Much More than Nutrients: The Protective Effects of Nutraceuticals on the Blood–Brain Barrier in Diseases
Abstract
:1. Importance of Nutraceuticals in Health and Disease
2. Major Properties of Nutraceuticals with Protective Effects on the Blood–Brain Barrier
| Nutraceutical | Properties | Source | Antioxidant | Efflux Pump Interaction Y/N | Influx Transport Interaction | BBB/Brain Penetration |
|---|---|---|---|---|---|---|
| Alkaloids | ||||||
| Caffeine | 194 Da A | Coffee, green and black tea, guarana berries | Yes [19] | No [20] | CNT2/SLC28A2 [21] OAT1/SLC22A6 [22] GLUT1/SLC2A1 [23] | Yes, high [24,25] |
| Capsaicin | 305 Da A | Chili pepper | Yes [26] | Yes [27,28] | ND | Yes, high [29] |
| Theophylline | 180 Da A | Cocoa beans, brewed tea | Yes [30] | No [31] | OAT1 [22] | Yes [32] |
| Anthocyanidines | ||||||
| Cyanidin/Cyanidin-3-O-beta-glucoside/Procyanidine | 287 Da H | Red wine, elderflower, berries, tea, apple, cinnamon | Yes * [33] | Yes [34,35] | GLUT1/SLC2A1 [36] | Yes [36,37] |
| Malvidin/Malvidin-3-O-glucoside | 331 Da H | Red wine, berries | Yes [33,38] | Yes [34] | GLUT1/SLC2A1, GLUT3/SLC2A3 [39] | Yes [40] |
| Carotenoids | ||||||
| Astaxanthin | 597 Da L | Seafood, salmon, trout, algae | Yes * | Yes [41] | ND | Yes * |
| β-Carotene | 537 Da L | Carrots, sweet potato, pumpkin | Yes * | Yes [31,42] | GLUT4/SLC2A4 [43] | No * |
| Fucoxanthin | 659 Da L | Brown algae | Yes * [44] | Yes [45] | SLC7A11 [44] | ND * |
| Lutein | 569 Da L | Kale, spinach, orange, egg yolk, avocado | Yes * | Yes [46] | SR-B1 [47] | Yes * |
| Lycopene | 537 Da L | Tomato, watermelon grapefruit, pomegranate | Yes * | Yes [48] | ND | Yes * |
| Diarylheptanoids | ||||||
| Curcumin | 368 Da L | Turmeric | Yes * [49] | Yes [50,51] | GLUT1/SLC2A1 [52] | No [53] |
| Flavonoids | ||||||
| Apigenin | 270 Da L | Parsley, celery, chamomile tea | Yes [54,55] | Yes [56,57] | GLUT1/SLC2A1 [58] | Yes, low [56,59] |
| Catechin/ Epicatechin | 290 Da L | Tea, red wine, cocoa | Yes [60,61] | Yes [62,63] | ASBT/SLC10A2 [64] | Yes [36] |
| Chrysin | 254 Da L | Chamomile, honey, propolis, passion fruit | Yes [65,66] | Yes [67,68] | OATP [69] | ND |
| Daidzein | 254 Da L | Soy, soy products | Yes [70] | Yes [71,72] | GLUT4/SLC2A4 [73] | Yes, high [59] |
| Fisetin | 286 Da L | Onions, leeks, broccoli | Yes [74] | Yes [75] | GLUT4/SLC2A4 [76] | Yes, low [59] |
| Genistein | 270 Da A | Soy, soy products | Yes * [77] | Yes [72,78] | Noradrenalin, serotonin transporter [79] GLUT1/SLC2A1 [80] | Yes, high [56,59] |
| Hesperetin | 302 Da L | Citrus fruit, herbs, wine | Yes * [81,82] | Yes [67,83] | ND | Yes [37] |
| Hesperidin | 611 Da H | Citrus fruit, herbs, wine | Yes [84,85] | Yes [68,86] | ND | Yes, low [56] |
| Kaempferol | 286 Da L | Onion, leeks, broccoli, ginkgo biloba | Yes [87,88] | Yes [67,71] | GLUT4/SLC2A4 [89] | Yes, low [56,90] |
| Luteolin | 286 Da L | Pepper, leafy greens, celery, broccoli | Yes [91] | Yes [92] | SLC7A11 [93] | Yes, low [59] |
| Myricetin | 318 Da L | Onions, leeks, broccoli | Yes [87] | Yes [94] | PCFT/SLC46A1 [95] | Yes [90] |
| Naringenin | 272 Da L | Citrus fruits, herbs, wine | Yes [96] | Yes [65] | ND | Yes [35] |
| Naringin | 581 Da H | Citrus fruits, herbs, wine | Yes [84] | Yes [68,97] | ND | ND |
| Quercetin | 302 Da L | Onion, broccoli, ginkgo biloba, apple | Yes * | Yes [67,98] | SLC7A11 [99] | Yes, low [40,56,90] |
| Rutin | 611 Da H | Citrus fruits, herbs, wine | Yes * | Yes [56,100] | OATP2B1/SLCO2B1 [101] GLUT4/SLC2A4 [102] | Yes, low [56,59] |
| Silybin/ Silymarin | 482 Da L | Milk thistle | Yes * [103] | Yes [68] | ND | ND |
| Tangeretin | 372 Da L | Tangerine, citrus peel | Yes [104,105] | Yes [106] | SGLT1/SLC5A1 [107] | Yes [108] |
| Monoterpenes | ||||||
| Borneol | 154 Da L | Coriander, ginger oil, rosemary, thyme | Yes [109] | Yes [110,111] | ND | Yes [112] |
| Carvacrol | 150 Da L | Oregano, thyme | Yes [113] | Yes [114] | ND | ND |
| Omega-3 fatty acids | ||||||
| Docosahexaenoic acid | 328 Da L | Oceanic fish oil, seaweed | Yes [115] | Yes [116] | MFSD2A [117] | Yes [117] |
| Eicosapentaenoic acid | 302 Da L | Fish oil, seaweed | Yes [118,119] | Yes [116] | VNUT/LC17A9 [120] | Yes [121] |
| Organosulfur compounds | ||||||
| α-Lipoic acid | 206 Da L | Broccoli, yeast, meat, kidney, heart, liver | Yes [122,123] | Yes [124] | SMVT/SLC5A6 [125] | Yes [126] |
| Sulforaphane | 177 Da L | Broccoli, kale, cauliflower | Yes * | Yes [127] | ND | Yes [128] |
| Phenolic acids | ||||||
| Caffeic acid | 180 Da L | Berries, kiwi, plum, apple | Yes [129] | Yes [130] | MCT1/SLC16A1, MCT4/SLC16A3 [131] | Yes [132] |
| Cinnamic acid | 148 Da L | Cinnamon, grape, cocoa | Yes [133] | Yes [134] | MCT1/SLC16A1, MCT4/SLC16A3 [131] | ND |
| p-Coumaric acid | 164 Da L | Berries, kiwi, plum, apple | Yes [135] | ND | MCT1/SLC16A1, MCT4/SLC16A3 [131] OAT3/SLC22A8 [136] | ND |
| Ferulic acid | 194 Da L | Grains, nuts, fruits, vegetables | Yes [137,138] | Yes [139] | MCT1/SLC16A1, MCT4/SLC16A3 [130,140] OAT3/SLC22A8 [136] | Yes, low [25] |
| Gallic acid | 170 Da L | Berries, kiwi, plum, apple | Yes [141] | Yes [134] | OAT3/SLC22A8 [136] | Yes [142] |
| Rosmarinic acid | 360 Da L | Berries, kiwi, plum, apple | Yes [143,144,145] | Yes [146] | OAT1/SLC22A6, OAT3/SLC22A8 [147] | Yes, low [132] |
| Stilbenes | ||||||
| Piceatannol | 244 Da L | Grape, white tea, passion fruit | Yes [148] | No [149] | MCT1/SLC16A1, MCT4/SLC16A3 [150] | ND |
| Polydatin | 390 Da L | Grapes, cocoa, peanuts | Yes [151] | Yes [152] | ND | Yes, low [153] |
| Pterostilbene | 256 Da L | Blueberries, grapes | Yes [154] | ND | MCT1/SLC16A1, MCT4/SLC16A3 [150] | Yes [155] |
| Resveratrol | 228 Da L | Grapes, wine, peanuts | Yes * [33] | Yes [156] | ND | Yes, low [59,157] |
| Vitamins | ||||||
| Vitamin C/ Ascorbic acid | 176 Da H | Fruits, vegetables | Yes * [158] | No [159] | SLC19A1, SLC23A2 [11] | Yes [160] |
| Vitamin B9/ Folic acid | 441 Da H | Fruits, vegetables, nuts | Yes [161] | ND | SLC19A1, SLC46A1 [11] | Yes [162] |
| Vitamin D3/ Cholecalciferol | 385 Da L | Fish, milk, meat | Yes [163] | ND | LRPs [11] | Yes [164] |
| Vitamin E/ α-Tocopherol | 431 Da L | Plant oil, nuts, nut oil, spinach, broccoli | Yes * | Yes [165] | SR-B1, αTTP, PLTP [162] | Yes [165] |
3. Protective Effects of Nutraceuticals on the BBB in CNS Diseases
3.1. In Vivo Investigations
| Compound | Disease Model | Effects on BBB Parameters | Reference |
|---|---|---|---|
| Apigenin | subarachnoid hemorrhage, rat | inflammation ↓, BBB disruption ↓, ZO-1, occludin ↑ | [189] |
| cerebral IR, MCAO, rat | vascularization/tube formation ↑, cerebral infarction ↓ | [190] | |
| Astaxanthin and derivatives | subarachnoid hemorrhage, mouse | BBB disruption ↓ | [191] |
| subarachnoid hemorrhage, rat | barrier integrity ↑, brain edema ↓, IL-1β, TNF-α, MMP-9 expression ↓ | [192] | |
| Borneol | - | R123 permeability in hippocampus ↑, PGP and MRP1 ↓, TJ disruption | [193] |
| cerebral IR - | blood pressure, cerebrovascular resistance ↓, edema ↓, BBB integrity, eNOS, CLDN-5, ZO-1 ↑, ET-1, iNOS, MMP-2/9, ICAM1, LFA-1 ↓ | [111,194] | |
| Caffeic acid phenethyl ester | TBI, rat and mouse | vascular integrity ↑, CLDN-5 ↑ | [195] |
| β-carotene | cerebral IR, MCAO, mouse | barrier integrity ↑, occludin, ZO-1 ↑ peroxynitrite generation ↓ | [196] |
| Carvacrol | TBI, rat | barrier integrity ↑, brain edema ↓, occludin, CLDN-5, ZO-1 ↑, MMP-9 ↓ | [113] |
| Catechin | TBI, rat | barrier integrity ↑, ZO-1, occludin ↑ | [197] |
| Chrysin | TBI, rat | barrier integrity ↑, brain EB content ↓ | [198] |
| p-Coumaric acid | hypoxia, mouse | barrier integrity ↑, brain edema ↓, occludin expression ↑ | [199] |
| Curcumin | cerebral IR, MCAO, rat | barrier integrity ↑, brain EB content ↓ | [200] |
| Hypoxia/hypercap-nia, rat | brain edema ↓, apoptosis ↓, AQP4 levels ↓, | [201] | |
| Daidzein | cerebral IR, MCAO, rat | barrier integrity ↑, astrocyte swelling ↓, cytoplasmic vacuolation ↓, edema ↓, vessel lumen ↑ | [202] |
| Docosahexaenoic acid | cerebral IR, MCAO, rat | barrier integrity ↑, brain edema ↓ | [203] |
| cerebral IR, MCAO, rat | barrier integrity ↑ | [204] | |
| cerebral IR, left CCAO, rat | barrier integrity ↑, brain edema ↓, occludin, CLDN-5, ZO-1 ↑, MMP-2/9 ↓ | [205] | |
| uremia + contrast media, mouse | CLDN-5, laminin α-4, -5 ↑ | [206] | |
| Eicosapentaenoic acid | cerebral IR, left CCAO, rat | barrier integrity ↑, brain edema ↓, occludin, CLDN-5, ZO-1 ↑, MMP-2/9 ↓ | [205] |
| uremia + contrast media, mouse | CLDN-5, laminin α-4, -5 ↑ | [206] | |
| Epigallo-catechin gallate | cerebral IR, MCAO, rat | barrier integrity ↑, TJ opening ↓ occludin, CLDN-5, ZO-1 expression ↑ | [207] |
| Ferulic acid + tetramethylpyrazine | cerebral IR, MCAO, rat | barrier integrity ↑, brain edema ↓, JAM-1, occludin ↑, MMP-9 expression ↓ | [208] |
| Fisetin | autism, valproic acid-induced, rat | barrier integrity ↑, CLDN-5 expression ↑ | [209] |
| Fucoxanthin | TBI, mouse | barrier integrity ↑, brain edema ↓, occludin, CLDN-5, ZO-1, VE cadherin ↑, MMP-9 ↓, apoptosis and ferroptosis ↓, BMEC mitophagy ↑ | [210] |
| Genistein | TBI, rat | barrier integrity ↑ | [211] |
| Hesperetin | TBI, mouse | barrier integrity ↑, brain edema ↓, ZO-1, occludin, CLDN-5 ↑, NLRP3 inflammasome ↓ | [212] |
| Hesperidin | cerebral IR, MCAO, mouse | barrier integrity ↑, brain edema ↓, disruption of CLDN-5 and ZO-1 ↓ | [85] |
| Kaempferol | neuroinflammation, LPS-induced, mouse | barrier integrity ↑, occludin, connexin-43 expression ↑ | [213] |
| neuroinflammation and BBB dysfunction, LPS-induced | BBB structure restored, brain edema ↓, occludin, connexin-43 expression ↑ | [214] | |
| Kaempferol-glucoside/Juglanin | cerebral IR, MCAO, mouse | BBB permeability ↓, VEGF and VEGFR2 ↓ ZO-1, occludin expression ↑ | [215] |
| α-Lipoic acid | TBI, rat | barrier integrity ↑, brain EB content ↓ | [216] |
| Lutein | subarachnoid hemorrhage, rat | vasospasm ↓ | [217] |
| TBI, rat | IL-1, IL-6, TNF-α, CCL2 ↓, ROS ↓, SOD, GSH ↑, ICAM-1, ET-1 ↓ | [218] | |
| Luteolin | AD, Aβ25–35-induced, mouse | BBB leakage ↓, astrocyte swelling ↓, CBF ↑ ZO-1, occludin, CLDN-5 expression ↑, | [219] |
| diabetes, high-fat diet and streptozotocin- induced, rat | ZO-1, occludin and GLUT-1 expression ↑ | [220] | |
| Lycopene | subarachnoid hemorrhage, rat | barrier integrity ↑, brain edema ↓ | [221] |
| hyperlipidemia, high fat diet induced, rat | VEGF, VCAM-1 ↓, CLDN-5 ↑, IL-1, IL-6, and TNF-α ↓ | [222] | |
| Malvidin | cerebral IR, BCCAO, rat | eNOS ↑, MMP-9 ↓ | [223] |
| Naringenin | cerebral IR, MCAO, mouse | BBB leakage ↓, ZO-1, occludin, CLDN-5, β-catenin ↑ | [224] |
| Polydatin | cerebral IR, MCAO, rat | barrier integrity ↑, brain edema ↓, CLDN-5 expression ↑ | [225] |
| cerebral IR, MCAO, rat | barrier integrity ↑, brain edema ↓, ZO-1, occludin, CLDN-5 ↑, TNF-α, IL-1β, IL-6, CCL2 levels ↓, ICAM-1 and VCAM-1 ↓ | [226] | |
| Procyanidin B2 | cerebral IR, MCAO, rat | barrier integrity ↑, brain edema ↓, ZO-1 expression ↑ | [227] |
| Pterostilbene | cerebral IR, MCAO, rat | barrier integrity ↑, CBF ↑, laminin ↑, ZO-1, occludin, CLDN-5, VE-cadherin ↑ | [228] |
| cerebral IR, MCAO, rat | barrier integrity ↑, brain edema ↓, MMP-2/9 expression ↓ | [229] | |
| Quercetin | AD, Aβ25–35-induced, mouse | barrier integrity ↑, CBF ↑ | [230] |
| cerebral ischemia, photothrombosis-induced, rat | barrier integrity ↑, MMP-9 activity ↓ | [231] | |
| cerebral IR, BCCAO, rat | barrier integrity ↑, brain endothelial cell swelling ↓, vesicles and vacuoles ↓, CLDN-5, ZO-1, β-catenin ↑, MMP-9 ↓ | [232] | |
| oxidative stress, PCB-induced, rat | occludin, CLDN-5, JAM-3, ZO-1, AF-6 ↑ | [233] | |
| cerebral IR, MCAO, rat | barrier integrity ↑, occludin, CLDN-5, ZO-1 expression ↑ | [234] | |
| Quercetin +/− hydroxylsafflor yellow A | cerebral IR, MCAO, mouse | barrier integrity ↑ | [235] |
| Resveratrol | recurrent ischemic stroke, rat | barrier integrity ↑, brain edema ↓, no change in CBF | [236] |
| Rosmarinic acid | MCAO + diabetes, STZ-induced, rat | barrier integrity ↑, brain edema ↓ | [237] |
| Tangeretin | cerebral IR, MCAO, rat | barrier integrity ↑ | [238] |
| Vitamin B9 | sepsis, cecal ligation and perforation, rat | barrier integrity ↑ | [239] |
| Vitamin C | cerebral IR, MCAO, rat | barrier integrity ↑, MMP-2/9 expression ↓, CLDN-1,CLDN-5, ZO-1 ↑ | [240] |
| Vitamin D3 | TBI, rat | barrier integrity ↑, brain edema ↓, ZO-1, occludin expression ↑ | [241] |
3.1.1. Traumatic Brain Injury
3.1.2. Cerebrovascular Disorders
3.1.3. Neurodegenerative Diseases
3.1.4. Neuroinflammation, Oxidative Stress, and Other Neurodevelopmental Diseases
3.2. In Vitro Investigations
| Compound | BBB Model | Injury | Effect on Brain Endothelial Cells | Reference |
|---|---|---|---|---|
| Apigenin | human BMEC | PMA | tube formation ↓, MMP-9 ↓ | [257] |
| human BMEC | OGD/R | cell viability ↑, cell migration and tube formation ↑, caveolin-1 ↑ | [190] | |
| Astaxanthin and derivatives | human HBMEC cell line | — | proliferation ↑, tube formation ↑ cell cycle G0/G1 phase ↓, S phase ↑, | [258] |
| OGD | cell viability ↑, LDH release ↓ | |||
| human BMEC | hemoglobin, collagenase | cell viability ↑, ROS ↓, VE-cadherin ↑ | [191] | |
| mouse bEnd.3 cell line | OGDR | cell viability ↑, apoptosis ↓, FD40 permeability ↓, CLDN-5, ZO-1 ↑ | [259] | |
| porcine BMEC | — | APP, ADAM10 ↑, BACE-1 ↓, PGP, ABCA1, LRP-1, Aβ1–40 uptake and transport ↑, cholesterol synthesis ↓ | [260] | |
| Borneol | rat BMEC, AC co-culture | — | PGP ↓, R123 accumulation ↑, digoxin, verapamil transport ↑ | [110] |
| mouse bEnd.3 cell line | — | puerarin, tetramethylpyrazine permeability ↑, ZO-1 ↓ | [246] | |
| rat BMEC | OGD | cell viability ↑, apoptosis ↓, CAT ↑, VEGF and VEGFR1 ↑ | [193] | |
| Caffeine | mouse BMEC | TNF-α + IFN-γ | VCAM-1 ↓, iNOS ↓ | [261] |
| Capsaicin | mouse cEND cell line | — | TEER ↓, CLDN5 ↓, ZO-1 dislocation | [262] |
| human hCMEC/D3 cell line | TNF-α | IL-1β, IL-6 ↓ | [263] | |
| Catechin/Epicatechin and derivatives | rat BMEC, AC, PC co-culture | TNF-α + Il-1β | CLDN-5, β-catenin staining ↑, ROS, NO ↓, leptin transporter LRP2 ↓ | [264] |
| human BMEC | Aβ1–42 | Aβ1–42 fibril formation ↓, ROS ↓ | [265] | |
| Chrysin | mouse bEnd.3 cell line | LPS | VCAM-1 ↓, monocyte adhesion ↓ | [266] |
| Cinnamic acid derivatives | human HBMEC-2 cell line | oxidative stress | cell damage ↓, cell viability ↑, mitochondrial transmembrane potential ↑ | [267] |
| Curcumin | bovine BMEC | oxidative stress | cell damage, LDH release ↓ | [200] |
| rat BMEC | OGD | LDH release ↓, IL-1β ↓ | [268] | |
| porcine BMEC | — | BCRP protein ↓, efflux transport ↓ | [68] | |
| Cyanidin metabolite | HBMEC | hypoxia | cell proliferation ↓, cell viability ↓, cyclin D1, CDK2, CDK4 ↓ | [269] |
| Docosahexaenoic acid | rat BMEC, PC, AC co-culture | oligomeric Aβ42 | cell viability ↑, ROS production ↓ barrier integrity ↑ SF, albumin permeability ↓, PGP ↑, R123 accumulation ↓ | [270] |
| BMEC | OGD | apoptosis ↓ | [271] | |
| porcine BMEC | IL-1β | Calcein-AM accumulation ↑ | [272] | |
| Fisetin | human BMEC | PMA | tube formation ↓, MMP-9 ↓ | [257] |
| Fucoxanthin | mouse bEnd.3 cell line | mechanical/ stretch | cell viability ↑, apoptosis↓, TEER ↑, γGT ↑, ACSL4 ↓, PINK1, LC3 ↑ | [210] |
| Gallic acid | rat BMEC, AC, PC co-culture | TNF-α + Il-1β | CLDN-5 and β-catenin staining ↑ | [264] |
| Genistein | human BMEC | TNF-α | TNF-α, IL-1β, CCL-1, IL-8, ICAM-1 ↓, leukocyte tr.migration ↓ | [273] |
| mouse bEnd.3 cell line | Aβ25–35 | cell viability ↑, ROS, and nitrotyrosine ↓, GSH ↑ | [274] | |
| Kaempferol and derivatives | rat RBE4 cell line | — | ecto-ALP ↑, MPP+ uptake ↑ | [275] |
| rat RBE4 cell line | — | ecto-ALP ↑, insulin uptake ↑ | [276] | |
| human BMEC | OGD/R | cell viability ↑, FD permeation ↓, occludin, ZO-1 ↑ | [215] | |
| human BMEC | hypoxia/reoxygenation | cell viability ↑, apoptosis ↓, mitochondrial membrane potential ↑, tube formation ↑, ICAM-1, VCAM-1, IL-1β ↓ | [277] | |
| α-Lipoic acid | bEnd.3, rat BMEC | OGD/R | LDH release ↓ | [278] |
| Luteolin | human BMEC | PMA | tube formation ↓, MMP-9 ↓ | [257] |
| human BMEC, AC co-culture | Aβ1–40 | cell viability ↑, TEER ↑, SF and albumin permeability ↓, TNF-α, IL-1β, IL-6, IL-8 release ↓ | [279] | |
| Lycopene | mouse bEnd.3 cell line | — | cell viability ↑ | [280] |
| Myricetin | human BMEC | OGD/R | FD70 permeation ↓, TEER ↑, TNF-α, IL-1β and IL-6 ↓, NO and eNOS activity ↑ | [281] |
| human BMEC | oxidative stress | cell viability ↑ | [90] | |
| Naringenin | mouse b.END5 rat RBE4 cell lines | — | concentration and time-dependent cellular uptake | [37] |
| Naringin | porcine BMEC | — | BCRP protein ↑, efflux transport ↑ | [68] |
| Piceatannol | mouse bEnd.3 cell line | LPS | ICAM-1 and VCAM-1 ↓, iNOS, ROS ↓ | [148] |
| Polydatin | primary rat BMEC | OGD | cell viability ↑, TNF-α, IL-6 ↓ CLDN-5, occludin, ZO-1 ↑ | [226] |
| Procyanidin | rat BMEC | — | PGP activity ↓, efflux transport↓, R123 accumulation ↑ | [35] |
| Pterostilbene | human BMEC | OGD | cell viability ↑, MMP-9 ↓, CLDN-5, ZO-1, VE-cadherin, occludin ↑, F/G actin ↓ | [228] |
| Quercetin and metabolites | rat RBEC1 cell line | — | concentration- and time-dependent cellular accumulation | [282] |
| human BMEC | Aβ1–40 | cell viability ↑, LDH release ↓ TEER ↑, albumin and SF permeability ↓, ROS ↓, γGT, ALP ↑ | [283] | |
| human BMEC | oxidative stress | cell viability ↑ | [90] | |
| porcine BMEC | — | BCRP protein ↑, efflux transport ↑ | [68] | |
| human BMEC | hypoxia/reoxygenetion | viability ↑, migration, angiogenesis ↑, CLDN-5 and ZO-1 ↑, VCAM-1 ↓, ROS ↓ | [284] | |
| mouse bEnd.3 cell line | Glaesserella parasuis infection | Il-6, Il-8, Il-18, TNF-α, MMP-9, ANG-2, ET-1 ↓, ZO-1, occludin, CLDN-5 ↑ | [285] | |
| Quercetin-biapigenin nanoparticles | human hCMEC/D3 cell line | oxidative stress | cell viability ↑, TEER ↓ | [286] |
| Quercetin +/− hydroxysafflor yellow A | human hCMEC/D3 cell line | OGD | cell viability ↑, TEER ↑ | [235] |
| Resveratrol | rat BMEC | OGD | cell viability ↑ | [236] |
| rat BMEC, AC, PC co-culture | TNF-α + Il-1β | albumin permeability ↓, CLDN-5 and β-catenin staining ↑, NO ↓ | [264] | |
| Rutin | HBMEC | hypoxia | cell proliferation ↓, cell viability ↓, cyclin D1, CDK2, CDK4 ↑ | [269] |
| Silymarin | human HBEC-5i cell line | AGE | cell migration ↓, tube formation ↓ | [287] |
| Sulforaphane | human hCMEC/D3 cell line | NRF2 gene silencing by siRNA | mitochondrial ABCB10 ↑ | [288] |
| mouse BMEC | - | GLUT1 ↑, HK1, PDK1, GSK, PKM2, ATP production ↑, NQO1, CAT, GSTs, TXN1, GSR ↑, ABCD3, ABCB6 ↑, ferroportin-1 ↑ | [127] | |
| Tangeretin | human HBMEC cell line | OGD | cell viability ↑, ROS and MDA ↓, SOD activity ↑, NO and iNOS ↓ | [289] |
| Theophylline | mouse BMEC | TNF-α + IFN-γ | VCAM-1 ↓, iNOS ↓ | [261] |
| Vitamin E | human HBEC-5i cell line | oxidative stress | cell viability ↑, apoptosis ↓, mitochondrial membrane potential ↑, ROS ↓, GSH ↑, SOD, GPX, CAT ↑, cytosolic HO-1 and NQO1 ↑ | [290] |
3.2.1. Cell Viability
3.2.2. Cell Proliferation, Migration, and Tube Formation
3.2.3. Barrier Integrity
3.2.4. Antioxidative and Anti-Inflammatory Effects
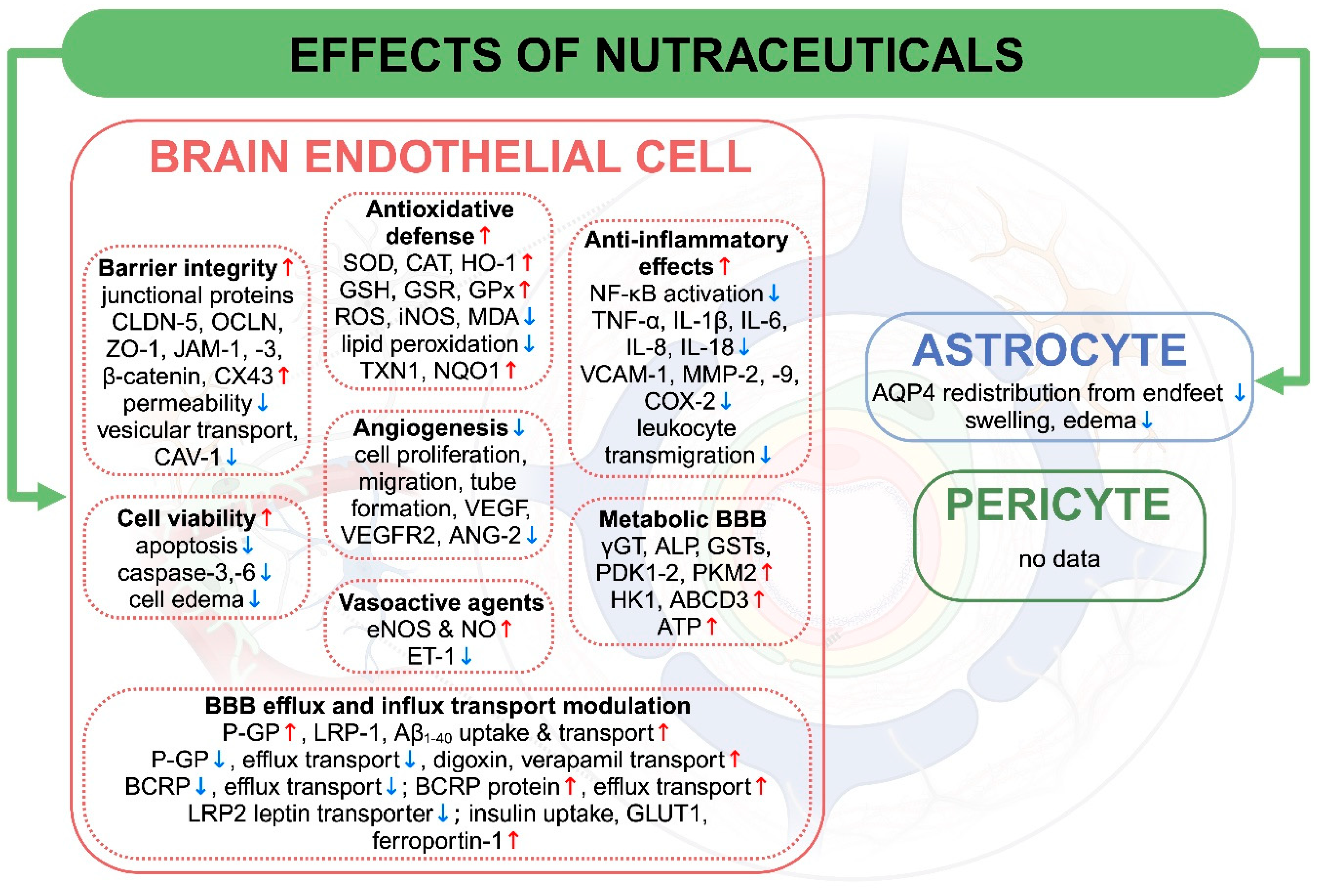
3.2.5. Vasoactive Agents
3.2.6. Effects on BBB Efflux and Influx Transport
3.2.7. BBB Enzymes and Metabolism
4. BBB Signaling Pathways Regulated by Nutraceuticals
| Nutraceutical | BBB Signaling Pathway Interactions | Reference |
|---|---|---|
| Apigenin | COX-2 ↓ TLR4, IκB, NF-κB ↓ BECN1 ↓, VEGF, mTOR ↑ | [257] [189] [190] |
| Astaxanthin | caspase-3 ↓, pGSK3β ↓ WNT7A, β-catenin, CCND1 ↑, ERK activation ↓ p75NTR ↓ PPAR-α activation ↑ | [258] [191] [259] [260] |
| Borneol | NF-κB activation ↑ A1AR, A2AR ↑ BCL-2 ↑, BAX ↓, Ca2+ ↓, VEGF ↑, VEGFR1 ↓ | [110] [246] [193] |
| Capsaicin | TRPV1 activity and Ca2+ ↑ NF-κB activity, nuclear translocation ↓ | [303] [263] |
| β-Carotene | AKT, FKHR, and ERK1/2 phosphorylation ↓ | [196] |
| Carvacrol | TRPM7 activation ↓ caspase-3 ↓, BAX ↓, BCL-2 ↑, NF-KB ↓ | [304] [113] |
| Catechin/Epicatechin/Epigallocatechin gallate | PKCα ↓ | [207] [197] |
| Chrysin | p38 MAPK and JNK activation ↓, NF-κB p65 translocation ↓ | [266] |
| Curcumin | p38 MAPK and NFκB activation ↓ | [268] |
| Cyanidin | AKT ↑, caspase-3 ↓, ERK1/2 ↓ | [301] |
| Docosahexaenoic acid | ANG2 ↓,VEGF ↑ PGE2, PGI2, COX-2 ↓ | [271] |
| Fisetin | COX-2 ↓ | [257] |
| Fucoxanthin | caspase-3 ↓ | [210] |
| Gallic acid | NF-κB nuclear translocation ↓ | [264] |
| Genistein | NRF2, PI3K ↑ | [274] |
| Hesperidin | FOXO3a nuclear translocation ↓ | [85] |
| Kaempferol and derivatives | VEGF and VEGFR2 ↓ VEGF ↑ | [215] [277] |
| α-Lipoic acid | AKT and mTOR phosphorylation ↑ | [278] |
| Luteolin | COX-2 ↓ NFκ-B activation ↓ | [257] [279] |
| Lycopene | AKT activation ↑, LXR-β ↑ | [280] |
| Myricetin | AKT and NRF2 activation ↑ | [281] |
| Naringenin | p-GSK-3β ↓ | [224] |
| Piceatannol | NF-κB, MAPK, p38, JNK ↓ p-IKKα/β, p-IκBα, p-p65 ↓ | [148] |
| Polydatin | CREB/PGC-1α/PPARγ ↑ COX-2 ↓ | [226] |
| Pterostilbene | c-Met, c-Jun and c-Myc proteins ↑ WNT pathway activation ↑ | [228] |
| Quercetin | KEAP1/NRF2 activation ↑, ATF6/GRP78 ↓ VEGF ↓, PI3K/AKT/ERK activation ↑ WNT ↑, GSK-3β expression ↓ NF-kB p65, RAGE ↓ | [284] [285] [232] [230] |
| Resveratrol | NF-κB nuclear translocation ↓ | [264] |
| Silymarin | VEGF release ↓ | [287] |
| Sulforaphan | NRF2 ↑, AKT phosphorylation ↑ NRF2 ↑ | [127,288] |
| Tangeretin | caspase-3 ↓, JNK activation ↓ | [289] |
| Vitamin E/α-Tocopherol | BAX, caspase-9/caspase-3 ↓, BCL-2 ↑, NRF2 ↑ | [290] |
5. Toxicity and Drug Interactions
6. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; International Natural Product Sciences Taskforce; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Puri, V.; Nagpal, M.; Singh, I.; Singh, M.; Dhingra, G.A.; Huanbutta, K.; Dheer, D.; Sharma, A.; Sangnim, T. A Comprehensive Review on Nutraceuticals: Therapy Support and Formulation Challenges. Nutrients 2022, 14, 4637. [Google Scholar] [CrossRef] [PubMed]
- Iriti, M.; Vitalini, S.; Fico, G.; Faoro, F. Neuroprotective herbs and foods from different traditional medicines and diets. Molecules 2010, 15, 3517–3555. [Google Scholar] [CrossRef] [PubMed]
- Castrogiovanni, P.; Trovato, F.M.; Loreto, C.; Nsir, H.; Szychlinska, M.A.; Musumeci, G. Nutraceutical Supplements in the Management and Prevention of Osteoarthritis. Int. J. Mol. Sci. 2016, 17, 2042. [Google Scholar] [CrossRef] [PubMed]
- Golla, U. Emergence of nutraceuticals as the alternative medications for pharmaceuticals. Int. J. Complement. Altern. Med. 2018, 11, 155–158. [Google Scholar] [CrossRef]
- Silva, R.F.M.; Pogačnik, L. Polyphenols from Food and Natural Products: Neuroprotection and Safety. Antioxidants 2020, 9, 61. [Google Scholar] [CrossRef]
- Zhu, T.; Wang, L.; Wang, L.P.; Wan, Q. Therapeutic targets of neuroprotection and neurorestoration in ischemic stroke: Applications for natural compounds from medicinal herbs. Biomed. Pharmacother. 2022, 148, 112719. [Google Scholar] [CrossRef]
- Banji, O.J.F.; Banji, D.; Makeen, H.A.; Alqahtani, S.S.; Alshahrani, S. Neuroinflammation: The Role of Anthocyanins as Neuroprotectants. Curr. Neuropharmacol. 2022, 20, 2156–2174. [Google Scholar] [CrossRef]
- Marino, P.; Pepe, G.; Basilicata, M.G.; Vestuto, V.; Marzocco, S.; Autore, G.; Procino, A.; Gomez-Monterrey, I.M.; Manfra, M.; Campiglia, P. Potential Role of Natural Antioxidant Products in Oncological Diseases. Antioxidants 2023, 12, 704. [Google Scholar] [CrossRef]
- Feng, J.; Zheng, Y.; Guo, M.; Ares, I.; Martínez, M.; Lopez-Torres, B.; Martínez-Larrañaga, M.R.; Wang, X.; Anadón, A.; Martínez, M.A. Oxidative stress, the blood-brain barrier and neurodegenerative diseases: The critical beneficial role of dietary antioxidants. Acta Pharm. Sin. B. 2023, 13, 3988–4024. [Google Scholar] [CrossRef]
- Campos-Bedolla, P.; Walter, F.R.; Veszelka, S.; Deli, M.A. Role of the blood-brain barrier in the nutrition of the central nervous system. Arch. Med. Res. 2014, 45, 610–638. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.D.; Zhao, Z.; Montagne, A.; Nelson, A.R.; Zlokovic, B.V. Blood-Brain Barrier: From Physiology to Disease and Back. Physiol. Rev. 2019, 99, 21–78. [Google Scholar] [CrossRef] [PubMed]
- Andjelkovic, A.V.; Stamatovic, S.M.; Phillips, C.M.; Martinez-Revollar, G.; Keep, R.F. Modeling blood-brain barrier pathology in cerebrovascular disease in vitro: Current and future paradigms. Fluids Barriers CNS 2020, 17, 44. [Google Scholar] [CrossRef] [PubMed]
- Archie, S.R.; Al Shoyaib, A.; Cucullo, L. Blood-Brain Barrier Dysfunction in CNS Disorders and Putative Therapeutic Targets: An Overview. Pharmaceutics 2021, 13, 1779. [Google Scholar] [CrossRef]
- Walter, F.R.; Santa-Maria, A.R.; Mészáros, M.; Veszelka, S.; Dér, A.; Deli, M.A. Surface charge, glycocalyx, and blood-brain barrier function. Tissue Barriers 2021, 9, 1904773. [Google Scholar] [CrossRef]
- de Rus Jacquet, A.; Layé, S.; Calon, F. How nutrients and natural products act on the brain: Beyond pharmacology. Cell Rep. Med. 2023, 4, 101243. [Google Scholar] [CrossRef]
- Wager, T.T.; Hou, X.; Verhoest, P.R.; Villalobos, A. Central Nervous System Multiparameter Optimization Desirability: Application in Drug Discovery. ACS Chem. Neurosci. 2016, 7, 767–775. [Google Scholar] [CrossRef]
- Lakey-Beitia, J.; Kumar, D.J.; Hegde, M.L.; Rao, K.S. Carotenoids as Novel Therapeutic Molecules Against Neurodegenerative Disorders: Chemistry and Molecular Docking Analysis. Int. J. Mol. Sci. 2019, 20, 5553. [Google Scholar] [CrossRef]
- Gao, L.; Sun, W.; Zhang, L.; Liang, C.; Zhang, D. Caffeine upregulates SIRT3 expression to ameliorate astrocytes-mediated HIV-1 Tat neurotoxicity via suppression of EGR1 signaling pathway. J. Neurovirol. 2024, 30, 286–302. [Google Scholar] [CrossRef]
- Netsch, M.I.; Gutmann, H.; Luescher, S.; Brill, S.; Schmidlin, C.B.; Kreuter, M.H.; Drewe, J. Inhibitory activity of a green tea extract and some of its constituents on multidrug resistance-associated protein 2 functionality. Planta Med. 2005, 71, 135–141. [Google Scholar] [CrossRef]
- McCall, A.L.; Millington, W.R.; Wurtman, R.J. Blood-brain barrier transport of caffeine: Dose-related restriction of adenine transport. Life Sci. 1982, 31, 2709–2715. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, M.; Mochizuki, T.; Takekuma, Y.; Miyazaki, K. Structure-affinity relationship in the interactions of human organic anion transporter 1 with caffeine, theophylline, theobromine and their metabolites. Biochim. Biophys. Acta 2005, 1714, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Gunnink, L.K.; Busscher, B.M.; Wodarek, J.A.; Rosette, K.A.; Strohbehn, L.E.; Looyenga, B.D.; Louters, L.L. Caffeine inhibition of GLUT1 is dependent on the activation state of the transporter. Biochimie 2017, 137, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, S.; Deli, M.A.; Kawaguchi, H.; Shimizudani, T.; Shimono, T.; Kittel, A.; Tanaka, K.; Niwa, M. A new blood-brain barrier model using primary rat brain endothelial cells, pericytes and astrocytes. Neurochem. Int. 2009, 54, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Lardeau, A.; Poquet, L. Phenolic acid metabolites derived from coffee consumption are unlikely to cross the blood-brain barrier. J. Pharm. Biomed. Anal. 2013, 76, 134–138. [Google Scholar] [CrossRef]
- Guo, S.Y.; Yang, G.P.; Jiang, D.J.; Wang, F.; Song, T.; Tan, X.H.; Sun, Z.Q. Protection of capsaicin against hypoxia-reoxygenation-induced apoptosis of rat hippocampal neurons. Can. J. Physiol. Pharmacol. 2008, 86, 785–792. [Google Scholar] [CrossRef]
- Nabekura, T.; Kamiyama, S.; Kitagawa, S. Effects of dietary chemopreventive phytochemicals on P-glycoprotein function. Biochem. Biophys. Res. Commun. 2005, 327, 866–870. [Google Scholar] [CrossRef]
- Okura, T.; Ibe, M.; Umegaki, K.; Shinozuka, K.; Yamada, S. Effects of dietary ingredients on function and expression of P-glycoprotein in human intestinal epithelial cells. Biol. Pharm. Bull. 2010, 33, 255–259. [Google Scholar] [CrossRef]
- Rollyson, W.D.; Stover, C.A.; Brown, K.C.; Perry, H.E.; Stevenson, C.D.; McNees, C.A.; Ball, J.G.; Valentovic, M.A.; Dasgupta, P. Bioavailability of capsaicin and its implications for drug delivery. J. Control. Release 2014, 196, 96–105. [Google Scholar] [CrossRef]
- Wu, F.; Liu, R.; Shen, X.; Xu, H.; Sheng, L. Study on the interaction and antioxidant activity of theophylline and theobromine with SOD by spectra and calculation. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 215, 354–362. [Google Scholar] [CrossRef]
- Kupsáková, I.; Rybár, A.; Docolomanský, P.; Drobná, Z.; Stein, U.; Walther, W.; Barancík, M.; Breier, A. Reversal of P-glycoprotein mediated vincristine resistance of L1210/VCR cells by analogues of pentoxifylline. A QSAR study. Eur. J. Pharm. Sci. 2004, 21, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.H.; Liu, M.C. Determination of unbound theophylline in rat blood and brain by microdialysis and liquid chromatography. J. Chromatogr. A 2004, 1032, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Dani, C.; Pasquali, M.A.; Oliveira, M.R.; Umezu, F.M.; Salvador, M.; Henriques, J.A.; Moreira, J.C. Protective effects of purple grape juice on carbon tetrachloride-induced oxidative stress in brains of adult Wistar rats. J. Med. Food. 2008, 11, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Dreiseitel, A.; Oosterhuis, B.; Vukman, K.V.; Schreier, P.; Oehme, A.; Locher, S.; Hajak, G.; Sand, P.G. Berry anthocyanins and anthocyanidins exhibit distinct affinities for the efflux transporters BCRP and MDR1. Br. J. Pharmacol. 2009, 158, 1942–1950. [Google Scholar] [CrossRef]
- He, L.; Zhao, C.; Yan, M.; Zhang, L.Y.; Xia, Y.Z. Inhibition of P-glycoprotein function by procyanidine on blood-brain barrier. Phytother. Res. 2009, 23, 933–937. [Google Scholar] [CrossRef]
- Faria, A.; Pestana, D.; Teixeira, D.; Azevedo, J.; De Freitas, V.; Mateus, N.; Calhau, C. Flavonoid transport across RBE4 cells: A blood-brain barrier model. Cell Mol. Biol. Lett. 2010, 15, 234–241. [Google Scholar] [CrossRef]
- Youdim, K.A.; Dobbie, M.S.; Kuhnle, G.; Proteggente, A.R.; Abbott, N.J.; Rice-Evans, C. Interaction between flavonoids and the blood-brain barrier: In vitro studies. J. Neurochem. 2003, 85, 180–192. [Google Scholar] [CrossRef]
- Xu, Y.; Ke, H.; Li, Y.; Xie, L.; Su, H.; Xie, J.; Mo, J.; Chen, W. Malvidin-3-O-Glucoside from Blueberry Ameliorates Nonalcoholic Fatty Liver Disease by Regulating Transcription Factor EB-Mediated Lysosomal Function and Activating the Nrf2/ARE Signaling Pathway. J. Agric. Food Chem. 2021, 69, 4663–4673. [Google Scholar] [CrossRef]
- Oliveira, H.; Roma-Rodrigues, C.; Santos, A.; Veigas, B.; Brás, N.; Faria, A.; Calhau, C.; de Freitas, V.; Baptista, P.V.; Mateus, N.; et al. GLUT1 and GLUT3 involvement in anthocyanin gastric transport- Nanobased targeted approach. Sci. Rep. 2019, 9, 789. [Google Scholar] [CrossRef]
- Faria, A.; Meireles, M.; Fernandes, I.; Santos-Buelga, C.; Gonzalez-Manzano, S.; Dueñas, M.; de Freitas, V.; Mateus, N.; Calhau, C. Flavonoid metabolites transport across a human BBB model. Food Chem. 2014, 149, 190–196. [Google Scholar] [CrossRef]
- Iizuka, M.; Ayaori, M.; Uto-Kondo, H.; Yakushiji, E.; Takiguchi, S.; Nakaya, K.; Hisada, T.; Sasaki, M.; Komatsu, T.; Yogo, M.; et al. Astaxanthin enhances ATP-binding cassette transporter A1/G1 expressions and cholesterol efflux from macrophages. J. Nutr. Sci. Vitaminol. 2012, 58, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.N.; Sheu, M.J.; Hsieh, Y.W.; Wang, R.Y.; Chiang, Y.C.; Hung, C.C. β-carotene reverses multidrug resistant cancer cells by selectively modulating human P-glycoprotein function. Phytomedicine 2016, 23, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, N.; Gao, Z. β-Carotene regulates glucose transport and insulin resistance in gestational diabetes mellitus by increasing the expression of SHBG. Clin. Exp. Pharmacol. Physiol. 2022, 49, 1307–1318. [Google Scholar] [CrossRef] [PubMed]
- Du, H.F.; Wu, J.W.; Zhu, Y.S.; Hua, Z.H.; Jin, S.Z.; Ji, J.C.; Wang, C.S.; Qian, G.Y.; Jin, X.D.; Ding, H.M. Fucoxanthin Induces Ferroptosis in Cancer Cells via Downregulation of the Nrf2/HO-1/GPX4 Pathway. Molecules 2024, 29, 2832. [Google Scholar] [CrossRef] [PubMed]
- Chandra, F.; Tania, T.F.; Nurcahyanti, A.D.R. Bixin and Fuxoxanthin Alone and in Combination with Cisplatin Regulate ABCC1 and ABCC2 Transcription in A549 Lung Cancer Cells. J. Pharm. Bioallied Sci. 2023, 15, 15–20. [Google Scholar] [CrossRef]
- Molnár, J.; Gyémánt, N.; Mucsi, I.; Molnár, A.; Szabó, M.; Körtvélyesi, T.; Varga, A.; Molnár, P.; Tóth, G. Modulation of multidrug resistance and apoptosis of cancer cells by selected carotenoids. In Vivo 2004, 18, 237–244. [Google Scholar]
- Sato, Y.; Kondo, Y.; Sumi, M.; Takekuma, Y.; Sugawara, M. Intracellular uptake mechanism of lutein in retinal pigment epithelial cells. J. Pharm. Pharm. Sci. 2013, 16, 494–501. [Google Scholar] [CrossRef]
- Palozza, P.; Simone, R.; Catalano, A.; Parrone, N.; Monego, G.; Ranelletti, F.O. Lycopene regulation of cholesterol synthesis and efflux in human macrophages. J. Nutr. Biochem. 2011, 22, 971–978. [Google Scholar] [CrossRef]
- Banji, D.; Banji, O.J.; Dasaroju, S.; Annamalai, A.R. Piperine and curcumin exhibit synergism in attenuating D-galactose induced senescence in rats. Eur. J. Pharmacol. 2013, 703, 91–99. [Google Scholar] [CrossRef]
- Chearwae, W.; Shukla, S.; Limtrakul, P.; Ambudkar, S.V. Modulation of the function of the multidrug resistance-linked ATP-binding cassette transporter ABCG2 by the cancer chemopreventive agent curcumin. Mol. Cancer Ther. 2006, 5, 1995–2006. [Google Scholar] [CrossRef]
- Shukla, S.; Zaher, H.; Hartz, A.; Bauer, B.; Ware, J.A.; Ambudkar, S.V. Curcumin inhibits the activity of ABCG2/BCRP1, a multidrug resistance-linked ABC drug transporter in mice. Pharm. Res. 2009, 26, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Gunnink, L.K.; Alabi, O.D.; Kuiper, B.D.; Gunnink, S.M.; Schuiteman, S.J.; Strohbehn, L.E.; Hamilton, K.E.; Wrobel, K.E.; Louters, L.L. Curcumin directly inhibits the transport activity of GLUT1. Biochimie 2016, 125, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Askarizadeh, A.; Barreto, G.E.; Henney, N.C.; Majeed, M.; Sahebkar, A. Neuroprotection by curcumin: A review on brain delivery strategies. Int. J. Pharm. 2020, 585, 119476. [Google Scholar] [CrossRef]
- Han, J.Y.; Ahn, S.Y.; Kim, C.S.; Yoo, S.K.; Kim, S.K.; Kim, H.C.; Hong, J.T.; Oh, K.W. Protection of apigenin against kainate-induced excitotoxicity by anti-oxidative effects. Biol. Pharm. Bull. 2012, 35, 1440–1446. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, J.L.; Wang, Y.R.; Fa, X.Z. Apigenin attenuates copper-mediated β-amyloid neurotoxicity through antioxidation, mitochondrion protection and MAPK signal inactivation in an AD cell model. Brain Res. 2013, 1492, 33–45. [Google Scholar] [CrossRef]
- Yang, Y.; Bai, L.; Li, X.; Xiong, J.; Xu, P.; Guo, C.; Xue, M. Transport of active flavonoids, based on cytotoxicity and lipophilicity: An evaluation using the blood-brain barrier cell and Caco-2 cell models. Toxicol. In Vitro 2014, 28, 388–396. [Google Scholar] [CrossRef]
- Ren, K.; Jiang, T.; Zhou, H.F.; Liang, Y.; Zhao, G.J. Apigenin Retards Atherogenesis by Promoting ABCA1-Mediated Cholesterol Efflux and Suppressing Inflammation. Cell Physiol. Biochem. 2018, 47, 2170–2184. [Google Scholar] [CrossRef]
- Melstrom, L.G.; Salabat, M.R.; Ding, X.Z.; Milam, B.M.; Strouch, M.; Pelling, J.C.; Bentrem, D.J. Apigenin inhibits the GLUT-1 glucose transporter and the phosphoinositide 3-kinase/Akt pathway in human pancreatic cancer cells. Pancreas 2008, 37, 426–431. [Google Scholar] [CrossRef]
- Shimazu, R.; Anada, M.; Miyaguchi, A.; Nomi, Y.; Matsumoto, H. Evaluation of Blood-Brain Barrier Permeability of Polyphenols, Anthocyanins, and Their Metabolites. J. Agric. Food Chem. 2021, 69, 11676–11686. [Google Scholar] [CrossRef]
- Werba, J.P.; Misaka, S.; Giroli, M.G.; Shimomura, K.; Amato, M.; Simonelli, N.; Vigo, L.; Tremoli, E. Update of green tea interactions with cardiovascular drugs and putative mechanisms. J. Food Drug Anal. 2018, 26, S72–S77. [Google Scholar] [CrossRef]
- Almajano, M.P.; Vila, I.; Gines, S. Neuroprotective effects of white tea against oxidative stress-induced toxicity in striatal cells. Neurotox. Res. 2011, 20, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Vaidyanathan, J.B.; Walle, T. Transport and metabolism of the tea flavonoid (-)-epicatechin by the human intestinal cell line Caco-2. Pharm. Res. 2001, 18, 1420–1425. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, T.; Hayashi, A.; Ikeda, N.; Morita, O.; Tasaki, J. Multidrug resistance-associated protein 2 (MRP2) is an efflux transporter of EGCG and its metabolites in the human small intestine. J. Nutr. Biochem. 2022, 107, 109071. [Google Scholar] [CrossRef] [PubMed]
- Annaba, F.; Kumar, P.; Dudeja, A.K.; Saksena, S.; Gill, R.K.; Alrefai, W.A. Green tea catechin EGCG inhibits ileal apical sodium bile acid transporter ASBT. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G467–G473. [Google Scholar] [CrossRef] [PubMed]
- He, X.L.; Wang, Y.H.; Bi, M.G.; Du, G.H. Chrysin improves cognitive deficits and brain damage induced by chronic cerebral hypoperfusion in rats. Eur. J. Pharmacol. 2012, 680, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, X.; Deng, L. Chrysin reduces inflammation and oxidative stress and improves ovarian function in D-gal-induced premature ovarian failure. Bioengineered 2022, 13, 8291–8301. [Google Scholar] [CrossRef]
- Mitsunaga, Y.; Takanaga, H.; Matsuo, H.; Naito, M.; Tsuruo, T.; Ohtani, H.; Sawada, Y. Effect of bioflavonoids on vincristine transport across blood-brain barrier. Eur. J. Pharmacol. 2000, 395, 193–201. [Google Scholar] [CrossRef]
- Kaur, M.; Badhan, R.K. Phytochemical mediated-modulation of the expression and transporter function of breast cancer resistance protein at the blood-brain barrier: An in-vitro study. Brain Res. 2017, 1654 Pt A, 9–23. [Google Scholar] [CrossRef]
- Mohos, V.; Fliszár-Nyúl, E.; Ungvári, O.; Bakos, É.; Kuffa, K.; Bencsik, T.; Zsidó, B.Z.; Hetényi, C.; Telbisz, Á.; Özvegy-Laczka, C.; et al. Effects of Chrysin and Its Major Conjugated Metabolites Chrysin-7-Sulfate and Chrysin-7-Glucuronide on Cytochrome P450 Enzymes and on OATP, P-gp, BCRP, and MRP2 Transporters. Drug Metab. Dispos. 2020, 48, 1064–1073. [Google Scholar] [CrossRef]
- Yu, Z.; Yang, L.; Deng, S.; Liang, M. Daidzein ameliorates LPS-induced hepatocyte injury by inhibiting inflammation and oxidative stress. Eur. J. Pharmacol. 2020, 885, 173399. [Google Scholar] [CrossRef]
- Limtrakul, P.; Khantamat, O.; Pintha, K. Inhibition of P-glycoprotein function and expression by kaempferol and quercetin. J. Chemother. 2005, 17, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Merino, G.; Perez, M.; Real, R.; Egido, E.; Prieto, J.G.; Alvarez, A.I. In vivo inhibition of BCRP/ABCG2 mediated transport of nitrofurantoin by the isoflavones genistein and daidzein: A comparative study in Bcrp1 (−/−) mice. Pharm. Res. 2010, 27, 2098–2105. [Google Scholar] [CrossRef] [PubMed]
- Cheong, S.H.; Furuhashi, K.; Ito, K.; Nagaoka, M.; Yonezawa, T.; Miura, Y.; Yagasaki, K. Daidzein promotes glucose uptake through glucose transporter 4 translocation to plasma membrane in L6 myocytes and improves glucose homeostasis in Type 2 diabetic model mice. J. Nutr. Biochem. 2014, 25, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, X.; Pi, W.; Zhang, Y.; Yu, L.; Xu, C.; Sun, Z.; Jiang, J. Fisetin Attenuates Doxorubicin-Induced Cardiomyopathy In Vivo and In Vitro by Inhibiting Ferroptosis Through SIRT1/Nrf2 Signaling Pathway Activation. Front. Pharmacol. 2022, 12, 808480. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, S.; Kawano, K.; Matsumura, R.; Sugihara, N.; Furuno, K. Inhibitory effect of flavonoids on the efflux of N-acetyl 5-aminosalicylic acid intracellularly formed in Caco-2 cells. J. Biomed. Biotechnol. 2009, 2009, 467489. [Google Scholar] [CrossRef]
- Watanabe, M.; Hisatake, M.; Fujimori, K. Fisetin Suppresses Lipid Accumulation in Mouse Adipocytic 3T3-L1 Cells by Repressing GLUT4-Mediated Glucose Uptake through Inhibition of mTOR-C/EBPα Signaling. J. Agric. Food Chem. 2015, 63, 4979–4987. [Google Scholar] [CrossRef]
- El-Far, Y.M.; Khodir, A.E.; Emarah, Z.A.; Ebrahim, M.A.; Al-Gayyar, M.M.H. Chemopreventive and hepatoprotective effects of genistein via inhibition of oxidative stress and the versican/PDGF/PKC signaling pathway in experimentally induced hepatocellular carcinoma in rats by thioacetamide. Redox Rep. 2022, 27, 9–20. [Google Scholar] [CrossRef]
- Kodaira, H.; Kusuhara, H.; Fujita, T.; Ushiki, J.; Fuse, E.; Sugiyama, Y. Quantitative evaluation of the impact of active efflux by p-glycoprotein and breast cancer resistance protein at the blood-brain barrier on the predictability of the unbound concentrations of drugs in the brain using cerebrospinal fluid concentration as a surrogate. J. Pharmacol. Exp. Ther. 2011, 339, 935–944. [Google Scholar] [CrossRef]
- Toyohira, Y.; Ueno, S.; Tsutsui, M.; Itoh, H.; Sakai, N.; Saito, N.; Takahashi, K.; Yanagihara, N. Stimulatory effects of the soy phytoestrogen genistein on noradrenaline transporter and serotonin transporter activity. Mol. Nutr. Food Res. 2010, 54, 516–524. [Google Scholar] [CrossRef]
- Vera, J.C.; Reyes, A.M.; Cárcamo, J.G.; Velásquez, F.V.; Rivas, C.I.; Zhang, R.H.; Strobel, P.; Iribarren, R.; Scher, H.I.; Slebe, J.C.; et al. Genistein is a natural inhibitor of hexose and dehydroascorbic acid transport through the glucose transporter, GLUT1. J. Biol. Chem. 1996, 271, 8719–8724. [Google Scholar] [CrossRef]
- Choi, E.J.; Ahn, W.S. Neuroprotective effects of chronic hesperetin administration in mice. Arch. Pharm. Res. 2008, 31, 1457–1462. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, T.; Liu, P.; Yang, F.; Wang, X.; Zheng, W.; Sun, W. Hesperetin ameliorates hepatic oxidative stress and inflammation via the PI3K/AKT-Nrf2-ARE pathway in oleic acid-induced HepG2 cells and a rat model of high-fat diet-induced NAFLD. Food Funct. 2021, 12, 3898–3918. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.E.; Zhang, S. Flavonoid-drug interactions: Effects of flavonoids on ABC transporters. Life Sci. 2006, 78, 2116–2130. [Google Scholar] [CrossRef]
- Ikemura, M.; Sasaki, Y.; Giddings, J.C.; Yamamoto, J. Preventive effects of hesperidin, glucosyl hesperidin and naringin on hypertension and cerebral thrombosis in stroke-prone spontaneously hypertensive rats. Phytother. Res. 2012, 26, 1272–1277. [Google Scholar] [CrossRef]
- Lee, B.K.; Hyun, S.W.; Jung, Y.S. Yuzu and Hesperidin Ameliorate Blood-Brain Barrier Disruption during Hypoxia via Antioxidant Activity. Antioxidants 2020, 9, 843. [Google Scholar] [CrossRef]
- El-Readi, M.Z.; Hamdan, D.; Farrag, N.; El-Shazly, A.; Wink, M. Inhibition of P-glycoprotein activity by limonin and other secondary metabolites from Citrus species in human colon and leukaemia cell lines. Eur. J. Pharmacol. 2010, 626, 139–145. [Google Scholar] [CrossRef]
- Lei, Y.; Chen, J.; Zhang, W.; Fu, W.; Wu, G.; Wei, H.; Wang, Q.; Ruan, J. In vivo investigation on the potential of galangin, kaempferol and myricetin for protection of D-galactose-induced cognitive impairment. Food Chem. 2012, 135, 2702–2707. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhai, Y.; Chen, J.; Xu, X.; Wang, H. Kaempferol Ameliorates Oxygen-Glucose Deprivation/Reoxygenation-Induced Neuronal Ferroptosis by Activating Nrf2/SLC7A11/GPX4 Axis. Biomolecules 2021, 11, 923. [Google Scholar] [CrossRef]
- Kitakaze, T.; Jiang, H.; Nomura, T.; Hironao, K.Y.; Yamashita, Y.; Ashida, H. Kaempferol Promotes Glucose Uptake in Myotubes through a JAK2-Dependent Pathway. J. Agric. Food Chem. 2020, 68, 13720–13729. [Google Scholar] [CrossRef]
- Figueira, I.; Garcia, G.; Pimpão, R.C.; Terrasso, A.P.; Costa, I.; Almeida, A.F.; Tavares, L.; Pais, T.F.; Pinto, P.; Ventura, M.R.; et al. Polyphenols journey through blood-brain barrier towards neuronal protection. Sci. Rep. 2017, 7, 11456. [Google Scholar] [CrossRef]
- Sharma, V.; Mishra, M.; Ghosh, S.; Tewari, R.; Basu, A.; Seth, P.; Sen, E. Modulation of interleukin-1beta mediated inflammatory response in human astrocytes by flavonoids: Implications in neuroprotection. Brain Res. Bull. 2007, 73, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Lee, K.; Kim, S.H.; Hong, M.J.; Jeong, N.J.; Kim, M.S. Luteolin improves hypercholesterolemia and glucose intolerance through LXRα-dependent pathway in diet-induced obese mice. J. Food Biochem. 2020, 44, e13358. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Batudeligen Chen, H.; Narisu Anda Xu, Y.; Xue, L. Luteolin attenuates CCl4-induced hepatic injury by inhibiting ferroptosis via SLC7A11. BMC Complement. Med. Ther. 2024, 24, 193. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.W.; Li, Y.; Paxton, J.W.; Birch, N.P.; Scheepens, A. Identification of novel dietary phytochemicals inhibiting the efflux transporter breast cancer resistance protein (BCRP/ABCG2). Food Chem. 2013, 138, 2267–2274. [Google Scholar] [CrossRef] [PubMed]
- Furumiya, M.; Inoue, K.; Nishijima, C.; Yamashiro, T.; Inaoka, E.; Ohta, K.; Hayashi, Y.; Yuasa, H. Noncompetitive inhibition of proton-coupled folate transporter by myricetin. Drug Metab. Pharmacokinet. 2014, 29, 312–316. [Google Scholar] [CrossRef]
- Li, Y.; He, B.; Zhang, C.; He, Y.; Xia, T.; Zeng, C. Naringenin Attenuates Isoprenaline-Induced Cardiac Hypertrophy by Suppressing Oxidative Stress through the AMPK/NOX2/MAPK Signaling Pathway. Nutrients 2023, 15, 1340. [Google Scholar] [CrossRef]
- Ali, M.M.; Agha, F.G.; El-Sammad, N.M.; Hassan, S.K. Modulation of anticancer drug-induced P-glycoprotein expression by naringin. Z. Naturforsch. C J. Biosci. 2009, 64, 109–116. [Google Scholar] [CrossRef]
- Ott, M.; Huls, M.; Cornelius, M.G.; Fricker, G. St. John’s Wort constituents modulate P-glycoprotein transport activity at the blood-brain barrier. Pharm. Res. 2010, 27, 811–822. [Google Scholar] [CrossRef]
- Lin, X.; Zhao, X.; Chen, Q.; Wang, X.; Wu, Y.; Zhao, H. Quercetin ameliorates ferroptosis of rat cardiomyocytes via activation of the SIRT1/p53/SLC7A11 signaling pathway to alleviate sepsis-induced cardiomyopathy. Int. J. Mol. Med. 2023, 52, 116. [Google Scholar] [CrossRef]
- Kumar, K.K.; Priyanka, L.; Gnananath, K.; Babu, P.R.; Sujatha, S. Pharmacokinetic drug interactions between apigenin, rutin and paclitaxel mediated by P-glycoprotein in rats. Eur. J. Drug Metab. Pharmacokinet. 2015, 40, 267–276. [Google Scholar] [CrossRef]
- Ogura, J.; Koizumi, T.; Segawa, M.; Yabe, K.; Kuwayama, K.; Sasaki, S.; Kaneko, C.; Tsujimoto, T.; Kobayashi, M.; Yamaguchi, H.; et al. Quercetin-3-rhamnoglucoside (rutin) stimulates transport of organic anion compounds mediated by organic anion transporting polypeptide 2B1. Biopharm. Drug Dispos. 2014, 35, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.Y.; Shih, H.Y.; Chia, Y.C.; Lee, C.H.; Ashida, H.; Lai, Y.K.; Weng, C.F. Rutin potentiates insulin receptor kinase to enhance insulin-dependent glucose transporter 4 translocation. Mol. Nutr. Food Res. 2014, 58, 1168–1176. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.J.; Liao, J.F.; Lin, D.Y.; Huang, M.C.; Liou, D.Y.; Yang, H.C.; Lee, H.J.; Chen, Y.T.; Chi, C.W.; Huang, W.C.; et al. Silymarin protects spinal cord and cortical cells against oxidative stress and lipopolysaccharide stimulation. Neurochem. Int. 2010, 57, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Huang, R.; Qin, Z.; Liu, F. Influence of Tangeretin on the Exponential Regression of Inflammation and Oxidative Stress in Streptozotocin-Induced Diabetic Nephropathy. Appl. Biochem. Biotechnol. 2022, 194, 3914–3929. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Hu, J.; Sun, Y.; Huang, Y.; Peng, Q.; Zhao, W.; Xu, W.; Zhu, L. Tangeretin alleviates inflammation and oxidative response induced by spinal cord injury by activating the Sesn2/Keap1/Nrf2 pathway. Phytother. Res. 2024, 38, 4555–4569. [Google Scholar] [CrossRef]
- Honda, Y.; Ushigome, F.; Koyabu, N.; Morimoto, S.; Shoyama, Y.; Uchiumi, T.; Kuwano, M.; Ohtani, H.; Sawada, Y. Effects of grapefruit juice and orange juice components on P-glycoprotein- and MRP2-mediated drug efflux. Br. J. Pharmacol. 2004, 143, 856–864. [Google Scholar] [CrossRef]
- Satsu, H.; Shibata, R.; Suzuki, H.; Kimura, S.; Shimizu, M. Inhibitory Effect of Tangeretin and Cardamonin on Human Intestinal SGLT1 Activity In Vitro and Blood Glucose Levels in Mice In Vivo. Nutrients 2021, 13, 3382. [Google Scholar] [CrossRef]
- Datla, K.P.; Christidou, M.; Widmer, W.W.; Rooprai, H.K.; Dexter, D.T. Tissue distribution and neuroprotective effects of citrus flavonoid tangeretin in a rat model of Parkinson’s disease. Neuroreport 2001, 12, 3871–3875. [Google Scholar] [CrossRef]
- Bansod, S.; Chilvery, S.; Saifi, M.A.; Das, T.J.; Tag, H.; Godugu, C. Borneol protects against cerulein-induced oxidative stress and inflammation in acute pancreatitis mice model. Environ. Toxicol. 2021, 36, 530–539. [Google Scholar] [CrossRef]
- Fan, X.; Chai, L.; Zhang, H.; Wang, Y.; Zhang, B.; Gao, X. Borneol Depresses P-Glycoprotein Function by a NF-κB Signaling Mediated Mechanism in a Blood Brain Barrier in Vitro Model. Int. J. Mol. Sci. 2015, 16, 27576–27588. [Google Scholar] [CrossRef]
- Chen, N.; Wen, J.; Wang, Z.; Wang, J. Multiple regulation and targeting effects of borneol in the neurovascular unit in neurodegenerative diseases. Basic Clin. Pharmacol. Toxicol. 2022, 130, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Li, W.R.; Chen, R.Y.; Yang, L.; Huang, T.L.; Xu, Q.W.; Mi, S.Q.; Wang, N.S. Pharmacokinetics of natural borneol after oral administration in mice brain and its effect on excitation ratio. Eur. J. Drug Metab. Pharmacokinet. 2012, 37, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Abbasloo, E.; Khaksari, M.; Sanjari, M.; Kobeissy, F.; Thomas, T.C. Carvacrol decreases blood-brain barrier permeability post-diffuse traumatic brain injury in rats. Sci. Rep. 2023, 13, 14546. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Barbosa, C.R.; Scherf, J.R.; de Freitas, T.S.; de Menezes, I.R.A.; Pereira, R.L.S.; Dos Santos, J.F.S.; de Jesus, S.S.P.; Lopes, T.P.; de Sousa Silveira, Z.; de Morais Oliveira-Tintino, C.D.; et al. Effect of Carvacrol and Thymol on NorA efflux pump inhibition in multidrug-resistant (MDR) Staphylococcus aureus strains. J. Bioenerg. Biomembr. 2021, 53, 489–498. [Google Scholar] [CrossRef]
- Li, G.; Li, Y.; Xiao, B.; Cui, D.; Lin, Y.; Zeng, J.; Li, J.; Cao, M.J.; Liu, J. Antioxidant Activity of Docosahexaenoic Acid (DHA) and Its Regulatory Roles in Mitochondria. J. Agric. Food Chem. 2021, 69, 1647–1655. [Google Scholar] [CrossRef]
- Kuan, C.Y.; Walker, T.H.; Luo, P.G.; Chen, C.F. Long-chain polyunsaturated fatty acids promote paclitaxel cytotoxicity via inhibition of the MDR1 gene in the human colon cancer Caco-2 cell line. J. Am. Coll. Nutr. 2011, 30, 265–273. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Ma, D.; Shui, G.; Wong, P.; Cazenave-Gassiot, A.; Zhang, X.; Wenk, M.R.; Goh, E.L.; Silver, D.L. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature 2014, 509, 503–506. [Google Scholar] [CrossRef]
- El Mahdy, R.N.; Nader, M.A.; Helal, M.G.; Abu-Risha, S.E.; Abdelmageed, M.E. Eicosapentaenoic acid mitigates ulcerative colitis-induced by acetic acid through modulation of NF-κB and TGF-β/ EGFR signaling pathways. Life Sci. 2023, 327, 121820. [Google Scholar] [CrossRef]
- Xiao, B.; Li, Y.; Lin, Y.; Lin, J.; Zhang, L.; Wu, D.; Zeng, J.; Li, J.; Liu, J.W.; Li, G. Eicosapentaenoic acid (EPA) exhibits antioxidant activity via mitochondrial modulation. Food Chem. 2022, 373 Pt A, 131389. [Google Scholar] [CrossRef]
- Kato, Y.; Ohsugi, K.; Fukuno, Y.; Iwatsuki, K.; Harada, Y.; Miyaji, T. Vesicular nucleotide transporter is a molecular target of eicosapentaenoic acid for neuropathic and inflammatory pain treatment. Proc. Natl. Acad. Sci. USA 2022, 119, e2122158119. [Google Scholar] [CrossRef]
- Edmond, J. Essential polyunsaturated fatty acids and the barrier to the brain: The components of a model for transport. J. Mol. Neurosci. 2001, 16, 181–193; discussion 215–221. [Google Scholar] [CrossRef] [PubMed]
- Qi, B.; Zheng, Y.; Gao, W.; Qi, Z.; Gong, Y.; Liu, Y.; Wang, Y.; Cheng, X.; Ning, M.; Lang, Y.; et al. Alpha-lipoic acid impedes myocardial ischemia-reperfusion injury, myocardial apoptosis, and oxidative stress by regulating HMGB1 expression. Eur. J. Pharmacol. 2022, 933, 175295. [Google Scholar] [CrossRef] [PubMed]
- Skibska, B.; Kochan, E.; Stanczak, A.; Lipert, A.; Skibska, A. Antioxidant and Anti-inflammatory Effects of α-Lipoic Acid on Lipopolysaccharide-induced Oxidative Stress in Rat Kidney. Arch. Immunol. Ther. Exp. 2023, 71, 16. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.C.; Su, K.H.; Kou, Y.R.; Shyue, S.K.; Ching, L.C.; Yu, Y.B.; Wu, Y.L.; Pan, C.C.; Lee, T.S. α-Lipoic acid ameliorates foam cell formation via liver X receptor α-dependent upregulation of ATP-binding cassette transporters A1 and G1. Free Radic. Biol. Med. 2011, 50, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Zehnpfennig, B.; Wiriyasermkul, P.; Carlson, D.A.; Quick, M. Interaction of α-Lipoic Acid with the Human Na+/Multivitamin Transporter (hSMVT). J. Biol. Chem. 2015, 290, 16372–16382. [Google Scholar] [CrossRef]
- Spector, R. Fatty acid transport through the blood-brain barrier. J. Neurochem. 1988, 50, 639–643. [Google Scholar] [CrossRef]
- Sajja, R.K.; Kaisar, M.A.; Vijay, V.; Desai, V.G.; Prasad, S.; Cucullo, L. In Vitro Modulation of Redox and Metabolism Interplay at the Brain Vascular Endothelium: Genomic and Proteomic Profiles of Sulforaphane Activity. Sci. Rep. 2018, 8, 12708. [Google Scholar] [CrossRef]
- Jazwa, A.; Rojo, A.I.; Innamorato, N.G.; Hesse, M.; Fernández-Ruiz, J.; Cuadrado, A. Pharmacological targeting of the transcription factor Nrf2 at the basal ganglia provides disease modifying therapy for experimental parkinsonism. Antioxid. Redox Signal. 2011, 14, 2347–2360. [Google Scholar] [CrossRef]
- He, J.; He, Z.; Wang, H.; Zhang, C.; Pei, T.; Yan, S.; Yan, Y.; Wang, F.; Chen, Y.; Yuan, N.; et al. Caffeic acid alleviates skeletal muscle atrophy in 5/6 nephrectomy rats through the TLR4/MYD88/NF-kB pathway. Biomed. Pharmacother. 2024, 174, 116556. [Google Scholar] [CrossRef]
- Wortelboer, H.M.; Usta, M.; van Zanden, J.J.; van Bladeren, P.J.; Rietjens, I.M.; Cnubben, N.H. Inhibition of multidrug resistance proteins MRP1 and MRP2 by a series of alpha,beta-unsaturated carbonyl compounds. Biochem. Pharmacol. 2005, 69, 1879–1890. [Google Scholar] [CrossRef]
- Konishi, Y.; Hagiwara, K.; Shimizu, M. Transepithelial transport of fluorescein in Caco-2 cell monolayers and use of such transport in in vitro evaluation of phenolic acid availability. Biosci. Biotechnol. Biochem. 2002, 66, 2449–2457. [Google Scholar] [CrossRef] [PubMed]
- Hase, T.; Shishido, S.; Yamamoto, S.; Yamashita, R.; Nukima, H.; Taira, S.; Toyoda, T.; Abe, K.; Hamaguchi, T.; Ono, K.; et al. Rosmarinic acid suppresses Alzheimer’s disease development by reducing amyloid β aggregation by increasing monoamine secretion. Sci. Rep. 2019, 9, 8711. [Google Scholar] [CrossRef] [PubMed]
- Kose, S.B.E.; Kocasari, F.S. Protective effect of cinnamic acid on orthophenylphenol-induced oxidative stress in rats. Vet. Res. Forum. 2022, 13, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Jana, S.; Patel, V.B.; Patel, H. Effects of piperine, cinnamic acid and gallic acid on rosuvastatin pharmacokinetics in rats. Phytother. Res. 2013, 27, 1548–1556. [Google Scholar] [CrossRef] [PubMed]
- Mani, A.; Kushwaha, K.; Khurana, N.; Gupta, J. p-Coumaric acid attenuates high-fat diet-induced oxidative stress and nephropathy in diabetic rats. J. Anim. Physiol. Anim. Nutr. 2022, 106, 872–880. [Google Scholar] [CrossRef]
- Wang, L.; Sweet, D.H. Potential for food-drug interactions by dietary phenolic acids on human organic anion transporters 1 (SLC22A6), 3 (SLC22A8), and 4 (SLC22A11). Biochem. Pharmacol. 2012, 84, 1088–1095. [Google Scholar] [CrossRef]
- Sun, S.; Ruan, Y.; Yan, M.; Xu, K.; Yang, Y.; Shen, T.; Jin, Z. Ferulic Acid Alleviates Oxidative Stress-Induced Cardiomyocyte Injury by the Regulation of miR-499-5p/p21 Signal Cascade. Evid. Based Complement. Altern. Med. 2021, 2021, 1921457. [Google Scholar] [CrossRef]
- Ermis, A.; Aritici Colak, G.; Acikel-Elmas, M.; Arbak, S.; Kolgazi, M. Ferulic Acid Treats Gastric Ulcer via Suppressing Oxidative Stress and Inflammation. Life 2023, 13, 388. [Google Scholar] [CrossRef]
- Muthusamy, G.; Balupillai, A.; Ramasamy, K.; Shanmugam, M.; Gunaseelan, S.; Mary, B.; Prasad, N.R. Ferulic acid reverses ABCB1-mediated paclitaxel resistance in MDR cell lines. Eur. J. Pharmacol. 2016, 786, 194–203. [Google Scholar] [CrossRef]
- Poquet, L.; Clifford, M.N.; Williamson, G. Transport and metabolism of ferulic acid through the colonic epithelium. Drug Metab. Dispos. 2008, 36, 190–197. [Google Scholar] [CrossRef]
- Lin, Y.; Luo, T.; Weng, A.; Huang, X.; Yao, Y.; Fu, Z.; Li, Y.; Liu, A.; Li, X.; Chen, D.; et al. Gallic Acid Alleviates Gouty Arthritis by Inhibiting NLRP3 Inflammasome Activation and Pyroptosis Through Enhancing Nrf2 Signaling. Front. Immunol. 2020, 11, 580593. [Google Scholar] [CrossRef] [PubMed]
- Pervin, M.; Unno, K.; Nakagawa, A.; Takahashi, Y.; Iguchi, K.; Yamamoto, H.; Hoshino, M.; Hara, A.; Takagaki, A.; Nanjo, F.; et al. Blood brain barrier permeability of (-)-epigallocatechin gallate, its proliferation-enhancing activity of human neuroblastoma SH-SY5Y cells, and its preventive effect on age-related cognitive dysfunction in mice. Biochem. Biophys. Rep. 2017, 9, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Joardar, S.; Dewanjee, S.; Bhowmick, S.; Dua, T.K.; Das, S.; Saha, A.; De Feo, V. Rosmarinic Acid Attenuates Cadmium-Induced Nephrotoxicity via Inhibition of Oxidative Stress, Apoptosis, Inflammation and Fibrosis. Int. J. Mol. Sci. 2019, 20, 2027. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Li, R.; Liu, D.; Long, L.; He, N. Rosmarinic acid alleviates acetaminophen-induced hepatotoxicity by targeting Nrf2 and NEK7-NLRP3 signaling pathway. Ecotoxicol. Environ. Saf. 2022, 241, 113773. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Tao, Y.; Chen, S.; Luo, H.; Li, X.; Qu, S.; Chen, K.; Zeng, C. Rosmarinic Acid Ameliorates Pulmonary Ischemia/Reperfusion Injury by Activating the PI3K/Akt Signaling Pathway. Front. Pharmacol. 2022, 13, 860944. [Google Scholar] [CrossRef]
- Nyandwi, J.B.; Ko, Y.S.; Jin, H.; Yun, S.P.; Park, S.W.; Kim, H.J. Rosmarinic Acid Increases Macrophage Cholesterol Efflux through Regulation of ABCA1 and ABCG1 in Different Mechanisms. Int. J. Mol. Sci. 2021, 22, 8791. [Google Scholar] [CrossRef]
- Wang, L.; Sweet, D.H. Competitive inhibition of human organic anion transporters 1 (SLC22A6), 3 (SLC22A8) and 4 (SLC22A11) by major components of the medicinal herb Salvia miltiorrhiza (Danshen). Drug Metab. Pharmacokinet. 2013, 28, 220–228. [Google Scholar] [CrossRef]
- Zhou, Y.; Khan, H.; Hoi, M.P.M.; Cheang, W.S. Piceatannol Protects Brain Endothelial Cell Line (bEnd.3) against Lipopolysaccharide-Induced Inflammation and Oxidative Stress. Molecules 2022, 27, 1206. [Google Scholar] [CrossRef]
- Rubic, T.; Lorenz, R.L. Downregulated CD36 and oxLDL uptake and stimulated ABCA1/G1 and cholesterol efflux as anti-atherosclerotic mechanisms of interleukin-10. Cardiovasc. Res. 2006, 69, 527–535. [Google Scholar] [CrossRef]
- Ibacache-Chía, A.P.; Sierralta, J.A.; Schüller, A. The Inhibitory Effects of the Natural Stilbene Piceatannol on Lactate Transport In Vitro Mediated by Monocarboxylate Transporters. Mol. Nutr. Food Res. 2024, 68, e2400414. [Google Scholar] [CrossRef]
- Shah, F.A.; Kury, L.A.; Li, T.; Zeb, A.; Koh, P.O.; Liu, F.; Zhou, Q.; Hussain, I.; Khan, A.U.; Jiang, Y.; et al. Polydatin Attenuates Neuronal Loss via Reducing Neuroinflammation and Oxidative Stress in Rat MCAO Models. Front. Pharmacol. 2019, 10, 663. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Xu, J.; Zeng, Y.; Chen, L.; Xu, X.L. Polydatin attenuates atherosclerosis in apolipoprotein E-deficient mice: Role of reverse cholesterol transport. Phytomedicine 2019, 62, 152935. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, Y.; Cai, X.; Qu, B.; Zhang, Y.; Sun, Z.; Yan, J. Comparative pharmacokinetics and tissue distribution of polydatin, resveratrol, and emodin after oral administration of Huzhang and Huzhang-Guizhi herb-pair extracts to rats. J. Ethnopharmacol. 2024, 318 Pt B, 117010. [Google Scholar] [CrossRef]
- Dos Santos Lacerda, D.; Türck, P.; Gazzi de Lima-Seolin, B.; Colombo, R.; Duarte Ortiz, V.; Poletto Bonetto, J.H.; Campos-Carraro, C.; Bianchi, S.E.; Belló-Klein, A.; Linck Bassani, V.; et al. Pterostilbene reduces oxidative stress, prevents hypertrophy and preserves systolic function of right ventricle in cor pulmonale model. Br. J. Pharmacol. 2017, 174, 3302–3314. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Su, Y.; Yang, Y.; Jin, H.; Wu, M.; Wang, Q.; Sun, P.; Zhang, J.; Yang, X.; Shu, X. Increased brain uptake of pterostilbene loaded folate modified micellar delivery system. Drug Deliv. 2022, 29, 3071–3086. [Google Scholar] [CrossRef] [PubMed]
- Planas, J.M.; Alfaras, I.; Colom, H.; Juan, M.E. The bioavailability and distribution of trans-resveratrol are constrained by ABC transporters. Arch. Biochem. Biophys. 2012, 527, 67–73. [Google Scholar] [CrossRef]
- Juan, M.E.; Maijó, M.; Planas, J.M. Quantification of trans-resveratrol and its metabolites in rat plasma and tissues by HPLC. J. Pharm. Biomed. Anal. 2010, 51, 391–398. [Google Scholar] [CrossRef]
- Yen, G.C.; Duh, P.D.; Tsai, H.L. Antioxidant and pro-oxidant properties of ascorbic acid and gallic acid. Food Chem. 2002, 79, 307–313. [Google Scholar] [CrossRef]
- Wartenberg, M.; Hoffmann, E.; Schwindt, H.; Grünheck, F.; Petros, J.; Arnold, J.R.; Hescheler, J.; Sauer, H. Reactive oxygen species-linked regulation of the multidrug resistance transporter P-glycoprotein in Nox-1 overexpressing prostate tumor spheroids. FEBS Lett. 2005, 579, 4541–4549. [Google Scholar] [CrossRef]
- May, J.M. Vitamin C transport and its role in the central nervous system. Subcell. Biochem. 2012, 56, 85–103. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, H.; Wang, Y.; Zheng, Y.; Sun, G. Effects of folate on arsenic toxicity in Chang human hepatocytes: Involvement of folate antioxidant properties. Toxicol. Lett. 2010, 195, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Spector, R.; Johanson, C.E. Vitamin transport and homeostasis in mammalian brain: Focus on Vitamins B and E. J. Neurochem. 2007, 103, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, Z.; Hekmatdoost, Z.; Nourian, M. Antioxidant efficacy of vitamin D. J. Parathyr. Dis. 2017, 5, 11–16. [Google Scholar]
- Pardridge, W.M.; Sakiyama, R.; Coty, W.A. Restricted transport of vitamin D and A derivatives through the rat blood-brain barrier. J. Neurochem. 1985, 44, 1138–1141. [Google Scholar] [CrossRef]
- Tang, J.; Fu, Q.; Wang, Y.; Racette, K.; Wang, D.; Liu, F. Vitamin E reverses multidrug resistance in vitro and in vivo. Cancer Lett. 2013, 336, 149–157. [Google Scholar] [CrossRef]
- Hunyadi, A. The mechanism(s) of action of antioxidants: From scavenging reactive oxygen/nitrogen species to redox signaling and the generation of bioactive secondary metabolites. Med. Res. Rev. 2019, 39, 2505–2533. [Google Scholar] [CrossRef]
- Ureña-Vacas, I.; Aznar de la Riera, M.B.; Serrano Dolores, R.; González-Burgos, E. A new frontier in neuropharmacology: Recent progress in natural products research for blood–brain barrier crossing. Curr. Res. Biotechnol. 2024, 8, 100235. [Google Scholar] [CrossRef]
- Munji, R.N.; Soung, A.L.; Weiner, G.A.; Sohet, F.; Semple, B.D.; Trivedi, A.; Gimlin, K.; Kotoda, M.; Korai, M.; Aydin, S.; et al. Profiling the mouse brain endothelial transcriptome in health and disease models reveals a core blood-brain barrier dysfunction module. Nat. Neurosci. 2019, 22, 1892–1902. [Google Scholar] [CrossRef]
- Sabbagh, M.F.; Heng, J.S.; Luo, C.; Castanon, R.G.; Nery, J.R.; Rattner, A.; Goff, L.A.; Ecker, J.R.; Nathans, J. Transcriptional and epigenomic landscapes of CNS and non-CNS vascular endothelial cells. eLife 2018, 7, e36187. [Google Scholar] [CrossRef]
- Vanlandewijck, M.; He, L.; Mäe, M.A.; Andrae, J.; Ando, K.; Del Gaudio, F.; Nahar, K.; Lebouvier, T.; Laviña, B.; Gouveia, L.; et al. A molecular atlas of cell types and zonation in the brain vasculature. Nature 2018, 554, 475–480. [Google Scholar] [CrossRef]
- Garcia, F.J.; Sun, N.; Lee, H.; Godlewski, B.; Mathys, H.; Galani, K.; Zhou, B.; Jiang, X.; Ng, A.P.; Mantero, J.; et al. Single-cell dissection of the human brain vasculature. Nature 2022, 603, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.C.; Vest, R.T.; Kern, F.; Lee, D.P.; Agam, M.; Maat, C.A.; Losada, P.M.; Chen, M.B.; Schaum, N.; Khoury, N.; et al. A human brain vascular atlas reveals diverse mediators of Alzheimer’s risk. Nature 2022, 603, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Kalari, K.R.; Thompson, K.J.; Nair, A.A.; Tang, X.; Bockol, M.A.; Jhawar, N.; Swaminathan, S.K.; Lowe, V.J.; Kandimalla, K.K. BBBomics-Human Blood Brain Barrier Transcriptomics Hub. Front. Neurosci. 2016, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Veszelka, S.; Tóth, A.; Walter, F.R.; Tóth, A.E.; Gróf, I.; Mészáros, M.; Bocsik, A.; Hellinger, É.; Vastag, M.; Rákhely, G.; et al. Comparison of a Rat Primary Cell-Based Blood-Brain Barrier Model With Epithelial and Brain Endothelial Cell Lines: Gene Expression and Drug Transport. Front. Mol. Neurosci. 2018, 11, 166. [Google Scholar] [CrossRef] [PubMed]
- Santa-Maria, A.R.; Heymans, M.; Walter, F.R.; Culot, M.; Gosselet, F.; Deli, M.A.; Neuhaus, W. Transport Studies Using Blood-Brain Barrier In Vitro Models: A Critical Review and Guidelines. Handb. Exp. Pharmacol. 2022, 273, 187–204. [Google Scholar] [CrossRef]
- Porkoláb, G.; Mészáros, M.; Szecskó, A.; Vigh, J.P.; Walter, F.R.; Figueiredo, R.; Kálomista, I.; Hoyk, Z.; Vizsnyiczai, G.; Gróf, I.; et al. Synergistic induction of blood-brain barrier properties. Proc. Natl. Acad. Sci. USA 2024, 121, e2316006121. [Google Scholar] [CrossRef]
- Zhou, S.; Lim, L.Y.; Chowbay, B. Herbal modulation of P-glycoprotein. Drug Metab. Rev. 2004, 36, 57–104. [Google Scholar] [CrossRef]
- Youdim, K.A.; Shukitt-Hale, B.; Joseph, J.A. Flavonoids and the brain: Interactions at the blood-brain barrier and their physiological effects on the central nervous system. Free Radic. Biol. Med. 2004, 37, 1683–1693. [Google Scholar] [CrossRef]
- Li, Y.; Revalde, J.; Paxton, J.W. The effects of dietary and herbal phytochemicals on drug transporters. Adv. Drug Deliv. Rev. 2017, 116, 45–62. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.; Tian, F.; Zhang, S.Q.; Jin, H. Advances in Pharmacokinetic Mechanisms of Transporter-Mediated Herb-Drug Interactions. Pharmaceuticals 2022, 15, 1126. [Google Scholar] [CrossRef]
- Fricker, G. Drug interactions with natural products at the blood brain barrier. Curr. Drug Metab. 2008, 9, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Pekdemir, B.; Raposo, A.; Saraiva, A.; Lima, M.J.; Alsharari, Z.D.; BinMowyna, M.N.; Karav, S. Mechanisms and Potential Benefits of Neuroprotective Agents in Neurological Health. Nutrients 2024, 16, 4368. [Google Scholar] [CrossRef] [PubMed]
- Auti, A.; Tathode, M.; Marino, M.M.; Vitiello, A.; Ballini, A.; Miele, F.; Mazzone, V.; Ambrosino, A.; Boccellino, M. Nature’s weapons: Bioactive compounds as anti-cancer agents. AIMS Public Health 2024, 11, 747–772. [Google Scholar] [CrossRef] [PubMed]
- Andrew, R.; Izzo, A.A. Principles of pharmacological research of nutraceuticals. Br. J. Pharmacol. 2017, 174, 1177–1194. [Google Scholar] [CrossRef]
- Lund, E.K. Health benefits of seafood; is it just the fatty acids? Food Chem. 2013, 140, 413–420. [Google Scholar] [CrossRef]
- Kulkarni, M.; Sawant, N.; Kolapkar, A.; Huprikar, A.; Desai, N. Borneol: A Promising Monoterpenoid in Enhancing Drug Delivery Across Various Physiological Barriers. AAPS PharmSciTech. 2021, 22, 145. [Google Scholar] [CrossRef]
- Profaci, C.P.; Munji, R.N.; Pulido, R.S.; Daneman, R. The blood-brain barrier in health and disease: Important unanswered questions. J. Exp. Med. 2020, 217, e20190062. [Google Scholar] [CrossRef]
- Mukherjee, P.; Roy, S.; Ghosh, D.; Nandi, S.K. Role of animal models in biomedical research: A review. Lab. Anim. Res. 2022, 38, 18. [Google Scholar] [CrossRef]
- Zhang, T.; Su, J.; Guo, B.; Wang, K.; Li, X.; Liang, G. Apigenin protects blood-brain barrier and ameliorates early brain injury by inhibiting TLR4-mediated inflammatory pathway in subarachnoid hemorrhage rats. Int. Immunopharmacol. 2015, 28, 79–87. [Google Scholar] [CrossRef]
- Pang, Q.; Zhao, Y.; Chen, X.; Zhao, K.; Zhai, Q.; Tu, F. Apigenin Protects the Brain against Ischemia/Reperfusion Injury via Caveolin-1/VEGF In Vitro and In Vivo. Oxid. Med. Cell Longev. 2018, 2018, 7017204. [Google Scholar] [CrossRef]
- Iwata, S.; Imai, T.; Shimazawa, M.; Ishibashi, T.; Hayashi, M.; Hara, H.; Nakamura, S. Protective effects of the astaxanthin derivative, adonixanthin, on brain hemorrhagic injury. Brain Res. 2018, 1698, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.S.; Zhang, X.; Zhang, Q.R.; Wu, Q.; Li, W.; Jiang, T.W.; Hang, C.H. Corrigendum to “Astaxanthin reduces matrix metalloproteinase-9 expression and activity in the brain after experimental subarachnoid hemorrhage in rats” [Brain Res. 1624 (2015) 113–124]. Brain Res. 2024, 1850, 149390. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Zhong, F.M.; Yao, Y.; Deng, S.Q.; Xu, H.Q.; Lu, J.F.; Ruan, M.; Shen, X.C. Synergistic protection of tetramethylpyrazine phosphate and borneol on brain microvascular endothelium cells injured by hypoxia. Am. J. Transl. Res. 2019, 11, 2168–2180. [Google Scholar]
- Li, Y.; Ren, M.; Wang, J.; Ma, R.; Chen, H.; Xie, Q.; Li, H.; Li, J.; Wang, J. Progress in Borneol Intervention for Ischemic Stroke: A Systematic Review. Front. Pharmacol. 2021, 12, 606682. [Google Scholar] [CrossRef]
- Zhao, J.; Pati, S.; Redell, J.B.; Zhang, M.; Moore, A.N.; Dash, P.K. Caffeic Acid phenethyl ester protects blood-brain barrier integrity and reduces contusion volume in rodent models of traumatic brain injury. J. Neurotrauma 2012, 29, 1209–1218. [Google Scholar] [CrossRef]
- Mahmood, Q.; Lu, N.; Wang, X.; Du, Y.; Ghori, M.U.; Tian, B.; Yang, H.; Han, F.; Jiang, G.; Lu, Y. Targeted delivery of β-carotene potentially prevents blood-brain barrier breakdown after stroke in mice. Phytomed. Plus 2023, 3, 100426. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhang, J.; Cai, Y.; Huang, J.; You, L. Catechin attenuates traumatic brain injury-induced blood-brain barrier damage and improves longer-term neurological outcomes in rats. Exp. Physiol. 2017, 102, 1269–1277. [Google Scholar] [CrossRef]
- Rashno, M.; Sarkaki, A.; Farbood, Y.; Rashno, M.; Khorsandi, L.; Naseri, M.K.G.; Dianat, M. Possible mechanisms involved in the neuroprotective effects of chrysin against mild traumatic brain injury-induced spatial cognitive decline: An in vivo study in a rat model. Brain Res. Bull. 2023, 204, 110779. [Google Scholar] [CrossRef]
- Li, Y.; Han, J.; Zhang, Y.; Chen, Y.; Zhang, Y. Prophylactic effect and mechanism of p-coumaric acid against hypoxic cerebral edema in mice. Respir. Physiol. Neurobiol. 2019, 260, 95–104. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, W.; Sun, Y.J.; Hu, M.; Li, F.; Zhu, D.Y. Neuroprotective effect of curcumin on focal cerebral ischemic rats by preventing blood-brain barrier damage. Eur. J. Pharmacol. 2007, 561, 54–62. [Google Scholar] [CrossRef]
- Yu, L.S.; Fan, Y.Y.; Ye, G.; Li, J.; Feng, X.P.; Lin, K.; Dong, M.; Wang, Z. Curcumin alleviates brain edema by lowering AQP4 expression levels in a rat model of hypoxia-hypercapnia-induced brain damage. Exp. Ther. Med. 2016, 11, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhong, X.; Zeng, J.; Huang, Z.; Li, X.; Xiao, H.; Chen, Q.; Li, D. 3′-Daidzein sulfonate sodium inhibits neuronal apoptosis induced by cerebral ischemia-reperfusion. Int. J. Mol. Med. 2017, 39, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.C.; Kao, T.K.; Ou, Y.C.; Yang, D.Y.; Yen, Y.J.; Wang, C.C.; Chuang, Y.H.; Liao, S.L.; Raung, S.L.; Wu, C.W.; et al. Protective effect of docosahexaenoic acid against brain injury in ischemic rats. J. Nutr. Biochem. 2009, 20, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Khoutorova, L.; Bazan, N.G.; Belayev, L. Docosahexaenoic acid improves behavior and attenuates blood-brain barrier injury induced by focal cerebral ischemia in rats. Exp. Transl. Stroke Med. 2015, 7, 3. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, H.; Mu, H.; Zhu, W.; Jiang, X.; Hu, X.; Shi, Y.; Leak, R.K.; Dong, Q.; Chen, J.; et al. Omega-3 polyunsaturated fatty acids mitigate blood-brain barrier disruption after hypoxic-ischemic brain injury. Neurobiol. Dis. 2016, 91, 37–46. [Google Scholar] [CrossRef]
- Shin, J.A.; Park, H.; Choi, H.; Chang, Y.K.; Kim, J.J.; Ham, Y.R.; Na, K.R.; Lee, K.W.; Choi, D.E. ω-3 Polyunsaturated Fatty Acids Improve the Blood-Brain-Barrier Integrity in Contrast-Induced Blood-Brain-Barrier Injury in Uremic Mice. Int. J. Mol. Sci. 2023, 24, 12168. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Z.; Wang, P.; Yu, B.; Liu, Y.; Xue, Y. Green tea polyphenols alleviate early BBB damage during experimental focal cerebral ischemia through regulating tight junctions and PKCalpha signaling. BMC Complement. Altern. Med. 2013, 13, 187. [Google Scholar] [CrossRef]
- Xu, S.H.; Yin, M.S.; Liu, B.; Chen, M.L.; He, G.W.; Zhou, P.P.; Cui, Y.J.; Yang, D.; Wu, Y.L. Tetramethylpyrazine-2′-O-sodium ferulate attenuates blood-brain barrier disruption and brain oedema after cerebral ischemia/reperfusion. Hum. Exp. Toxicol. 2017, 36, 670–680. [Google Scholar] [CrossRef]
- Mehra, S.; Ahsan, A.U.; Sharma, M.; Budhwar, M.; Chopra, M. Gestational Fisetin Exerts Neuroprotection by Regulating Mitochondria-Directed Canonical Wnt Signaling, BBB Integrity, and Apoptosis in Prenatal VPA-Induced Rodent Model of Autism. Mol. Neurobiol. 2024, 61, 4001–4020. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, Z.; Bai, W.; Peng, Y.; Lin, Y.; Cong, Z. Fucoxanthin ameliorates traumatic brain injury by suppressing the blood-brain barrier disruption. iScience 2023, 26, 108270. [Google Scholar] [CrossRef]
- Soltani, Z.; Khaksari, M.; Jafari, E.; Iranpour, M.; Shahrokhi, N. Is genistein neuroprotective in traumatic brain injury? Physiol. Behav. 2015, 152 Pt A, 26–31. [Google Scholar] [CrossRef]
- Song, H.; Ding, Z.; Chen, J.; Chen, T.; Wang, T.; Huang, J. The AMPK-SIRT1-FoxO1-NF-κB signaling pathway participates in hesperetin-mediated neuroprotective effects against traumatic brain injury via the NLRP3 inflammasome. Immunopharmacol. Immunotoxicol. 2022, 44, 970–983. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Yang, Y.L.; Yang, H.; Wang, Y.H.; Du, G.H. Kaempferol alleviates LPS-induced neuroinflammation and BBB dysfunction in mice via inhibiting HMGB1 release and down-regulating TLR4/MyD88 pathway. Int. Immunopharmacol. 2018, 56, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.L.; Cheng, X.; Li, W.H.; Liu, M.; Wang, Y.H.; Du, G.H. Kaempferol Attenuates LPS-Induced Striatum Injury in Mice Involving Anti-Neuroinflammation, Maintaining BBB Integrity, and Down-Regulating the HMGB1/TLR4 Pathway. Int. J. Mol. Sci. 2019, 20, 491. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, L.; Zhang, X.; Pan, L.; Jiang, L. The Protective Effects of Juglanin in Cerebral Ischemia Reduce Blood-Brain Barrier Permeability via Inhibition of VEGF/VEGFR2 Signaling. Drug Des. Dev. Ther. 2020, 14, 3165–3175. [Google Scholar] [CrossRef] [PubMed]
- Toklu, H.Z.; Hakan, T.; Biber, N.; Solakoğlu, S.; Oğünç, A.V.; Sener, G. The protective effect of alpha lipoic acid against traumatic brain injury in rats. Free Radic. Res. 2009, 43, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Turk, C.; Camlar, M.; Diniz, G.; Arslan, F.D.; Oren, M.M.; Ozer, F. Effects of Lutein on Brain Damage and Vasospasm in an Experimental Subarachnoid Hemorrhage Model. World Neurosurg. 2020, 143, e450–e455. [Google Scholar] [CrossRef]
- Tan, D.; Yu, X.; Chen, M.; Chen, J.; Xu, J. Lutein protects against severe traumatic brain injury through anti-inflammation and antioxidative effects via ICAM-1/Nrf-2. Mol. Med. Rep. 2017, 16, 4235–4240. [Google Scholar] [CrossRef]
- Liu, R.; Gao, M.; Qiang, G.F.; Zhang, T.T.; Lan, X.; Ying, J.; Du, G.H. The anti-amnesic effects of luteolin against amyloid beta(25-35) peptide-induced toxicity in mice involve the protection of neurovascular unit. Neuroscience 2009, 162, 1232–1243. [Google Scholar] [CrossRef]
- Moustafa, E.M.; Moawed, F.S.M.; Elmaghraby, D.F. Luteolin/ZnO nanoparticles attenuate neuroinflammation associated with diabetes via regulating MicroRNA-124 by targeting C/EBPA. Environ. Toxicol. 2023, 38, 2691–2704. [Google Scholar] [CrossRef]
- Wu, A.; Liu, R.; Dai, W.; Jie, Y.; Yu, G.; Fan, X.; Huang, Q. Lycopene attenuates early brain injury and inflammation following subarachnoid hemorrhage in rats. Int. J. Clin. Exp. Med. 2015, 8, 14316–14322. [Google Scholar] [PubMed]
- Wang, W.; Yang, W.; Shen, Z.; Wen, S.; Hu, M. The Dose-Response Effect of Lycopene on Cerebral Vessel and Neuron Impairment Induced by Hyperlipidemia. J. Agric. Food Chem. 2018, 66, 13173–13182. [Google Scholar] [CrossRef] [PubMed]
- Lapi, D.; Chiurazzi, M.; Di Maro, M.; Mastantuono, T.; Battiloro, L.; Sabatino, L.; Ricci, S.; Di Carlo, A.; Starita, N.; Guida, B.; et al. Malvidin’s Effects on Rat Pial Microvascular Permeability Changes Due to Hypoperfusion and Reperfusion Injury. Front. Cell Neurosci. 2016, 10, 153. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, L.; Yu, L.; Xia, Y.; Fang, Z.; Wang, S. Naringenin Protected Against Blood Brain Barrier Breakdown after Ischemic Stroke through GSK-3β/β-Catenin Pathway. Neurochem. Res. 2024, 50, 17. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Zhang, X.; Du, Y.; Liu, H.; Li, S.; Li, L. Polydatin modulates inflammation by decreasing NF-κB activation and oxidative stress by increasing Gli1, Ptch1, SOD1 expression and ameliorates blood-brain barrier permeability for its neuroprotective effect in pMCAO rat brain. Brain Res. Bull. 2012, 87, 50–59. [Google Scholar] [CrossRef]
- Ruan, W.; Li, J.; Xu, Y.; Wang, Y.; Zhao, F.; Yang, X.; Jiang, H.; Zhang, L.; Saavedra, J.M.; Shi, L.; et al. MALAT1 Up-Regulator Polydatin Protects Brain Microvascular Integrity and Ameliorates Stroke Through C/EBPβ/MALAT1/CREB/PGC-1α/PPARγ Pathway. Cell Mol. Neurobiol. 2019, 39, 265–286. [Google Scholar] [CrossRef]
- Wu, S.; Yue, Y.; Li, J.; Li, Z.; Li, X.; Niu, Y.; Xiang, J.; Ding, H. Procyanidin B2 attenuates neurological deficits and blood-brain barrier disruption in a rat model of cerebral ischemia. Mol. Nutr. Food Res. 2015, 59, 1930–1941. [Google Scholar] [CrossRef]
- Yang, Z.H.; Liu, Y.J.; Ban, W.K.; Liu, H.B.; Lv, L.J.; Zhang, B.Y.; Liu, A.L.; Hou, Z.Y.; Lu, J.; Chen, X.; et al. Pterostilbene alleviated cerebral ischemia/reperfusion-induced blood-brain barrier dysfunction via inhibiting early endothelial cytoskeleton reorganization and late basement membrane degradation. Food Funct. 2023, 14, 8291–8308. [Google Scholar] [CrossRef]
- Yan, W.; Ren, D.; Feng, X.; Huang, J.; Wang, D.; Li, T.; Zhang, D. Neuroprotective and Anti-Inflammatory Effect of Pterostilbene Against Cerebral Ischemia/Reperfusion Injury via Suppression of COX-2. Front. Pharmacol. 2021, 12, 770329. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, T.T.; Zhou, D.; Bai, X.Y.; Zhou, W.L.; Huang, C.; Song, J.K.; Meng, F.R.; Wu, C.X.; Li, L.; et al. Quercetin protects against the Aβ(25-35)-induced amnesic injury: Involvement of inactivation of rage-mediated pathway and conservation of the NVU. Neuropharmacology 2013, 67, 419–431. [Google Scholar] [CrossRef]
- Lee, J.K.; Kwak, H.J.; Piao, M.S.; Jang, J.W.; Kim, S.H.; Kim, H.S. Quercetin reduces the elevated matrix metalloproteinases-9 level and improves functional outcome after cerebral focal ischemia in rats. Acta Neurochir. 2011, 153, 1321–1329. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Ke, J.; Guo, P.; Wang, Y.; Wu, H. Quercetin improves blood-brain barrier dysfunction in rats with cerebral ischemia reperfusion via Wnt signaling pathway. Am. J. Transl. Res. 2019, 11, 4683–4695. [Google Scholar] [PubMed]
- Selvakumar, K.; Prabha, R.L.; Saranya, K.; Bavithra, S.; Krishnamoorthy, G.; Arunakaran, J. Polychlorinated biphenyls impair blood-brain barrier integrity via disruption of tight junction proteins in cerebrum, cerebellum and hippocampus of female Wistar rats: Neuropotential role of quercetin. Hum. Exp. Toxicol. 2013, 32, 706–720. [Google Scholar] [CrossRef]
- Yang, R.; Shen, Y.J.; Chen, M.; Zhao, J.Y.; Chen, S.H.; Zhang, W.; Song, J.K.; Li, L.; Du, G.H. Quercetin attenuates ischemia reperfusion injury by protecting the blood-brain barrier through Sirt1 in MCAO rats. J. Asian Nat. Prod. Res. 2022, 24, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, Y.; Feng, P.; Wang, H.; Zheng, M.; Zhu, Y.; Zhong, K.; Hu, J.; Ye, Y.; Lu, L.; et al. Quercetin improves the protection of hydroxysafflor yellow a against cerebral ischemic injury by modulating of blood-brain barrier and src-p-gp-mmp-9 signalling. Heliyon 2024, 10, e31002. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.; Tuor, U.I.; Thompson, R.; Institoris, A.; Kulynych, A.; Zhang, X.; Kinniburgh, D.W.; Bari, F.; Busija, D.W.; Barber, P.A. Protection against recurrent stroke with resveratrol: Endothelial protection. PLoS ONE 2012, 7, e47792. [Google Scholar] [CrossRef]
- Luan, H.; Kan, Z.; Xu, Y.; Lv, C.; Jiang, W. Rosmarinic acid protects against experimental diabetes with cerebral ischemia: Relation to inflammation response. J. Neuroinflamm. 2013, 10, 28. [Google Scholar] [CrossRef]
- Yang, T.; Feng, C.; Wang, D.; Qu, Y.; Yang, Y.; Wang, Y.; Sun, Z. Neuroprotective and Anti-inflammatory Effect of Tangeretin Against Cerebral Ischemia-Reperfusion Injury in Rats. Inflammation 2020, 43, 2332–2343. [Google Scholar] [CrossRef]
- Novochadlo, M.; Goldim, M.P.; Bonfante, S.; Joaquim, L.; Mathias, K.; Metzker, K.; Machado, R.S.; Lanzzarin, E.; Bernades, G.; Bagio, E.; et al. Folic acid alleviates the blood brain barrier permeability and oxidative stress and prevents cognitive decline in sepsis-surviving rats. Microvasc. Res. 2021, 137, 104193. [Google Scholar] [CrossRef]
- Chang, C.Y.; Chen, J.Y.; Wu, M.H.; Hu, M.L. Therapeutic treatment with vitamin C reduces focal cerebral ischemia-induced brain infarction in rats by attenuating disruptions of blood brain barrier and cerebral neuronal apoptosis. Free Radic. Biol. Med. 2020, 155, 29–36. [Google Scholar] [CrossRef]
- Yang, J.; Wang, K.; Hu, T.; Wang, G.; Wang, W.; Zhang, J. Vitamin D3 Supplement Attenuates Blood-Brain Barrier Disruption and Cognitive Impairments in a Rat Model of Traumatic Brain Injury. Neuromol. Med. 2021, 23, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Shlosberg, D.; Benifla, M.; Kaufer, D.; Friedman, A. Blood-brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat. Rev. Neurol. 2010, 6, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Cash, A.; Theus, M.H. Mechanisms of Blood-Brain Barrier Dysfunction in Traumatic Brain Injury. Int. J. Mol. Sci. 2020, 21, 3344. [Google Scholar] [CrossRef] [PubMed]
- Keep, R.F.; Zhou, N.; Xiang, J.; Andjelkovic, A.V.; Hua, Y.; Xi, G. Vascular disruption and blood-brain barrier dysfunction in intracerebral hemorrhage. Fluids Barriers CNS 2014, 11, 18. [Google Scholar] [CrossRef]
- Bernardo-Castro, S.; Sousa, J.A.; Brás, A.; Cecília, C.; Rodrigues, B.; Almendra, L.; Machado, C.; Santo, G.; Silva, F.; Ferreira, L.; et al. Pathophysiology of Blood-Brain Barrier Permeability Throughout the Different Stages of Ischemic Stroke and Its Implication on Hemorrhagic Transformation and Recovery. Front. Neurol. 2020, 11, 594672. [Google Scholar] [CrossRef]
- Wu, J.Y.; Li, Y.J.; Yang, L.; Hu, Y.Y.; Hu, X.B.; Tang, T.T.; Wang, J.M.; Liu, X.Y.; Xiang, D.X. Borneol and A-asarone as adjuvant agents for improving blood-brain barrier permeability of puerarin and tetramethylpyrazine by activating adenosine receptors. Drug Deliv. 2018, 25, 1858–1864. [Google Scholar] [CrossRef]
- Bjelik, A.; Bereczki, E.; Gonda, S.; Juhász, A.; Rimanóczy, A.; Zana, M.; Csont, T.; Pákáski, M.; Boda, K.; Ferdinandy, P.; et al. Human apoB overexpression and a high-cholesterol diet differently modify the brain APP metabolism in the transgenic mouse model of atherosclerosis. Neurochem. Int. 2006, 49, 393–400. [Google Scholar] [CrossRef]
- Hoyk, Z.; Tóth, M.E.; Lénárt, N.; Nagy, D.; Dukay, B.; Csefová, A.; Zvara, Á.; Seprényi, G.; Kincses, A.; Walter, F.R.; et al. Cerebrovascular Pathology in Hypertriglyceridemic APOB-100 Transgenic Mice. Front. Cell Neurosci. 2018, 12, 380. [Google Scholar] [CrossRef]
- Barabási, B.; Barna, L.; Santa-Maria, A.R.; Harazin, A.; Molnár, R.; Kincses, A.; Vigh, J.P.; Dukay, B.; Sántha, M.; Tóth, M.E.; et al. Role of interleukin-6 and interleukin-10 in morphological and functional changes of the blood-brain barrier in hypertriglyceridemia. Fluids Barriers CNS 2023, 20, 15. [Google Scholar] [CrossRef]
- Galea, I. The blood-brain barrier in systemic infection and inflammation. Cell Mol. Immunol. 2021, 18, 2489–2501. [Google Scholar] [CrossRef]
- Gozal, E.; Jagadapillai, R.; Cai, J.; Barnes, G.N. Potential crosstalk between sonic hedgehog-WNT signaling and neurovascular molecules: Implications for blood-brain barrier integrity in autism spectrum disorder. J. Neurochem. 2021, 159, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Tesse, A.; Grossini, E.; Tamma, G.; Brenner, C.; Portincasa, P.; Marinelli, R.A.; Calamita, G. Aquaporins as Targets of Dietary Bioactive Phytocompounds. Front. Mol. Biosci. 2018, 5, 30. [Google Scholar] [CrossRef] [PubMed]
- Langen, U.H.; Ayloo, S.; Gu, C. Development and Cell Biology of the Blood-Brain Barrier. Annu. Rev. Cell Dev. Biol. 2019, 35, 591–613. [Google Scholar] [CrossRef] [PubMed]
- Stresser, D.M.; Kopec, A.K.; Hewitt, P.; Hardwick, R.N.; Van Vleet, T.R.; Mahalingaiah, P.K.S.; O’Connell, D.; Jenkins, G.J.; David, R.; Graham, J.; et al. Towards in vitro models for reducing or replacing the use of animals in drug testing. Nat. Biomed. Eng. 2024, 8, 930–935. [Google Scholar] [CrossRef]
- Ingber, D.E. Human organs-on-chips for disease modelling, drug development and personalized medicine. Nat. Rev. Genet. 2022, 23, 467–491. [Google Scholar] [CrossRef]
- Helms, H.C.; Abbott, N.J.; Burek, M.; Cecchelli, R.; Couraud, P.O.; Deli, M.A.; Förster, C.; Galla, H.J.; Romero, I.A.; Shusta, E.V.; et al. In vitro models of the blood-brain barrier: An overview of commonly used brain endothelial cell culture models and guidelines for their use. J. Cereb. Blood Flow. Metab. 2016, 36, 862–890. [Google Scholar] [CrossRef]
- Tahanian, E.; Sanchez, L.A.; Shiao, T.C.; Roy, R.; Annabi, B. Flavonoids targeting of IκB phosphorylation abrogates carcinogen-induced MMP-9 and COX-2 expression in human brain endothelial cells. Drug Des. Dev. Ther. 2011, 5, 299–309. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, J.; Jiang, W.; Zhang, S. Astaxanthin induces angiogenesis through Wnt/β-catenin signaling pathway. Phytomedicine 2015, 22, 744–751. [Google Scholar] [CrossRef]
- Kuo, M.H.; Lee, H.F.; Tu, Y.F.; Lin, L.H.; Cheng, Y.Y.; Lee, H.T. Astaxanthin Ameliorates Ischemic-Hypoxic-Induced Neurotrophin Receptor p75 Upregulation in the Endothelial Cells of Neonatal Mouse Brains. Int. J. Mol. Sci. 2019, 20, 6168. [Google Scholar] [CrossRef]
- Fanaee-Danesh, E.; Gali, C.C.; Tadic, J.; Zandl-Lang, M.; Carmen Kober, A.; Agujetas, V.R.; de Dios, C.; Tam-Amersdorfer, C.; Stracke, A.; Albrecher, N.M.; et al. Astaxanthin exerts protective effects similar to bexarotene in Alzheimer’s disease by modulating amyloid-beta and cholesterol homeostasis in blood-brain barrier endothelial cells. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 2224–2245. [Google Scholar] [CrossRef]
- Bereta, M.; Bereta, J.; Georgoff, I.; Coffman, F.D.; Cohen, S.; Cohen, M.C. Methylxanthines and calcium-mobilizing agents inhibit the expression of cytokine-inducible nitric oxide synthase and vascular cell adhesion molecule-1 in murine microvascular endothelial cells. Exp. Cell Res. 1994, 212, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, M.; Burek, M.; Britz, S.; Lankamp, F.; Ketelhut, S.; Kemper, B.; Förster, C.; Gorzelanny, C.; Goycoolea, F.M. The Influence of Capsaicin on the Integrity of Microvascular Endothelial Cell Monolayers. Int. J. Mol. Sci. 2018, 20, 122. [Google Scholar] [CrossRef] [PubMed]
- Jamornwan, S.; Chokpanuwat, T.; Uppakara, K.; Laorob, T.; Wichai, U.; Ketsawatsomkron, P.; Saengsawang, W. Nitro Capsaicin Suppressed Microglial Activation and TNF-α-Induced Brain Microvascular Endothelial Cell Damage. Biomedicines 2022, 10, 2680. [Google Scholar] [CrossRef] [PubMed]
- Ardid-Ruiz, A.; Harazin, A.; Barna, L.; Walter, F.R.; Bladé, C.; Suárez, M.; Deli, M.A.; Aragonès, G. The effects of Vitis vinifera L. phenolic compounds on a blood-brain barrier culture model: Expression of leptin receptors and protection against cytokine-induced damage. J. Ethnopharmacol. 2020, 247, 112253. [Google Scholar] [CrossRef]
- Lee, S.B.; Choi, E.H.; Jeong, K.H.; Kim, K.S.; Shim, S.M.; Kim, G.H. Effect of catechins and high-temperature-processed green tea extract on scavenging reactive oxygen species and preventing Aβ1-42 fibrils’ formation in brain microvascular endothelium. Nutr. Neurosci. 2020, 23, 363–373. [Google Scholar] [CrossRef]
- Lee, B.K.; Lee, W.J.; Jung, Y.S. Chrysin Attenuates VCAM-1 Expression and Monocyte Adhesion in Lipopolysaccharide-Stimulated Brain Endothelial Cells by Preventing NF-κB Signaling. Int. J. Mol. Sci. 2017, 18, 1424. [Google Scholar] [CrossRef]
- Zhang, W.X.; Wang, H.; Cui, H.R.; Guo, W.B.; Zhou, F.; Cai, D.S.; Xu, B.; Jia, X.H.; Huang, X.M.; Yang, Y.Q.; et al. Design, synthesis and biological evaluation of cinnamic acid derivatives with synergetic neuroprotection and angiogenesis effect. Eur. J. Med. Chem. 2019, 183, 111695. [Google Scholar] [CrossRef]
- Dong, H.J.; Shang, C.Z.; Peng, D.W.; Xu, J.; Xu, P.X.; Zhan, L.; Wang, P. Curcumin attenuates ischemia-like injury induced IL-1β elevation in brain microvascular endothelial cells via inhibiting MAPK pathways and nuclear factor-κB activation. Neurol. Sci. 2014, 35, 1387–1392. [Google Scholar] [CrossRef]
- Lamy, S.; Muhire, É.; Annabi, B. Antiproliferative efficacy of elderberries and elderflowers (Sambucus canadensis) on glioma and brain endothelial cells under normoxic and hypoxic conditions. J. Funct. Foods 2018, 40, 164–179. [Google Scholar] [CrossRef]
- Veszelka, S.; Tóth, A.E.; Walter, F.R.; Datki, Z.; Mózes, E.; Fülöp, L.; Bozsó, Z.; Hellinger, E.; Vastag, M.; Orsolits, B.; et al. Docosahexaenoic acid reduces amyloid-β induced toxicity in cells of the neurovascular unit. J. Alzheimer’s Dis. 2013, 36, 487–501. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Q.; Zhan, L.; Shu, A. Effects and mechanisms of docosahexaenoic acid on the generation of angiopoietin-2 by rat brain microvascular endothelial cells under an oxygen- and glucose-deprivation environment. SpringerPlus 2016, 5, 1518. [Google Scholar] [CrossRef] [PubMed]
- Torres-Vergara, P.; Penny, J. Pro-inflammatory and anti-inflammatory compounds exert similar effects on P-glycoprotein in blood-brain barrier endothelial cells. J. Pharm. Pharmacol. 2018, 70, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.W.; Lee, W.H. Protective effects of genistein on proinflammatory pathways in human brain microvascular endothelial cells. J. Nutr. Biochem. 2008, 19, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.D.; Yu, H.L.; Ding, J.; Ma, W.W.; Yuan, L.H.; Feng, J.F.; Xiao, Y.X.; Xiao, R. Flavonoids protect cerebrovascular endothelial cells through Nrf2 and PI3K from β-amyloid peptide-induced oxidative damage. Curr. Neurovasc. Res. 2012, 9, 32–41. [Google Scholar] [CrossRef]
- Calhau, C.; Martel, F.; Soares-da-Silva, P.; Hipólito-Reis, C.; Azevedo, I. Regulation of [(3)H]MPP(+) transport by phosphorylation/dephosphorylation pathways in RBE4 cells: Role of ecto-alkaline phosphatase. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2002, 365, 349–356. [Google Scholar] [CrossRef]
- Calhau, C.; Martel, F.; Pinheiro-Silva, S.; Pinheiro, H.; Soares-da-Silva, P.; Hipólito-Reis, C.; Azevedo, I. Modulation of insulin transport in rat brain microvessel endothelial cells by an ecto-phosphatase activity. J. Cell Biochem. 2002, 84, 389–400. [Google Scholar] [CrossRef]
- Li, M.T.; Ke, J.; Guo, S.F.; Shan, L.L.; Gong, J.H.; Qiao, T.C.; Tian, H.Y.; Wu, Y.; Peng, Z.Y.; Zeng, X.Q.; et al. Huzhangqingmaiyin protected vascular endothelial cells against cerebral small vessel disease through inhibiting inflammation. J. Ethnopharmacol. 2024, 318 Pt A, 116905. [Google Scholar] [CrossRef]
- Xie, R.; Li, X.; Ling, Y.; Shen, C.; Wu, X.; Xu, W.; Gao, X. Alpha-lipoic acid pre- and post-treatments provide protection against in vitro ischemia-reperfusion injury in cerebral endothelial cells via Akt/mTOR signaling. Brain Res. 2012, 1482, 81–90. [Google Scholar] [CrossRef]
- Zhang, J.X.; Xing, J.G.; Wang, L.L.; Jiang, H.L.; Guo, S.L.; Liu, R. Luteolin Inhibits Fibrillary β-Amyloid1-40-Induced Inflammation in a Human Blood-Brain Barrier Model by Suppressing the p38 MAPK-Mediated NF-κB Signaling Pathways. Molecules 2017, 22, 334. [Google Scholar] [CrossRef]
- Xu, X.D.; Teng, Y.; Zou, J.Y.; Ye, Y.; Song, H.; Wang, Z.Y. Effects of lycopene on vascular remodeling through the LXR-PI3K-AKT signaling pathway in APP/PS1 mice. Biochem. Biophys. Res. Commun. 2020, 526, 699–705. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, X.; Guo, S.; Shi, L.; He, Q.; Zhang, P.; Yu, S.; Zhao, R. Myricetin ameliorated ischemia/reperfusion-induced brain endothelial permeability by improvement of eNOS uncoupling and activation eNOS/NO. J. Pharmacol. Sci. 2019, 140, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Ishisaka, A.; Ichikawa, S.; Sakakibara, H.; Piskula, M.K.; Nakamura, T.; Kato, Y.; Ito, M.; Miyamoto, K.; Tsuji, A.; Kawai, Y.; et al. Accumulation of orally administered quercetin in brain tissue and its antioxidative effects in rats. Free Radic. Biol. Med. 2011, 51, 1329–1336. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, S.; Li, J.; Sun, Y.; Hasimu, H.; Liu, R.; Zhang, T. Quercetin protects human brain microvascular endothelial cells from fibrillar β-amyloid1-40-induced toxicity. Acta Pharm. Sin. B 2015, 5, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Li, M.T.; Ke, J.; Guo, S.F.; Wu, Y.; Bian, Y.F.; Shan, L.L.; Liu, Q.Y.; Huo, Y.J.; Guo, C.; Liu, M.Y.; et al. The Protective Effect of Quercetin on Endothelial Cells Injured by Hypoxia and Reoxygenation. Front. Pharmacol. 2021, 12, 732874. [Google Scholar] [CrossRef]
- Sun, P.; Yang, Y.; Yang, L.; Qian, Y.; Liang, M.; Chen, H.; Zhang, J.; Qiu, Y.; Guo, L.; Fu, S. Quercetin Protects Blood-Brain Barrier Integrity via the PI3K/Akt/Erk Signaling Pathway in a Mouse Model of Meningitis Induced by Glaesserella parasuis. Biomolecules 2024, 14, 696. [Google Scholar] [CrossRef]
- Oliveira, A.I.; Pinho, C.; Sarmento, B.; Dias, A.C.P. Quercetin-biapigenin nanoparticles are effective to penetrate the blood-brain barrier. Drug Deliv. Transl. Res. 2022, 12, 267–281. [Google Scholar] [CrossRef]
- Alhusban, A.; Alkhazaleh, E.; El-Elimat, T. Silymarin Ameliorates Diabetes-Induced Proangiogenic Response in Brain Endothelial Cells through a GSK-3β Inhibition-Induced Reduction of VEGF Release. J. Diabetes Res. 2017, 2017, 2537216. [Google Scholar] [CrossRef]
- Sajja, R.K.; Prasad, S.; Tang, S.; Kaisar, M.A.; Cucullo, L. Blood-brain barrier disruption in diabetic mice is linked to Nrf2 signaling deficits: Role of ABCB10? Neurosci. Lett. 2017, 653, 152–158. [Google Scholar] [CrossRef]
- Wu, C.; Zhao, J.; Chen, Y.; Li, T.; Zhu, R.; Zhu, B.; Zhang, Y. Tangeretin protects human brain microvascular endothelial cells against oxygen-glucose deprivation-induced injury. J. Cell Biochem. 2019, 120, 4883–4891. [Google Scholar] [CrossRef]
- Shieh, P.; Hsu, S.S.; Liang, W.Z. Mechanisms underlying protective effects of vitamin E against mycotoxin deoxynivalenol-induced oxidative stress and its related cytotoxicity in primary human brain endothelial cells. Environ. Toxicol. 2021, 36, 1375–1388. [Google Scholar] [CrossRef]
- Deli, M.A. Potential use of tight junction modulators to reversibly open membranous barriers and improve drug delivery. Biochim. Biophys. Acta 2009, 1788, 892–910. [Google Scholar] [CrossRef] [PubMed]
- Greene, C.; Campbell, M. Tight junction modulation of the blood brain barrier: CNS delivery of small molecules. Tissue Barriers 2016, 4, e1138017. [Google Scholar] [CrossRef] [PubMed]
- Tavakkoli, A.; Iranshahi, M.; Hasheminezhad, S.H.; Hayes, A.W.; Karimi, G. The neuroprotective activities of natural products through the Nrf2 upregulation. Phytother. Res. 2019, 33, 2256–2273. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A.; Kovac, A.; Morofuji, Y. Neurovascular unit crosstalk: Pericytes and astrocytes modify cytokine secretion patterns of brain endothelial cells. J. Cereb. Blood Flow. Metab. 2018, 38, 1104–1118. [Google Scholar] [CrossRef] [PubMed]
- Deli, M.A.; Porkoláb, G.; Kincses, A.; Mészáros, M.; Szecskó, A.; Kocsis, A.E.; Vigh, J.P.; Valkai, S.; Veszelka, S.; Walter, F.R.; et al. Lab-on-a-chip models of the blood-brain barrier: Evolution, problems, perspectives. Lab Chip 2024, 24, 1030–1063. [Google Scholar] [CrossRef] [PubMed]
- Scarpellino, G.; Brunetti, V.; Berra-Romani, R.; De Sarro, G.; Guerra, G.; Soda, T.; Moccia, F. The Unexpected Role of the Endothelial Nitric Oxide Synthase at the Neurovascular Unit: Beyond the Regulation of Cerebral Blood Flow. Int. J. Mol. Sci. 2024, 25, 9071. [Google Scholar] [CrossRef] [PubMed]
- Black, K.L.; Baba, T.; Pardridge, W.M. Enzymatic barrier protects brain capillaries from leukotriene C4. J. Neurosurg. 1994, 81, 745–751. [Google Scholar] [CrossRef]
- Gosslau, A.; Chen, K.Y. Nutraceuticals, apoptosis, and disease prevention. Nutrition 2004, 20, 95–102. [Google Scholar] [CrossRef]
- Rattner, A.; Wang, Y.; Nathans, J. Signaling Pathways in Neurovascular Development. Annu. Rev. Neurosci. 2022, 45, 87–108. [Google Scholar] [CrossRef]
- Dragoni, S.; Turowski, P. Polarised VEGFA signalling at vascular blood–neural barriers. Int. J. Mol. Sci. 2018, 19, 1378. [Google Scholar] [CrossRef]
- Xu, J.W.; Ikeda, K.; Yamori, Y. Inhibitory effect of polyphenol cyanidin on TNF-alpha-induced apoptosis through multiple signaling pathways in endothelial cells. Atherosclerosis 2007, 193, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Scuto, M.; Majzúnová, M.; Torcitto, G.; Antonuzzo, S.; Rampulla, F.; Di Fatta, E.; Trovato Salinaro, A. Functional Food Nutrients, Redox Resilience Signaling and Neurosteroids for Brain Health. Int. J. Mol. Sci. 2024, 25, 12155. [Google Scholar] [CrossRef] [PubMed]
- Golech, S.A.; McCarron, R.M.; Chen, Y.; Bembry, J.; Lenz, F.; Mechoulam, R.; Shohami, E.; Spatz, M. Human brain endothelium: Coexpression and function of vanilloid and endocannabinoid receptors. Brain Res. Mol. Brain Res. 2004, 132, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Park, C.S.; Lee, J.Y.; Choi, H.Y.; Yune, T.Y. Suppression of Transient Receptor Potential Melastatin 7 by Carvacrol Protects against Injured Spinal Cord by Inhibiting Blood-Spinal Cord Barrier Disruption. J. Neurotrauma 2022, 39, 735–749. [Google Scholar] [CrossRef] [PubMed]
- Vezzani, A.; Balosso, S.; Varvel, N.H.; Dingledine, R. Anti-inflammatory strategies for disease modification: Focus on therapies close to clinical translation. In Jasper’s Basic Mechanisms of the Epilepsies, 5th ed.; Noebels, J.L., Avoli, M., Rogawski, M.A., Vezzani, A., Delgado-Escueta, A.V., Eds.; Oxford University Press: New York, NY, USA, 2024; Chapter 74. [Google Scholar]
- Rodak, K.; Kokot, I.; Kratz, E.M. Caffeine as a Factor. Influencing the Functioning of the Human. Body-Friend or Foe? Nutrients 2021, 13, 3088. [Google Scholar] [CrossRef]
- van Hameren, G.; Aboghazleh, R.; Parker, E.; Dreier, J.P.; Kaufer, D.; Friedman, A. From spreading depolarization to blood-brain barrier dysfunction: Navigating traumatic brain injury for novel diagnosis and therapy. Nat. Rev. Neurol. 2024, 20, 408–425. [Google Scholar] [CrossRef]
- Li, Y.; Luo, Z.; Li, G.; Belwal, T.; Li, L.; Xu, Y.; Su, B.; Lin, X. Interference-free Detection of Caffeine in Complex Matrices Using a Nanochannel Electrode Modified with Binary Hydrophilic-Hydrophobic PDMS. ACS Sens. 2021, 6, 1604–1612. [Google Scholar] [CrossRef]
- Sharma, N.; Phan, H.T.T.; Yoda, T.; Shimokawa, N.; Vestergaard, M.C.; Takagi, M. Effects of Capsaicin on Biomimetic Membranes. Biomimetics 2019, 4, 17. [Google Scholar] [CrossRef]
- Konovalova, I.S.; Shishkina, S.V.; Wyshusek, M.; Patzer, M.; Reiss, G.J. Supramolecular architecture of theophylline polymorphs, monohydrate and co-crystals with iodine: Study from the energetic viewpoint. RSC Adv. 2024, 14, 29774–29788. [Google Scholar] [CrossRef]
- Whaley, W.L.; Rummel, J.D.; Kastrapeli, N. Interactions of genistein and related isoflavones with lipid micelles. Langmuir 2006, 22, 7175–7184. [Google Scholar] [CrossRef]

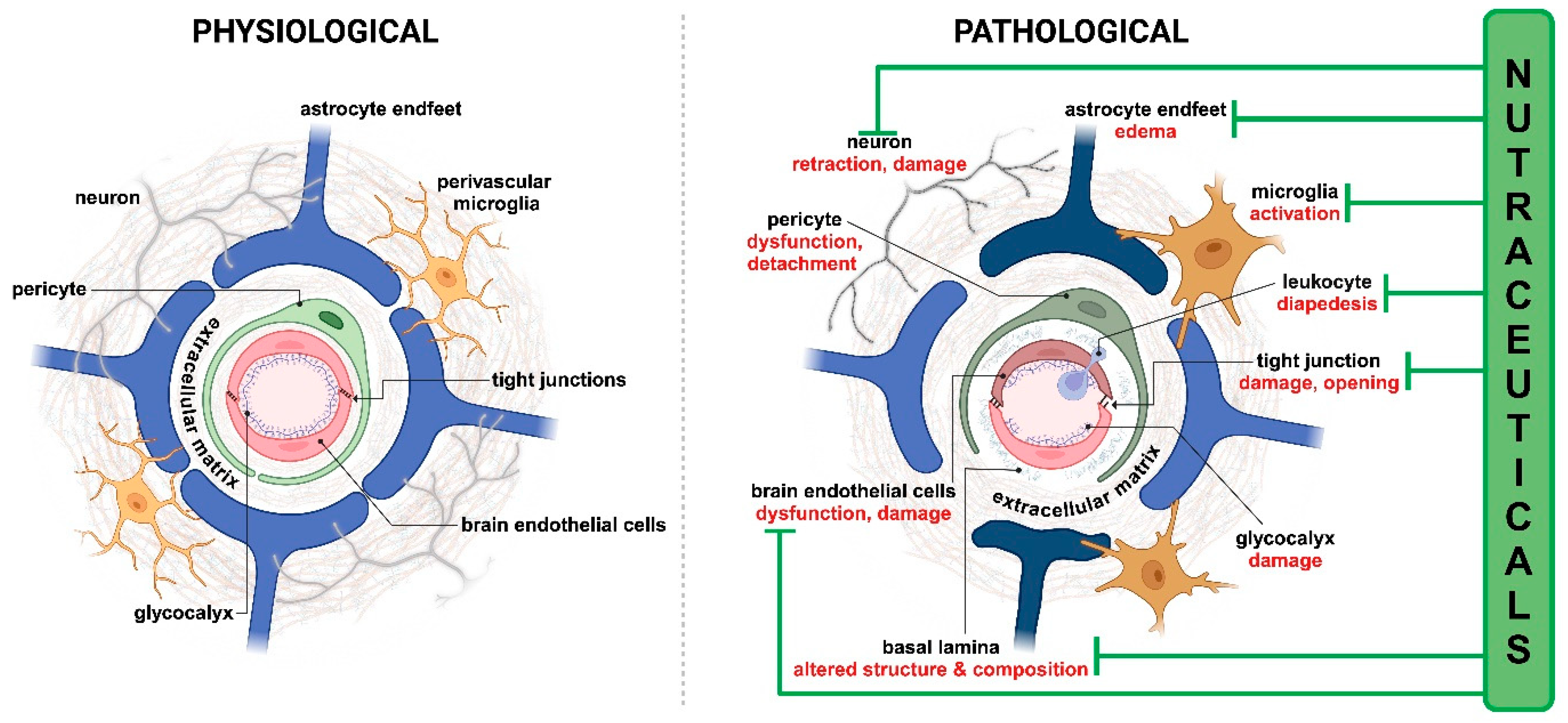
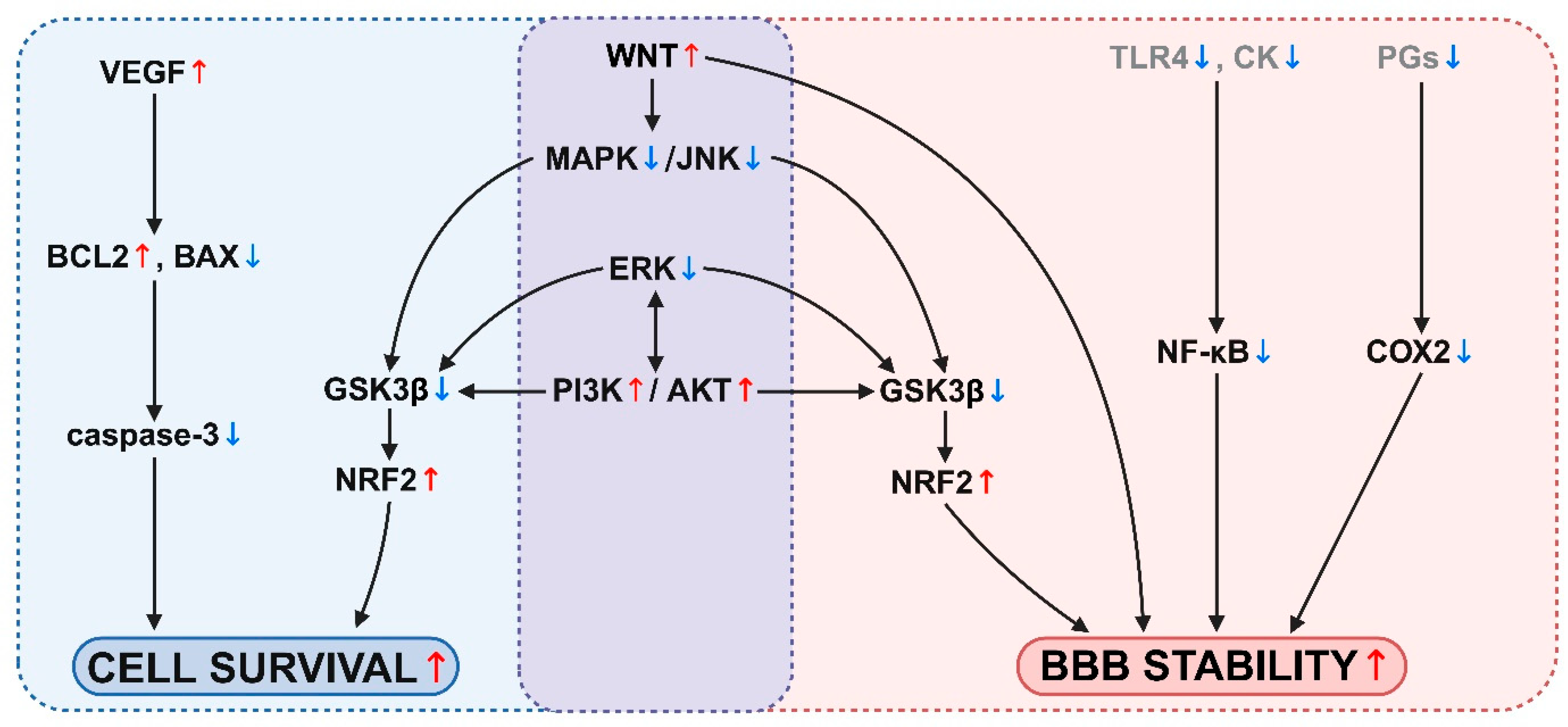
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kocsis, A.E.; Kucsápszky, N.; Santa-Maria, A.R.; Hunyadi, A.; Deli, M.A.; Walter, F.R. Much More than Nutrients: The Protective Effects of Nutraceuticals on the Blood–Brain Barrier in Diseases. Nutrients 2025, 17, 766. https://doi.org/10.3390/nu17050766
Kocsis AE, Kucsápszky N, Santa-Maria AR, Hunyadi A, Deli MA, Walter FR. Much More than Nutrients: The Protective Effects of Nutraceuticals on the Blood–Brain Barrier in Diseases. Nutrients. 2025; 17(5):766. https://doi.org/10.3390/nu17050766
Chicago/Turabian StyleKocsis, Anna E., Nóra Kucsápszky, Ana Raquel Santa-Maria, Attila Hunyadi, Mária A. Deli, and Fruzsina R. Walter. 2025. "Much More than Nutrients: The Protective Effects of Nutraceuticals on the Blood–Brain Barrier in Diseases" Nutrients 17, no. 5: 766. https://doi.org/10.3390/nu17050766
APA StyleKocsis, A. E., Kucsápszky, N., Santa-Maria, A. R., Hunyadi, A., Deli, M. A., & Walter, F. R. (2025). Much More than Nutrients: The Protective Effects of Nutraceuticals on the Blood–Brain Barrier in Diseases. Nutrients, 17(5), 766. https://doi.org/10.3390/nu17050766











