Lactobacillus acidophilus TW01 Mitigates PM2.5-Induced Lung Injury and Improves Gut Health in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacteria Preparation
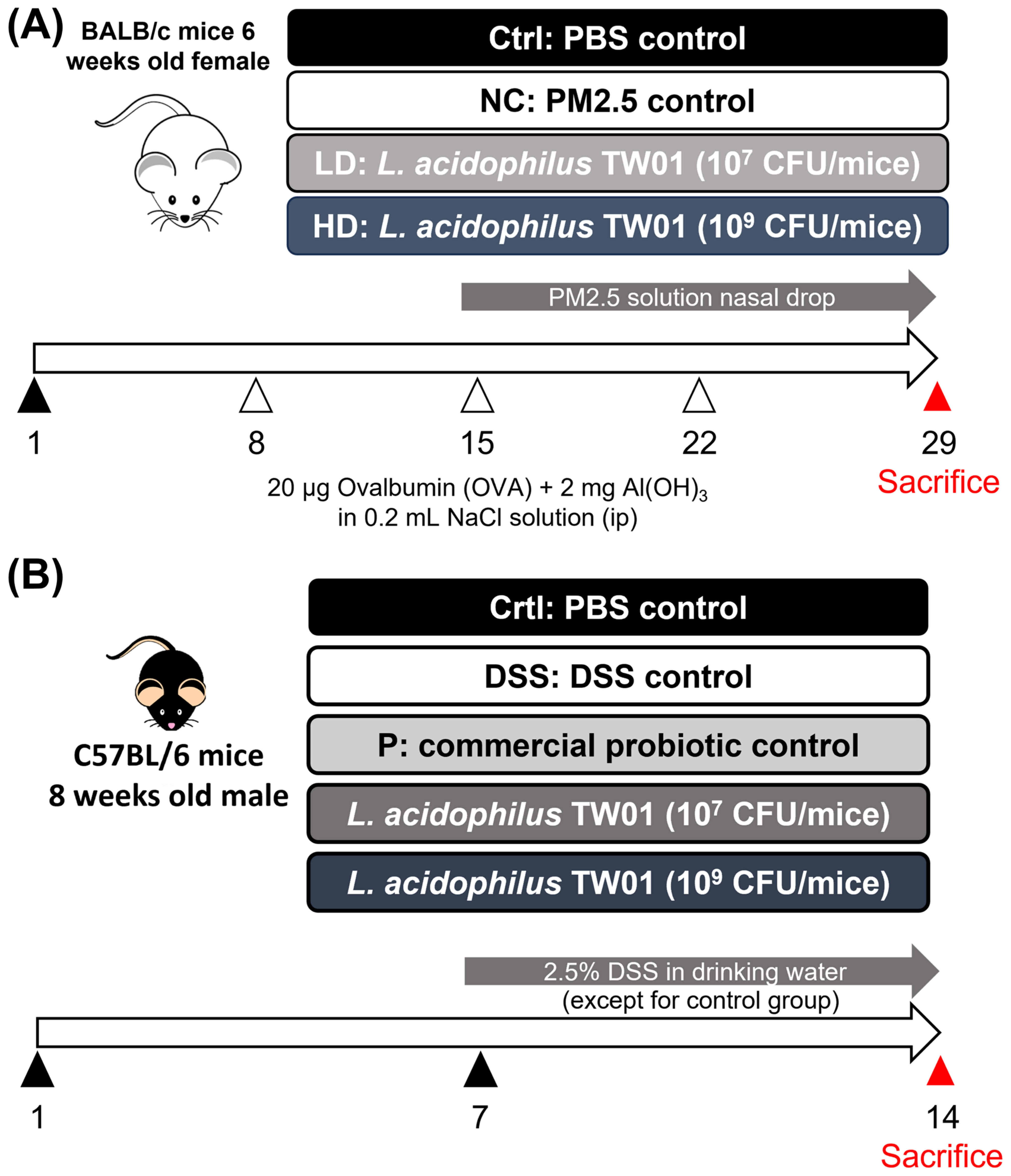
2.2. Animal Models
2.3. PM2.5-Induced Lung Injury
2.3.1. Bronchoalveolar Lavage Fluid (BALF) PM2.5-Induced Lung Injury Mice
2.3.2. Detection of Fibrosis Markers in PM2.5-Induced Lung Injury in Mice
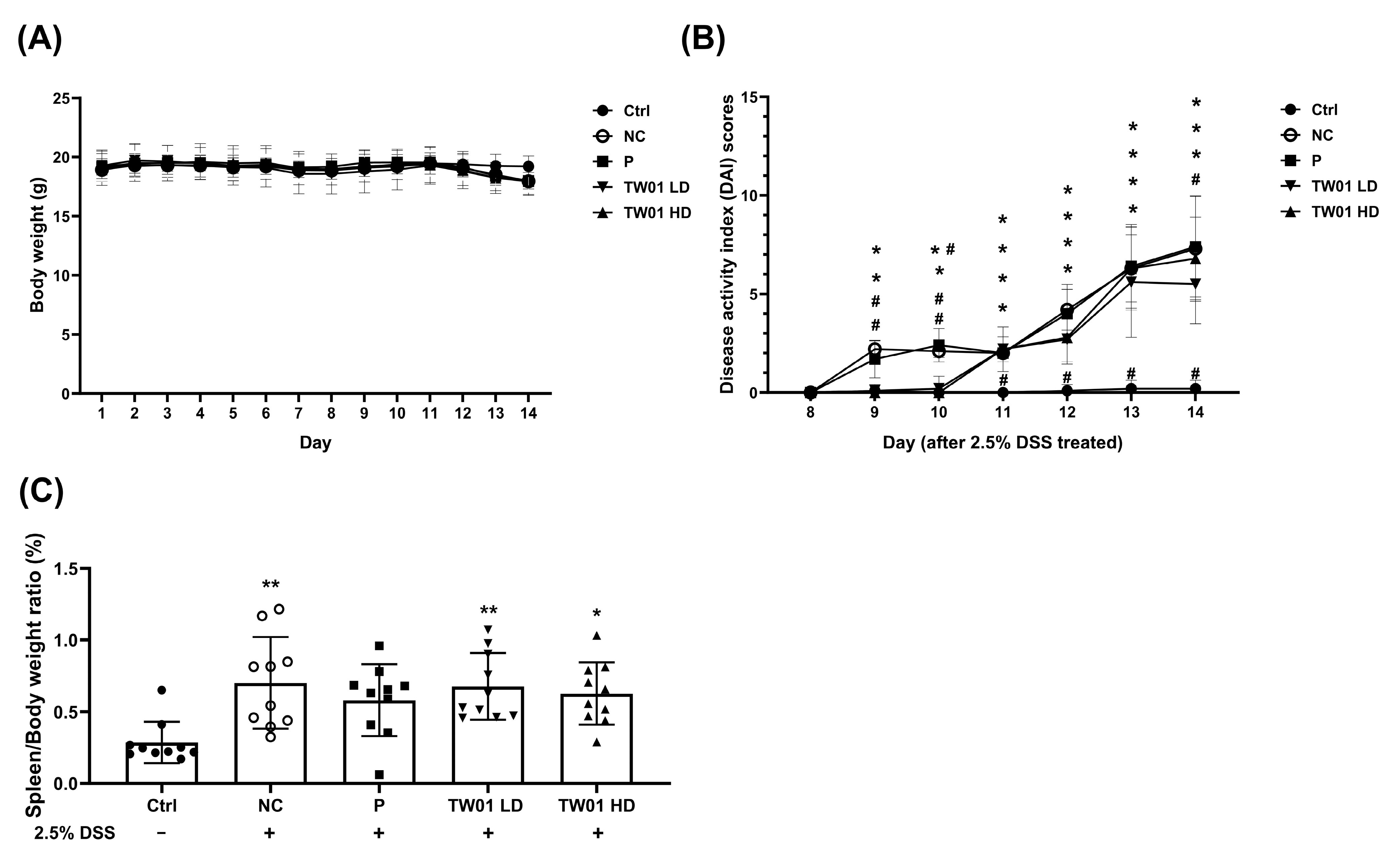
2.4. Dextran Sulfate Sodium Salt (DSS)—Colitis Treatment
2.4.1. The Determination of Occult Stool Blood and Scoring the Disease Activity Index (DAI) in DSS-Colitis Mice
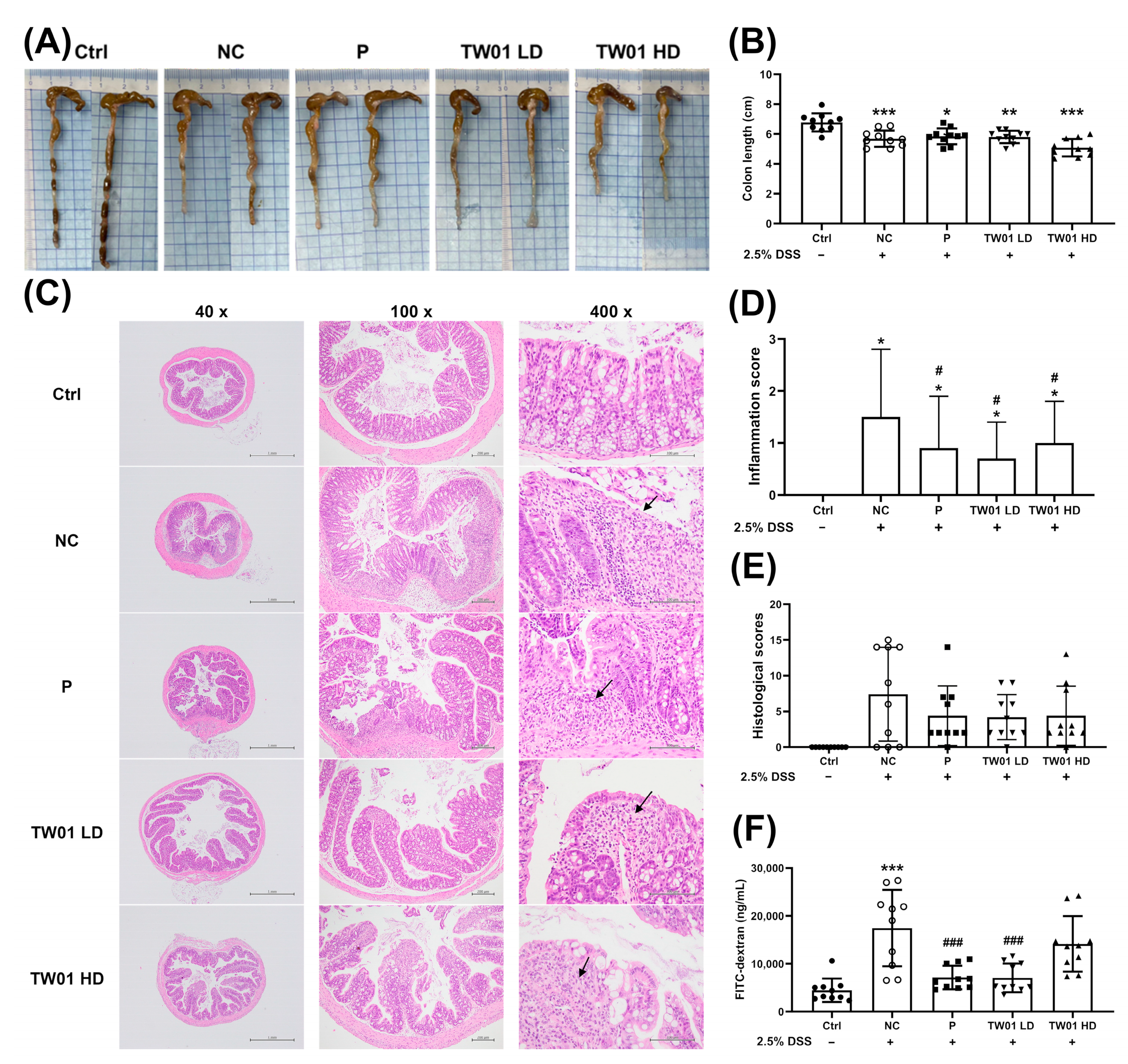
2.4.2. Hematoxylin and Eosin (HE) Stain for Histological Evaluation
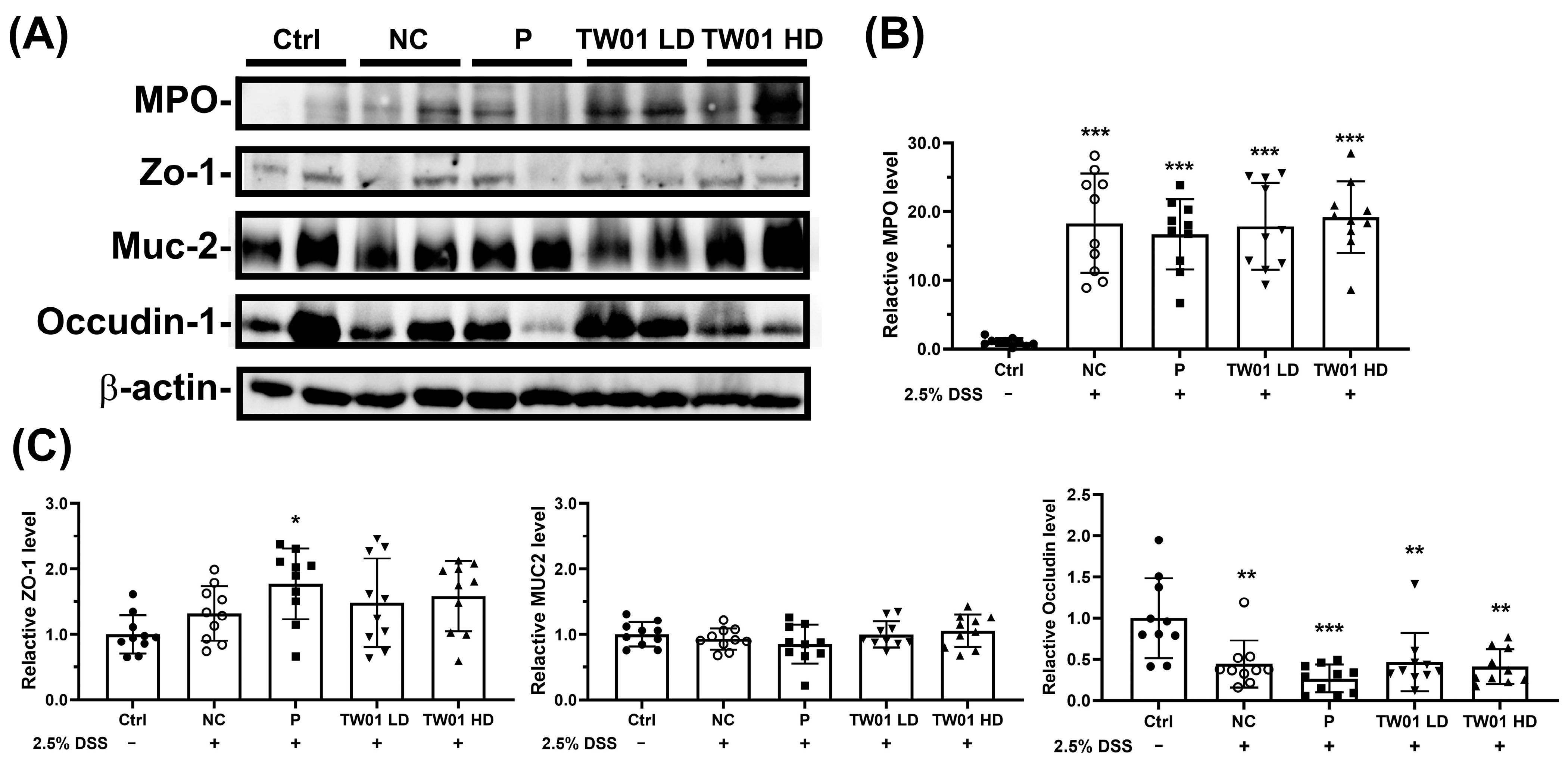
2.4.3. Detection of the Mucosal Barrier Protein Expression
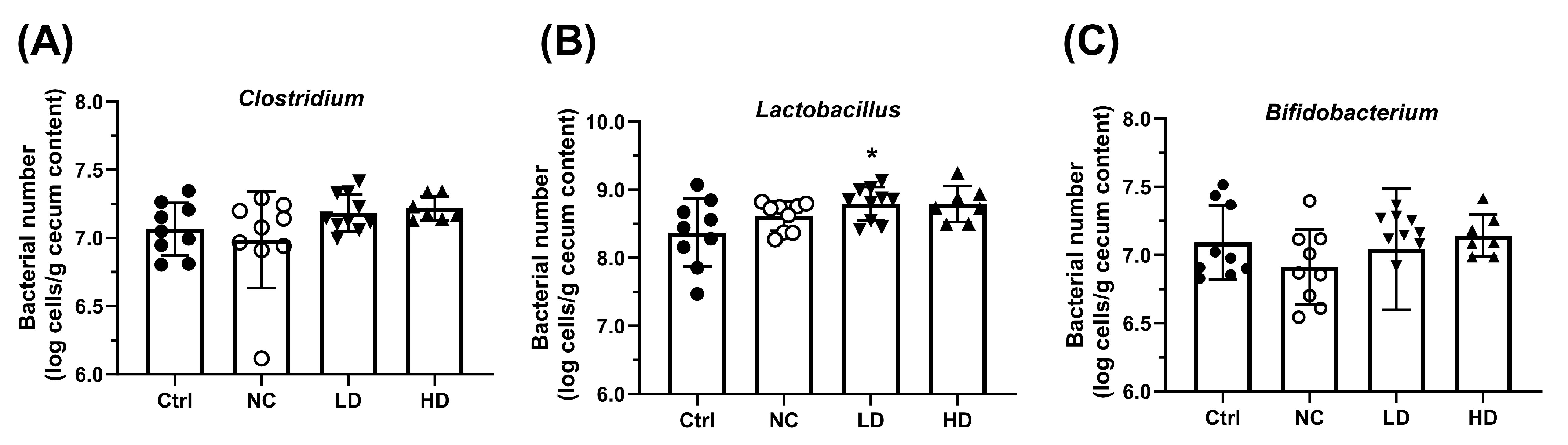
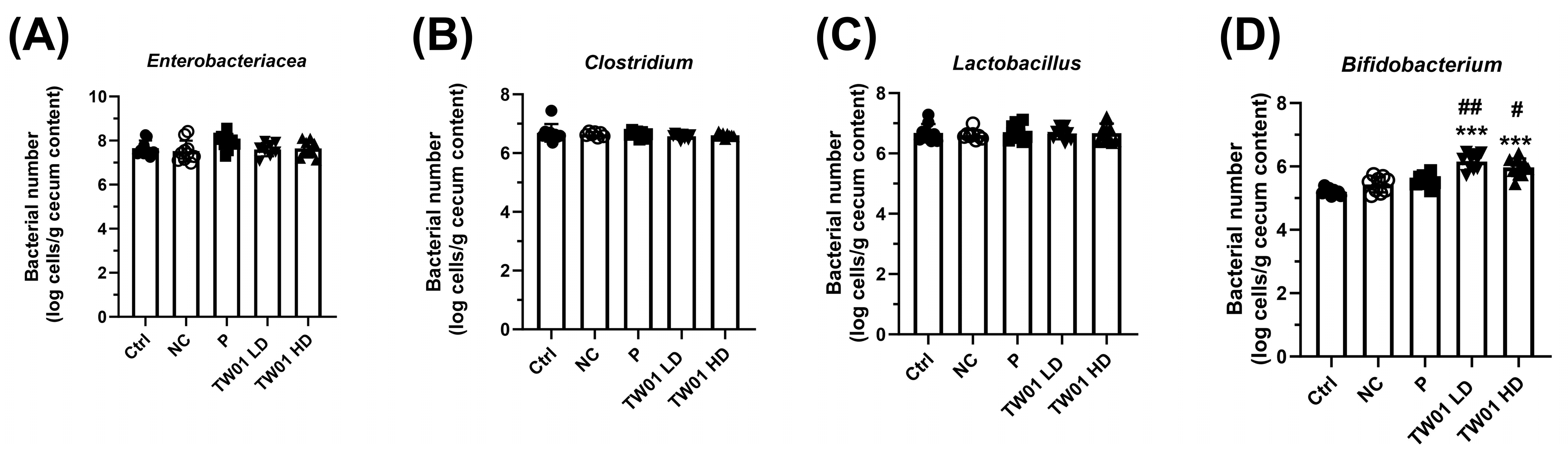
2.5. Specific Cecal Bacteria Determination
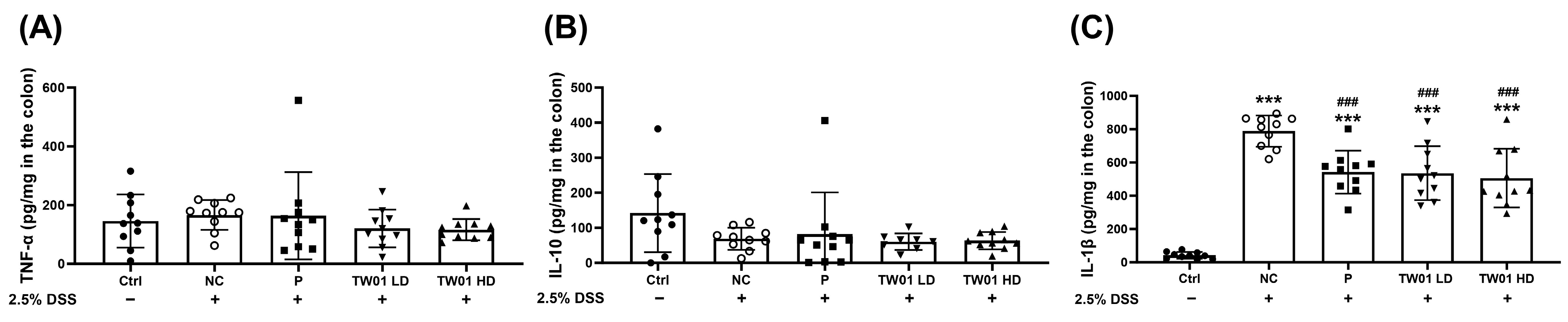
2.6. The Concentration of Cytokines
2.7. Statistical Analysis
3. Results
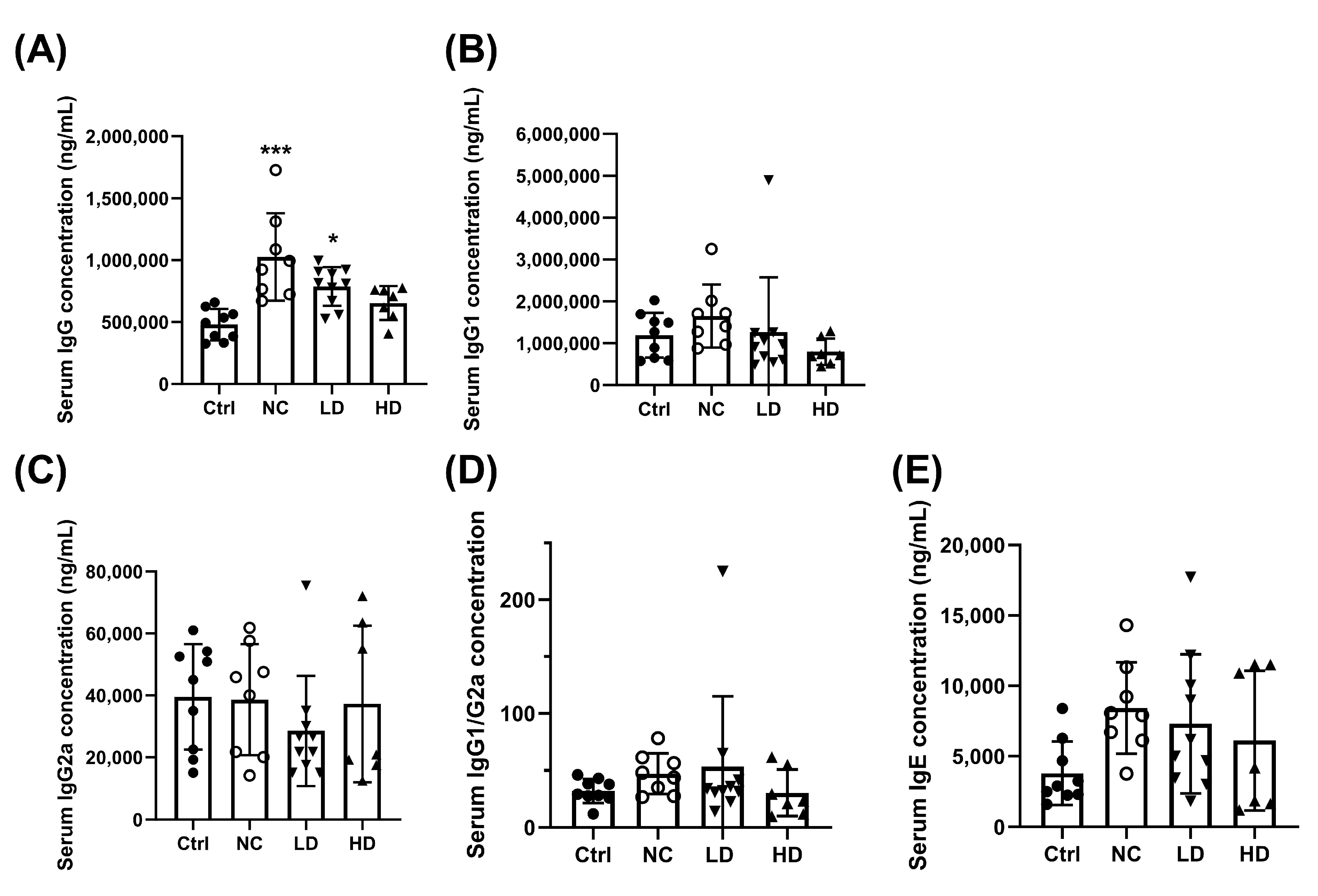
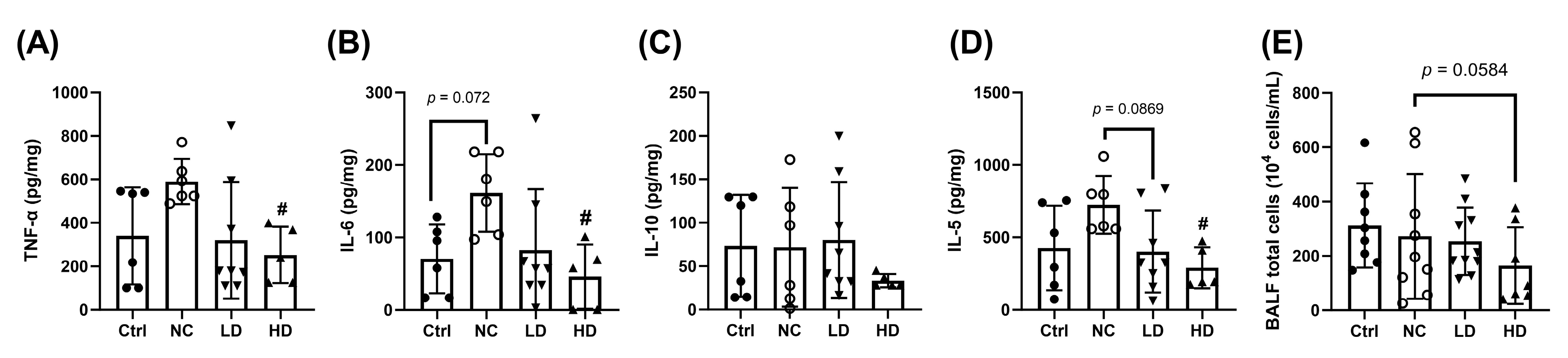
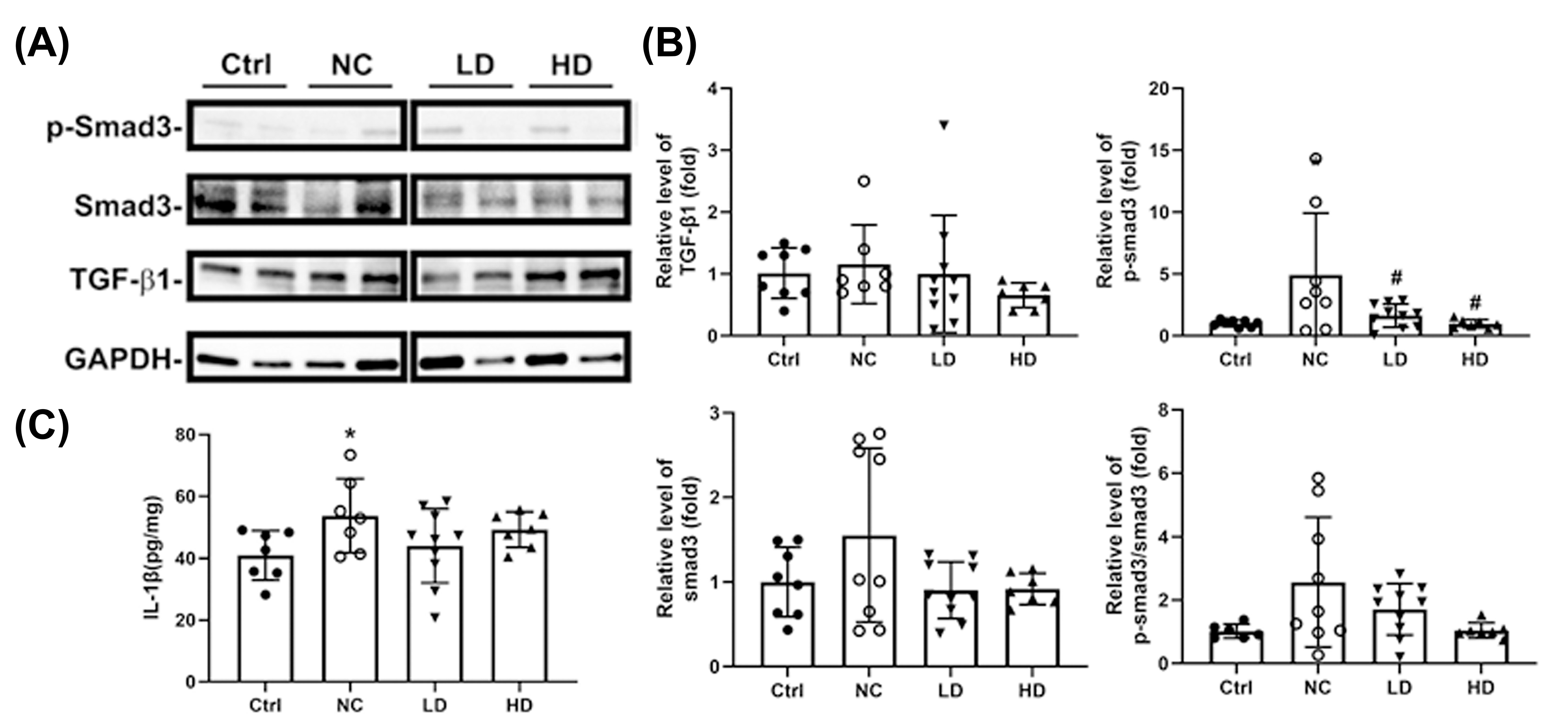
3.1. Effects of Lactobacillus acidophilus TW01 on Physiological Responses in PM2.5-Induced Lung Injury in Mice
3.2. Lactobacillus acidophilus TW01 Ameliorates DSS-Induced Colitis by Modulating Cytokines, Improving Gut Barrier Integrity, and Upregulating Bifidobacterium
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PM2.5 | particulate matter 2.5 |
| L. acidophilus TW01 | Lactobacillus acidophilus TW01 |
| SPF | Specific Pathogen-Free |
| IACUC | Institutional Animal Care and Use Committee |
| OVA | ovalbumin |
| BALF | bronchoalveolar lavage fluid |
| DSS | dextran sulfate sodium salt |
| IBD | inflammatory bowel disease |
| Ctrl | control group |
| NC | negative group |
| P | commercial probiotic control |
| LD | low-dose L. acidophilus TW01 |
| HD | high-dose L. acidophilus TW01 |
| DAI | disease activity index |
| HE | hematoxylin and eosin |
| SDS-PAGE | sodium dodecyl sulfate polyacrylamide gel electrophoresis |
| Smad3 | SMAD family member 3 |
| MPO | myeloperoxidase |
| MUC2 | mucin 2 |
| ZO-1 | zonula occludens-1 |
| GAPDH | glyceraldehyde 3-phosphate dehydrogenase |
References
- Nam, W.; Kim, H.; Bae, C.; Kim, J.; Nam, B.; Lee, Y.; Kim, J.; Park, S.; Lee, J.; Sim, J. Lactobacillus HY2782 and Bifidobacterium HY8002 Decrease Airway Hyperresponsiveness Induced by Chronic PM2.5 Inhalation in Mice. J. Med. Food 2020, 23, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, S. The Effects and Pathogenesis of PM2.5 and Its Components on Chronic Obstructive Pulmonary Disease. Int. J. Chronic Obstr. Pulm. Dis. 2023, 18, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yang, D.; Liu, Y.; Piao, H.; Zhang, T.; Li, X.; Zhao, E.; Zhang, D.; Zheng, Y.; Tang, X. The effect of ambient PM2.5 exposure on survival of lung cancer patients after lobectomy. Environ. Health 2023, 22, 23. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.Y.; Chang, C.C.; Luo, C.S.; Chen, K.Y.; Yeh, Y.K.; Zheng, J.Q.; Wu, S.M. Targeting Lung-Gut Axis for Regulating Pollution Particle-Mediated Inflammation and Metabolic Disorders. Cells 2023, 12, 901. [Google Scholar] [CrossRef]
- Håglin, L.M.; Törnkvist, B.; Bäckman, L.O. High serum phosphate and triglyceride levels in smoking women and men with CVD risk and type 2 diabetes. Diabetol. Metab. Syndr. 2014, 6, 39. [Google Scholar] [CrossRef]
- Deng, X.; Zhang, F.; Rui, W.; Long, F.; Wang, L.; Feng, Z.; Chen, D.; Ding, W. PM2.5-induced oxidative stress triggers autophagy in human lung epithelial A549 cells. Toxicol. Vitro 2013, 27, 1762–1770. [Google Scholar] [CrossRef]
- Piao, C.H.; Fan, Y.; Nguyen, T.V.; Song, C.H.; Kim, H.T.; Chai, O.H. PM2.5 exposure regulates Th1/Th2/Th17 cytokine production through NF-kappaB signaling in combined allergic rhinitis and asthma syndrome. Int. Immunopharmacol. 2023, 119, 110254. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, J.; Chen, M.; Huang, X.; Xie, X.; Li, W.; Cao, Q.; Kan, H.; Xu, Y.; Ying, Z. Exposure to concentrated ambient PM2.5 alters the composition of gut microbiota in a murine model. Part. Fibre Toxicol. 2018, 15, 17. [Google Scholar] [CrossRef]
- Hernandez, A.F.; Tsatsakis, A.M. Human exposure to chemical mixtures: Challenges for the integration of toxicology with epidemiology data in risk assessment. Food Chem. Toxicol. 2017, 103, 188–193. [Google Scholar] [CrossRef]
- Chen, D.; Xiao, C.; Jin, H.; Yang, B.; Niu, J.; Yan, S.; Sun, Y.; Zhou, Y.; Wang, X. Exposure to atmospheric pollutants is associated with alterations of gut microbiota in spontaneously hypertensive rats. Exp. Ther. Med. 2019, 18, 3484–3492. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, H.; Fu, T.; Zhao, J.; Nowak, J.K.; Kalla, R.; Wellens, J.; Yuan, S.; Noble, A.; Ventham, N.T.; et al. Exposure to air pollution increases susceptibility to ulcerative colitis through epigenetic alterations in CXCR2 and MHC class III region. EBioMedicine 2024, 110, 105443. [Google Scholar] [CrossRef] [PubMed]
- Li, F.R.; Wu, K.Y.; Fan, W.D.; Chen, G.C.; Tian, H.; Wu, X.B. Long-term exposure to air pollution and risk of incident inflammatory bowel disease among middle and old aged adults. Ecotoxicol. Environ. Saf. 2022, 242, 113835. [Google Scholar] [CrossRef] [PubMed]
- Bener, A.; Ozturk, A.E.; Dasdelen, M.F.; Barisik, C.C.; Dasdelen, Z.B.; Agan, A.F.; De La Rosette, J.; Day, A.S. Colorectal cancer and associated genetic, lifestyle, cigarette, nargileh-hookah use and alcohol consumption risk factors: A comprehensive case-control study. Oncol. Rev. 2024, 18, 1449709. [Google Scholar] [CrossRef] [PubMed]
- Craver, A.; Luo, J.; Kibriya, M.G.; Randorf, N.; Bahl, K.; Connellan, E.; Powell, J.; Zakin, P.; Jones, R.R.; Argos, M.; et al. Air quality and cancer risk in the All of Us Research Program. Cancer Causes Control 2024, 35, 749–760. [Google Scholar] [CrossRef]
- Wu, Y.; Pei, C.; Wang, X.; Wang, Y.; Huang, D.; Shi, S.; Shen, Z.; Li, S.; He, Y.; Wang, Z.; et al. Probiotics ameliorates pulmonary inflammation via modulating gut microbiota and rectifying Th17/Treg imbalance in a rat model of PM2.5 induced lung injury. Ecotoxicol. Environ. Saf. 2022, 244, 114060. [Google Scholar] [CrossRef]
- Dang, A.T.; Marsland, B.J. Microbes, metabolites, and the gut-lung axis. Mucosal Immunol. 2019, 12, 843–850. [Google Scholar] [CrossRef]
- Enaud, R.; Prevel, R.; Ciarlo, E.; Beaufils, F.; Wieers, G.; Guery, B.; Delhaes, L. The Gut-Lung Axis in Health and Respiratory Diseases: A Place for Inter-Organ and Inter-Kingdom Crosstalks. Front. Cell Infect. Microbiol. 2020, 10, 9. [Google Scholar] [CrossRef]
- Keulers, L.; Dehghani, A.; Knippels, L.; Garssen, J.; Papadopoulos, N.; Folkerts, G.; Braber, S.; van Bergenhenegouwen, J. Probiotics, prebiotics, and synbiotics to prevent or combat air pollution consequences: The gut-lung axis. Environ. Pollut. 2022, 302, 119066. [Google Scholar] [CrossRef]
- Chiu, Y.H.; Chiu, H.P.; Lin, M.Y. Synergistic effect of probiotic and postbiotic on attenuation of PM2.5-induced lung damage and allergic response. J. Food Sci. 2023, 88, 513–522. [Google Scholar] [CrossRef]
- Luo, S.; Chen, M. Systematic Investigation of the Effect of Lactobacillus acidophilus TW01 on Potential Prevention of Particulate Matter (PM)2.5-Induced Damage Using a Novel In Vitro Platform. Foods 2023, 12, 3278. [Google Scholar] [CrossRef]
- SRM 1649b; Certificate of Analysis Standard Reference Material 1649b: Urban Dust. National Institutes of Science & Technology Department of Commerce: Gaithersburg, MD, USA, 2016.
- Huang, H.; Li, K.; Lee, Y.; Chen, M. Preventive Effects of Lactobacillus Mixture against Chronic Kidney Disease Progression through Enhancement of Beneficial Bacteria and Downregulation of Gut-Derived Uremic Toxins. J. Agric. Food Chem. 2021, 69, 7353–7366. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Chen, Y.; Wang, G.; Yang, Y.; Song, X.; Xiong, Z.; Zhang, H.; Lai, P.; Wang, S.; Ai, L. Lactobacillus plantarum AR113 alleviates DSS-induced colitis by regulating the TLR4/MyD88/NF-κB pathway and gut microbiota composition. J. Funct. Foods 2020, 67, 3854. [Google Scholar] [CrossRef]
- Shackelford, C.; Long, G.; Wolf, J.; Okerberg, C.; Herbert, R. Qualitative and quantitative analysis of nonneoplastic lesions in toxicology studies. Toxicol Pathol. 2002, 30, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Matsuki, T.; Watanabe, K.; Fujimoto, J.; Takada, T.; Tanaka, R. Use of 16S rRNA gene-targeted group-specific primers for real-time PCR analysis of predominant bacteria in human feces. Appl. Environ. Microbiol. 2004, 70, 7220–7228. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.; Tsuji, H.; Asahara, T.; Kado, Y.; Nomoto, K. Sensitive quantitative detection of commensal bacteria by rRNA-targeted reverse transcription-PCR. Appl. Environ. Microbiol. 2007, 73, 32–39. [Google Scholar] [CrossRef]
- Rinttila, T.; Kassinen, A.; Malinen, E.; Krogius, L.; Palva, A. Development of an extensive set of 16S rDNA-targeted primers for quantification of pathogenic and indigenous bacteria in faecal samples by real-time PCR. J. Appl. Microbiol. 2004, 97, 1166–1177. [Google Scholar] [CrossRef]
- Kikuchi, E.; Miyamoto, Y.; Narushima, S.; Itoh, K. Design of species-specific primers to identify 13 species of Clostridium harbored in human intestinal tracts. Microbiol. Immunol. 2002, 46, 353–358. [Google Scholar] [CrossRef]
- Li, B.; Ma, Y.; Zhou, Y.; Chai, E. Research progress of different components of PM2.5 and ischemic stroke. Sci. Rep. 2023, 13, 15965. [Google Scholar] [CrossRef]
- Yan, M.; Ge, H.; Zhang, L.; Chen, X.; Yang, X.; Liu, F.; Shan, A.; Liang, F.; Li, X.; Ma, Z.; et al. Long-term PM2.5 exposure in association with chronic respiratory diseases morbidity: A cohort study in Northern China. Ecotoxicol. Environ. Saf. 2022, 244, 114025. [Google Scholar] [CrossRef]
- Wang, X.; Hui, Y.; Zhao, L.; Hao, Y.; Guo, H.; Ren, F. Oral administration of Lactobacillus paracasei L9 attenuates PM2.5-induced enhancement of airway hyperresponsiveness and allergic airway response in murine model of asthma. PLoS ONE 2017, 12, e0171721. [Google Scholar] [CrossRef]
- Lin, C.H.; Tseng, C.Y.; Chao, M.W. Administration of Lactobacillus paracasei HB89 mitigates PM2.5-induced enhancement of inflammation and allergic airway response in murine asthma model. PLoS ONE 2020, 15, e0243062. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Abd El-Gawaad, N.S.; Osman Abdallah, S.A.; Al-Dossari, M. Possible modulating functions of probiotic Lactiplantibacillus plantarum in particulate matter-associated pulmonary inflammation. Front. Cell Infect. Microbiol. 2023, 13, 1290914. [Google Scholar] [CrossRef] [PubMed]
- Ostro, B.; Feng, W.Y.; Broadwin, R.; Green, S.; Lipsett, M. The effects of components of fine particulate air pollution on mortality in california: Results from CALFINE. Environ. Health Perspect. 2007, 115, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.Y.; Chou, C.C.; Liu, S.; Zhang, Y. The Characteristics of PM2.5 and Its Chemical Compositions between Different Prevailing Wind Patterns in Guangzhou. Aerosol Air Qual. Res. 2013, 13, 1373–1383. [Google Scholar] [CrossRef]
- Huang, R.J.; Zhang, Y.; Bozzetti, C.; Ho, K.F.; Cao, J.J.; Han, Y.; Daellenbach, K.R.; Slowik, J.G.; Platt, S.M.; Canonaco, F.; et al. High secondary aerosol contribution to particulate pollution during haze events in China. Nature 2014, 514, 218–222. [Google Scholar] [CrossRef]
- Ma, J.; Chiu, Y.F.; Kao, C.C.; Chuang, C.N.; Chen, C.Y.; Lai, C.H.; Kuo, M.L. Fine particulate matter manipulates immune response to exacerbate microbial pathogenesis in the respiratory tract. Eur. Respir. Rev. 2024, 33, 230259. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Ferreira-Gomes, M.; Kruglov, A.; Durek, P.; Heinrich, F.; Tizian, C.; Heinz, G.A.; Pascual-Reguant, A.; Du, W.; Mothes, R.; Fan, C.; et al. SARS-CoV-2 in severe COVID-19 induces a TGF-beta-dominated chronic immune response that does not target itself. Nat. Commun. 2021, 12, 1961. [Google Scholar] [CrossRef]
- Al-Qahtani, A.A.; Alhamlan, F.S.; Al-Qahtani, A.A. Pro-Inflammatory and Anti-Inflammatory Interleukins in Infectious Diseases: A Comprehensive Review. Trop. Med. Infect. Dis. 2024, 9, 13. [Google Scholar] [CrossRef]
- Kita, H. Eosinophils: Multifaceted biological properties and roles in health and disease. Immunol. Rev. 2011, 242, 161–177. [Google Scholar] [CrossRef]
- Verrecchia, F.; Chu, M.L.; Mauviel, A. Identification of novel TGF-beta/Smad gene targets in dermal fibroblasts using a combined cDNA microarray/promoter transactivation approach. J. Biol. Chem. 2001, 276, 17058–17062. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Muragaki, Y.; Saika, S.; Roberts, A.B.; Ooshima, A. Targeted disruption of TGF-beta1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J. Clin. Investig. 2003, 112, 1486–1494. [Google Scholar] [CrossRef] [PubMed]
- Derynck, R.; Zhang, Y.E. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 2003, 425, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, Y.; Sumitomo, S.; Ishigaki, K.; Suzuki, A.; Kochi, Y.; Tsuchiya, H.; Ota, M.; Komai, T.; Inoue, M.; Morita, K.; et al. TGF-beta3 Inhibits Antibody Production by Human B Cells. PLoS ONE 2017, 12, e0169646. [Google Scholar] [CrossRef]
- Keshteli, A.H.; Madsen, K.L.; Dieleman, L.A. Diet in the Pathogenesis and Management of Ulcerative Colitis; A Review of Randomized Controlled Dietary Interventions. Nutrients 2019, 11, 1498. [Google Scholar] [CrossRef]
- Ratajczak, W.; Ryl, A.; Mizerski, A.; Walczakiewicz, K.; Sipak, O.; Laszczynska, M. Immunomodulatory potential of gut microbiome-derived short-chain fatty acids (SCFAs). Acta Biochim. Pol. 2019, 66, 1–12. [Google Scholar] [CrossRef]
- Markowiak-Kopec, P.; Slizewska, K. The Effect of Probiotics on the Production of Short-Chain Fatty Acids by Human Intestinal Microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef]
- Sharon, G.; Garg, N.; Debelius, J.; Knight, R.; Dorrestein, P.C.; Mazmanian, S.K. Specialized metabolites from the microbiome in health and disease. Cell Metab. 2014, 20, 719–730. [Google Scholar] [CrossRef]
- Garrett, W.S.; Gordon, J.I.; Glimcher, L.H. Homeostasis and inflammation in the intestine. Cell 2010, 140, 859–870. [Google Scholar] [CrossRef]
- Dai, S.; Wang, Z.; Yang, Y.; Du, P.; Li, X. PM2.5 induced weight loss of mice through altering the intestinal microenvironment: Mucus barrier, gut microbiota, and metabolic profiling. J. Hazard. Mater. 2022, 431, 128653. [Google Scholar] [CrossRef]
- Dai, S.; Wang, Z.; Cai, M.; Guo, T.; Mao, S.; Yang, Y. A multi-omics investigation of the lung injury induced by PM2.5 at environmental levels via the lung-gut axis. Sci. Total Environ. 2024, 926, 172027. [Google Scholar] [CrossRef]

| Score | Weight Loss (%) | Stool Consistency | Blood in Stool |
|---|---|---|---|
| 0 | 0 | Normal | Negative (no bleeding) |
| 1 | 1–5 | - | No blood trance on fecal but slightly positive |
| 2 | 5–10 | Loose stool | No blood trance on fecal but positive |
| 3 | 10–15 | - | Blood fecal with moderate positive |
| 4 | >15 | Diarrhea | Blood fecal with strong positive |
| Score | Scribe |
|---|---|
| 1 | Lost, crypt |
| 2 | Regeneration, crypt |
| 3 | Edema, submucosa |
| 4 | Inflammation, mononuclear cells |
| 5 | Ulcer, with fibroblast cell infiltration |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, S.-M.; Chen, M.-J. Lactobacillus acidophilus TW01 Mitigates PM2.5-Induced Lung Injury and Improves Gut Health in Mice. Nutrients 2025, 17, 831. https://doi.org/10.3390/nu17050831
Luo S-M, Chen M-J. Lactobacillus acidophilus TW01 Mitigates PM2.5-Induced Lung Injury and Improves Gut Health in Mice. Nutrients. 2025; 17(5):831. https://doi.org/10.3390/nu17050831
Chicago/Turabian StyleLuo, Siou-Min, and Ming-Ju Chen. 2025. "Lactobacillus acidophilus TW01 Mitigates PM2.5-Induced Lung Injury and Improves Gut Health in Mice" Nutrients 17, no. 5: 831. https://doi.org/10.3390/nu17050831
APA StyleLuo, S.-M., & Chen, M.-J. (2025). Lactobacillus acidophilus TW01 Mitigates PM2.5-Induced Lung Injury and Improves Gut Health in Mice. Nutrients, 17(5), 831. https://doi.org/10.3390/nu17050831






