Protective Effects of Bifidobacterium Breve MCC1274 as a Novel Therapy for Alzheimer’s Disease
Abstract
:1. Alzheimer’s Disease
2. Role of Gut Microbiota in Neurodegenerative Diseases
3. Gut–Brain Axis
4. Bifidobacterium breve MCC1274 as a Multifaceted Probiotic for the Gut and Brain
4.1. Characterization of Bifidobacterium breve MCC1274 and Its Benefits on Gut Integrity
4.2. Probiotic Properties of B. breve MCC1274
5. Mechanisms of Action of B. breve MCC1274 in Neurodegenerative Disease
5.1. Potential Effects of B. breve MCC1274 Supplementation in Modulating Neuroinflammation
5.2. Modulation of Oxidative Stress and Chronic Stress Responses by B. breve MCC1274 Supplementation
5.3. Potential Effects of B. breve MCC1274 Supplementation in Modulating Aβ and Tau Pathology
5.4. Modulation of Gut Microbiota and Metabolic Function by breve MCC1274 Supplementation
5.5. The Impacts of B. breve MCC1274 Supplementation on BBB Integrity
5.6. The Effects of B. breve MCC1274 Supplementation on Cellular Proliferation and Neuronal Cell Loss
5.7. The Effects of B. breve MCC1274 Supplementation on Synaptic Protein Levels
6. B. breve MCC1274 as a Potential Treatment for Cognitive Behavioral Abnormalities in Neurodegenerative Diseases
7. Comparison of B. breve MCC1274 with Other Probiotic Strains in AD Progression and Brain Health
8. Future Directions and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dubois, B.; Hampel, H.; Feldman, H.H.; Scheltens, P.; Aisen, P.; Andrieu, S.; Bakardjian, H.; Benali, H.; Bertram, L.; Blennow, K.; et al. Preclinical Alzheimer’s disease: Definition, natural history, and diagnostic criteria. Alzheimer’s Dement. 2016, 12, 292–323. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R., Jr.; Knopman, D.S.; Jagust, W.J.; Shaw, L.M.; Aisen, P.S.; Weiner, M.W.; Petersen, R.C.; Trojanowski, J.Q. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010, 9, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Weiner, M.W.; Veitch, D.P.; Aisen, P.S.; Beckett, L.A.; Cairns, N.J.; Green, R.C.; Harvey, D.; Jack, C.R.; Jagust, W.; Liu, E.; et al. The Alzheimer’s Disease Neuroimaging Initiative: A review of papers published since its inception. Alzheimer’s Dement. 2013, 9, e111–e194. [Google Scholar] [CrossRef]
- Koblinsky, N.D.; Power, K.A.; Middleton, L.; Ferland, G.; Anderson, N.D. The Role of the Gut Microbiome in Diet and Exercise Effects on Cognition: A Review of the Intervention Literature. J. Gerontol. Ser. A 2022, 78, 195–205. [Google Scholar] [CrossRef]
- Pedroza Matute, S.; Iyavoo, S. Exploring the gut microbiota: Lifestyle choices, disease associations, and personal genomics. Front. Nutr. 2023, 10, 1225120. [Google Scholar] [CrossRef]
- Wen, L.; Duffy, A. Factors Influencing the Gut Microbiota, Inflammation, and Type 2 Diabetes. J. Nutr. 2017, 147, 1468s–1475s. [Google Scholar] [CrossRef]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid-β Pathway in Alzheimer’s Disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef]
- Selkoe, D.J. Alzheimer’s Disease: A Central Role for Amyloid. J. Neuropathol. Exp. Neurol. 1994, 53, 438–447. [Google Scholar] [CrossRef]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef]
- Ballatore, C.; Lee, V.M.Y.; Trojanowski, J.Q. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat. Rev. Neurosci. 2007, 8, 663–672. [Google Scholar] [CrossRef]
- Heneka, M.T.; Carson, M.J.; Khoury, J.E.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef] [PubMed]
- Terry, R.D.; Masliah, E.; Salmon, D.P.; Butters, N.; DeTeresa, R.; Hill, R.; Hansen, L.A.; Katzman, R. Physical basis of cognitive alterations in alzheimer’s disease: Synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 1991, 30, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Weller, J.; Budson, A. Current understanding of Alzheimer’s disease diagnosis and treatment. F1000Research 2018, 7, 1161. [Google Scholar] [CrossRef] [PubMed]
- Cappa, S.F. The Quest for an Alzheimer Therapy. Front. Neurol. 2018, 9, 108. [Google Scholar] [CrossRef]
- Karakaya, T.; Fußer, F.; Schroder, J.; Pantel, J. Pharmacological Treatment of Mild Cognitive Impairment as a Prodromal Syndrome of Alzheimer’s Disease. Curr. Neuropharmacol. 2013, 11, 102–108. [Google Scholar] [CrossRef]
- Schneider, L.S.; Insel, P.S.; Weiner, M.W.; Initiative, A.s.D.N. Treatment With Cholinesterase Inhibitors and Memantine of Patients in the Alzheimer’s Disease Neuroimaging Initiative. Arch. Neurol. 2011, 68, 58–66. [Google Scholar] [CrossRef]
- Rutsch, A.; Kantsjö, J.B.; Ronchi, F. The Gut-Brain Axis: How Microbiota and Host Inflammasome Influence Brain Physiology and Pathology. Front. Immunol. 2020, 11, 604179. [Google Scholar] [CrossRef]
- Wang, W.Y.; Tan, M.S.; Yu, J.T.; Tan, L. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann. Transl. Med. 2015, 3, 136. [Google Scholar] [CrossRef]
- Miao, J.; Ma, H.; Yang, Y.; Liao, Y.; Lin, C.; Zheng, J.; Yu, M.; Lan, J. Microglia in Alzheimer’s disease: Pathogenesis, mechanisms, and therapeutic potentials. Front. Aging Neurosci. 2023, 15, 1201982. [Google Scholar] [CrossRef]
- Wu, J.-J.; Wei, Z. Advances in the study of the effects of gut microflora on microglia in Alzheimer’s disease. Front. Mol. Neurosci. 2023, 16, 1295916. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Fock, E.; Parnova, R. Mechanisms of Blood–Brain Barrier Protection by Microbiota-Derived Short-Chain Fatty Acids. Cells 2023, 12, 657. [Google Scholar] [CrossRef] [PubMed]
- Cavaliere, G.; Catapano, A.; Trinchese, G.; Cimmino, F.; Penna, E.; Pizzella, A.; Cristiano, C.; Lama, A.; Crispino, M.; Mollica, M.P. Butyrate Improves Neuroinflammation and Mitochondrial Impairment in Cerebral Cortex and Synaptic Fraction in an Animal Model of Diet-Induced Obesity. Antioxidants 2023, 12, 4. [Google Scholar] [CrossRef]
- Jaworska, J.; Zalewska, T.; Sypecka, J.; Ziemka-Nalecz, M. Effect of the HDAC Inhibitor, Sodium Butyrate, on Neurogenesis in a Rat Model of Neonatal Hypoxia–Ischemia: Potential Mechanism of Action. Mol. Neurobiol. 2019, 56, 6341–6370. [Google Scholar] [CrossRef]
- Wasén, C.; Beauchamp, L.C.; Vincentini, J.; Li, S.; LeServe, D.S.; Gauthier, C.; Lopes, J.R.; Moreira, T.G.; Ekwudo, M.N.; Yin, Z.; et al. Bacteroidota inhibit microglia clearance of amyloid-beta and promote plaque deposition in Alzheimer’s disease mouse models. Nat. Commun. 2024, 15, 3872. [Google Scholar] [CrossRef]
- Dhami, M.; Raj, K.; Singh, S. Relevance of gut microbiota to Alzheimer’s Disease (AD): Potential effects of probiotic in management of AD. Aging Health Res. 2023, 3, 100128. [Google Scholar] [CrossRef]
- Zhu, X.; Li, B.; Lou, P.; Dai, T.; Chen, Y.; Zhuge, A.; Yuan, Y.; Li, L. The Relationship Between the Gut Microbiome and Neurodegenerative Diseases. Neurosci. Bull. 2021, 37, 1510–1522. [Google Scholar] [CrossRef]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V.; et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell 2016, 167, 1469–1480.e1412. [Google Scholar] [CrossRef]
- Harach, T.; Marungruang, N.; Duthilleul, N.; Cheatham, V.; Mc Coy, K.D.; Frisoni, G.; Neher, J.J.; Fåk, F.; Jucker, M.; Lasser, T.; et al. Reduction of Abeta amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Sci. Rep. 2017, 7, 41802. [Google Scholar] [CrossRef]
- Knox, E.G.; Aburto, M.R.; Clarke, G.; Cryan, J.F.; O’Driscoll, C.M. The blood-brain barrier in aging and neurodegeneration. Mol. Psychiatry 2022, 27, 2659–2673. [Google Scholar] [CrossRef]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P.; et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014, 6, 263ra158. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Du, W.; Hu, X.; Yu, X.; Guo, C.; Jin, X.; Wang, W. Targeting the blood–brain barrier to delay aging-accompanied neurological diseases by modulating gut microbiota, circadian rhythms, and their interplays. Acta Pharm. Sin. B 2023, 13, 4667–4687. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Bruggeman, A.; De Nolf, C.; Vandendriessche, C.; Van Imschoot, G.; Van Wonterghem, E.; Vereecke, L.; Vandenbroucke, R.E. Gut microbiota regulates blood-cerebrospinal fluid barrier function and Aβ pathology. EMBO J. 2023, 42, e111515. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Gao, J.; Liu, K.; Zhang, H.-L. Microbiota-gut-brain axis and Alzheimer’s disease: Implications of the blood-brain barrier as an intervention target. Mech. Ageing Dev. 2021, 199, 111560. [Google Scholar] [CrossRef]
- Montanari, M.; Imbriani, P.; Bonsi, P.; Martella, G.; Peppe, A. Beyond the Microbiota: Understanding the Role of the Enteric Nervous System in Parkinson’s Disease from Mice to Human. Biomedicines 2023, 11, 1560. [Google Scholar] [CrossRef]
- Margolis, K.G.; Cryan, J.F.; Mayer, E.A. The Microbiota-Gut-Brain Axis: From Motility to Mood. Gastroenterology 2021, 160, 1486–1501. [Google Scholar] [CrossRef]
- Breit, S.; Kupferberg, A.; Rogler, G.; Hasler, G. Vagus Nerve as Modulator of the Brain–Gut Axis in Psychiatric and Inflammatory Disorders. Front. Psychiatry 2018, 9, 44. [Google Scholar] [CrossRef]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous Bacteria from the Gut Microbiota Regulate Host Serotonin Biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef]
- Ashique, S.; Mohanto, S.; Ahmed, M.G.; Mishra, N.; Garg, A.; Chellappan, D.K.; Omara, T.; Iqbal, S.; Kahwa, I. Gut-brain axis: A cutting-edge approach to target neurological disorders and potential synbiotic application. Heliyon 2024, 10, 13. [Google Scholar] [CrossRef]
- Vogt, N.M.; Kerby, R.L.; Dill-McFarland, K.A.; Harding, S.J.; Merluzzi, A.P.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Zetterberg, H.; Blennow, K.; et al. Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 2017, 7, 13537. [Google Scholar] [CrossRef]
- Tooley, K.L. Effects of the Human Gut Microbiota on Cognitive Performance, Brain Structure and Function: A Narrative Review. Nutrients 2020, 12, 3009. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wei, J.; Liu, X.; Li, D.; Pang, X.; Chen, F.; Cao, H.; Lei, P. Gut microbiota–astrocyte axis: New insights into age-related cognitive decline. Neural Regen. Res. 2025, 20, 990–1008. [Google Scholar] [CrossRef] [PubMed]
- Loh, J.S.; Mak, W.Q.; Tan, L.K.S.; Ng, C.X.; Chan, H.H.; Yeow, S.H.; Foo, J.B.; Ong, Y.S.; How, C.W.; Khaw, K.Y. Microbiota–gut–brain axis and its therapeutic applications in neurodegenerative diseases. Signal Transduct. Target. Ther. 2024, 9, 37. [Google Scholar] [CrossRef]
- de Leon-Derecho, C.M.P. Microbiome-based therapeutics. In Human Microbiome Drug Targets; Elsevier: Amsterdam, The Netherlands, 2025; pp. 233–244. [Google Scholar]
- Ruwatia, R.; Sarkar, S.R.; Panda, S.; Banerjee, S. Gut microbiome and neurodegenerative disorders: Therapeutic Implications. Aging Pathobiol. Ther. 2024, 7, 1. [Google Scholar]
- Bercik, P.; Denou, E.; Collins, J.; Jackson, W.; Lu, J.; Jury, J.; Deng, Y.; Blennerhassett, P.; Macri, J.; McCoy, K.D.; et al. The Intestinal Microbiota Affect Central Levels of Brain-Derived Neurotropic Factor and Behavior in Mice. Gastroenterology 2011, 141, 599–609.e593. [Google Scholar] [CrossRef]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef]
- Sun, M.; Wu, W.; Liu, Z.; Cong, Y. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J. Gastroenterol. 2017, 52, 1–8. [Google Scholar] [CrossRef]
- Du, Y.; He, C.; An, Y.; Huang, Y.; Zhang, H.; Fu, W.; Wang, M.; Shan, Z.; Xie, J.; Yang, Y.; et al. The Role of Short Chain Fatty Acids in Inflammation and Body Health. Int. J. Mol. Sci. 2024, 25, 7379. [Google Scholar] [CrossRef]
- Kim, C.H. Complex regulatory effects of gut microbial short-chain fatty acids on immune tolerance and autoimmunity. Cell. Mol. Immunol. 2023, 20, 341–350. [Google Scholar] [CrossRef]
- Duan, H.; Wang, L.; Huangfu, M.; Li, H. The impact of microbiota-derived short-chain fatty acids on macrophage activities in disease: Mechanisms and therapeutic potentials. Biomed. Pharmacother. 2023, 165, 115276. [Google Scholar] [CrossRef]
- Guo, T.-T.; Zhang, Z.; Sun, Y.; Zhu, R.-Y.; Wang, F.-X.; Ma, L.-J.; Jiang, L.; Liu, H.-D. Neuroprotective Effects of Sodium Butyrate by Restoring Gut Microbiota and Inhibiting TLR4 Signaling in Mice with MPTP-Induced Parkinson’s Disease. Nutrients 2023, 15, 930. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Tillisch, K.; Gupta, A. Gut/brain axis and the microbiota. J. Clin. Investig. 2015, 125, 926–938. [Google Scholar] [CrossRef] [PubMed]
- Mhanna, A.; Martini, N.; Hmaydoosh, G.; Hamwi, G.; Jarjanazi, M.; Zaifah, G.; Kazzazo, R.; Haji Mohamad, A.; Alshehabi, Z. The correlation between gut microbiota and both neurotransmitters and mental disorders: A narrative review. Medicine 2024, 103, e37114. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, T.A.; Nguyen, J.C.; Polglaze, K.E.; Bertrand, P.P. Influence of Tryptophan and Serotonin on Mood and Cognition with a Possible Role of the Gut-Brain Axis. Nutrients 2016, 8, 56. [Google Scholar] [CrossRef]
- Messaoudi, M.; Violle, N.; Bisson, J.F.; Desor, D.; Javelot, H.; Rougeot, C. Beneficial psychological effects of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in healthy human volunteers. Gut Microbes 2011, 2, 256–261. [Google Scholar] [CrossRef]
- Arboleya, S.; Watkins, C.; Stanton, C.; Ross, R.P. Gut Bifidobacteria Populations in Human Health and Aging. Front. Microbiol. 2016, 7, 1204. [Google Scholar] [CrossRef]
- Fernández, L.; Langa, S.; Martín, V.; Maldonado, A.; Jiménez, E.; Martín, R.; Rodríguez, J.M. The human milk microbiota: Origin and potential roles in health and disease. Pharmacol. Res. 2013, 69, 1–10. [Google Scholar] [CrossRef]
- Butta, H.; Sardana, R.; Vaishya, R.; Singh, K.N.; Mendiratta, L. Bifidobacterium: An Emerging Clinically Significant Metronidazole-resistant Anaerobe of Mixed Pyogenic Infections. Cureus 2017, 9, e1134. [Google Scholar] [CrossRef]
- Prasanna, P.H.P.; Grandison, A.S.; Charalampopoulos, D. Bifidobacteria in milk products: An overview of physiological and biochemical properties, exopolysaccharide production, selection criteria of milk products and health benefits. Food Res. Int. 2014, 55, 247–262. [Google Scholar] [CrossRef]
- Pokusaeva, K.; Fitzgerald, G.F.; van Sinderen, D. Carbohydrate metabolism in Bifidobacteria. Genes Nutr. 2011, 6, 285–306. [Google Scholar] [CrossRef]
- Bernier, F.; Ohno, K.; Katsumata, N.; Shimizu, T.; Xiao, J. Association of Plasma Hemoglobin A1c with Improvement of Cognitive Functions by Probiotic Bifidobacterium breve Supplementation in Healthy Adults with Mild Cognitive Impairment. J. Alzheimer’s Dis. 2021, 81, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, Y.; Hand, T.W. Role of the Microbiota in Immunity and Inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Wasan, A.; Sharma, R.K. Recent developments in probiotics: An emphasis on Bifidobacterium. Food Biosci. 2021, 41, 100993. [Google Scholar] [CrossRef]
- Saturio, S.; Nogacka, A.M.; Alvarado-Jasso, G.M.; Salazar, N.; de Los Reyes-Gavilán, C.G.; Gueimonde, M.; Arboleya, S. Role of Bifidobacteria on Infant Health. Microorganisms 2021, 9, 2415. [Google Scholar] [CrossRef]
- Melini, F.; Melini, V.; Luziatelli, F.; Ficca, A.G.; Ruzzi, M. Health-Promoting Components in Fermented Foods: An Up-to-Date Systematic Review. Nutrients 2019, 11, 1189. [Google Scholar] [CrossRef]
- Shah, A.M.; Tarfeen, N.; Mohamed, H.; Song, Y. Fermented Foods: Their Health-Promoting Components and Potential Effects on Gut Microbiota. Fermentation 2023, 9, 118. [Google Scholar] [CrossRef]
- Niu, M.M.; Guo, H.X.; Cai, J.W.; Meng, X.C. Bifidobacterium breve Alleviates DSS-Induced Colitis in Mice by Maintaining the Mucosal and Epithelial Barriers and Modulating Gut Microbes. Nutrients 2022, 14, 3671. [Google Scholar] [CrossRef]
- Bozzi Cionci, N.; Baffoni, L.; Gaggìa, F.; Di Gioia, D. Therapeutic Microbiology: The Role of Bifidobacterium breve as Food Supplement for the Prevention/Treatment of Paediatric Diseases. Nutrients 2018, 10, 1723. [Google Scholar] [CrossRef]
- Abdulqadir, R.; Engers, J.; Al-Sadi, R. Role of Bifidobacterium in Modulating the Intestinal Epithelial Tight Junction Barrier: Current Knowledge and Perspectives. Curr. Dev. Nutr. 2023, 7, 102026. [Google Scholar] [CrossRef]
- Mazziotta, C.; Tognon, M.; Martini, F.; Torreggiani, E.; Rotondo, J.C. Probiotics Mechanism of Action on Immune Cells and Beneficial Effects on Human Health. Cells 2023, 12, 184. [Google Scholar] [CrossRef]
- Slavin, J. Fiber and prebiotics: Mechanisms and health benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, M.; Zhou, C.; Jung, C.G.; Michikawa, M. Probiotic Bifidobacterium breve MCC1274 Mitigates Alzheimer’s Disease-Related Pathologies in Wild-Type Mice. Nutrients 2022, 14, 2543. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, M.; Zhou, C.; Ohno, K.; Kuhara, T.; Taslima, F.; Abdullah, M.; Jung, C.-G.; Michikawa, M. Probiotic Bifidobacterium breve Prevents Memory Impairment Through the Reduction of Both Amyloid-β Production and Microglia Activation in APP Knock-In Mouse 1. J. Alzheimer’s Dis. 2022, 85, 1555–1571. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Sugahara, H.; Shimada, K.; Mitsuyama, E.; Kuhara, T.; Yasuoka, A.; Kondo, T.; Abe, K.; Xiao, J.-Z. Therapeutic potential of Bifidobacterium breve strain A1 for preventing cognitive impairment in Alzheimer’s disease. Sci. Rep. 2017, 7, 13510. [Google Scholar] [CrossRef]
- Abdelhamid, M.; Jung, C.G.; Zhou, C.; Inoue, R.; Chen, Y.; Sento, Y.; Hida, H.; Michikawa, M. Potential Therapeutic Effects of Bifidobacterium breve MCC1274 on Alzheimer’s Disease Pathologies in App(NL-G-F) Mice. Nutrients 2024, 16, 538. [Google Scholar] [CrossRef]
- Bernier, F.; Kuhara, T.; Xiao, J. Probiotic Bifidobacterium breve MCC1274 Protects against Oxidative Stress and Neuronal Lipid Droplet Formation via PLIN4 Gene Regulation. Microorganisms 2023, 11, 791. [Google Scholar] [CrossRef]
- Ohno, K.; Abdelhamid, M.; Zhou, C.; Jung, C.G.; Michikawa, M. Bifidobacterium breve MCC1274 Supplementation Increased the Plasma Levels of Metabolites with Potential Anti-Oxidative Activity in APP Knock-In Mice. J. Alzheimers Dis. 2022, 89, 1413–1425. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Kinoshita, T.; Matsumoto, A.; Yoshino, K.; Saito, I.; Xiao, J.Z. Bifidobacterium Breve A1 Supplementation Improved Cognitive Decline in Older Adults with Mild Cognitive Impairment: An Open-Label, Single-Arm Study. J. Prev. Alzheimers Dis. 2019, 6, 70–75. [Google Scholar] [CrossRef]
- Asaoka, D.; Xiao, J.; Takeda, T.; Yanagisawa, N.; Yamazaki, T.; Matsubara, Y.; Sugiyama, H.; Endo, N.; Higa, M.; Kasanuki, K.; et al. Effect of Probiotic Bifidobacterium breve in Improving Cognitive Function and Preventing Brain Atrophy in Older Patients with Suspected Mild Cognitive Impairment: Results of a 24-Week Randomized, Double-Blind, Placebo-Controlled Trial. J. Alzheimers Dis. 2022, 88, 75–95. [Google Scholar] [CrossRef]
- Xiao, J.; Katsumata, N.; Bernier, F.; Ohno, K.; Yamauchi, Y.; Odamaki, T.; Yoshikawa, K.; Ito, K.; Kaneko, T. Probiotic Bifidobacterium breve in Improving Cognitive Functions of Older Adults with Suspected Mild Cognitive Impairment: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Alzheimers Dis. 2020, 77, 139–147. [Google Scholar] [CrossRef]
- Okubo, R.; Koga, M.; Katsumata, N.; Odamaki, T.; Matsuyama, S.; Oka, M.; Narita, H.; Hashimoto, N.; Kusumi, I.; Xiao, J.; et al. Effect of bifidobacterium breve A-1 on anxiety and depressive symptoms in schizophrenia: A proof-of-concept study. J. Affect. Disord. 2019, 245, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Day, C.M.; Abdella, S.; Garg, S. Alzheimer’s disease current therapies, novel drug delivery systems and future directions for better disease management. J. Control. Release 2024, 367, 402–424. [Google Scholar] [CrossRef] [PubMed]
- Holz, R.W.; Fisher, S.K. Chapter 12—Synaptic Transmission and Cellular Signaling: An Overview. In Basic Neurochemistry, 8th ed.; Brady, S.T., Siegel, G.J., Albers, R.W., Price, D.L., Eds.; Academic Press: New York, NY, USA, 2012; pp. 235–257. [Google Scholar]
- Ishii, T.; Furuoka, H.; Kaya, M.; Kuhara, T. Oral Administration of Probiotic Bifidobacterium breve Improves Facilitation of Hippocampal Memory Extinction via Restoration of Aberrant Higher Induction of Neuropsin in an MPTP-Induced Mouse Model of Parkinson’s Disease. Biomedicines 2021, 9, 167. [Google Scholar] [CrossRef] [PubMed]
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef]
- Wang, C.; Zong, S.; Cui, X.; Wang, X.; Wu, S.; Wang, L.; Liu, Y.; Lu, Z. The effects of microglia-associated neuroinflammation on Alzheimer’s disease. Front. Immunol. 2023, 14, 1117172. [Google Scholar] [CrossRef]
- Wong-Guerra, M.; Calfio, C.; Maccioni, R.B.; Rojo, L.E. Revisiting the neuroinflammation hypothesis in Alzheimer’s disease: A focus on the druggability of current targets. Front. Pharmacol. 2023, 14, 1161850. [Google Scholar] [CrossRef]
- Shin, Y.; Han, S.; Kwon, J.; Ju, S.; Choi, T.G.; Kang, I.; Kim, S.S. Roles of Short-Chain Fatty Acids in Inflammatory Bowel Disease. Nutrients 2023, 15, 4466. [Google Scholar] [CrossRef]
- Yamamura, R.; Okubo, R.; Katsumata, N.; Odamaki, T.; Hashimoto, N.; Kusumi, I.; Xiao, J.; Matsuoka, Y.J. Lipid and Energy Metabolism of the Gut Microbiota Is Associated with the Response to Probiotic Bifidobacterium breve Strain for Anxiety and Depressive Symptoms in Schizophrenia. J. Pers. Med. 2021, 11, 987. [Google Scholar] [CrossRef]
- Sakurai, T.; Odamaki, T.; Xiao, J.Z. Production of Indole-3-Lactic Acid by Bifidobacterium Strains Isolated fromHuman Infants. Microorganisms 2019, 7, 340. [Google Scholar] [CrossRef]
- Wong, C.B.; Tanaka, A.; Kuhara, T.; Xiao, J.Z. Potential Effects of Indole-3-Lactic Acid, a Metabolite of Human Bifidobacteria, on NGF-induced Neurite Outgrowth in PC12 Cells. Microorganisms 2020, 8, 398. [Google Scholar] [CrossRef]
- Meng, D.; Sommella, E.; Salviati, E.; Campiglia, P.; Ganguli, K.; Djebali, K.; Zhu, W.; Walker, W.A. Indole-3-lactic acid, a metabolite of tryptophan, secreted by Bifidobacterium longum subspecies infantis is anti-inflammatory in the immature intestine. Pediatr. Res. 2020, 88, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, B. Oxidative stress and the pathogenesis of Alzheimer’s disease. Oxid. Med. Cell. Longev. 2013, 2013, 316523. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D.S.A.; Oliver, P.L. ROS Generation in Microglia: Understanding Oxidative Stress and Inflammation in Neurodegenerative Disease. Antioxidants 2020, 9, 743. [Google Scholar] [CrossRef] [PubMed]
- Bisht, K.; Sharma, K.; Tremblay, M. Chronic stress as a risk factor for Alzheimer’s disease: Roles of microglia-mediated synaptic remodeling, inflammation, and oxidative stress. Neurobiol. Stress 2018, 9, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Lyons, C.E.; Bartolomucci, A. Stress and Alzheimer’s disease: A senescence link? Neurosci. Biobehav. Rev. 2020, 115, 285–298. [Google Scholar] [CrossRef]
- Justice, N.J. The relationship between stress and Alzheimer’s disease. Neurobiol. Stress 2018, 8, 127–133. [Google Scholar] [CrossRef]
- Sharan, P.; Vellapandian, C. Hypothalamic-Pituitary-Adrenal (HPA) Axis: Unveiling the Potential Mechanisms Involved in Stress-Induced Alzheimer’s Disease and Depression. Cureus 2024, 16, e67595. [Google Scholar] [CrossRef]
- Weng, C.-Y.; Lin, T.-K.; Hsu, B.-C. Chapter 25—Psychopathophysiology and compassion-based cognitive-behavior group therapy for patients with coronary artery disease. In Handbook of Cognitive Behavioral Therapy by Disorder; Martin, C.R., Patel, V.B., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 307–320. [Google Scholar]
- Cole, A.B.; Montgomery, K.; Bale, T.L.; Thompson, S.M. What the hippocampus tells the HPA axis: Hippocampal output attenuates acute stress responses via disynaptic inhibition of CRF+ PVN neurons. Neurobiol. Stress 2022, 20, 100473. [Google Scholar] [CrossRef]
- Clemens, V.; Bürgin, D.; Eckert, A.; Kind, N.; Dölitzsch, C.; Fegert, J.M.; Schmid, M. Hypothalamic-pituitary-adrenal axis activation in a high-risk sample of children, adolescents and young adults in residential youth care—Associations with adverse childhood experiences and mental health problems. Psychiatry Res. 2020, 284, 112778. [Google Scholar] [CrossRef]
- Chen, X.; Guo, C.; Kong, J. Oxidative stress in neurodegenerative diseases. Neural Regen. Res. 2012, 7, 376–385. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Gulisano, W.; Maugeri, D.; Baltrons, M.A.; Fà, M.; Amato, A.; Palmeri, A.; D’Adamio, L.; Grassi, C.; Devanand, D.P.; Honig, L.S.; et al. Role of Amyloid-β and Tau Proteins in Alzheimer’s Disease: Confuting the Amyloid Cascade. J. Alzheimers Dis. 2018, 64, S611–S631. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Hong, F.; Yang, S. Amyloidosis in Alzheimer’s Disease: Pathogeny, Etiology, and Related Therapeutic Directions. Molecules 2022, 27, 1210. [Google Scholar] [CrossRef]
- Azargoonjahromi, A. The duality of amyloid-β: Its role in normal and Alzheimer’s disease states. Mol. Brain 2024, 17, 44. [Google Scholar] [CrossRef]
- Weglinski, C.; Jeans, A. Amyloid-β in Alzheimer’s disease—Front and centre after all? Neuronal Signal. 2023, 7, Ns20220086. [Google Scholar] [CrossRef]
- Musiek, E.S.; Bennett, D.A. Aducanumab and the “post-amyloid” era of Alzheimer research? Neuron 2021, 109, 3045–3047. [Google Scholar] [CrossRef]
- Ramanan, V.K.; Day, G.S. Anti-amyloid therapies for Alzheimer disease: Finally, good news for patients. Mol. Neurodegener. 2023, 18, 42. [Google Scholar] [CrossRef]
- Shi, M.; Chu, F.; Zhu, F.; Zhu, J. Impact of Anti-amyloid-β Monoclonal Antibodies on the Pathology and Clinical Profile of Alzheimer’s Disease: A Focus on Aducanumab and Lecanemab. Front. Aging Neurosci. 2022, 14, 870517. [Google Scholar] [CrossRef]
- Kinney, J.W.; Bemiller, S.M.; Murtishaw, A.S.; Leisgang, A.M.; Salazar, A.M.; Lamb, B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2018, 4, 575–590. [Google Scholar] [CrossRef]
- Yao, R.; Wong, C.B.; Nakamura, K.; Mitsuyama, E.; Tanaka, A.; Kuhara, T.; Odamaki, T.; Xiao, J.Z. Bifidobacterium breve MCC1274 with glycosidic activity enhances in vivo isoflavone bioavailability. Benef. Microbes 2019, 10, 521–531. [Google Scholar] [CrossRef]
- Dionisio-Santos, D.A.; Karaahmet, B.; Belcher, E.K.; Owlett, L.D.; Trojanczyk, L.A.; Olschowka, J.A.; O’Banion, M.K. Evaluating Effects of Glatiramer Acetate Treatment on Amyloid Deposition and Tau Phosphorylation in the 3xTg Mouse Model of Alzheimer’s Disease. Front. Neurosci. 2021, 15, 758677. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, R.; Baglietto-Vargas, D.; LaFerla, F.M. The role of tau in Alzheimer’s disease and related disorders. CNS Neurosci. Ther. 2011, 17, 514–524. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Dong, S.; Gu, F.; Hu, Y.; Zhao, Z. Advances in the Pathogenesis of Alzheimer’s Disease: Focusing on Tau-Mediated Neurodegeneration. Transl. Neurodegener. 2012, 1, 24. [Google Scholar] [CrossRef] [PubMed]
- Congdon, E.E.; Ji, C.; Tetlow, A.M.; Jiang, Y.; Sigurdsson, E.M. Tau-targeting therapies for Alzheimer disease: Current status and future directions. Nat. Rev. Neurol. 2023, 19, 715–736. [Google Scholar] [CrossRef]
- Gong, C.-X.; Grundke-Iqbal, I.; Iqbal, K. Targeting Tau Protein in Alzheimer’s Disease. Drugs Aging 2010, 27, 351–365. [Google Scholar] [CrossRef]
- Rafii, M.S. Targeting tau protein in Alzheimer’s disease. Lancet 2016, 388, 2842–2844. [Google Scholar] [CrossRef]
- Rawat, P.; Sehar, U.; Bisht, J.; Selman, A.; Culberson, J.; Reddy, P.H. Phosphorylated Tau in Alzheimer’s Disease and Other Tauopathies. Int. J. Mol. Sci. 2022, 23, 12841. [Google Scholar] [CrossRef]
- Barbier, P.; Zejneli, O.; Martinho, M.; Lasorsa, A.; Belle, V.; Smet-Nocca, C.; Tsvetkov, P.O.; Devred, F.; Landrieu, I. Role of Tau as a Microtubule-Associated Protein: Structural and Functional Aspects. Front. Aging Neurosci. 2019, 11, 204. [Google Scholar] [CrossRef]
- Kitagishi, Y.; Nakanishi, A.; Ogura, Y.; Matsuda, S. Dietary regulation of PI3K/AKT/GSK-3β pathway in Alzheimer’s disease. Alzheimer’s Res. Ther. 2014, 6, 35. [Google Scholar] [CrossRef]
- Limantoro, J.; de Liyis, B.G.; Sutedja, J.C. Akt signaling pathway: A potential therapy for Alzheimer’s disease through glycogen synthase kinase 3 beta inhibition. Egypt. J. Neurol. Psychiatry Neurosurg. 2023, 59, 147. [Google Scholar] [CrossRef]
- Sayas, C.L.; Ávila, J. GSK-3 and Tau: A Key Duet in Alzheimer’s Disease. Cells 2021, 10, 721. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.; Nishimura, T.; Yoshimoto, S.; Yoshida, K.; Gotoh, A.; Katoh, T.; Yoneda, Y.; Hashimoto, T.; Xiao, J.-Z.; Katayama, T.; et al. Comprehensive analysis of metabolites produced by co-cultivation of Bifidobacterium breve MCC1274 with human iPS-derived intestinal epithelial cells. Front. Microbiol. 2023, 14, 1155438. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, K.; Ogawa, A.; Mizoguchi, E.; Shimomura, Y.; Andoh, A.; Bhan, A.K.; Blumberg, R.S.; Xavier, R.J.; Mizoguchi, A. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J. Clin. Invest. 2008, 118, 534–544. [Google Scholar] [CrossRef]
- Zenewicz, L.A.; Yin, X.; Wang, G.; Elinav, E.; Hao, L.; Zhao, L.; Flavell, R.A. IL-22 deficiency alters colonic microbiota to be transmissible and colitogenic. J. Immunol. 2013, 190, 5306–5312. [Google Scholar] [CrossRef]
- Ren, T.; Gao, Y.; Qiu, Y.; Jiang, S.; Zhang, Q.; Zhang, J.; Wang, L.; Zhang, Y.; Wang, L.; Nie, K. Gut Microbiota Altered in Mild Cognitive Impairment Compared With Normal Cognition in Sporadic Parkinson’s Disease. Front. Neurol. 2020, 11, 137. [Google Scholar] [CrossRef]
- Iljazovic, A.; Roy, U.; Gálvez, E.J.C.; Lesker, T.R.; Zhao, B.; Gronow, A.; Amend, L.; Will, S.E.; Hofmann, J.D.; Pils, M.C.; et al. Perturbation of the gut microbiome by Prevotella spp. enhances host susceptibility to mucosal inflammation. Mucosal Immunol. 2021, 14, 113–124. [Google Scholar] [CrossRef]
- Wu, F.; Guo, X.; Zhang, J.; Zhang, M.; Ou, Z.; Peng, Y. Phascolarctobacterium faecium abundant colonization in human gastrointestinal tract. Exp. Ther. Med. 2017, 14, 3122–3126. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Wang, D.; Chen, F.; Han, Z.; Yin, Z.; Ge, X.; Lei, P. Relationship Between Amyloid-β Deposition and Blood-Brain Barrier Dysfunction in Alzheimer’s Disease. Front. Cell. Neurosci. 2021, 15, 695479. [Google Scholar] [CrossRef]
- Chen, Y.; He, Y.; Han, J.; Wei, W.; Chen, F. Blood-brain barrier dysfunction and Alzheimer’s disease: Associations, pathogenic mechanisms, and therapeutic potential. Front. Aging Neurosci. 2023, 15, 1258640. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of neuroinflammation in neurodegeneration development. Signal Transduct. Target. Ther. 2023, 8, 267. [Google Scholar] [CrossRef] [PubMed]
- Adamu, A.; Li, S.; Gao, F.; Xue, G. The role of neuroinflammation in neurodegenerative diseases: Current understanding and future therapeutic targets. Front. Aging Neurosci. 2024, 16. [Google Scholar] [CrossRef] [PubMed]
- Brandl, S.; Reindl, M. Blood-Brain Barrier Breakdown in Neuroinflammation: Current In Vitro Models. Int. J. Mol. Sci. 2023, 24, 12699. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Gage, F.H. Adult hippocampal neurogenesis and its role in Alzheimer’s disease. Mol. Neurodegener. 2011, 6, 85. [Google Scholar] [CrossRef]
- Herrup, K. The involvement of cell cycle events in the pathogenesis of Alzheimer’s disease. Alzheimer’s Res. Ther. 2010, 2, 13. [Google Scholar] [CrossRef]
- Raina, A.K.; Monteiro, M.J.; McShea, A.; Smith, M.A. The role of cell cycle-mediated events in Alzheimer’s disease. Int. J. Exp. Pathol. 1999, 80, 71–76. [Google Scholar] [CrossRef]
- Abdelhamid, M.; (Department of Translational Neuroscience, College of Human Medicine, Michigan State University, 400 Monroe Avenue NW, Grand Rapids, MI, USA); Jung, C.G.; (Center for Nursing International Promotion, Nagoya City University Graduate School of Nursing, Nagoya, Japan). Unpublished work, 2024.
- Masliah, E.; Terry, R. The role of synaptic proteins in the pathogenesis of disorders of the central nervous system. Brain Pathol. 1993, 3, 77–85. [Google Scholar] [CrossRef]
- Michetti, C.; Falace, A.; Benfenati, F.; Fassio, A. Synaptic genes and neurodevelopmental disorders: From molecular mechanisms to developmental strategies of behavioral testing. Neurobiol. Dis. 2022, 173, 105856. [Google Scholar] [CrossRef]
- Shankar, G.M.; Walsh, D.M. Alzheimer’s disease: Synaptic dysfunction and Abeta. Mol. Neurodegener. 2009, 4, 48. [Google Scholar] [CrossRef]
- Marsh, J.; Alifragis, P. Synaptic dysfunction in Alzheimer’s disease: The effects of amyloid beta on synaptic vesicle dynamics as a novel target for therapeutic intervention. Neural Regen. Res. 2018, 13, 616–623. [Google Scholar] [CrossRef]
- Tu, S.; Okamoto, S.-i.; Lipton, S.A.; Xu, H. Oligomeric Aβ-induced synaptic dysfunction in Alzheimer’s disease. Mol. Neurodegener. 2014, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Francis, P.T. The interplay of neurotransmitters in Alzheimer’s disease. CNS Spectr. 2005, 10, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Tammineni, P. Mitochondrial Aspects of Synaptic Dysfunction in Alzheimer’s Disease. J. Alzheimers Dis. 2017, 57, 1087–1103. [Google Scholar] [CrossRef] [PubMed]
- Pelucchi, S.; Gardoni, F.; Di Luca, M.; Marcello, E. Chapter 28—Synaptic dysfunction in early phases of Alzheimer’s Disease. In Handbook of Clinical Neurology; Quartarone, A., Ghilardi, M.F., Boller, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 184, pp. 417–438. [Google Scholar]
- Lovinger, D.M. Communication networks in the brain: Neurons, receptors, neurotransmitters, and alcohol. Alcohol. Res. Health 2008, 31, 196–214. [Google Scholar]
- Wu, Q.; Sun, M.; Bernard, L.P.; Zhang, H. Postsynaptic density 95 (PSD-95) serine 561 phosphorylation regulates a conformational switch and bidirectional dendritic spine structural plasticity. J. Biol. Chem. 2017, 292, 16150–16160. [Google Scholar] [CrossRef]
- Coley, A.A.; Gao, W.-J. PSD-95 deficiency disrupts PFC-associated function and behavior during neurodevelopment. Sci. Rep. 2019, 9, 9486. [Google Scholar] [CrossRef]
- Shiosaka, S.; Ishikawa, Y. Neuropsin—A possible modulator of synaptic plasticity. J. Chem. Neuroanat. 2011, 42, 24–29. [Google Scholar] [CrossRef]
- Kinoshita, K.-i.; Muroi, Y.; Unno, T.; Ishii, T. Rolipram improves facilitation of contextual fear extinction in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced mouse model of Parkinson’s disease. J. Pharmacol. Sci. 2017, 134, 55–58. [Google Scholar] [CrossRef]
- Miranda, M.; Morici, J.F.; Zanoni, M.B.; Bekinschtein, P. Brain-Derived Neurotrophic Factor: A Key Molecule for Memory in the Healthy and the Pathological Brain. Front. Cell. Neurosci. 2019, 13, 472800. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Kuhara, T.; Oki, M.; Xiao, J.Z. Effects of Bifidobacterium breve A1 on the cognitive function of older adults with memory complaints: A randomised, double-blind, placebo-controlled trial. Benef. Microbes 2019, 10, 511–520. [Google Scholar] [CrossRef]
- Mårtensson, G.; Håkansson, C.; Pereira, J.B.; Palmqvist, S.; Hansson, O.; van Westen, D.; Westman, E. Medial temporal atrophy in preclinical dementia: Visual and automated assessment during six year follow-up. Neuroimage Clin. 2020, 27, 102310. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, M.A.; Wichmann, A.K.; Torgerson, B.M.; Ward, M.A.; Schmitz, T.W.; Ries, M.L.; Koscik, R.L.; Asthana, S.; Johnson, S.C. Structural MRI discriminates individuals with Mild Cognitive Impairment from age-matched controls: A combined neuropsychological and voxel based morphometry study. Alzheimers Dement. 2006, 2, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, S.; Zhang, Q.; He, C.; Fu, C.; Wei, Q. The role of the gut microbiota in health and cardiovascular diseases. Mol. Biomed. 2022, 3, 30. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Jin, G.; Pang, X.; Mo, Q.; Bao, J.; Liu, T.; Wu, J.; Xie, R.; Liu, X.; Liu, J.; et al. Lactobacillus rhamnosus GG colonization in early life regulates gut-brain axis and relieves anxiety-like behavior in adulthood. Pharmacol. Res. 2022, 177, 106090. [Google Scholar] [CrossRef]
- Wang, H.; Braun, C.; Murphy, E.F.; Enck, P. Bifidobacterium longum 1714™ Strain Modulates Brain Activity of Healthy Volunteers During Social Stress. Am. J. Gastroenterol. 2019, 114, 1152–1162. [Google Scholar] [CrossRef]
- Heydari, R.; Khosravifar, M.; Abiri, S.; Dashtbin, S.; Alvandi, A.; Nedaei, S.E.; Salimi, Z.; Zarei, F.; Abiri, R. A domestic strain of Lactobacillus rhamnosus attenuates cognitive deficit and pro-inflammatory cytokine expression in an animal model of Alzheimer’s disease. Behav. Brain Res. 2025, 476, 115277. [Google Scholar] [CrossRef]
- Tette, F.M.; Kwofie, S.K.; Wilson, M.D. Therapeutic Anti-Depressant Potential of Microbial GABA Produced by Lactobacillus rhamnosus Strains for GABAergic Signaling Restoration and Inhibition of Addiction-Induced HPA Axis Hyperactivity. Curr. Issues Mol. Biol. 2022, 44, 1434–1451. [Google Scholar] [CrossRef]
- Bharwani, A.; Mian, M.F.; Surette, M.G.; Bienenstock, J.; Forsythe, P. Oral treatment with Lactobacillus rhamnosus attenuates behavioural deficits and immune changes in chronic social stress. BMC Med. 2017, 15, 7. [Google Scholar] [CrossRef]
- Groeger, D.; O’Mahony, L.; Murphy, E.F.; Bourke, J.F.; Dinan, T.G.; Kiely, B.; Shanahan, F.; Quigley, E.M. Bifidobacterium infantis 35624 modulates host inflammatory processes beyond the gut. Gut Microbes 2013, 4, 325–339. [Google Scholar] [CrossRef]
- Carbuhn, A.F.; Reynolds, S.M.; Campbell, C.W.; Bradford, L.A.; Deckert, J.A.; Kreutzer, A.; Fry, A.C. Effects of Probiotic (Bifidobacterium longum 35624) Supplementation on Exercise Performance, Immune Modulation, and Cognitive Outlook in Division I Female Swimmers. Sports 2018, 6, 116. [Google Scholar] [CrossRef]
- Cazzola, M.; Tompkins, T.A.; Matera, M.G. Immunomodulatory impact of a synbiotic in T(h)1 and T(h)2 models of infection. Ther. Adv. Respir. Dis. 2010, 4, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, S.; Park, S.-j.; Park, G.; Shin, H.; Park, M.S.; Kim, J. Administration of Bifidobacterium bifidum BGN4 and Bifidobacterium longum BORI Improves Cognitive and Memory Function in the Mouse Model of Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 709091. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-J.; Chen, J.-L.; Liao, J.-F.; Chen, Y.-H.; Chieu, M.-W.; Ke, Y.-Y.; Hsu, C.-C.; Tsai, Y.-C.; Hsieh-Li, H.M. Lactobacillus plantarum PS128 prevents cognitive dysfunction in Alzheimer’s disease mice by modulating propionic acid levels, glycogen synthase kinase 3 beta activity, and gliosis. BMC Complement. Med. Ther. 2021, 21, 259. [Google Scholar] [CrossRef] [PubMed]
- Agahi, A.; Hamidi, G.A.; Daneshvar, R.; Hamdieh, M.; Soheili, M.; Alinaghipour, A.; Esmaeili Taba, S.M.; Salami, M. Does Severity of Alzheimer’s Disease Contribute to Its Responsiveness to Modifying Gut Microbiota? A Double Blind Clinical Trial. Front. Neurol. 2018, 9, 662. [Google Scholar] [CrossRef] [PubMed]
- Akbari, E.; Asemi, Z.; Daneshvar Kakhaki, R.; Bahmani, F.; Kouchaki, E.; Tamtaji, O.R.; Hamidi, G.A.; Salami, M. Effect of Probiotic Supplementation on Cognitive Function and Metabolic Status in Alzheimer’s Disease: A Randomized, Double-Blind and Controlled Trial. Front. Aging Neurosci. 2016, 8, 256. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Heuman, D.M.; Hylemon, P.B.; Sanyal, A.J.; Puri, P.; Sterling, R.K.; Luketic, V.; Stravitz, R.T.; Siddiqui, M.S.; Fuchs, M.; et al. Randomised clinical trial: Lactobacillus GG modulates gut microbiome, metabolome and endotoxemia in patients with cirrhosis. Aliment. Pharmacol. Ther. 2014, 39, 1113–1125. [Google Scholar] [CrossRef]
- Benton, D.; Williams, C.; Brown, A. Impact of consuming a milk drink containing a probiotic on mood and cognition. Eur. J. Clin. Nutr. 2007, 61, 355–361. [Google Scholar] [CrossRef]
- Kelly, J.R.; Allen, A.P.; Temko, A.; Hutch, W.; Kennedy, P.J.; Farid, N.; Murphy, E.; Boylan, G.; Bienenstock, J.; Cryan, J.F.; et al. Lost in translation? The potential psychobiotic Lactobacillus rhamnosus (JB-1) fails to modulate stress or cognitive performance in healthy male subjects. Brain Behav. Immun. 2017, 61, 50–59. [Google Scholar] [CrossRef]
- Lew, L.-C.; Hor, Y.-Y.; Yusoff, N.A.A.; Choi, S.-B.; Yusoff, M.S.B.; Roslan, N.S.; Ahmad, A.; Mohammad, J.A.M.; Abdullah, M.F.I.L.; Zakaria, N.; et al. Probiotic Lactobacillus plantarum P8 alleviated stress and anxiety while enhancing memory and cognition in stressed adults: A randomised, double-blind, placebo-controlled study. Clin. Nutr. 2019, 38, 2053–2064. [Google Scholar] [CrossRef]
- Ruiz-Gonzalez, C.; Cardona, D.; Rueda-Ruzafa, L.; Rodriguez-Arrastia, M.; Ropero-Padilla, C.; Roman, P. Cognitive and Emotional Effect of a Multi-species Probiotic Containing Lactobacillus rhamnosus and Bifidobacterium lactis in Healthy Older Adults: A Double-Blind Randomized Placebo-Controlled Crossover Trial. Probiotics Antimicrob. Proteins 2024. [Google Scholar] [CrossRef]
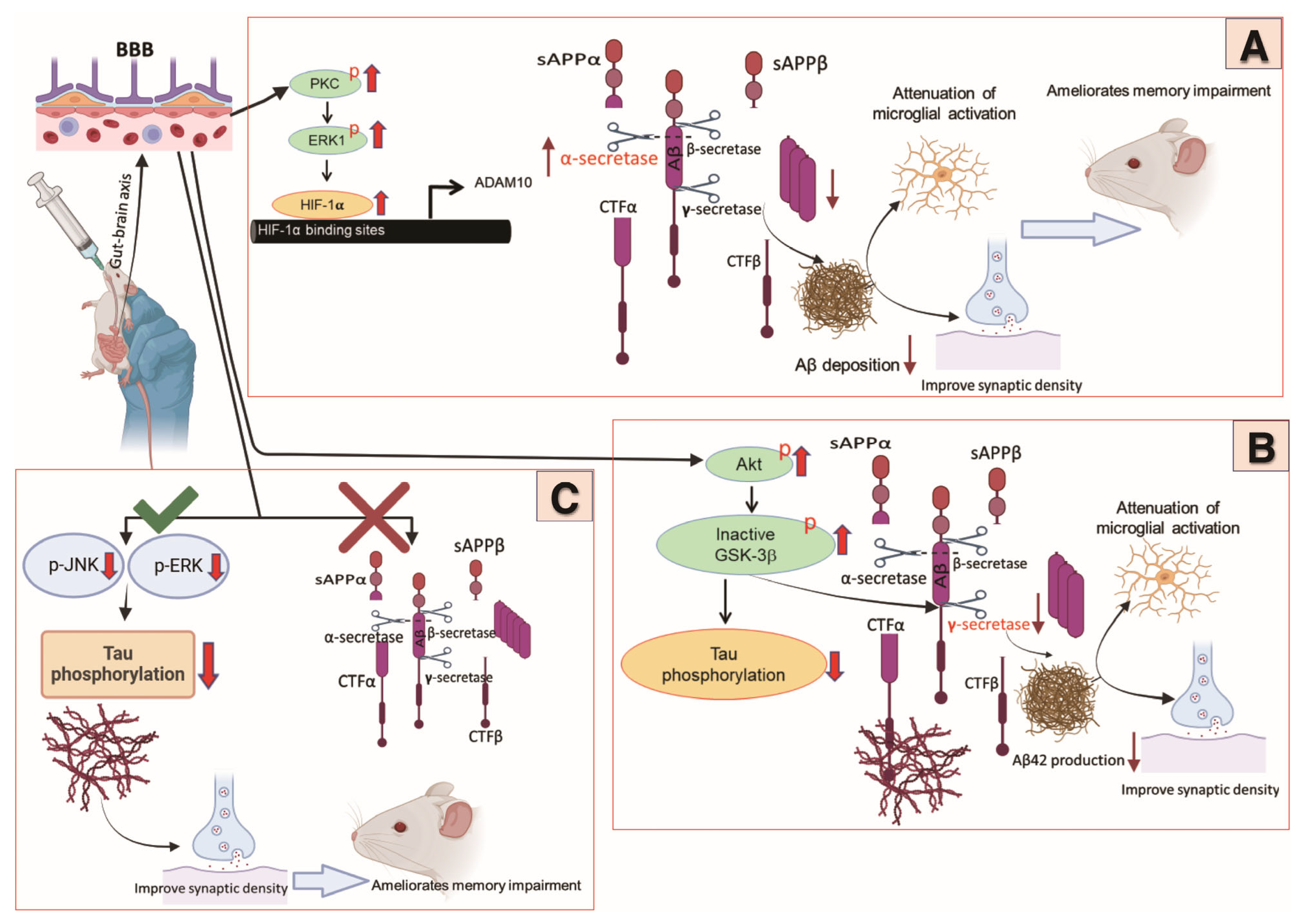
| Effect | Mouse Model | Description | Findings/Mechanism | Reference |
|---|---|---|---|---|
| Neuroinflammation Modulation | Aβ–injected mice | Suppressed inflammation and immune–reactive genes. | DEGs observed in Aβ–injected mice returned to normal expression levels in the hippocampus. | [71] |
| 6-month–old AppNL–G–F mice | Shift in microglial phenotype from M1 to M2 (reduced inflammation around Aβ plaques). | Decreased IL–1β, TNF–α, Iba1 protein, and Iba1+ cells and increased anti–inflammatory cytokines like TGF–β1. | [70] | |
| WT mice | Attenuated microglial activation | Reduced protein and the number of Iba1+ cells by Akt/GSK–3β pathway. | [69] | |
| Oxidative Stress & Chronic Stress Response | 17–month–old AppNL–G–F mice | Reduction of chronic stress markers | Decreased protein levels of p–JNK, p–ERK1/2, and HSP90 | [72] |
| In vitro cell culture | Reduction of oxidative stress | Reduced perilipin 4 and lipid droplet accumulation in neurons | [73] | |
| Amyloid Pathology Modulation | Aβ–injected mice | Lowering Aβ toxicity | Production of beneficial SCFAs such as acetate | [71] |
| 6–month–old AppNL–G–F mice | Reduction in Aβ deposition | Increased ADAM10 enzyme activity | [70] | |
| WT mice | Reduction in soluble Aβ42 | Decreased PS1 enzyme activity | [69] | |
| 17–month–old AppNL–G–F mice | No effect on amyloid pathology | No significant reduction in Aβ levels | [72] | |
| Tau Pathology Modulation | 6-month–old AppNL–G–F | No effect on tau phosphorylation | Phosphorylated tau levels at Ser202/Thr205 and Thr231 had no change | [70] |
| WT and 17–month–old AppNL–G–F mice | Reduction in tau phosphorylation | Reduced phosphorylated tau levels at Thr231 and Ser202/Thr205 (WT) as well as Thr231 and Ser396/Ser404 (AppNL–G–F) | [69,72] | |
| Gut Microbiota | Aβ-injected and AppNL–G–F mice | No change in the overall gut microbiota | No increase or decrease in the abundance of other bacterial species | [70,71] |
| Female Wistar rats | Did not restore microbial diversity after antibiotic treatments | Increase the relative abundance of Actinobacteria and the total counts of B. breve | [83] | |
| Blood–Brain Barrier Integrity | Aβ-injected, 6–month–old AppNL–G–F and WT mice | Potential protection of BBB from neuroinflammation | Reduced neuroinflammation and Aβ accumulation which can protect the BBB | [69,71] |
| Synaptic Protein Levels | 6–month–old AppNL–G–F | Increase in synaptic protein expression | Increase in SYT and PSD–95 protein levels | [70] |
| WT mice | Increase in synaptic protein expression | Increase in SYT, SYP, syntaxin, and PSD–95 protein levels | [69] | |
| 17–month–old AppNL–G–F | Increase in synaptic protein expression | Increase in SYP and PSD–95 protein levels | [72] | |
| PD mice | Improved synaptic plasticity | mRNA expression levels of SYP and PSD–95 were restored | [84] | |
| Behavioral Improvements | Aβ–injected mice | Improvement in cognitive function and behavior | Improved working memory (Y–maze) and memory retention (passive avoidance test) | [71] |
| 6–and 17–month–old AppNL–G–F | Ameliorated cognitive decline and improved memory | Increased exploration of novel object and discrimination index in NOR test. | [70,72] | |
| Cellular Proliferation & Neuronal Cell Loss | 6–month–old AppNL–G–F and WT mice | No effect on neurogenesis | No significant increase in cellular proliferation (BrdU–positive cells) | [69,70] |
| 17–month–old AppNL–G–F | No effect on neuronal loss | No significant change in mature neuronal marker NeuN levels | Not published |
| Disease and Duration of Supplementation | Effects | Test(s) Used for Assessment | Effect on Inflammation | Effect on Gut Microbiota | Reference |
|---|---|---|---|---|---|
| Mild Cognitive Impairment (12 weeks) | Significant improvement in immediate and delayed memory in low-score subgroup. | BRANS and MMSE | Reduction in systemic inflammation, likely via SCFAs and anti–inflammatory metabolites. | No significant changes in gut microbiota composition. | [85] |
| Mild Cognitive Impairment (24 weeks) | Significant improvement in MMSE scores after 16 weeks, with some reaching cognitive normality. | MMSE and DSST | Potential reduction in inflammation, as observed in improved cognitive scores. | No significant changes in gut microbiota composition. | [75] |
| Mild Cognitive Impairment (16 weeks) | Improvement in memory, especially in RBANS domains such as immediate and delayed memory. | RBANS | Potential reduction in inflammation in hippocampus, linked to cognitive improvement. | No significant changes in gut microbiota composition. | [77] |
| Mild Cognitive Impairment (16 weeks) | Improvement in cognitive function, as reflected in RBANS total scores | RBANS | Lower serum levels of hemoglobin A1c correlate to reducing inflammation | Not evaluated | [58] |
| Mild Cognitive Impairment (12 weeks) | Improvement in the orientation subdomains of ADAS–Jcog and MMSE; slower progression of brain atrophy. | ADAS–Jcog, MMSE, and VSRAD brain MRI | Slower progression of brain atrophy, indicating reduced neuroinflammation | No significant changes in gut microbiota composition. | [76] |
| Schizophrenia (4 weeks) | Significant improvement in anxiety and depression symptoms. | Anxiety and depression scores | Significant reduction in anxiety/depression linked to reduced inflammation via improved gut barrier function by increased IL–22 and TRANCE | No changes in gut microbiota composition but related to gut barrier function | [78] |
| Schizophrenia (4 weeks) | Significant improvement in anxiety and depressive symptoms, linked to lipid metabolism. | Anxiety and depression scores | Enhanced lipid metabolism linked to reduced inflammation, improving anxiety/depression symptoms | No changes in gut microbiota composition, but related to metabolic effects | [86] |
| Metabolite | Model/System | Influence | References |
|---|---|---|---|
| Genistein | AppNL–G–F and WT mice | Increases plasma levels; potential anti–inflammatory and antioxidative activities, reduces inflammation oxidative stress in the brain | [74] |
| Acetate | Aβ-injected and AppNL–G–F mice, and MCI | Alleviate neuroinflammation and can decrease amyloid pathology by lowering the levels of both soluble and insoluble fractions of hippocampal Aβ1–42 | [71,74,77] |
| 5–methoxyindoleacetic acid | AppNL–G–F mice | Exhibits antioxidative activity; reduces oxidative stress not only in the brain but throughout the body, providing broader protective effects | [74] |
| Indole–3–lactic acid | AppNL–G–F mice and MCI | Impact intestinal barrier function and immune regulation, rather than causing substantial changes to bacterial diversity | [74] |
| Daidzein | Wistar rats (antibiotic pre-treated) | Reduce inflammation and oxidative stress by increasing bioavailability by hydrolyzing daidzin into a more absorbable form | [83] |
| SCFAs | AppNL–G–F mice, MCI, and Schizophrenia | Promotes gut health, modulates inflammation and microglial activity | [74,76,86] |
| Brain-derived neurotrophic factor | Aβ–injected mice | Ameliorate memory dysfunction in mice administered Aβ | [71] |
| Lipid Droplet Metabolites | SH-SY5Y cells | Reduces lipid droplet formation and expression of perilipin 4 in neurons | [73] |
| IL–1β, TNF–α | AppNL–G–F mice | Reducing pro–inflammatory cytokines like IL-1β and TNF–α directly contributes to reducing inflammation in the body | [70] |
| TGF–β1 | AppNL–G–F mice | Increased anti–inflammatory cytokines like TGF–β1 help to dampen excessive immune responses and reduce inflammation in the body | [70] |
| Lipopolysaccharides | MCI | Elevated gut inflammation levels, but it reduced with B. breve supplementation | [77] |
| Bacterial Strain | Effect on AD Progression | Effect on Aβ Production | Effect on Neuroinflammation | Effect on Brain Health | Reference |
|---|---|---|---|---|---|
| B. breve MCC1274 | Potential to reduce AD progression by modulating gut–brain axis and improving cognitive function | Reduce Aβ levels in AD model mice, possibly by altering APP processing enzymes or via effects on neuroinflammation | Reduces neuroinflammation, likely through modulation of microglial activation and inflammatory cytokines | Improves cognitive function, reduces anxiety and depression, and enhances memory | [69,70,71,72] |
| Lactobacillus rhamnosus GG | Enhances cognitive function and reduces anxiety–like behaviors, potentially benefiting AD patients | Some evidence suggests a potential effect on GABA receptors but no direct data on Aβ reduction in AD. | Regulates immune response, increases IL–10 and regulatory T cells, and decreases pro-inflammatory cytokines. | Improves anxiety, stress–related behaviors, and cognitive function. | [155,157,158,159] |
| Bifidobacterium longum 1714 | Potential cognitive benefits with improvements in mental health. | No direct evidence on Aβ reduction but may influence gut–brain communication | Modulates neuroinflammation by influencing cytokine production and reducing pro-inflammatory signals. | Enhances cognitive function, reduces anxiety, and boosts mental well-being. | [156,161] |
| Bifidobacterium infantis 35624 | May have neuroprotective effects, particularly in improving mood and cognitive function. | Some studies suggest immunomodulatory effects that could indirectly affect Aβ | Reduces plasma IL–6 and TNF–α levels, suggesting a reduction in neuroinflammation. | Improves mood and cognitive function by modulating gut–brain axis. | [160] |
| Bifidobacterium bifidum BGN4 | Reduces amyloidosis and apoptotic processes in AD model mice. | Effectively suppresses amyloid deposition in AD model mice. | Potential to reduce neuroinflammation and related damage in the brain. | Shows promise in improving brain health in preclinical AD models. | [163] |
| Bifidobacterium longum BORI | Suppresses amyloidosis and mitigates neuronal damage in AD models | Suppresses Aβ accumulation in AD mouse models | Reduces neuroinflammation, likely via modulation of the gut–brain axis. | Shows improvements in cognitive health in AD models | [163] |
| Lactobacillus plantarum PS12 | Potential neuroprotective effects in AD models | Inhibits Aβ deposition in 3×Tg–AD mouse models by inhibiting BACE1 and GSK3β | Modulates neuroinflammation by affecting cytokine and immune responses | Enhances brain health and reduces cognitive decline in AD models | [164] |
| Lactobacillus helveticus R0052, Bifidobacterium longum R0033, Bifidobacterium bifidum R0071 | Shows promising preclinical effects in improving cognition and reducing AD progression | No direct evidence on Aβ reduction, but likely modulates brain health through gut-brain interactions | Reduces neuroinflammation in AD models by improving gut microbiome balance | Improves cognitive function and reduces AD–like pathology in preclinical models | [162] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelhamid, M.; Counts, S.E.; Zhou, C.; Hida, H.; Kim, J.-I.; Michikawa, M.; Jung, C.-G. Protective Effects of Bifidobacterium Breve MCC1274 as a Novel Therapy for Alzheimer’s Disease. Nutrients 2025, 17, 558. https://doi.org/10.3390/nu17030558
Abdelhamid M, Counts SE, Zhou C, Hida H, Kim J-I, Michikawa M, Jung C-G. Protective Effects of Bifidobacterium Breve MCC1274 as a Novel Therapy for Alzheimer’s Disease. Nutrients. 2025; 17(3):558. https://doi.org/10.3390/nu17030558
Chicago/Turabian StyleAbdelhamid, Mona, Scott E. Counts, Chunyu Zhou, Hideki Hida, Jae-Il Kim, Makoto Michikawa, and Cha-Gyun Jung. 2025. "Protective Effects of Bifidobacterium Breve MCC1274 as a Novel Therapy for Alzheimer’s Disease" Nutrients 17, no. 3: 558. https://doi.org/10.3390/nu17030558
APA StyleAbdelhamid, M., Counts, S. E., Zhou, C., Hida, H., Kim, J.-I., Michikawa, M., & Jung, C.-G. (2025). Protective Effects of Bifidobacterium Breve MCC1274 as a Novel Therapy for Alzheimer’s Disease. Nutrients, 17(3), 558. https://doi.org/10.3390/nu17030558







