L-Theanine Mitigates Acute Alcoholic Intestinal Injury by Activating the HIF-1 Signaling Pathway to Regulate the TLR4/NF-κB/HIF-1α Axis in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Animals and Treatments
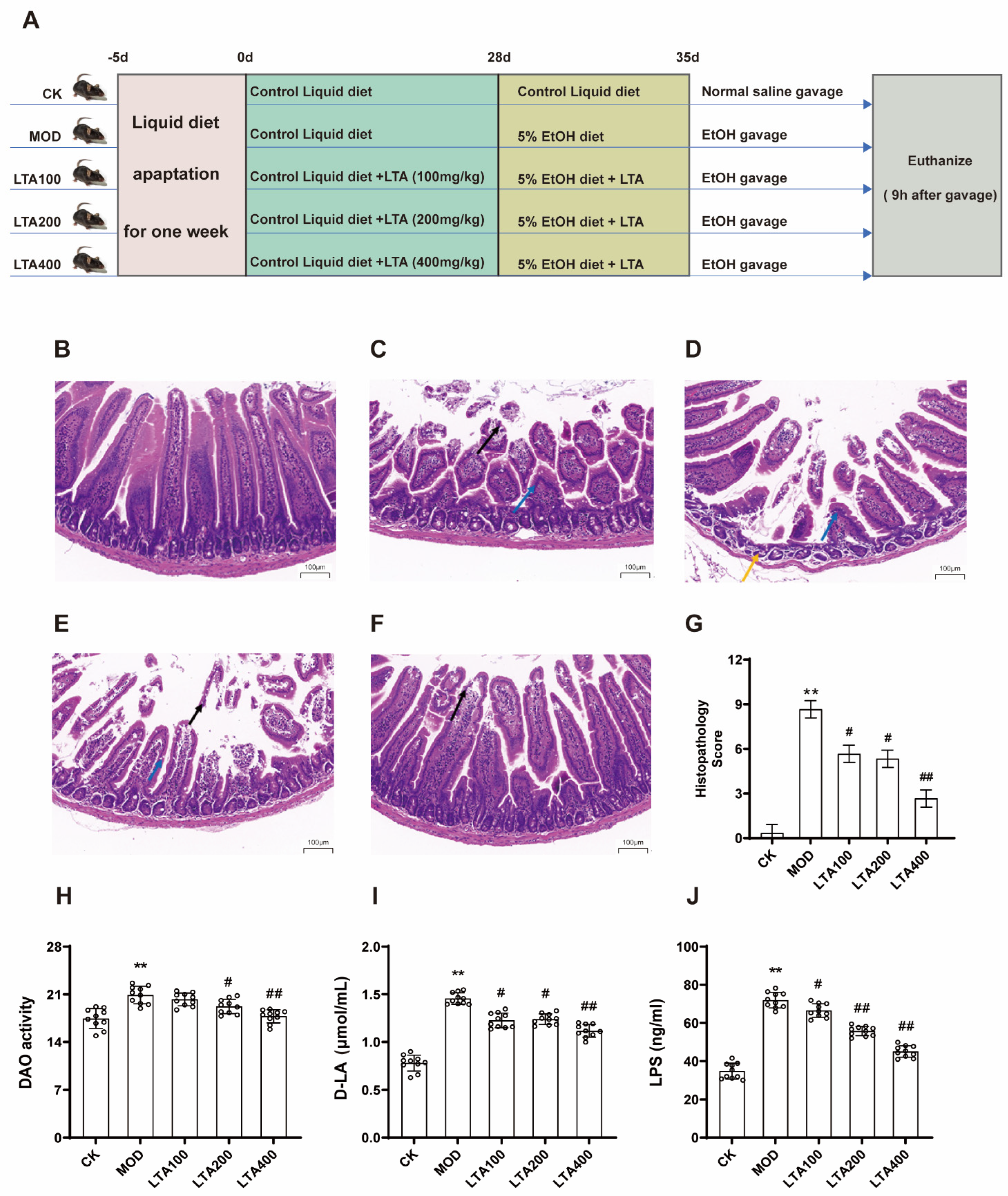
2.3. Acute Alcoholic Intestinal Injury Model
2.4. Treatments
2.5. Histopathological Assessment
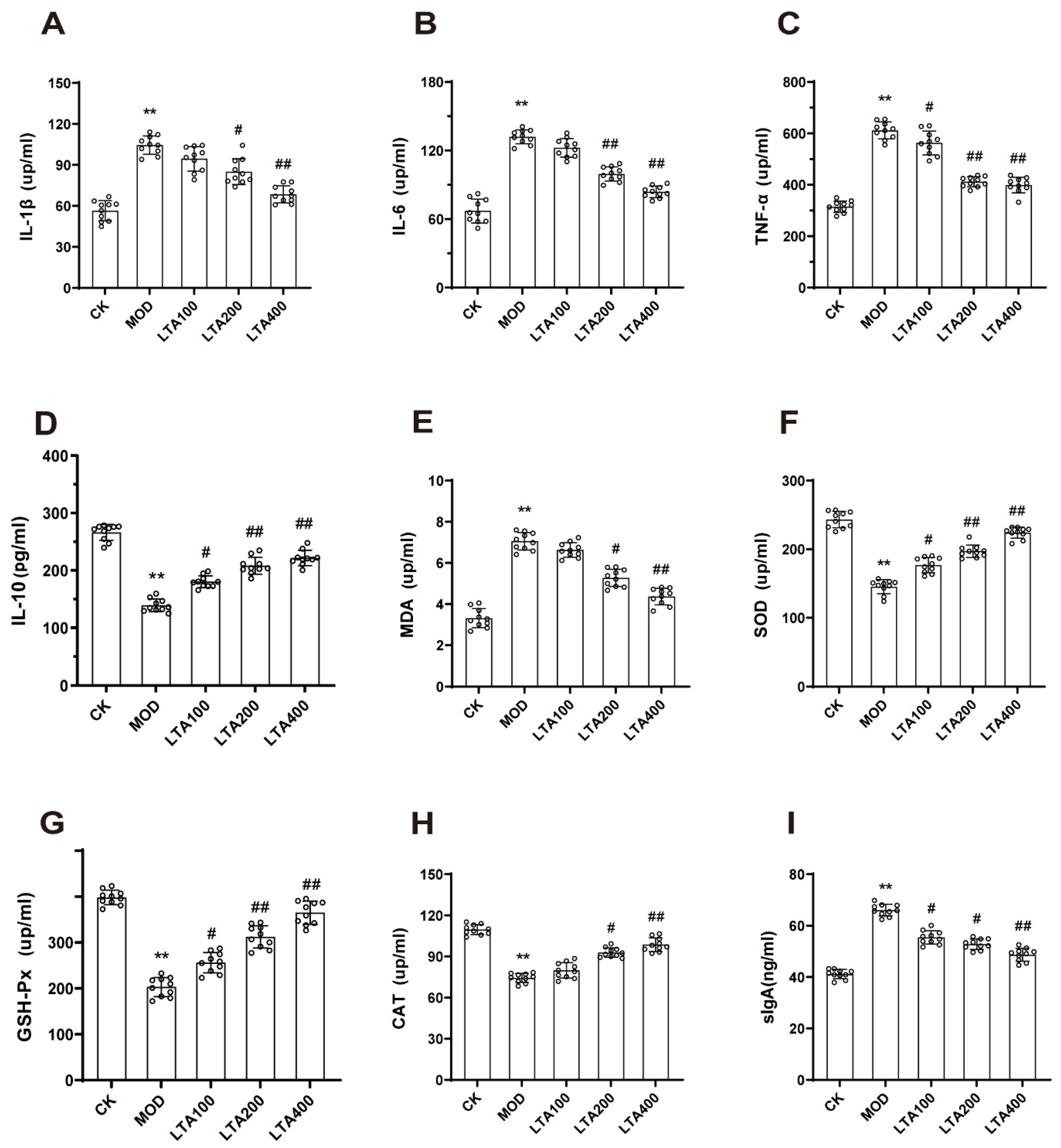
2.6. Biochemical Analysis of Serum and Intestinal Tissue Samples
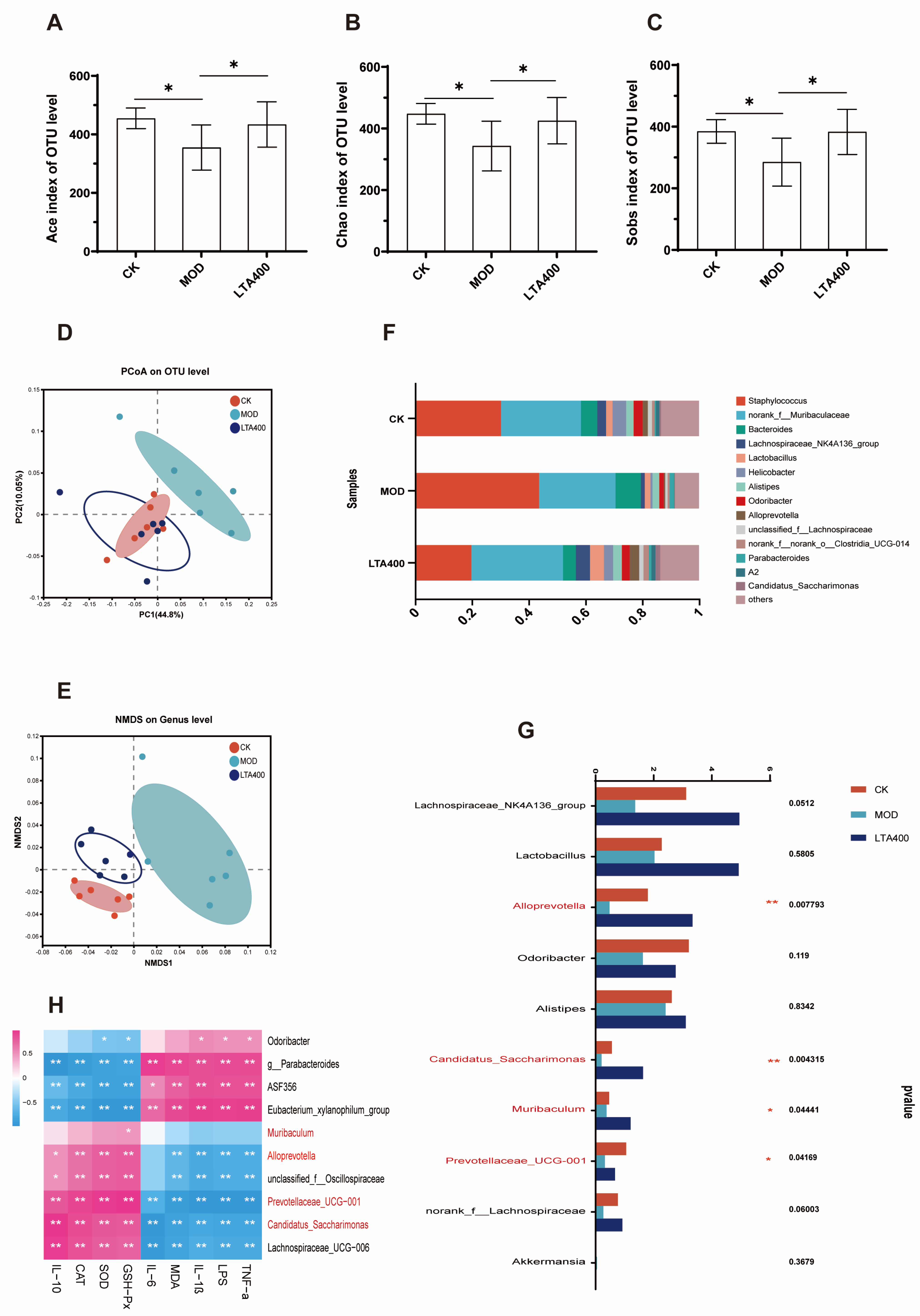
2.7. Gut Microbiota Determination
2.8. LC/MS-Based Gut Metabolomics
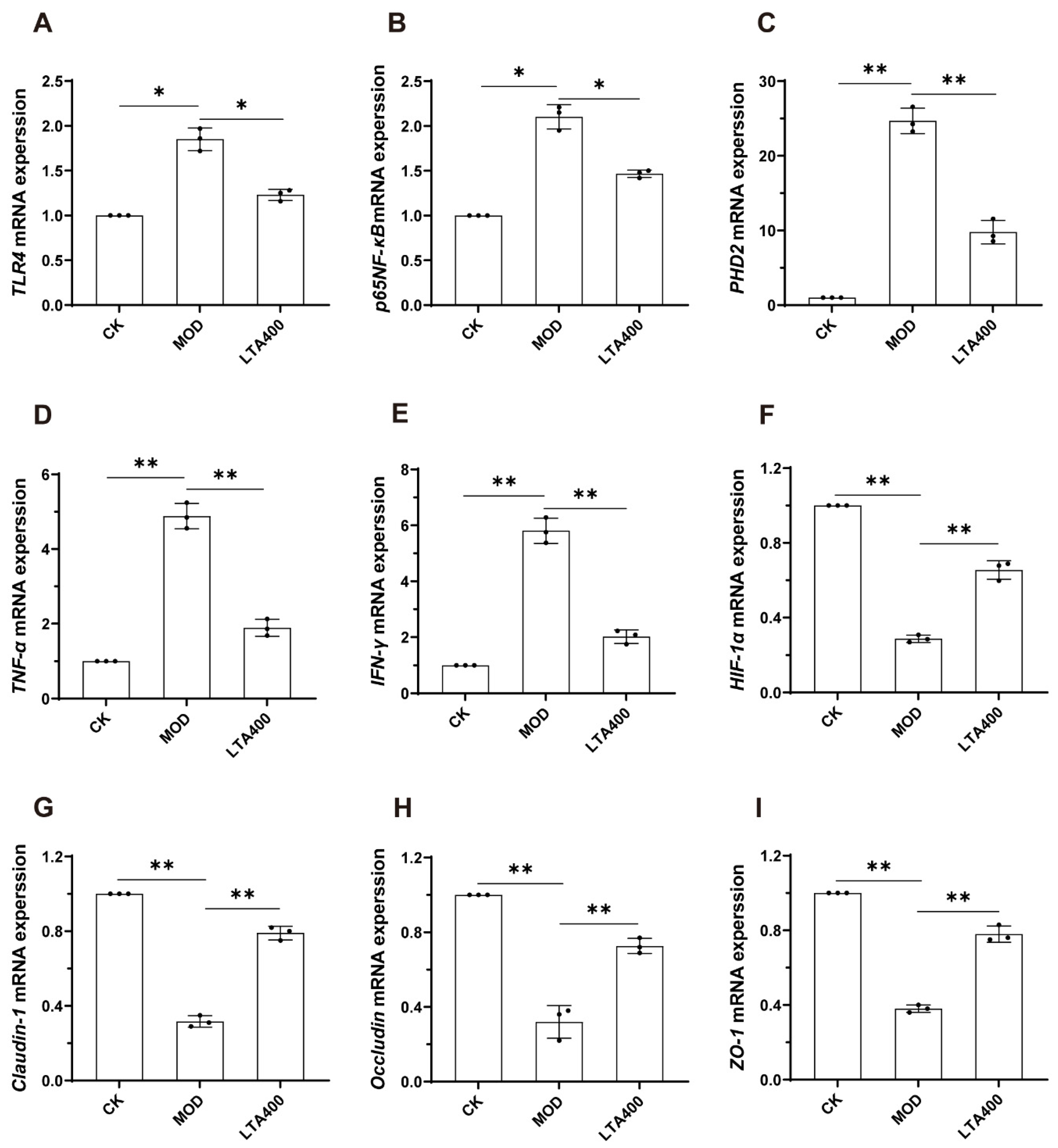
2.9. Quantitative Real-Time PCR
2.10. Western Blotting
2.11. Statistical Analysis
3. Results
3.1. LTA Attenuates Duodenal Pathology and Intestinal Permeability Injury in Mice with Acute Alcoholic Intestinal Injury
3.2. LTA Improved Intestinal Inflammatory Injury and Oxidative Stress in Mice with Acute Alcoholic Intestinal Injury
3.3. LTA Regulates the Intestinal Flora Structure in Mice with Acute Alcoholic Intestinal Injury
3.4. LTA Maintains Intestinal Metabolic Balance in Mice with Acute Alcoholic Intestinal Injury
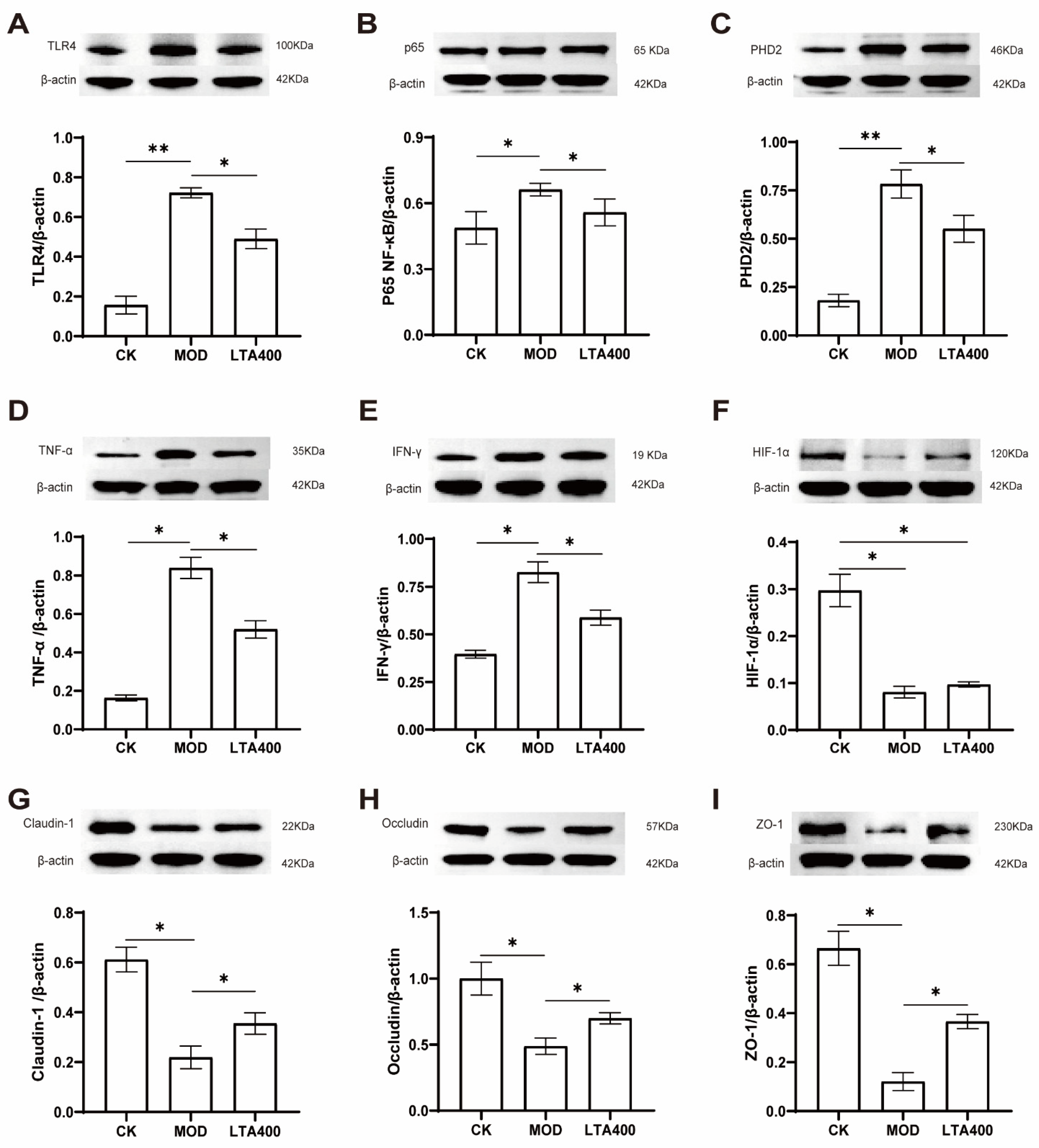
3.5. Activation of the HIF-1 Signaling Pathway to Regulate the TLR4/NF-κB/HIF-1α Axis by LTA and the Consequent Enhancement of the Intestinal Mechanical and Immune Barrier in Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, X.; Yan, C.; Zhang, H.; Chen, L.; Jiang, R.; Zheng, K.; Jin, W.; Ma, H.; Liu, X.; Dong, M. Oral Probiotic Expressing Human Ethanol Dehydrogenase Attenuates Damage Caused by Acute Alcohol Consumption in Mice. Microbiol. Spectr. 2023, 11, e04294-22. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-P.; Lei, F.; Du, F.; Chai, Y.-S.; Jiang, J.-F.; Wang, Y.-G.; Yu, X.; Yan, X.-J.; Xing, D.-M.; Du, L.-J. Protection of gastrointestinal mucosa from acute heavy alcohol consumption: The effect of berberine and its correlation with TLR2, 4/IL1β-TNFα signaling. PLoS ONE 2015, 10, e0134044. [Google Scholar] [CrossRef] [PubMed]
- Ming, L.; Qiao, X.; Yi, L.; Siren, D.; He, J.; Hai, L.; Guo, F.; Xiao, Y.; Ji, R. Camel milk modulates ethanol-induced changes in the gut microbiome and transcriptome in a mouse model of acute alcoholic liver disease. J. Dairy Sci. 2020, 103, 3937–3949. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, S.; Dong, S.; Zhang, Y.; Ismael, M.; Wang, S.; Shi, C.; Yang, J.; Wang, X.; Lü, X. Interaction of Companilactobacillus crustorum MN047-derived bacteriocins with gut microbiota. Food Chem. 2022, 396, 133730. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, S.; Chen, G.; Liu, C.; Zhang, M.; Wang, X. Nomilin Attenuates Lipopolysaccharide-Induced Inflammatory Response by Binding with Myeloid Differentiation Protein-2. Comb. Chem. High Throughput Screen. 2023, 26, 2469–2475. [Google Scholar] [CrossRef]
- Obianwuna, U.E.; Agbai Kalu, N.; Wang, J.; Zhang, H.; Qi, G.; Qiu, K.; Wu, S. Recent Trends on Mitigative Effect of Probiotics on Oxidative-Stress-Induced Gut Dysfunction in Broilers under Necrotic Enteritis Challenge: A Review. Antioxidants 2023, 12, 911. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, Q.-Y.; Jiang, Q.-Y.; Kang, X.-M.; Zhao, L. Polysaccharides derived from Lycium barbarum suppress IGF-1-induced angiogenesis via PI3K/HIF-1α/VEGF signalling pathways in MCF-7 cells. Food Chem. 2012, 131, 1479–1484. [Google Scholar] [CrossRef]
- Powell-Coffman, J.A. Hypoxia signaling and resistance in C. elegans. Trends Endocrinol. Metab. 2010, 21, 435–440. [Google Scholar] [CrossRef]
- Morris, N.L.; Yeligar, S.M. Role of HIF-1α in alcohol-mediated multiple organ dysfunction. Biomolecules 2018, 8, 170. [Google Scholar] [CrossRef]
- Ouyang, X.; Mehal, W.Z. The multi-dimensional role of intestinal HIFs in liver pathobiology. J. Hepatol. 2018, 69, 772–773. [Google Scholar] [CrossRef]
- Singh, D.; Srivastava, S.; Pradhan, M.; Kanwar, J.R.; Singh, M. Inflammatory bowel disease: Pathogenesis, causative factors, issues, drug treatment strategies, and delivery approaches. Crit. Rev. ™ Ther. Drug Carr. Syst. 2015, 32, 181–214. [Google Scholar] [CrossRef] [PubMed]
- Triantafyllidi, A.; Xanthos, T.; Papalois, A.; Triantafillidis, J.K. Herbal and plant therapy in patients with inflammatory bowel disease. Ann. Gastroenterol. Q. Publ. Hell. Soc. Gastroenterol. 2015, 28, 210. [Google Scholar]
- Tavares, E.d.A.; Guerra, G.C.B.; da Costa Melo, N.M.; Dantas-Medeiros, R.; da Silva, E.C.S.; Andrade, A.W.L.; de Souza Araújo, D.F.; da Silva, V.C.; Zanatta, A.C.; de Carvalho, T.G. Toxicity and Anti-Inflammatory Activity of Phenolic-Rich Extract from Nopalea cochenillifera (Cactaceae): A Preclinical Study on the Prevention of Inflammatory Bowel Diseases. Plants 2023, 12, 594. [Google Scholar] [CrossRef]
- Vuong, Q.V.; Bowyer, M.C.; Roach, P.D. L-Theanine: Properties, synthesis and isolation from tea. J. Sci. Food Agric. 2011, 91, 1931–1939. [Google Scholar] [CrossRef]
- Ben, P.; Zhang, Z.; Zhu, Y.; Xiong, A.; Gao, Y.; Mu, J.; Yin, Z.; Luo, L. l-Theanine attenuates cadmium-induced neurotoxicity through the inhibition of oxidative damage and tau hyperphosphorylation. Neurotoxicology 2016, 57, 95–103. [Google Scholar] [CrossRef]
- Duygu, T.; Nevin, Ş. L-theanine, unique amino acid of tea, and its metabolism, health effects, and safety. Crit. Rev. Food Sci. Nutr. 2017, 57, 1681–1687. [Google Scholar]
- Lin, L.; Zeng, L.; Liu, A.; Peng, Y.; Yuan, D.; Zhang, S.; Li, Y.; Chen, J.; Xiao, W.; Gong, Z. l-Theanine regulates glucose, lipid, and protein metabolism via insulin and AMP-activated protein kinase signaling pathways. Food Funct. 2020, 11, 1798–1809. [Google Scholar] [CrossRef]
- Wang, B.; Liu, S.; Lin, L.; Xu, W.; Gong, Z.; Xiao, W. The protective effect of l-theanine on the intestinal barrier in heat-stressed organisms. Food Funct. 2024, 15, 3036–3049. [Google Scholar] [CrossRef]
- Liu, M.Y.; Xu, K.H.; Liu, S.; Xiao, W.J. Protective Effect and Mechanism of l-Theanine on Acute Alcoholic Liver Injury in Mice. Mol. Nutr. Food Res. 2024, 68, 2400766. [Google Scholar] [CrossRef]
- Bertola, A.; Mathews, S.; Ki, S.H.; Wang, H.; Gao, B. Mouse model of chronic and binge ethanol feeding (the NIAAA model). Nat. Protoc. 2013, 8, 627–637. [Google Scholar] [CrossRef]
- Wang, H.; Shen, H.; Seo, W.; Hwang, S. Experimental models of fatty liver diseases: Status and appraisal. Hepatol. Commun. 2023, 7, e00200. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Gu, L.; Liu, X.; Yan, X.; Bi, X.; Fan, X.; Zhou, J.; Lu, S.; Luo, L.; Yin, Z. L-theanine prevents progression of nonalcoholic hepatic steatosis by regulating hepatocyte lipid metabolic pathways via the CaMKKβ-AMPK signaling pathway. Nutr. Metab. 2022, 19, 29. [Google Scholar] [CrossRef] [PubMed]
- Qu, Q.-Y.; Song, X.-Y.; Lin, L.; Gong, Z.-H.; Xu, W.; Xiao, W.-J. L-theanine modulates intestine-specific immunity by regulating the differentiation of CD4+ T cells in ovalbumin-sensitized mice. J. Agric. Food Chem. 2022, 70, 14851–14863. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, B.; Lin, L.; Xu, W.; Gong, Z.H.; Xiao, W.J. L-Theanine alleviates heat stress through modulation of gut microbiota and immunity. J. Sci. Food Agric. 2023, 104, 2059–2072. [Google Scholar] [CrossRef]
- Gong, Z.; Lin, L.; Liu, Z.; Zhang, S.; Liu, A.; Chen, L.; Liu, Q.; Deng, Y.; Xiao, W. Immune-modulatory effects and mechanism of action of L-theanine on ETEC-induced immune-stressed mice via nucleotide-binding oligomerization domain-like receptor signaling pathway. J. Funct. Foods 2019, 54, 32–40. [Google Scholar] [CrossRef]
- Bolte, L.A.; Vila, A.V.; Imhann, F.; Collij, V.; Gacesa, R.; Peters, V.; Wijmenga, C.; Kurilshikov, A.; Campmans-Kuijpers, M.J.; Fu, J. Long-term dietary patterns are associated with pro-inflammatory and anti-inflammatory features of the gut microbiome. Gut 2021, 70, 1287–1298. [Google Scholar] [CrossRef]
- Joshi, A.U.; Van Wassenhove, L.D.; Logas, K.R.; Minhas, P.S.; Andreasson, K.I.; Weinberg, K.I.; Chen, C.-H.; Mochly-Rosen, D. Aldehyde dehydrogenase 2 activity and aldehydic load contribute to neuroinflammation and Alzheimer’s disease related pathology. Acta Neuropathol. Commun. 2019, 7, 190. [Google Scholar] [CrossRef]
- Han, H.; He, N.; Pan, E.; Tan, X.; Yang, Z.; Li, X.; Shi, D.; Dong, J. Disruption of the intestinal barrier by avermectin in carp involves oxidative stress and apoptosis and leads to intestinal inflammation. Pestic. Biochem. Physiol. 2023, 195, 105531. [Google Scholar] [CrossRef]
- Wang, G.; Ma, F.; Xie, K.; Li, X.; Tan, X.; Xia, Y.; Wang, Y.; Dong, J. Liensinine alleviates mouse intestinal injury induced by sepsis through inhibition of oxidative stress, inflammation, and cell apoptosis. Int. Immunopharmacol. 2024, 127, 111335. [Google Scholar] [CrossRef]
- Zhou, S.-K.; Xu, J.-D.; Gao, X.-Q.; Zhang, R.-J.; Cheng, F.-F.; Yao, W.-F.; Zhang, Y.; Geng, T.; Zhang, L. Fructus Jujubae cooperated with water-expelling members in Shizao decoction alleviated intestinal injury and malignant ascites by modulating gut microbiota and metabolic homeostasis. Phytomedicine 2024, 133, 155895. [Google Scholar] [CrossRef]
- Gu, M.; Jia, Q.; Zhang, Z.; Bai, N.; Xu, X.; Xu, B. Soya-saponins induce intestinal inflammation and barrier dysfunction in juvenile turbot (Scophthalmus maximus). Fish Shellfish Immunol. 2018, 77, 264–272. [Google Scholar] [CrossRef]
- Yan, Y.; Kolachala, V.; Dalmasso, G.; Nguyen, H.; Laroui, H.; Sitaraman, S.V.; Merlin, D. Temporal and spatial analysis of clinical and molecular parameters in dextran sodium sulfate induced colitis. PLoS ONE 2009, 4, e6073. [Google Scholar] [CrossRef]
- Wang, D.; Cai, M.; Wang, T.; Liu, T.; Huang, J.; Wang, Y.; Granato, D. Ameliorative effects of L-theanine on dextran sulfate sodium induced colitis in C57BL/6J mice are associated with the inhibition of inflammatory responses and attenuation of intestinal barrier disruption. Food Res. Int. 2020, 137, 109409. [Google Scholar] [CrossRef]
- Liang, W.; Zhao, Y.-J.; Yang, H.; Shen, L.-H. Effects of antioxidant system on coronary artery lesions in patients with abnormal glucose metabolism. Aging Clin. Exp. Res. 2017, 29, 141–146. [Google Scholar] [CrossRef]
- Hwang, J.; Jin, J.; Jeon, S.; Moon, S.H.; Park, M.Y.; Yum, D.-Y.; Kim, J.H.; Kang, J.-E.; Park, M.H.; Kim, E.-J. SOD1 suppresses pro-inflammatory immune responses by protecting against oxidative stress in colitis. Redox Biol. 2020, 37, 101760. [Google Scholar] [CrossRef]
- Liu, T.; Xia, Y.; Li, J.; Li, S.; Feng, J.; Wu, L.; Zhang, R.; Xu, S.; Cheng, K.; Zhou, Y. Shikonin attenuates concanavalin A-induced acute liver injury in mice via inhibition of the JNK pathway. Mediat. Inflamm. 2016, 2016, 2748367. [Google Scholar] [CrossRef]
- Jiang, X.; Zhou, Y.; Zhang, Y.; Tian, D.; Jiang, S.; Tang, Y. Hepatoprotective effect of pyrroloquinoline quinone against alcoholic liver injury through activating Nrf2-mediated antioxidant and inhibiting TLR4-mediated inflammation responses. Process Biochem. 2020, 92, 303–312. [Google Scholar] [CrossRef]
- Chen, L.; Xiao, W.-j.; Yan, Q.-x.; Gong, Z.-h.; Zhang, S.; Zeng, L.; Yang, M.; Zhou, Y.-h. Protective effects of L-theanine on rats with dextran sulfate sodium-induced inflammatory bowel disease. Arch. Pharmacal Res. 2020, 43, 821–862. [Google Scholar] [CrossRef]
- Xu, W.; Liu, A.-X.; Liu, K.-H.; Zhang, S.; Gong, Z.-H.; Xiao, W.-J. l-Theanine Alleviates Ulcerative Colitis by Regulating Colon Immunity via the Gut Microbiota in an MHC-II-Dependent Manner. J. Agric. Food Chem. 2024, 72, 19852–19868. [Google Scholar] [CrossRef]
- Lee, E.; Lee, J.-E. Impact of drinking alcohol on gut microbiota: Recent perspectives on ethanol and alcoholic beverage. Curr. Opin. Food Sci. 2021, 37, 91–97. [Google Scholar] [CrossRef]
- Dubinkina, V.B.; Tyakht, A.V.; Odintsova, V.Y.; Yarygin, K.S.; Kovarsky, B.A.; Pavlenko, A.V.; Ischenko, D.S.; Popenko, A.S.; Alexeev, D.G.; Taraskina, A.Y. Links of gut microbiota composition with alcohol dependence syndrome and alcoholic liver disease. Microbiome 2017, 5, 141. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Lin, H.; Luo, F.; Wu, X.; Zhu, L.; Chen, S.; Luo, H.; Ye, F.; Peng, X.; Zhang, Y. Oryzanol ameliorates dss-stimulated gut barrier damage via targeting the gut microbiota accompanied by the tlr4/nf-κb/nlrp3 cascade response in vivo. J. Agric. Food Chem. 2022, 70, 15747–15762. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.; Cai, Z.; Chai, J.; Liu, J.; Liu, B.; Yu, Y.; Liu, J.; Zhang, T. Egg white peptides ameliorate dextran sulfate sodium-induced acute colitis symptoms by inhibiting the production of pro-inflammatory cytokines and modulation of gut microbiota composition. Food Chem. 2021, 360, 129981. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, Q.; Mi, X.; Qiu, L.; Tao, X.; Zhang, Z.; Xia, J.; Wu, Q.; Wei, H. Ripened Pu-erh tea extract promotes gut microbiota resilience against dextran sulfate sodium induced colitis. J. Agric. Food Chem. 2021, 69, 2190–2203. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Jin, L.; Xia, D.; Zhang, Q.; Ma, L.; Zheng, H.; Xu, T.; Chang, S.; Li, X.; Xun, Z. Nitrate ameliorates dextran sodium sulfate-induced colitis by regulating the homeostasis of the intestinal microbiota. Free Radic. Biol. Med. 2020, 152, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-Z.; Zhang, K.-K.; He, J.-T.; Chen, L.-J.; Ding, J.-F.; Liu, J.-L.; Li, J.-H.; Liu, Y.; Li, X.-W.; Zhao, D. Obeticholic acid protects against methamphetamine-induced anxiety-like behavior by ameliorating microbiota-mediated intestinal barrier impairment. Toxicology 2023, 486, 153447. [Google Scholar] [CrossRef]
- Wu, D.; Fan, Z.; Li, J.; Zhang, Y.; Wang, C.a.; Xu, Q.; Wang, L. Evaluation of alpha-ketoglutarate supplementation on the improvement of intestinal antioxidant capacity and immune response in songpu mirror carp (Cyprinus carpio) after infection with aeromonas hydrophila. Front. Immunol. 2021, 12, 690234. [Google Scholar] [CrossRef]
- Li, X.-Y.; Meng, L.; Shen, L.; Ji, H.-F. Regulation of gut microbiota by vitamin C, vitamin E and β-carotene. Food Res. Int. 2023, 169, 112749. [Google Scholar] [CrossRef]
- Koukoulas, K.; Giakountis, A.; Karagiota, A.; Samiotaki, M.; Panayotou, G.; Simos, G.; Mylonis, I. ERK signaling controls productive HIF-1 binding to chromatin and cancer cell adaptation to hypoxia through HIF-1α interaction with NPM1. Mol. Oncol. 2021, 15, 3468–3489. [Google Scholar] [CrossRef]
- Saeedi, B.; Glover, L. P-212 A Role for Hypoxia-Inducible Factor (HIF) in Intestinal Epithelial Junctional Assembly and Barrier Function. Inflamm. Bowel Dis. 2013, 19 (Suppl. S1), S109–S110. [Google Scholar] [CrossRef]
- Shah, Y.M. The role of hypoxia in intestinal inflammation. Mol. Cell. Pediatr. 2016, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Huang, Z.; Jia, G.; Zhao, H.; Liu, G.; Chen, X. Naringin mitigates LPS-induced intestinal barrier injury in mice. Food Funct. 2023, 14, 1617–1626. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, S.; Pan, X.; Zhang, M.; Lv, Z.; Pan, L.-L.; Sun, J. Recombinant CRAMP-producing Lactococcus lactis attenuates dextran sulfate sodium-induced colitis by colonic colonization and inhibiting p38/NF-κB signaling. Food Nutr. Res. 2021, 65, 1–11. [Google Scholar] [CrossRef]
- Peng, Z.; Liu, X.; Jin, M.; Zhan, Y.; Zhang, X.; Bao, Y.; Liu, M. Hypoxia Activates HIF-1α and Affects Gene Expression and Transcriptional Regulation of PHD in Tegillarca granosa. Fishes 2023, 8, 359. [Google Scholar] [CrossRef]
- Rius, J.; Guma, M.; Schachtrup, C.; Akassoglou, K.; Zinkernagel, A.S.; Nizet, V.; Johnson, R.S.; Haddad, G.G.; Karin, M. NF-κB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1α. Nature 2008, 453, 807–811. [Google Scholar] [CrossRef]
- Guan, Z.; Ding, C.; Du, Y.; Zhang, K.; Zhu, J.N.; Zhang, T.; He, D.; Xu, S.; Wang, X.; Fan, J. HAF drives the switch of HIF-1α to HIF-2α by activating the NF-κB pathway, leading to malignant behavior of T24 bladder cancer cells. Int. J. Oncol. 2014, 44, 393–402. [Google Scholar] [CrossRef]
- Chen, M.-H.; Wang, Y.-H.; Sun, B.-J.; Yu, L.-M.; Chen, Q.-Q.; Han, X.-X.; Liu, Y.-H. HIF-1α activator DMOG inhibits alveolar bone resorption in murine periodontitis by regulating macrophage polarization. Int. Immunopharmacol. 2021, 99, 107901. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, S.; Gu, J.; Yang, J.; Dang, X.; Liu, K.; Gong, Z.; Xiao, W. L-Theanine Mitigates Acute Alcoholic Intestinal Injury by Activating the HIF-1 Signaling Pathway to Regulate the TLR4/NF-κB/HIF-1α Axis in Mice. Nutrients 2025, 17, 720. https://doi.org/10.3390/nu17040720
Tan S, Gu J, Yang J, Dang X, Liu K, Gong Z, Xiao W. L-Theanine Mitigates Acute Alcoholic Intestinal Injury by Activating the HIF-1 Signaling Pathway to Regulate the TLR4/NF-κB/HIF-1α Axis in Mice. Nutrients. 2025; 17(4):720. https://doi.org/10.3390/nu17040720
Chicago/Turabian StyleTan, Simin, Jiayou Gu, Jiahao Yang, Xuhui Dang, Kehong Liu, Zhihua Gong, and Wenjun Xiao. 2025. "L-Theanine Mitigates Acute Alcoholic Intestinal Injury by Activating the HIF-1 Signaling Pathway to Regulate the TLR4/NF-κB/HIF-1α Axis in Mice" Nutrients 17, no. 4: 720. https://doi.org/10.3390/nu17040720
APA StyleTan, S., Gu, J., Yang, J., Dang, X., Liu, K., Gong, Z., & Xiao, W. (2025). L-Theanine Mitigates Acute Alcoholic Intestinal Injury by Activating the HIF-1 Signaling Pathway to Regulate the TLR4/NF-κB/HIF-1α Axis in Mice. Nutrients, 17(4), 720. https://doi.org/10.3390/nu17040720





