Anti-Inflammatory Effects of Curcumin-Based Nanoparticles Containing α-Linolenic Acid in a Model of Psoriasis In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Treatments
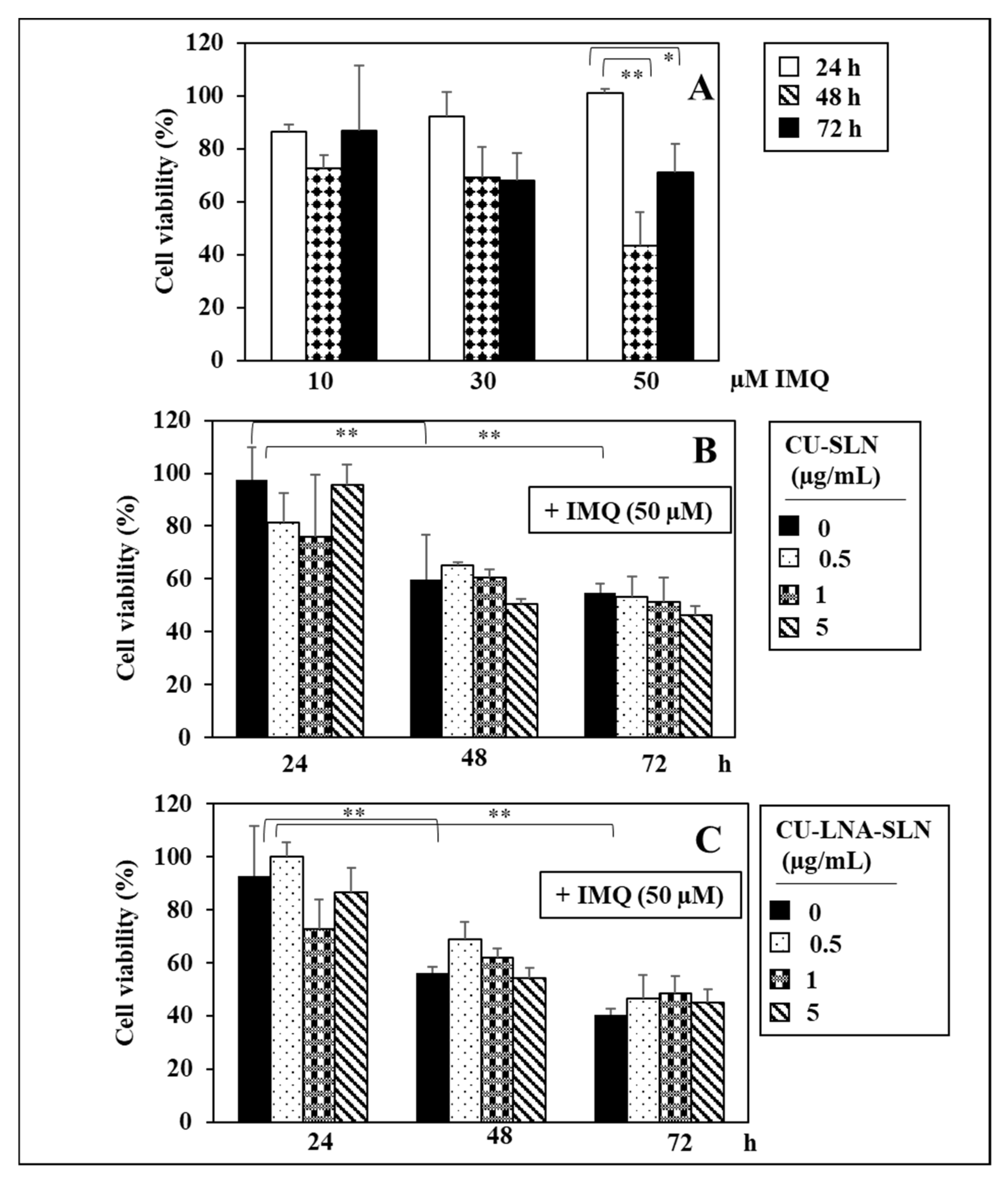
2.2. MTT Cell Viability Assay
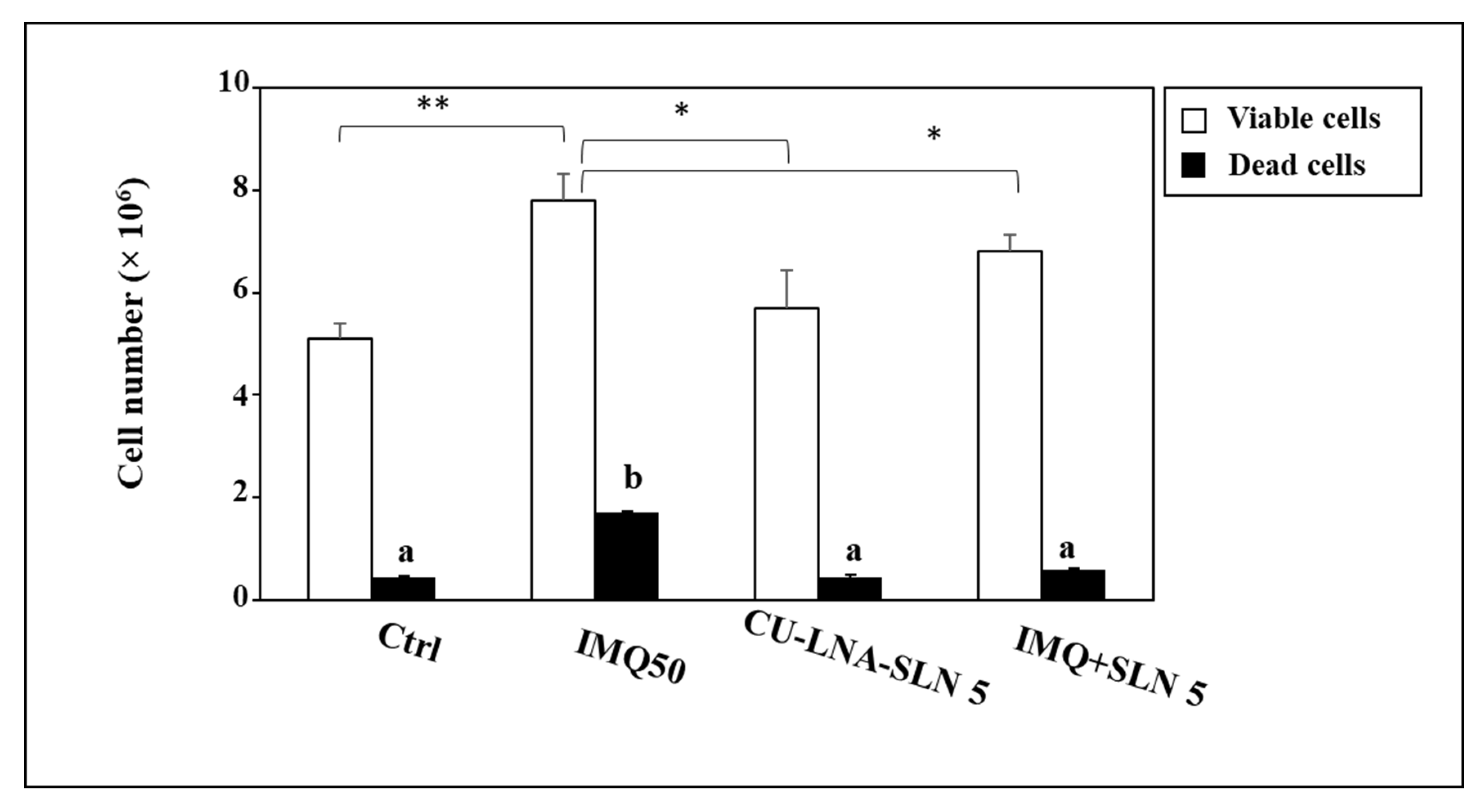
2.3. Trypan Blue Cytotoxicity Assay
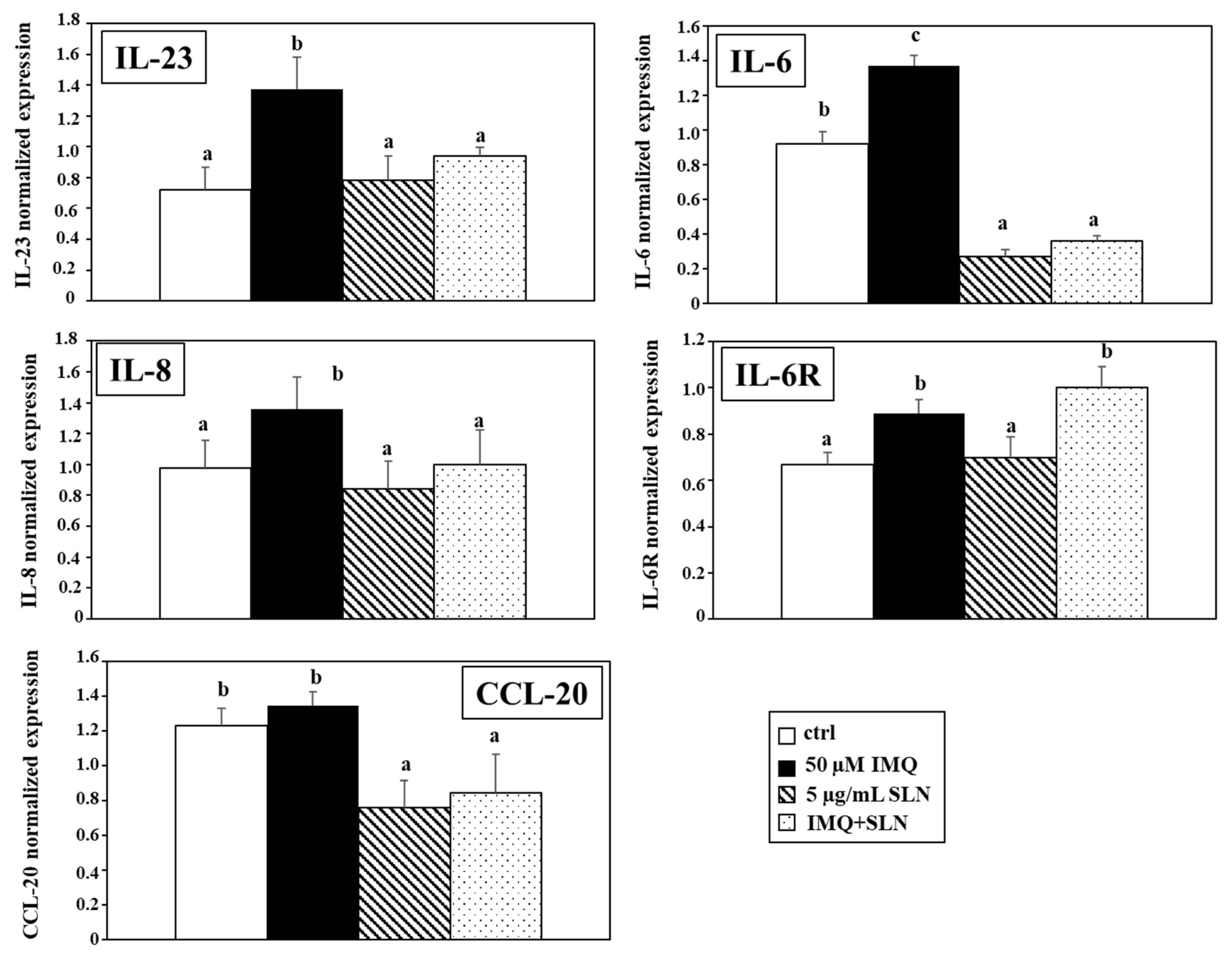
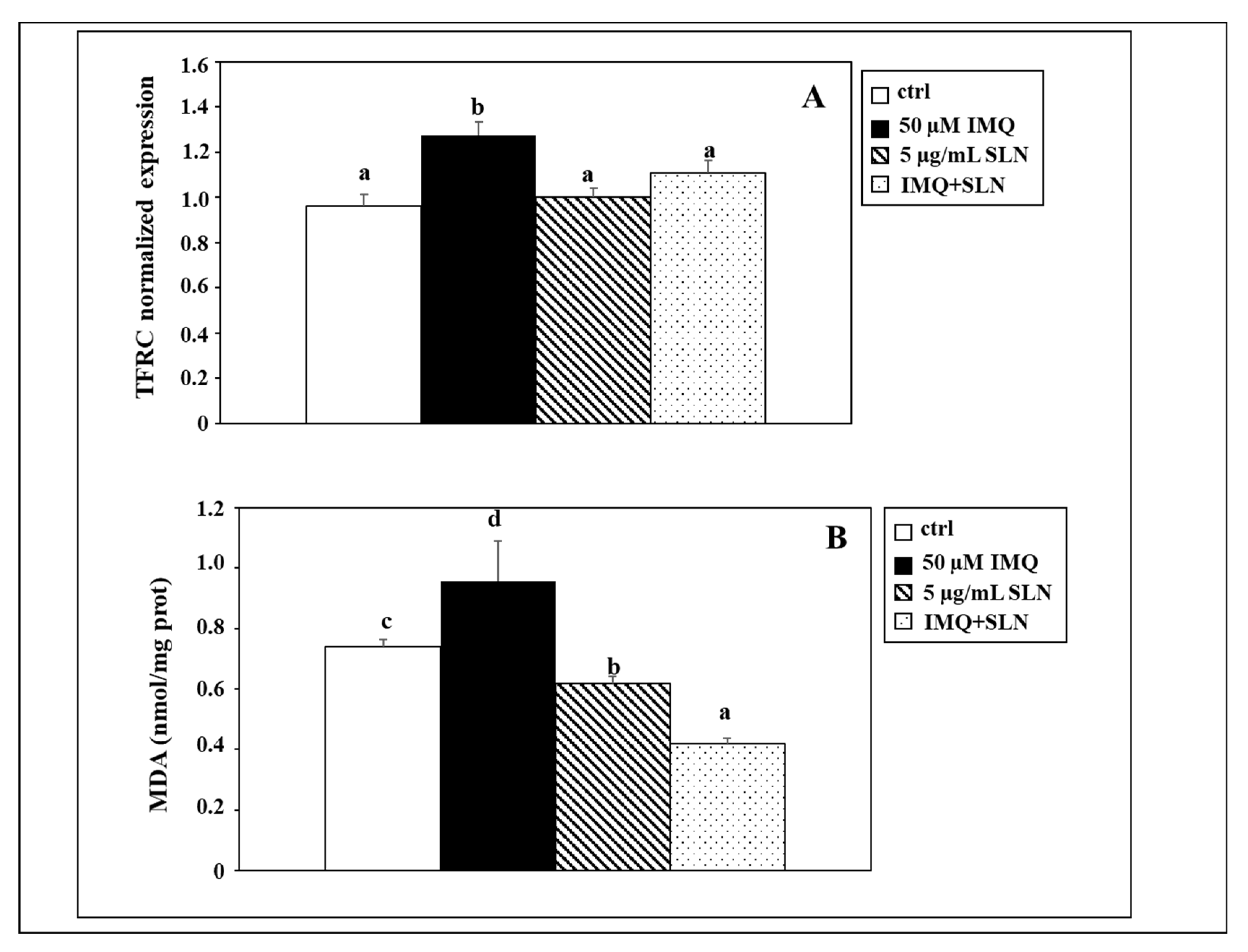
2.4. Real-Time Quantitative PCR Analysis of IL-23, IL-8, IL-6, IL-6R, and TFRC Expression
2.5. MDA Assay for the Evaluation of Lipid Peroxidation
- A = MDA nmoles of samples extrapolated for standard curve;
- mg = protein amount in the sample (in mg); 4 = correction factor (from the original reaction mix, only 200 µL were utilized);
- D = potential dilution factor used before the analysis.
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weigle, N.; McBane, S. Psoriasis. Am. Fam. Physician 2013, 87, 626–633. [Google Scholar]
- World Health Organization. Global Report on Psoriasis; World Health Organization: Geneva, Switzerland, 2016; Available online: https://www.who.int/publications/i/item/9789241565189 (accessed on 22 November 2024).
- Rendon, A.; Schäkel, K. Psoriasis pathogenesis and treatment. Int. J. Mol. Sci. 2019, 20, 1475. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.W.; Read, C. Pathophysiology, clinical presentation, and treatment of psoriasis: A review. JAMA 2020, 323, 1945–1960. [Google Scholar] [CrossRef]
- Dascălu, R.C.; Bărbulescu, A.L.; Stoica, L.E.; Dinescu, Ș.C.; Biță, C.E.; Popoviciu, H.V.; Ionescu, R.A.; Vreju, F.A. Review: A Contemporary, Multifaced Insight into Psoriasis Pathogenesis. J. Pers. Med. 2024, 14, 535. [Google Scholar] [CrossRef]
- Ni, X.; Lai, Y. Keratinocyte: A trigger or an executor of psoriasis? J. Leukoc. Biol. 2020, 108, 485–491. [Google Scholar] [CrossRef]
- Ippagunta, S.K.; Gangwar, R.; Finkelstein, D.; Vogel, P.; Pelletier, S.; Gingras, S.; Redecke, V.; Häcker, H. Keratinocytes contribute intrinsically to psoriasis upon loss of Tnip1 function. Proc. Natl. Acad. Sci. USA 2016, 113, E6162–E6171. [Google Scholar] [CrossRef] [PubMed]
- Furue, M.; Furue, K.; Tsuji, G.; Nakahara, T. Interleukin-17A and keratinocytes in psoriasis. Int. J. Mol Sci. 2020, 21, 1275. [Google Scholar] [CrossRef] [PubMed]
- Kamata, M.; Tada, Y. Crosstalk: Keratinocytes and immune cells in psoriasis. Front. Immunol. 2023, 14, 1286344. [Google Scholar] [CrossRef]
- Lowes, M.A.; Suárez-Fariñas, M.; Krueger, J.G. Immunology of psoriasis. Annu. Rev. Immunol. 2014, 32, 227–255. [Google Scholar] [CrossRef] [PubMed]
- Nazimek, K.; Bryniarski, K. Macrophage Functions in Psoriasis: Lessons from Mouse Models. Int. J. Mol. Sci. 2024, 25, 5306. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Liu, X.; Liu, N.; Huang, Y.; Jin, Z.; Zhang, S.; Ming, Z.; Chen, H. Inhibition of keratinocyte necroptosis mediated by RIPK1/RIPK3/MLKL provides a protective effect against psoriatic inflammation. Cell Death Dis. 2020, 11, 134. [Google Scholar] [CrossRef]
- Kastelan, M.; Prpić-Massari, L.; Brajac, I. Apoptosis in psoriasis. Acta Dermatovenerol. Croat. 2009, 17, 182–186. [Google Scholar] [PubMed]
- Honda, T.; Kabashima, K. Involvement of necroptosis in the development of imiquimod-induced psoriasis-like dermatitis (BA3P.135). J. Immunol. 2014, 192, 44.5. [Google Scholar] [CrossRef]
- Vats, K.; Tian, H.; Singh, K.; Tyurina, Y.Y.; Sparvero, L.J.; Tyurin, V.A.; Kruglov, O.; Chang, A.; Wang, J.; Green, F.; et al. Ferroptosis of select skin epithelial cells initiates and maintains chronic systemic immune-mediated psoriatic disease. J. Clin. Investig. 2024, 135, e183219. [Google Scholar] [CrossRef] [PubMed]
- Shou, Y.; Yang, L.; Yang, Y.; Xu, J. Inhibition of keratinocyte ferroptosis suppresses psoriatic inflammation. Cell Death Dis. 2021, 12, 1009. [Google Scholar] [CrossRef]
- Liu, L.; Kang, X.X. ACSL4 is overexpressed in psoriasis and enhances inflammatory responses by activating ferroptosis. Biochem. Biophys. Res. Commun. 2022, 623, 1–8. [Google Scholar] [CrossRef]
- Dobrică, E.C.; Cozma, M.A.; Găman, M.A.; Voiculescu, V.M.; Găman, A.M. The Involvement of Oxidative Stress in Psoriasis: A Systematic Review. Antioxidants 2022, 11, 282. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Meng, Y.; Le, J.; Sun, Y.; Dian, Y.; Yao, L.; Xiong, Y.; Zeng, F.; Chen, X.; Deng, G. Ferroptosis: Mechanisms and therapeutic targets. MedComm 2024, 5, e70010. [Google Scholar] [CrossRef] [PubMed]
- Kshirsagar, S.J.; Adhav, P.S.; Laddha, U.D.; Ganore, J.S.; Pagar, C.S.; Bambal, V.R. Navigating psoriasis: From immune mechanisms to natural healing approaches. Int. Immunopharmacol. 2025, 144, 113626. [Google Scholar] [CrossRef] [PubMed]
- Pinter, A.; Tsianakas, A.; Eichner, A. ScaTAC study group. Efficacy and Safety of Topical Tacrolimus Microemulsion Applied Twice Daily in Patients with Mild to Moderate Scalp Psoriasis. Dermatol. Ther. 2024, 14, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Fujiyama, T.; Ito, T.; Umayahara, T.; Ikeya, S.; Tatsuno, K.; Funakoshi, A.; Hashizume, H.; Tokura, Y. Topical application of a vitamin D3 analogue and corticosteroid to psoriasis plaques decreases skin infiltration of TH17 cells and their ex vivo expansion. J. Allergy Clin. Immunol. 2016, 138, 517–528.e5. [Google Scholar] [CrossRef]
- Shalaby, R.A.; El-Gazayerly, O.; Abdallah, M. Cubosomal Betamethasone-Salicylic Acid Nano Drug Delivery System for Enhanced Management of Scalp Psoriasis. Int. J. Nanomed. 2022, 17, 1659–1677. [Google Scholar] [CrossRef]
- Pender, E.K.; Kirby, B. An update on topical therapies for psoriasis. Curr. Opin. Rheumatol. 2024, 36, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Chat, V.S.; Uppal, S.K.; Kearns, D.G.; Han, G.; Wu, J.J. Translating the 2020 AAD-NPF Guidelines of Care for the Management of Psoriasis with Systemic Nonbiologics to Clinical Practice. Cutis 2021, 107, 99–103. [Google Scholar] [CrossRef]
- Demirel Öğüt, N.; Ayanoğlu, M.A.; Koç Yıldırım, S.; Erbağcı, E.; Ünal, S.; Gökyayla, E. Are IL-17 inhibitors superior to IL-23 inhibitors in reducing systemic inflammation in moderate-to-severe plaque psoriasis? A retrospective cohort study. Arch. Dermatol. Res. 2025, 317, 232. [Google Scholar] [CrossRef] [PubMed]
- Blauvelt, A.; Papp, K.; Trivedi, M.; Barragan, C.; Chow, V.; Mytych, D.T.; Yamauchi, P.; Crowley, J.; Franklin, J. Efficacy and safety of the ustekinumab biosimilar ABP 654 in patients with moderate-to-severe plaque psoriasis: A randomized, double-blinded, active-controlled, comparative clinical study over 52 weeks. Br. J. Dermatol. 2024, ljae402. [Google Scholar] [CrossRef] [PubMed]
- Karamova, A.; Znamenskaya, L.; Vorontsova, A.; Obraztsova, O.; Nikonorov, A.; Nikonorova, E.; Deryabin, D.; Kubanov, A. Plasma Cytokines for the Prediction of the Effectiveness of TNFα Inhibitors Etanercept, Infliximab, and Adalimumab in the Treatment of Psoriasis. J. Clin. Med. 2024, 13, 3895. [Google Scholar] [CrossRef] [PubMed]
- Egeberg, A.; Skov, L. Drug survival of secukinumab for moderate-to-severe plaque psoriasis: Reply from authors. Br. J. Dermatol. 2018, 179, 222–223. [Google Scholar] [CrossRef] [PubMed]
- Egeberg, A.; Wu, J.J.; Korman, N.; Solomon, J.A.; Goldblum, O.; Zhao, F.; Mallbris, L. Ixekizumab treatment shows a neutral impact on cardiovascular parameters in patients with moderate-to-severe plaque psoriasis: Results from UNCOVER-1, UNCOVER-2, and UNCOVER-3. J. Am. Acad. Dermatol. 2018, 79, 104–109.e8. [Google Scholar] [CrossRef] [PubMed]
- Sarda, A.; Vaidyanathan, V.; Das, S.; De, A. Laser and Lights in Psoriasis. Indian J. Dermatol. 2024, 69, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Hartmann Schatloff, D.; Retamal Altbir, C.; Valenzuela, F. The role of excimer light in dermatology: A review. An. Bras. Dermatol. 2024, 99, 887–894. [Google Scholar] [CrossRef]
- Radu, A.; Tit, D.M.; Endres, L.M.; Radu, A.F.; Vesa, C.M.; Bungau, S.G. Naturally derived bioactive compounds as precision modulators of immune and inflammatory mechanisms in psoriatic conditions. Inflammopharmacology 2024. [Google Scholar] [CrossRef] [PubMed]
- Rippon, M.; Perrin, A.; Darwood, R.; Ousey, K. The potential benefits of using aloe vera in stoma patient skin care. Br. J. Nurs. 2017, 26, S12–S19. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.U.; Kang, Y.J.; Park, M.K.; Lee, Y.S.; Seo, H.G.; Kim, T.S.; Kim, C.H.; Chang, K.C. Effects of 13-alkyl-substituted berberine alkaloids on the expression of COX-II, TNF-alpha, iNOS, and IL-12 production in LPS-stimulated macrophages. Life Sci. 2003, 73, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Song, S.; Wang, Y.; Zhang, X.; Zhang, J.; Wu, L.; Wu, J.; Li, X. Topical application of berberine ameliorates imiquimod-induced psoriasis-like dermatitis in BALB/c mice via suppressing JAK1/STAT1 signaling pathway. Arab. J. Chem. 2024, 17, 105612. [Google Scholar] [CrossRef]
- Antiga, E.; Bonciolini, V.; Volpi, W.; Del Bianco, E.; Caproni, M. Oral Curcumin (Meriva) Is Effective as an Adjuvant Treatment and Is Able to Reduce IL-22 Serum Levels in Patients with Psoriasis Vulgaris. Biomed. Res. Int. 2015, 2015, 283634. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shao, X.; Xu, J.; Wei, Y.; Xu, F.; Wang, H. Tea tree oil exhibits antifungal activity against Botrytis cinerea by affecting mitochondria. Food Chem. 2017, 234, 62–67. [Google Scholar] [CrossRef]
- Ellis, C.N.; Berberian, B.; Sulica, V.I.; Dodd, W.A.; Jarratt, M.T.; Katz, H.I.; Prawer, S.; Krueger, G.; Rex, I.H., Jr.; Wolf, J.E. A double-blind evaluation of topical capsaicin in pruritic psoriasis. J. Am. Acad. Dermatol. 1993, 29, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Nickoloff, B.J.; Karabin, G.D.; Barker, J.N.; Griffiths, C.E.; Sarma, V.; Mitra, R.S.; Elder, J.T.; Kunkel, S.L.; Dixit, V.M. Cellular localization of interleukin-8 and its inducer, tumor necrosis factor-alpha in psoriasis. Am. J. Pathol. 1991, 138, 129–140. [Google Scholar]
- Yuan, Q.; Xie, F.; Huang, W.; Hu, M.; Yan, Q.; Chen, Z.; Zheng, Y.; Liu, L. The review of alpha-linolenic acid: Sources, metabolism, and pharmacology. Phytother. Res. 2022, 36, 164–188. [Google Scholar] [CrossRef]
- Ayub, H.; Islam, M.; Saeed, M.; Ahmad, H.; Al-Asmari, F.; Ramadan, M.F.; Alissa, M.; Arif, M.A.; Rana, M.U.J.; Subtain, M.; et al. On the health effects of curcumin and its derivatives. Food Sci. Nutr. 2024, 12, 8623–8650. [Google Scholar] [CrossRef]
- Serini, S.; Fasano, E.; Celleno, L.; Cittadini, A.; Calviello, G. Potential of long-chain n-3 polyunsaturated fatty acids in melanoma prevention. Nutr. Rev. 2014, 72, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Serini, S.; Ottes Vasconcelos, R.; Fasano, E.; Calviello, G. Epigenetic regulation of gene expression and M2 macrophage polarization as new potential omega-3 polyunsaturated fatty acid targets in colon inflammation and cancer. Expert Opin. Ther. Targets 2016, 20, 843–858. [Google Scholar] [CrossRef] [PubMed]
- Serini, S.; Cassano, R.; Trombino, S.; Calviello, G. Nanomedicine-based formulations containing ω-3 polyunsaturated fatty acids: Potential application in cardiovascular and neoplastic diseases. Int. J. Nanomed. 2019, 14, 2809–2828. [Google Scholar] [CrossRef] [PubMed]
- Serini, S.; Cassano, R.; Facchinetti, E.; Amendola, G.; Trombino, S.; Calviello, G. Anti-Irritant and Anti-Inflammatory Effects of DHA Encapsulated in Resveratrol-Based Solid Lipid Nanoparticles in Human Keratinocytes. Nutrients 2019, 11, 1400. [Google Scholar] [CrossRef]
- McCusker, M.M.; Grant-Kels, J.M. Healing fats of the skin: The structural and immunologic roles of the ω-6 and ω-3 fatty acids. Clin. Dermatol. 2010, 28, 440–451. [Google Scholar] [CrossRef]
- Upala, S.; Yong, W.C.; Theparee, T.; Sanguankeo, A. Effect of omega-3 fatty acids on disease severity in patients with psoriasis: A systematic review. Int. J. Rheum. Dis. 2017, 20, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Sarandi, E.; Krueger-Krasagakis, S.; Tsoukalas, D.; Evangelou, G.; Sifaki, M.; Kyriakakis, M.; Paramera, E.; Papakonstantinou, E.; Rudofsky, G.; Tsatsakis, A. Novel Fatty Acid Biomarkers in Psoriasis and the Role of Modifiable Factors: Results from the METHAP Clinical Study. Biomolecules 2024, 14, 1114. [Google Scholar] [CrossRef] [PubMed]
- Trombino, S.; Serini, S.; Cassano, R.; Calviello, G. Xanthan gum-based materials for omega-3 PUFA delivery: Preparation, characterization and antineoplastic activity evaluation. Carbohydr. Polym. 2019, 208, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Feingold, K.R. Thematic review series: Skin lipids. The role of epidermal lipids in cutaneous permeability barrier homeostasis. J. Lipid Res. 2007, 48, 2531–2546. [Google Scholar] [CrossRef]
- Barceló-Coblijn, G.; Murphy, E.J. Alpha-linolenic acid and its conversion to longer chain n-3 fatty acids: Benefits for human health and a role in maintaining tissue n-3 fatty acid levels. Prog. Lipid Res. 2009, 48, 355–374. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Etherton, T.D.; Martin, K.R.; Vanden Heuvel, J.P.; Gillies, P.J.; West, S.G.; Kris-Etherton, P.M. Anti-inflammatory effects of polyunsaturated fatty acids in THP-1 cells. Biochem. Biophys. Res. Commun. 2005, 336, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Siebenlist, U.; Franzoso, G.; Brown, K. Structure, regulation and function of NFkappa B. Annu. Rev. Cell Biol. 1994, 10, 405–455. [Google Scholar] [CrossRef]
- Arterburn, L.M.; Hall, E.B.; Oken, H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am. J. Clin. Nutr. 2006, 83, 1467S–1476S. [Google Scholar] [CrossRef]
- Cambiaggi, L.; Chakravarty, A.; Noureddine, N.; Hersberger, M. The Role of α-Linolenic Acid and Its Oxylipins in Human Cardiovascular Diseases. Int. J. Mol. Sci. 2023, 24, 6110. [Google Scholar] [CrossRef] [PubMed]
- Kocaadam, B.; Şanlier, N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2889–2895. [Google Scholar] [CrossRef] [PubMed]
- Chopra, H.; Dey, P.S.; Das, D.; Bhattacharya, T.; Shah, M.; Mubin, S.; Maishu, S.P.; Akter, R.; Rahman, M.H.; Karthika, C.; et al. Curcumin nanoparticles as promising therapeutic agents for drug targets. Molecules 2021, 26, 4998. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Harikumar, K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int. J. Biochem. Cell Biol. 2009, 41, 40–59. [Google Scholar] [CrossRef]
- Walker, B.C.; Mittal, S. Antitumor activity of curcumin in glioblastoma. Int. J. Mol. Sci. 2020, 21, E9435. [Google Scholar] [CrossRef] [PubMed]
- Mahjoob, M.; Stochaj, U. Curcumin nanoformulations to combat aging-related diseases. Ageing Res. Rev. 2021, 69, 101364. [Google Scholar] [CrossRef]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic roles of curcumin: Lessons learned from clinical trials. AAPS J. 2013, 15, 195–218. [Google Scholar] [CrossRef] [PubMed]
- Pourbagher-Shahri, A.M.; Farkhondeh, T.; Ashrafizadeh, M.; Talebi, M.; Samargahndian, S. Curcumin and cardiovascular diseases: Focus on cellular targets and cascades. Biomed. Pharmacother. 2021, 136, 111214. [Google Scholar] [CrossRef]
- Rahmayunita, G.; Jacoeb, T.N.A.; Novianto, E.; Indriatmi, W.; Rihatmadja, R.; Pusponegoro, E.H.D. A double-blind randomized controlled trial of topical Curcuma xanthorrhiza roxb. on mild psoriasis: Clinical manifestations, histopathological features, and K6 expressions. Med. J. Indones. 2018, 27, 178–184. [Google Scholar] [CrossRef]
- Mata, I.R.D.; Mata, S.R.D.; Menezes, R.C.R.; Faccioli, L.S.; Bandeira, K.K.; Bosco, S.M.D. Benefits of turmeric supplementation for skin health in chronic diseases: A systematic review. Crit. Rev. Food Sci. Nutr. 2021, 61, 3421–3435. [Google Scholar] [CrossRef]
- Marton, L.T.; Barbalho, S.M.; Sloan, K.P.; Sloan, L.A.; Goulart, R.A.; Araújo, A.C.; Bechara, M.D. Curcumin, autoimmune and inflammatory diseases: Going beyond conventional therapy—A systematic review. Crit. Rev. Food Sci. Nutr. 2022, 62, 2140–2157. [Google Scholar] [CrossRef] [PubMed]
- Elmets, C.A.; Korman, N.J.; Prater, E.F.; Wong, E.B.; Rupani, R.N.; Kivelevitch, D.; Armstrong, A.W.; Connor, C.; Cordoro, K.M.; Davis, D.M.R.; et al. Joint AAD-NPF Guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J. Am. Acad. Dermatol. 2021, 84, 432–470. [Google Scholar] [CrossRef]
- Cassano, R.; Serini, S.; Curcio, F.; Trombino, S.; Calviello, G. Preparation and Study of Solid Lipid Nanoparticles Based on Curcumin, Resveratrol and Capsaicin Containing Linolenic Acid. Pharmaceutics 2022, 14, 1593. [Google Scholar] [CrossRef]
- Chuang, S.Y.; Lin, C.H.; Sung, C.T.; Fang, J.Y. Murine models of psoriasis and their usefulness for drug discovery. Expert Opin. Drug Discov. 2018, 13, 551–562. [Google Scholar] [CrossRef]
- Chuang, S.Y.; Chen, C.Y.; Yang, S.C.; Alalaiwe, A.; Lin, C.H.; Fang, J.Y. 2,4-Dimethoxy-6-Methylbenzene-1,3-diol, a Benzenoid From Antrodia cinnamomea, Mitigates Psoriasiform Inflammation by Suppressing MAPK/NF-κB Phosphorylation and GDAP1L1/Drp1 Translocation. Front. Immunol. 2021, 12, 664425. [Google Scholar] [CrossRef]
- Serini, S.; Donato, V.; Piccioni, E.; Trombino, S.; Monego, G.; Toesca, A.; Innocenti, I.; Missori, M.; De Spirito, M.; Celleno, L.; et al. Docosahexaenoic acid reverts resistance to UV-induced apoptosis in human keratinocytes: Involvement of COX-2 and HuR. J. Nutr. Biochem. 2011, 22, 874–885. [Google Scholar] [CrossRef]
- Burlando, B.; Parodi, A.; Volante, A.; Bassi, A.M. Comparison of the irritation potentials of Boswellia serrata gum resin and of acetyl-11-keto-β-boswellic acid by in vitro cytotoxicity tests on human skin-derived cell lines. Toxicol. Lett. 2008, 177, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Hinchliffe, T.E.; Wu, T. Biomarkers of An Autoimmune Skin Disease—Psoriasis. Genom. Proteom. Bioinform. 2015, 13, 224–233. [Google Scholar] [CrossRef]
- Wang, Y.; Edelmayer, R.; Wetter, J.; Salte, K.; Gauvin, D.; Leys, L.; Paulsboe, S.; Su, Z.; Weinberg, I.; Namovic, M.; et al. Monocytes/Macrophages play a pathogenic role in IL-23 mediated psoriasis-like skin inflammation. Sci. Rep. 2019, 9, 5310. [Google Scholar] [CrossRef]
- Tokuyama, M.; Mabuchi, T. New Treatment Addressing the Pathogenesis of Psoriasis. Int. J. Mol. Sci. 2020, 21, 7488. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.F.; Chuang, S.Y.; Huang, T.H.; Nguyen, T.M.H.; Wang, P.W.; Alalaiwe, A.; Fang, J.Y. A systematic comparison of the effect of topically applied anthraquinone aglycones to relieve psoriasiform lesion: The evaluation of percutaneous absorption and anti-inflammatory potency. Biomed. Pharmacother. 2022, 145, 112482. [Google Scholar] [CrossRef] [PubMed]
- Bai, F.; Zheng, W.; Dong, Y.; Wang, J.; Garstka, M.A.; Li, R.; An, J.; Ma, H. Serum levels of adipokines and cytokines in psoriasis patients: A systematic review and meta-analysis. Oncotarget 2017, 9, 1266–1278. [Google Scholar] [CrossRef] [PubMed]
- Krueger, J.; Ray, A.; Tamm, I.; Sehgal, P.B. Expression and function of interleukin-6 in epithelial cells. J. Cell Biochem. 1991, 45, 327–334. [Google Scholar] [CrossRef]
- Nickoloff, B.J.; Xin, H.; Nestle, F.O.; Qin, J.Z. The cytokine and chemokine network in psoriasis. Clin. Dermatol. 2007, 25, 568–573. [Google Scholar] [CrossRef]
- Lee, C.H.; Hwang, S.T.Y. Pathophysiology of Chemokines and Chemokine Receptors in Dermatological Science: A Focus on Psoriasis and Cutaneous T-cell Lymphoma. Dermatol. Sin. 2012, 30, 128–135. [Google Scholar] [CrossRef]
- Aggarwal, P.; Fleischer, A.B., Jr. IL-17 and IL-23 Inhibitors Have the Fastest Time to Meaningful Clinical Response for Plaque Psoriasis: A Network Meta-Analysis. J. Clin. Med. 2024, 13, 5139. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.M.; Kuo, Y.Z.; Chang, C.Y.; Chang, C.H.; Fang, W.Y.; Chang, C.N.; Pan, S.C.; Lin, J.Y.; Wu, L.W. The anti-TH17 polarization effect of Indigo naturalis and tryptanthrin by differentially inhibiting cytokine expression. J. Ethnopharmacol. 2020, 255, 112760. [Google Scholar] [CrossRef]
- Tabbarah, S.; Sulaiman, H.; Ansah Owusu, F.; Rajeev Joshi, M.; Marepalli, N.R.; Pino, N.; Saleem Azam, S.; Ali Ahmed, A.; Abraham Suárez Álvarez, J. Shared Pathophysiology of Inflammatory Bowel Disease and Psoriasis: Unraveling the Connection. Cureus 2024, 16, e70148. [Google Scholar] [CrossRef]
- Stein, P.; Gogoll, K.; Tenzer, S.; Schild, H.; Stevanovic, S.; Langguth, P.; Radsak, M.P. Efficacy of imiquimod-based transcutaneous immunization using a nano-dispersed emulsion gel formulation. PLoS ONE 2014, 9, e102664. [Google Scholar] [CrossRef] [PubMed]
- Uttarkar, S.; Brembilla, N.C.; Boehncke, W.H. Regulatory cells in the skin: Pathophysiologic role and potential targets for anti-inflammatory therapies. J. Allergy Clin. Immunol. 2019, 143, 1302–1310. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, R.; Rykowski, P.; Pasierb, A.; Bartoszewicz, Z.; Stakun, M.; Rykowska, M.; Karoń, K.; Rudnicka, L.; Czuwara, J. Adrenocorticotropin/cortisol ratio—A marker of psoriasis severity. Postepy Dermatol. Alergol. 2020, 37, 746–750. [Google Scholar] [CrossRef]
- Reddy, V.K.K.; Shiddapur, G.; Jagdale, N.; Kondapalli, M.P.; Adapa, S. Investigating Interleukin-6 Levels in Type 2 Diabetes Mellitus Patients with and Without Diabetic Nephropathy. Cureus 2024, 16, e67014. [Google Scholar] [CrossRef] [PubMed]
- Kutsuna, T.; Hino, K.; Hasegawa, H.; Watamori, K.; Kidani, T.; Imai, H.; Miura, H. Psoriatic arthritis successfully treated with second-line anti-interleukin-6 treatment: A case report and review of the literature. J. Med. Case Rep. 2022, 16, 402. [Google Scholar] [CrossRef]
- Luo, Y.; Luo, Y.; Chang, J.; Xiao, Z.; Zhou, B. Identification of candidate biomarkers and pathways associated with psoriasis using bioinformatics analysis. Hereditas 2020, 157, 30. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.P.; Lee, C.H.; Farber, J.M. Chemokine receptors in psoriasis. Expert Opin. Ther. Targets 2013, 17, 1405–1422. [Google Scholar] [CrossRef]
- Kumari, S.; Bonnet, M.C.; Ulvmar, M.H.; Wolk, K.; Karagianni, N.; Witte, E.; Uthoff-Hachenberg, C.; Renauld, J.C.; Kollias, G.; Toftgard, R.; et al. Tumor necrosis factor receptor signaling in keratinocytes triggers interleukin-24-dependent psoriasis-like skin inflammation in mice. Immunity 2013, 39, 899–911. [Google Scholar] [CrossRef]
- Schuster, C.; Huard, A.; Sirait-Fischer, E.; Dillmann, C.; Brüne, B.; Weigert, A. S1PR4-dependent CCL2 production promotes macrophage recruitment in a murine psoriasis model. Eur. J. Immunol. 2020, 50, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Simard, M.; Rioux, G.; Morin, S.; Martin, C.; Guérin, S.L.; Flamand, N.; Julien, P.; Fradette, J.; Pouliot, R. Investigation of Omega-3 Polyunsaturated Fatty Acid Biological Activity in a Tissue-Engineered Skin Model Involving Psoriatic Cells. J. Investig. Dermatol. 2021, 141, 2391–2401. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, B.R.; Friedmann Angeli, J.P.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A regulated cell death nexus linking metabolism, redox biology, and disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Ursini, F.; Maiorino, M. Lipid peroxidation and ferroptosis: The role of GSH and GPx4. Free Radic. Biol. Med. 2020, 152, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Rochette, L.; Dogon, G.; Rigal, E.; Zeller, M.; Cottin, Y.; Vergely, C. Lipid Peroxidation and Iron Metabolism: Two Corner Stones in the Homeostasis Control of Ferroptosis. Int. J. Mol. Sci. 2022, 24, 449. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serini, S.; Trombino, S.; Cassano, R.; Marino, M.; Calviello, G. Anti-Inflammatory Effects of Curcumin-Based Nanoparticles Containing α-Linolenic Acid in a Model of Psoriasis In Vitro. Nutrients 2025, 17, 692. https://doi.org/10.3390/nu17040692
Serini S, Trombino S, Cassano R, Marino M, Calviello G. Anti-Inflammatory Effects of Curcumin-Based Nanoparticles Containing α-Linolenic Acid in a Model of Psoriasis In Vitro. Nutrients. 2025; 17(4):692. https://doi.org/10.3390/nu17040692
Chicago/Turabian StyleSerini, Simona, Sonia Trombino, Roberta Cassano, Mariapaola Marino, and Gabriella Calviello. 2025. "Anti-Inflammatory Effects of Curcumin-Based Nanoparticles Containing α-Linolenic Acid in a Model of Psoriasis In Vitro" Nutrients 17, no. 4: 692. https://doi.org/10.3390/nu17040692
APA StyleSerini, S., Trombino, S., Cassano, R., Marino, M., & Calviello, G. (2025). Anti-Inflammatory Effects of Curcumin-Based Nanoparticles Containing α-Linolenic Acid in a Model of Psoriasis In Vitro. Nutrients, 17(4), 692. https://doi.org/10.3390/nu17040692









