The Impact of a Commercial Electrolyte Beverage on the Hydration Status of Active Men and Women
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Overview
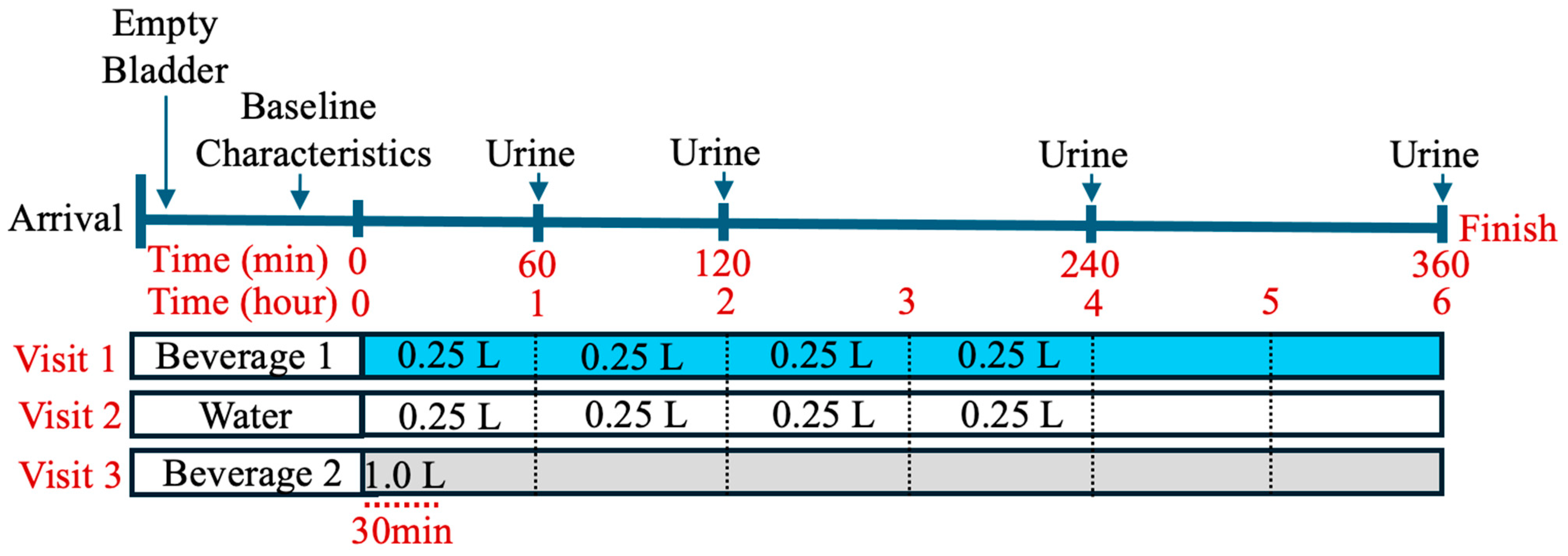
| Water | Beverage 1 | Beverage 2 | |
|---|---|---|---|
| Total Volume | 1 L | 1 L | 1 L |
| Sodium (mg) | Negligible | 600 | 600 |
| Potassium (mg) | Negligible | 120 | 300 |
| Chloride (mg) | Negligible | 960 | 80 |
| Magnesium (mg) | Negligible | 6 | 50 |
| Calcium (mg) | Negligible | 6 | 26 |
| Calories (kcal/L) | 0 | 0 | 30 |
| Sugars (g) | 0 | 0 | 2 |
| Carbohydrates | 0 | 0 | 8 |
3.2. Baseline Physical Characteristics
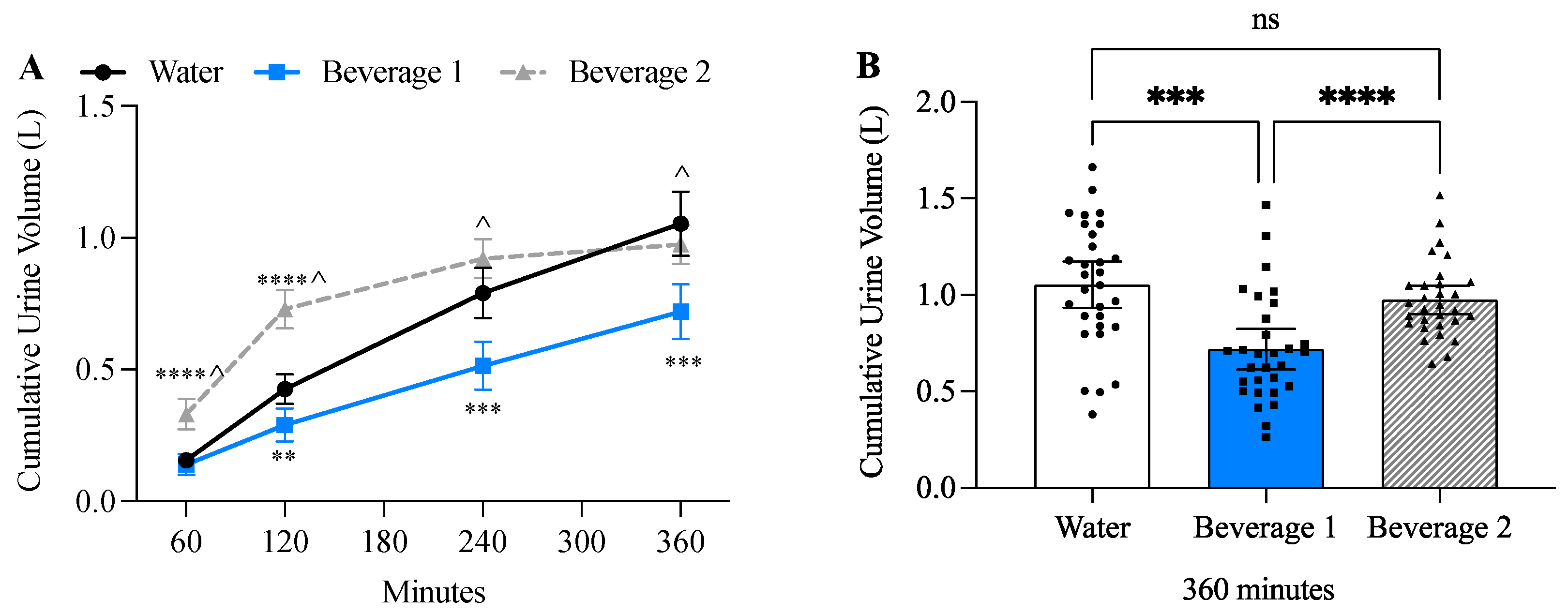
3.3. Beverage 1 Significantly Decreased Urine Volume
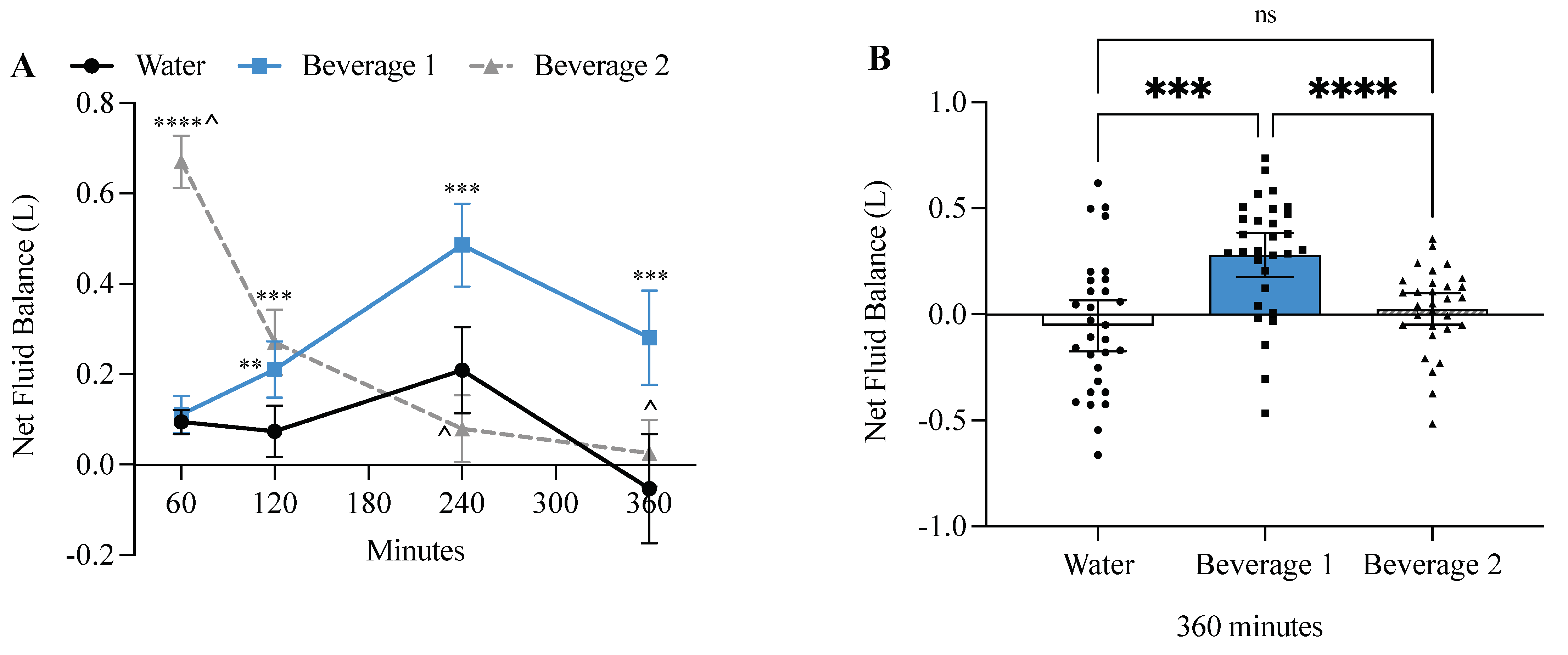
3.4. Beverage 1 Significantly Increased Net Fluid Balance
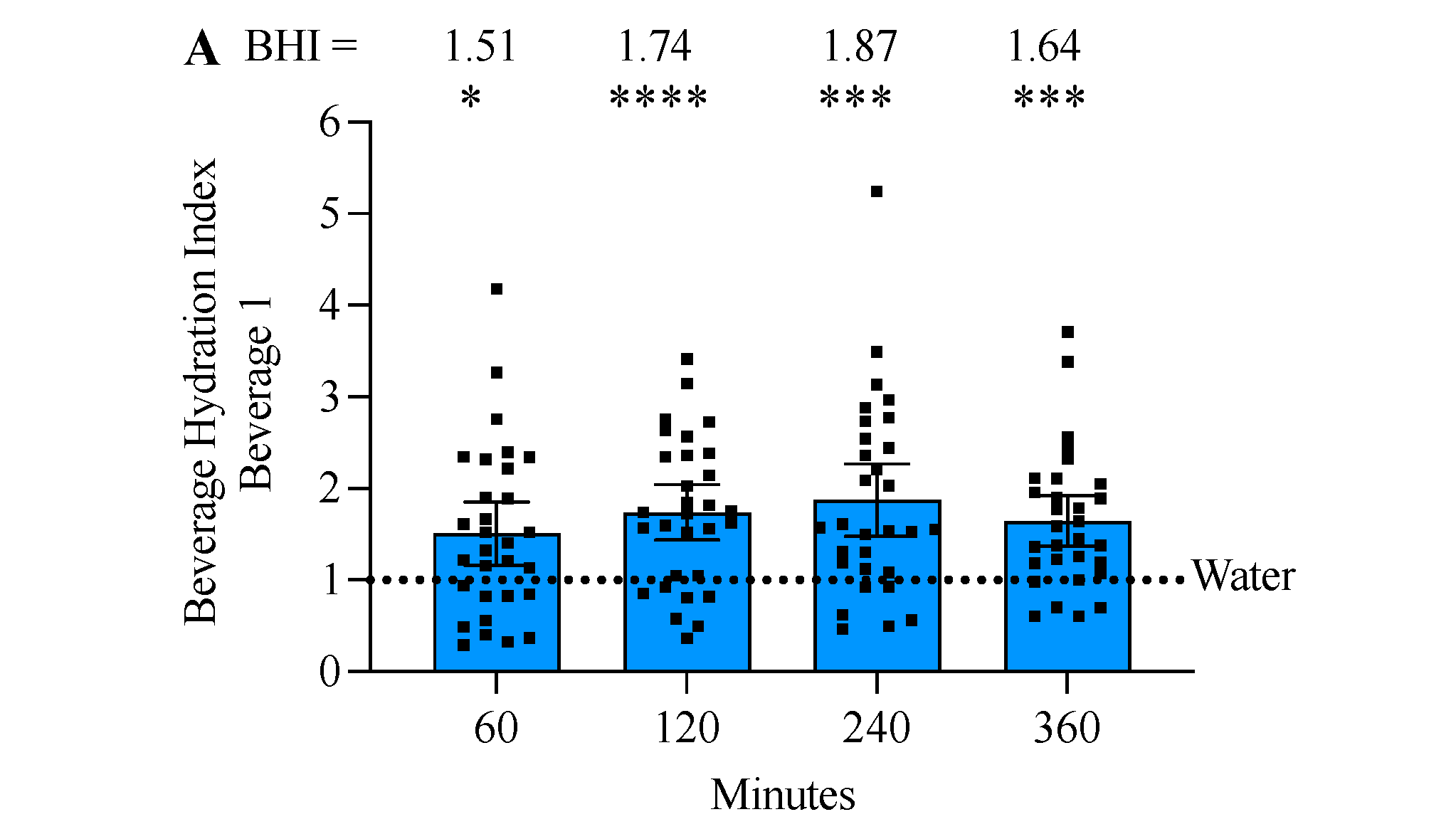
3.5. Beverage 1 Significantly Increased the Beverage Hydration Index
3.6. Beverage 1 Increased Cumulative Urine Osmolality
3.7. Beverage 1 Decreased Cumulative Urine Sodium and Chloride Compared to Water-Alone
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tobias, A.; Ballard, B.D.; Mohiuddin, S.S. Physiology, Water Balance. In StatPearls Physiology, Water Balance; StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar]
- American College of Sports Medicine; Sawka, M.N.; Burke, L.M.; Eichner, E.R.; Maughan, R.J.; Montain, S.J.; Stachenfeld, N.S. American College of Sports Medicine position stand. Exercise and fluid replacement. Med. Sci. Sports Exerc. 2007, 39, 377–390. [Google Scholar] [CrossRef]
- Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Panel on Dietary Reference Intakes for Electrolytes, and Water. Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate; National Academies Press: Cambridge, MA, USA, 2005. [Google Scholar]
- Bhave, G.; Neilson, E.G. Volume depletion versus dehydration: How understanding the difference can guide therapy. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2011, 58, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Evans, G.H.; James, L.J.; Shirreffs, S.M.; Maughan, R.J. Optimizing the restoration and maintenance of fluid balance after exercise-induced dehydration. J. Appl. Physiol. (1985) 2017, 122, 945–951. [Google Scholar] [CrossRef]
- Popkin, B.M.; D’Anci, K.E.; Rosenberg, I.H. Water, hydration, and health. Nutr. Rev. 2010, 68, 439–458. [Google Scholar] [CrossRef] [PubMed]
- Murray, R. Dehydration, hyperthermia, and athletes: Science and practice. J. Athl. Train. 1996, 31, 248–252. [Google Scholar] [PubMed]
- Shirreffs, S.M.; Watson, P.; Maughan, R.J. Milk as an effective post-exercise rehydration drink. Br. J. Nutr. 2007, 98, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Kenefick, R.W. Drinking Strategies: Planned Drinking Versus Drinking to Thirst. Sports Med. 2018, 48, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Love, T.D.; Baker, D.F.; Healey, P.; Black, K.E. Measured and perceived indices of fluid balance in professional athletes. The use and impact of hydration assessment strategies. Eur. J. Sport Sci. 2018, 18, 349–356. [Google Scholar] [CrossRef]
- Kim, S. Preventable hospitalizations of dehydration: Implications of inadequate primary health care in the United States. Ann. Epidemiol. 2007, 17, 736. [Google Scholar] [CrossRef]
- Nose, H.; Mack, G.W.; Shi, X.R.; Nadel, E.R. Role of osmolality and plasma volume during rehydration in humans. J. Appl. Physiol. (1985) 1988, 65, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Vroomen Durning, M. The Science of Hydration. Physiol. Mag. 2021, 64, 24–28. [Google Scholar]
- Kovacs, E.M.; Schmahl, R.M.; Senden, J.M.; Brouns, F. Effect of high and low rates of fluid intake on post-exercise rehydration. Int. J. Sport Nutr. Exerc. Metab. 2002, 12, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.J.; Bishop, P.A.; Green, J.M.; Richardson, M.T. Effects of metered versus bolus water consumption on urine production and rehydration. Int. J. Sport Nutr. Exerc. Metab. 2010, 20, 139–144. [Google Scholar] [CrossRef]
- McBride, C.; Boy, T.; Green, M.; O’Neal, E.; Renfroe, L. Hydration Efficiency of a Protein Beverage Consumed in a Bolus vs. Metered Pattern during Recovery. Int. J. Exerc. Sci. 2020, 13, 1476–1486. [Google Scholar] [CrossRef] [PubMed]
- Millard-Stafford, M.; Snow, T.K.; Jones, M.L.; Suh, H. The Beverage Hydration Index: Influence of Electrolytes, Carbohydrate and Protein. Nutrients 2021, 13, 2933. [Google Scholar] [CrossRef]
- Maughan, R.J.; Owen, J.H.; Shirreffs, S.M.; Leiper, J.B. Post-exercise rehydration in man: Effects of electrolyte addition to ingested fluids. Eur. J. Appl. Physiol. Occup. Physiol. 1994, 69, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Evans, G.H.; Shirreffs, S.M.; Maughan, R.J. Postexercise rehydration in man: The effects of osmolality and carbohydrate content of ingested drinks. Nutrition 2009, 25, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Maughan, R.J.; Watson, P.; Cordery, P.A.; Walsh, N.P.; Oliver, S.J.; Dolci, A.; Rodriguez-Sanchez, N.; Galloway, S.D. A randomized trial to assess the potential of different beverages to affect hydration status: Development of a beverage hydration index. Am. J. Clin. Nutr. 2016, 103, 717–723. [Google Scholar] [CrossRef]
- Maughan, R.J.; Watson, P.; Cordery, P.A.A.; Walsh, N.P.; Oliver, S.J.; Dolci, A.; Rodriguez-Sanchez, N.; Galloway, S.D.R. Sucrose and Sodium but not Caffeine Content Influence the Retention of Beverages in Humans Under Euhydrated Conditions. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Shirreffs, S.M.; Taylor, A.J.; Leiper, J.B.; Maughan, R.J. Post-exercise rehydration in man: Effects of volume consumed and drink sodium content. Med. Sci. Sports Exerc. 1996, 28, 1260–1271. [Google Scholar] [CrossRef]
- Clarke, M.M.; Stanhewicz, A.E.; Wolf, S.T.; Cheuvront, S.N.; Kenefick, R.W.; Kenney, W.L. A randomized trial to assess beverage hydration index in healthy older adults. Am. J. Clin. Nutr. 2019, 109, 1640–1647. [Google Scholar] [CrossRef] [PubMed]
- Peden, D.L.; Funnell, M.P.; Reynolds, K.M.; Kenefick, R.W.; Cheuvront, S.N.; Mears, S.A.; James, L.J. Post-exercise rehydration: Comparing the efficacy of three commercial oral rehydration solutions. Front. Sports Act. Living 2023, 5, 1158167. [Google Scholar] [CrossRef]
- Pence, J.; Bloomer, R.J. Impact of Nuun Electrolyte Tablets on Fluid Balance in Active Men and Women. Nutrients 2020, 12, 3030. [Google Scholar] [CrossRef]
- Fountain, J.H.; Kaur, J.; Lappin, S.L. Physiology, Renin Angiotensin System; StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar]
- Meneton, P.; Jeunemaitre, X.; de Wardener, H.E.; MacGregor, G.A. Links between dietary salt intake, renal salt handling, blood pressure, and cardiovascular diseases. Physiol. Rev. 2005, 85, 679–715. [Google Scholar] [CrossRef]
- Vasold, K.L.; Parks, A.C.; Phelan, D.M.L.; Pontifex, M.B.; Pivarnik, J.M. Reliability and Validity of Commercially Available Low-Cost Bioelectrical Impedance Analysis. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Pribyl, M.I.; Smith, J.D.; Grimes, G.R. Accuracy of the Omron HBF-500 body composition monitor in male and female college students. Int. J. Exerc. Sci. 2011, 4, 2. [Google Scholar] [CrossRef]
- Sollanek, K.J.; Tsurumoto, M.; Vidyasagar, S.; Kenefick, R.W.; Cheuvront, S.N. Neither body mass nor sex influences beverage hydration index outcomes during randomized trial when comparing 3 commercial beverages. Am. J. Clin. Nutr. 2018, 107, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Perrier, E.T. Shifting Focus: From Hydration for Performance to Hydration for Health. Ann. Nutr. Metab. 2017, 70 (Suppl. S1), 4–12. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, S.M. Water: An essential but overlooked nutrient. J. Am. Diet. Assoc. 1999, 99, 200–206. [Google Scholar] [CrossRef]
- Clayton, D.J.; Evans, G.H.; James, L.J. Effect of drink carbohydrate content on postexercise gastric emptying, rehydration, and the calculation of net fluid balance. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 79–89. [Google Scholar] [CrossRef]
- Perez-Castillo, I.M.; Williams, J.A.; Lopez-Chicharro, J.; Mihic, N.; Rueda, R.; Bouzamondo, H.; Horswill, C.A. Compositional Aspects of Beverages Designed to Promote Hydration Before, During, and After Exercise: Concepts Revisited. Nutrients 2023, 16, 17. [Google Scholar] [CrossRef]
- Pfeiffer, B.; Stellingwerff, T.; Hodgson, A.B.; Randell, R.; Pottgen, K.; Res, P.; Jeukendrup, A.E. Nutritional intake and gastrointestinal problems during competitive endurance events. Med. Sci. Sports Exerc. 2012, 44, 344–351. [Google Scholar] [CrossRef]
- West, E.S.; Burget, G.E. Sorbitol as a Diuretic. Proc. Soc. Exp. Biol. Med. 1936, 35, 105–107. [Google Scholar] [CrossRef]
- Berend, K.; van Hulsteijn, L.H.; Gans, R.O. Chloride: The queen of electrolytes? Eur. J. Intern. Med. 2012, 23, 203–211. [Google Scholar] [CrossRef]
- Siegler, J.C.; Carr, A.J.; Jardine, W.T.; Convit, L.; Cross, R.; Chapman, D.; Burke, L.M.; Ross, M. The Hyperhydration Potential of Sodium Bicarbonate and Sodium Citrate. Int. J. Sport. Nutr. Exerc. Metab. 2022, 32, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Liska, D.; Mah, E.; Brisbois, T.; Barrios, P.L.; Baker, L.B.; Spriet, L.L. Narrative Review of Hydration and Selected Health Outcomes in the General Population. Nutrients 2019, 11, 70. [Google Scholar] [CrossRef] [PubMed]
- Frangeskou, M.; Lopez-Valcarcel, B.; Serra-Majem, L. Dehydration in the Elderly: A Review Focused on Economic Burden. J. Nutr. Health Aging 2015, 19, 619–627. [Google Scholar] [CrossRef]
- Pershad, J. A systematic data review of the cost of rehydration therapy. Appl. Health Econ. Health Policy 2010, 8, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Chitwood, H.; Hampton, D.; Patel, R. The effect of amino acid-oral rehydration solution (Enterade(R)) on chemotherapy related diarrhea and quality of life in solid tumor cancer patients: A non-randomized experimental study. Eur. J. Oncol. Nurs. 2022, 60, 102186. [Google Scholar] [CrossRef] [PubMed]
- Hwa, Y.L.; Kull, M.R. The why and how of maintaining hydration during cancer therapy. Curr. Opin. Support. Palliat. Care 2020, 14, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Horinouchi, H.; Kubota, K.; Miyanaga, A.; Nakamichi, S.; Seike, M.; Gemma, A.; Yamane, Y.; Kurimoto, F.; Sakai, H.; Kanda, S.; et al. Oral rehydration solution (OS-1) as a substitute of intravenous hydration after cisplatin administration in patients with lung cancer: A prospective multicenter trial. ESMO Open 2018, 3, e000288. [Google Scholar] [CrossRef] [PubMed]
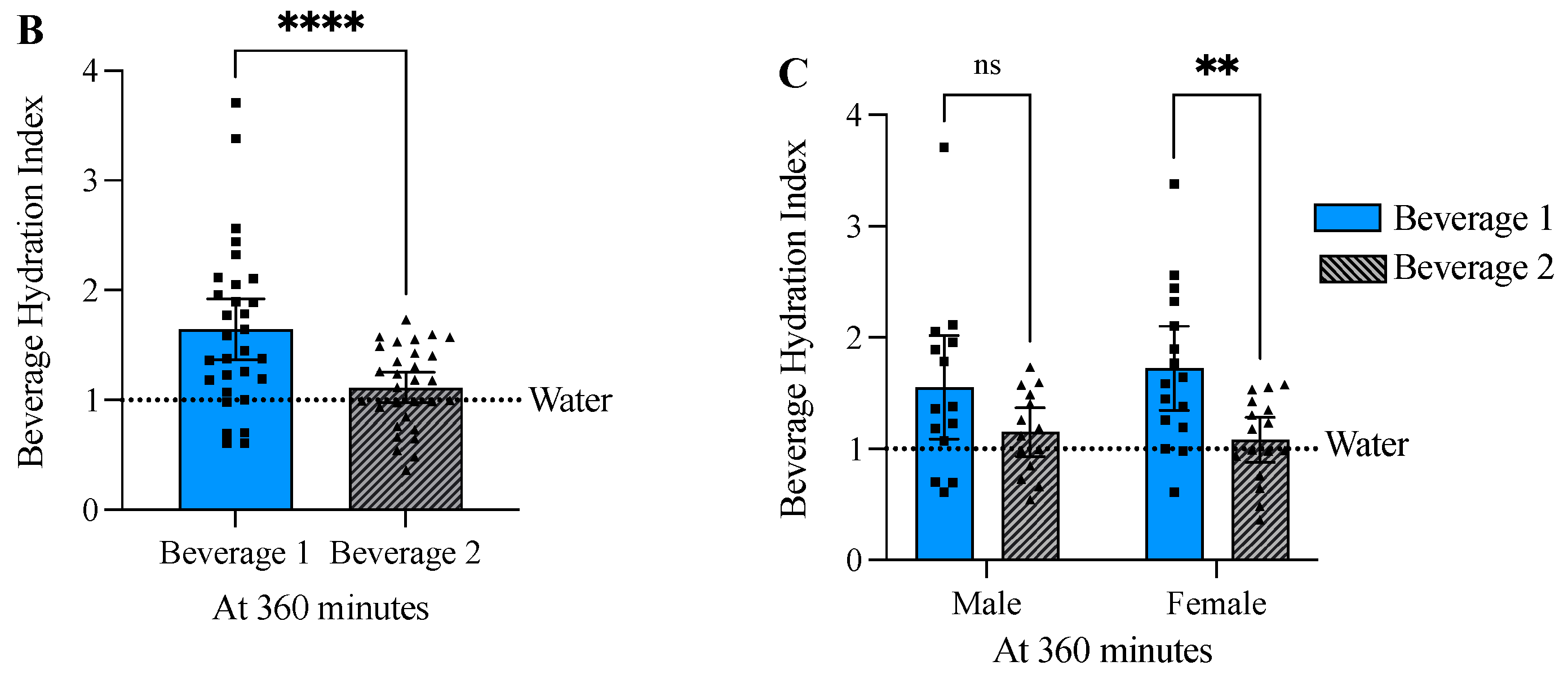
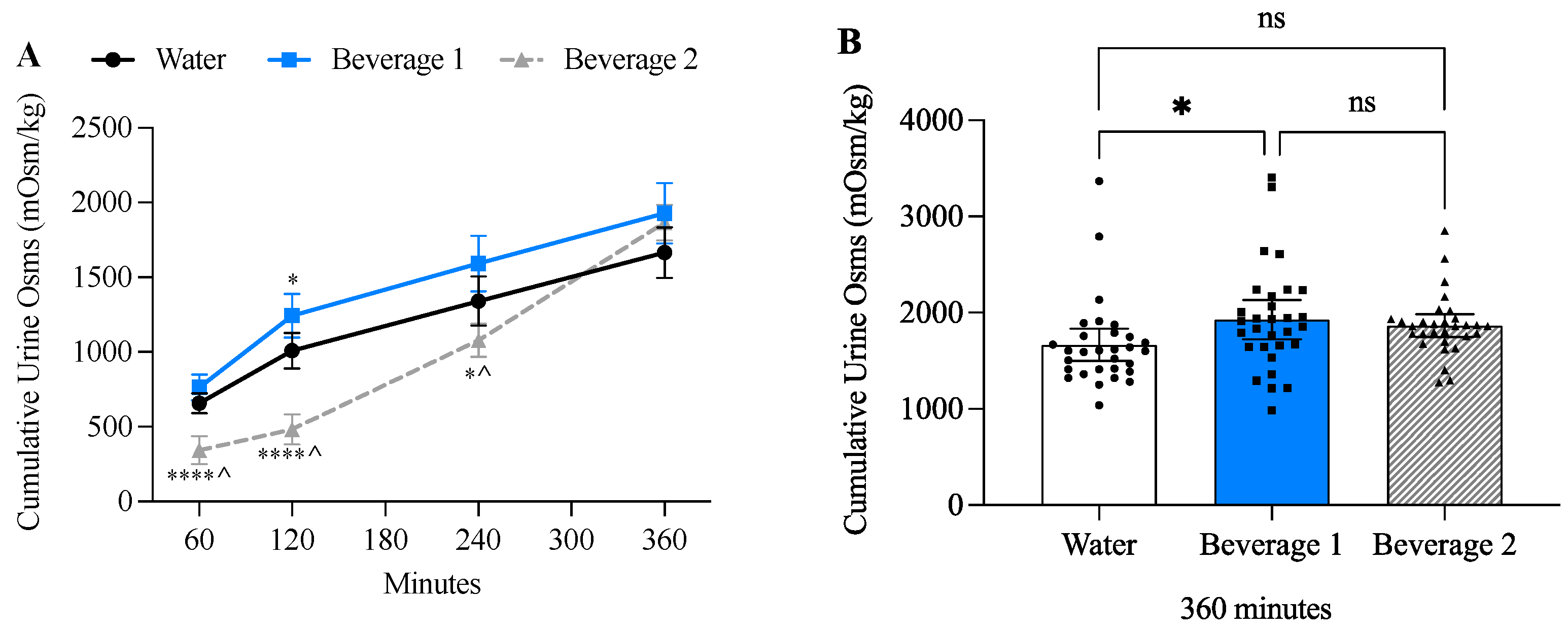
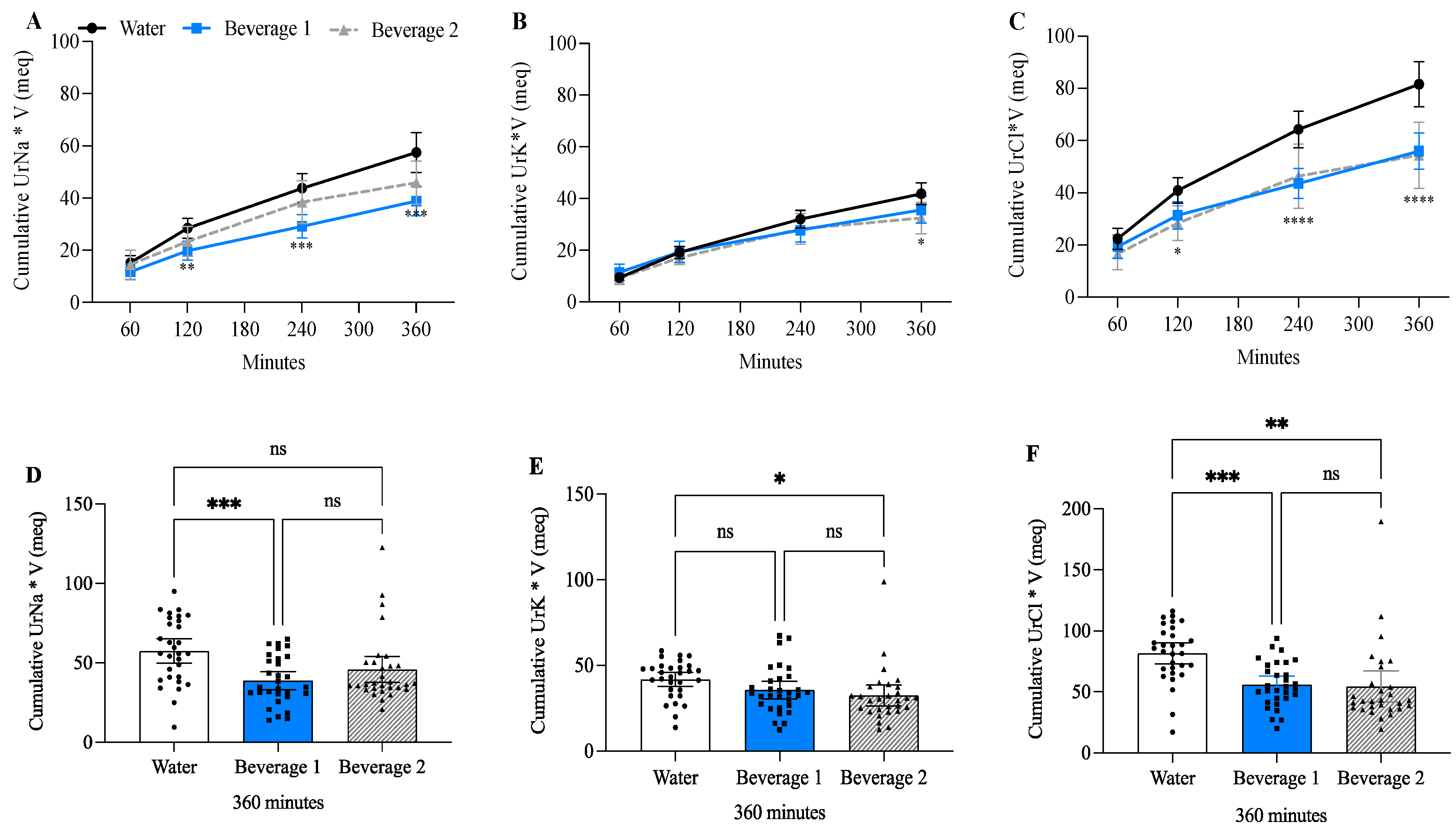
| Male ± SD (n = 14) | Female ± SD (n = 16) | Combined ± SD (n = 30) | |
|---|---|---|---|
| Age (years) | 31.6 (±6.6) | 28.3 (±4.3) | 29.9 (±5.6) |
| Body Weight (kg) | 88.96 (±12.8) | 76.9 (±18.5) * | 82.6 (±16.9) |
| BMI | 27.1 (±3.8) | 28.1 (±7.6) | 27.7 (±6.1) |
| Body Fat % | 23.4 (±8.6) | 33.7 (±9.3) ** | 28.7 (±10.2) |
| Skeletal Muscle % | 36.6 (±6.0) | 28.6 (±4.8) *** | 32.5 (±6.7) |
| Resting Metabolism (kcal) | 1868 (±166) | 1535 (±199) *** | 1695 (±248) |
| Visceral Fat | 7.4 (±3.5) | 6.5 (±2.8) | 7.0 (±3.2) |
| Systolic Blood Pressure | 120.4 (±3.4) | 119.7 (±2.9) | 120.0 (±3.1) |
| Diastolic Blood Pressure | 77.1 (±2.0) | 77.3 (±1.8) | 77.2 (±1.9) |
| Heart Rate | 77.9 (±7.5) | 80.0 (±11.3) | 79.0 (±9.6) |
| Serum Creatinine | 0.94 (±0.09) | 0.97 (±0.12) | 0.95 (±0.11) |
| SD) (n = 30) | SD) (n = 30) | SD) (n = 30) | |
|---|---|---|---|
| 60 min | 0.156 (0.074) | 0.139 (0.109) | 0.330 (0.155) ****^ |
| 120 min | 0.270 (0.139) | 0.151 (0.084) *** | 0.399 (0.105) ***^ |
| 240 min | 0.365 (0.170) | 0.225 (0.143) ** | 0.191 (0.172) *** |
| 360 min | 0.262 (0.125) | 0.205 (0.065) | 0.054 (0.038) ****^ |
| SD) (n = 30) | SD) (n = 30) | SD) (n = 30) | |
|---|---|---|---|
| 60 min | 658 (180) | 764 (230) | 343 (251) ****^ |
| 120 min | 352 (179) | 479 (210) * | 140 (40) ****^ |
| 240 min | 331 (164) | 351 (181) | 595 (168) ****^ |
| 360 min | 325 (68) | 335 (91) | 789 (111) ****^ |
| Urine Na+ Concentration (meq/L) | Urine Na+ Normalized to Urine Volume (UNa·V) (meq) | |||||
|---|---|---|---|---|---|---|
| Water ( SD) | Beverage 1 ( SD) | Beverage 2 ( SD) | Water ( SD) | Beverage 1 ( SD) | Beverage 2 ( SD) | |
| 60 min | 103.7 (37.0) | 92.2 (34.0) | 48.5 (47.8) ****^ | 15.2 (7.3) | 11.8 (8.0) | 14.5 (14.9) |
| 120 min | 55.2 (33.5) | 63.7 (42.9) | 22.6 (7.5) ****^ | 13.2 (7.5) | 8.0 (4.1) ** | 8.9 (3.6) * |
| 240 min | 48.5 (28.0) | 46.3 (35.2) | 87.3 (35.2) ****^ | 15.4 (6.5) | 9.4 (7.5) ** | 15.1 (14.2) |
| 360 min | 49.1 (20.3) | 46.4 (19.4) | 142.7 (34.9) ****^ | 13.7 (10.2) | 9.7 (5.1) | 7.4 (4.6) * |
| Urine K+ concentration (meq/L) | Urine K+ normalized to urine volume (UK·V) (meq) | |||||
| Water (SD) | Beverage 1 (SD) | Beverage 2 (SD) | Water (SD) | Beverage 1 (SD) | Beverage 2 (SD) | |
| 60 min | 65.2 (16.3) | 91.1 (45.4) * | 29.5 (19.3) ****^ | 9.5 (3.9) | 11.5 (8.6) | 9.2 (6.3) |
| 120 min | 41.7 (20.9) | 57.7 (26.7) * | 19.0 (4.6) ****^ | 9.7 (4.8) | 7.9 (4.7) | 7.6 (2.8) * |
| 240 min | 41.1 (19.9) | 42.8 (16.2) | 61.7 (31.2) *^ | 12.9 (4.6) | 8.4 (3.8) *** | 11.1 (13.4) |
| 360 min | 37.3 (11.2) | 39.4 (13.1) | 78.3 (20.6) ****^ | 9.8 (5.1) | 7.8 (3.0) | 4.0 (2.4) ****^ |
| Urine Cl− concentration (meq/L) | Urine Cl− normalized to urine volume (UCl·V) (meq) | |||||
| Water (SD) | Beverage 1 (SD) | Beverage 2 (SD) | Water (SD) | Beverage 1 (SD) | Beverage 2 (SD) | |
| 60 min | 149.8 (43.4) | 154.7 (54.9) | 56.4 (55.7) ****^ | 22.4 (10.7) | 19.3 (12.0) | 16.6 (16.5) |
| 120 min | 78.7 (42.3) | 92.0 (44.4) | 28.6 (9.9) ****^ | 18.5 (8.5) | 12.1 (5.0) ** | 11.3 (4.6) *** |
| 240 min | 74.0 (39.7) | 61.9 (36.5) | 96.6 (36.3) ^ | 23.4 (8.9) | 12.1 (6.9) **** | 18.5 (24.1) |
| 360 min | 63.9 (20.9) | 60.3 (19.5) | 152.8 (35.3) ****^ | 17.2 (10.6) | 12.5 (5.7) | 7.8 (4.4) ***^ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boyd-Shiwarski, C.; Ray, E.; Subramanian, H.; Zharichenko, N.; Monroe, A.; Mahajan, A. The Impact of a Commercial Electrolyte Beverage on the Hydration Status of Active Men and Women. Nutrients 2025, 17, 585. https://doi.org/10.3390/nu17030585
Boyd-Shiwarski C, Ray E, Subramanian H, Zharichenko N, Monroe A, Mahajan A. The Impact of a Commercial Electrolyte Beverage on the Hydration Status of Active Men and Women. Nutrients. 2025; 17(3):585. https://doi.org/10.3390/nu17030585
Chicago/Turabian StyleBoyd-Shiwarski, Cary, Evan Ray, Harikesh Subramanian, Nicole Zharichenko, Amy Monroe, and Aman Mahajan. 2025. "The Impact of a Commercial Electrolyte Beverage on the Hydration Status of Active Men and Women" Nutrients 17, no. 3: 585. https://doi.org/10.3390/nu17030585
APA StyleBoyd-Shiwarski, C., Ray, E., Subramanian, H., Zharichenko, N., Monroe, A., & Mahajan, A. (2025). The Impact of a Commercial Electrolyte Beverage on the Hydration Status of Active Men and Women. Nutrients, 17(3), 585. https://doi.org/10.3390/nu17030585






