; Amengual, J.; Lietz, G.; Landrier, J.F. Beta-Carotene conversion products and their effects on adipose tissue. Genes Nutr. 2009, 4, 179–187. [Google Scholar] [CrossRef]
- Leung, W.C.; Hessel, S.; Méplan, C.; Flint, J.; Oberhauser, V.; Tourniaire, F.; Hesketh, J.E.; von Lintig, J.; Lietz, G. Two common single nucleotide polymorphisms in the gene encoding beta-carotene 15,15′-monoxygenase alter beta-carotene metabolism in female volunteers. FASEB J. 2009, 23, 1041–1053. [Google Scholar] [CrossRef]
- Lindqvist, A.; Sharvill, J.; Sharvill, D.E.; Andersson, S. Loss-of-function mutation in carotenoid 15,15′-monooxygenase identified in a patient with hypercarotenemia and hypovitaminosis A. J. Nutr. 2007, 137, 2346–2350. [Google Scholar] [CrossRef]
- Berni, P.; Chitchumroonchokchai, C.; Canniatti-Brazaca, S.G.; De Moura, F.F.; Failla, M.L. Comparison of content and in vitro bioaccessibility of provitamin A carotenoids in home cooked and commercially processed orange fleshed sweet potato (Ipomea batatas Lam). Plant Foods Hum. Nutr. 2015, 70, 1–8. [Google Scholar] [CrossRef]
- Crupi, P.; Faienza, M.F.; Naeem, M.Y.; Corbo, F.; Clodoveo, M.L.; Muraglia, M. Overview of the potential beneficial effects of carotenoids on consumer health and well-being. Antioxidants 2023, 12, 1069. [Google Scholar] [CrossRef] [PubMed]
- Neufingerl, N.; Eilander, A. Nutrient intake and status in adults consuming plant-based diets compared to meat-eaters: A systematic review. Nutrients 2021, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Unlu, N.Z.; Bohn, T.; Clinton, S.K.; Schwartz, S.J. Carotenoid absorption from salad and salsa by humans is enhanced by the addition of avocado or avocado oil. J. Nutr. 2005, 135, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Nankumbi, J.; Grant, F.; Sibeko, L.; Mercado, E.; O’Neil, K.; Cordeiro, L.S. Effects of food-based approaches on vitamin A status of women and children: A systematic review. Adv. Nutr. 2023, 14, 1436–1452. [Google Scholar] [CrossRef] [PubMed]
- Gijsbers, B.L.; Jie, K.S.; Vermeer, C. Effect of food composition on vitamin K absorption in human volunteers. Br. J. Nutr. 1996, 76, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Schurgers, L.J.; Teunissen, K.J.; Hamulyák, K.; Knapen, M.H.; Vik, H.; Vermeer, C. Vitamin K-containing dietary supplements: Comparison of synthetic vitamin K1 and natto-derived menaquinone-7. Blood 2007, 109, 3279–3283. [Google Scholar] [CrossRef] [PubMed]
- Schurgers, L.J.; Vermeer, C. Determination of phylloquinone and menaquinones in food. Effect of food matrix on circulating vitamin K concentrations. Haemostasis 2000, 30, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Schurgers, L.J.; Vermeer, C. Differential lipoprotein transport pathways of K-vitamins in healthy subjects. Biochim. Biophys. Acta 2002, 1570, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, L.; Liu, C.; Sun, Y.; Zhang, D. New aspects of microbial vitamin K2 production by expanding the product spectrum. Microb. Cell Fact. 2021, 20, 84. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.; Wynne, H.; Wood, P.; Torrance, A.; Hankey, C.; Avery, P.; Kesteven, P.; Kamali, F. Dietary vitamin K influences intra-individual variability in anticoagulant response to warfarin. Br. J. Haematol. 2004, 124, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Hirota, Y.; Sawada, N.; Yuge, N.; Watanabe, M.; Uchino, Y.; Okuda, N.; Shimomura, Y.; Suhara, Y.; Okano, T. Identification of UBIAD1 as a novel human menaquinone-4 biosynthetic enzyme. Nature 2010, 468, 117–121. [Google Scholar] [CrossRef]
- Sato, T.; Schurgers, L.J.; Uenishi, K. Comparison of menaquinone-4 and menaquinone-7 bioavailability in healthy women. Nutr. J. 2012, 11, 93. [Google Scholar] [CrossRef]
- Okano, T.; Shimomura, Y.; Yamane, M.; Suhara, Y.; Kamao, M.; Sugiura, M.; Nakagawa, K. Conversion of phylloquinone (Vitamin K1) into menaquinone-4 (Vitamin K2) in mice: Two possible routes for menaquinone-4 accumulation in cerebra of mice. J. Biol. Chem. 2008, 283, 11270–11279. [Google Scholar] [CrossRef] [PubMed]
- Binkley, N.; Harke, J.; Krueger, D.; Engelke, J.; Vallarta-Ast, N.; Gemar, D.; Checovich, M.; Chappell, R.; Suttie, J. Vitamin K treatment reduces undercarboxylated osteocalcin but does not alter bone turnover, density, or geometry in healthy postmenopausal North American women. J. Bone Miner. Res. 2009, 24, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhang, Z.L.; Zhang, Z.L.; Zhu, H.M.; Wu, Y.Y.; Cheng, Q.; Wu, F.L.; Xing, X.P.; Liu, J.L.; Yu, W.; et al. Menatetrenone versus alfacalcidol in the treatment of Chinese postmenopausal women with osteoporosis: A multicenter, randomized, double-blinded, double-dummy, positive drug-controlled clinical trial. Clin. Interv. Aging 2014, 9, 121–127. [Google Scholar] [CrossRef]
- Shiraki, M.; Shiraki, Y.; Aoki, C.; Miura, M. Vitamin K2 (menatetrenone) effectively prevents fractures and sustains lumbar bone mineral density in osteoporosis. J. Bone Miner. Res. 2000, 15, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Giri, T.K.; Newton, D.; Chaudhary, O.; Deych, E.; Napoli, N.; Villareal, R.; Diemer, K.; Milligan, P.E.; Gage, B.F. Maximal dose-response of vitamin-K2 (menaquinone-4) on undercarboxylated osteocalcin in women with osteoporosis. Int. J. Vitam. Nutr. Res. 2020, 90, 42–48. [Google Scholar] [CrossRef]
- Knapen, M.H.; Drummen, N.E.; Smit, E.; Vermeer, C.; Theuwissen, E. Three-year low-dose menaquinone-7 supplementation helps decrease bone loss in healthy postmenopausal women. Osteoporos. Int. 2013, 24, 2499–2507. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Z.; Duan, L.; Ji, Y.; Yang, S.; Zhang, Y.; Li, H.; Wang, Y.; Wang, P.; Chen, J.; et al. Effect of low-dose vitamin K2 supplementation on bone mineral density in middle-aged and elderly Chinese: A randomized controlled study. Calcif. Tissue Int. 2020, 106, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Rønn, S.H.; Harsløf, T.; Oei, L.; Pedersen, S.B.; Langdahl, B.L. The effect of vitamin MK-7 on bone mineral density and microarchitecture in postmenopausal women with osteopenia, a 3-year randomized, placebo-controlled clinical trial. Osteoporos. Int. 2021, 32, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Scheiber, D.; Veulemans, V.; Horn, P.; Chatrou, M.L.; Potthoff, S.A.; Kelm, M.; Schurgers, L.J.; Westenfeld, R. High-dose menaquinone-7 supplementation reduces cardiovascular calcification in a murine model of extraosseous calcification. Nutrients 2015, 7, 6991–7011. [Google Scholar] [CrossRef]
- Bellinge, J.W.; Dalgaard, F.; Murray, K.; Connolly, E.; Blekkenhorst, L.C.; Bondonno, C.P.; Lewis, J.R.; Sim, M.; Croft, K.D.; Gislason, G.; et al. Vitamin K intake and atherosclerotic cardiovascular disease in the Danish Diet Cancer and Health Study. J. Am. Heart Assoc. 2021, 10, e020551. [Google Scholar] [CrossRef] [PubMed]
- Gast, G.C.; de Roos, N.M.; Sluijs, I.; Bots, M.L.; Beulens, J.W.; Geleijnse, J.M.; Witteman, J.C.; Grobbee, D.E.; Peeters, P.H.; van der Schouw, Y.T. A high menaquinone intake reduces the incidence of coronary heart disease. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Li, Y.; Sun, Q.; Pan, A.; Manson, J.E.; Rexrode, K.M.; Willett, W.C.; Rimm, E.B.; Hu, F.B. Dairy fat and risk of cardiovascular disease in 3 cohorts of US adults. Am. J. Clin. Nutr. 2016, 104, 1209–1217. [Google Scholar] [CrossRef]
- Soerensen, K.V.; Thorning, T.K.; Astrup, A.; Kristensen, M.; Lorenzen, J.K. Effect of dairy calcium from cheese and milk on fecal fat excretion, blood lipids, and appetite in young men. Am. J. Clin. Nutr. 2014, 99, 984–991. [Google Scholar] [CrossRef] [PubMed]
- McFarlin, B.K.; Henning, A.L.; Venable, A.S. Oral consumption of vitamin K2 for 8 weeks associated with increased maximal cardiac output during exercise. Altern. Ther. Health Med. 2017, 23, 26–32. [Google Scholar]
- Kim, H.; Caulfield, L.E.; Garcia-Larsen, V.; Steffen, L.M.; Coresh, J.; Rebholz, C.M. Plant-based diets are associated with a lower risk of incident cardiovascular disease, cardiovascular disease mortality, and all-cause mortality in a general population of middle-aged adults. J. Am. Heart Assoc. 2019, 8, e012865. [Google Scholar] [CrossRef] [PubMed]
- Theuwissen, E.; Cranenburg, E.C.; Knapen, M.H.; Magdeleyns, E.J.; Teunissen, K.J.; Schurgers, L.J.; Smit, E.; Vermeer, C. Low-dose menaquinone-7 supplementation improved extra-hepatic vitamin K status, but had no effect on thrombin generation in healthy subjects. Br. J. Nutr. 2012, 108, 1652–1657. [Google Scholar] [CrossRef] [PubMed]
- Stephens, F.B.; Marimuthu, K.; Cheng, Y.; Patel, N.; Constantin, D.; Simpson, E.J.; Greenhaff, P.L. Vegetarians have a reduced skeletal muscle carnitine transport capacity. Am. J. Clin. Nutr. 2011, 94, 938–944. [Google Scholar] [CrossRef]
- Flanagan, J.L.; Simmons, P.A.; Vehige, J.; Willcox, M.D.; Garrett, Q. Role of carnitine in disease. Nutr. Metab. 2010, 7, 30. [Google Scholar] [CrossRef]
- Rebouche, C.J. Carnitine. In Modern Nutrition in Health and Disease; Ross, A.C., Caballero, B., Cousins, R.J., Tucker, K.L., Ziegler, T.R., Eds.; Lippincott Williams & Wilkins: Baltimore, MD, USA, 2014; pp. 252–253. [Google Scholar]
- Rebouche, C.J. Carnitine. In Nutrition in Health and Disease; Shils, M.E., Olson, J.A., Shike, M., Ross, A.C., Eds.; Lippincott Williams & Wilkins: Baltimore, MD, USA, 1999; pp. 505–512. [Google Scholar]
- Evans, A.M.; Fornasini, G. Pharmacokinetics of L-carnitine. Clin. Pharmacokinet. 2003, 42, 941–967. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Park, Y.O.; Cha, Y.S. Carnitine content of common Korean foods. Nutraceuticals Food 2002, 7, 293–298. [Google Scholar] [CrossRef]
- Leroy, F.; Cofnas, N. Should dietary guidelines recommend low red meat intake? Crit. Rev. Food Sci. Nutr. 2020, 60, 2763–2772. [Google Scholar] [CrossRef] [PubMed]
- Novakova, K.; Kummer, O.; Bouitbir, J.; Stoffel, S.D.; Hoerler-Koerner, U.; Bodmer, M.; Roberts, P.; Urwyler, A.; Ehrsam, R.; Krähenbühl, S. Effect of L-carnitine supplementation on the body carnitine pool, skeletal muscle energy metabolism and physical performance in male vegetarians. Eur. J. Nutr. 2016, 55, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Lombard, K.A.; Olson, A.L.; Nelson, S.E.; Rebouche, C.J. Carnitine status of lactoovovegetarians and strict vegetarian adults and children. Am. J. Clin. Nutr. 1989, 50, 301–306. [Google Scholar] [CrossRef]
- Delanghe, J.; De Slypere, J.P.; De Buyzere, M.; Robbrecht, J.; Wieme, R.; Vermeulen, A. Normal reference values for creatine, creatinine, and carnitine are lower in vegetarians. Clin. Chem. 1989, 35, 1802–1803. [Google Scholar] [CrossRef]
- Rebouche, C.J.; Lombard, K.A.; Chenard, C.A. Renal adaptation to dietary carnitine in humans. Am. J. Clin. Nutr. 1993, 58, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Gnoni, A.; Longo, S.; Gnoni, G.V.; Giudetti, A.M. Carnitine in human muscle bioenergetics: Can carnitine supplementation improve physical exercise? Molecules 2020, 25, 182. [Google Scholar] [CrossRef] [PubMed]
- Talenezhad, N.; Mohammadi, M.; Ramezani-Jolfaie, N.; Mozaffari-Khosravi, H.; Salehi-Abargouei, A. Effects of L-carnitine supplementation on weight loss and body composition: A systematic review and meta-analysis of 37 randomized controlled clinical trials with dose-response analysis. Clin. Nutr. ESPEN 2020, 37, 9–23. [Google Scholar] [CrossRef]
- Hudson, S.; Tabet, N. Acetyl-L-carnitine for dementia. Cochrane Database Syst. Rev. 2003, 2003, Cd003158. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; Lavie, C.J.; Fares, H.; Menezes, A.R.; O’Keefe, J.H. L-carnitine in the secondary prevention of cardiovascular disease: Systematic review and meta-analysis. Mayo Clin. Proc. 2013, 88, 544–551. [Google Scholar] [CrossRef]
- Thompson, W.G.; Hensrud, D.D.; Murad, M.H. Regarding L-carnitine and cardiovascular disease. Mayo Clin. Proc. 2013, 88, 899–900. [Google Scholar] [CrossRef] [PubMed]
- Johri, A.M.; Hétu, M.F.; Heyland, D.K.; Herr, J.E.; Korol, J.; Froese, S.; Norman, P.A.; Day, A.G.; Matangi, M.F.; Michos, E.D.; et al. Progression of atherosclerosis with carnitine supplementation: A randomized controlled trial in the metabolic syndrome. Nutr. Metab. 2022, 19, 26. [Google Scholar] [CrossRef]
- Zhao, J.V.; Burgess, S.; Fan, B.; Schooling, C.M. L-carnitine, a friend or foe for cardiovascular disease? A Mendelian randomization study. BMC Med. 2022, 20, 272. [Google Scholar] [CrossRef]
- Dinu, M.; Abbate, R.; Gensini, G.F.; Casini, A.; Sofi, F. Vegetarian, vegan diets and multiple health outcomes: A systematic review with meta-analysis of observational studies. Crit. Rev. Food Sci. Nutr. 2017, 57, 3640–3649. [Google Scholar] [CrossRef]
- Tsai, J.H.; Huang, C.F.; Lin, M.N.; Chang, C.E.; Chang, C.C.; Lin, C.L. Taiwanese vegetarians are associated with lower dementia risk: A prospective cohort study. Nutrients 2022, 14, 588. [Google Scholar] [CrossRef] [PubMed]
- de Crom, T.O.E.; Steur, M.; Ikram, M.K.; Ikram, M.A.; Voortman, T. Plant-based dietary patterns and the risk of dementia: A population-based study. Age Ageing 2023, 52, afad178. [Google Scholar] [CrossRef] [PubMed]
- Iguacel, I.; Huybrechts, I.; Moreno, L.A.; Michels, N. Vegetarianism and veganism compared with mental health and cognitive outcomes: A systematic review and meta-analysis. Nutr. Rev. 2021, 79, 361–381. [Google Scholar] [CrossRef] [PubMed]
- Gatto, N.M.; Garcia-Cano, J.; Irani, C.; Jaceldo-Siegl, K.; Liu, T.; Chen, Z.; Paul, J.; Fraser, G.; Wang, C.; Lee, G.J. Vegetarian dietary patterns and cognitive function among older adults: The Adventist Health Study-2. J. Nutr. Gerontol Geriatr. 2021, 40, 197–214. [Google Scholar] [CrossRef]
- Wu, H.; Gu, Y.; Meng, G.; Wu, H.; Zhang, S.; Wang, X.; Zhang, J.; Huang, T.; Niu, K. Quality of plant-based diet and the risk of dementia and depression among middle-aged and older population. Age Ageing 2023, 52, afad070. [Google Scholar] [CrossRef] [PubMed]
- Gan, Z.H.; Cheong, H.C.; Tu, Y.K.; Kuo, P.H. Association between plant-based dietary patterns and risk of cardiovascular disease: A systematic review and meta-analysis of prospective cohort studies. Nutrients 2021, 13, 3952. [Google Scholar] [CrossRef]
- Quek, J.; Lim, G.; Lim, W.H.; Ng, C.H.; So, W.Z.; Toh, J.; Pan, X.H.; Chin, Y.H.; Muthiah, M.D.; Chan, S.P.; et al. The association of plant-based diet with cardiovascular disease and mortality: A meta-analysis and systematic review of prospect cohort studies. Front. Cardiovasc. Med. 2021, 8, 756810. [Google Scholar] [CrossRef]
- Bonilla, D.A.; Kreider, R.B.; Stout, J.R.; Forero, D.A.; Kerksick, C.M.; Roberts, M.D.; Rawson, E.S. Metabolic basis of creatine in health and disease: A bioinformatics-assisted review. Nutrients 2021, 13, 1238. [Google Scholar] [CrossRef] [PubMed]
- Goldman, R.; Moss, J.X. Creatine synthesis after creatinine loading and after nephrectomy. Proc. Soc. Exp. Biol. Med. 1960, 105, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Lanza, I.R.; Befroy, D.E.; Kent-Braun, J.A. Age-related changes in ATP-producing pathways in human skeletal muscle in vivo. J. Appl. Physiol. (1985) 2005, 99, 1736–1744. [Google Scholar] [CrossRef]
- Kuchukashvili, Z.; Burjanadze, G.; Menabde, K.; Chachua, M.; Dachanidze, N.; Mikadze, M.; Koshoridze, N. Long-lasting stress, quantitative changes in nitric oxide concentration and functional state of brain mitochondria. Acta Neurobiol. Exp. 2012, 72, 40–50. [Google Scholar] [CrossRef]
- Koshoridze, N.I.; Menabde, K.O.; Kuchukashvili, Z.T.; Chachua, M.V.; Chipashvili, M.D. Quantitative alterations in the products of lipid peroxidation under stress. J. Stress Physiol. Biochem. 2010, 6, 4–9. [Google Scholar]
- Mecrimek-Andrews, S.; Salomons, G. Creatine Deficiency Disorders. In Gene Reviews; Adam, M.P., Feldman, J., Mirzaa, G.M., et al., Eds.; University of Washington: Seattle, WA, USA, 2009. [Google Scholar]
- Brosnan, M.E.; Brosnan, J.T. The role of dietary creatine. Amino Acids 2016, 48, 1785–1791. [Google Scholar] [CrossRef] [PubMed]
- Solis, M.Y.; Artioli, G.G.; Otaduy, M.C.G.; Leite, C.D.C.; Arruda, W.; Veiga, R.R.; Gualano, B. Effect of age, diet, and tissue type on PCr response to creatine supplementation. J. Appl. Physiol. (1985) 2017, 123, 407–414. [Google Scholar] [CrossRef]
- Blancquaert, L.; Baguet, A.; Bex, T.; Volkaert, A.; Everaert, I.; Delanghe, J.; Petrovic, M.; Vervaet, C.; De Henauw, S.; Constantin-Teodosiu, D.; et al. Changing to a vegetarian diet reduces the body creatine pool in omnivorous women, but appears not to affect carnitine and carnosine homeostasis: A randomised trial. Br. J. Nutr. 2018, 119, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Watt, K.K.; Garnham, A.P.; Snow, R.J. Skeletal muscle total creatine content and creatine transporter gene expression in vegetarians prior to and following creatine supplementation. Int. J. Sport Nutr. Exerc. Metab. 2004, 14, 517–531. [Google Scholar] [CrossRef] [PubMed]
- Lukaszuk, J.M.; Robertson, R.J.; Arch, J.E.; Moore, G.E.; Yaw, K.M.; Kelley, D.E.; Rubin, J.T.; Moyna, N.M. Effect of creatine supplementation and a lacto-ovo-vegetarian diet on muscle creatine concentration. Int. J. Sport Nutr. Exerc. Metab. 2002, 12, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Lukaszuk, J.M.; Robertson, R.J.; Arch, J.E.; Moyna, N.M. Effect of a defined lacto-ovo-vegetarian diet and oral creatine monohydrate supplementation on plasma creatine concentration. J. Strength Cond. Res. 2005, 19, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Burke, D.G.; Chilibeck, P.D.; Parise, G.; Candow, D.G.; Mahoney, D.; Tarnopolsky, M. Effect of creatine and weight training on muscle creatine and performance in vegetarians. Med. Sci. Sports Exerc. 2003, 35, 1946–1955. [Google Scholar] [CrossRef]
- Yazigi Solis, M.; de Salles Painelli, V.; Giannini Artioli, G.; Roschel, H.; Concepción Otaduy, M.; Gualano, B. Brain creatine depletion in vegetarians? A cross-sectional ¹H-magnetic resonance spectroscopy (¹H-MRS) study. Br. J. Nutr. 2014, 111, 1272–1274. [Google Scholar] [CrossRef]
- Sandkühler, J.F.; Kersting, X.; Faust, A.; Königs, E.K.; Altman, G.; Ettinger, U.; Lux, S.; Philipsen, A.; Müller, H.; Brauner, J. The effects of creatine supplementation on cognitive performance-a randomised controlled study. BMC Med. 2023, 21, 440. [Google Scholar] [CrossRef] [PubMed]
- Antonio, J.; Brown, A.F.; Candow, D.G.; Chilibeck, P.D.; Ellery, S.J.; Forbes, S.C.; Gualano, B.; Jagim, A.R.; Kerksick, C.; Kreider, R.B.; et al. Part II. Common questions and misconceptions about creatine supplementation: What does the scientific evidence really show? J. Int. Soc. Sports Nutr. 2025, 22, 2441760. [Google Scholar] [CrossRef] [PubMed]
- Benton, D.; Donohoe, R. The influence of creatine supplementation on the cognitive functioning of vegetarians and omnivores. Br. J. Nutr. 2011, 105, 1100–1105. [Google Scholar] [CrossRef]
- Forbes, S.C.; Cordingley, D.M.; Cornish, S.M.; Gualano, B.; Roschel, H.; Ostojic, S.M.; Rawson, E.S.; Roy, B.D.; Prokopidis, K.; Giannos, P.; et al. Effects of creatine supplementation on brain function and health. Nutrients 2022, 14, 921. [Google Scholar] [CrossRef]
- Chan, H.; Ribeiro, R.V.; Haden, S.; Hirani, V. Plant-based dietary patterns, body composition, muscle strength and function in middle and older age: A systematic review. J. Nutr. Health Aging 2021, 25, 1012–1022. [Google Scholar] [CrossRef]
- Presti, N.; Mansouri, T.; Maloney, M.K.; Hostler, D. The impact plant-based diets have on athletic performance and body composition: A systematic review. J. Am. Nutr. Assoc. 2024, 43, 636–643. [Google Scholar] [CrossRef]
- Damasceno, Y.O.; Leitão, C.; de Oliveira, G.M.; Andrade, F.A.B.; Pereira, A.B.; Viza, R.S.; Correia, R.C.; Campos, H.O.; Drummond, L.R.; Leite, L.H.R.; et al. Plant-based diets benefit aerobic performance and do not compromise strength/power performance: A systematic review and meta-analysis. Br. J. Nutr. 2024, 131, 829–840. [Google Scholar] [CrossRef]
- Smillie, L.; Minehan, M.; Knight-Agarwal, C.R.; Oliver, C.; Turner, M. A systematic review of the impact of vegetarian diets on muscle mass and muscle strength in community-dwelling, healthy adults. JCSM Commun. 2024, 7, 173–185. [Google Scholar] [CrossRef]
- Kaviani, M.; Shaw, K.; Chilibeck, P.D. Benefits of creatine supplementation for vegetarians compared to omnivorous athletes: A systematic review. Int. J. Environ. Res. Public Health 2020, 17, 3041. [Google Scholar] [CrossRef] [PubMed]
- Goldman, D.M.; Stiegmann, R.A.; Craddock, J.C. Supplemental creatine, not dietary creatine, appears to improve exercise performance in individuals following omnivorous or meat-free diets: A narrative review. Int. J. Dis. Rev. Prev. 2022, 4, 15. [Google Scholar] [CrossRef]
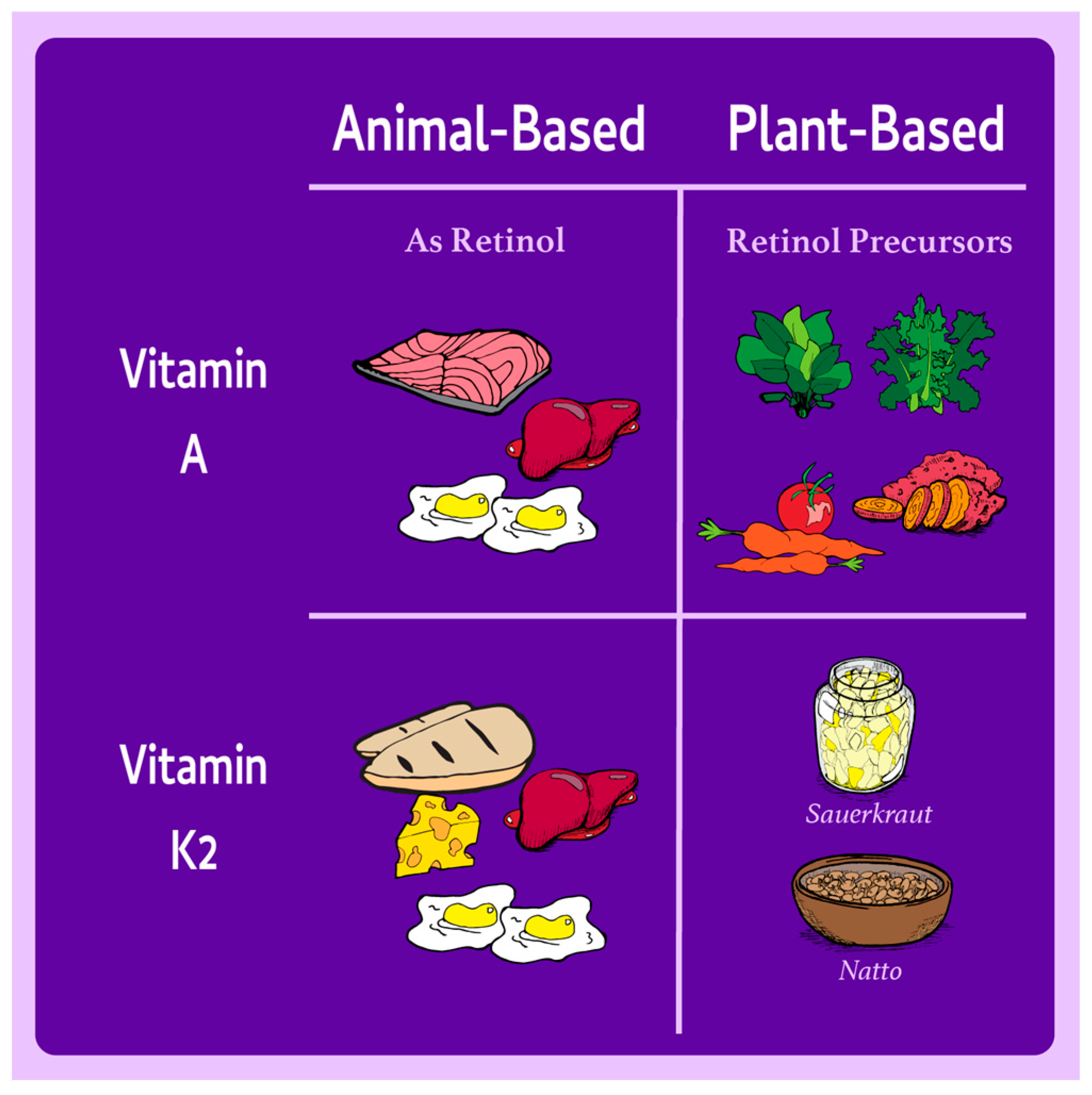
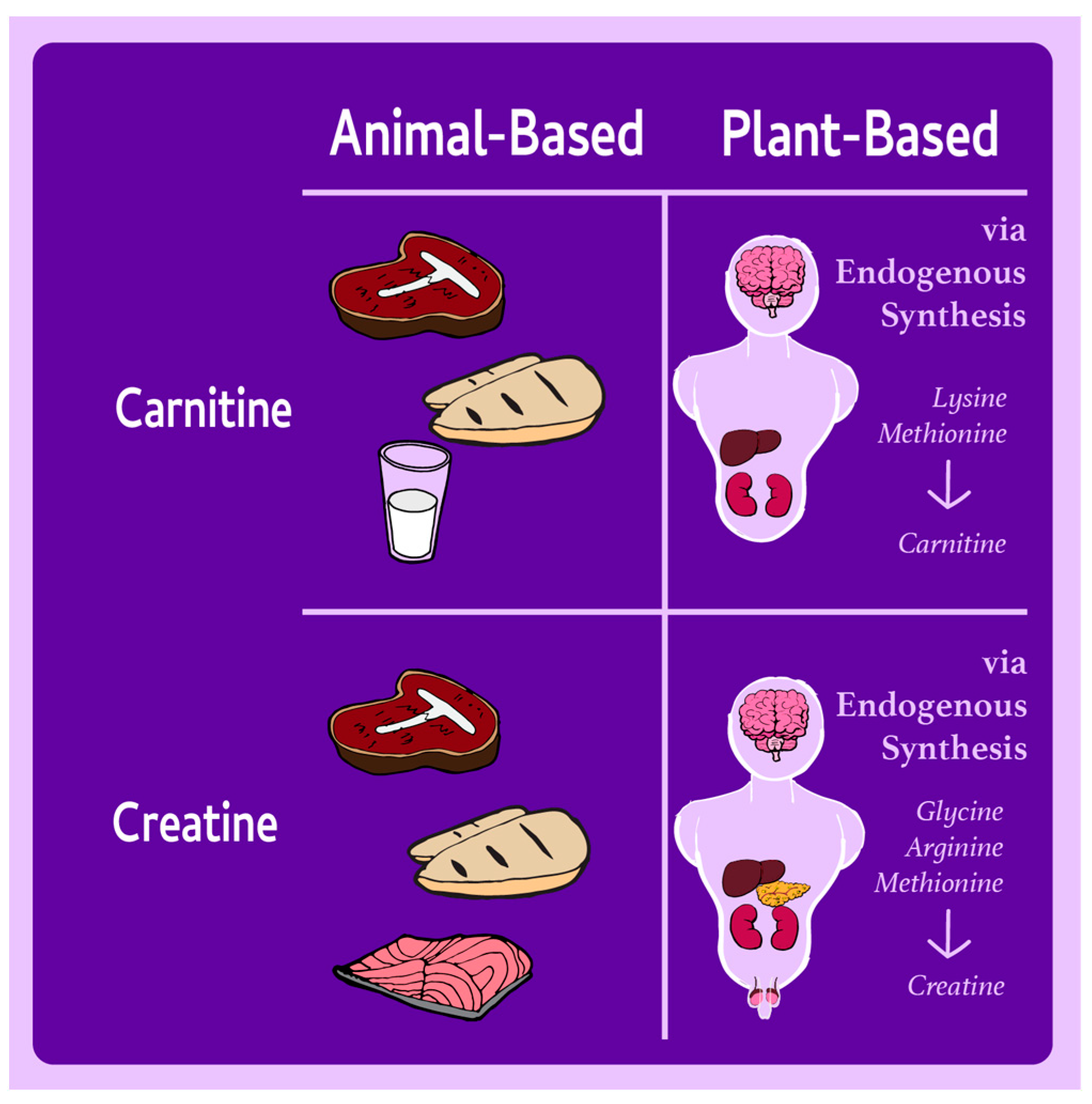
| Nutrient | Primary Roles in the Body | Dietary Sources in Omnivorous Diets | Dietary Sources in Plant-Based Diets | Health Implications |
|---|---|---|---|---|
| Retinol | Vision, immune function, cell differentiation, embryonic development | Liver, eggs, dairy | Provitamin A carotenoids (e.g., carrots, sweet potatoes, spinach), which can be paired with fat for enhanced absorption | Conversion efficiency varies, but adequate intakes can meet requirements in plant-based diets, even for low converters. |
| Vitamin K2 | Bone health (activates osteocalcin), cardiovascular health (inhibits vascular calcification) | Animal products (MK-4), fermented dairy | Natto (fermented soy), plant-derived MK-7 supplements | MK-4 is poorly bioavailable; plant-based sources like MK-7 are effective and reliable for supplementation if needed. |
| Carnitine | Fatty acid metabolism, energy production, detoxification | Red meat, poultry, fish | Trace amounts in mushrooms; synthesized endogenously from lysine and methionine | No evidence of deficiency in plant-based diets; endogenous synthesis is sufficient in healthy individuals. |
| Creatine | Rapid ATP recycling, cognitive function, muscle contraction | Meat, fish | None; synthesized endogenously from glycine, arginine, and methionine | Lower muscle stores in plant-based dieters, but no adverse impact on health. Supplementation can benefit high-performance populations if needed. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goldman, D.M.; Warbeck, C.B.; Barbaro, R.; Khambatta, C.; Nagra, M. Assessing the Roles of Retinol, Vitamin K2, Carnitine, and Creatine in Plant-Based Diets: A Narrative Review of Nutritional Adequacy and Health Implications. Nutrients 2025, 17, 525. https://doi.org/10.3390/nu17030525
Goldman DM, Warbeck CB, Barbaro R, Khambatta C, Nagra M. Assessing the Roles of Retinol, Vitamin K2, Carnitine, and Creatine in Plant-Based Diets: A Narrative Review of Nutritional Adequacy and Health Implications. Nutrients. 2025; 17(3):525. https://doi.org/10.3390/nu17030525
Chicago/Turabian StyleGoldman, David M., Cassandra B. Warbeck, Robby Barbaro, Cyrus Khambatta, and Matthew Nagra. 2025. "Assessing the Roles of Retinol, Vitamin K2, Carnitine, and Creatine in Plant-Based Diets: A Narrative Review of Nutritional Adequacy and Health Implications" Nutrients 17, no. 3: 525. https://doi.org/10.3390/nu17030525
APA StyleGoldman, D. M., Warbeck, C. B., Barbaro, R., Khambatta, C., & Nagra, M. (2025). Assessing the Roles of Retinol, Vitamin K2, Carnitine, and Creatine in Plant-Based Diets: A Narrative Review of Nutritional Adequacy and Health Implications. Nutrients, 17(3), 525. https://doi.org/10.3390/nu17030525










