Association of Homocysteine, S-Adenosylhomocysteine and S-Adenosylmethionine with Cardiovascular Events in Chronic Kidney Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Exposure Definition
2.3. Outcome Assessment
2.4. Baseline Characteristics
2.5. Statistical Analysis
3. Results
3.1. Participants’ Characteristics
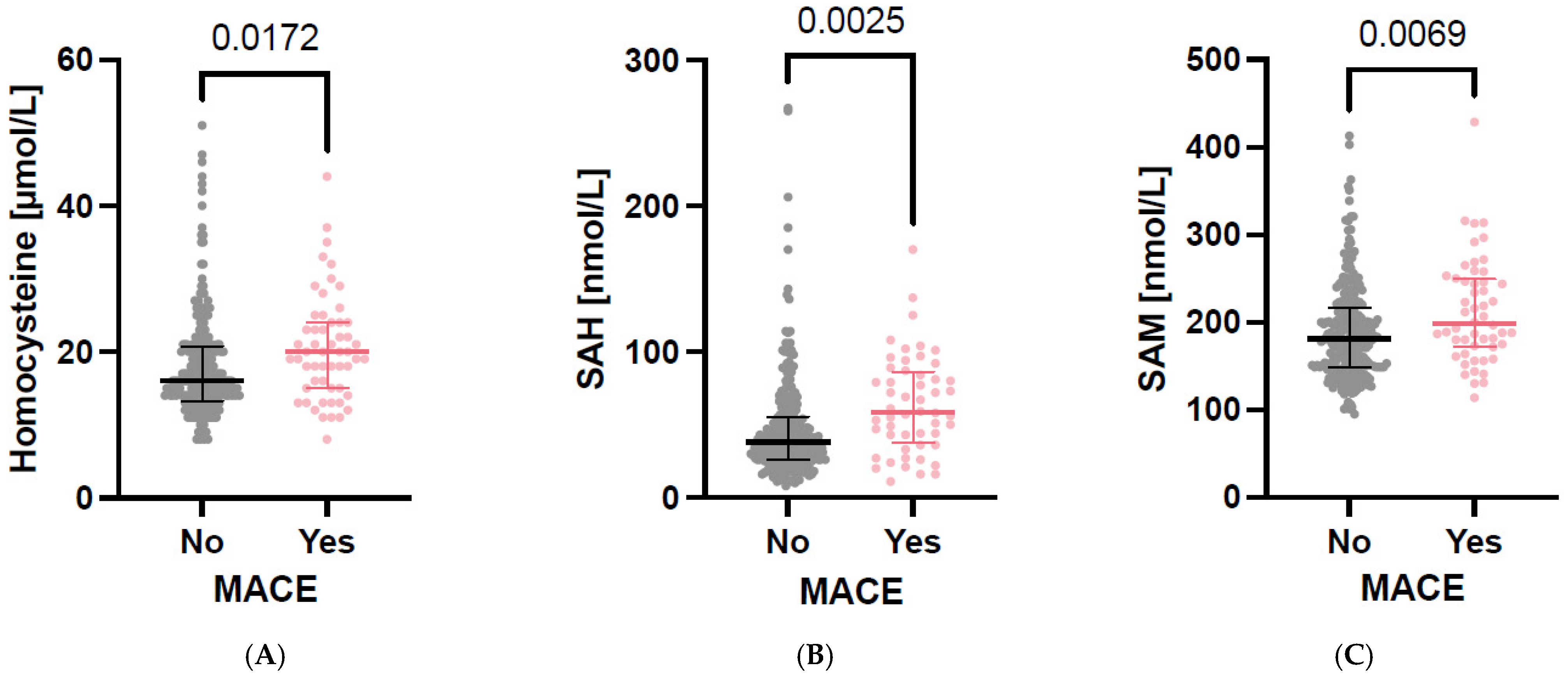
3.2. Parameters of One-Carbon Metabolism in Cross-Sectional Analyses
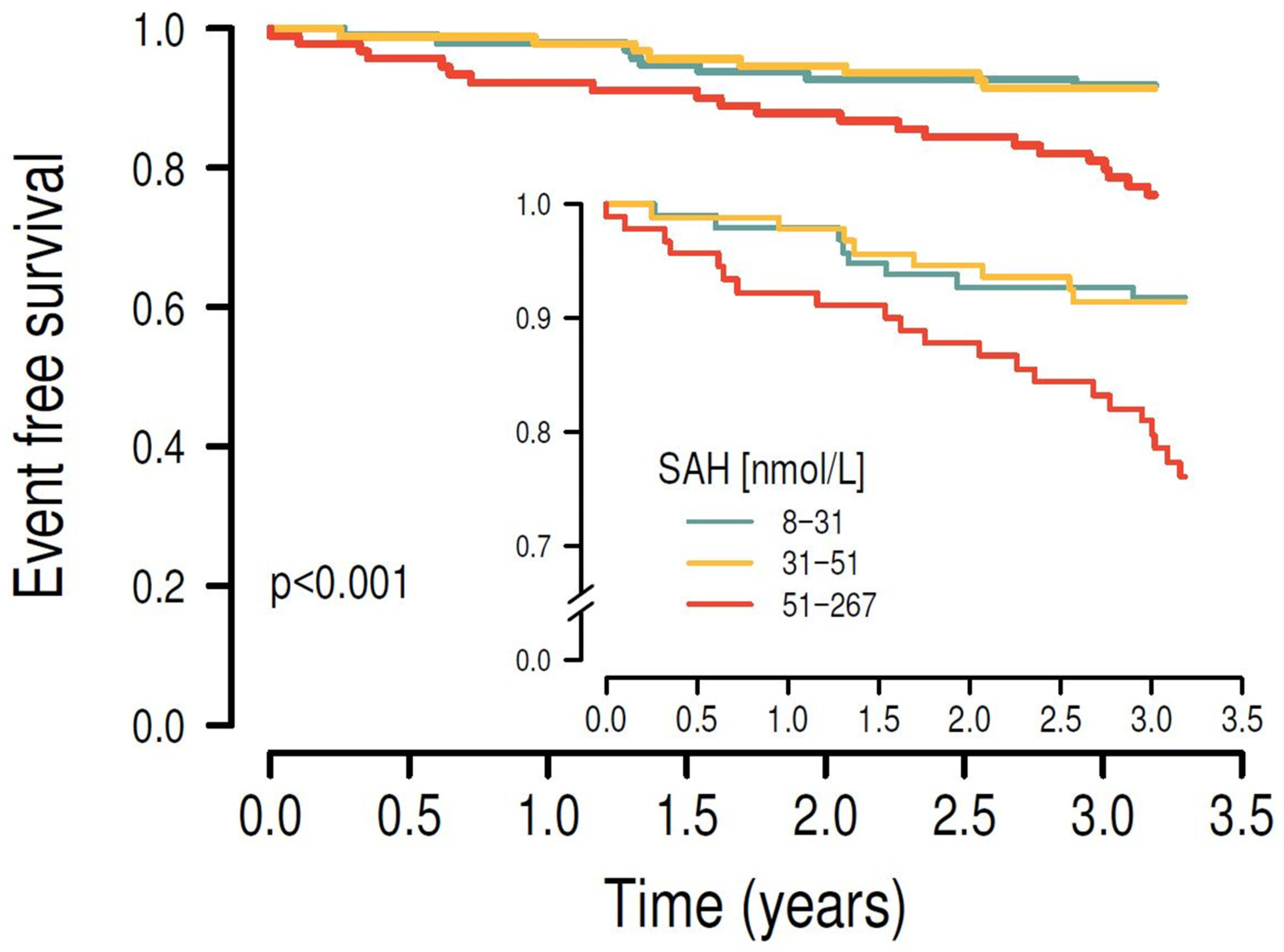
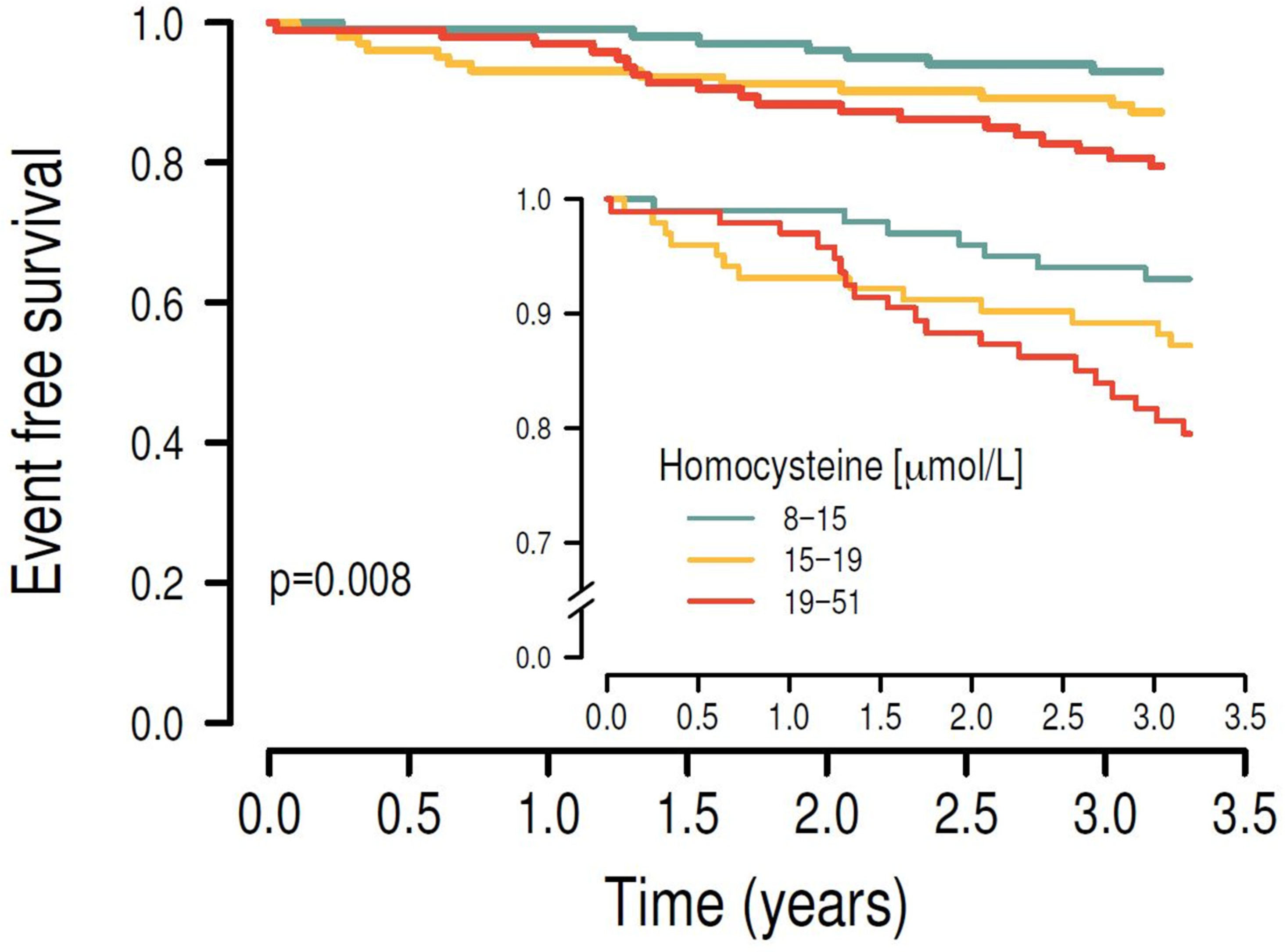
3.3. Associations Between C1 Metabolites and Cardiovascular Outcomes in Longitudinal Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cockwell, P.; Fisher, L.A. The global burden of chronic kidney disease. Lancet 2020, 395, 662–664. [Google Scholar] [CrossRef] [PubMed]
- Go, A.S.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Hsu, C. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 2004, 351, 1296–1305. [Google Scholar] [CrossRef]
- Sarnak, M.J.; Levey, A.S.; Schoolwerth, A.C.; Coresh, J.; Culleton, B.; Hamm, L.L.; McCullough, P.A.; Kasiske, B.L.; Kelepouris, E.; Klag, M.J.; et al. Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 2003, 108, 2154–2169. [Google Scholar] [CrossRef] [PubMed]
- Castro, R.; Rivera, I.; Struys, E.A.; Jansen, E.E.; Ravasco, P.; Camilo, M.E.; Blom, H.J.; Jakobs, C.; de Almeida, I.T. Increased homocysteine and S-adenosylhomocysteine concentrations and DNA hypomethylation in vascular disease. Clin. Chem. 2003, 49, 1292–1296. [Google Scholar] [CrossRef] [PubMed]
- Mallamaci, F.; Zoccali, C.; Tripepi, G.; Fermo, I.; Benedetto, F.A.; Cataliotti, A.; Bellanuova, I.; Malatino, L.S.; Soldarini, A. Hyperhomocysteinemia predicts cardiovascular outcomes in hemodialysis patients. Kidney Int. 2002, 61, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Zawada, A.M.; Rogacev, K.S.; Heine, G.H. Clinical relevance of epigenetic dysregulation in chronic kidney disease-associated cardiovascular disease. Nephrol. Dial. Transplant. 2013, 28, 1663–1671. [Google Scholar] [CrossRef]
- Garibotto, G.; Valli, A.; Anderstam, B.; Eriksson, M.; Suliman, M.E.; Balbi, M.; Rollando, D.; Vigo, E.; Lindholm, B. The kidney is the major site of S-adenosylhomocysteine disposal in humans. Kidney Int. 2009, 76, 293–296. [Google Scholar] [CrossRef]
- Dai, X.; Liu, S.; Cheng, L.; Huang, T.; Guo, H.; Wang, D.; Xia, M.; Ling, W.; Xiao, Y. Epigenetic Upregulation of H19 and AMPK Inhibition Concurrently Contribute to S-Adenosylhomocysteine Hydrolase Deficiency-Promoted Atherosclerotic Calcification. Circ. Res. 2022, 130, 1565–1582. [Google Scholar] [CrossRef]
- Xiao, Y.; Su, X.; Huang, W.; Zhang, J.; Peng, C.; Huang, H.; Wu, X.; Huang, H.; Xia, M.; Ling, W. Role of S-adenosylhomocysteine in cardiovascular disease and its potential epigenetic mechanism. Int. J. Biochem. Cell Biol. 2015, 67, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zhang, Y.; Wang, M.; Li, X.; Su, D.; Qiu, J.; Li, D.; Yang, Y.; Xia, M.; Ling, W. Plasma S-adenosylhomocysteine is associated with the risk of cardiovascular events in patients undergoing coronary angiography: A cohort study. Am. J. Clin. Nutr. 2013, 98, 1162–1169. [Google Scholar] [CrossRef] [PubMed]
- Emrich, I.E.; Vodovar, N.; Feuer, L.; Untersteller, K.; Nougue, H.; Seiler-Mussler, S.; Fliser, D.; Launay, J.-M.; Heine, G.H. Do plasma neprilysin activity and plasma neprilysin concentration predict cardiac events in chronic kidney disease patients? Nephrol. Dial. Transplant. 2018, 34, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, S.H.; Knapp, J.P.; Geisel, J.; Herrmann, W.; Obeid, R. Simultaneous quantification of S-adenosyl methionine and S-adenosyl homocysteine in human plasma by stable-isotope dilution ultra performance liquid chromatography tandem mass spectrometry. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2009, 877, 3865–3870. [Google Scholar] [CrossRef] [PubMed]
- Heart Outcomes Prevention Evaluation (HOPE) 2 Investigators. Homocysteine lowering with folic acid and B vitamins in vascular disease. N. Engl. J. Med. 2006, 354, 1567–1577. [Google Scholar] [CrossRef] [PubMed]
- Green, T.J.; Skeaff, C.M.; McMahon, J.A.; Venn, B.J.; Williams, S.M.; Devlin, A.M.; Innis, S.M. Homocysteine-lowering vitamins do not lower plasma S-adenosylhomocysteine in older people with elevated homocysteine concentrations. Br. J. Nutr. 2010, 103, 1629–1634. [Google Scholar] [CrossRef] [PubMed]
- Zawada, A.M.; Rogacev, K.S.; Hummel, B.; Berg, J.T.; Friedrich, A.; Roth, H.J.; Obeid, R.; Geisel, J.; Fliser, D.; Heine, G.H. S-adenosylhomocysteine is associated with subclinical atherosclerosis and renal function in a cardiovascular low-risk population. Atherosclerosis 2014, 234, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Jabs, K.; Koury, M.J.; Dupont, W.D.; Wagner, C. Relationship between plasma S-adenosylhomocysteine concentration and glomerular filtration rate in children. Metabolism 2006, 55, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Stühlinger, M.C.; Tsao, P.S.; Her, J.H.; Kimoto, M.; Balint, R.F.; Cooke, J.P. Homocysteine impairs the nitric oxide synthase pathway: Role of asymmetric dimethylarginine. Circulation 2001, 104, 2569–2575. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liao, R.; Dai, X.; Guo, H.; Wang, D.; Xia, M.; Ling, W.; Xiao, Y. Association between plasma S-adenosylmethionine and risk of mortality in patients with coronary artery disease: A cohort study. Am. J. Clin. Nutr. 2021, 114, 1360–1370. [Google Scholar] [CrossRef]
- Kielstein, J.T.; Salpeter, S.R.; Buckley, N.S.; Cooke, J.P.; Fliser, D. Two cardiovascular risk factors in one? Homocysteine and its relation to glomerular filtration rate. A meta-analysis of 41 studies with 27,000 participants. Kidney Blood Press. Res. 2008, 31, 259–267. [Google Scholar] [CrossRef] [PubMed]


| Total [n = 297] | CKD G2 [n = 58] | CKD G3a [n = 92] | CKD G3b [n = 79] | CKD G4/5 ND [n = 60] | CKD G5D [n = 8] | p | |
|---|---|---|---|---|---|---|---|
| Patient demographics | |||||||
| Age [years] | 69.1 [60.4; 76.8] | 60.8 [50.5; 67.9] | 67.2 [58.8; 74.3] | 75.7 [65.9; 79.8] | 75.6 [69.1; 80.0] | 63.5 [53.3; 77.2] | <0.001 |
| Gender [female] | 117 [39.4%] | 23 [39.7%] | 31 [33.7%] | 36 [45.6%] | 25 [41.7%] | 2 [25.0%] | 0.503 |
| Smoking [yes] | 32 [10.8%] | 12 [20.7%] | 11 [12.0%] | 4 [5.1%] | 4 [6.7%] | 1 [12.5%] | 0.044 |
| Family’s history of CVD [yes] | 61 [20.5%] | 12 [20.7%] | 21 [22.8%] | 15 [19.0%] | 8 [13.3%] | 5 [62.5%] | 0.027 |
| CVD [prevalent] | 95 [32.0%] | 10 [17.2%] | 25 [27.2%] | 32 [40.5%] | 23 [38.3%] | 5 [62.5%] | 0.007 |
| Diabetes mellitus [yes] | 106 [35.7%] | 20 [34.5%] | 30 [32.6%] | 28 [35.4%] | 25 [41.7%] | 3 [37.5%] | 0.850 |
| BMI [kg/m2] | 30 [27; 34] | 30 [28; 33] | 30 [26; 33] | 30 [28; 34] | 30 [27; 34] | 28 [25; 35] | 0.606 |
| SBP [mmHg] | 144 [132; 159] | 139 [129; 155] | 142 [132; 159] | 144 [133; 155] | 153 [133; 166] | 152 [125; 181] | 0.085 |
| DBP [mmHg] | 84 [76; 91] | 86 [79; 94] | 87 [79; 92] | 80 [72; 89] | 83 [75; 88] | 82 [75; 84] | <0.001 |
| Laboratory parameters | |||||||
| Total Cholesterol [mg/dL] | 179.8 ± 41.6 | 186.3 ± 36.4 | 185.4 ± 44.2 | 170.1 ± 39.0 | 177.6 ± 44.0 | * | 0.054 |
| LDL-C [mg/dL] | 102.7 ± 35.5 | 106.3 ± 32.1 | 107.3 ± 38.0 | 95.8 ± 31.7 | 101.4 ± 38.4 | * | 0.143 |
| HDL-C [mg/dL] | 53.2 ± 16.4 | 55.3 ± 15.8 | 55.8 ± 18.9 | 51.6 ± 15.8 | 49.3 ± 12.7 | * | 0.013 |
| Triglyceride [mg/dL] | 157.6 ± 84.2 | 167.4 ± 104.7 | 146.3 ± 79.9 | 150.7 ± 74.6 | 174.6 ± 78.6 | * | 0.532 |
| CRP [mg/L] | 2.5 [1.2; 5.5] | 1.9 [1.1; 3.8] | 2.6 [1.2; 6.0] | 2.8 [1.3; 5.4] | 3.0 [1.4; 6.0] | * | 0.632 |
| Renal parameters | |||||||
| eGFR [mL/min/1.73 m2] | 45 [32; 57] | 68 [62; 75] | 51 [47; 55] | 38 [33; 40] | 23 [19; 25] | * | <0.001 |
| Plasma creatinine [mg/dL] | 1.64 ± 0.72 | 1.04 ± 0.15 | 1.32 ± 0.21 | 1.62 ± 0.28 | 2.72 ± 0.78 | * | <0.001 |
| Albuminuria [mg/g crea] | 38 [10;198] | 18 [7; 110] | 20 [8; 120] | 39 [11;180] | 124 [36; 508] | * | 0.001 |
| Phosphate [mg/dL] | 3.36 ± 0.67 | 3.07 ± 0.58 | 3.18 ± 0.58 | 3.38 ± 0.58 | 3.90 ± 0.70 | * | <0.001 |
| One-carbon metabolites | |||||||
| SAH [nmol/L] | 41.5 [26.6; 63.9] | 21.2 [16.1; 31.1] | 32.2 [26.3; 42.0] | 45.7 [40.4; 61.7] | 82.3 [68.7; 100.3] | 113.5 [92.2; 184.7] | <0.001 |
| SAM [nmol/L] | 183.4 [151.1; 223.5] | 146.7 [129.7; 174.0] | 165.4 [148.8 184.3] | 197.4 [176.7; 222.7] | 242.9 [205.4; 276.5] | 291.1 [262.7; 317.2] | <0.001 |
| Homocysteine [µmol/L] | 16.6 [13.5; 21.2] | 12.9 [11.3; 15.8] | 15.7 [13.6; 18.0] | 18.3 [15.3; 22.9] | 22.4 [19.6; 29.4] | 21.0 [16.4; 25.5] | <0.001 |
| SAH | SAM | Homocysteine | SAM/SAH | |
|---|---|---|---|---|
| age | 0.263 *** | 0.191 *** | 0.285 *** | −0.221 *** |
| BMI | −0.036 | −0.165 *** | −0.020 | 0.048 |
| SBP | 0.043 | 0.023 | 0.046 | −0.041 |
| Total Cholesterol | −0.120 ** | −0.126 ** | −0.111 ** | 0.074 |
| Albuminuria | 0.250 *** | 0.208 *** | 0.110 ** | −0.210 *** |
| eGFR CKD-EPIcrea | −0.588 *** | −0.497 *** | −0.424 *** | 0.442 *** |
| Crude Model | Model 2 | Model 3 | Model 4 | |||||
|---|---|---|---|---|---|---|---|---|
| Exposure Variable | HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value | HR (95% CI) | p-Value |
| Categories | ||||||||
| SAH | ||||||||
| First tertile (8–31 nmol/L; n = 99) | 1 | 1 | 1 | 1 | ||||
| Second tertile (31–51 nmol/L; n = 99) | 1.286 (0.531–3.196) | 0.577 | 1.074 (0.440–2.687) | 0.874 | 0.7609 (0.291–2.018) | 0.574 | 1.443 (1.012–2.056) | 0.042 |
| Third tertile (51–267 nmol/L; n = 99) | 3.544 (1.751–7.935) | <0.001 | 2.696 (1.293–6.170) | 0.001 | 1.645 (0.654–4.411) | 0.303 | 1.209 (0.780–1.865) | 0.393 |
| SAM | ||||||||
| First tertile (95–160 nmol/L; n = 99) | 1 | 1 | 1 | 1 | ||||
| Second tertile (160–20 nmol/L; n = 99) | 1.862 (0.846–4.380) | 0.132 | 1.357 (0.602–3.251) | 0.471 | 1.159 (0.497–2.855) | 0.737 | 1.124 (0.477–2.788) | 0.792 |
| Third tertile (201–429 nmol/L; n = 99) | 3.073 (1.474–7.017) | 0.004 | 2.769 (1.324–6.335) | 0.009 | 1.920 (0.764–5.138) | 0.176 | 1.604 (0.605–4.470) | 0.351 |
| Homocysteine | ||||||||
| First tertile (8–15 µmol/L; n = 99) | 1 | 1 | 1 | 1 | ||||
| Second tertile (15–19 µmol/L; n = 99) | 1.534 (0.689–3.503) | 0.296 | 1.171 (0.515–2.735) | 0.707 | 1.131 (0.499–2.630) | 0.768 | 1.396 (0.593–3.379) | 0.447 |
| Third tertile (19–51 µmol/L; n = 99) | 2.76 (1.409–5.804) | 0.004 | 1.804 (0.868–3.974) | 0.124 | 1.115 (0.521–2.546) | 0.786 | 1.212 (0.56–2.786) | 0.635 |
| Continuous predictors | ||||||||
| log SAH | 7.379 (2.512–21.92) | <0.001 | 5.771 (1.797–18.66) | 0.003 | 2.237 (0.351–14.37) | 0.396 | 1.312 (0.204–8.446) | 0.775 |
| log SAM | 40.59 (3.968–413.6) | 0.002 | 34.73 (3.015–390.8) | 0.004 | 7.126 (0.271–186.9) | 0.239 | 2.935 (0.086–103.1) | 0.552 |
| log homocysteine | 11.42 (2.238–55.69) | 0.002 | 4.709 (0.759–27.19) | 0.089 | 1.572 (0.243–10.18) | 0.634 | 1.615 (0.244–10.78) | 0.618 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emrich, I.E.; Obeid, R.; Geisel, J.; Fliser, D.; Böhm, M.; Heine, G.H.; Zawada, A.M. Association of Homocysteine, S-Adenosylhomocysteine and S-Adenosylmethionine with Cardiovascular Events in Chronic Kidney Disease. Nutrients 2025, 17, 626. https://doi.org/10.3390/nu17040626
Emrich IE, Obeid R, Geisel J, Fliser D, Böhm M, Heine GH, Zawada AM. Association of Homocysteine, S-Adenosylhomocysteine and S-Adenosylmethionine with Cardiovascular Events in Chronic Kidney Disease. Nutrients. 2025; 17(4):626. https://doi.org/10.3390/nu17040626
Chicago/Turabian StyleEmrich, Insa E., Rima Obeid, Jürgen Geisel, Danilo Fliser, Michael Böhm, Gunnar H. Heine, and Adam M. Zawada. 2025. "Association of Homocysteine, S-Adenosylhomocysteine and S-Adenosylmethionine with Cardiovascular Events in Chronic Kidney Disease" Nutrients 17, no. 4: 626. https://doi.org/10.3390/nu17040626
APA StyleEmrich, I. E., Obeid, R., Geisel, J., Fliser, D., Böhm, M., Heine, G. H., & Zawada, A. M. (2025). Association of Homocysteine, S-Adenosylhomocysteine and S-Adenosylmethionine with Cardiovascular Events in Chronic Kidney Disease. Nutrients, 17(4), 626. https://doi.org/10.3390/nu17040626








