Impact of Vegan and Vegetarian Diets on Neurological Health: A Critical Review
Abstract
1. Introduction
2. Nutritional Composition of Vegan and Vegetarian Diets
| Nutrient/Component | Presence in Plant-Based Diets | Impact on Mental Health | References |
|---|---|---|---|
| Carbohydrates and Fiber | High presence due to the abundance of whole grains, legumes, fruits, and vegetables. | They promote prebiotic effects, support gut microbiota diversity, and help regulate glucose and lipid levels, benefiting brain function. | Craig & Mangels, 2009 [3] |
| Protein Intake | Adequate when combining various plant sources; however, it may lack a complete amino acid profile. | Tryptophan acts as a precursor to serotonin, essential for mood regulation. | Leitzmann, 2014 [25] |
| Omega-3 Fatty Acids | Low levels of EPA and DHA; ALA is found in foods such as flaxseeds, chia, and walnuts, though conversion to EPA/DHA is low. | Essential for neuronal health and cognitive function; deficiency may affect membrane fluidity and cognitive performance. | Saunders et al., 2021 [26] |
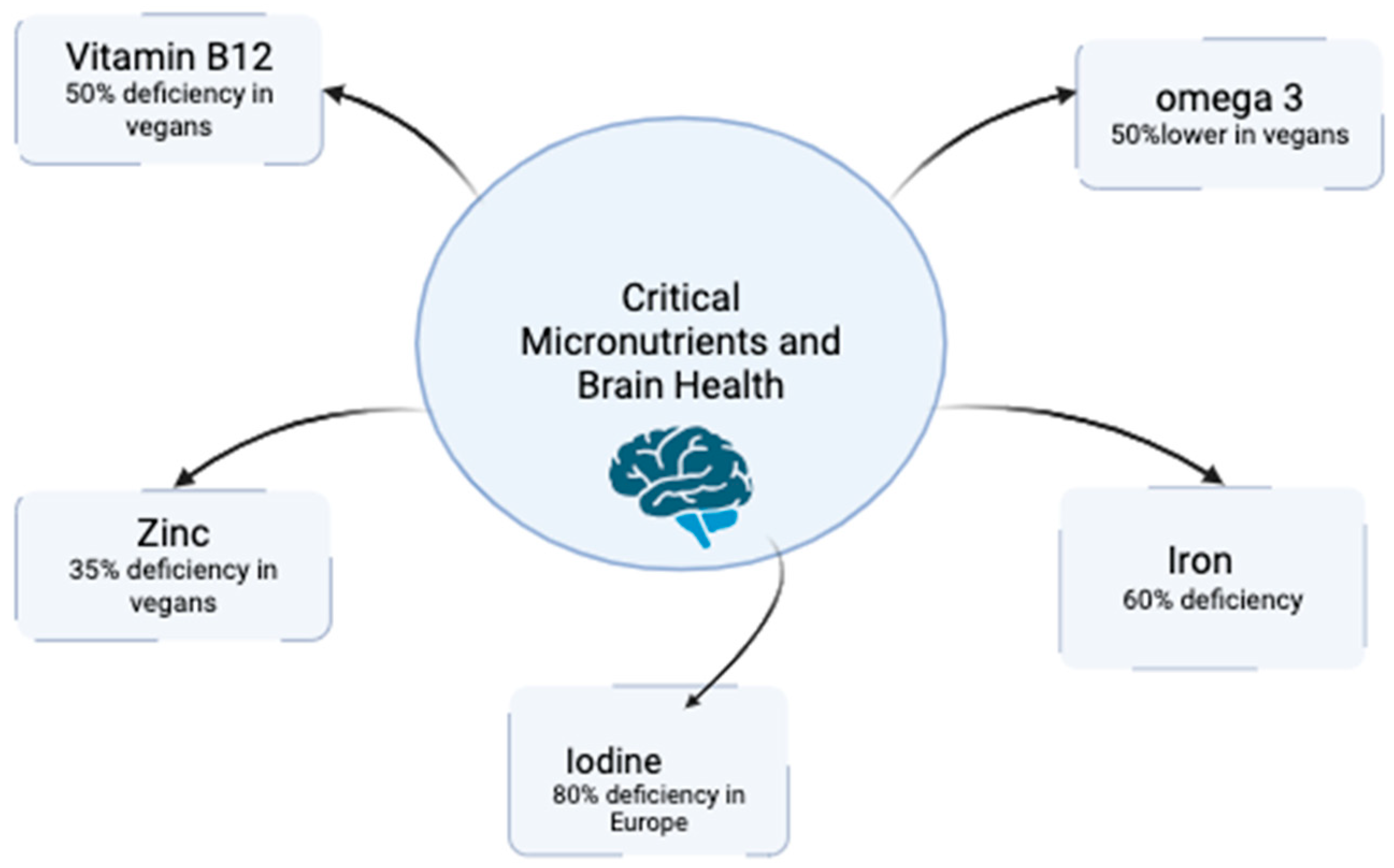
| Vitamin B12 | Absent in non-fortified plant foods; present in vegetarian diets (through dairy and eggs), but insufficient in vegans without fortification or supplements. | Crucial for DNA synthesis, myelin formation, and neurotransmitter metabolism; deficiency can lead to cognitive decline and mood disorders. | Pawlak et al., 2013 [27] |
| Iron | Found as non-heme iron, which has lower bioavailability, with absorption inhibited by phytates; absorption can be improved with vitamin C intake. | Fundamental for oxygen transport and brain metabolism; deficiency is associated with cognitive decline and anemia. | Hunt, 2003 [28] |
| Zinc | Available in legumes, seeds, and nuts, although absorption is reduced due to the presence of phytates. | Vital for enzymatic activity, synaptic plasticity, and neurogenesis; deficiency can affect memory, learning, and other cognitive processes. | Weaver, 2013 [29] |
| Calcium | May be insufficient in vegan diets without the use of fortified products (plant milks, tofu) or supplements. | Important for neuronal excitability and synaptic transmission; deficiency can affect neuronal signaling and increase neurovascular risks. | Peneau et al., 2008 [30] |
| Iodine | Frequently low due to the absence of sources such as fish, dairy, or iodized salt in vegan diets. | Essential for the production of thyroid hormones, which influence cognitive function and mood regulation; deficiency can lead to hypothyroidism. | Peneau et al., 2008 [30] |
| Selenium | Usually deficient in vegan diets; acts as a cofactor in antioxidant enzymes such as glutathione peroxidase. | Involved in neurotransmitter synthesis and mood regulation; deficiency can increase vulnerability to neurological disorders. | Peneau et al., 2008 [30] |
| Phytonutrients | High presence of bioactive compounds (polyphenols, flavonoids, carotenoids) from fruits, vegetables, and whole grains. | Possess anti-inflammatory and antioxidant properties, protecting against neurodegeneration and supporting cognitive function. | Middleton et al., 2000 [31] |
| Inflammation and Oxidative Stress | Characterized by a low intake of pro-inflammatory foods and a high intake of antioxidants, although no exact percentages are specified. | Associated with reduced levels of inflammatory markers (such as C-reactive protein), which correlates with a lower risk of neurodegenerative diseases and mood disorders. | Tonstad et al., 2013 [32] |
3. Antinutrients in Plants
3.1. Plant Secondary Metabolites as Antinutrients
3.2. Antinutrients in Plant-Based Foods
3.3. Biological Role and Processing Benefits of Plant Antinutrients
3.4. Dual Role of Antinutrients in Human Health
4. Effects of Antinutrients on the Nervous System
- -
- Soaking and Sprouting:This reduces phytates and tannins by soaking legumes, grains, and seeds before cooking. Additionally, sprouting enhances the bioavailability of critical nutrients like iron and zinc, which are essential for neurological health.
- -
- Cooking at High Temperatures:This deactivates lectins and protease inhibitors in beans and legumes through boiling or steaming. This method ensures safe consumption and reduces gastrointestinal and neurological stressors.
- -
- Incorporating Vitamin C-Rich Foods:Pairing iron-rich plant foods with vitamin C sources (e.g., spinach with citrus fruits) enhances iron absorption, counteracting the inhibitory effects of phytates.
- -
- Supplementation:Using algae-based DHA/EPA supplements offsets the limited conversion of ALA in plant-based diets, while vitamin B12 supplementation supports myelin synthesis and cognitive function.
- -
- Fortified Foods:Consuming fortified plant-based alternatives (e.g., fortified cereals or milks) addresses common deficiencies that are exacerbated by antinutrient interactions.
5. Micronutrient Deficiencies of Vegan and Vegetarian Diets in Neurological Health
6. Plant Phytonutrients and Neurological Health
- 1.
- Oxidative Stress Reduction: Flavonoids and polyphenols act as potent antioxidants, neutralizing reactive oxygen species (ROS) and reducing lipid peroxidation in neuronal membranes. This action is critical for maintaining cellular integrity and preventing damage associated with neurodegenerative diseases like Alzheimer’s and Parkinson’s.
- 2.
- Inflammatory Pathway Modulation: These phytonutrients activate the Nrf2/ARE signaling pathway, which upregulates the expression of antioxidant enzymes such as superoxide dismutase and catalase. Additionally, they inhibit pro-inflammatory pathways mediated by NF-κB, reducing chronic inflammation that is often implicated in cognitive decline.
- 3.
- Gut–Brain Axis Interaction: Polyphenols, such as curcumin and epigallocatechin gallate (EGCG), enhance gut microbiota diversity by promoting beneficial bacteria. This improvement in gut health indirectly supports neurological function by reducing systemic inflammation and improving neurotransmitter synthesis, including serotonin and dopamine.
- 4.
- Direct Neuroprotective Effects: Certain flavonoids, like quercetin and resveratrol, exhibit direct neuroprotective effects by crossing the blood–brain barrier and protecting neurons from apoptosis. Their roles in enhancing synaptic plasticity and memory formation have been substantiated in recent research.
7. Inflammation and Oxidative Stress in Vegan and Vegetarian Diets
8. Vegetarian Diets on Mood Regulation and Mental Well-Being
8.1. Positive Influences
8.2. Negative Influences
9. Influence of Vegan and Vegetarian Diets on Neurodegenerative Diseases
9.1. Positive Effects
9.2. Detrimental Effects
10. Benefits of Vegan and Vegetarian Diets
- 1.
- Reduction in Oxidative Stress and Neuroinflammation
- 2.
- Support for Cognitive Function and Mood Regulation
- 3.
- Potential for Lowering the Risk of Neurodegenerative Diseases
- 4.
- Cardiovascular Benefits and Cerebral Perfusion
- 5.
- Neuroprotective Role of Specific Nutrients in Vegan and Vegetarian Diets
- Polyphenols found in berries, green tea, and cocoa have been linked to improved cognitive performance and memory retention.
- Flavonoids, particularly those in citrus fruits and dark chocolate, enhance neurogenesis and synaptic plasticity.
- Carotenoids, such as lutein and zeaxanthin from leafy greens, support visual processing and cognitive resilience in aging populations.
Practical Applications
11. Conclusions
Key messages:
- Nutritional deficiencies in vegan and vegetarian diets, such as vitamin B12 and omega-3s, pose risks for cognitive function and neurological health.
- Antinutritional factors can impair nutrient absorption, but can be mitigated through preparation techniques like fermentation and sprouting.
- Vegan and vegetarian diets influence the gut–brain axis, emphasizing the importance of microbiota in cognitive and emotional health.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The Vegan Society. Worldwide Veganism Statistics. Available online: https://www.vegansociety.com/news/media/statistics/worldwide (accessed on 24 February 2025).
- Sofi, F.; Dinu, M.; Pagliai, G.; Cesari, F.; Marcucci, R.; Casini, A. Mediterranean versus Vegetarian Diet for Cardiovascular Disease Prevention (the CARDIVEG Study): Study Protocol for a Randomized Controlled Trial. Trials 2016, 17, 233. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.J.; Mangels, A.R.; Fresán, U.; Marsh, K.; Miles, F.L.; Saunders, A.V.; Haddad, E.H.; Heskey, C.E.; Johnston, P.; Larson-Meyer, E.; et al. The Safe and Effective Use of Plant-Based Diets with Guidelines for Health Professionals. Nutrients 2021, 13, 4144. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, F.; Samman, S. Vitamin B12 in Health and Disease. Nutrients 2010, 2, 299–316. [Google Scholar] [CrossRef] [PubMed]
- Rogers, P.J.; Appleton, K.M.; Kessler, D.; Peters, T.J.; Gunnell, D.; Hayward, R.C.; Heatherley, S.V.; Christian, L.M.; McNaughton, S.A.; Ness, A.R. No Effect of N-3 Long-Chain Polyunsaturated Fatty Acid (EPA and DHA) Supplementation on Depressed Mood and Cognitive Function: A Randomised Controlled Trial. Br. J. Nutr. 2008, 99, 421–431. [Google Scholar] [CrossRef]
- Gibson, R.S.; Bailey, K.B.; Gibbs, M.; Ferguson, E.L. A Review of Phytate, Iron, Zinc, and Calcium Concentrations in Plant-Based Complementary Foods Used in Low-Income Countries and Implications for Bioavailability. Food Nutr. Bull. 2010, 31, S134–S146. [Google Scholar] [CrossRef]
- Cordain, L.; Toohey, L.; Smith, M.J.; Hickey, M.S. Modulation of Immune Function by Dietary Lectins in Rheumatoid Arthritis. Br. J. Nutr. 2000, 83, 207–217. [Google Scholar] [CrossRef]
- Noonan, S.C.; Savage, G.P. Oxalate Content of Foods and Its Effect on Humans. Asia Pac. J. Clin. Nutr. 1999, 8, 64–74. [Google Scholar]
- Łuszczki, E.; Boakye, F.; Zielińska, M.; Dereń, K.; Bartosiewicz, A.; Oleksy, Ł.; Stolarczyk, A. Vegan diet: Nutritional components, implementation, and effects on adults’ health. Front. Nutr. 2023, 10, 64–1294497, Erratum in Front. Nutr. 2024, 10, 1354336. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barbaresko, J.; Koch, M.; Schulze, M.B.; Nöthlings, U. Dietary Pattern Analysis and Biomarkers of Low-Grade Inflammation: A Systematic Literature Review. Nutr. Rev. 2013, 71, 511–527. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Importance of the Omega-6/Omega-3 Balance in Health and Disease: Evolutionary Aspects of Diet. World Rev. Nutr. Diet. 2011, 102, 10–21. [Google Scholar] [CrossRef]
- Lauer, A.A.; Grimm, H.S.; Apel, B.; Golobrodska, N.; Kruse, L.; Ratanski, E.; Schulten, N.; Schwarze, L.; Slawik, T.; Sperlich, S.; et al. Mechanistic Link between Vitamin B12 and Alzheimer’s Disease. Biomolecules 2022, 12, 129. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Clarys, P.; Deliens, T.; Huybrechts, I.; Deriemaeker, P.; Vanaelst, B.; De Keyzer, W.; Hebbelinck, M.; Mullie, P. Comparison of nutritional quality of the vegan, vegetarian, semi-vegetarian, pesco-vegetarian and omnivorous diet. Nutrients 2014, 6, 1318–1332. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Deleu, S.; Machiels, K.; Raes, J.; Verbeke, K.; Vermeire, S. Short Chain Fatty Acids and Its Producing Organisms: An Overlooked Therapy for IBD? EBioMedicine 2021, 66, 103293. [Google Scholar] [CrossRef] [PubMed]
- Conlon, M.A.; Bird, A.R. The Impact of Diet and Lifestyle on Gut Microbiota and Human Health. Nutrients 2014, 7, 17–44. [Google Scholar] [CrossRef]
- Tansey, M.G.; Wallings, R.L.; Houser, M.C.; Herrick, M.K.; Keating, C.E.; Joers, V. Inflammation and immune dysfunction in Parkinson disease. Nat. Rev. Immunol. 2022, 22, 657–673. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dhakal, S.; Kushairi, N.; Phan, C.W.; Adhikari, B.; Sabaratnam, V.; Macreadie, I. Dietary Polyphenols: A Multifactorial Strategy to Target Alzheimer’s Disease. Int. J. Mol. Sci. 2019, 20, 5090. [Google Scholar] [CrossRef]
- Dinu, M.; Tristan Asensi, M.; Pagliai, G.; Lotti, S.; Martini, D.; Colombini, B.; Sofi, F. Consumption of Ultra-Processed Foods Is Inversely Associated with Adherence to the Mediterranean Diet: A Cross-Sectional Study. Nutrients 2022, 14, 2073. [Google Scholar] [CrossRef]
- Pawlak, R.; Parrott, S.J.; Raj, S.; Cullum-Dugan, D.; Lucus, D. How Prevalent Is Vitamin B(12) Deficiency among Vegetarians? Nutr. Rev. 2013, 71, 110–117. [Google Scholar] [CrossRef]
- Zhang, X.-W.; Chen, J.-Y.; Ouyang, D.; Lu, J.-H. Quercetin in Animal Models of Alzheimer’s Disease: A Systematic Review of Preclinical Studies. Int. J. Mol. Sci. 2020, 21, 493. [Google Scholar] [CrossRef]
- Kciuk, M.; Kruczkowska, W.; Gałęziewska, J.; Wanke, K.; Kałuzińska-Kołat, Ż.; Aleksandrowicz, M.; Kontek, R. Alzheimer’s Disease as Type 3 Diabetes: Understanding the Link and Implications. Int. J. Mol. Sci. 2024, 25, 11955. [Google Scholar] [CrossRef]
- Satija, A.; Bhupathiraju, S.N.; Rimm, E.B.; Spiegelman, D.; Chiuve, S.E.; Borgi, L.; Willett, W.C.; Manson, J.E.; Sun, Q.; Hu, F.B. Plant-Based Dietary Patterns and Incidence of Type 2 Diabetes in US Men and Women: Results from Three Prospective Cohort Studies. PLoS Med. 2016, 13, e1002039. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, G.; Baroni, L.; Bonetto, C.; Visaggi, P.; Orazzini, M.; Solinas, I.; Guidi, G.; Pugliese, J.; Scaramuzza, G.; Ovidi, F.; et al. The Role of a Plant-Only (Vegan) Diet in Gastroesophageal Reflux Disease: Online Survey of the Italian General Population. Nutrients 2023, 15, 4725. [Google Scholar] [CrossRef]
- Weaver, C.; Marr, E.T. White Vegetables: A Forgotten Source of Nutrients: Purdue Roundtable Executive Summary. Adv. Nutr. 2013, 4, 318S–326S. [Google Scholar] [CrossRef] [PubMed]
- Leitzmann, C. Vegetarian Nutrition: Past, Present, Future. Am. J. Clin. Nutr. 2014, 100 (Suppl. S1), 496S–502S. [Google Scholar] [CrossRef] [PubMed]
- Saunders, A.V.; Davis, B.C.; Garg, M.L. Omega-3 Polyunsaturated Fatty Acids and Vegetarian Diets. Med. J. Aust. 2013, 199, S22–S26. [Google Scholar] [CrossRef]
- Pawlak, R.; Lester, S.E.; Babatunde, T. The Prevalence of Cobalamin Deficiency among Vegetarians Assessed by Serum Vitamin B12: A Review of Literature. Eur. J. Clin. Nutr. 2014, 68, 541–548. [Google Scholar] [CrossRef]
- Hunt, J.R. Bioavailability of Iron, Zinc, and Other Trace Minerals from Vegetarian Diets. Am. J. Clin. Nutr. 2003, 78, 633S–639S. [Google Scholar] [CrossRef]
- Giudici, K.V.; Weaver, C.M. Plant-Based Diets and Risk of Osteoporosis. In Vegetarian Nutrition and Wellness; CRC Press: Boca Raton, FL, USA, 2018; ISBN 978-1-315-26701-2. [Google Scholar]
- Péneau, S.; Dauchet, L.; Vergnaud, A.-C.; Estaquio, C.; Kesse-Guyot, E.; Bertrais, S.; Latino-Martel, P.; Hercberg, S.; Galan, P. Relationship between Iron Status and Dietary Fruit and Vegetables Based on Their Vitamin C and Fiber Content. Am. J. Clin. Nutr. 2008, 87, 1298–1305. [Google Scholar] [CrossRef]
- Middleton, E.; Kandaswami, C.; Theoharides, T.C. The Effects of Plant Flavonoids on Mammalian Cells:Implications for Inflammation, Heart Disease, and Cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar]
- Tonstad, S.; Stewart, K.; Oda, K.; Batech, M.; Herring, R.P.; Fraser, G.E. Vegetarian Diets and Incidence of Diabetes in the Adventist Health Study-2. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 292–299. [Google Scholar] [CrossRef]
- Koch, K.; Bhushan, B.; Barthlott, W. Multifunctional Surface Structures of Plants: An Inspiration for Biomimetics. Prog. Mater. Sci. 2009, 54, 137–178. [Google Scholar] [CrossRef]
- Yan, Y.; Li, X.; Zhang, C.; Lv, L.; Gao, B.; Li, M. Research Progress on Antibacterial Activities and Mechanisms of Natural Alkaloids: A Review. Antibiotics 2021, 10, 318. [Google Scholar] [CrossRef] [PubMed]
- Mithen, R.F.; Dekker, M.; Verkerk, R.; Rabot, S.; Johnson, I.T. The Nutritional Significance, Biosynthesis and Bioavailability of Glucosinolates in Human Foods. J. Sci. Food Agric. 2000, 80, 967–984. [Google Scholar] [CrossRef]
- Hurrell, R.; Egli, I. Iron Bioavailability and Dietary Reference Values. Am. J. Clin. Nutr. 2010, 91, 1461S–1467S. [Google Scholar] [CrossRef] [PubMed]
- López, B.; Ravassa, S.; Moreno, M.U.; José, G.S.; Beaumont, J.; González, A.; Díez, J. Diffuse Myocardial Fibrosis: Mechanisms, Diagnosis and Therapeutic Approaches. Nat. Rev. Cardiol. 2021, 18, 479–498. [Google Scholar] [CrossRef]
- Massey, L.K. Food Oxalate: Factors Affecting Measurement, Biological Variation, and Bioavailability. J. Am. Diet. Assoc. 2007, 107, 1191–1194. [Google Scholar] [CrossRef]
- Vasconcelos, I.M.; Oliveira, J.T.A. Antinutritional Properties of Plant Lectins. Toxicon Off. J. Int. Soc. Toxinology 2004, 44, 385–403. [Google Scholar] [CrossRef]
- Hurrell, R.F.; Reddy, M.; Cook, J.D. Inhibition of Non-Haem Iron Absorption in Man by Polyphenolic-Containing Beverages. Br. J. Nutr. 1999, 81, 289–295. [Google Scholar] [CrossRef]
- Augustin, J.M.; Kuzina, V.; Andersen, S.B.; Bak, S. Molecular Activities, Biosynthesis and Evolution of Triterpenoid Saponins. Phytochemistry 2011, 72, 435–457. [Google Scholar] [CrossRef]
- Friedman, W.E.; Moore, R.C.; Purugganan, M.D. The Evolution of Plant Development. Am. J. Bot. 2004, 91, 1726–1741. [Google Scholar] [CrossRef]
- Lopez, H.W.; Leenhardt, F.; Coudray, C.; Remesy, C. Minerals and Phytic Acid Interactions: Is It a Real Problem for Human Nutrition? Int. J. Food Sci. Technol. 2002, 37, 727–739. [Google Scholar] [CrossRef]
- Traka, M.; Mithen, R. Glucosinolates, Isothiocyanates and Human Health. Phytochem. Rev. 2009, 8, 269–282. [Google Scholar] [CrossRef]
- Sharon, N.; Lis, H. History of Lectins: From Hemagglutinins to Biological Recognition Molecules. Glycobiology 2004, 14, 53R–62R. [Google Scholar] [CrossRef]
- Scalbert, A.; Johnson, I.T.; Saltmarsh, M. Polyphenols: Antioxidants and Beyond. Am. J. Clin. Nutr. 2005, 81, 215S–217S. [Google Scholar] [CrossRef] [PubMed]
- Nath, H.; Samtiya, M.; Dhewa, T. Beneficial Attributes and Adverse Effects of Major Plant-Based Foods Anti-Nutrients on Health: A Review. Hum. Nutr. Metab. 2022, 28, 200147. [Google Scholar] [CrossRef]
- Petroski, W.; Minich, D.M. Is There Such a Thing as “Anti-Nutrients”? A Narrative Review of Perceived Problematic Plant Compounds. Nutrients 2020, 12, 2929. [Google Scholar] [CrossRef]
- Badaeva, A.V.; Danilov, A.B.; Clayton, P.; Moskalev, A.A.; Karasev, A.V.; Tarasevich, A.F.; Vorobyeva, Y.D.; Novikov, V.N. Perspectives on Neuronutrition in Prevention and Treatment of Neurological Disorders. Nutrients 2023, 15, 2505. [Google Scholar] [CrossRef]
- Beam, A.; Clinger, E.; Hao, L. Effect of Diet and Dietary Components on the Composition of the Gut Microbiota. Nutrients 2021, 13, 2795. [Google Scholar] [CrossRef]
- Gamelin, L.; Capitain, O.; Morel, A.; Dumont, A.; Traore, S.; Anne, L.B.; Gilles, S.; Boisdron-Celle, M.; Gamelin, E. Predictive Factors of Oxaliplatin Neurotoxicity: The Involvement of the Oxalate Outcome Pathway. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2007, 13, 6359–6368. [Google Scholar] [CrossRef]
- Scaglia, F.; Northrop, J.L. The Mitochondrial Myopathy Encephalopathy, Lactic Acidosis with Stroke-like Episodes (MELAS) Syndrome: A Review of Treatment Options. CNS Drugs 2006, 20, 443–464. [Google Scholar] [CrossRef]
- Nio-Kobayashi, J.; Itabashi, T. Galectins and Their Ligand Glycoconjugates in the Central Nervous System Under Physiological and Pathological Conditions. Front. Neuroanat. 2021, 15, 767330. [Google Scholar] [CrossRef] [PubMed]
- López-Moreno, M.; Garcés-Rimón, M.; Miguel, M. Antinutrients: Lectins, Goitrogens, Phytates and Oxalates, Friends or Foe? J. Funct. Foods 2022, 89, 104938. [Google Scholar] [CrossRef]
- Robles-Vera, I.; Jarit-Cabanillas, A.; Brandi, P.; Martínez-López, M.; Martínez-Cano, S.; González-Correa, C.; Moleón, J.; Duarte, J.; Conejero, L.; Mata-Martínez, P.; et al. Translocation of Gut Enterococcus Faecalis Trains Myeloid Bone Marrow Progenitors via the C-Type Lectin Receptor Mincle. bioRxiv 2024. [Google Scholar] [CrossRef]
- Mahbub, N.U.; Islam, M.M.; Hong, S.-T.; Chung, H.-J. Dysbiosis of the Gut Microbiota and Its Effect on α-Synuclein and Prion Protein Misfolding: Consequences for Neurodegeneration. Front. Cell. Infect. Microbiol. 2024, 14, 1348279. [Google Scholar] [CrossRef]
- Aryasa, I.A.; Widyantara, I.W.; Kusuma Putra, I.B. Dysbiosis of Gut Microbiome in Neurocritically Ill Patients in Intensive Care: Systematic Review. Int. J. Sci. Healthc. Res. 2023, 8, 294–301. [Google Scholar] [CrossRef]
- Upadhyay, J.; Tiwari, N.; Durgapal, S.; Jantwal, A.; Kumar, A. Chapter4.13—Phytic Acid: As a Natural Antioxidant. In Antioxidants Effects in Health; Nabavi, S.M., Silva, A.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 437–450. ISBN 978-0-12-819096-8. [Google Scholar]
- Tiwari, A.P.; Albin, B.; Qubbaj, K.; Adhikari, P.; Yang, I.H. Phytic Acid Maintains Peripheral Neuron Integrity and Enhances Survivability against Platinum-Induced Degeneration via Reducing Reactive Oxygen Species and Enhancing Mitochondrial Membrane Potential. ACS Chem. Neurosci. 2024, 15, 1157–1168. [Google Scholar] [CrossRef]
- Ullah, R.; Ali, G.; Baseer, A.; Irum Khan, S.; Akram, M.; Khan, S.; Ahmad, N.; Farooq, U.; Kanwal Nawaz, N.; Shaheen, S.; et al. Tannic Acid Inhibits Lipopolysaccharide-Induced Cognitive Impairment in Adult Mice by Targeting Multiple Pathological Features. Int. Immunopharmacol. 2022, 110, 108970. [Google Scholar] [CrossRef]
- Antinutritional Factors in Plant Based Foods. Available online: https://www.researchgate.net/publication/370136589_Antinutritional_factors_in_plant_based_foods (accessed on 6 December 2024).
- Kong, X.; Li, Y.; Liu, X. A Review of Thermosensitive Antinutritional Factors in Plant-Based Foods. J. Food Biochem. 2022, 46, e14199. [Google Scholar] [CrossRef]
- Fehlbaum, S.; Prudence, K.; Kieboom, J.; Heerikhuisen, M.; Van den Broek, T.; Schuren, F.H.J.; Steinert, R.E.; Raederstorff, D. In vitro fermentation of selected prebiotics and their effects on the composition and activity of the adult gut microbiota. Int. J. Mol. Sci. 2018, 19, 3097. [Google Scholar] [CrossRef]
- Predescu, N.C.; Stefan, G.; Rosu, M.P.; Papuc, C. Fermented Feed in Broiler Diets Reduces the Antinutritional Factors, Improves Productive Performances and Modulates Gut Microbiome—A Review. Agriculture 2024, 14, 1752. [Google Scholar] [CrossRef]
- Clower, L.; Fleshman, T.; Geldenhuys, W.J.; Santanam, N. Targeting Oxidative Stress Involved in Endometriosis and Its Pain. Biomolecules 2022, 12, 1055. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.; Oliveira, L.; Pereira, A.; Costa, M.d.C.; Raposo, A.; Saraiva, A.; Magalhães, B. Exploring Vitamin B12 Supplementation in the Vegan Population: A Scoping Review of the Evidence. Nutrients 2024, 16, 1442. [Google Scholar] [CrossRef] [PubMed]
- Krajčovičová-Kudláčková, M.; Bucková, K.; Klimeš, I.; Béderová, A. Homocysteine Levels in Vegetarians versus Omnivores. Ann. Nutr. Metab. 2000, 44, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Vogel, R.A.; Corretti, M.C.; Plotnick, G.D. The Postprandial Effect of Components of the Mediterranean Diet on Endothelial Function. J. Am. Coll. Cardiol. 2000, 36, 1455–1460. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Li, X.; Zhang, H.; Wang, C.; Liu, Y. Association Between Homocysteine, Vitamin B12, and Alzheimer’s Disease: A Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2024, 13, 1023. [Google Scholar]
- Bourre, J.-M.; Gueant, J.-L.; Cailliez, R.; Galland, F.; Rocher, M.; Deharo, D.; Levy, R. The Role of Vitamin B12 in Cognition and Neurodegenerative Diseases: Clinical Implications. Nutr. Neurosci. 2023, 26, 12712. [Google Scholar]
- Miller, A.; Korem, M.; Almog, R.; Galboiz, Y. Vitamin B12, Cognition, and Neurological Disorders: Insights from Clinical and Experimental Studies. Front. Neurol. 2018, 9, 325. [Google Scholar] [CrossRef]
- Kumar, S.B.; Arnipalli, S.R.; Mehta, P.; Carrau, S.; Ziouzenkova, O. Iron Deficiency Anemia: Efficacy and Limitations of Nutritional and Comprehensive Mitigation Strategies. Nutrients 2022, 14, 2976. [Google Scholar] [CrossRef]
- Andrieu, S.; Guyonnet, S.; Coley, N.; Cantet, C.; Bonnefoy, M.; Bordes, S.; Bories, L.; Cufi, M.-N.; Dantoine, T.; Dartigues, J.-F.; et al. Effect of Long-Term Omega 3 Polyunsaturated Fatty Acid Supplementation with or without Multidomain Intervention on Cognitive Function in Elderly Adults with Memory Complaints (MAPT): A Randomised, Placebo-Controlled Trial. Lancet Neurol. 2017, 16, 377–389. [Google Scholar] [CrossRef]
- Białek-Dratwa, A.; Stoń, W.; Staśkiewicz-Bartecka, W.; Grajek, M.; Krupa-Kotara, K.; Kowalski, O. The Psychosocial Aspects of Vegetarian Diets: A Cross-Sectional Study of the Motivations, Risks, and Limitations in Daily Life. Nutrients 2024, 16, 2504. [Google Scholar] [CrossRef]
- Akhtar, S.; Ismail, T.; Atukorala, S.; Arlappa, N. Micronutrient Deficiencies in South Asia—Current Status and Strategies. Trends Food Sci. Technol. 2013, 31, 55–62. [Google Scholar] [CrossRef]
- Loef, M.; Walach, H. Fruit, Vegetables and Prevention of Cognitive Decline or Dementia: A Systematic Review of Cohort Studies. J. Nutr. Health Aging 2012, 16, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Jaberi, K.R.; Alamdari-Palangi, V.; Savardashtaki, A.; Vatankhah, P.; Jamialahmadi, T.; Tajbakhsh, A.; Sahebkar, A. Modulatory Effects of Phytochemicals on Gut-Brain Axis: Therapeutic Implication. Curr. Dev. Nutr. 2024, 8, 103785. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Sancheti, H.; Patil, I.; Cadenas, E. Energy Metabolism and Inflammation in Brain Aging and Alzheimer’s Disease. Free Radic. Biol. Med. 2016, 100, 108–122. [Google Scholar] [CrossRef]
- Puspita, L.; Chung, S.Y.; Shim, J.-W. Oxidative Stress and Cellular Pathologies in Parkinson’s Disease. Mol. Brain 2017, 10, 53. [Google Scholar] [CrossRef]
- Dong-Chen, X.; Yong, C.; Yang, X.; Chen-Yu, S.; Li-Hua, P. Signaling Pathways in Parkinson’s Disease: Molecular Mechanisms and Therapeutic Interventions. Signal Transduct. Target. Ther. 2023, 8, 73. [Google Scholar] [CrossRef]
- Arts, I.C.W.; Hollman, P.C.H. Polyphenols and Disease Risk in Epidemiologic Studies. Am. J. Clin. Nutr. 2005, 81, 317S–325S. [Google Scholar] [CrossRef]
- Sun, H.; Chen, Y.; Cheng, M.; Zhang, X.; Zheng, X.; Zhang, Z. The modulatory effect of polyphenols from green tea, oolong tea and black tea on human intestinal microbiota in vitro. J. Food Sci. Technol. 2018, 55, 399–407. [Google Scholar] [CrossRef]
- Salisbury, D.; Bronas, U. Reactive Oxygen and Nitrogen Species: Impact on Endothelial Dysfunction. Nurs. Res. 2015, 64, 53–66. [Google Scholar] [CrossRef]
- Venigalla, M.; Sonego, S.; Gyengesi, E.; Sharman, M.J.; Münch, G. Novel Promising Therapeutics against Chronic Neuroinflammation and Neurodegeneration in Alzheimer’s Disease. Neurochem. Int. 2016, 95, 63–74. [Google Scholar] [CrossRef]
- Cirillo, G.; Curcio, M.; Vittorio, O.; Iemma, F.; Restuccia, D.; Spizzirri, U.G.; Puoci, F.; Picci, N. Polyphenol Conjugates and Human Health: A Perspective Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.M.; Murphy, E.A.; Carmichael, M.D. Effects of the Dietary Flavonoid Quercetin upon Performance and Health. Curr. Sports Med. Rep. 2009, 8, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.H.; Akter, R.; Bhattacharya, T.; Abdel-Daim, M.M.; Alkahtani, S.; Arafah, M.W.; Al-Johani, N.S.; Alhoshani, N.M.; Alkeraishan, N.; Alhenaky, A.; et al. Resveratrol and Neuroprotection: Impact and Its Therapeutic Potential in Alzheimer’s Disease. Front. Pharmacol. 2020, 11, 619024. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.; Mazzitelli, S.; Arciello, M.; Capo, C.R.; Rotilio, G. Benefits from Dietary Polyphenols for Brain Aging and Alzheimer’s Disease. Neurochem. Res. 2008, 33, 2390–2400. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Venkidasamy, B.; Subramanian, U.; Samynathan, R.; Ali Shariati, M.; Rebezov, M.; Girish, S.; Thangavel, S.; Dhanapal, A.R.; Fedoseeva, N.; et al. Bioactive Compounds in Oxidative Stress-Mediated Diseases: Targeting the NRF2/ARE Signaling Pathway and Epigenetic Regulation. Antioxidants 2021, 10, 1859. [Google Scholar] [CrossRef]
- Chodari, L.; Dilsiz Aytemir, M.; Vahedi, P.; Alipour, M.; Vahed, S.Z.; Khatibi, S.M.H.; Ahmadian, E.; Ardalan, M.; Eftekhari, A. Targeting Mitochondrial Biogenesis with Polyphenol Compounds. Oxidative Med. Cell. Longev. 2021, 2021, e4946711. [Google Scholar] [CrossRef]
- Lin, X.; Bai, D.; Wei, Z.; Zhang, Y.; Huang, Y.; Deng, H.; Huang, X. Curcumin Attenuates Oxidative Stress in RAW264.7 Cells by Increasing the Activity of Antioxidant Enzymes and Activating the Nrf2-Keap1 Pathway. PLoS ONE 2019, 14, e0216711. [Google Scholar] [CrossRef]
- Cho, J.A.; Park, E. Curcumin Utilizes the Anti-Inflammatory Response Pathway to Protect the Intestine against Bacterial Invasion. Nutr. Res. Pract. 2015, 9, 117–122. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, S.; Li, J.; Wang, R.; Xie, X.; Yu, X.; Pan, J.; Xu, Y.; Zheng, L. The Effect of Curcumin on the Brain-Gut Axis in Rat Model of Irritable Bowel Syndrome: Involvement of 5-HT-Dependent Signaling. Metab. Brain Dis. 2015, 30, 47–55. [Google Scholar] [CrossRef]
- Shandilya, S.; Kumar, S.; Kumar Jha, N.; Kumar Kesari, K.; Ruokolainen, J. Interplay of Gut Microbiota and Oxidative Stress: Perspective on Neurodegeneration and Neuroprotection. J. Adv. Res. 2022, 38, 223–244. [Google Scholar] [CrossRef]
- Calis, Z.; Mogulkoc, R.; Baltaci, A.K. The Roles of Flavonols/Flavonoids in Neurodegeneration and Neuroinflammation. Mini Rev. Med. Chem. 2020, 20, 1475–1488. [Google Scholar] [CrossRef] [PubMed]
- Management of Oxidative Stress and Other Pathologies in Alzheimer’s Disease. Available online: https://pubmed.ncbi.nlm.nih.gov/31440798/ (accessed on 10 December 2024).
- Zamanian, M.Y.; Soltani, A.; Khodarahmi, Z.; Alameri, A.A.; Alwan, A.M.R.; Ramírez-Coronel, A.A.; Obaid, R.F.; Abosaooda, M.; Heidari, M.; Golmohammadi, M.; et al. Targeting Nrf2 Signaling Pathway by Quercetin in the Prevention and Treatment of Neurological Disorders: An Overview and Update on New Developments. Fundam. Clin. Pharmacol. 2023, 37, 1050–1064. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.J.; Spencer, J.P.E.; Rice-Evans, C. Flavonoids: Antioxidants or Signalling Molecules? Free Radic. Biol. Med. 2004, 36, 838–849. [Google Scholar] [CrossRef]
- Wang, T.; Masedunskas, A.; Willett, W.C.; Fontana, L. Vegetarian and Vegan Diets: Benefits and Drawbacks. Eur. Heart J. 2023, 44, 3423–3439. [Google Scholar] [CrossRef] [PubMed]
- Guyon, C.; Meynier, A.; de Lamballerie, M. Protein and Lipid Oxidation in Meat: A Review with Emphasis on High-Pressure Treatments. Trends Food Sci. Technol. 2016, 50, 131–143. [Google Scholar] [CrossRef]
- Ahn-Jarvis, J.H.; Parihar, A.; Doseff, A.I. Dietary Flavonoids for Immunoregulation and Cancer: Food Design for Targeting Disease. Antioxidants 2019, 8, 202. [Google Scholar] [CrossRef]
- Capozzi, A.; Saucier, C.; Bisbal, C.; Lambert, K. Grape Polyphenols in the Treatment of Human Skeletal Muscle Damage Due to Inflammation and Oxidative Stress during Obesity and Aging: Early Outcomes and Promises. Molecules 2022, 27, 6594. [Google Scholar] [CrossRef]
- Bitler, C.M.; Viale, T.M.; Damaj, B.; Crea, R. Hydrolyzed Olive Vegetation Water in Mice Has Anti-Inflammatory Activity. J. Nutr. 2005, 135, 1475–1479. [Google Scholar] [CrossRef]
- Nam, N.-H. Naturally Occurring NF-kappaB Inhibitors. Mini Rev. Med. Chem. 2006, 6, 945–951. [Google Scholar] [CrossRef]
- Gomez-Cabrera, M.-C.; Domenech, E.; Romagnoli, M.; Arduini, A.; Borras, C.; Pallardo, F.V.; Sastre, J.; Viña, J. Oral Administration of Vitamin C Decreases Muscle Mitochondrial Biogenesis and Hampers Training-Induced Adaptations in Endurance Performance. Am. J. Clin. Nutr. 2008, 87, 142–149. [Google Scholar] [CrossRef]
- Niki, E. Role of Vitamin E as a Lipid-Soluble Peroxyl Radical Scavenger: In Vitro and in Vivo Evidence. Free Radic. Biol. Med. 2014, 66, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Elorinne, A.-L.; Alfthan, G.; Erlund, I.; Kivimäki, H.; Paju, A.; Salminen, I.; Turpeinen, U.; Voutilainen, S.; Laakso, J. Food and Nutrient Intake and Nutritional Status of Finnish Vegans and Non-Vegetarians. PLoS ONE 2016, 11, e0148235. [Google Scholar] [CrossRef] [PubMed]
- Askari, M.; Daneshzad, E.; Darooghegi Mofrad, M.; Bellissimo, N.; Suitor, K.; Azadbakht, L. Vegetarian Diet and the Risk of Depression, Anxiety, and Stress Symptoms: A Systematic Review and Meta-Analysis of Observational Studies. Crit. Rev. Food Sci. Nutr. 2022, 62, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Abumweis, S.S.; Barake, R.; Jones, P.J.H. Plant Sterols/Stanols as Cholesterol Lowering Agents: A Meta-Analysis of Randomized Controlled Trials. Food Nutr. Res. 2008, 52, 1811. [Google Scholar] [CrossRef]
- Theuwissen, E.; Mensink, R.P. Water-Soluble Dietary Fibers and Cardiovascular Disease. Physiol. Behav. 2008, 94, 285–292. [Google Scholar] [CrossRef]
- la Torre Fabiola, V.-D.; Ralf, K.; Gabriel, B.; Victor Ermilo, A.-A.; Martha, M.-G.; Mirbella, C.-F.; Rocio, B.-A. Anti-Inflammatory and Immunomodulatory Effects of Critonia Aromatisans Leaves: Downregulation of pro-Inflammatory Cytokines. J. Ethnopharmacol. 2016, 190, 174–182. [Google Scholar] [CrossRef]
- Dwaraka, V.B.; Aronica, L.; Carreras-Gallo, N.; Robinson, J.L.; Hennings, T.; Carter, M.M.; Corley, M.J.; Lin, A.; Turner, L.; Smith, R.; et al. Unveiling the epigenetic impact of vegan vs. omnivorous diets on aging: Insights from the Twins Nutrition Study (TwiNS). BMC Med. 2024, 22, 301. [Google Scholar] [CrossRef]
- Capodici, A.; Mocciaro, G.; Gori, D.; Landry, M.J.; Masini, A.; Sanmarchi, F.; Fiore, M.; Coa, A.A.; Castagna, G.; Gardner, C.D.; et al. Cardiovascular Health and Cancer Risk Associated with Plant Based Diets: An Umbrella Review. PLoS ONE 2024, 19, e0300711. [Google Scholar] [CrossRef]
- Trevithick-Sutton, C.C.; Foote, C.S.; Collins, M.; Trevithick, J.R. The Retinal Carotenoids Zeaxanthin and Lutein Scavenge Superoxide and Hydroxyl Radicals: A Chemiluminescence and ESR Study. Mol. Vis. 2006, 12, 1127–1135. [Google Scholar]
- Abdull Razis, A.F.; Noor, N.M. Sulforaphane Is Superior to Glucoraphanin in Modulating Carcinogen-Metabolising Enzymes in Hep G2 Cells. Asian Pac. J. Cancer Prev. APJCP 2013, 14, 4235–4238. [Google Scholar] [CrossRef]
- Kensler, T.W.; Qian, G.-S.; Chen, J.-G.; Groopman, J.D. Translational Strategies for Cancer Prevention in Liver. Nat. Rev. Cancer 2003, 3, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Chiba, T.; Takahashi, S.; Ishii, T.; Igarashi, K.; Katoh, Y.; Oyake, T.; Hayashi, N.; Satoh, K.; Hatayama, I.; et al. An Nrf2/Small Maf Heterodimer Mediates the Induction of Phase II Detoxifying Enzyme Genes through Antioxidant Response Elements. Biochem. Biophys. Res. Commun. 1997, 236, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, F.H.; Johnson, L.K.; Zeng, H. Magnesium Supplementation Improves Indicators of Low Magnesium Status and Inflammatory Stress in Adults Older than 51 Years with Poor Quality Sleep. Magnes. Res. 2010, 23, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Bakaloudi, D.R.; Halloran, A.; Rippin, H.L.; Oikonomidou, A.C.; Dardavesis, T.I.; Williams, J.; Wickramasinghe, K.; Breda, J.; Chourdakis, M. Intake and Adequacy of the Vegan Diet. A systematic review of the evidence. Clin. Nutr. 2021, 40, 3503–3521. [Google Scholar] [CrossRef]
- Hargreaves, S.M.; Raposo, A.; Saraiva, A.; Zandonadi, R.P. Vegetarian Diet: An Overview through the Perspective of Quality of Life Domains. Int. J. Environ. Res. Public Health 2021, 18, 4067. [Google Scholar] [CrossRef]
- Iguacel, I.; Huybrechts, I.; Moreno, L.A.; Michels, N. Vegetarianism and Veganism Compared with Mental Health and Cognitive Outcomes: A Systematic Review and Meta-Analysis. Nutr. Rev. 2021, 79, 361–381. [Google Scholar] [CrossRef]
- Mayrhofer, R.; Roberts, L.M.; Hackl, J.M.; Frischholz, K. Psychological Differences and Similarities between Vegans, Prospective Vegans, and Vegetarians. Motivation, Knowledge, Vegan Literacy—And Cheese. Front. Psychol. 2024, 15, 1163869. [Google Scholar] [CrossRef]
- Plante, C.N.; Rosenfeld, D.L.; Plante, M.; Reysen, S. The Role of Social Identity Motivation in Dietary Attitudes and Behaviors among Vegetarians. Appetite 2019, 141, 104307. [Google Scholar] [CrossRef]
- Rosenfeld, D.L.; Burrow, A.L. Vegetarian on Purpose: Understanding the Motivations of Plant-Based Dieters. Appetite 2017, 116, 456–463. [Google Scholar] [CrossRef]
- Rosenfeld, D.L.; Burrow, A.L. The Unified Model of Vegetarian Identity: A Conceptual Framework for Understanding Plant-Based Food Choices. Appetite 2017, 112, 78–95. [Google Scholar] [CrossRef]
- Krizanova, J.; Guardiola, J. Conceptualizations of Happiness and Vegetarianism: Empirical Evidence from University Students in Spain. J. Happiness Stud. 2023, 24, 1483–1503. [Google Scholar] [CrossRef]
- Bertella, G. The Vegan Food Experience: Searching for Happiness in the Norwegian Foodscape. Societies 2020, 10, 95. [Google Scholar] [CrossRef]
- Dobersek, U.; Bender, M.; Etienne, A.; Fernandez Gil, G.E.; Hostetter, C. Meat Consumption & Positive Mental Health: A Scoping Review. Prev. Med. Rep. 2024, 37, 102556. [Google Scholar] [CrossRef] [PubMed]
- Beezhold, B.L.; Johnston, C.S. Restriction of Meat, Fish, and Poultry in Omnivores Improves Mood: A Pilot Randomized Controlled Trial. Nutr. J. 2012, 11, 9. [Google Scholar] [CrossRef]
- Beezhold, B.L.; Johnston, C.S.; Daigle, D.R. Vegetarian Diets Are Associated with Healthy Mood States: A Cross-Sectional Study in Seventh Day Adventist Adults. Nutr. J. 2010, 9, 26. [Google Scholar] [CrossRef]
- Beezhold, B.; Radnitz, C.; Rinne, A.; DiMatteo, J. Vegans Report Less Stress and Anxiety than Omnivores. Nutr. Neurosci. 2015, 18, 289–296. [Google Scholar] [CrossRef]
- Khaledi-Paveh, B.; Abdi, A.; Heydarpour, S.; Dehghan, F.; Haghparast, R.; Ghasemi, H. The Perceived Experience of Adhering to Vegan Diet: A Descriptive Phenomenological Study. BMC Public Health 2024, 24, 753. [Google Scholar] [CrossRef]
- Marrone, G.; Guerriero, C.; Palazzetti, D.; Lido, P.; Marolla, A.; Di Daniele, F.; Noce, A. Vegan Diet Health Benefits in Metabolic Syndrome. Nutrients 2021, 13, 817. [Google Scholar] [CrossRef]
- Bali, A.; Naik, R. The Impact of a Vegan Diet on Many Aspects of Health: The Overlooked Side of Veganism. Cureus 2023, 15, e35148. [Google Scholar] [CrossRef]
- Null, G.; Pennesi, L. Diet and Lifestyle Intervention on Chronic Moderate to Severe Depression and Anxiety and Other Chronic Conditions. Complement. Ther. Clin. Pract. 2017, 29, 189–193. [Google Scholar] [CrossRef]
- Raman, M.; Vishnubhotla, R.; Ramay, H.R.; Gonçalves, M.C.B.; Shin, A.S.; Pawale, D.; Subramaniam, B.; Sadhasivam, S. Isha Yoga Practices, Vegan Diet, and Participation in Samyama Meditation Retreat: Impact on the Gut Microbiome & Metabolome—A Non-Randomized Trial. BMC Complement. Med. Ther. 2023, 23, 107. [Google Scholar] [CrossRef]
- Hevia-Larraín, V.; Gualano, B.; Longobardi, I.; Gil, S.; Fernandes, A.L.; Costa, L.A.R.; Pereira, R.M.R.; Artioli, G.G.; Phillips, S.M.; Roschel, H. High-Protein Plant-Based Diet Versus a Protein-Matched Omnivorous Diet to Support Resistance Training Adaptations: A Comparison Between Habitual Vegans and Omnivores. Sports Med. 2021, 51, 1317–1330. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Suárez, V.J.; Bustamante-Sanchez, Á.; Mielgo-Ayuso, J.; Martínez-Guardado, I.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Antioxidants and Sports Performance. Nutrients 2023, 15, 2371. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.S.; Calle, M.; Fernandez, M.L. Healthy Plant-Based Diets Improve Dyslipidemias, Insulin Resistance, and Inflammation in Metabolic Syndrome. A Narrative Review. Adv. Nutr. 2023, 14, 44–54. [Google Scholar] [CrossRef]
- Chai, B.; Gao, F.; Wu, R.; Dong, T.; Gu, C.; Lin, Q.; Zhang, Y. Vitamin D Deficiency as a Risk Factor for Dementia and Alzheimer’s Disease: An Updated Meta-Analysis. BMC Neurol. 2019, 19, 284. [Google Scholar] [CrossRef]
- Müller, P. Vegan Diet in Young Children. Nestle Nutr. Inst. Workshop Ser. 2020, 93, 103–110. [Google Scholar] [CrossRef]
- Haghighatdoost, F.; Mahdavi, A.; Mohammadifard, N.; Hassannejad, R.; Najafi, F.; Farshidi, H.; Lotfizadeh, M.; Kazemi, T.; Karimi, S.; Roohafza, H.; et al. The Relationship between a Plant-Based Diet and Mental Health: Evidence from a Cross-Sectional Multicentric Community Trial (LIPOKAP Study). PLoS ONE 2023, 18, e0284446. [Google Scholar] [CrossRef]
- Williams, K.A.; Patel, H. Healthy Plant-Based Diet: What Does It Really Mean?*. J. Am. Coll. Cardiol. 2017, 70, 423–425. [Google Scholar] [CrossRef]
- Fazelian, S.; Sadeghi, E.; Firouzi, S.; Haghighatdoost, F. Adherence to the Vegetarian Diet May Increase the Risk of Depression: A Systematic Review and Meta-Analysis of Observational Studies. Nutr. Rev. 2022, 80, 242–254. [Google Scholar] [CrossRef]
- Jin, Y.; Kandula, N.R.; Kanaya, A.M.; Talegawkar, S.A. Vegetarian Diet Is Inversely Associated with Prevalence of Depression in Middle-Older Aged South Asians in the United States. Ethn. Health 2021, 26, 504–511. [Google Scholar] [CrossRef]
- Hibbeln, J.R.; Northstone, K.; Evans, J.; Golding, J. Vegetarian Diets and Depressive Symptoms among Men. J. Affect. Disord. 2018, 225, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Baş, M.; Karabudak, E.; Kiziltan, G. Vegetarianism and Eating Disorders: Association between Eating Attitudes and Other Psychological Factors among Turkish Adolescents. Appetite 2005, 44, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Larsuphrom, P.; Degremont, A.; Latunde-Dada, G.O.; Philippou, E. Association between Vegetarian and Vegan Diets and Depression: A Systematic Review. Nutr. Bull. 2022, 47, 27–49. [Google Scholar] [CrossRef] [PubMed]
- Walsh, H.; Lee, M.; Best, T. The Association between Vegan, Vegetarian, and Omnivore Diet Quality and Depressive Symptoms in Adults: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2023, 20, 3258. [Google Scholar] [CrossRef]
- Li, X.; Cao, H.-J.; Xie, S.-Y.; Li, K.-C.; Tao, F.-B.; Yang, L.-S.; Zhang, J.-Q.; Bao, Y.-S. Adhering to a Vegetarian Diet May Create a Greater Risk of Depressive Symptoms in the Elderly Male Chinese Population. J. Affect. Disord. 2019, 243, 182–187. [Google Scholar] [CrossRef]
- Shen, Y.-C.; Chang, C.-E.; Lin, M.-N.; Lin, C.-L. Vegetarian Diet Is Associated with Lower Risk of Depression in Taiwan. Nutrients 2021, 13, 1059. [Google Scholar] [CrossRef]
- Matta, J.; Czernichow, S.; Kesse-Guyot, E.; Hoertel, N.; Limosin, F.; Goldberg, M.; Zins, M.; Lemogne, C. Depressive Symptoms and Vegetarian Diets: Results from the Constances Cohort. Nutrients 2018, 10, 1695. [Google Scholar] [CrossRef]
- Lee, M.F.; Eather, R.; Best, T. Plant-Based Dietary Quality and Depressive Symptoms in Australian Vegans and Vegetarians: A Cross-Sectional Study. BMJ Nutr. Prev. Health 2021, 4, 479–486. [Google Scholar] [CrossRef]
- Katonova, A.; Sheardova, K.; Amlerova, J.; Angelucci, F.; Hort, J. Effect of a Vegan Diet on Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 14924. [Google Scholar] [CrossRef]
- Nichols, E.; Steinmetz, J.D.; Vollset, S.E.; Fukutaki, K.; Chalek, J.; Abd-Allah, F.; Abdoli, A.; Abualhasan, A.; Abu-Gharbieh, E.; Akram, T.T.; et al. Estimation of the Global Prevalence of Dementia in 2019 and Forecasted Prevalence in 2050: An Analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022, 7, e105–e125. [Google Scholar] [CrossRef]
- Anderson, R.M.; Hadjichrysanthou, C.; Evans, S.; Wong, M.M. Why Do so Many Clinical Trials of Therapies for Alz-Heimer’s Disease Fail? Lancet 2017, 390, 2327–2329. [Google Scholar] [CrossRef] [PubMed]
- Chrysohoou, C.; Panagiotakos, D.B.; Pitsavos, C.; Das, U.N.; Stefanadis, C. Adherence to the Mediterranean Diet Attenuates Inflammation and Coagulation Process in Healthy Adults. J. Am. Coll. Cardiol. 2004, 44, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Tosti, V.; Bertozzi, B.; Fontana, L. Health Benefits of the Mediterranean Diet: Metabolic and Molecular Mechanisms. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Chareonrungrueangchai, K.; Wongkawinwoot, K.; Anothaisintawee, T.; Reutrakul, S. Dietary Factors and Risks of Cardiovascular Diseases: An Umbrella Review. Nutrients 2020, 12, 1088. [Google Scholar] [CrossRef]
- Neufingerl, N.; Eilander, A. Nutrient Intake and Status in Adults Consuming Plant-Based Diets Compared to Meat-Eaters: A Systematic Review. Nutrients 2021, 14, 29. [Google Scholar] [CrossRef]
- Jiang, X.; Huang, J.; Song, D.; Deng, R.; Wei, J.; Zhang, Z. Increased Consumption of Fruit and Vegetables Is Related to a Reduced Risk of Cognitive Impairment and Dementia: Meta-Analysis. Front. Aging Neurosci. 2017, 9, 18. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, J.; Cao, L.; Shi, M.; Liu, H.; Zhao, Y.; Xia, Y. Fruit and Vegetable Consumption and Cognitive Disorders in Older Adults: A Meta-Analysis of Observational Studies. Front. Nutr. 2022, 9, 871061. [Google Scholar] [CrossRef]
- Weikert, C.; Trefflich, I.; Menzel, J.; Obeid, R.; Longree, A.; Dierkes, J.; Meyer, K.; Herter-Aeberli, I.; Mai, K.; Stangl, G.I.; et al. Vitamin and Mineral Status in a Vegan Diet. Dtsch. Arztebl. Int. 2020, 117, 575–582. [Google Scholar] [CrossRef]
- McCarty, M.F. Does a Vegan Diet Reduce Risk for Parkinson’s Disease? Med. Hypotheses 2001, 57, 318–323. [Google Scholar] [CrossRef]
- Obesity, Diabetes, and Risk of Parkinson’s Disease—Palacios—2011—Movement Disorders—Wiley Online Library. Available online: https://movementdisorders.onlinelibrary.wiley.com/doi/abs/10.1002/mds.23855 (accessed on 3 December 2024).
- Kivipelto, M.; Helkala, E.-L.; Laakso, M.P.; Hänninen, T.; Hallikainen, M.; Alhainen, K.; Iivonen, S.; Mannermaa, A.; Tuomilehto, J.; Nissinen, A.; et al. Apolipoprotein E Ε4 Allele, Elevated Midlife Total Cholesterol Level, and High Midlife Systolic Blood Pressure Are Independent Risk Factors for Late-Life Alzheimer Disease. Ann. Intern. Med. 2002, 137, 149–155. [Google Scholar] [CrossRef]
- Profenno, L.A.; Porsteinsson, A.P.; Faraone, S.V. Meta-Analysis of Alzheimer’s Disease Risk with Obesity, Diabetes, and Related Disorders. Biol. Psychiatry 2010, 67, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Gao, J.; Zhu, M.; Liu, K.; Zhang, H.-L. Gut Microbiota and Dysbiosis in Alzheimer’s Disease: Implications for Pathogenesis and Treatment. Mol. Neurobiol. 2020, 57, 5026–5043. [Google Scholar] [CrossRef] [PubMed]
- Kohnert, E.; Kreutz, C.; Binder, N.; Hannibal, L.; Gorkiewicz, G.; Müller, A.; Storz, M.A.; Huber, R.; Lederer, A.-K. Changes in Gut Microbiota after a Four-Week Intervention with Vegan vs. Meat-Rich Diets in Healthy Participants: A Randomized Controlled Trial. Microorganisms 2021, 9, 727. [Google Scholar] [CrossRef] [PubMed]
- Losno, E.A.; Sieferle, K.; Perez-Cueto, F.J.A.; Ritz, C. Vegan Diet and the Gut Microbiota Composition in Healthy Adults. Nutrients 2021, 13, 2402. [Google Scholar] [CrossRef]
- Mehta, V.; Parashar, A.; Udayabanu, M. Quercetin Prevents Chronic Unpredictable Stress Induced Behavioral Dysfunction in Mice by Alleviating Hippocampal Oxidative and Inflammatory Stress. Physiol. Behav. 2017, 171, 69–78. [Google Scholar] [CrossRef]
- Kahleova, H.; Petersen, K.F.; Shulman, G.I.; Alwarith, J.; Rembert, E.; Tura, A.; Hill, M.; Holubkov, R.; Barnard, N.D. Effect of a Low-Fat Vegan Diet on Body Weight, Insulin Sensitivity, Postprandial Metabolism, and Intramyocellular and Hepatocellular Lipid Levels in Overweight Adults: A Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e2025454. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Martín-Rodríguez, A.; Redondo-Flórez, L.; López-Mora, C.; Yáñez-Sepúlveda, R.; Tornero-Aguilera, J.F. New Insights and Potential Therapeutic Interventions in Metabolic Diseases. Int. J. Mol. Sci. 2023, 24, 10672. [Google Scholar] [CrossRef]
- Miao, Z.; Du, W.; Xiao, C.; Su, C.; Gou, W.; Shen, L.; Zhang, J.; Fu, Y.; Jiang, Z.; Wang, Z.; et al. Gut Microbiota Signatures of Long-Term and Short-Term Plant-Based Dietary Pattern and Cardiometabolic Health: A Prospective Cohort Study. BMC Med. 2022, 20, 204. [Google Scholar] [CrossRef]
- Anbari-Nogyni, Z.; Bidaki, R.; Madadizadeh, F.; Sangsefidi, Z.S.; Fallahzadeh, H.; Karimi-Nazari, E.; Nadjarzadeh, A. Relationship of Zinc Status with Depression and Anxiety among Elderly Population. Clin. Nutr. ESPEN 2020, 37, 233–239. [Google Scholar] [CrossRef]
- Selinger, E.; Kühn, T.; Procházková, M.; Anděl, M.; Gojda, J. Vitamin B12 Deficiency Is Prevalent Among Czech Vegans Who Do Not Use Vitamin B12 Supplements. Nutrients 2019, 11, 3019. [Google Scholar] [CrossRef]
- Lederer, A.-K.; Hannibal, L.; Hettich, M.; Behringer, S.; Spiekerkoetter, U.; Steinborn, C.; Gründemann, C.; Zimmermann-Klemd, A.M.; Müller, A.; Simmet, T.; et al. Vitamin B12 Status Upon Short-Term Intervention with a Vegan Diet—A Randomized Controlled Trial in Healthy Participants. Nutrients 2019, 11, 2815. [Google Scholar] [CrossRef] [PubMed]
- Scalabrino, G. The Multi-Faceted Basis of Vitamin B12 (Cobalamin) Neurotrophism in Adult Central Nervous System: Lessons Learned from Its Deficiency. Prog. Neurobiol. 2009, 88, 203–220. [Google Scholar] [CrossRef] [PubMed]
- Tangney, C.C.; Aggarwal, N.T.; Li, H.; Wilson, R.S.; DeCarli, C.; Evans, D.A.; Morris, M.C. Vitamin B12, Cognition, and Brain MRI Measures. Neurology 2011, 77, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- de Jager, C.A.; Oulhaj, A.; Jacoby, R.; Refsum, H.; Smith, A.D. Cognitive and Clinical Outcomes of Homocysteine-Lowering B-Vitamin Treatment in Mild Cognitive Impairment: A Randomized Controlled Trial. Int. J. Geriatr. Psychiatry 2012, 27, 592–600. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, J.; Chang, H.; Liu, X.; Zhu, R. Homocysteine and Folic Acid: Risk Factors for Alzheimer’s Disease—An Updated Meta-Analysis. Front. Aging Neurosci. 2021, 13, 665114. [Google Scholar] [CrossRef]
- Mavraki, E.; Ioannidis, P.; Tripsianis, G.; Gioka, T.; Kolousi, M.; Vadikolias, K. Vitamin D in Mild Cognitive Impairment and Alzheimer’s Disease. A Study Older Greek Adults. Hippokratia 2020, 24, 120–126. [Google Scholar]
- Annweiler, C.; Llewellyn, D.J.; Beauchet, O. Low Serum Vitamin D Concentrations in Alzheimer’s Disease: A Systematic Review and Meta-Analysis. J. Alzheimers Dis. 2013, 33, 659–674. [Google Scholar] [CrossRef]
- Full Article: Bioavailability and Conversion of Plant Based Sources of Omega-3 Fatty Acids—A Scoping Review to Update Supplementation Options for Vegetarians and Vegans. Available online: https://www.tandfonline.com/doi/full/10.1080/10408398.2021.1880364 (accessed on 3 December 2024).
- Freund-Levi, Y.; Eriksdotter-Jönhagen, M.; Cederholm, T.; Basun, H.; Faxén-Irving, G.; Garlind, A.; Vedin, I.; Vessby, B.; Wahlund, L.-O.; Palmblad, J. ω-3 Fatty Acid Treatment in 174 Patients With Mild to Moderate Alzheimer Disease: OmegAD Study: A Randomized Double-Blind Trial. Arch. Neurol. 2006, 63, 1402–1408. [Google Scholar] [CrossRef]


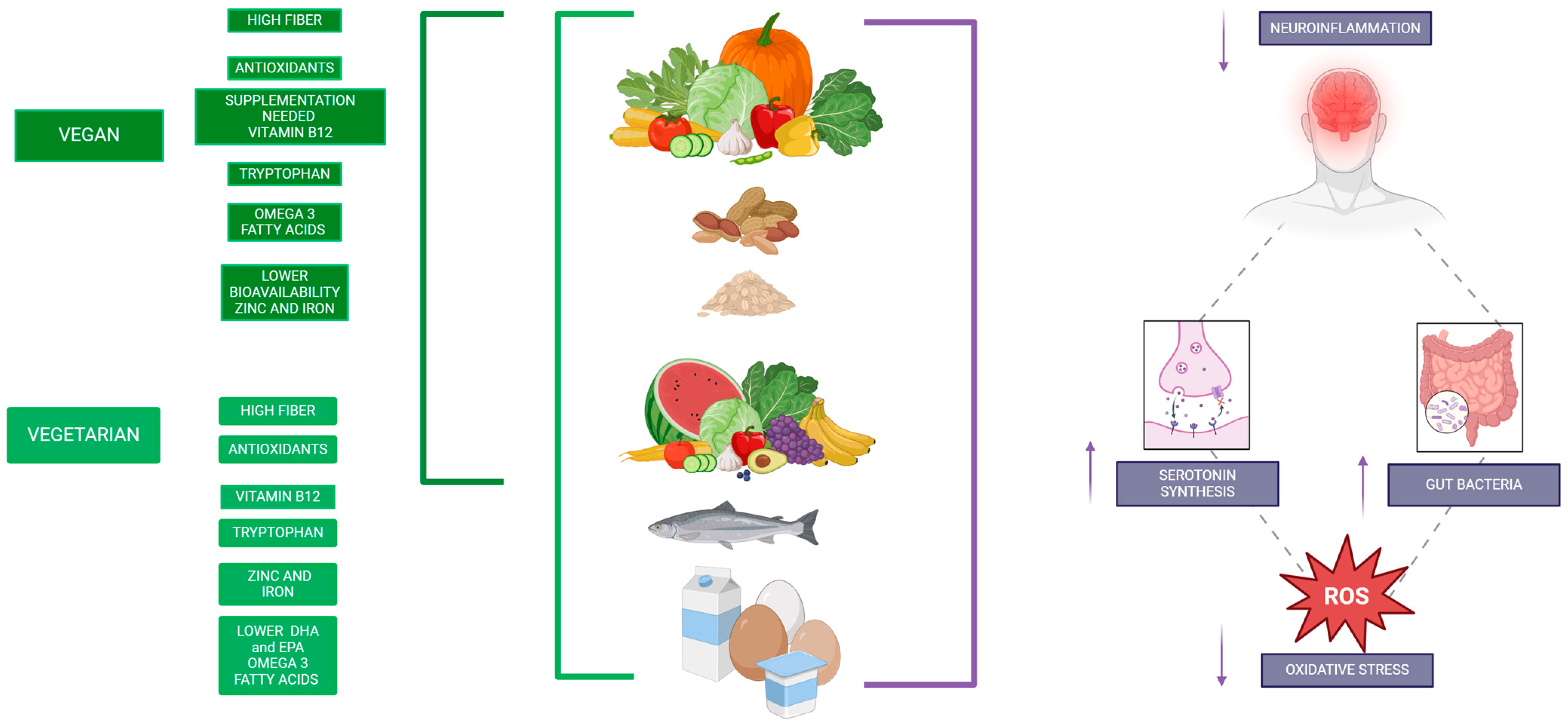
| Category | Strategy | Key Recommendations |
|---|---|---|
| Supplementation | Vitamin B12 | - Essential for all vegans/vegetarians (absent in unfortified plant-based foods). - Use supplements or fortified foods (plant-based milks, breakfast cereals, nutritional yeast) and regular monitoring to prevent deficiencies linked to cognitive decline, mood disorders, and neuropathy. |
| Omega-3 Fatty Acids (DHA/EPA) | - Use algae-based supplements, the most effective and sustainable option, critical for maintaining neuronal integrity, synaptic plasticity, and reducing neuroinflammation. | |
| Iron and Zinc | - Non-heme iron and zinc from plant sources have lower bioavailability: pairing with vitamin C can enhance absorption. Iron supplementation is important for those at risk of anemia, while zinc supports immune function and cognitive processes. | |
| Dietary Strategies | Emphasize Whole Nutrient-Dense Foods | - Focus on ALA-rich foods (flaxseeds, chia seeds, hemp seeds, walnuts) as precursors to omega-3 fatty acids. - Incorporate antioxidant-rich fruits and vegetables (e.g., berries, leafy greens) to combat oxidative stress and support neurological health. |
| Improve Mineral Bioavailability | - Utilize methods such as soaking, sprouting, and fermenting to reduce antinutrients (e.g., phytates). - Pair iron-rich foods (e.g., legumes, tofu) with vitamin C-rich options (e.g., bell peppers, citrus fruits) to optimize nutrient uptake. | |
| Include Protein Variety | - Ensure a diverse intake of plant-based proteins (e.g., lentils, quinoa, soy products) to provide a complete amino acid profile and support neurotransmitter production. | |
| Fortified Foods and Monitoring | Incorporate Fortified Products | - Regularly consume fortified foods containing B12, calcium, iodine, and other critical nutrients (e.g., fortified plant-based milks, cereals, iodized salt). |
| Regular Biomarker Assessments | - Periodically test levels of key biomarkers (B12, DHA/EPA, iron, zinc). Monitor cognitive function (e.g., memory and executive processing) to identify early signs of nutrient deficiencies, particularly in high-risk groups (pregnant individuals, children, older adults). | |
| Education and Awareness | Nutritional Guidance | - Provide comprehensive education on balanced vegan and vegetarian diets, including meal planning, proper supplementation, and food preparation techniques. - Offer tailored advice for specific populations (e.g., athletes, children, older adults) who may have heightened nutrient requirements. |
| Community and Healthcare Involvement | - Encourage collaboration with healthcare providers to develop personalized dietary plans and ensure ongoing support for individuals transitioning to or maintaining plant-based diets. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clemente-Suárez, V.J.; Redondo-Flórez, L.; Martín-Rodríguez, A.; Curiel-Regueros, A.; Rubio-Zarapuz, A.; Tornero-Aguilera, J.F. Impact of Vegan and Vegetarian Diets on Neurological Health: A Critical Review. Nutrients 2025, 17, 884. https://doi.org/10.3390/nu17050884
Clemente-Suárez VJ, Redondo-Flórez L, Martín-Rodríguez A, Curiel-Regueros A, Rubio-Zarapuz A, Tornero-Aguilera JF. Impact of Vegan and Vegetarian Diets on Neurological Health: A Critical Review. Nutrients. 2025; 17(5):884. https://doi.org/10.3390/nu17050884
Chicago/Turabian StyleClemente-Suárez, Vicente Javier, Laura Redondo-Flórez, Alexandra Martín-Rodríguez, Agustín Curiel-Regueros, Alejandro Rubio-Zarapuz, and José Francisco Tornero-Aguilera. 2025. "Impact of Vegan and Vegetarian Diets on Neurological Health: A Critical Review" Nutrients 17, no. 5: 884. https://doi.org/10.3390/nu17050884
APA StyleClemente-Suárez, V. J., Redondo-Flórez, L., Martín-Rodríguez, A., Curiel-Regueros, A., Rubio-Zarapuz, A., & Tornero-Aguilera, J. F. (2025). Impact of Vegan and Vegetarian Diets on Neurological Health: A Critical Review. Nutrients, 17(5), 884. https://doi.org/10.3390/nu17050884








