Metabolic Syndrome in the Amazon: Customizing Diagnostic Methods for Urban Communities
Abstract
:1. Introduction
1.1. Metabolic Syndrome: Definition and Global Relevance
1.2. Prevalence and Risk Factors for MetS in Peru and Latin America
1.3. MetS in the Peruvian Amazon and the Impact of Urbanisation
1.4. San Juan Bautista: Characterisation of an Urban Amazonian District
1.5. The Harmonised MetS Diagnostic Model and Its Application in Specific Populations
2. Materials and Methods
2.1. Eligibility Criteria
- ○
- Study variables and measurementThe dependent variable of the study was the diagnosis of MetS according to the NCEP ATP III criteria [28]. These criteria establish the presence of STEM when three or more of the following risk factors are present: Obesity Central. CC ≥ 94 cm for males o ≥88 cm for females.
- High blood pressure. SBP ≥ 130 mmHg and/or DBP ≥ 85 mmHg or antihypertensive treatment.
- High triglycerides. TG ≥ 150 mg/dL or lipid-lowering treatment.
- High blood glucose. FG ≥ 100 mg/dL or hypoglycaemic treatment.
- Low HDL. HDL < 40 mg/dL in women or HDL < 50 mg/dL in men or pharmacological treatment to address it.
- ○
- The independent variables of the study were:
- Sociodemographics: Age (years) and sex (male/female).
- Anthropometric. Height (cm), weight (kg), BMI (kg/m2), waist circumference (WC, cm), body fat percentage (FP%), muscle mass (MM, kg), basal metabolic rate (BMR, kcal), waist-to-height ratio (WHtR), A New Body Shape Index (ABSI) (113) and Body Adiposity Index (BAI) (114). In addition, the BMI was used to assess nutritional status according to the cut-off points established by the WHO (111) for underweight (≤18.49), normal weight (18.5–24.99 kg/m2), overweight (25.00–29.99 kg/m2) and obesity (≥30.00 kg/m2). WHtR was categorised as healthy (Males (M): 0.43 to 0.52 and Females (F): 0.42 to 0.48); overweight (M: 0.53 to 0.57 and F: 0.49 to 0.53); elevated overweight (M: 0.58 to 0.62 and F: 0.54 to 0.57), and obese (M: ≥0.63 and F: ≥0.58) [29,30].
- Laboratory tests. FG (mg/dL), HDL cholesterol (mg/dL), TG (mg/dL), VLDL cholesterol according to Friedewald’s formula (mg/dL) [31].
2.2. Data Collection
2.3. Statistical Analysis
2.4. Ethical Considerations
3. Results
3.1. Characteristics of the Sample
3.2. Bivariate Analysis and Logistic Regression for MetS
| Variable | No MS 84 (33.5%) | Yes MS 167 (66.5%) | Raw OR | p | Adjusted OR | p |
|---|---|---|---|---|---|---|
| Age (years) | 43.74 (14.4) | 50.04 (14.51) | 1.03 (1.01–1.05) | p < 0.05 | NS | |
| Sex | ||||||
| Women | 73 (86.9%) | 91 (54.5%) | 1 | |||
| Men | 11 (13.1%) | 76 (45.5%) | 5.54 (2.74–11.19) | p < 0.001 | NS | |
| Anthropometry | ||||||
| WC (cm) | 96.31 (8.55) | 101.31 (9.76) | 1.06 (1.02–1.09) | p < 0.001 | NS | |
| Level of WC | ||||||
| Low | 5 (6%) | 2 (1.2%) | 1 | |||
| High | 79 (94%) | 165 (98.8%) | 5.22 (0.99–27.5) | p = 0.51 | NS | |
| Weight (kg) | 86.91 (11.75) | 76.29(16.1) | 1.03 (1.01–1.05) | p < 0.001 | NS | |
| Height (cm) | 152 (6.2) | 155 (8.6) | 1.08 (1.02–1.14) | p < 0.001 | NS | |
| WHtR | 0.63 (0.05) | 0.64 (0.07) | 1.24 (0.77–2.01) | NS | ||
| Level of WHtR | ||||||
| Low | 3 (3.6%) | 2 (1.2%) | 1 | |||
| High | 81 (96.4%) | 163 (98.8%) | 3.01 (0.49– 18.42) | NS | ||
| BMI (kg/m2) | 29.45 (4.19) | 30.81 (5.12) | 1.06 (1.001–1.12) | p < 0.05 | NS | |
| Level of BMI | ||||||
| Underweight | 1 (1.3%) | 1 (0.6%) | NS | |||
| Healthy weight | 8 (10%) | 15 (9.4%) | ||||
| Overweight | 40 (50%) | 56 (35%) | ||||
| Obesity | 31 (38.8%) | 88 (55%) | ||||
| Variable | MetS No 84 (33.5%) | MetS Yes 167 (66.5%) | Raw OR | p | Adjusted OR | p |
| Bioimpedance variables | ||||||
| FP (%) | 34.97 (7.94) | 31.61 (6.489) | 1.22 (1.05–1.43) | p < 0.05 | NS | |
| MM (kg) | 42.34 (6.96) | 49.03 (10.38) | 1.09 (1.05–1.12) | p < 0.001 | NS | |
| BM (kcal) | 1364 (189.73) | 1556.31 (319.31) | 1.003 (1.002–1.004) | p < 0.001 | NS | |
| MA (years) | 46.61 (10.18) | 53.19 (10.63) | 1.06 (1.03–1.09) | p < 0.001 | NS | |
| Clinical variables | ||||||
| SBP (mmHg) | 115.27 (17) | 137.96 (22.91) | 1.06 (1.04–108) | p < 0.001 | 1.08 (1.05–1.11) | p < 0.001 |
| DBP (mmHg) | 72.61 (8.66) | 82.3 (13.72) | 1.07 (1.04–1.11) | p < 0.001 | 1.23 (1.12–1.28) | p < 0.001 |
| HDL-C: (mg/dL) | 43.96 (12.54) | 35.26 (11.15) | 0.91 (0.89–0.95) | p < 0.001 | NS | |
| Level of HDL-C | ||||||
| Low | 52 (61.9%) | 150 (89.8%) | 1 | |||
| High | 32 (38.1%) | 17 (10.2%) | 0.18 (0.09–0.35) | p < 0.001 | NS | |
| TG (mg/dL) | 98.7 (31.02) | 170.75 (71.04) | 1.03 (1.02–1.04) | p < 0.001 | 1.03 (1.02–1.04) | p < 0.001 |
| TG level | ||||||
| Low | 82 (97.6%) | 65 (38.9%) | 1 | |||
| High | 2 (2.4%) | 102 (61.1%) | 64.38 (15.29–270.64) | p < 0.001 | NS | |
| FG (mg/dL) | 90.29 (19.74) | 110.02 (48) | 1.03 (1.01–1.05) | p < 0.001 | 1.02 (1.01–1.09) | p < 0.001 |
| Level of FG | ||||||
| Low | 80 (95.2%) | 89 (53.3%) | 1 | |||
| High | 4 (4.8%) | 78 (46.7%) | 17.52 (6.13–50.04) | p < 0.001 | NS | |
| VLDL | 45.48 (14.29) | 82.48 (39.87) | 1.06 (1.043–1.08) | p < 0.001 | 1.04 (1.01–1.07) | p < 0.001 |
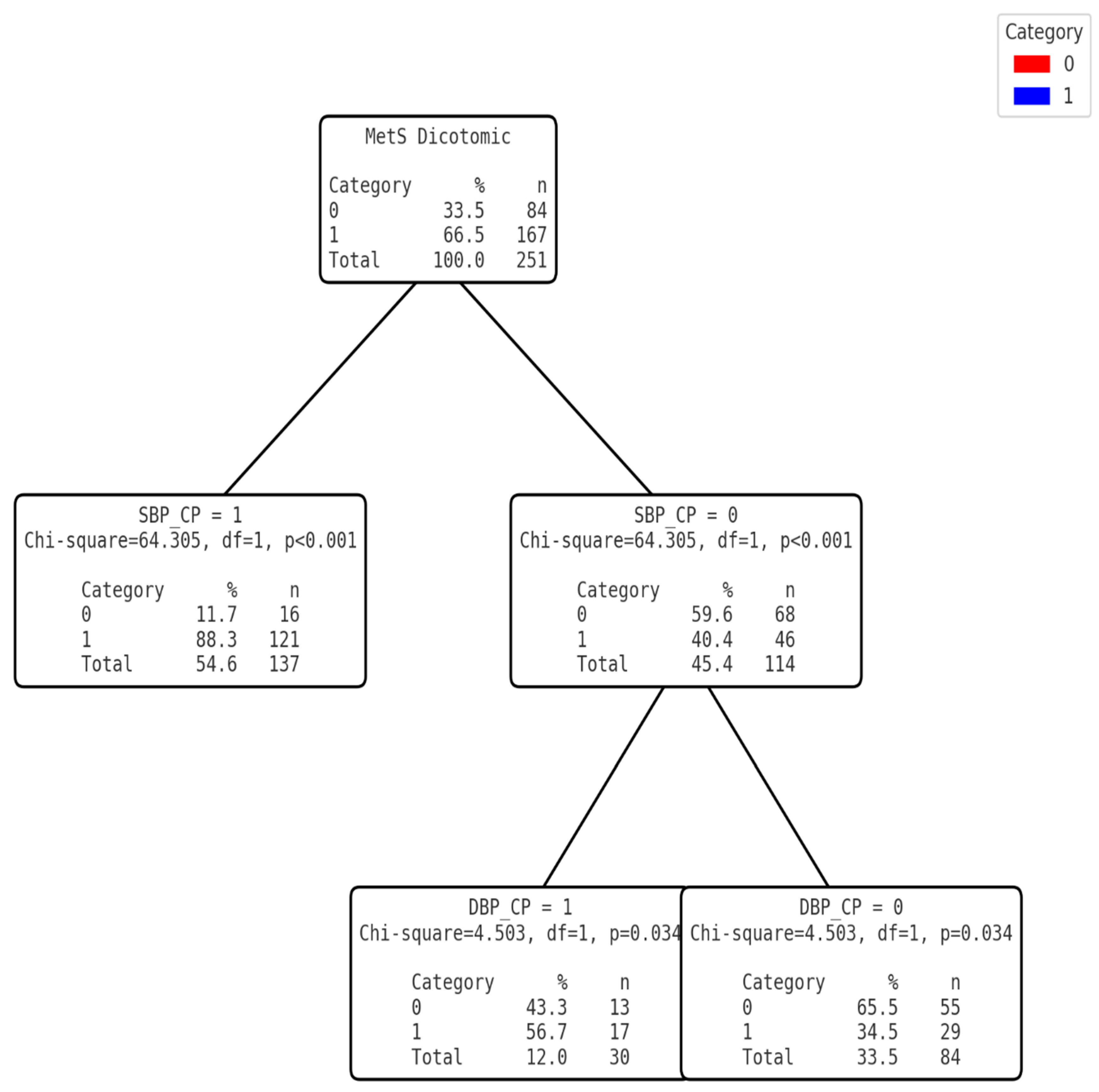
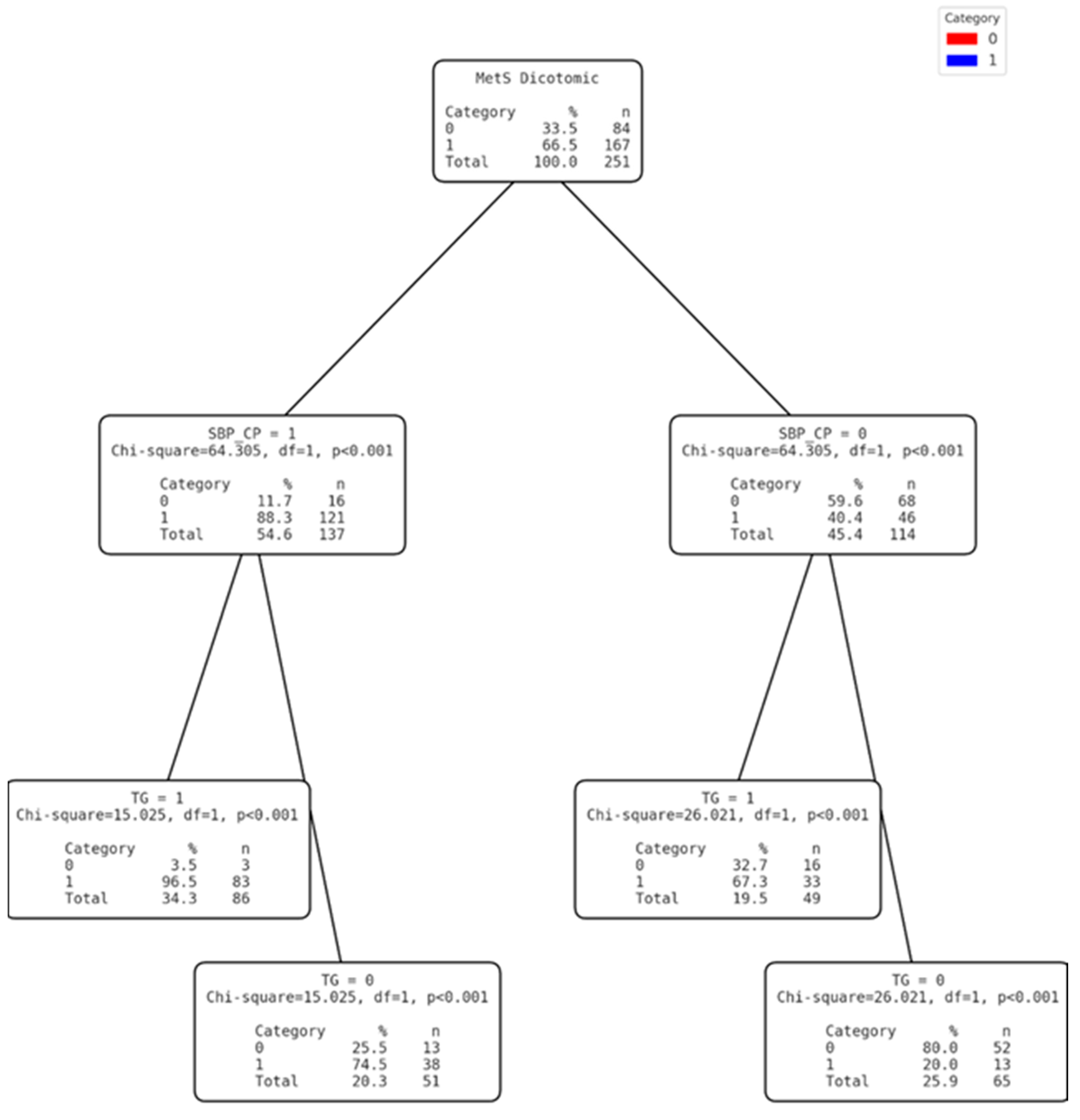
3.3. Development of the Clinical Decision Tree
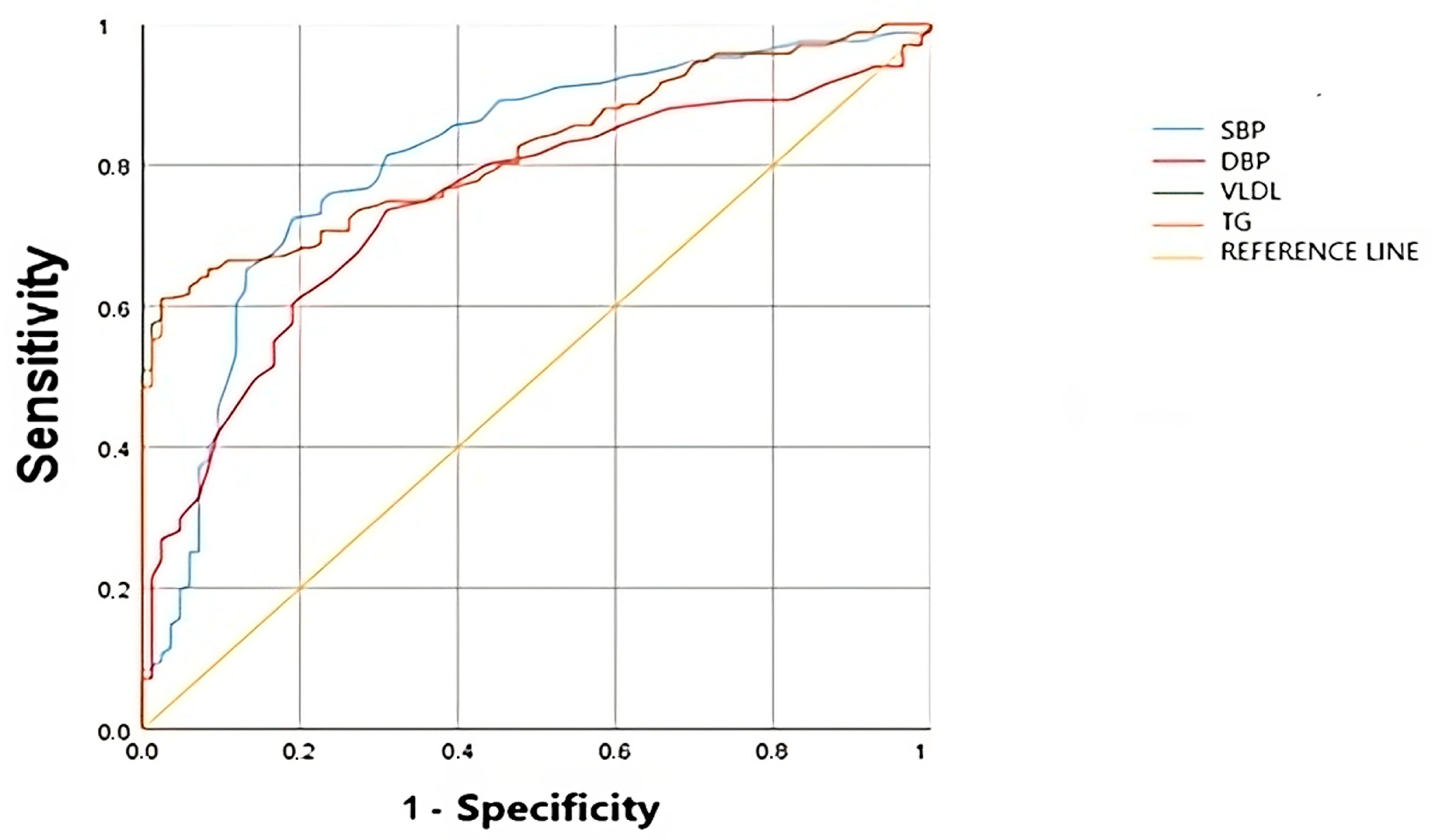
3.4. Comparison of the Diagnostic Efficacy of Clinical Decision Trees
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alberti, K.; Zimmet, P.; Shaw, J.E. Metabolic Syndrome—A New World-wide Definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Mottillo, S.; Filion, K.B.; Genest, J.; Joseph, L.; Pilote, L.; Poirier, P.; Rinfret, S.; Schiffrin, E.L.; Eisenberg, M.J. The Metabolic Syndrome and Cardiovascular Risk: A Systematic Review and Meta-Analysis. J. Am. Coll. Cardiol. 2010, 56, 1113–1132. [Google Scholar] [CrossRef] [PubMed]
- Reaven, G.M. Role of Insulin Resistance in Human Disease. Diabetes 1988, 37, 1595–1607. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; de Ferranti, S.; Després, J.P.; Fullerton, H.J.; Howard, V.J. Heart Disease and Stroke Statistics—2016 Update: A Report from the American Heart Association. Circulation 2016, 133, e38–e360. [Google Scholar] [CrossRef] [PubMed]
- Popkin, B.M. The Nutrition Transition: An Overview of World Patterns of Change. Nutr. Rev. 2004, 62, S140–S143. [Google Scholar] [CrossRef]
- Organización Panamericana de la Salud (OPS). Obesity and Overweight in the Americas; Pan American Health Organization: Washington, DC, USA, 2021. [Google Scholar]
- Ministerio de Salud del Perú (MINSA). Encuesta Demográfica y de Salud Familiar 2021; Ministerio de Salud del Perú: Lima, Peru, 2021.
- International Diabetes Federation (IDF). IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- Chan, J.C.; Malik, V.; Jia, W.; Kadowaki, T.; Yajnik, C.S.; Yoon, K.H.; Hu, F.B. Diabetes in Asia: Epidemiology, Risk Factors, and Pathophysiology. JAMA 2009, 301, 2129–2140. [Google Scholar] [CrossRef]
- Popkin, B.M. The Nutrition Transition and Obesity in the Developing World. J. Nutr. 2001, 131, 871S–873S. [Google Scholar] [CrossRef]
- Lee, C.M.; Huxley, R.; Wildman, R.; Woodward, M. Indices of Abdominal Obesity Are Better Discriminators of Cardiovascular Risk Factors than BMI: A Meta-Analysis. J. Clin. Epidemiol. 2008, 61, 646–653. [Google Scholar] [CrossRef]
- Nyamdorj, R.; Qiao, Q.; Söderberg, S.; Pitkäniemi, J.; Zimmet, P.; Shaw, J.; Alberti, K.G. BMI Compared with Central Obesity Indicators in an Urban Amazonian Context. Int. J. Obes. 2021, 32, 801–808. [Google Scholar] [CrossRef]
- Alberti, K.g.m.m.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, S.C. Harmonizing the Metabolic Syndrome. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef]
- Misra, A.; Khurana, L. Obesity-Related Non-Communicable Diseases: South Asians vs White Caucasians. Int. J. Obes. 2011, 35, 167–187. [Google Scholar] [CrossRef] [PubMed]
- Chirif, A.C. Biodiversidad Amazónica y Gastronomía Regional. Folia Amaz. 2005, 14, 91–98. [Google Scholar] [CrossRef]
- Browning, L.M.; Hsieh, S.D.; Ashwell, M. A Systematic Review of Waist-to-Height Ratio as a Screening Tool for the Prediction of Cardiovascular Disease and Diabetes: 0·5 Could Be a Suitable Global Boundary Value. Nutr. Res. Rev. 2010, 23, 247–269. [Google Scholar] [CrossRef] [PubMed]
- Huxley, R.; Mendis, S.; Zheleznyakov, E.; Reddy, S.; Chan, J. Body Mass Index, Waist Circumference and Waist–Hip Ratio as Predictors of Cardiovascular Risk—A Review of the Literature. Eur. J. Clin. Nutr. 2010, 64, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Lear, S.A.; Humphries, K.H.; Kohli, S.; Birmingham, C.L. The Use of BMI and Waist Circumference as Surrogates of Body Fat Differs by Ethnicity. Obesity 2007, 15, 2817–2824. [Google Scholar] [CrossRef]
- Misra, A.; Khurana, L. The Metabolic Syndrome in South Asians: Epidemiology, Determinants, and Prevention. Metab. Syndr. Relat. Disord. 2009, 7, 497–514. [Google Scholar] [CrossRef]
- Lear, S.A.; James, P.T.; Ko, G.T.; Kumanyika, S. Appropriateness of Waist Circumference and Waist-to-Hip Ratio Cutoffs for Different Ethnic Groups. Eur. J. Clin. Nutr. 2010, 64, 42–61. [Google Scholar] [CrossRef]
- Popkin, B.M. Global Nutrition Dynamics: The World Is Shifting Rapidly toward a Diet Linked with Noncommunicable Diseases. Am. J. Clin. Nutr. 2006, 84, 289–298. [Google Scholar] [CrossRef]
- Marquez-Sandoval, F.; Macedo-Ojeda, G.; Viramontes-Hörner, D.; Fernández Ballart, J.D.; Salas-Salvadó, J.; Vizmanos, B. The Prevalence of Metabolic Syndrome in Latin America: A Systematic Review. Public Health Nutr. 2011, 14, 1702–1713. [Google Scholar] [CrossRef]
- Popkin, B.M.; Adair, L.S.; Ng, S.W. Global Nutrition Transition and the Pandemic of Obesity in Developing Countries. Nutr. Rev. 2012, 70, 3–21. [Google Scholar] [CrossRef]
- Rodríguez, M.G.; Saldaña, M.R.; Leyva, J.M.A.; Rojas, R.M.; Molina-Recio, G. The FINDRISC Questionnaire Capacity to Predict Diabetes Mellitus II, Arterial Hypertension and Comorbidity in Women from Low-and-Middle-Income Countries. Health Care Women Int. 2020, 41, 205–226. [Google Scholar] [CrossRef] [PubMed]
- Alcaide-Leyva, J.M.; Romero-Saldaña, M.; García-Rodríguez, M.; Molina-Luque, R.; Jiménez-Mérida, R.; Molina-Recio, G. Development of a Predictive Model of Cardiovascular Risk in a Male Population from the Peruvian Amazon. J. Clin. Med. 2023, 12, 3199. [Google Scholar] [CrossRef] [PubMed]
- Simental-Mendía, L.E.; Rodríguez-Morán, M.; Guerrero-Romero, F. A New Lipid Index for Identifying Metabolic Syndrome in the Mexican Population. Eur. J. Intern. Med. 2008, 19, 442–447. [Google Scholar] [CrossRef]
- Guerrero-Romero, F.; Villalobos-Molina, R.; Jiménez-Flores, J.R.; Simental-Mendía, L.E. The TyG Index as a Cardiometabolic Risk Marker in Healthy Young Adults. Acta Diabetol. 2016, 53, 461–467. [Google Scholar] [CrossRef]
- Rubio, M.; Moreno, C.; Cabrerizo, L. Guías Para El Tratamiento de Las Dislipemias En El Adulto: Adult Treatment Panel III (ATP-III). Endocrinol. Nutr. 2004, 51, 254–265. [Google Scholar] [CrossRef]
- Schneider, H.J.; Klotsche, J.; Silber, S.; Stalla, G.; Wittchen, H. Measuring Abdominal Obesity: Effects of Height on Distribution of Cardiometabolic Risk Factors Risk Using Waist Circumference and Waist-to-Height Ratio. Diabetes Care 2011, 34, e7. [Google Scholar] [CrossRef]
- Ashwell, M.; Gunn, P.; Gibson, S. Waist-to-height Ratio Is a Better Screening Tool than Waist Circumference and BMI for Adult Cardiometabolic Risk Factors: Systematic Review and Meta-analysis. Obes. Rev. 2012, 13, 275–286. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma, without Use of the Preparative Ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Norton, K.I. Standards for Anthropometry Assessment. In Kinanthropometry and Exercise Physiology; Routledge: Boca Raton, FL, USA, 2018; pp. 68–137. ISBN 1-315-38566-X. [Google Scholar]
- Ladwig, G.B.; Ackley, B.J.; Makic, M.B.F. Mosby’s Guide to Nursing Diagnosis, Revised Reprint with 2021–2023 NANDA-I® Updates-E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2021; ISBN 0-323-87547-5. [Google Scholar]
- Manzini, J.L. Declaración de Helsinki: Principios Éticos Para La Investigación Médica Sobre Sujetos Humanos. Acta Bioethica 2000, 6, 321–334. [Google Scholar] [CrossRef]
- Perú: Ley No 26.842 de 1997—Ley General de Salud. Available online: https://www.refworld.org/es/leg/legis/pleg/1997/es/130591 (accessed on 15 January 2025).
- European Union. Reglamento (UE) 2016/679 del Parlamento Europeo y del Consejo—De 27 de Abril de 2016—Relativo a la Protección de las Personas Físicas en lo Que Respecta al Tratamiento de Datos Personales y a la Libre Circulación de Estos Datos y Por el Que se Deroga la Directiva 95/46/CE (Reglamento General de Protección de Datos); European Union: Brussels, Belgium, 2016. [Google Scholar]
- Pajuelo, J.; Sánchez-Abanto, J.; Torres, H.L.; Miranda, M. Prevalencia Del Síndrome Metabólico En Pobladores Peruanos Por Debajo de 1 000 y Por Encima de Los 3 000 Msnm. An. Fac. Med. 2012, 73, 101–106. [Google Scholar] [CrossRef]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- França, S.L.; Lima, S.S.; Vieira, J.R.D.S. Metabolic Syndrome and Associated Factors in Adults of the Amazon Region. PLOS ONE 2016, 11, e0167320. [Google Scholar] [CrossRef]
- Bernabé-Ortiz, A.; Carrillo-Larco, R.M.; Gilman, R.H.; Checkley, W.; Smeeth, L.; Miranda, J.J.; CRONICAS Cohort Study Group. Impact of Urbanisation and Altitude on the Incidence of, and Risk Factors for, Hypertension. Heart 2017, 103, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Fornari Laurindo, L.; Minniti, G.; José Tofano, R.; Quesada, K.; Federighi Baisi Chagas, E.; Maria Barbalho, S. Detection of Metabolic Syndrome Using Insulin Resistance Indexes: A Cross-Sectional Observational Cohort Study. Endocrines 2023, 4, 257–268. [Google Scholar] [CrossRef]
- Farinola, M.G.; Sganga, M. Puntos de Corte de Indicadores Antropométricos Para Hipertensión e Hiperglucemia En Adultos Argentinos. Rev. Fac. Cienc. Médicas Univ. Nac. Córdoba 2022, 79, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Xie, E.; Ye, Z.; Wu, Y.; Zhao, X.; Li, Y.; Shen, N.; Gao, Y.; Zheng, J. The Triglyceride-Glucose Index Predicts 1-Year Major Adverse Cardiovascular Events in End-Stage Renal Disease Patients with Coronary Artery Disease. Cardiovasc. Diabetol. 2023, 22, 292. [Google Scholar] [CrossRef]
- Sghaireen, M.G.; Al-Smadi, Y.; Al-Qerem, A.; Srivastava, K.C.; Ganji, K.K.; Alam, M.K.; Khader, Y. Machine Learning Approach for Metabolic Syndrome Diagnosis Using Explainable Data-Augmentation-Based Classification. Diagnostics 2022, 12, 3117. [Google Scholar] [CrossRef]
- Guzmán, A.; Navarro, E.; Obando, L.; Pacheco, J.; Quirós, K.; Vásquez, L.; Ramírez, F. Efectividad de Las Intervenciones Para Revertir El Diagnóstico Del Síndrome Metabólico: Actualización de Un Metaanálisis de Comparación Mixta de Tratamientos. Biomédica 2019, 39, 647–662. [Google Scholar] [CrossRef]
- Houser, D.S.; Champagne, C.D.; Crocker, D.E. A Non-Traditional Model of the Metabolic Syndrome: The Adaptive Significance of Insulin Resistance in Fasting-Adapted Seals. Front. Endocrinol. 2013, 4, 164. [Google Scholar] [CrossRef]
- Cho, Y.; Lee, S.Y. Useful Biomarkers of Metabolic Syndrome. Int. J. Environ. Res. Public. Health 2022, 19, 15003. [Google Scholar] [CrossRef]
- Falahi, E.; Khalkhali, H.R.; Bagherniya, M.; Hashemi, R. Systematic Review of Metabolic Syndrome Biomarkers: A Panel for Early Detection, Management, and Risk Stratification. Int. J. Endocrinol. Metab. 2015, 13, e22848. [Google Scholar]
- Lee, J.H.; Lee, K.H.; Kim, H.J.; Youk, H.; Lee, H.Y. Effective Prevention and Management Tools for Metabolic Syndrome Based on Digital Health-Based Lifestyle Interventions Using Healthcare Devices. Diagnostics 2022, 12, 1730. [Google Scholar] [CrossRef] [PubMed]
- Croci, S.; D’Apolito, L.I.; Gasperi, V.; Catani, M.V.; Savini, I. Dietary Strategies for Management of Metabolic Syndrome: Role of Gut Microbiota Metabolites. Nutrients 2021. [CrossRef] [PubMed]

| Variable | Total n = 251 | Women 164 (65.3%) | Men 87 (34.7%) | p |
|---|---|---|---|---|
| Age (years) | 47.93 (SD 15.71) | 47.68 (SD15.68) | 48.39 (1SD3.96) | |
| Anthropometry | ||||
| WC (cm) | 99.64 (SD9.65) | 96.53 (53 (8.2) | 105.49 (SD9.51) | <0.001 |
| Level of WC | ||||
| Low | 7 (2.8%) | 3 (42.9%) | 4 (57.1%) | |
| High | 244 (97.2%) | 161 (66%) | 83 (34%) | NS |
| Weight (kg) | 73.83 (SD15.17) | 67.98 (SD11.92) | 71.57(SD6.48) | <0.001 |
| Height (cm) | 154.76 (SD1.54) | 1.51 (SD0.05) | 1.62 (SD0.06) | <0.001 |
| WHtR | 0.64 (SD0.07) | 0.63 (SD0.07) | 0.65 (SD0.05) | |
| Level of WHtR | ||||
| Low | 5 (2) | 4 (80) | 1 (20%) | NS |
| High | 244 (98) | 158 (64.8) | 86 (35.2%) | |
| BMI (kg/m2) | 30.360 (SD4.86) | 29.83(SD 3.12) | 32 (SD4.44) | <0.001 |
| Level of BMI | ||||
| Underweight | 2 (0.8%) | 2 (100%) | 0 | <0.001 |
| Healthy weight | 23.8 (9.2%) | 20 (87%) | 3 (13%) | |
| Overweight | 96 (38.2%) | 69 (71.1%) | 27 (28.1%) | |
| Obesity | 119 (47.4%) | 66 (55.5%) | 52 (44.5%) | |
| Variable | Total | Women 164 (65.3%) | Men 87 (34.7%) | p |
|---|---|---|---|---|
| Bioimpedance variables | ||||
| FP (%) | 32.73 (SD7.17) | 35.21 (SD6.67) | 28.06 (SD5.61) | <0.001 |
| MM (kg) | 46.86 (SD 4.05) | 47.26 (SD5.53) | 52.39 (SD4.27) | <0.001 |
| BMR (kcal) | 1492.53 (SD296.48) | 1334.61 (SD162.31) | 1791.02 (SD260.85) | <0.001 |
| MA (years) | 51 (SD10.91) | 49 (SD10.8) | 54.8 (SD10.13) | <0.001 |
| Clinical variables | ||||
| SBP (mmHg) | 130.37 (DE 23.65) | 125.41 (SD 23.44) | 139.70 (SD 21.21) | <0.001 |
| DBP (mmHg) | 79.06 (DE 13.07) | 75.68 (SD 12.83) | 85.43 (SD 11.04) | <0.001 |
| HDL-C: (mg/dL) | 38.18 (DE 13.31) | 42.38 (SD 12.61) | 30.24 (SD 6.479) | <0.001 |
| Level of HDL-C | ||||
| Low | 49 (19.5%) | 39 (79.6%) | 10 (20.4%) | <0.05 |
| High | 202 (80.5%) | 125 (61.9%) | 77 (38.1%) | |
| TG (mg/dL) | 152.12 (SD 69.5) | 134.01 (SD 124.01) | 170.44 (SD 76.17) | <0.001 |
| TG level | ||||
| Low | 147 (58.6%) | 106 (72.1%) | 41 (27.9%) | <0.05 |
| High | 104 (41.4%) | 58 (55.8%) | 46 (44.2%) | |
| FG (mg/dL) | 103.42 (SD 41.79) | 102.63 (SD 39.42) | 104.91 (SD 46.13) | NS |
| Low | 169 (67.3%) | 112 (66.3%) | 57 (33.7%) | NS |
| High | 82 (32.7%) | 52 (63.4%) | 30 (36.6%) | |
| VLDL | 70.10 (SD 37.81) | 45.48(SD 14.29) | 82.45 (SD 39.87) | <0.001 |
| Variable | AUC | p | 95%IC | Cut-Off Point | Sensitivity | Specificity | J |
|---|---|---|---|---|---|---|---|
| TG | 0.83 | <0.001 | 0.78 –0.88 | 149.5 | 0.611 | 0.976 | 0.587 |
| VLDL | 0.82 | <0.001 | 0.77–0.872 | 68.89 | 0.591 | 0.914 | 0.612 |
| SBP | 0.819 | <0.001 | 0.71–0.83 | 125.5 | 0.725 | 0.81 | 0.534 |
| DBP | 0.756 | <0.001 | 0.69–0.82 | 75.5 | 0.737 | 0.69 | 0.424 |
| Sensitivity (%) | Specificity (%) | Validity Index (%) | PPV (%) | NVP (%) | Youden | |
|---|---|---|---|---|---|---|
| Model 1 | 84.43 (78.63–90.23) | 65.48 (54.71–76.24) | 78.09 (72.77–83.40) | 83.94 (76.99–88.89) | 67.90 (57.12–79.69) | 0.5 (0.38–0.61) |
| Model 2 | 92.22 (87.85–96.58) | 61.90 (50.92–72.88) | 82.07 (77.13–87.02) | 82.80 (77.10–88.49) | 80 (69.51–90.49) | 0.54 (0.43–0.65) |
| Model 3 | 91.62 (87.11–96.12) | 78.57 (69.2–87.94) | 87.25 (82.93–91.58) | 89.47(84.58–94.37) | 82.50(73.55–91.45) | 0.7(0.6–0.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alcaide-Leyva, J.M.; Romero-Saldaña, M.; García-Rodríguez, M.; Molina-Luque, R.; Jiménez-Mérida, M.d.R.; Molina-Recio, G. Metabolic Syndrome in the Amazon: Customizing Diagnostic Methods for Urban Communities. Nutrients 2025, 17, 538. https://doi.org/10.3390/nu17030538
Alcaide-Leyva JM, Romero-Saldaña M, García-Rodríguez M, Molina-Luque R, Jiménez-Mérida MdR, Molina-Recio G. Metabolic Syndrome in the Amazon: Customizing Diagnostic Methods for Urban Communities. Nutrients. 2025; 17(3):538. https://doi.org/10.3390/nu17030538
Chicago/Turabian StyleAlcaide-Leyva, José M., Manuel Romero-Saldaña, María García-Rodríguez, Rafael Molina-Luque, María del Rocío Jiménez-Mérida, and Guillermo Molina-Recio. 2025. "Metabolic Syndrome in the Amazon: Customizing Diagnostic Methods for Urban Communities" Nutrients 17, no. 3: 538. https://doi.org/10.3390/nu17030538
APA StyleAlcaide-Leyva, J. M., Romero-Saldaña, M., García-Rodríguez, M., Molina-Luque, R., Jiménez-Mérida, M. d. R., & Molina-Recio, G. (2025). Metabolic Syndrome in the Amazon: Customizing Diagnostic Methods for Urban Communities. Nutrients, 17(3), 538. https://doi.org/10.3390/nu17030538








