Different Chemical Forms of Thiamine, Riboflavin, and Folate in Human Milk as a Function of Lactation Stages—A Cohort Study on Breastfeeding Women from Beijing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Study Population
2.2. Sample Collection
2.3. Information Collection
2.4. Materials
2.5. Analysis Procedure
2.6. Statistical Analyses
3. Results
3.1. Characteristics of the Study Population and Human Milk Collection
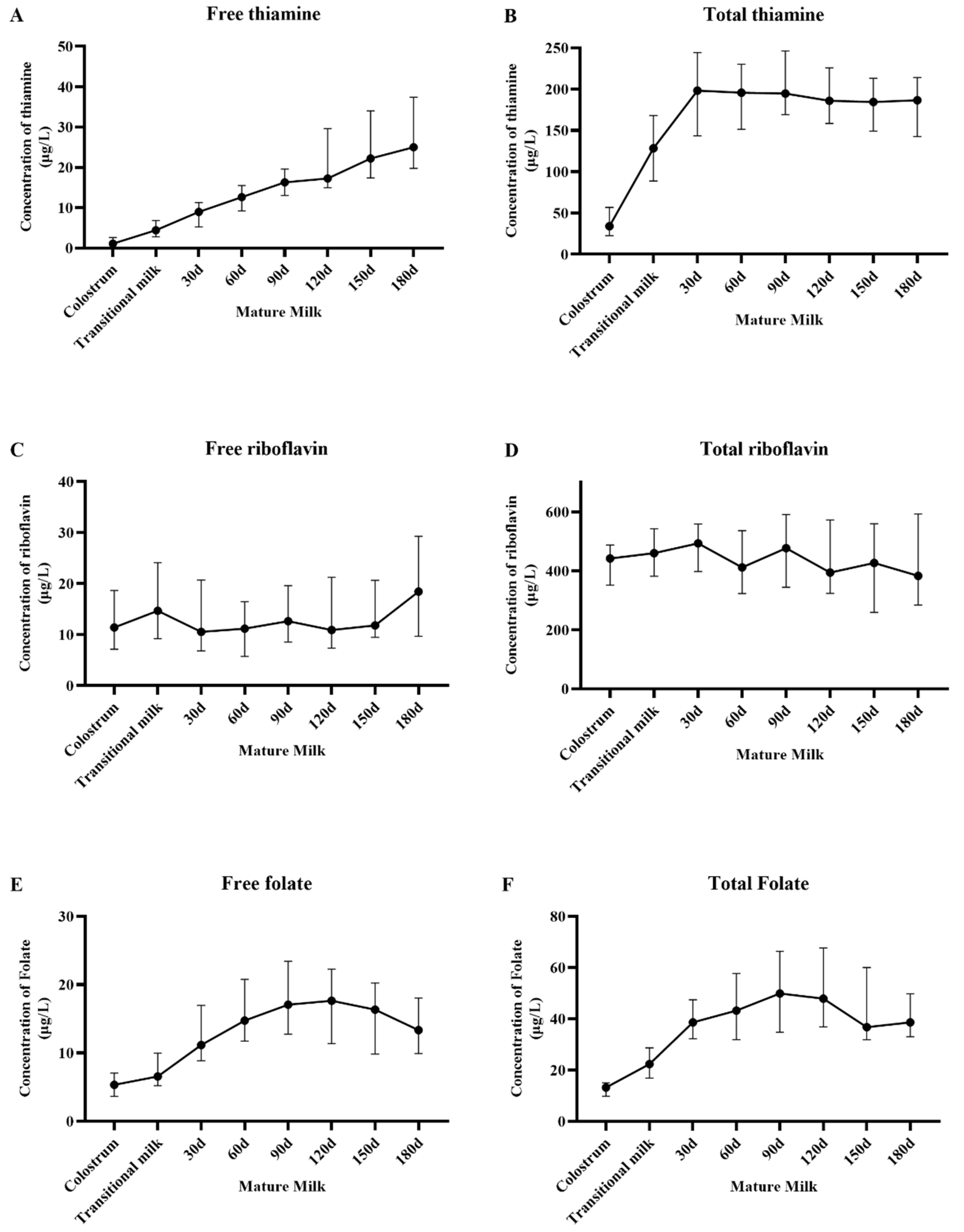
3.2. Vitamin Concentration and Change Trend in HM Milk
4. Discussion
4.1. Thiamine
4.2. Riboflavin
4.3. Folate
4.4. Reasons for Using Cohort Study to Investigate the HM
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dunlap, B.J. A joint WHO/UNICEF statement. Protecting, Promoting and Supporting Breastfeeding: The Special Role of the Maternity Services. Int. J. Gynaecol. Obstet. 1990, 31 (Suppl. S1), 171–183. [Google Scholar]
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef]
- Rindi, G. Metabolism of thiamin and its phosphoric esters in different regions of the nervous system: A new approach. Acta Vitaminol. Enzymol. 1982, 4, 59–68. [Google Scholar] [PubMed]
- Powers, H.J. Riboflavin (vitamin B-2) and health. Am. J. Clin. Nutr. 2003, 77, 1352–1360. [Google Scholar] [CrossRef] [PubMed]
- Redeuil, K.; Lévêques, A.; Oberson, J.M.; Bénet, S.; Tissot, E.; Longet, K.; de Castro, A.; Romagny, C.; Beauport, L.; Fumeaux, C.J.F.; et al. Vitamins and carotenoids in human milk delivering preterm and term infants: Implications for preterm nutrient requirements and human milk fortification strategies. Clin. Nutr. 2021, 40, 222–228. [Google Scholar] [CrossRef]
- Ortega, R.M.; Martínez, R.M.; Andrés, P.; Marín-Arias, L.; López-Sobaler, A.M. Thiamin status during the third trimester of pregnancy and its influence on thiamin concentrations in transition and mature breast milk. Br. J. Nutr. 2004, 92, 129–135. [Google Scholar] [CrossRef]
- Stuetz, W.; Carrara, V.I.; Mcgready, R.; Lee, S.J.; Biesalski, H.K.; Nosten, F.H. Thiamine diphosphate in whole blood, thiamine and thiamine monophosphate in breast milk in a refugee population. PLoS ONE 2012, 7, e36280. [Google Scholar] [CrossRef]
- Ren, X.; Yang, Z.; Shao, B.; Yin, S.A.; Yang, X. B-vitamin levels in human milk among different lactation stages and areas in China. PLoS ONE 2015, 10, e0133285. [Google Scholar] [CrossRef]
- Hampel, D.; Shahab-Ferdows, S.; Adair, L.S.; Bentley, M.E.; Flax, V.L.; Jamieson, D.J.; Ellington, S.R.; Tegha, G.; Chasela, C.S.; Kamwendo, D.; et al. Thiamin and Riboflavin in Human Milk: Effects of Lipid-Based Nutrient Supplementation and Stage of Lactation on Vitamer Secretion and Contributions to Total Vitamin Content. PLoS ONE 2016, 11, e0149479. [Google Scholar] [CrossRef]
- Su, Y.; Mao, Y.; Tian, F.; Cai, X.; Chen, R.; Li, N.; Qian, C.; Li, X.; Zhao, Y.; Wang, Y. Profile of Folate in Breast Milk from Chinese Women over 1–400 Days Postpartum. Nutrients 2022, 14, 2962. [Google Scholar] [CrossRef] [PubMed]
- Houghton, L.A.; Sherwood, K.L.; Pawlosky, R.; Ito, S.; O’Connor, D.L. [6S]-5-Methyltetrahydrofolate is at least as effective as folic acid in preventing a decline in blood folate concentrations during lactation. Am. J. Clin. Nutr. 2006, 83, 842–850. [Google Scholar] [CrossRef]
- Xue, Y.; Redeuil, K.M.; Giménez, E.C.; Vinyes-Pares, G.; Zhao, A.; He, T.; Yang, X.; Zheng, Y.; Zhang, Y.; Wang, P.; et al. Regional, socioeconomic, and dietary factors influencing B-vitamins in human milk of urban Chinese lactating women at different lactation stages. BMC Nutr. 2017, 3, 22. [Google Scholar] [CrossRef]
- Shi, Y.; Sun, G.; Zhang, Z.; Deng, X.; Kang, X.-H.; Liu, Z.-D.; Ma, Y.; Sheng, Q.-H. The chemical composition of human milk from Inner Mongolia of China. Food Chem. 2011, 127, 1193–1198. [Google Scholar] [CrossRef] [PubMed]
- Edwards, K.A.; Tu-Maung, N.; Cheng, K.; Wang, B.; Baeumner, A.J.; Kraft, C.E. Thiamine Assays-Advances, Challenges, and Caveats. Chem. Open 2017, 6, 178–191. [Google Scholar] [CrossRef] [PubMed]
- Nabokina, S.M.; Said, H.M. A high-affinity and specific carrier-mediated mechanism for uptake of thiamine pyrophosphate by human colonic epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G389–G395. [Google Scholar] [CrossRef]
- Said, H.M.; Ortiz, A.; Subramanian, V.S.; Neufeld, E.J.; Moyer, M.P.; Dudeja, P.K. Mechanism of thiamine uptake by human colonocytes: Studies with cultured colonic epithelial cell line NCM460. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 281, G144–G150. [Google Scholar] [CrossRef] [PubMed]
- Gangolf, M.; Czerniecki, J.; Radermecker, M.; Detry, O.; Nisolle, M.; Jouan, C.; Martin, D.; Chantraine, F.; Lakaye, B.; Wins, P.; et al. Thiamine status in humans and content of phosphorylated thiamine derivatives in biopsies and cultured cells. PLoS ONE 2010, 5, e13616. [Google Scholar] [CrossRef]
- Zhao, R.; Gao, F.; Goldman, I.D. Reduced folate carrier transports thiamine monophosphate: An alternative route for thiamine delivery into mammalian cells. Am. J. Physiol. Cell Physiol. 2002, 282, C1512–C1517. [Google Scholar] [CrossRef]
- Ganapathy, V.; Smith, S.B.; Prasad, P.D. SLC19: The folate/thiamine transporter family. Pflug. Arch. Eur. J. Physiol. 2004, 447, 641–646. [Google Scholar] [CrossRef]
- Mosegaard, S.; Dipace, G.; Bross, P.; Carlsen, J.; Gregersen, N.; Olsen, R.K.J. Riboflavin Deficiency—Implications for General Human Health and Inborn Errors of Metabolism. Int. J. Mol. Sci. 2020, 21, 3847. [Google Scholar] [CrossRef]
- Cochrane, K.M.; Elango, R.; Devlin, A.M.; Hutcheon, J.A.; Karakochuk, C.D. Human milk unmetabolized folic acid is increased following supplementation with synthetic folic acid as compared to (6S)-5-methyltetrahydrofolic acid. Sci. Rep. 2023, 13, 11298. [Google Scholar] [CrossRef] [PubMed]
- Menezo, Y.; Elder, K.; Clement, A.; Clement, P. Folic Acid, Folinic Acid, 5 Methyl TetraHydroFolate Supplementation for Mutations That Affect Epigenesis through the Folate and One-Carbon Cycles. Biomolecules 2022, 12, 197. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.R.; Mcpartlin, J.; Weir, D.G.; Daly, S.; Pentieva, K.; Daly, L.; Scott, J.M. Evidence of unmetabolised folic acid in cord blood of newborn and serum of 4-day-old infants. Br. J. Nutr. 2005, 94, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.T.T.; Kim, J.; Lee, H.; Won, S.; Kim, Y.; Jung, J.A.; Li, D.; To, X.H.M.; Huynh, K.T.N.; Van Le, T.; et al. A Comparison of Vitamin and Lutein Concentrations in Breast Milk from Four Asian Countries. Nutrients 2020, 12, 1794. [Google Scholar] [CrossRef]
- Page, R.; Robichaud, A.; Arbuckle, T.E.; Fraser, W.D.; Macfarlane, A.J. Total folate and unmetabolized folic acid in the breast milk of a cross-section of Canadian women. Am. J. Clin. Nutr. 2017, 105, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, T.; Furukawa, M.; Asoh, M.; Kanno, T.; Kojima, T.; Yonekubo, A. Fat-soluble and water-soluble vitamin contents of breast milk from Japanese women. J. Nutr. Sci. Vitaminol. 2005, 51, 239–247. [Google Scholar] [CrossRef]
- Samuel, T.M.; De-Castro, C.A.; Dubascoux, S.; Affolter, M.; Giuffrida, F.; Billeaud, C.; Picaud, J.-C.; Agosti, M.; Al-Jashi, I.; Pereira, A.B.; et al. Subclinical Mastitis in a European Multicenter Cohort: Prevalence, Impact on Human Milk (HM) Composition, and Association with Infant HM Intake and Growth. Nutrients 2019, 12, e105. [Google Scholar] [CrossRef]
- Allen, L.H.; Hampel, D.; Shahab-Ferdows, S.; Andersson, M.; Barros, E.; Doel, A.M.; Eriksen, K.G.; Christensen, S.H.; Islam, M.; Kac, G.; et al. The Mothers, Infants, and Lactation Quality (MILQ) Study: A Multi-Center Collaboration. Curr. Dev. Nutr. 2021, 5, nzab116. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Duan, Y.; Yang, J.; Li, J.; Li, F.; Zhou, P.; Liu, C.; Zhao, Y.; Gu, X.; Yuan, C.; et al. Cohort profile: The Taicang and Wuqiang mother-child cohort study (TAWS) in China. BMJ Open 2022, 12, e060868. [Google Scholar] [CrossRef]
- Qiao, W.; Chen, J.; Zhang, M.; Wang, Y.; Yang, B.; Zhao, J.; Jiang, T.; Chen, L. A cohort study of vitamins contents in human milk from maternal-infant factors. Front. Nutr. 2022, 6, 9. [Google Scholar] [CrossRef] [PubMed]

| Participant (n = 28) | |
|---|---|
| Age, years | 28.7 ± 3.2 |
| Education, n (%) | |
| Senior middle school and below | 5 (18%) |
| College and above | 23 (82%) |
| Household income per year, CNY, n (%) | |
| <20,000 | 21 (75%) |
| ≥20,000 | 7 (25%) |
| Pre-pregnancy BMI (kg/m2) | 21.70 (20.14, 21.92) |
| Gestational weight gain (GWG) (kg) | 15.29 ± 6.26 |
| Inadequate GWG, n (%) * | 2 (7%) |
| Appropriate GWG, n (%) * | 8 (29%) |
| Excessive GWG, n (%) * | 18 (64%) |
| Weeks of gestation at delivery, weeks ** | 40.4 ± 1.2 |
| Offspring gender | |
| Male, n (%) | 13 (46%) |
| Female, n (%) | 15 (54%) |
| Offspring birth weight, g | 3449.0 ± 436.3 |
| SGA, n (%) *** | 3 (11%) |
| AGA, n (%) *** | 19 (68%) |
| LGA, n (%) *** | 6 (21%) |
| Offspring birth length, cm | 51.2 ± 1.5 |
| Free Thiamine | Total Thiamine | Free Riboflavin | Total Riboflavin | Free Folate | Total Folate | |
|---|---|---|---|---|---|---|
| P(0–7d vs. 14d) | 0.1437 | <0.0001 * | 0.7798 | 0.2339 | 0.0796 | 0.0006 * |
| P(14d vs. 30d) | 0.1024 | <0.0001 * | 0.7851 | 0.5855 | 0.0006 * | <0.0001 * |
| P(30d vs. 60d) | 0.0348 * | 0.9734 | 0.8708 | 0.0732 | 0.0151 * | 0.2946 |
| P(60d vs. 90d) | 0.0034 * | 0.4343 | 0.8990 | 0.0829 | 0.0757 | 0.0330 * |
| P(90d vs. 120d) | 0.1463 | 0.3389 | 0.8713 | 0.2200 | 0.9104 | 0.8007 |
| P(120d vs. 150d) | 0.0003 * | 0.9694 | 0.0076 * | 0.9249 | 0.0197 * | 0.0174 * |
| P(150d vs. 180d) | 0.1616 | 0.8157 | 0.3059 | 0.6142 | 0.2698 | 0.7193 |
| Pall | <0.0001 | 0.5355 | 0.1710 | 0.8896 | <0.0001 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Xing, X.; Ren, X.; Jiang, S.; Yang, Z.; Lai, J. Different Chemical Forms of Thiamine, Riboflavin, and Folate in Human Milk as a Function of Lactation Stages—A Cohort Study on Breastfeeding Women from Beijing. Nutrients 2025, 17, 624. https://doi.org/10.3390/nu17040624
Wang Y, Xing X, Ren X, Jiang S, Yang Z, Lai J. Different Chemical Forms of Thiamine, Riboflavin, and Folate in Human Milk as a Function of Lactation Stages—A Cohort Study on Breastfeeding Women from Beijing. Nutrients. 2025; 17(4):624. https://doi.org/10.3390/nu17040624
Chicago/Turabian StyleWang, Ye, Xinxin Xing, Xiangnan Ren, Shan Jiang, Zhenyu Yang, and Jianqiang Lai. 2025. "Different Chemical Forms of Thiamine, Riboflavin, and Folate in Human Milk as a Function of Lactation Stages—A Cohort Study on Breastfeeding Women from Beijing" Nutrients 17, no. 4: 624. https://doi.org/10.3390/nu17040624
APA StyleWang, Y., Xing, X., Ren, X., Jiang, S., Yang, Z., & Lai, J. (2025). Different Chemical Forms of Thiamine, Riboflavin, and Folate in Human Milk as a Function of Lactation Stages—A Cohort Study on Breastfeeding Women from Beijing. Nutrients, 17(4), 624. https://doi.org/10.3390/nu17040624






