Vitamin D Deficiency Meets Hill’s Criteria for Causation in SARS-CoV-2 Susceptibility, Complications, and Mortality: A Systematic Review
Abstract
:1. Preamble
1.1. Establishing Optimal 25(OH)D Levels for Disease Prevention
1.2. Definitions of Vitamin D Statuses
- (a)
- Vitamin D Sufficiency:
- (b)
- Hypovitaminosis D:
- (c)
- Vitamin D Deficiency:
- (d)
- Severe Vitamin D Deficiency:
2. Systematic Review Procedure
2.1. Criteria Evaluated and Related Analyses of the Systematic Review
2.2. Methods Used in This Systematic Review
2.2.1. Meta-Analyses: Addressing Selection Bias and Unscientific Study Designs
2.2.2. Data Sources and Search Strategy
2.2.3. PICOS Process
2.3. The Area Focussed in the Systematic Review
2.3.1. Generalizability and Applicability of Data to Broader Population
- (A)
- Geographical Diversity—Incorporating studies from various countries and latitudes to account for differences in sunlight exposure and vitamin D synthesis.
- (B)
- Ethnic and Population Diversity—Including diverse ethnic groups and populations to confirm relevance across demographic segments.
- (C)
- Study Settings—Differentiating between community, outpatient, and in-hospital settings to evaluate the impact of vitamin D supplementation across diverse healthcare environments.
- (D)
- Inverse Associations—Presenting evidence of an inverse relationship between serum 25(OH)D concentrations and disease vulnerability, severity, and mortality rates from infections, including SARS-CoV-2.
2.3.2. Evaluation of Study Designs and Quality of Clinical Studies, Including RCTs
2.3.3. Mechanisms and Mechanical Insights
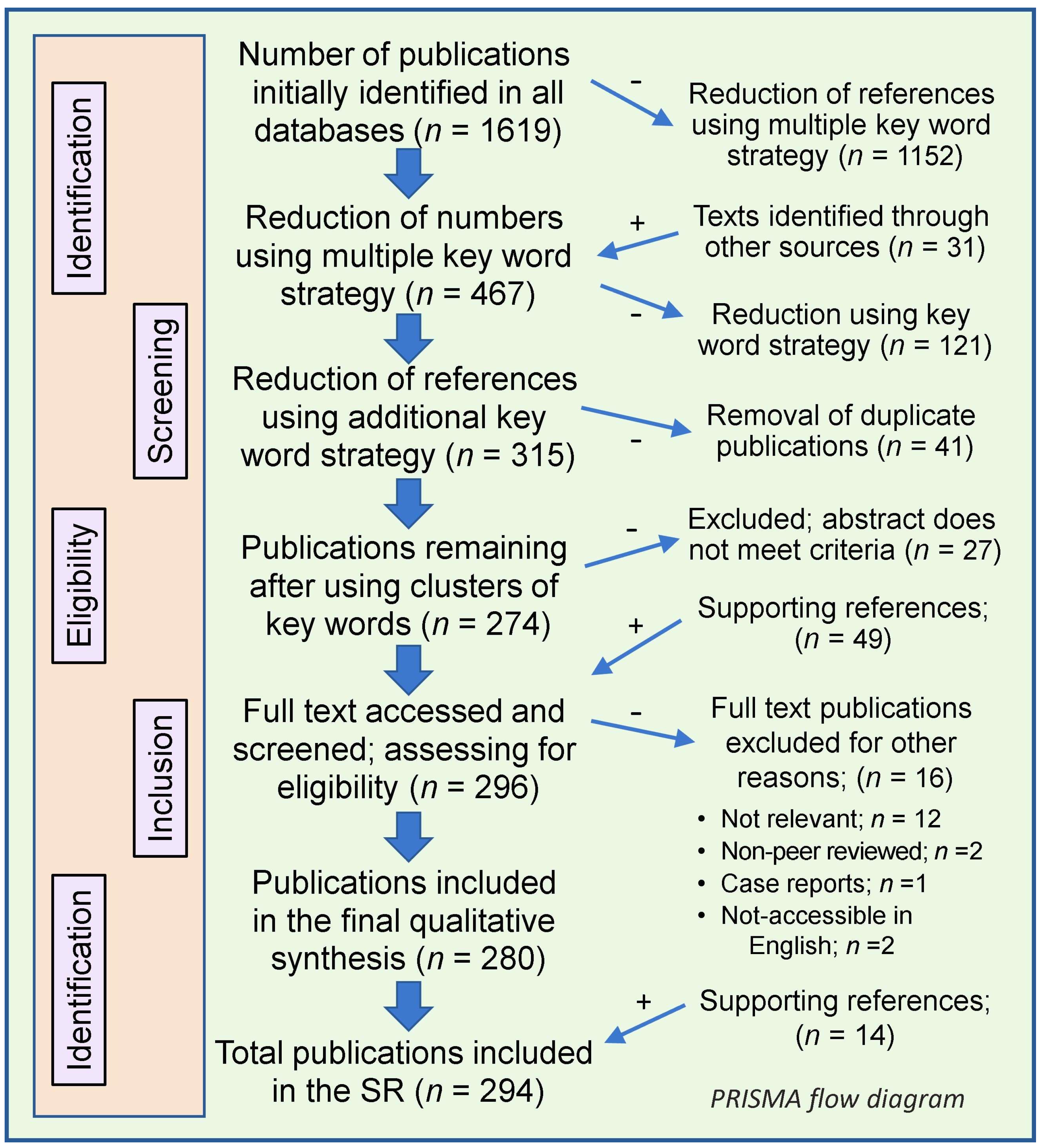
2.4. Results from the SR
Manuscript Selection, Screening, and Data Accumulation
2.5. Scope and Synthesis of Systematic Reviews on Vitamin D and COVID-19: Data and Limitations
2.6. Study Findings
3. Introduction
3.1. Benefits of Maintaining Steady Levels of Vitamin D and 25(OH)D for Infections
3.2. Benefits of Adequate Vitamin D Supplementation in Infections
3.3. Evidence Related to Respiratory Viral Infections, Including SARS-CoV-2
4. The Importance of Proper Designs of RCTs
4.1. Conflicts of Interest and Study Design Errors
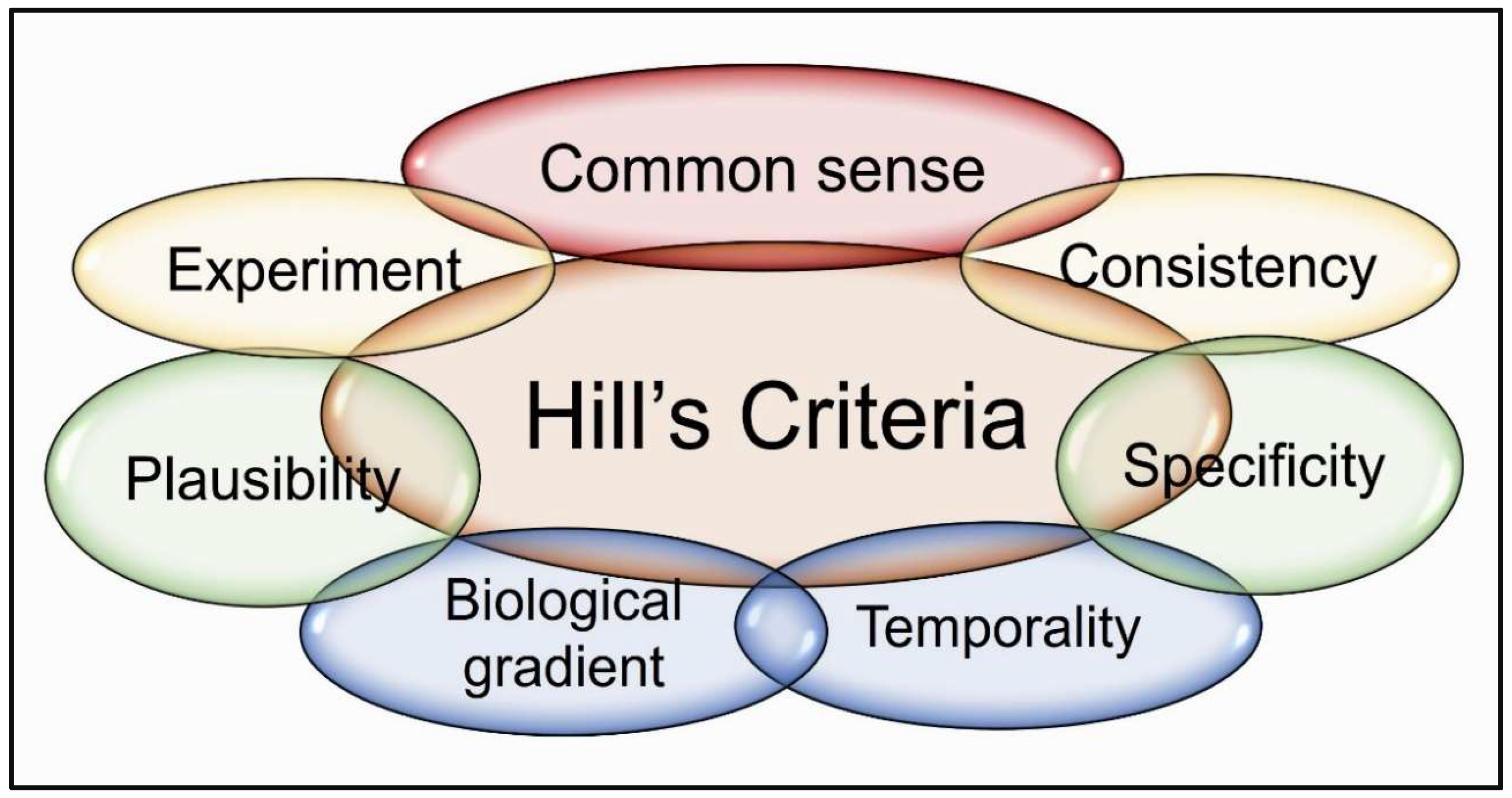
4.2. Hill’s Criteria—Linking Hypovitaminosis D to COVID-19 Clinical Outcomes
4.3. Importance of Real-Time Meta-Analysis to Understand the Efficacy
5. Clinical Trial Design Failures Led to Erroneous Data and Conclusions
5.1. Objectives of Clinical Trials Should Be:
5.2. Lessons Learned from Large Pre-Pandemic Vitamin D RCTs
5.3. Key Causes of Failures in Vitamin D RCTs
5.4. The Ways to Minimize Study Design Errors
5.5. Faulty Study Designs Mislead Vitamin D–SARS-CoV-2 Trial Conclusions
5.6. Failed COVID-19 Pandemic—Related Vitamin D RCTs
6. Enhancing Natural Immunity to Overcome SARS-CoV-2 Infections
6.1. Validated Disorders Associated with Vitamin D Deficiency Based on Hill’s Criteria
6.2. Vitamin D Deficiency and SARS-CoV-2 Risk: Evidence Supporting Bradford Hill’s Causation Criteria
6.3. Do We Always Need RCTs to Establish Efficacy and Causality?
6.4. Why Are RCTs Unsuitable for Testing Micronutrient Efficacy?
6.5. Applying Hill’s Criteria for Vitamin D Deficiency as a Major Risk Factor for SARS-CoV-2
6.6. Vitamin D Insufficiency Meets Bradford Hill Criteria for SARS-CoV-2 Susceptibility—Clinical Implications
7. Discussion
8. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Charoenngam, N.; Sriussadaporn, S. Darker skin color measured by Von Luschan Chromatic Scale and increased sunlight exposure time are independently associated with decreased odds of vitamin D deficiency in Thai ambulatory patients. J. Nutr. Metab. 2021, 2021, 8899931. [Google Scholar] [CrossRef]
- Islamoska, S.; Petersen, J.H.; Benfield, T.; Norredam, M. Socioeconomic and demographic risk factors in COVID-19 hospitalization among immigrants and ethnic minorities. Eur. J. Public Health 2022, 32, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.M.; Bateman, J.; Viswanath, A.; Klaire, V.; Mahmud, S.; Nevill, A.; Dunmore, S.J. Risk of COVID-19 hospital admission and COVID-19 mortality during the first COVID-19 wave with a special emphasis on ethnic minorities: An observational study of a single, deprived, multiethnic UK health economy. BMJ Open 2021, 11, e046556. [Google Scholar] [CrossRef] [PubMed]
- Hardy, A. Commentary: Bread and alum, syphilis and sunlight: Rickets in the nineteenth century. Int. J. Epidemiol. 2003, 32, 337–340. [Google Scholar] [CrossRef]
- Zhang, M.; Shen, F.; Petryk, A.; Tang, J.; Chen, X.; Sergi, C. “English disease”: Historical notes on rickets, the bone-lung link and child neglect issues. Nutrients 2016, 8, 722. [Google Scholar] [CrossRef]
- NHANES. Analytical Note for 25-Hydroxyvitamin D Data Analysis Using NHANES III (1988–1994), NHANES 2001–2006, and NHANES 2007–2010. Available online: https://wwwn.cdc.gov/nchs/nhanes/vitamind/analyticalnote.aspx (accessed on 10 January 2024).
- Yu, G.; Lin, Y.; Dai, H.; Xu, J.; Liu, J. Association between serum 25-hydroxyvitamin D and osteoarthritis: A national population-based analysis of NHANES 2001–2018. Front. Nutr. 2023, 10, 1016809. [Google Scholar] [CrossRef] [PubMed]
- Alloubani, A.; Akhu-Zaheya, L.; Samara, R.; Abdulhafiz, I.; Saleh, A.; Altowijri, A. Relationship between vitamin D deficiency, diabetes, and obesity. Diabetes Metab. Syndr. 2019, 13, 1457–1461. [Google Scholar] [CrossRef]
- Li, Y.X.; Zhou, L. Vitamin D deficiency, obesity and diabetes. Cell. Mol. Biol. 2015, 61, 35–38. [Google Scholar] [PubMed]
- Brenner, H.; Kuznia, S.; Laetsch, C.; Niedermaier, T.; Schottker, B. Prevention of advanced cancer by vitamin D(3) supplementation: Interaction by body mass index revisited. Nutrients 2021, 13, 1408. [Google Scholar] [CrossRef]
- Rhodes, A.; Evans, L.E.; Alhazzani, W.; Levy, M.M.; Antonelli, M.; Ferrer, R.; Kumar, A.; Sevransky, J.E.; Sprung, C.L.; Nunnally, M.E.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017, 43, 304–377. [Google Scholar] [CrossRef]
- Watkins, R.R.; Yamshchikov, A.V.; Lemonovich, T.L.; Salata, R.A. The role of vitamin D deficiency in sepsis and potential therapeutic implications. J. Infect. 2011, 63, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Malinverni, S.; Ochogavia, Q.; Lecrenier, S.; Scorpinniti, M.; Preiser, J.C.; Cotton, F.; Mols, P.; Bartiaux, M. Severe vitamin D deficiency in patients admitted to the emergency department with severe sepsis is associated with an increased 90-day mortality. Emerg. Med. J. 2022, 40, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Martineau, A.R.; Hanifa, Y.; Witt, K.D.; Barnes, N.C.; Hooper, R.L.; Patel, M.; Stevens, N.; Enayat, Z.; Balayah, Z.; Syed, A.; et al. Double-blind randomised controlled trial of vitamin D3 supplementation for the prevention of acute respiratory infection in older adults and their carers (ViDiFlu). Thorax 2015, 70, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef]
- Charoenngam, N.; Holick, M.F. Immunologic effects of vitamin D on human health and sisease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef]
- Charoenngam, N.; Shirvani, A.; Reddy, N.; Vodopivec, D.M.; Apovian, C.M.; Holick, M.F. Association of Vitamin D Status With Hospital Morbidity and Mortality in Adult Hospitalized Patients With COVID-19. Endocr. Pract. 2021, 27, 271–278. [Google Scholar] [CrossRef]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Kostenberger, M.; Tmava Berisha, A.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Unlocking insights: Navigating COVID-19 challenges and Emulating future pandemic Resilience strategies with strengthening natural immunity. Heliyon 2024, 10, e34691. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Physiology of Vitamin D-Focusing on Disease Prevention. Nutrients 2024, 16, 1666. [Google Scholar] [CrossRef] [PubMed]
- Polonowita, A.; Wimalawansa, S.J. Molecular quantum and logic process of consciousness—Vitamin D big-data in COVID-19—A case for incorporating machine learning in medicine. Euro. J. Biomed. Pharma. Sci. 2023, 10, 24–43. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Decoding the paradox: Understanding elevated hospitalization and reduced mortality in SARS-CoV-2 variants. Int. J. Front. Sci. Technol. Res. 2024, 6, 1–20. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Physiological Basis for Using Vitamin D to Improve Health. Biomedicines 2023, 11, 1542. [Google Scholar] [CrossRef]
- Wimalawansa, S. Enhancing the design of nutrient clinical trials for disease prevention: A focus on vitamin D: A systematic review. Nutr. Rev. 2025; in press. [Google Scholar] [CrossRef]
- Wimalawansa, S. Overcoming infections including COVID-19, by maintaining circulating 25(OH)D concentrations above 50 ng/mL. Pathol. Lab. Med. Int. 2022, 14, 37–60. [Google Scholar] [CrossRef]
- McDonnell, S.L.; Baggerly, C.; French, C.B.; Baggerly, L.L.; Garland, C.F.; Gorham, E.D.; Lappe, J.M.; Heaney, R.P. Serum 25-hydroxyvitamin D concentrations >/=40 ng/ml are associated with >65% lower cancer risk: Pooled analysis of randomized trial and prospective cohort study. PLoS ONE 2016, 11, e0152441. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.; Wimalawansa, S.J.; Pludowski, P.; Cheng, R. Vitamin D: Evidence-based health benefits and recommendations for population guidelines. Nutrients 2025, 17, 277. [Google Scholar] [CrossRef]
- Demay, M.B.; Pittas, A.G.; Bikle, D.D.; Diab, D.L.; Kiely, M.E.; Lazaretti-Castro, M.; Lips, P.; Mitchell, D.M.; Murad, M.H.; Powers, S.; et al. Vitamin D for the prevention of disease: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2024, 109, 1907–1947. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Rapidly Increasing Serum 25(OH)D Boosts the Immune System, against Infections-Sepsis and COVID-19. Nutrients 2022, 14, 2997. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, S.L.; Baggerly, K.A.; Baggerly, C.A.; Aliano, J.L.; French, C.B.; Baggerly, L.L.; Ebeling, M.D.; Rittenberg, C.S.; Goodier, C.G.; Mateus Nino, J.F.; et al. Maternal 25(OH)D concentrations >/=40 ng/mL associated with 60% lower preterm birth risk among general obstetrical patients at an urban medical center. PLoS ONE 2017, 12, e0180483. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Infections and Autoimmunity-The Immune System and Vitamin D: A Systematic Review. Nutrients 2023, 15, 3842. [Google Scholar] [CrossRef]
- Wimalawansa, S.J.; Weiss, S.T.; Hollis, B.W. Integrating Endocrine, Genomic, and Extra-Skeletal Benefits of Vitamin D into National and Regional Clinical Guidelines. Nutrients 2024, 16, 3969. [Google Scholar] [CrossRef] [PubMed]
- Razzaque, M.S. Wimalawansa. S.J. Minerals and Human Health: From Deficiency to Toxicity. Nutrients 2025, 17, 454. [Google Scholar] [CrossRef]
- Murai, I.H.; Fernandes, A.L.; Sales, L.P.; Pinto, A.J.; Goessler, K.F.; Duran, C.S.C.; Silva, C.B.R.; Franco, A.S.; Macedo, M.B.; Dalmolin, H.H.H.; et al. Effect of a Single High Dose of Vitamin D3 on Hospital Length of Stay in Patients With Moderate to Severe COVID-19: A Randomized Clinical Trial. JAMA 2021, 325, 1053–1060. [Google Scholar] [CrossRef]
- Jaun, F.; Boesing, M.; Luthi-Corridori, G.; Abig, K.; Makhdoomi, A.; Bloch, N.; Lins, C.; Raess, A.; Grillmayr, V.; Haas, P.; et al. High-dose vitamin D substitution in patients with COVID-19: Study protocol for a randomized, double-blind, placebo-controlled, multi-center study-VitCov Trial. Trials 2022, 23, 114. [Google Scholar] [CrossRef] [PubMed]
- Zangeneh, M.; Valeh, T.; Sharifi, A. Survival analysis based on body mass index in patients with Covid-19 admitted to the intensive care unit of Amir Al-Momenin Hospital in Arak—2021. Obes. Med. 2022, 32, 100420. [Google Scholar] [CrossRef]
- Cannata-Andia, J.B.; Diaz-Sottolano, A.; Fernandez, P.; Palomo-Antequera, C.; Herrero-Puente, P.; Mouzo, R.; Carrillo-Lopez, N.; Panizo, S.; Ibanez, G.H.; Cusumano, C.A.; et al. A single-oral bolus of 100,000 IU of cholecalciferol at hospital admission did not improve outcomes in the COVID-19 disease: The COVID-VIT-D-a randomised multicentre international clinical trial. BMC Med. 2022, 20, 83. [Google Scholar] [CrossRef]
- Annweiler, C.; Beaudenon, M.; Gautier, J.; Gonsard, J.; Boucher, S.; Chapelet, G.; Darsonval, A.; Fougere, B.; Guerin, O.; Houvet, M.; et al. High-dose versus standard-dose vitamin D supplementation in older adults with COVID-19 (COVIT-TRIAL): A multicenter, open-label, randomized controlled superiority trial. PLoS Med. 2022, 19, e1003999. [Google Scholar] [CrossRef]
- Mariani, J.; Antonietti, L.; Tajer, C.; Ferder, L.; Inserra, F.; Sanchez Cunto, M.; Brosio, D.; Ross, F.; Zylberman, M.; Lopez, D.E.; et al. High-dose vitamin D versus placebo to prevent complications in COVID-19 patients: Multicentre randomized controlled clinical trial. PLoS ONE 2022, 17, e0267918. [Google Scholar] [CrossRef] [PubMed]
- Al Sulaiman, K.; Korayem, G.B.; Aljuhani, O.; Altebainawi, A.F.; Shawaqfeh, M.S.; Alarfaj, S.J.; Alharbi, R.A.; Ageeli, M.M.; Alissa, A.; Vishwakarma, R.; et al. Survival implications vs. complications: Unraveling the impact of vitamin D adjunctive use in critically ill patients with COVID-19-A multicenter cohort study. Front Med. 2023, 10, 1237903. [Google Scholar] [CrossRef]
- Manson, J.E.; Cook, N.R.; Lee, I.M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Albert, C.M.; Gordon, D.; Copeland, T.; et al. Marine n-3 Fatty Acids and Prevention of Cardiovascular Disease and Cancer. N. Engl. J. Med. 2019, 380, 23–32. [Google Scholar] [CrossRef]
- Manson, J.E.; Cook, N.R.; Lee, I.M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Gordon, D.; Copeland, T.; D’Agostino, D.; et al. Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease. N. Engl. J. Med. 2018, 380, 33–44. [Google Scholar] [CrossRef]
- National Heart, L.; Blood Institute, P.C.T.N.; Ginde, A.A.; Brower, R.G.; Caterino, J.M.; Finck, L.; Banner-Goodspeed, V.M.; Grissom, C.K.; Hayden, D.; Hough, C.L.; et al. Early high-dose vitamin D3 for critically ill, vitamin D-deficient patients. N. Engl. J. Med. 2019, 381, 2529–2540. [Google Scholar] [CrossRef]
- Desouza, C.; Chatterjee, R.; Vickery, E.M.; Nelson, J.; Johnson, K.C.; Kashyap, S.R.; Lewis, M.R.; Margolis, K.; Pratley, R.; Rasouli, N.; et al. The effect of vitamin D supplementation on cardiovascular risk in patients with prediabetes: A secondary analysis of the D2d study. J. Diabetes Its Complicat. 2022, 36, 108230. [Google Scholar] [CrossRef]
- Scragg, R.K.R. Overview of results from the Vitamin D Assessment (ViDA) study. J. Endocrinol. Investig. 2019, 42, 1391–1399. [Google Scholar] [CrossRef]
- Zittermann, A.; Ernst, J.B.; Prokop, S.; Fuchs, U.; Dreier, J.; Kuhn, J.; Knabbe, C.; Birschmann, I.; Schulz, U.; Berthold, H.K.; et al. Effect of vitamin D on all-cause mortality in heart failure (EVITA): A 3-year randomized clinical trial with 4000 IU vitamin D daily. Eur Heart J. 2017, 38, 2279–2286. [Google Scholar] [CrossRef]
- Infante, M.; Ricordi, C.; Baidal, D.A.; Alejandro, R.; Lanzoni, G.; Sears, B.; Caprio, M.; Fabbri, A. VITAL study: An incomplete picture? Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 3142–3147. [Google Scholar] [CrossRef]
- Borsche, L.; Glauner, B.; von Mendel, J. COVID-19 mortality risk correlates inversely with vitamin D3 status, and a mortality rate close to zero could theoretically be achieved at 50 ng/mL 25(OH)D3: Results of a systematic review and meta-analysis. Nutrients 2021, 13, 3596. [Google Scholar] [CrossRef]
- Essalim, S.; Tachet, C.; Demingo, S.; Bruel, S.; Gagneux-Brunon, A.; Botelho-Nevers, E. Vaccination during febrile illness, what do we know? A systematic-narrative hybrid review of the literature and international recommendations. Vaccine 2024, 42, 126473. [Google Scholar] [CrossRef]
- Khan, N.; Kurnik-Lucka, M.; Latacz, G.; Gil, K. Systematic-narrative hybrid lterature review: Crosstalk between gastrointestinal renin-angiotensin and dopaminergic systems in the regulation of intestinal permeability by tight junctions. Int. J. Mol. Sci. 2024, 25, 5566. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Group, P.-P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 350, g7647. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Group, P.-P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Welch, V.; Petticrew, M.; Tugwell, P.; Moher, D.; O’Neill, J.; Waters, E.; White, H.; group, P.R.-E.B. PRISMA-Equity 2012 extension: Reporting guidelines for systematic reviews with a focus on health equity. PLoS Med 2012, 9, e1001333. [Google Scholar] [CrossRef]
- Group-C19.com. Vitamin D for COVID-19: Real-Time Analysis of All 300 Studies. Available online: https://c19early.org/d (accessed on 25 March 2024).
- Polonowita, A.; Wimalawansa, S.J. The impact of withholding cost-effective early treatments, such as vitamin D, on COVID-19: An analysis using an innovative logical paradigm. World J. Adv. Pharma Life Sci. 2023, 5, 013–034. [Google Scholar] [CrossRef]
- Wacker, M.; Holick, M.F. Sunlight and Vitamin D: A global perspective for health. Derm. Endocrinol. 2013, 5, 51–108. [Google Scholar] [CrossRef]
- Shoemaker, M.E.; Huynh, L.M.; Smith, C.M.; Mustad, V.A.; Duarte, M.O.; Cramer, J.T. Immunomodulatory effects of vitamin D and prevention of respiratory tract infections and COVID-19. Top. Clin. Nutr. 2022, 37, 203–217. [Google Scholar] [CrossRef]
- Merzon, E.; Tworowski, D.; Gorohovski, A.; Vinker, S.; Golan Cohen, A.; Green, I.; Frenkel-Morgenstern, M. Low plasma 25(OH) vitamin D level is associated with increased risk of COVID-19 infection: An Israeli population-based study. FEBS J. 2020, 287, 3693–3702. [Google Scholar] [CrossRef]
- Dror, A.A.; Morozov, N.; Daoud, A.; Namir, Y.; Yakir, O.; Shachar, Y.; Lifshitz, M.; Segal, E.; Fisher, L.; Mizrachi, M.; et al. Pre-infection 25-hydroxyvitamin D3 levels and association with severity of COVID-19 illness. PLoS ONE 2022, 17, e0263069. [Google Scholar] [CrossRef]
- Bayrak, H.; Ozturk, D.; Bolat, A.; Unay, B. Association between vitamin D levels and COVID-19 infection in children: A case-control study. Turk. Arch. Pediatr. 2023, 58, 250–255. [Google Scholar] [CrossRef]
- Wimalawansa, S.J.; Polonowita, A. Boosting immunity with vitamin D for preventing complications and deaths from COVID-19. In Proceedings of the COVID 19: Impact, Mitigation, Opportunities and Building Resilience “From Adversity to Serendipity”, Perspectives of Global Relevance Based on Research, Experience and Successes in Combating COVID-19 in Sri Lanka, Colombo, Sri Lanka, 27–28 January 2021; National Science Foundation: Colombo, Sri Lanka, 2021; pp. 171–198. [Google Scholar]
- Rustecka, A.; Maret, J.; Drab, A.; Leszczynska, M.; Tomaszewska, A.; Lipinska-Opalka, A.; Bedzichowska, A.; Kalicki, B.; Kubiak, J.Z. The Impact of COVID-19 pandemic during 2020-2021 on the vitamin D serum levels in the paediatric population in Warsaw, Poland. Nutrients 2021, 13, 1990. [Google Scholar] [CrossRef]
- Radujkovic, A.; Hippchen, T.; Tiwari-Heckler, S.; Dreher, S.; Boxberger, M.; Merle, U. Vitamin D deficiency and outcome of COVID-19 patients. Nutrients 2020, 12, 2757. [Google Scholar] [CrossRef]
- Baktash, V.; Hosack, T.; Patel, N.; Shah, S.; Kandiah, P.; Van den Abbeele, K.; Mandal, A.K.J.; Missouris, C.G. Vitamin D status and outcomes for hospitalised older patients with COVID-19. Postgrad. Med. J. 2021, 97, 442–447. [Google Scholar] [CrossRef]
- Smaha, J.; Jackuliak, P.; Kuzma, M.; Max, F.; Binkley, N.; Payer, J. Vitamin D deficiency prevalence in hospitalized patients with COVID-19 significantly decreased during the pandemic in Slovakia from 2020 to 2022 Which Was associated with decreasing mortality. Nutrients 2023, 15, 1132. [Google Scholar] [CrossRef]
- Shiravi, A.A.; Saadatkish, M.; Abdollahi, Z.; Miar, P.; Khanahmad, H.; Zeinalian, M. Vitamin D can be effective on the prevention of COVID-19 complications: A narrative review on molecular aspects. Int. J. Vitam. Nutr. Res. 2022, 92, 134–146. [Google Scholar] [CrossRef]
- Maghbooli, Z.; Sahraian, M.A.; Jamalimoghadamsiahkali, S.; Asadi, A.; Zarei, A.; Zendehdel, A.; Varzandi, T.; Mohammadnabi, S.; Alijani, N.; Karimi, M.; et al. Treatment With 25-hydroxyvitamin D(3) (calcifediol) is associated with a reduction in the blood neutrophil-to-lymphocyte ratio marker of disease severity in hospitalized patients with COVID-19: A pilot multicenter, randomized, placebo-controlled, double-blinded clinical trial. Endocr. Pract. 2021, 27, 1242–1251. [Google Scholar] [CrossRef]
- Garg, M.; Al-Ani, A.; Mitchell, H.; Hendy, P.; Christensen, B. Editorial: Low population mortality from COVID-19 in countries south of latitude 35 degrees North-supports vitamin D as a factor determining severity. Authors’ reply. Aliment. Pharmacol. Ther. 2020, 51, 1438–1439. [Google Scholar] [CrossRef]
- Quesada-Gomez, J.M.; Lopez-Miranda, J.; Entrenas-Castillo, M.; Casado-Diaz, A.; Nogues, Y.S.X.; Mansur, J.L.; Bouillon, R. Vitamin D endocrine system and COVID-19: Treatment with calcifediol. Nutrients 2022, 14, 2716. [Google Scholar] [CrossRef]
- AlSafar, H.; Grant, W.B.; Hijazi, R.; Uddin, M.; Alkaabi, N.; Tay, G.; Mahboub, B.; Al Anouti, F. COVID-19 disease severity and death in relation to vitamin D status among SARS-CoV-2-positive UAE residents. Nutrients 2021, 13, 1714. [Google Scholar] [CrossRef]
- Bianconi, V.; Mannarino, M.R.; Figorilli, F.; Cosentini, E.; Batori, G.; Marini, E.; Lombardini, R.; Gargaro, M.; Fallarino, F.; Scarponi, A.M.; et al. Prevalence of vitamin D deficiency and its prognostic impact on patients hospitalized with COVID-19. Nutrition 2021, 91–92, 111408. [Google Scholar] [CrossRef]
- Kazemi, A.; Mohammadi, V.; Aghababaee, S.K.; Golzarand, M.; Clark, C.C.T.; Babajafari, S. Association of vitamin D status with SARS-CoV-2 infection or COVID-19 severity: A systematic review and meta-analysis. Adv. Nutr. 2021, 12, 1636–1658. [Google Scholar] [CrossRef]
- Kaufman, H.W.; Niles, J.K.; Kroll, M.H.; Bi, C.; Holick, M.F. SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels. PLoS ONE 2020, 15, e0239252. [Google Scholar] [CrossRef] [PubMed]
- Alcala-Diaz, J.F.; Limia-Perez, L.; Gomez-Huelgas, R.; Martin-Escalante, M.D.; Cortes-Rodriguez, B.; Zambrana-Garcia, J.L.; Entrenas-Castillo, M.; Perez-Caballero, A.I.; Lopez-Carmona, M.D.; Garcia-Alegria, J.; et al. Calcifediol treatment and hospital mortality due to COVID-19: A cohort study. Nutrients 2021, 13, 1760. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.B.; McCartney, D.M.; Laird, E.; McCarroll, K.; Byrne, D.G.; Healy, M.; O’Shea, P.M.; Kenny, R.A.; Faul, J.L. Title: Understanding a Low Vitamin D State in the Context of COVID-19. Front. Pharmacol. 2022, 13, 835480. [Google Scholar] [CrossRef]
- Verstuyf, A.; Carmeliet, G.; Bouillon, R.; Mathieu, C. Vitamin D: A pleiotropic hormone. Kidney Int. 2010, 78, 140–145. [Google Scholar] [CrossRef]
- Cancela, L.; Nemere, I.; Norman, A.W. 1 alpha,25(OH)2 vitamin D3: A steroid hormone capable of producing pleiotropic receptor-mediated biological responses by both genomic and nongenomic mechanisms. J. Steroid Biochem. 1988, 30, 33–39. [Google Scholar] [CrossRef]
- Manson, J.E.; Bassuk, S.S.; Lee, I.M.; Cook, N.R.; Albert, M.A.; Gordon, D.; Zaharris, E.; Macfadyen, J.G.; Danielson, E.; Lin, J.; et al. The VITamin D and OmegA-3 TriaL (VITAL): Rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemp. Clin. Trials 2012, 33, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Neale, R.E.; Baxter, C.; Romero, B.D.; McLeod, D.S.A.; English, D.R.; Armstrong, B.K.; Ebeling, P.R.; Hartel, G.; Kimlin, M.G.; O’Connell, R.; et al. The D-Health Trial: A randomised controlled trial of the effect of vitamin D on mortality. Lancet. Diabetes Endocrinol. 2022, 10, 120–128. [Google Scholar] [CrossRef]
- Cauley, J.A.; LaCroix, A.Z.; Wu, L.; Horwitz, M.; Danielson, M.E.; Bauer, D.C.; Lee, J.S.; Jackson, R.D.; Robbins, J.A.; Wu, C.; et al. Serum 25 hydroxyvitamin D concentrations and the risk of hip Fractures: The women’s health initiative. Ann. Intern. Med. 2008, 149, 242–250. [Google Scholar] [CrossRef]
- Grant, W.B.; Boucher, B.J.; Bhattoa, H.P.; Lahore, H. Why vitamin D clinical trials should be based on 25-hydroxyvitamin D concentrations. J. Steroid Biochem. Mol. Biol. 2018, 177, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Unveiling the interplay-vitamin D and ACE-2 molecular interactions in mitigating complications and deaths from SARS-CoV-2. Biology 2024, 13, 831. [Google Scholar] [CrossRef]
- Ismailova, A.; White, J.H. Vitamin D, infections and immunity. Rev. Endocr. Metab. Disord. 2022, 23, 265–277. [Google Scholar] [CrossRef]
- Sarau, O.S.; Rachabattuni, H.C.; Gadde, S.T.; Daruvuri, S.P.; Marusca, L.M.; Horhat, F.G.; Fildan, A.P.; Tanase, E.; Prodan-Barbulescu, C.; Horhat, D.I. Exploring the preventive potential of vitamin D against respiratory infections in preschool-age children: A cross-sectional study. Nutrients 2024, 16, 1595. [Google Scholar] [CrossRef]
- Tenali, N.; Babu, G.R.M. A systematic literature review and future perspectives for handling big data analytics in COVID-19 diagnosis. New Gener. Comput. 2023, 41, 243–280. [Google Scholar] [CrossRef] [PubMed]
- Diani, S.; Leonardi, E.; Cavezzi, A.; Ferrari, S.; Iacono, O.; Limoli, A.; Bouslenko, Z.; Natalini, D.; Conti, S.; Mantovani, M.; et al. SARS-CoV-2-The role of natural immunity: A narrative review. J. Clin. Med. 2022, 11, 6272. [Google Scholar] [CrossRef] [PubMed]
- Sciscent, B.Y.; Eisele, C.D.; Ho, L.; King, S.D.; Jain, R.; Golamari, R.R. COVID-19 reinfection: The role of natural immunity, vaccines, and variants. J. Community Hosp. Intern. Med. Perspect. 2021, 11, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Nafilyan, V.; Bermingham, C.R.; Ward, I.L.; Morgan, J.; Zaccardi, F.; Khunti, K.; Stanborough, J.; Banerjee, A.; Doidge, J.C. Risk of death following COVID-19 vaccination or positive SARS-CoV-2 test in young people in England. Nat. Commun. 2023, 14, 1541. [Google Scholar] [CrossRef] [PubMed]
- Quraishi, S.A.; De Pascale, G.; Needleman, J.S.; Nakazawa, H.; Kaneki, M.; Bajwa, E.K.; Camargo, C.A., Jr.; Bhan, I. Effect of cholecalciferol supplementation on vitamin D status and cathelicidin levels in sepsis: A randomized, placebo-controlled trial. Crit. Care Med. 2015, 43, 1928–1937. [Google Scholar] [CrossRef]
- Quraishi, S.A.; Bittner, E.A.; Blum, L.; Hutter, M.M.; Camargo, C.A., Jr. Association between preoperative 25-hydroxyvitamin D level and hospital-acquired infections following Roux-en-Y gastric bypass surgery. JAMA Surg. 2014, 149, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Charoenngam, N.; Shirvani, A.; Holick, M.F. Vitamin D and Its Potential Benefit for the COVID-19 Pandemic. Endocr. Pract. 2021, 27, 484–493. [Google Scholar] [CrossRef]
- Sempos, C.T.; Durazo-Arvizu, R.A.; Binkley, N.; Jones, J.; Merkel, J.M.; Carter, G.D. Developing vitamin D dietary guidelines and the lack of 25-hydroxyvitamin D assay standardization: The ever-present past. J. Steroid Biochem. Mol. Biol. 2016, 164, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Biology of vitamin D. J. Steroids Horm. Sci. 2019, 10, 1–8. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Non-musculoskeletal benefits of vitamin D. J. Steroid Biochem. Mol. Biol. 2018, 175, 60–81. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Al Anouti, F.; Boucher, B.J.; Fakhoury, H.M.A.; Moukayed, M.; Pilz, S.; Al-Daghri, N.M. Evidence That Increasing Serum 25(OH)D Concentrations to 30 ng/mL in the Kingdom of Saudi Arabia and the United Arab Emirates Could Greatly Improve Health Outcomes. Biomedicines 2023, 11, 994. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine, S. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Holick, M.F. Revisiting vitamin D guidelines: A critical appraisal of the literature. Endocr. Pract. 2024, 30, 1227–1241. [Google Scholar] [CrossRef] [PubMed]
- Barker-Davies, R.M.; O’Sullivan, O.; Senaratne, K.P.P.; Baker, P.; Cranley, M.; Dharm-Datta, S.; Ellis, H.; Goodall, D.; Gough, M.; Lewis, S.; et al. The Stanford Hall consensus statement for post-COVID-19 rehabilitation. Br. J. Sports Med. 2020, 54, 949–959. [Google Scholar] [CrossRef] [PubMed]
- de Sire, A.; Andrenelli, E.; Negrini, F.; Lazzarini, S.G.; Patrini, M.; Ceravolo, M.G.; Kiekens, C.; Arienti, C.; Maria, G.C.; Côté, P.; et al. Rehabilitation and COVID-19: The Cochrane Rehabilitation 2020 rapid living systematic review. Update as of August 31st, 2020. Eur. J. Phys. Rehabil. Med. 2020, 56, 839–845. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, J.; Hua, L.; Li, P.; Wu, J.; Shang, S.; Deng, F.; Luo, J.; Liao, M.; Wang, N.; et al. Vitamin D receptor (VDR) on the cell membrane of mouse macrophages participates in the formation of lipopolysaccharide tolerance: mVDR is related to the effect of artesunate to reverse LPS tolerance. Cell Commun. Signal 2023, 21, 124. [Google Scholar] [CrossRef]
- Guo, Y.; Li, X.; Geng, C.; Song, S.; Xie, X.; Wang, C. Vitamin D receptor involves in the protection of intestinal epithelial barrier function via up-regulating SLC26A3. J. Steroid Biochem. Mol. Biol. 2023, 227, 106231. [Google Scholar] [CrossRef] [PubMed]
- Ledderose, C.; Bao, Y.; Zhang, J.; Junger, W.G. Novel method for real-time monitoring of ATP release reveals multiple phases of autocrine purinergic signalling during immune cell activation. Acta Physiol. 2015, 213, 334–345. [Google Scholar] [CrossRef] [PubMed]
- Chauss, D.; Freiwald, T.; McGregor, R.; Yan, B.; Wang, L.; Nova-Lamperti, E.; Kumar, D.; Zhang, Z.; Teague, H.; West, E.E.; et al. Autocrine vitamin D signaling switches off pro-inflammatory programs of TH1 cells. Nat. Immunol. 2022, 23, 62–74. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Hii, C.S.; Ferrante, A. The non-genomic actions of vitamin D. Nutrients 2016, 8, 135. [Google Scholar] [CrossRef] [PubMed]
- Zmijewski, M.A. Nongenomic activities of vitamin D. Nutrients 2022, 14, 5104. [Google Scholar] [CrossRef]
- Sun, L.; Arbesman, J.; Piliang, M. Vitamin D, autoimmunity and immune-related adverse events of immune checkpoint inhibitors. Arch. Dermatol. Res. 2021, 313, 1–10. [Google Scholar] [CrossRef]
- Athanassiou, L.; Kostoglou-Athanassiou, I.; Koutsilieris, M.; Shoenfeld, Y. Vitamin D and autoimmune rheumatic diseases. Biomolecules 2023, 13, 709. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.L.; Wu, J. Role of vitamin D in immune responses and autoimmune diseases, with emphasis on its role in multiple sclerosis. Neurosci. Bull. 2010, 26, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Pender, M.P. CD8+ T-cell deficiency, Epstein-Barr virus infection, vitamin D deficiency, and steps to autoimmunity: A unifying hypothesis. Autoimmune Dis. 2012, 2012, 189096. [Google Scholar] [CrossRef] [PubMed]
- Quraishi, S.A.; Bittner, E.A.; Blum, L.; McCarthy, C.M.; Bhan, I.; Camargo, C.A., Jr. Prospective study of vitamin D status at initiation of care in critically ill surgical patients and risk of 90-day mortality. Crit. Care Med. 2014, 42, 1365–1371. [Google Scholar] [CrossRef]
- Fernandez, G.J.; Ramirez-Mejia, J.M.; Castillo, J.A.; Urcuqui-Inchima, S. Vitamin D modulates expression of antimicrobial peptides and proinflammatory cytokines to restrict Zika virus infection in macrophages. Int. Immunopharmacol. 2023, 119, 110232. [Google Scholar] [CrossRef] [PubMed]
- Kalantari, N.; Sepidarkish, M.; Ghaffari, S.; Rostami-Mansoor, S. Does vitamin D reduce the mortality rate of Plasmodium infection?: A systematic review and meta-analysis. Malar. J. 2023, 22, 173. [Google Scholar] [CrossRef]
- Sposito, F.; Pennington, S.H.; David, C.A.W.; Duggan, J.; Northey, S.; Biagini, G.A.; Liptrott, N.J.; Charras, A.; McNamara, P.S.; Hedrich, C.M. Age-differential CD13 and interferon expression in airway epithelia affect SARS-CoV-2 infection—Effects of vitamin D. Mucosal Immunol. 2023, 16, 776–787. [Google Scholar] [CrossRef]
- Georgakopoulou, V.E.; Gkoufa, A.; Tsakanikas, A.; Makrodimitri, S.; Karamanakos, G.; Basoulis, D.; Voutsinas, P.M.; Eliadi, I.; Bougea, A.; Spandidos, D.A.; et al. Predictors of COVID-19-associated mortality among hospitalized elderly patients with dementia. Exp. Ther. Med. 2023, 26, 395. [Google Scholar] [CrossRef]
- Trecarichi, E.M.; Mazzitelli, M.; Serapide, F.; Pelle, M.C.; Tassone, B.; Arrighi, E.; Perri, G.; Fusco, P.; Scaglione, V.; Davoli, C.; et al. Clinical characteristics and predictors of mortality associated with COVID-19 in elderly patients from a long-term care facility. Sci. Rep. 2020, 10, 20834. [Google Scholar] [CrossRef]
- Asaduzzaman; Alam, Z.H.M.N.; Bari, M.Z.J.; Alam, M.M.J.; Chakraborty, S.R.; Ferdousi, T. Clinical Characteristics and Predictors of Mortality in Elderly Patients Hospitalized with COVID-19 in Bangladesh: A Multicenter, Retrospective Study. Interdiscip. Perspect. Infect. Dis. 2022, 2022, 1–10. [Google Scholar] [CrossRef]
- Gan, Y.; You, S.; Ying, J.; Mu, D. The association between serum vitamin D levels and urinary tract infection risk in children: A systematic review and meta-analysis. Nutrients 2023, 15, 2690. [Google Scholar] [CrossRef]
- Raju, A.; Luthra, G.; Shahbaz, M.; Almatooq, H.; Foucambert, P.; Esbrand, F.D.; Zafar, S.; Panthangi, V.; Cyril Kurupp, A.R.; Khan, S. Role of vitamin D deficiency in increased susceptibility to respiratory infections among children: A systematic review. Cureus 2022, 14, e29205. [Google Scholar] [CrossRef]
- Charpy, J. The clinical treatment of tuberculosis lupus and certain tuberculosis by vitamin D 2 (calciferol). Med. Cir. Farm. 1947, 11, 145–159. [Google Scholar]
- Cadranel, J.L.; Garabedian, M.; Milleron, B.; Guillozzo, H.; Valeyre, D.; Paillard, F.; Akoun, G.; Hance, A.J. Vitamin D metabolism by alveolar immune cells in tuberculosis: Correlation with calcium metabolism and clinical manifestations. Eur. Respir. J. 1994, 7, 1103–1110. [Google Scholar] [CrossRef]
- Huang, S.J.; Wang, X.H.; Liu, Z.D.; Cao, W.L.; Han, Y.; Ma, A.G.; Xu, S.F. Vitamin D deficiency and the risk of tuberculosis: A meta-analysis. Drug Des. Dev. Ther. 2017, 11, 91–102. [Google Scholar] [CrossRef]
- Bekele, A.; Gebreselassie, N.; Ashenafi, S.; Kassa, E.; Aseffa, G.; Amogne, W.; Getachew, M.; Aseffa, A.; Worku, A.; Raqib, R.; et al. Daily adjunctive therapy with vitamin D(3) and phenylbutyrate supports clinical recovery from pulmonary tuberculosis: A randomized controlled trial in Ethiopia. J. Intern. Med. 2018, 284, 292–306. [Google Scholar] [CrossRef]
- Salahuddin, N.; Ali, F.; Hasan, Z.; Rao, N.; Aqeel, M.; Mahmood, F. Vitamin D accelerates clinical recovery from tuberculosis: Results of the SUCCINCT Study [Supplementary Cholecalciferol in recovery from tuberculosis]. A randomized, placebo-controlled, clinical trial of vitamin D supplementation in patients with pulmonary tuberculosis’. BMC Infect. Dis. 2013, 13, 22. [Google Scholar] [CrossRef]
- Fu, G.; Wu, R.; Zhang, R.; Chen, D.; Li, H.; Zheng, Q.; Ma, Y. Preoperative vitamin D deficiency is associated with increased one-year mortality in Chinese geriatric hip fracture patients—A propensity score matching study. Clin. Interv. Aging 2023, 18, 263–272. [Google Scholar] [CrossRef]
- Ashique, S.; Gupta, K.; Gupta, G.; Mishra, N.; Singh, S.K.; Wadhwa, S.; Gulati, M.; Dureja, H.; Zacconi, F.; Oliver, B.G.; et al. Vitamin D—A prominent immunomodulator to prevent COVID-19 infection. Int. J. Rheum. Dis. 2023, 26, 13–30. [Google Scholar] [CrossRef]
- Dudenkov, D.V.; Yawn, B.P.; Oberhelman, S.S.; Fischer, P.R.; Singh, R.J.; Cha, S.S.; Maxson, J.A.; Quigg, S.M.; Thacher, T.D. Changing incidence of serum 25-hydroxyvitamin D values above 50 ng/mL: A 10-year population-based study. Mayo Clin. Proc. 2015, 90, 577–586. [Google Scholar] [CrossRef]
- Argano, C.; Mallaci Bocchio, R.; Natoli, G.; Scibetta, S.; Lo Monaco, M.; Corrao, S. Protective effect of vitamin D supplementation on COVID-19-related intensive care hospitalization and mortality: Sefinitive evidence from meta-analysis and trial sequential analysis. Pharmaceuticals 2023, 16, 130. [Google Scholar] [CrossRef]
- Greiller, C.L.; Martineau, A.R. Modulation of the immune response to respiratory viruses by vitamin D. Nutrients 2015, 7, 4240–4270. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Fogacci, F.; Borghi, C. Vitamin D supplementation and COVID-19 outcomes: Mounting evidence and fewer doubts. Nutrients 2022, 14, 3584. [Google Scholar] [CrossRef]
- Gonen, M.S.; Alaylioglu, M.; Durcan, E.; Ozdemir, Y.; Sahin, S.; Konukoglu, D.; Nohut, O.K.; Urkmez, S.; Kucukece, B.; Balkan, I.I.; et al. Rapid and effective vitamin D supplementation may present better clinical outcomes in COVID-19 (SARS-CoV-2) patients by altering serum INOS1, IL1B, IFNg, cathelicidin-LL37, and ICAM1. Nutrients 2021, 13, 4047. [Google Scholar] [CrossRef]
- Jolliffe, D.A.; Camargo, C.A., Jr.; Sluyter, J.D.; Aglipay, M.; Aloia, J.F.; Ganmaa, D.; Bergman, P.; Bischoff-Ferrari, H.A.; Borzutzky, A.; Damsgaard, C.T.; et al. Vitamin D supplementation to prevent acute respiratory infections: A systematic review and meta-analysis of aggregate data from randomised controlled trials. Lancet. Diabetes Endocrinol. 2021, 9, 276–292. [Google Scholar] [CrossRef]
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ 2017, 356, i6583. [Google Scholar] [CrossRef]
- Jolliffe, D.A.; Griffiths, C.J.; Martineau, A.R. Vitamin D in the prevention of acute respiratory infection: Systematic review of clinical studies. J. Steroid Biochem. Mol. Biol. 2013, 136, 321–329. [Google Scholar] [CrossRef]
- Zhuang, Y.; Zhu, Z.; Chi, P.; Zhou, H.; Peng, Z.; Cheng, H.; Xin, X.; Luo, W.; Si, S.; Mo, M.; et al. Efficacy of intermittent versus daily vitamin D supplementation on improving circulating 25(OH)D concentration: A Bayesian network meta-analysis of randomized controlled trials. Front. Nutr. 2023, 10, 1168115. [Google Scholar] [CrossRef]
- Hong, M.; Xiong, T.; Huang, J.; Wu, Y.; Lin, L.; Zhang, Z.; Huang, L.; Gao, D.; Wang, H.; Kang, C.; et al. Association of vitamin D supplementation with respiratory tract infection in infants. Matern. Child. Nutr. 2020, 16, e12987. [Google Scholar] [CrossRef]
- Molloy, E.J.; Murphy, N. Vitamin D, Covid-19 and Children. Ir. Med. J. 2020, 113, 64. [Google Scholar]
- Stohs, S.J.; Aruoma, O.I. Vitamin D and Wellbeing beyond Infections: COVID-19 and Future Pandemics. J. Am. Coll. Nutr. 2020, 40, 41–42. [Google Scholar] [CrossRef]
- Ling, S.F.; Broad, E.; Murphy, R.; Pappachan, J.M.; Pardesi-Newton, S.; Kong, M.F.; Jude, E.B. High-dose cholecalciferol booster therapy is associated with a reduced risk of mortality in patients with COVID-19: A cross-sectional multi-centre observational study. Nutrients 2020, 12, 3799. [Google Scholar] [CrossRef]
- Entrenas Castillo, M.; Entrenas Costa, L.M.; Vaquero Barrios, J.M.; Alcala Diaz, J.F.; Lopez Miranda, J.; Bouillon, R.; Quesada Gomez, J.M. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study. J. Steroid Biochem. Mol. Biol. 2020, 203, 105751. [Google Scholar] [CrossRef]
- Ebrahimzadeh, A.; Mohseni, S.; Narimani, B.; Ebrahimzadeh, A.; Kazemi, S.; Keshavarz, F.; Yaghoubi, M.J.; Milajerdi, A. Association between vitamin D status and risk of covid-19 in-hospital mortality: A systematic review and meta-analysis of observational studies. Crit. Rev. Food Sci. Nutr. 2021, 63, 5033–5043. [Google Scholar] [CrossRef]
- Brown, R.; Sakar, A. Vitamin D Deficiency: A Factor in COVID-19, Progression, Severity and Mortality?—An Urgent Call for Research. 2020. Available online: https://www.mitofit.org/images/e/ec/Brown_et_al_2020_MitoFit_Preprint_Arch_doi_10.26214_mitofit_200001.pdf (accessed on 10 November 2024).
- Davies, G.; Mazess, R.B.; Benskin, L.L. Letter to the editor in response to the article: “Vitamin D concentrations and COVID-19 infection in UK biobank” (Hastie et al.). Diabetes Metab. Syndr. 2021, 15, 643–644. [Google Scholar] [CrossRef]
- Hastie, C.E.; Pell, J.P.; Sattar, N. Vitamin D and COVID-19 infection and mortality in UK Biobank. Eur. J. Nutr. 2020, 60, 545–548. [Google Scholar] [CrossRef] [PubMed]
- Raisi-Estabragh, Z.; McCracken, C.; Bethell, M.S.; Cooper, J.; Cooper, C.; Caulfield, M.J.; Munroe, P.B.; Harvey, N.C.; Petersen, S.E. Greater risk of severe COVID-19 in Black, Asian and Minority Ethnic populations is not explained by cardiometabolic, socioeconomic or behavioural factors, or by 25(OH)-vitamin D status: Study of 1326 cases from the UK Biobank. J. Public Health 2020, 42, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Hastie, C.E.; Mackay, D.F.; Ho, F.; Celis-Morales, C.A.; Katikireddi, S.V.; Niedzwiedz, C.L.; Jani, B.D.; Welsh, P.; Mair, F.S.; Gray, S.R.; et al. Vitamin D concentrations and COVID-19 infection in UK Biobank. Diabetes Metab. Syndr. 2020, 14, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.B. The environment and disease: Association or causation? Proc. R. Soc. Med. 1965, 58, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Gallelli, L.; Mannino, G.C.; Luciani, F.; de Sire, A.; Mancuso, E.; Gangemi, P.; Cosco, L.; Monea, G.; Averta, C.; Minchella, P.; et al. Vitamin D serum Levels in subjects tested for SARS-CoV-2: What are the differences among acute, healed, and negative COVID-19 patients? A multicenter real-practice study. Nutrients 2021, 13, 3932. [Google Scholar] [CrossRef]
- Charoenporn, V.; Tungsukruthai, P.; Teacharushatakit, P.; Hanvivattanakul, S.; Sriyakul, K.; Sukprasert, S.; Kamalashiran, C.; Tungsukruthai, S.; Charernboon, T. Effects of an 8-week high-dose vitamin D supplementation on fatigue and neuropsychiatric manifestations in post-COVID syndrome: A randomized controlled trial. Psychiatry Clin. Neurosci. 2024, 78, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Wobke, T.K.; Sorg, B.L.; Steinhilber, D. Vitamin D in inflammatory diseases. Front. Physiol. 2014, 5, 244. [Google Scholar] [CrossRef]
- Wilding, P.M. Cardiovascular disease, statins and vitamin D. Br. J. Nurs. 2012, 21, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Ulitsky, A.; Ananthakrishnan, A.N.; Naik, A.; Skaros, S.; Zadvornova, Y.; Binion, D.G.; Issa, M. Vitamin D deficiency in patients with inflammatory bowel disease: Association with disease activity and quality of life. JPEN J. Parenter. Enter. Nutr. 2011, 35, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Bergman, P.; Lindh, A.U.; Bjorkhem-Bergman, L.; Lindh, J.D. Vitamin D and Respiratory Tract Infections: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. PLoS ONE 2013, 8, e65835. [Google Scholar] [CrossRef]
- Al-Khalil, O. High-dose vitamin D—No benefit for postmenopausal women. Praxis 2015, 104, 1289–1290. [Google Scholar] [CrossRef] [PubMed]
- Haines, N.; Kempton, L.B.; Seymour, R.B.; Bosse, M.J.; Churchill, C.; Hand, K.; Hsu, J.R.; Keil, D.; Kellam, J.; Rozario, N.; et al. The effect of a single early high-dose vitamin D supplement on fracture union in patients with hypovitaminosis D: A prospective randomised trial. Bone Jt. J. 2017, 99-B, 1520–1525. [Google Scholar] [CrossRef]
- Evans, M.; Lewis, E.D.; Antony, J.M.; Crowley, D.C.; Guthrie, N.; Blumberg, J.B. Breaking new frontiers: Assessment and re-evaluation of clinical trial design for nutraceuticals. Front. Nutr. 2022, 9, 958753. [Google Scholar] [CrossRef]
- Chandler, P.D.; Chen, W.Y.; Ajala, O.N.; Hazra, A.; Cook, N.; Bubes, V.; Lee, I.M.; Giovannucci, E.L.; Willett, W.; Buring, J.E.; et al. Effect of vitamin D3 supplements on development of advanced cancer: A secondary analysis of the VITAL randomized clinical trial. JAMA Netw. Open 2020, 3, e2025850. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Trummer, C.; Theiler-Schwetz, V.; Grubler, M.R.; Verheyen, N.D.; Odler, B.; Karras, S.N.; Zittermann, A.; Marz, W. Critical appraisal of large vitamin D randomized controlled trials. Nutrients 2022, 14, 303. [Google Scholar] [CrossRef] [PubMed]
- Lappe, J.M.; Heaney, R.P. Why randomized controlled trials of calcium and vitamin D sometimes fail. Derm.-Endocrinol. 2012, 4, 95–100. [Google Scholar] [CrossRef]
- Grant, W.B.; Boucher, B.J. Randomized controlled trials of vitamin D and cancer incidence: A modeling study. PLoS ONE 2017, 12, e0176448. [Google Scholar] [CrossRef] [PubMed]
- Heath, A.K.; Hodge, A.M.; Ebeling, P.R.; Kvaskoff, D.; Eyles, D.W.; Giles, G.G.; English, D.R.; Williamson, E.J. Circulating 25-hydroxyvitamin D concentration and cause-specific mortality in the Melbourne Collaborative Cohort Study. J. Steroid Biochem. Mol. Biol. 2020, 198, 105612. [Google Scholar] [CrossRef]
- Grant, W.B.; Al Anouti, F.; Boucher, B.J.; Dursun, E.; Gezen-Ak, D.; Jude, E.B.; Karonova, T.; Pludowski, P. A Narrative Review of the Evidence for Variations in Serum 25-Hydroxyvitamin D Concentration Thresholds for Optimal Health. Nutrients 2022, 14, 639. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B. Vitamin D Acceptance Delayed by Big Pharma Following the Disinformation Playbook. Available online: http://orthomolecular.org/resources/omns/v14n22.shtml (accessed on 22 March 2022).
- Slominski, A.T.; Slominski, R.M.; Goepfert, P.A.; Kim, T.K.; Holick, M.F.; Jetten, A.M.; Raman, C. Reply to Jakovac and to Rocha et al.: Can vitamin D prevent or manage COVID-19 illness? Am. J. Physiol. Endocrinol. Metab. 2020, 319, E455–E457. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Controlling chronic diseases and acute Infections with vitamin D sufficiency. Nutrients 2023, 15, 3623. [Google Scholar] [CrossRef]
- Jodar, E.; Campusano, C.; de Jongh, T.; Holick, M. Calcifediol: A review of its pharmacological characteristics and clinical use in correcting vitamin D deficiency. Eur. J. Nutr. 2023, 62, 1579–1597. [Google Scholar] [CrossRef]
- Nogues, X.; Ovejero, D.; Pineda-Moncusí, M.; Bouillon, R.; Arenas, D.; Pascual, J.; Ribes, A.; Guerri-Fernandez, R.; Villar-Garcia, J.; Rial, A.; et al. Calcifediol treatment and COVID-19-related outcomes. J. Clin. Endocrinol. Metab. 2021, 106, e4017–e4027. [Google Scholar] [CrossRef]
- Stagi, S.; Rigante, D.; Lepri, G.; Matucci Cerinic, M.; Falcini, F. Severe vitamin D deficiency in patients with Kawasaki disease: A potential role in the risk to develop heart vascular abnormalities? Clin. Rheumatol. 2016, 35, 1865–1872. [Google Scholar] [CrossRef]
- Xu, Y.; Baylink, D.J.; Chen, C.S.; Reeves, M.E.; Xiao, J.; Lacy, C.; Lau, E.; Cao, H. The importance of vitamin D metabolism as a potential prophylactic, immunoregulatory and neuroprotective treatment for COVID-19. J. Transl. Med. 2020, 18, 322. [Google Scholar] [CrossRef]
- Perez-Castrillon, J.L.; Duenas-Laita, A.; Brandi, M.L.; Jodar, E.; Del Pino-Montes, J.; Quesada-Gomez, J.M.; Cereto Castro, F.; Gomez-Alonso, C.; Gallego Lopez, L.; Olmos Martinez, J.M.; et al. Calcifediol is superior to cholecalciferol in improving vitamin D status in postmenopausal women: A randomized trial. J. Bone Min. Res. 2021, 36, 1967–1978. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Maintaining optimum health requires longer-term stable vitamin D concentrations. Int. J. Regenr Med. 2020, 3, 1–5. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Achieving population vitamin D sufficiency will markedly reduce healthcare costs. EJBPS 2020, 7, 136–141. [Google Scholar]
- Lemen, R.A. Chrysotile asbestos as a cause of mesothelioma: Application of the Hill causation model. Int. J. Occup. Env. Health 2004, 10, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Hanwell, H.E.; Banwell, B. Assessment of evidence for a protective role of vitamin D in multiple sclerosis. Biochim. Biophys. Acta 2011, 1812, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Boucher, B.J. Are Hill’s criteria for causality satisfied for vitamin D and periodontal disease? Dermatoendocrinol 2010, 2, 30–36. [Google Scholar] [CrossRef]
- Ben-Eltriki, M.; Hopefl, R.; Wright, J.M.; Deb, S. Association between vitamin D status and risk of developing severe COVID-19 infection: A meta-analysis of observational studies. J. Am. Coll. Nutr. 2021, 41, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.O.; Pamukcu, E.; Yakar, B. The role of vitamin D deficiency on COVID-19: A systematic review and meta-analysis of observational studies. Epidemiol. Health 2021, 43, e2021074. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. More than 50 long-term effects of COVID-19: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 16144. [Google Scholar] [CrossRef] [PubMed]
- D’Ecclesiis, O.; Gavioli, C.; Martinoli, C.; Raimondi, S.; Chiocca, S.; Miccolo, C.; Bossi, P.; Cortinovis, D.; Chiaradonna, F.; Palorini, R.; et al. Vitamin D and SARS-CoV2 infection, severity and mortality: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0268396. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, B.; El Abd, A.; Ducharme, F.M. Effects of Vitamin D Supplementation on COVID-19 Related Outcomes: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 2134. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.; Dantas Damascena, A.; Galvao Azevedo, L.M.; de Almeida Oliveira, T.; da Mota Santana, J. Vitamin D deficiency aggravates COVID-19: Systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2022, 62, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Bilezikian, J.P.; Bikle, D.; Hewison, M.; Lazaretti-Castro, M.; Formenti, A.M.; Gupta, A.; Madhavan, M.V.; Nair, N.; Babalyan, V.; Hutchings, N.; et al. Mechanisums in Endocrinology: Vitamin D and COVID-19. Eur. J. Endocrinol. 2020, 183, R133–R147. [Google Scholar] [CrossRef]
- Hewison, M. Vitamin D and innate and adaptive immunity. Vitam. Horm. 2011, 86, 23–62. [Google Scholar] [CrossRef] [PubMed]
- Bishop, E.; Ismailova, A.; Dimeloe, S.K.; Hewison, M.; White, J.H. Vitamin D and immune regulation: Antibacterial, antiviral, anti-inflammatory. JBMR Plus 2020. [Google Scholar] [CrossRef] [PubMed]
- Shahini, E.; Pesce, F.; Argentiero, A.; Solimando, A.G. Can vitamin D status influence seroconversion to SARS-COV2 vaccines? Front. Immunol. 2022, 13, 1038316. [Google Scholar] [CrossRef] [PubMed]
- Werneke, U.; Gaughran, F.; Taylor, D.M. Vitamin D in the time of the coronavirus (COVID-19) pandemic - a clinical review from a public health and public mental health perspective. Ther. Adv. Psychopharmacol. 2021, 11, 20451253211027699. [Google Scholar] [CrossRef]
- Dancer, R.C.; Parekh, D.; Lax, S.; D’Souza, V.; Zheng, S.; Bassford, C.R.; Park, D.; Bartis, D.G.; Mahida, R.; Turner, A.M.; et al. Vitamin D deficiency contributes directly to the acute respiratory distress syndrome (ARDS). Thorax 2015, 70, 617–624. [Google Scholar] [CrossRef]
- Zdrenghea, M.T.; Makrinioti, H.; Bagacean, C.; Bush, A.; Johnston, S.L.; Stanciu, L.A. Vitamin D modulation of innate immune responses to respiratory viral infections. Rev. Med. Virol. 2017, 27, e1909. [Google Scholar] [CrossRef]
- Barrea, L.; Verde, L.; Grant, W.B.; Frias-Toral, E.; Sarno, G.; Vetrani, C.; Ceriani, F.; Garcia-Velasquez, E.; Contreras-Briceno, J.; Savastano, S.; et al. Vitamin D: A role also in long COVID-19? Nutrients 2022, 14, 1625. [Google Scholar] [CrossRef] [PubMed]
- Ben Mohamed, D.; Zouari, R.; Ketata, J.; Nabli, F.; Blel, S.; Ben Sassi, S. Myoclonus status revealing COVID 19 infection. Seizure 2023, 104, 12–14. [Google Scholar] [CrossRef]
- Xu, J.; Yang, J.; Chen, J.; Luo, Q.; Zhang, Q.; Zhang, H. Vitamin D alleviates lipopolysaccharide-induced acute lung injury via regulation of the renin-angiotensin system. Mol. Med. Rep. 2017, 16, 7432–7438. [Google Scholar] [CrossRef]
- McGregor, R.; Chauss, D.; Freiwald, T.; Yan, B.; Wang, L.; Nova-Lamperti, E.; Zhang, Z.; Teague, H.; West, E.E.; Bibby, J.; et al. An Autocrine Vitamin D-Driven Th1 Shutdown Program Can Be Exploited for COVID-19. Available online: https://www.biorxiv.org/content/10.1101/2020.07.18.210161v1 (accessed on 10 November 2024).
- Akbar, M.R.; Wibowo, A.; Pranata, R.; Setiabudiawan, B. Low Serum 25-hydroxyvitamin D (Vitamin D) Level Is Associated With Susceptibility to COVID-19, Severity, and Mortality: A Systematic Review and Meta-Analysis. Front. Nutr. 2021, 8, 660420. [Google Scholar] [CrossRef] [PubMed]
- Sirbe, C.; Rednic, S.; Grama, A.; Pop, T.L. An update on the effects of vitamin D on the immune system and autoimmune diseases. Int. J. Mol. Sci. 2022, 23, 9784. [Google Scholar] [CrossRef]
- Ghanaati, S.; Choukroun, J.; Volz, U.; Hueber, R.; Mourão, C.F.A.B.; Sader, R.; Kawase-Koga, Y.; Mazhari, R.; Amrein, K.; Maybohm, P.; et al. One Hundred Years after Vitamin D Discovery: Is There Clinical Evidence for Supplementation Doses? Int. J. Growth Factors Stem Cells Dent. 2020, 3, 3–11. [Google Scholar] [CrossRef]
- Gospodarska, E.; Ghosh Dastidar, R.; Carlberg, C. Intervention Approaches in Studying the Response to Vitamin D(3) Supplementation. Nutrients 2023, 15, 3382. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Prophylactic use of vitamin D to maintain a robust immune system against infections like SARS-CoV-2. Glob. J. Endocrinol. Metab. GJEM. 2023, 3, 1–9. [Google Scholar]
- Smaha, J.; Kuzma, M.; Brazdilova, K.; Nachtmann, S.; Jankovsky, M.; Pastirova, K.; Gazova, A.; Jackuliak, P.; Killinger, Z.; Kyselovic, J.; et al. Patients with COVID-19 pneumonia with 25(OH)D levels lower than 12 ng/ml are at increased risk of death. Int. J. Infect. Dis. 2022, 116, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.R. Fundamentals of clinical trial design. J. Exp. Stroke Transl. Med. 2010, 3, 19–27. [Google Scholar] [CrossRef]
- Heaney, R.P. Guidelines for optimizing design and analysis of clinical studies of nutrient effects. Nutr. Rev. 2014, 72, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Zurita-Cruz, J.; Fonseca-Tenorio, J.; Villasis-Keever, M.; Lopez-Alarcon, M.; Parra-Ortega, I.; Lopez-Martinez, B.; Miranda-Novales, G. Efficacy and safety of vitamin D supplementation in hospitalized COVID-19 pediatric patients: A randomized controlled trial. Front. Pediatr. 2022, 10, 943529. [Google Scholar] [CrossRef]
- Uwitonze, A.M.; Razzaque, M.S. Role of magnesium in vitamin D activation and function. J. Am. Osteopath. Assoc. 2018, 118, 181–189. [Google Scholar] [CrossRef]
- Azem, R.; Daou, R.; Bassil, E.; Anvari, E.M.; Taliercio, J.J.; Arrigain, S.; Schold, J.D.; Vachharajani, T.; Nally, J.; Na Khoul, G.N. Serum magnesium, mortality and disease progression in chronic kidney disease. BMC Nephrol. 2020, 21, 49. [Google Scholar] [CrossRef]
- Andrade, C. Research design: Cohort studies. Indian. J. Psychol. Med. 2022, 44, 189–191. [Google Scholar] [CrossRef]
- Grant, W.B. The Institute of Medicine did not find the vitamin D-cancer link because it ignored UV-B dose studies. Public Health Nutr. 2011, 14, 745–746. [Google Scholar] [CrossRef]
- Grant, W.B.; Boucher, B.J.; Pludowski, P.; Wimalawansa, S.J. The emerging evidence for non-skeletal health benefits of vitamin D supplementation in adults. Nat. Rev. Endocrinol. 2022, 18, 323. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Vitamin D: An essential component for skeletal health. Ann. N. Y Acad. Sci. 2011, 1240, E1–E12. [Google Scholar] [CrossRef]
- Sharma, M.; Kumar, M.; Dutta, D. Vitamin D Supplementation, Insulin Resistance, and Cardiovascular Risk Factors: Who are Likely to Benefit the Most? Indian. J. Endocrinol. Metab. 2019, 23, 650–652. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Kunutsor, S.; Franco, O.H.; Chowdhury, R. Vitamin D, type 2 diabetes and other metabolic outcomes: A systematic review and meta-analysis of prospective studies. Proc. Nutr. Soc. 2013, 72, 89–97. [Google Scholar] [CrossRef]
- Garland, C.F.; Gorham, E.D.; Mohr, S.B.; Garland, F.C. Vitamin D for cancer prevention: Global perspective. Ann. Epidemiol. 2009, 19, 468–483. [Google Scholar] [CrossRef]
- Leung, H.W.; Muo, C.H.; Liu, C.F.; Chan, A.L. Vitamin D3 Intake Dose and Common Cancer: A Population-Based Case Control Study in a Chinese Population. J. Cancer 2016, 7, 2028–2034. [Google Scholar] [CrossRef]
- Hollis, B.W.; Marshall, D.T.; Savage, S.J.; Garrett-Mayer, E.; Kindy, M.S.; Gattoni-Celli, S. Vitamin D3 supplementation, low-risk prostate cancer, and health disparities. J. Steroid Biochem. Mol. Biol. 2013, 136, 233–237. [Google Scholar] [CrossRef]
- Guven, M.; Gultekin, H. The effect of high-dose parenteral vitamin D(3) on COVID-19-related inhospital mortality in critical COVID-19 patients during intensive care unit admission: An observational cohort study. Eur. J. Clin. Nutr. 2021, 75, 1383–1388. [Google Scholar] [CrossRef]
- Cervero, M.; Lopez-Wolf, D.; Casado, G.; Novella-Mena, M.; Ryan-Murua, P.; Taboada-Martinez, M.L.; Rodriguez-Mora, S.; Vigon, L.; Coiras, M.; Torres, M. Beneficial effect of short-term supplementation of high dose of vitamin D(3) in hospitalized patients with COVID-19: A multicenter, single-blinded, prospective randomized pilot clinical trial. Front. Pharmacol. 2022, 13, 863587. [Google Scholar] [CrossRef]
- Fairfield, K.M.; Murray, K.A.; Anzalone, A.J.; Beasley, W.; Khodaverdi, M.; Hodder, S.L.; Harper, J.; Santangelo, S.; Rosen, C.J.; On Behalf Of The, N.C.C. Association of vitamin D prescribing and clinical outcomes in adults hospitalized with COVID-19. Nutrients 2022, 14, 3073. [Google Scholar] [CrossRef]
- Dayem Ullah, A.Z.M.; Sivapalan, L.; Kocher, H.M.; Chelala, C. COVID-19 in patients with hepatobiliary and pancreatic diseases: A single-centre cross-sectional study in East London. BMJ Open 2021, 11, e045077. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, A.; Bhansali, A.; Khare, N.; Suri, V.; Yaddanapudi, N.; Sachdeva, N.; Puri, G.D.; Malhotra, P. Short term, high-dose vitamin D supplementation for COVID-19 disease: A randomised, placebo-controlled, study (SHADE study). Postgrad. Med. J. 2022, 98, 87–90. [Google Scholar] [CrossRef]
- Brustad, N.; Yousef, S.; Stokholm, J.; Bonnelykke, K.; Bisgaard, H.; Chawes, B.L. Safety of high-dose vitamin D supplementation among children aged 0 to 6 years: A systematic review and meta-analysis. JAMA Netw. Open 2022, 5, e227410. [Google Scholar] [CrossRef] [PubMed]
- La Carrubba, A.; Veronese, N.; Di Bella, G.; Cusumano, C.; Di Prazza, A.; Ciriminna, S.; Ganci, A.; Naro, L.; Dominguez, L.J.; Barbagallo, M.; et al. Prognostic Value of Magnesium in COVID-19: Findings from the COMEPA Study. Nutrients 2023, 15, 830. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Boucher, B.J. Why secondary analyses in vitamin D clinical trials are important and how to improve vitamin D clinical trial outcome analyses-A momment on “extra-skeletal effects of vitamin D. Nutrients 2019, 11, 2182. [Google Scholar] [CrossRef]
- Grant, W.B.; Al Anouti, F.; Moukayed, M. Targeted 25-hydroxyvitamin D concentration measurements and vitamin D3 supplementation can have important patient and public health benefits. Eur. J. Clin. Nutr. 2020, 74, 366–376. [Google Scholar] [CrossRef]
- Fletcher, J.; Brown, M.; Hewison, M.; Swift, A.; Cooper, S.C. Prevalence of vitamin D deficiency and modifiable risk factors in patients with Crohn’s disease: A prospective observational study. J. Adv. Nurs. 2023, 79, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Castano, L.; Madariaga, L.; Grau, G.; Garcia-Castano, A. 25(OH)Vitamin D deficiency and calcifediol treatment in pediatrics. Nutrients 2022, 14, 1854. [Google Scholar] [CrossRef] [PubMed]
- Donati, S.; Palmini, G.; Aurilia, C.; Falsetti, I.; Miglietta, F.; Iantomasi, T.; Brandi, M.L. Rapid Nontranscriptional Effects of Calcifediol and Calcitriol. Nutrients 2022, 14, 1291. [Google Scholar] [CrossRef] [PubMed]
- FAES-Pharma. Calcifediol Raises 25(OH)D Levels from 18 ng/mL to over 55 ng/mL in 4 Hours; FAES-Pharma: Leioa, Spain, 2018. [Google Scholar]
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA 2020, 323, 1574–1581. [Google Scholar] [CrossRef]
- Alexander, J.; Tinkov, A.; Strand, T.A.; Alehagen, U.; Skalny, A.; Aaseth, J. Early nutritional interventions with zinc, selenium and vitamin D for raising anti-viral resistance against progressive COVID-19. Nutrients 2020, 12, 2358. [Google Scholar] [CrossRef] [PubMed]
- Caccialanza, R.; Laviano, A.; Lobascio, F.; Montagna, E.; Bruno, R.; Ludovisi, S.; Corsico, A.G.; Di Sabatino, A.; Belliato, M.; Calvi, M.; et al. Early nutritional supplementation in non-critically ill patients hospitalized for the 2019 novel coronavirus disease (COVID-19): Rationale and feasibility of a shared pragmatic protocol. Nutrition 2020, 74, 110835. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, M.; Vernocchi, V.; Martini, S.; Marino, F.; Allasino, B.; Balzola, M.A.; Burigana, F.; Dallari, A.; Pagano, C.S.F.; Palma, A.; et al. Early outpatient treatment of COVID-19: A retrospective analysis of 392 cases in Italy. J. Clin. Med. 2022, 11, 6138. [Google Scholar] [CrossRef]
- Holick, M.F. The vitamin D epidemic and its health consequences. J. Nutr. 2005, 135, 2739S–2748S. [Google Scholar] [CrossRef]
- Zhong, Z.; Zhao, L.; Zhao, Y.; Xia, S. High-dose vitamin D supplementation in patients with COVID-19: A meta-analysis of randomized controlled trials. Food Sci. Nutr. 2024, 12, 1808–1817. [Google Scholar] [CrossRef]
- Song, Z.; Shi, S.; Zhang, Y. Ivermectin for treatment of COVID-19: A systematic review and meta-analysis. Heliyon 2024, 10, e27647. [Google Scholar] [CrossRef]
- Wimalawansa, S.J.; Whittle, R. Vitamin D: A single initial dose is not bogus if followed by an appropriate maintenance intake. JBMR Plus 2022, 6, e10606. [Google Scholar] [CrossRef]
- World Council of Health. At Home Treatment Guide: Early COVID-19 Treatment Guidelines: A Practical Approach to Home-Based Care for Healthy Families. 2021. Available online: https://www.worldcouncilforhealth.org/early-covid-19-treatment-guide/ (accessed on 10 December 2024).
- Beltran-Garcia, J.; Osca-Verdegal, R.; Pallardo, F.V.; Ferreres, J.; Rodriguez, M.; Mulet, S.; Sanchis-Gomar, F.; Carbonell, N.; Garcia-Gimenez, J.L. Oxidative stress and Inflammation in COVID-19-associated sepsis: The potential role of anti-oxidant therapy in avoiding disease progression. Antioxidants 2020, 9, 936. [Google Scholar] [CrossRef] [PubMed]
- Ramezani-Jolfaie, N.; Eftekhar, E.; Dadinasab, M.; Hesarooeyeh, Z.G.; Pakdaman, P.; Razmpour, F.; Javedan, G.; Khayatian, M.; Azad, M.H.; Davoodian, P.; et al. The effect of vitamin D and magnesium supplementation on clinical symptoms and serum inflammatory and oxidative stress markers in patients with COVID-19: A structured summary of a study protocol for a randomized controlled trial. Trials 2023, 24, 87. [Google Scholar] [CrossRef] [PubMed]
- Luong, K.; Nguyen, L.T. Impact of vitamin D in the treatment of tuberculosis. Am. J. Med. Sci. 2011, 341, 493–498. [Google Scholar] [CrossRef]
- Nnoaham, K.E.; Clarke, A. Low serum vitamin D levels and tuberculosis: A systematic review and meta-analysis. Int. J. Epidemiol. 2008, 37, 113–119. [Google Scholar] [CrossRef]
- Selvaraj, P. Vitamin D, vitamin D receptor, and cathelicidin in the treatment of tuberculosis. Vitam. Horm. 2011, 86, 307–325. [Google Scholar] [CrossRef]
- Nielsen, N.M.; Junker, T.G.; Boelt, S.G.; Cohen, A.S.; Munger, K.L.; Stenager, E.; Ascherio, A.; Boding, L.; Hviid, A. Vitamin D status and severity of COVID-19. Sci. Rep. 2022, 12, 1–9. [Google Scholar] [CrossRef]
- Meltzer, D.O.; Best, T.J.; Zhang, H.; Vokes, T.; Arora, V.; Solway, J. Association of vitamin D status and other clinical characteristics with COVID-19 test results. JAMA Netw. Open 2020, 3, e2019722. [Google Scholar] [CrossRef] [PubMed]
- Chiodini, I.; Gatti, D.; Soranna, D.; Merlotti, D.; Mingiano, C.; Fassio, A.; Adami, G.; Falchetti, A.; Eller-Vainicher, C.; Rossini, M.; et al. Vitamin D Status and SARS-CoV-2 Infection and COVID-19 Clinical Outcomes. Front Public Health. 2021, 9, 736665. [Google Scholar] [CrossRef]
- Sobczak, M.; Pawliczak, R. Effect of vitamin D3 supplementation on severe COVID-19: A meta-analysis of randomized clinical trials. Nutrients 2024, 16, 1402. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhang, Y.; Liu, Z.; Pei, Y.; Xu, P.; Chong, W.; Hai, Y.; He, L.; He, Y.; Yu, J.; et al. Association between Vitamin D Supplementation and Cancer Mortality: A Systematic Review and Meta-Analysis. Cancers 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Huang, M.; Fan, D.; Hong, Y.; Zhao, M.; Ding, R.; Cheng, Y.; Duan, S. Association between vitamin D supplementation and cancer incidence and mortality: A trial sequential meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2022, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B. How strong is the evidence that solar ultraviolet B and vitamin D reduce the risk of cancer?: An examination using Hill’s criteria for causality. Derm.-Endocrinol. 2009, 1, 17–24. [Google Scholar] [CrossRef]
- Mohr, S.B.; Gorham, E.D.; Alcaraz, J.E.; Kane, C.I.; Macera, C.A.; Parsons, J.K.; Wingard, D.L.; Garland, C.F. Does the evidence for an inverse relationship between serum vitamin D status and breast cancer risk satisfy the Hill criteria? Derm.-Endocrinol. 2012, 4, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Weyland, P.G.; Grant, W.B.; Howie-Esquivel, J. Does sufficient evidence exist to support a causal association between vitamin D status and cardiovascular disease risk? An assessment using Hill’s criteria for causality. Nutrients 2014, 6, 3403–3430. [Google Scholar] [CrossRef]
- Fernandes de Souza, W.D.; Fonseca, D.M.D.; Sartori, A. COVID-19 and Multiple Sclerosis: A Complex Relationship Possibly Aggravated by Low Vitamin D Levels. Cells 2023, 12. [Google Scholar] [CrossRef]
- Akhtar, A.; Neupane, R.; Singh, A.; Khan, M. Radiological Association Between Multiple Sclerosis Lesions and Serum Vitamin D Levels. Cureus 2022, 14, e31824. [Google Scholar] [CrossRef]
- Sami, A.; Abrahamsen, B. The latest evidence from vitamin D intervention trials for skeletal and non-skeletal outcomes. Calcif. Tissue Int. 2020, 106, 88–93. [Google Scholar] [CrossRef]
- White, J.H. Regulation of intracrine production of 1,25-dihydroxyvitamin D and its role in innate immune defense against infection. Arch. Biochem. Biophys. 2012, 523, 58–63. [Google Scholar] [CrossRef]
- Hewison, M. Vitamin D and immune function: Autocrine, paracrine or endocrine? Scand. J. Clin. Lab. Investig. Suppl. 2012, 243, 92–102. [Google Scholar] [CrossRef]
- Liu, K.; Meng, H.; Hou, J. Characterization of the autocrine/paracrine function of vitamin D in human gingival fibroblasts and periodontal ligament cells. PLoS ONE 2012, 7, e39878. [Google Scholar] [CrossRef] [PubMed]
- Morris, H.A.; Anderson, P.H. Autocrine and paracrine actions of vitamin d. Clin. Biochem. Rev. 2010, 31, 129–138. [Google Scholar]
- Jain, A.; Chaurasia, R.; Sengar, N.S.; Singh, M.; Mahor, S.; Narain, S. Analysis of vitamin D level among asymptomatic and critically ill COVID-19 patients and its correlation with inflammatory markers. Sci. Rep. 2020, 10, 20191. [Google Scholar] [CrossRef] [PubMed]
- Annweiler, G.; Corvaisier, M.; Gautier, J.; Dubee, V.; Legrand, E.; Sacco, G.; Annweiler, C. Vitamin D supplementation associated to better survival in hospitalized frail elderly COVID-19 patients: The GERIA-COVID Quasi-Experimental Study. Nutrients 2020, 12, 3377. [Google Scholar] [CrossRef]
- Dilokpattanamongkol, P.; Yan, C.; Jayanama, K.; Ngamjanyaporn, P.; Sungkanuparph, S.; Rotjanapan, P. Impact of vitamin D supplementation on the clinical outcomes of COVID-19 pneumonia patients: A single-center randomized controlled trial. BMC Complement. Med. Ther. 2024, 24, 97. [Google Scholar] [CrossRef]
- Rachman, A.; Rahmaniyah, R.; Khomeini, A.; Iriani, A. The association between vitamin D deficiency and the clinical outcomes of hospitalized COVID-19 patients. F1000Res 2023, 12, 394. [Google Scholar] [CrossRef]
- Wan Nik, W.; Zulkeflee, H.A.; Ab Rahim, S.N.; Tuan Ismail, T.S. Association of vitamin D and magnesium with insulin sensitivity and their influence on glycemic control. World J. Diabetes 2023, 14, 26–34. [Google Scholar] [CrossRef]
- Wolf, F.I.; Trapani, V. Magnesium and vitamin D in long COVID syndrome; do they help? Magnes. Res. 2024, 36. [Google Scholar] [CrossRef]
- Dai, Q.; Zhu, X.; Manson, J.E.; Song, Y.; Li, X.; Franke, A.A.; Costello, R.B.; Rosanoff, A.; Nian, H.; Fan, L.; et al. Magnesium status and supplementation influence vitamin D status and metabolism: Results from a randomized trial. Am. J. Clin. Nutr. 2018, 108, 1249–1258. [Google Scholar] [CrossRef]
- Wimalawansa, S. Part 4: How does one determine the right amount and type of vitamin D to take? Available online: https://www.grassrootshealth.net/blog/one-determine-right-amount-type-vitamin-d-take/ (accessed on 10 December 2024).
- Lopez-Caleya, J.F.; Ortega-Valin, L.; Fernandez-Villa, T.; Delgado-Rodriguez, M.; Martin-Sanchez, V.; Molina, A.J. The role of calcium and vitamin D dietary intake on risk of colorectal cancer: Systematic review and meta-analysis of case-control studies. Cancer Causes Control 2022, 33, 167–182. [Google Scholar] [CrossRef]
- Shah, K.; Varna, V.P.; Sharma, U.; Mavalankar, D. Does vitamin D supplementation reduce COVID-19 severity?: A systematic review. QJM 2022, 115, 665–672. [Google Scholar] [CrossRef]
- Doll, R.; Peto, R. Mortality in relation to smoking: 20 years’ observations on male British doctors. Br. Med. J. 1976, 2, 1525–1536. [Google Scholar] [CrossRef]
- Doll, R.; Hill, A.B. Mortality in relation to smoking: Ten years’ observations of british doctors. Br. Med. J. 1964, 1, 1399–1410. [Google Scholar] [CrossRef]
- Buechner, J.S.; Constantine, H.; Gjelsvik, A. John Snow and the Broad Street pump: 150 years of epidemiology. Med. Health R. I 2004, 87, 314–315. [Google Scholar]
- Tulchinski, T. John Snow, cholera, the Broad Street pump; Waterborne diseases then and now. Case Stud. Public Health 2018, 30, 77–99. [Google Scholar] [CrossRef]
- Underwood, B.R.; White, V.L.; Baker, T.; Law, M.; Moore-Gillon, J.C. Contact tracing and population screening for tuberculosis—Who should be assessed? J. Public Health Med. 2003, 25, 59–61. [Google Scholar] [CrossRef]
- Wortham, J.M.; Li, R.; Althomsons, S.P.; Kammerer, S.; Haddad, M.B.; Powell, K.M. Tuberculosis genotype clusters and transmission in the U.S., 2009-2018. Am. J. Prev. Med. 2021, 61, 201–208. [Google Scholar] [CrossRef]
- Olivier, J.; Boufous, S.; Grzebieta, R. The impact of bicycle helmet legislation on cycling fatalities in Australia. Int. J. Epidemiol. 2019, 48, 1197–1203. [Google Scholar] [CrossRef]
- Lee, L.K.; Flaherty, M.R.; Blanchard, A.M.; Agarwal, M.; Council on Injury, Violence, and Poison Prevention. Helmet use in preventing head injuries in bicycling, snow sports, and other recreational activities and sports. Pediatrics 2022, 150, e2022058877. [Google Scholar] [CrossRef]
- Fouda Mbarga, N.; Abubakari, A.R.; Aminde, L.N.; Morgan, A.R. Seatbelt use and risk of major injuries sustained by vehicle occupants during motor-vehicle crashes: A systematic review and meta-analysis of cohort studies. BMC Public Health 2018, 18, 1413. [Google Scholar] [CrossRef]
- Sarwahi, V.; Atlas, A.M.; Galina, J.; Satin, A.; Dowling, T.J., 3rd; Hasan, S.; Amaral, T.D.; Lo, Y.; Christopherson, N.; Prince, J. Seatbelts save Lives, and spines, in motor vehicle accidents: A review of the national trauma data bank in the pediatric population. Spine 2021, 46, 1637–1644. [Google Scholar] [CrossRef]
- Wimalawansa, S.J.; Dissanayake, C.B. Factors Affecting the Environmentally Induced, Chronic Kidney Disease of Unknown Aetiology in Dry Zonal Regions in Tropical Countries—Novel Findings. Environments 2019, 7, 2. [Google Scholar] [CrossRef]
- Wimalawansa, S.J.; Dissanayake, C.B. Nanocrystal-induced chronic tubular-nephropathy in tropical countries: Diagnosis, mitigation, and eradication. Eur. J. Med. Res. 2023, 28, 221. [Google Scholar] [CrossRef]
- Jukes, T.H. The prevention and conquest of scurvy, beri-beri, and pellagra. Prev. Med. 1989, 18, 877–883. [Google Scholar] [CrossRef]
- Tanaka, K.; Ao, M.; Kuwabara, A. Insufficiency of B vitamins with its possible clinical implications. J. Clin. Biochem. Nutr. 2020, 67, 19–25. [Google Scholar] [CrossRef]
- Wald, N.J. Folic acid and neural tube defects: Discovery, debate and the need for policy change. J. Med. Screen. 2022, 29, 138–146. [Google Scholar] [CrossRef]
- Holick, M.F. Resurrection of vitamin D deficiency and rickets. J. Clin. Investig. 2006, 116, 2062–2072. [Google Scholar] [CrossRef]
- Pittas, A.G.; Dawson-Hughes, B.; Li, T.; Van Dam, R.M.; Willett, W.C.; Manson, J.E.; Hu, F.B. Vitamin D and calcium intake in relation to type 2 diabetes in women. Diabetes Care 2006, 29, 650–656. [Google Scholar] [CrossRef]
- Euser, A.M.; Zoccali, C.; Jager, K.J.; Dekker, F.W. Cohort studies: Prospective versus retrospective. Nephron Clin. Pract. 2009, 113, c214–c217. [Google Scholar] [CrossRef]
- Pittas, A.G.; Dawson-Hughes, B.; Sheehan, P.; Ware, J.H.; Knowler, W.C.; Aroda, V.R.; Brodsky, I.; Ceglia, L.; Chadha, C.; Chatterjee, R.; et al. Vitamin D supplementation and prevention of type 2 diabetes. N. Engl. J. Med. 2019, 381, 520–530. [Google Scholar] [CrossRef]
- Virtanen, J.K.; Nurmi, T.; Aro, A.; Bertone-Johnson, E.R.; Hypponen, E.; Kroger, H.; Lamberg-Allardt, C.; Manson, J.E.; Mursu, J.; Mantyselka, P.; et al. Vitamin D supplementation and prevention of cardiovascular disease and cancer in the Finnish vitamin D trial: A randomized controlled trial. Am. J. Clin. Nutr. 2022, 115, 1300–1310. [Google Scholar] [CrossRef]
- Scragg, R.; Khaw, K.T.; Toop, L.; Sluyter, J.; Lawes, C.M.M.; Waayer, D.; Giovannucci, E.; Camargo, C.A., Jr. Monthly high-dose vitamin D supplementation and cancer risk: A post hoc analysis of the vitamin D assessment randomized clinical trial. JAMA Oncol. 2018, 4, e182178. [Google Scholar] [CrossRef]
- Bischoff-Ferrari, H.A.; Vellas, B.; Rizzoli, R.; Kressig, R.W.; da Silva, J.A.P.; Blauth, M.; Felson, D.T.; McCloskey, E.V.; Watzl, B.; Hofbauer, L.C.; et al. Effect of vitamin D supplementation, omega-3 fatty acid supplementation, or a strength-training exercise program on clinical outcomes in older adults: The DO-HEALTH randomized clinical trial. JAMA 2020, 324, 1855–1868. [Google Scholar] [CrossRef]
- Klebanoff, M.A.; Snowden, J.M. Historical (retrospective) cohort studies and other epidemiologic study designs in perinatal research. Am. J. Obs. Gynecol. 2018, 219, 447–450. [Google Scholar] [CrossRef]
- Annweiler, C.; Hanotte, B.; Grandin de l’Eprevier, C.; Sabatier, J.M.; Lafaie, L.; Celarier, T. Vitamin D and survival in COVID-19 patients: A quasi-experimental study. J. Steroid Biochem. Mol. Biol. 2020, 204, 105771. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, D.O.; Best, T.J.; Zhang, H.; Vokes, T.; Arora, V.; Solway, J. Association of vitamin D deficiency and treatment with COVID-19 incidence. medRxiv 2020, 13, 2020.05.08.20095893. [Google Scholar] [CrossRef]
- Barrett, R.; Youssef, M.; Shah, I.; Ioana, J.; Lawati, A.A.; Bukhari, A.; Hegarty, S.; Cormican, L.J.; Judge, E.; Burke, C.M.; et al. Vitamin D status and mortality from SARS CoV-2: A prospective study of unvaccinated caucasian adults. Nutrients 2022, 14, 3252. [Google Scholar] [CrossRef]
- Smolders, J.; Torkildsen, O.; Camu, W.; Holmoy, T. An update on vitamin D and disease activity in multiple sclerosis. CNS Drugs 2019, 33, 1187–1199. [Google Scholar] [CrossRef]
- Pludowski, P.; Marcinowska-Suchowierska, E.; Togizbayev, G.; Belaya, Z.; Grant, W.B.; Pilz, S.; Holick, M.F. Daily and Weekly “High Doses” of Cholecalciferol for the Prevention and Treatment of Vitamin D Deficiency for Obese or Multi-Morbidity and Multi-Treatment Patients Requiring Multi-Drugs-A Narrative Review. Nutrients 2024, 16, 2541. [Google Scholar] [CrossRef]

| PICOS Criteria | Conditions | |
|---|---|---|
| 1 | Participants | Adults aged 18 to 75 (males and females) infected with SARS-CoV-2; clinical trials. Including RCTs using vitamin D3 (cholecalciferol) and/or calcifediol (25(OH)D) as an intervention for those with SARS-CoV-2 infection. |
| 2 | Intervention | Observational and retrospective clinical studies or interventions and RCTs using vitamin D or calcifediol—focusing on vulnerability to infection and disease severity (hospitalization intensive care unit (ICU) admission) and deaths. |
| 3 | Comparison/control | Observational, community-based/ecological, RCTs, and meta-analyses were investigated with and without providing vitamin D or calcifediol, including outcomes from control groups. |
| 4 | Outcome elements | Focused on hard endpoints—morbidity, complications, comorbidities, hospitalization and ICU admissions, death, and all-cause mortality. |
| 5 | Study designphilosophies | RCTs, non-randomized controlled clinical trials, non-randomized non-controlled trials, and prospective and observational studies, including ecological studies that have used vitamin D or calcifediol as an intervention prior to or after the diagnosis of SARS-CoV-2 infection. |
| Criteria | Evidence | Evaluation and Explanation |
| Strength of the association | Based on RCTs, meta-analysis, and cohort studies, hypovitaminosis D is associated with increased vulnerability to SARS-CoV-2 infection. The lower the serum 25(OH)D concentrations, the higher the severity and rate of deaths. | The stronger an association, the more likely it contributes to disease causality. While COVID-19 stems from a single pathogen, susceptibility increases with factors like obesity, diabetes, dark skin, overcrowding, and air pollution—factors known to reduce vitamin D levels. Adjusting for these causation analyses is complex due to their multifactorial nature. Despite the robust inverse associations of COVID-19 risks with pre-pandemic and pre-infection vitamin D status, these data were overlooked by regulators and health authorities [60,116,117,118,169]. In COVID-19, when vitamin D was used prophylactically [170], early in the treatment [129,131,132,144,145,146], or when patients treated with calcifediol [141,168,171], most RCTs reported greater efficacy with a large effect size associated with significant p-values. |
| Consistency | Properly conducted observational evidence from cross-sectional and longitudinal studies and RCTs confirmed that hypovitaminosis D increased the risks and complications from SARS-CoV-2. Vitamin D status is a biological determinant of immunity. | The available evidence from the pre-COVID era and prospective and retrospective clinical studies of vitamin D status concerning SARS-CoV-2 risks in different situations in multiple ethnic groups provides similar associations and outcomes in different locations. It has continued to do so over time. Inverse vitamin D status–SARS-CoV-2 relationships have been found repeatedly in multiple circumstances (community, outpatients, and in-hospital) [131,141,168,170,171] by many research groups in many different countries [129,169], which provides additional evidence for the consistency of the association [129,132,144,145,146], and also, in the pre-COVID [120,133,134,169,172,173] and post-COVID era [60,65,71,72,73,74,129,132,142,143,144,145,147]. |
| Specificity | Hypovitaminosis D is prevalent among vulnerable populations, such as in nursing homes, disability centers, and dark-skinned people in temperate climates, leading to high death rates in these groups. | Does the experimental evidence point to a specific agent, location, or disease for the outcome? Differential exposure may give rise to a single result in certain situations [169,174], such as CKD arising from diabetes, hypertension, or exposure to toxins. However, this is not relevant to a single-cause disease like SARS-CoV-2. As discussed above, several factors increase susceptibility to viral illness. Aging and comorbidities increase the prevalence of hypovitaminosis D, which is the critical factor increasing COVID-19 risks or susceptibility to developing complications and deaths across all the groups studied. Chronic diseases like CVD and CKD often have multiple causes, including multigene factors, as seen in obesity and diabetes, leading to varied clinical outcomes. These diseases may lack one-to-one causality. In contrast, infections like COVID-19 primarily result from a single cause—the SARS-CoV-2 virus [62,129,131,144,145,146,170]. |
| Temporality | Hypovitaminosis D precedes the onset of SARS-CoV-2 infections in the studied cohort. | Temporality is an essential criterion for establishing a causal association between exposure and outcome [29,73,127,142,175,176,177,178,179,180,181,182]—i.e., the exposure (SARS-CoV-2) must precede the outcome (COVID-19) [62,131,169,170]. Evidence suggests that clinical outcomes often remain unaffected by the intake of a single nutrient, like vitamin D, or the evaluation of hard outcomes. Chronic conditions such as cancer, CKD, or chemotherapy-induced appetite loss can independently influence outcomes. The overall nutrient status changes are more likely to affect these processes than the “experimental” nutrient, potentially worsening outcomes. Distinguishing cause–effect relationships from reverse causation is possible in some cases. Unlike severe bacterial pneumonia and septicemia, any slight reduction in serum 25(OH)D levels before COVID-19 hospitalization is clinically insignificant [59,74,144,145,146,147], not affecting Hill’s criteria. Pre-infection serum 25(OH)D levels are preferable [60,74,116,117,118]; studies recording vitamin D status pre-pandemic or pre-illness are the most relevant [74,120]. |
| Biological gradient | Increased 25(OH)D concentrations are linked to better clinical outcomes. As a threshold nutrient, vitamin D intake does not have a linear relationship with serum 25(OH)D levels, making it unreliable to estimate serum levels based on oral dose. Effective serum 25(OH)D levels are 40–80 ng/mL without adverse effects. | This requires a demonstration of the dose–response association—curve: the greater the severity of the causal factor (the lower the serum 25(OH)D concentrations), the higher the risk of adverse health effects and outcomes (hospitalizations, complications, and death). Similarly, longer exposure and/or greater accumulation of a toxic agent (e.g., the SARS-CoV-2 viral load) increase the harmful effect. Moreover, when a condition is in its early stages, before irreversible structural damage occurs, eliminating the exposure should reduce adverse outcomes [62,131,141,168,170,171]. While the dose–response relationship of orally supplemented vitamin D to serum 25(OH)D concentrations achieved is not linear, a robust inverse relationship exists between them—i.e., serum 25(OH)D concentrations are higher with higher oral intakes of vitamin D, which protect from infections [169,183,184]. The indicated therapeutic levels of above 50 ng/mL also prevent symptomatic disease, complications, and deaths from SARS-CoV-2 [59,74,129,132,144,145,146,147]. The dose–response relationship between oral vitamin D intake and serum levels is complex, with the response influenced more by the degree of deficiency and body weight (including fat and muscle mass) than by the administered dose. Therefore, clinical outcomes should be correlated with the achieved serum levels rather than the oral dose. |
| Plausibility (mechanisms) | Vitamin D participates in the biology and physiology of the immune system. Thus, it is unsurprising that vitamin D sufficiency leads to a robust immune system and infection protection. | Probability or likelihood assumptions rely on prior beliefs, reports, or expectations rather than logic or data [185]. Developing plausible explanations is easier than empirically evaluating them [120,169]. For known mechanisms, such as cathelicidin and defensins in infections (which could also serve as biomarkers of severity or responses), their concentrations increase with increasing serum 25(OH)D concentration D, which enhances both innate and acquired immunity, e.g., preventing cytokine storms and reducing the risk of acute respiratory distress syndrome [60,65,71,72,73,74,76,129,142,143,147,186,187]. A multitude of published data related to vitamin D confirmed that low vitamin D status [i.e., circulating 25(OH)D cocentrations] pre-infection or at the time of hospitalization increases the risk of infection [60,74,116,117,118,134,183,185,188,189], similar to contracting COVID-19 as well [129,144,145,146,186,187]. Post-COVID syndrome, a chronic process, is initiated mainly in those with severe hypovitaminosis D [190], followed by the dissemination of infection into the central nervous system [191]. This prolonged infectious process continues, which consumes 25(OH)D, keeping it even lower (this is compatible with a reverse causality but is a separate entity). |
| Coherence (being logical and consistent) | A clear relationship between the two variables. Robust evidence that serum 25(OH)D levels are a key biological determinant that increases the vulnerability to viral infections and deaths, especially SARS-CoV-2. | Coherence and biological plausibility share typical constraints [76,187]. When evaluating an association, cause-and-effect interpretations must align with known facts of the disease’s natural history and biology [74,120,131,169,185]. This requires examining exposure patterns and biological effects of the observed disease patterns and outcomes. Trials, sequential analyses, and meta-analyses show that proper vitamin D supplementation during illness significantly reduces risks, including hospitalization [132] and ICU admissions [129,144,145,146]. In addition, in vitro and ex vivo data from Chausse et al. [104] and Xu et al. [192], pulmonary lymphocytes from patients with COVID-19 [193], and animal studies further support the role of vitamin D in activating T-cell immunity by intracellular calcitriol (i.e., worse pulmonary inflammatory in response to the intratracheal challenges of lipopolysaccharide) in vitamin D deficiency. |
| Experiments | Vitamin D supplementation reduces symptomatic disease incidence, complications, and mortality. | Empirical data: Examined whether preventive actions based on a demonstrated “cause-and-effect” association would modify the expected health outcomes (Koch’s postulates). Would the experimental data strongly support causal relationships with a larger effect size? The overall answer is yes, regarding vitamin D [60,65,71,72,73,74,129,142,143,147,186]. Unlike ecological (observational/epidemiological) studies, well-designed experiments (laboratory or clinical trials/RCTs) control variables and confounders or modify exposure. The data’s value depends on the study design and conduct. Multiple meta-analyses and trial sequential analyses confirm this relationship [73,129,142,177,178,179,180,181,182,194]. |
| Trial Authors/Year | Faulty Study Design (Example) | Reference |
|---|---|---|
| Using a one-time, high dose of vitamin D | One-dose, oral administration of vitamin D, “late” in the disease | |
| Murai et al., 2021 | A single large bolus dose of 200,000 IU (oral) vitamin D in moderately ill hospitalized patients. | [34] |
| Guven et al., 2021 | A single 300,000 IU bolus dose (IM) of vitamin D in critically ill late-stage patients in the ICU. | [214] |
| Juan et al., 2022 | A single 140,000 IU bolus dose (oral) of vitamin D in males, age > 65, critical patients in the ICU. | [35] |
| Zangeneh et al., 2022 | Severely ill, late-stage COVID-19 patients (ICU; n = 193) with a single bolus of 100,000 IU of D3 showed no benefit from vitamin D. | [36] |
| Cannata-Andia et al., 2022 | A single dose of 100,000 IU D3 administered to severely ill, late-stage COVID-19 patients (n = 274) failed to improve progress or ICU admissions (COVID-VIT-D). | [37] |
| Mariano et al., 2022 | A single oral dose of 500,000 IU was compared to a placebo (n = 115) in patients with mild to moderate illness. They reported no difference in mortality and progression of the disease. | [39] |
| Cervero et al., 2022 | Compared to 10,000 vs. 2000 IU: the higher dose was marginally better. | [215] |
| Fairfield et al., 2022 | Vitamin D treatment was associated with greater odds of extended hospitalization and mechanical ventilation—the retrospective, unbalanced study used small doses of OTC vitamin D (unquantified). Besides, participants in the vitamin-D-treated group were older and had more comorbidities and higher BMI. | [216] |
| Ullah et al., 2021 | A cross-sectional uncontrolled study showed no benefit. No improvement in mortality. | [217] |
| Al Sulaiman et al., 2023 | Moderate to severely ill patients (n = 177) had unknown amounts of vitamin D compared to unmatched participants (random: n = 288) who did not receive vitamin D. No information was provided on the dose of vitamin D, and the study was not standardized. There was no difference in ICU admissions, ventilation, or mortality. | [40] |
| Comparator Trials: | Similar study designs, but vitamin D was administered “early” in the disease. | |
| Annweilier et al., 2022 | Compared 400,000 vs. 50,000 IU single dose administered early: significant improvement in mortality. | [38] |
| Zhong et al., 2023 | Meta-analysis: single high doses (100,000 IU), analysis of five clinical trials (Murai; Cerero; Mariani; Rasogi; Annweilwer). | [232] |
| Rastgoli et al., 2022 | Early therapy with 60,000 IU daily for 7 days that maintained serum 25(OH)D above 50 ng/mL for a few weeks showed positive outcomes in patients with mild to moderate illness. | [218] |
| Elements That Needed Root-Cause Identification | The Method Used to Identify the Root Cause | Reference |
|---|---|---|
| Tobacco smoking and lung cancer | The causality that smoking causes lung cancer was established through epidemiological and observational studies. | [267,268] |
| Identification of the source of cholera by Dr. John Snow | Dr. Snow used basic ecological approaches to identify the source of cholera outbreaks | [269,270] |
| Tuberculosis—identifications of clusters helping control the disease | Contact tracing has been pivotal in understanding and controlling the disease and identifying index cases. | [271,272] |
| Use of helmets to prevent head injuries | Demonstrated effectiveness in reducing head injuries and fatalities among cyclists and motorcyclists. | [273,274] |
| Seat-belt use in vehicles, saving lives | Proven to reduce significant injuries and deaths through observational studies. | [275,276] |
| Chronic kidney disease of unknown etiology (CKDu, now called CKD–crystal tubular nephropathy; CKD-CTN) | The cause was identified through field observations, followed by laboratory water testing for common ions and electron microscopic studies. | [236,277,278] |
| Observations revealed that vitamin B, C, D, B12, and folic acid deficiencies caused specific nutritional disorders like scurvy and beriberi | Careful observations and documentation led to the identification of several specific nutritional disorders. For example, scurvy due to vitamin C deficiency among submarine staff led to identifying root causes such as deficiencies in vitamin C. | [279,280,281,282] |
| Criteria | Supporting Statistical Correlations and Clinical Outcomes |
|---|---|
| Consistency | Multiple studies across different populations and locations consistently show an inverse association between vitamin D levels and the risk and severity of SARS-CoV-2 infections. |
| Strength of association | Strong statistical associations are observed, with significant differences in infection rates and clinical outcomes between vitamin-D-deficient and -sufficient individuals. |
| Temporality | Evidence indicates that low vitamin D levels precede the onset of infection, establishing a temporal relationship necessary for causality. |
| Biological gradient | There is a precise dose–response relationship where lower levels of vitamin D correlate with higher risks and severity of infections, supporting the causality. |
| Plausibility | Biological mechanisms explain how vitamin D modulates immune responses, reducing the risk of infection and severity through effects on immune cell function and inflammation control. |
| Coherence | The association fits well with the current knowledge of vitamin D’s role in immune function, supporting a coherent narrative that aligns with known biological processes. |
| Experimental evidence | Intervention studies show that correcting vitamin D deficiency can improve clinical outcomes in viral infections, including SARS-CoV-2. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wimalawansa, S.J. Vitamin D Deficiency Meets Hill’s Criteria for Causation in SARS-CoV-2 Susceptibility, Complications, and Mortality: A Systematic Review. Nutrients 2025, 17, 599. https://doi.org/10.3390/nu17030599
Wimalawansa SJ. Vitamin D Deficiency Meets Hill’s Criteria for Causation in SARS-CoV-2 Susceptibility, Complications, and Mortality: A Systematic Review. Nutrients. 2025; 17(3):599. https://doi.org/10.3390/nu17030599
Chicago/Turabian StyleWimalawansa, Sunil J. 2025. "Vitamin D Deficiency Meets Hill’s Criteria for Causation in SARS-CoV-2 Susceptibility, Complications, and Mortality: A Systematic Review" Nutrients 17, no. 3: 599. https://doi.org/10.3390/nu17030599
APA StyleWimalawansa, S. J. (2025). Vitamin D Deficiency Meets Hill’s Criteria for Causation in SARS-CoV-2 Susceptibility, Complications, and Mortality: A Systematic Review. Nutrients, 17(3), 599. https://doi.org/10.3390/nu17030599






