The Impact of Tartrazine on DNA Methylation, Histone Deacetylation, and Genomic Stability in Human Cell Lines
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Tartrazine Treatment
2.3. RNA Extraction and Quantitative Real-Time PCR Analysis
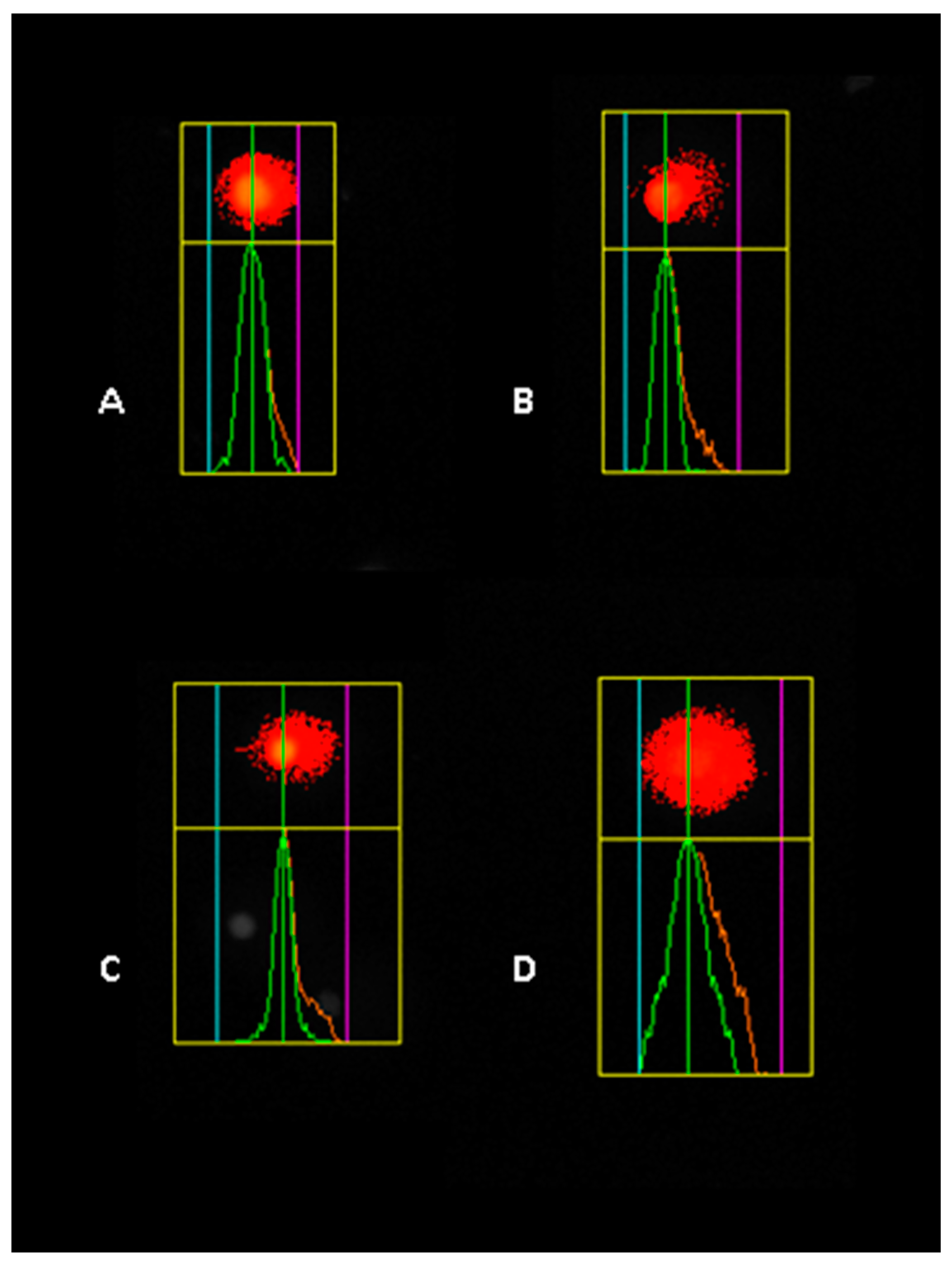
2.4. Assessment of Genomic DNA Stability
2.5. Statistical Analysis
3. Results
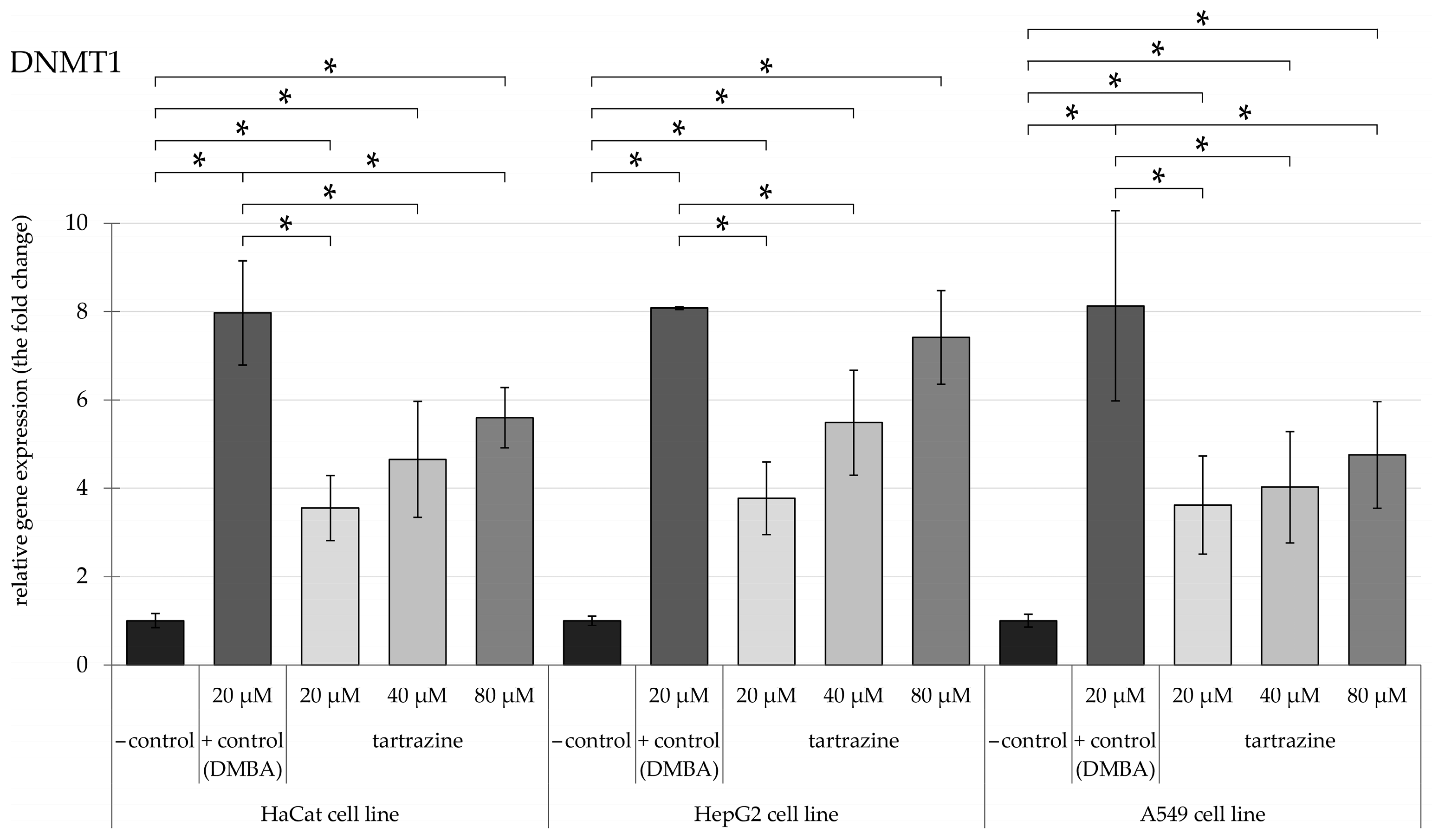
3.1. TRZ Exposure Significantly Upregulates DNMT1, DNMT3a, and DNMT3b Gene Expression in a Dose-Dependent Manner Across HaCaT, HepG2, and A549 Cell Lines
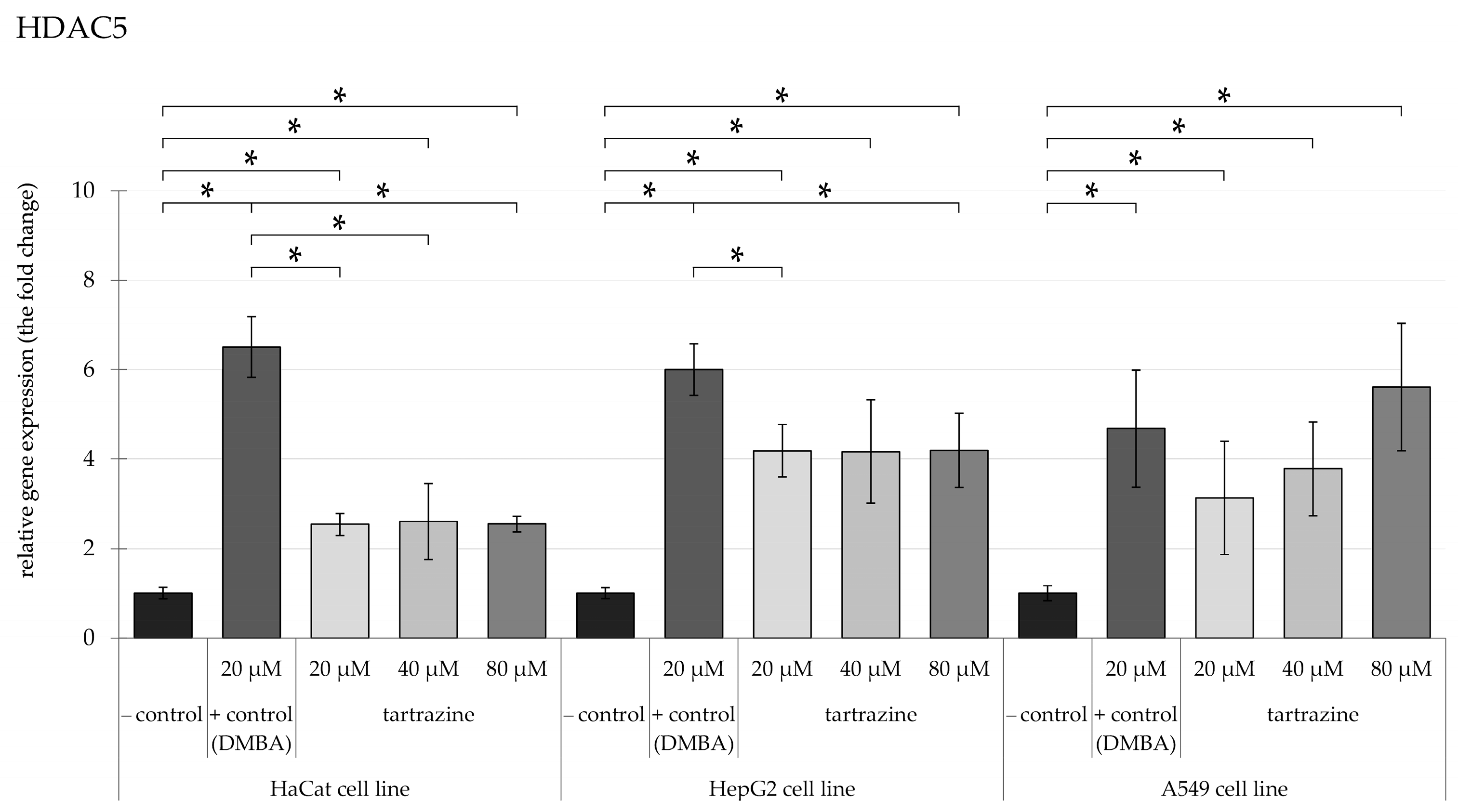
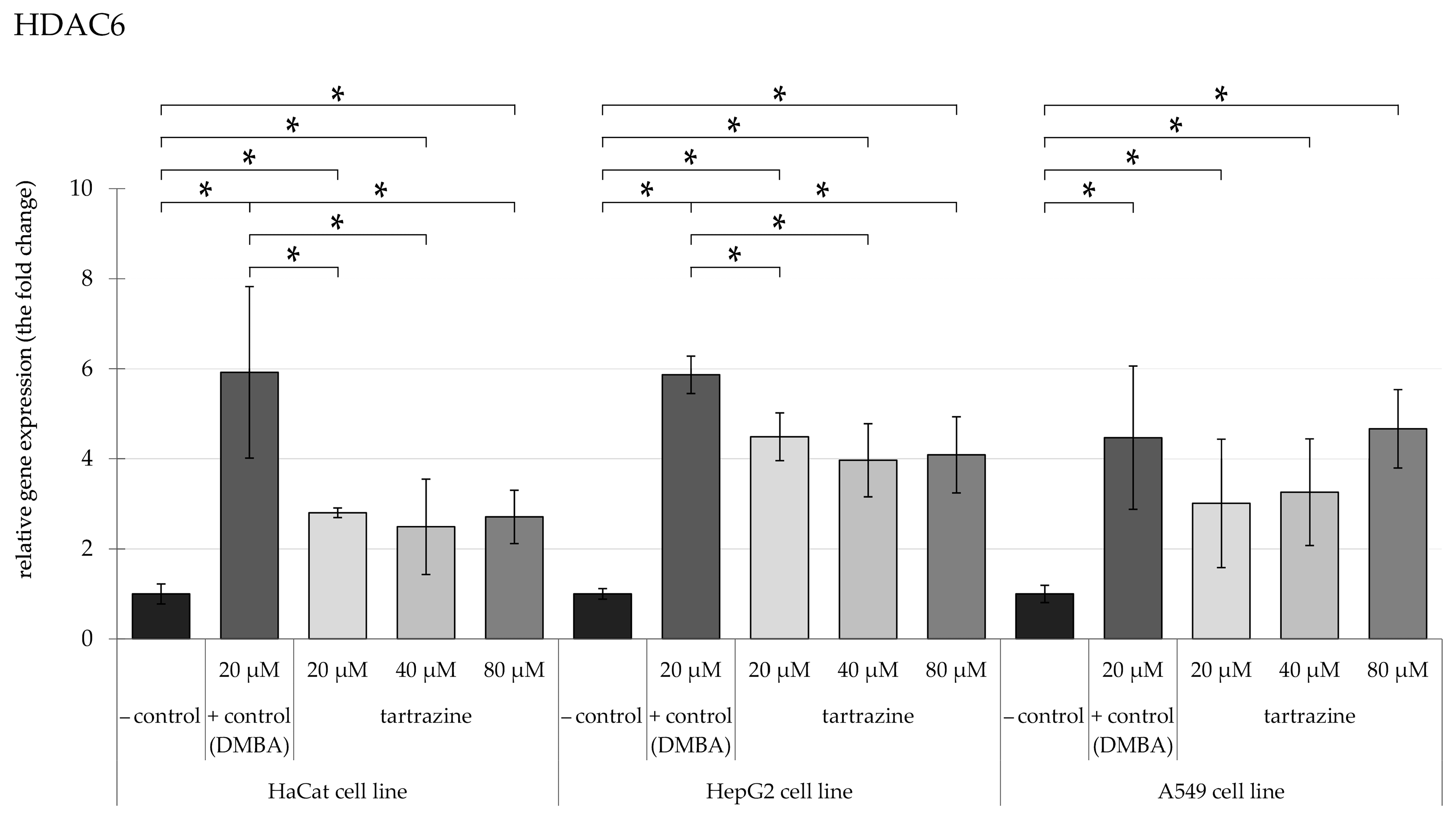
3.2. TRZ Exposure Induces Significant Overexpression of HDAC5 and HDAC6 Genes in HaCaT, HepG2, and A549 Cell Lines
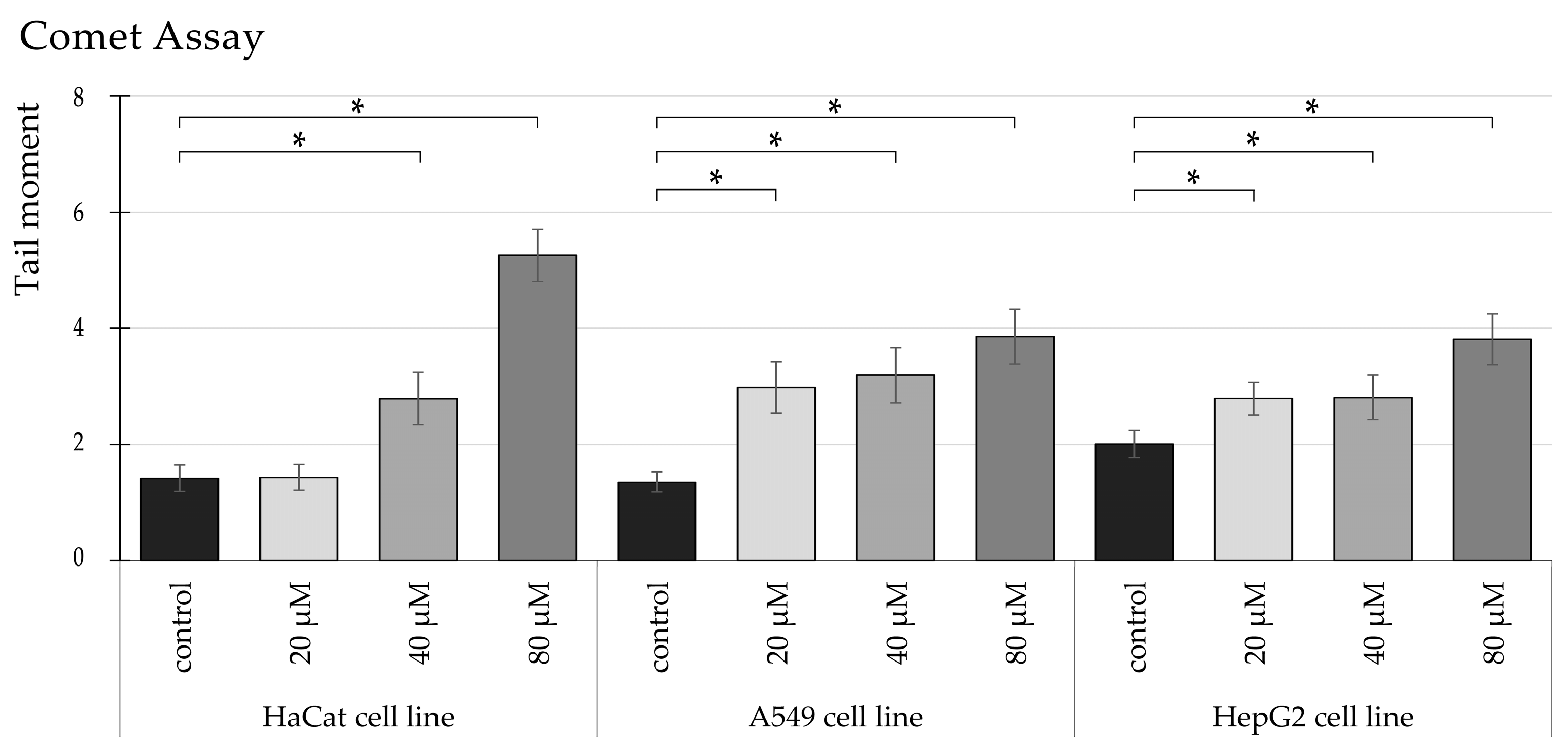
3.3. TRZ Induces Dose-Dependent DNA Damage in HaCaT, A549, and HepG2 Cell Lines
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADI | linear dichroism |
| EFSA | European Food Safety Authority |
| FDA | Food and Drug Administration |
| A549 | human lung adenocarcinoma |
| Ach | acetylcholine |
| ALT | alanine aminotransferase |
| AST | aspartate aminotransferase |
| Cox-2 | cyclooxygenase-2 |
| DMBA | dimethyl-benzanthracene |
| DMEM | Dulbecco’s altered Eagle’s medium |
| DMSO | dimethyl sulfoxide |
| DNMT | DNA methyltransferases |
| DNMT1 | DNA methyltransferases 1 |
| DNMT3 | DNA methyltransferases 3b |
| DNMT3a | DNA methyltransferases 3a |
| EGCG | (−)-epigallocatechin-3-gallate |
| FBS | fetal bovine serum |
| GABA | gamma-aminobutyric acid |
| GSH | glutathione |
| HaCaT | immortalized human keratinocyte |
| HepG2 | human hepatocellular carcinoma |
| HPRT1 | hypoxanthine-guanine Phosphoribosyl transferase 1 |
| MDA | malondialdehyde |
| NOAEL | No observed adverse effect level |
| ROS | reactive oxygen species |
| RT-qPCR | reverse transcription-quantitative polymerase chain reaction |
| TRZ | tartrazine |
| TSGs | tumor suppressor gene |
References
- Color Additives in Foods|FDA. Available online: https://www.fda.gov/food/color-additives-information-consumers/color-additives-foods (accessed on 18 March 2024).
- EFSA Panel on Food Additives and Nutrient Sources AddTives Nutrient Sources Added to Food. Scientific Opinion on the Re-Evaluation Tartrazine (E 102). EFSA J. 2009, 7, 1331. [Google Scholar] [CrossRef]
- Leo, L.; Loong, C.; Ho, X.L.; Raman, M.F.B.; Suan, M.Y.T.; Loke, W.M. Occurrence of Azo Food Dyes and Their Effects on Cellular Inflammatory Responses. Nutrition 2018, 46, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Revankar, M.S.; Lele, S.S. Synthetic Dye Decolorization by White Rot Fungus, Ganoderma Sp. WR-1. Bioresour. Technol. 2007, 98, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Amin, K.A.; Al-Shehri, F.S. Toxicological and Safety Assessment of Tartrazine as a Synthetic Food Additive on Health Biomarkers: A Review. Afr. J. Biotechnol. 2018, 17, 139–149. [Google Scholar] [CrossRef]
- Alsalman, N.; Aljafari, A.; Wani, T.A.; Zargar, S. High-Dose Aspirin Reverses Tartrazine-Induced Cell Growth Dysregulation Independent of P53 Signaling and Antioxidant Mechanisms in Rat Brain. BioMed Res. Int. 2019, 2019, 9096404. [Google Scholar] [CrossRef]
- Meyer, S.K.; Probert, P.M.E.; Lakey, A.F.; Axon, A.R.; Leitch, A.C.; Williams, F.M.; Jowsey, P.A.; Blain, P.G.; Kass, G.E.N.; Wright, M.C. Hepatic Effects of Tartrazine (E 102) after Systemic Exposure Are Independent of Oestrogen Receptor Interactions in the Mouse. Toxicol. Lett. 2017, 273, 55–68. [Google Scholar] [CrossRef]
- Amin, K.A.; Abdel Hameid, H.; Abd Elsttar, A.H. Effect of Food Azo Dyes Tartrazine and Carmoisine on Biochemical Parameters Related to Renal, Hepatic Function and Oxidative Stress Biomarkers in Young Male Rats. Food Chem. Toxicol. 2010, 48, 2994–2999. [Google Scholar] [CrossRef]
- Moutinho, I.L.D.; Bertges, L.C.; Assis, R.V.C. Prolonged Use of the Food Dye Tartrazine (FD&C Yellow N° 5) and Its Effects on the Gastric Mucosa of Wistar Rats. Braz. J. Biol. 2007, 67, 141–145. [Google Scholar] [CrossRef]
- Chung, K. Mutagenicity and Carcinogenicity of Aromatic Amines Metabolically Produced from Azo Dyes. J. Environ. Sci. Health Part C 2000, 18, 51–74. [Google Scholar] [CrossRef]
- Walton, K.; Walker, R.; Van De Sandt, J.J.M.; Castell, J.V.; Knapp, A.G.A.A.; Kozianowski, G.; Roberfroid, M.; Schilter, B. The Application of in Vitro Data in the Derivation of the Acceptable Daily Intake of Food Additives. Food Chem. Toxicol. 1999, 37, 1175–1197. [Google Scholar] [CrossRef]
- Ardern, K.; Ram, F.S.F.; Lasserson, T.J. Tartrazine Exclusion for Allergic Asthma. Cochrane Database Syst. Rev. 2001, 2001, CD000460. [Google Scholar] [CrossRef]
- Mohamed, A.A.R.; Galal, A.A.A.; Elewa, Y.H.A. Comparative Protective Effects of Royal Jelly and Cod Liver Oil against Neurotoxic Impact of Tartrazine on Male Rat Pups Brain. Acta Histochem. 2015, 117, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Joint FAO/WHO Expert Committee on Food Additives. Evaluation of Certain Food Additives. World Health Organ. Technol. Rep. Ser. 2009, 1–208. [Google Scholar]
- Sasaki, Y.F.; Kawaguchi, S.; Kamaya, A.; Ohshita, M.; Kabasawa, K.; Iwama, K.; Taniguchi, K.; Tsuda, S. The Comet Assay with 8 Mouse Organs: Results with 39 Currently Used Food Additives. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2002, 519, 103–119. [Google Scholar] [CrossRef] [PubMed]
- McCann, D.; Barrett, A.; Cooper, A.; Crumpler, D.; Dalen, L.; Grimshaw, K.; Kitchin, E.; Lok, K.; Porteous, L.; Prince, E.; et al. Food Additives and Hyperactive Behaviour in 3-Year-Old and 8/9-Year-Old Children in the Community: A Randomised, Double-Blinded, Placebo-Controlled Trial. Lancet 2007, 370, 1560–1567. [Google Scholar] [CrossRef]
- Novembre, E.; Dini, L.; Bernardini, R.; Resti, M.; Vierucci, A. Manifestazioni cliniche “inusuali” da additivi alimentari. [Unusual Reactions to Food Additives]. Pediatr. Med. Chir. 1992, 14, 39–42. (In Italian) [Google Scholar]
- Bhatia, M.S. Allergy to Tartrazine in Psychotropic Drugs. J. Clin. Psychiatry 2000, 61, 473–476. [Google Scholar] [CrossRef]
- Nettis, E.; Colanardi, M.; Ferrannini, A.; Tursi, A. Suspected Tartrazine—Induced Acute Urticaria/Angioedema Is Only Rarely Reproducible by Oral Rechallenge. Clin. Exp. Allergy 2003, 33, 1725–1729. [Google Scholar] [CrossRef]
- Worm, M.; Vieth, W.; Ehlers, I.; Sterry, W.; Zuberbier, T. Increased Leukotriene Production by Food Additives in Patients with Atopic Dermatitis and Proven Food Intolerance. Clin. Exp. Allergy 2001, 31, 265–273. [Google Scholar] [CrossRef]
- Inomata, N.; Osuna, H.; Fujita, H.; Ogawa, T.; Ikezawa, Z. Multiple Chemical Sensitivities Following Intolerance to Azo Dye in Sweets in a 5-Year-Old Girl. Allergol. Int. 2006, 55, 203–205. [Google Scholar] [CrossRef][Green Version]
- Khayyat, L.; Essawy, A.; Sorour, J.; Soffar, A. Tartrazine Induces Structural and Functional Aberrations and Genotoxic Effects in Vivo. PeerJ 2017, 5, e3041. [Google Scholar] [CrossRef]
- dos Santos, J.R.; de Sousa Soares, L.; Soares, B.M.; de Gomes Farias, M.; de Oliveira, V.A.; de Sousa, N.A.B.; Negreiros, H.A.; da Silva, F.C.C.; Peron, A.P.; Pacheco, A.C.L.; et al. Cytotoxic and Mutagenic Effects of the Food Additive Tartrazine on Eukaryotic Cells. BMC Pharmacol. Toxicol. 2022, 23, 95. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, C.; Shen, J.; Yin, H.; An, X.; Jin, H. Effect of Food Azo Dye Tartrazine on Learning and Memory Functions in Mice and Rats, and the Possible Mechanisms Involved. J. Food Sci. 2011, 76, T125–T129. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.W.; Turnbull, D.M. Mitochondrial DNA Mutations in Human Disease. Nat. Rev. Genet. 2005, 6, 389–402. [Google Scholar] [CrossRef]
- Esteller, M. Epigenetics in Cancer. N. Engl. J. Med. 2008, 358, 1148–1159. [Google Scholar] [CrossRef] [PubMed]
- Poh, W.J.; Wee, C.P.P.; Gao, Z. DNA Methyltransferase Activity Assays: Advances and Challenges. Theranostics 2016, 6, 369–391. [Google Scholar] [CrossRef]
- De Ruijter, A.J.M.; Van Gennip, A.H.; Caron, H.N.; Kemp, S.; Van Kuilenburg, A.B.P. Histone Deacetylases (HDACs): Characterization of the Classical HDAC Family. Biochem. J. 2003, 370, 737–749. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, J.S. A Short Guide to Histone Deacetylases Including Recent Progress on Class II Enzymes. Exp. Mol. Med. 2020, 52, 204–212. [Google Scholar] [CrossRef]
- Glozak, M.A.; Seto, E. Histone Deacetylases and Cancer. Oncogene 2007, 26, 5420–5432. [Google Scholar] [CrossRef]
- Şenkal, S.; Burukçu, D.; Bartu Hayal, T.; Kiratli, B.; Burcu Şişli, H.; Sağraç, D.; Burçin Asutay, A.; Sümer, E.; Şahin, F.; Doğan, A.; et al. 3D CULTURE OF HaCaT KERATINOCYTE CELL LINE AS AN in Vitro TOXICITY MODEL. Trak. Univ. J. Nat. Sci. 2022, 23, 211–220. [Google Scholar] [CrossRef]
- Aden, D.; Fogel, A.; Plotkin, S.; Damjanov, I.; Knowles, B.B. Controlled Synthesis of HBsAg in a Differentiated Human Liver Carcinoma-Derived Cell Line. Nature 1979, 282, 615–616. [Google Scholar] [CrossRef] [PubMed]
- Donato, M.T.; Tolosa, L.; Gómez-Lechón, M.J. Culture and Functional Characterization of Human Hepatoma HepG2 Cells. Methods Mol. Biol. 2015, 1250, 77–93. [Google Scholar] [CrossRef]
- Cooper, J.R.; Abdullatif, M.B.; Burnett, E.C.; Kempsell, K.E.; Conforti, F.; Tolley, H.; Collins, J.E.; Davies, D.E. Long Term Culture of the A549 Cancer Cell Line Promotes Multilamellar Body Formation and Differentiation towards an Alveolar Type II Pneumocyte Phenotype. PLoS ONE 2016, 11, e0164438. [Google Scholar] [CrossRef]
- Zand, A.; Enkhbilguun, S.; Macharia, J.M.; Varajti, K.; Szabó, I.; Gerencsér, G.; Tisza, B.B.; Raposa, B.L.; Gyöngyi, Z.; Varjas, T. Betanin Attenuates Epigenetic Mechanisms and UV-Induced DNA Fragmentation in HaCaT Cells: Implications for Skin Cancer Chemoprevention. Nutrients 2024, 16, 860. [Google Scholar] [CrossRef] [PubMed]
- Gene Quantification & Real Time PCR Quantification Strategy. Available online: https://www.gene-quantification.com/strategy.html (accessed on 18 February 2025).
- Hermann, A.; Gowher, H.; Jeltsch, A. Biochemistry and Biology of Mammalian DNA Methyltransferases. Cell. Mol. Life Sci. 2004, 61, 2571–2587. [Google Scholar] [CrossRef]
- Tuorto, F.; Herbst, F.; Alerasool, N.; Bender, S.; Popp, O.; Federico, G.; Reitter, S.; Liebers, R.; Stoecklin, G.; Gröne, H.; et al. The tRNA Methyltransferase Dnmt2 Is Required for Accurate Polypeptide Synthesis during Haematopoiesis. EMBO J. 2015, 34, 2350–2362. [Google Scholar] [CrossRef]
- Jurkowska, R.Z.; Anspach, N.; Urbanke, C.; Jia, D.; Reinhardt, R.; Nellen, W.; Cheng, X.; Jeltsch, A. Formation of Nucleoprotein Filaments by Mammalian DNA Methyltransferase Dnmt3a in Complex with Regulator Dnmt3L. Nucleic Acids Res. 2008, 36, 6656–6663. [Google Scholar] [CrossRef] [PubMed]
- Al-Seeni, M.; Rabey, H.E.; Al-Hamed, A.M.; Zamazami, M.A. Nigella sativa Oil Protects against Tartrazine Toxicity in Male Rats. Toxicol. Rep. 2017, 5, 146–155. [Google Scholar] [CrossRef]
- Saxena, B.; Sharma, S. Food Color Induced Hepatotoxicity in Swiss Albino Rats, Rattus Norvegicus. Toxicol Int. 2015, 22, 152–157. [Google Scholar] [CrossRef]
- Demirkol, O.; Zhang, X.; Ercal, N. Oxidative Effects of Tartrazine (CAS No. 1934-21-0) and New Coccin (CAS No. 2611-82-7) Azo Dyes on CHO Cells. J. Consum. Prot. Food Saf. 2012, 7, 229–236. [Google Scholar] [CrossRef]
- Shakoor, S.; Ismail, A.; Zia-Ur-Rahman; Sabran, M.R.; Mohtarrudin, N. Effect of Food Colorants Supplementation on Reactive Oxygen Species, Antioxidant Vitamins Level and DNA Damage. Sains Malays. 2021, 50, 1343–1356. [Google Scholar] [CrossRef]
- Prasad, R.; Katiyar, S.K. Prostaglandin E2 Promotes UV Radiation-Induced Immune Suppression through DNA Hypermethylation. Neoplasia 2013, 15, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Serup, J.; Kluger, N.; Bäumler, W. Absorption, Distribution, Metabolism and Excretion of Tattoo Colorants and Ingredients in Mouse and Man: The Known and the Unknown. Curr. Probl. Dermatol. 2015, 48, 176–184. [Google Scholar] [CrossRef]
- Wang, Q.; Liang, N.; Yang, T.; Li, Y.; Li, J.; Huang, Q.; Wu, C.; Sun, L.; Zhou, X.; Cheng, X.; et al. DNMT1-Mediated Methylation of BEX1 Regulates Stemness and Tumorigenicity in Liver Cancer. J. Hepatol. 2021, 75, 1142–1153. [Google Scholar] [CrossRef]
- Saito, Y.; Kanai, Y.; Nakagawa, T.; Sakamoto, M.; Saito, H.; Ishi, H.; Hirohashi, S. Increased Protein Expression of DNA Methyltransferase (DNMT) 1 Is Significantly Correlated with the Malignant Potential and Poor Prognosis of Human Hepatocellular Carcinomas. Int. J. Cancer 2003, 105, 527–532. [Google Scholar] [CrossRef]
- Lin, R.K.; Wu, C.Y.; Chang, J.W.; Juan, L.J.; Hsu, H.S.; Chen, C.Y.; Lu, Y.Y.; Tang, Y.A.; Yang, Y.C.; Yang, P.C.; et al. Dysregulation of P53/Sp1 Control Leads to DNA Methyltransferase-1 Overexpression in Lung Cancer. Cancer Res. 2010, 70, 5807–5817. [Google Scholar] [CrossRef]
- Micevic, G.; Theodosakis, N.; Bosenberg, M. Aberrant DNA Methylation in Melanoma: Biomarker and Therapeutic Opportunities. Clin. Epigenet. 2017, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, F.Q.; Sun, Y.H.; Zhou, S.Y.; Li, T.Y.; Chen, R. Effects of DNMT1 Silencing on Malignant Phenotype and Methylated Gene Expression in Cervical Cancer Cells. J. Exp. Clin. Cancer Res. 2011, 30, 98. [Google Scholar] [CrossRef]
- Li, Y.; Tollefsbol, T.O. Impact on DNA Methylation in Cancer Prevention and Therapy by Bioactive Dietary Components. Curr. Med. Chem. 2010, 17, 2141–2151. [Google Scholar] [CrossRef]
- Ragusa, M.A.; Naselli, F.; Cruciata, I.; Volpes, S.; Schimmenti, C.; Serio, G.; Mauro, M.; Librizzi, M.; Luparello, C.; Chiarelli, R.; et al. Indicaxanthin Induces Autophagy in Intestinal Epithelial Cancer Cells by Epigenetic Mechanisms Involving DNA Methylation. Nutrients 2023, 15, 3495. [Google Scholar] [CrossRef]
- Yang, H.; Salz, T.; Zajac-Kaye, M.; Liao, D.; Huang, S.; Qiu, Y. Overexpression of Histone Deacetylases in Cancer Cells Is Controlled by Interplay of Transcription Factors and Epigenetic Modulators. FASEB J. 2014, 28, 4265–4279. [Google Scholar] [CrossRef]
- Duarte, J.H. Inflammation Feeds Inflammation—HDAC5 Downregulation Leads to Activation of Fibroblast-like Synoviocytes in RA. Nat. Rev. Rheumatol. 2014, 11, 64. [Google Scholar] [CrossRef]
- Özdaǧ, H.; Teschendorff, A.E.; Ahmed, A.A.; Hyland, S.J.; Blenkiron, C.; Bobrow, L.; Veerakumarasivam, A.; Burtt, G.; Subkhankulova, T.; Arends, M.J.; et al. Differential Expression of Selected Histone Modifier Genes in Human Solid Cancers. BMC Genom. 2006, 7, 90. [Google Scholar] [CrossRef] [PubMed]
- Osada, H.; Tatematsu, Y.; Saito, H.; Yatabe, Y.; Mitsudomi, T.; Takahashi, T. Reduced Expression of Class II Histone Deacetylase Genes Is Associated with Poor Prognosis in Lung Cancer Patients. Int. J. Cancer 2004, 112, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Liu, Z.; Li, M.; Zhou, S.; Xu, Y.; Xiao, Y.; Yang, W. HDAC5, a Potential Therapeutic Target and Prognostic Biomarker, Promotes Proliferation, Invasion and Migration in Human Breast Cancer. Oncotarget 2016, 7, 37966–37978. [Google Scholar] [CrossRef]
- Collins, A.; Koppen, G.; Valdiglesias, V.; Dusinska, M.; Kruszewski, M.; Møller, P.; Rojas, E.; Dhawan, A.; Benzie, I.; Coskun, E.; et al. The Comet Assay as a Tool for Human Biomonitoring Studies: The ComNet Project. Mutat. Res. Rev. Mutat. Res. 2014, 759, 27–39. [Google Scholar] [CrossRef]
- Araldi, R.P.; de Melo, T.C.; Mendes, T.B.; de Sá Júnior, P.L.; Nozima, B.H.N.; Ito, E.T.; de Carvalho, R.F.; de Souza, E.B.; de Cassia Stocco, R. Using the Comet and Micronucleus Assays for Genotoxicity Studies: A Review. Biomed. Pharmacother. 2015, 72, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric Oxide and Peroxynitrite in Health and Disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef]
- Himri, I.; Souna, F.; Belmekki, F.; Aziz, M.; Bnouham, M.; Zoheir, J.; Berkia, Z.; Mekhfi, H.; Saalaoui, E. A 90 day Oral Toxicity Study of Tartrazine, a Synthetic Food Dye, in Wistar Rats. Int. J. Pharm. Pharm. Sci. 2011, 3 (Suppl. 3), 159–169. [Google Scholar]
- El-Desoky, G.E.; Wabaidur, S.M.; Alothman, Z.A.; Habila, M.A. Regulatory Role of Nano-Curcumin against Tartrazine-Induced Oxidative Stress, Apoptosis-Related Genes Expression, and Genotoxicity in Rats. Molecules 2020, 25, 5801. [Google Scholar] [CrossRef]
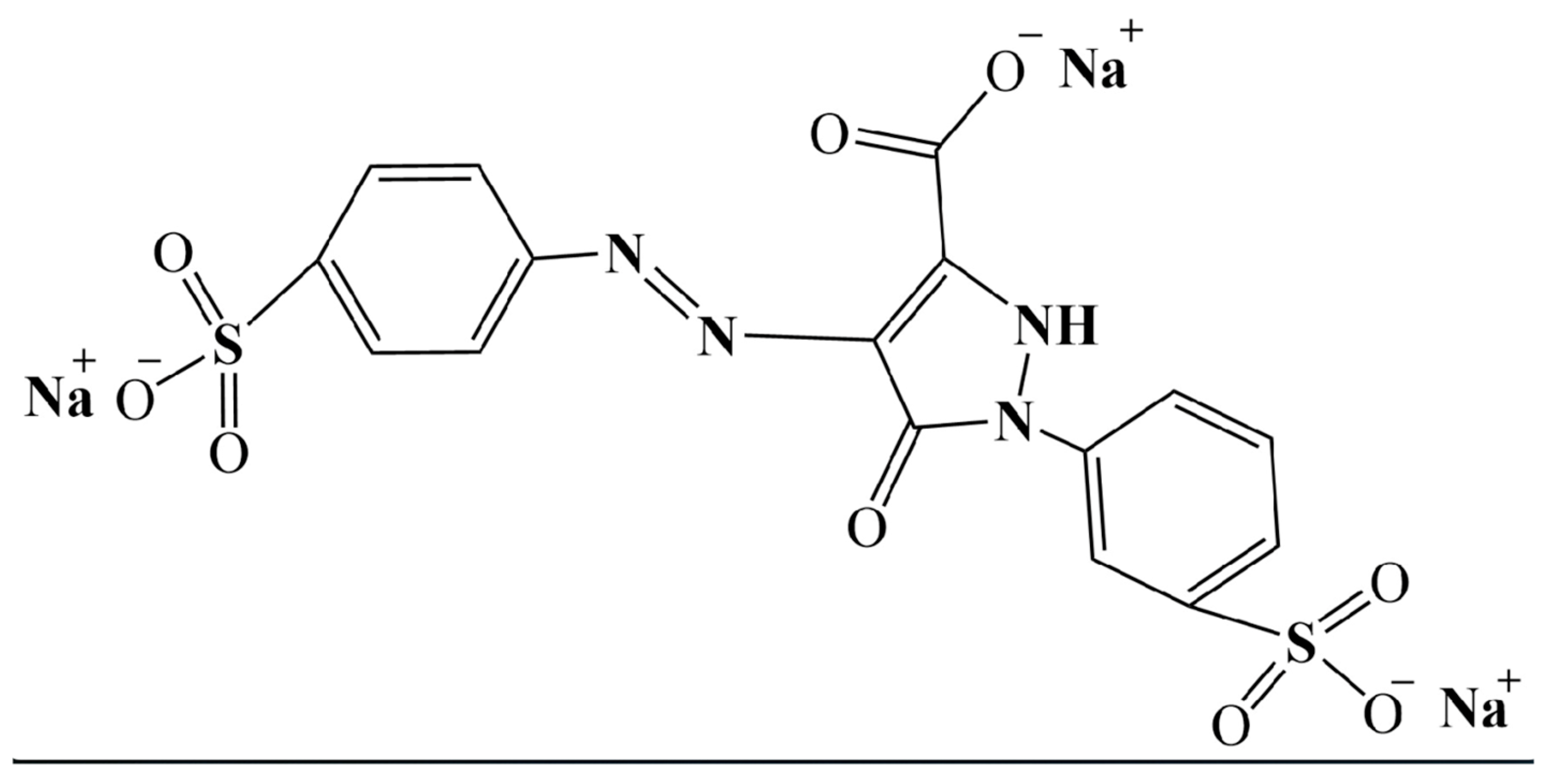




| Group ID | Group Name | Food Colorant Concentration (μM) | Description |
|---|---|---|---|
| 1 | Negative Control (Medium) | 0 | Baseline control group with no treatment |
| 2 | Treated Group 1 (Low Dose) | 20 | Low-concentration treatment group |
| 3 | Treated Group 2 (Medium Dose) | 40 | Medium concentration treatment group |
| 4 | Treated Group 3 (High Dose) | 80 | High-concentration treatment group |
| 5 | Positive Control (DMBA) | - | Control group treated with DMBA as a positive reference |
| 6 | Untreated Control (1% DMSO) | - | Control group treated with DMSO as a solvent control |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zand, A.; Macharia, J.M.; Szabó, I.; Gerencsér, G.; Molnár, Á.; Raposa, B.L.; Varjas, T. The Impact of Tartrazine on DNA Methylation, Histone Deacetylation, and Genomic Stability in Human Cell Lines. Nutrients 2025, 17, 913. https://doi.org/10.3390/nu17050913
Zand A, Macharia JM, Szabó I, Gerencsér G, Molnár Á, Raposa BL, Varjas T. The Impact of Tartrazine on DNA Methylation, Histone Deacetylation, and Genomic Stability in Human Cell Lines. Nutrients. 2025; 17(5):913. https://doi.org/10.3390/nu17050913
Chicago/Turabian StyleZand, Afshin, John M. Macharia, Istvan Szabó, Gellért Gerencsér, Ádám Molnár, Bence L. Raposa, and Timea Varjas. 2025. "The Impact of Tartrazine on DNA Methylation, Histone Deacetylation, and Genomic Stability in Human Cell Lines" Nutrients 17, no. 5: 913. https://doi.org/10.3390/nu17050913
APA StyleZand, A., Macharia, J. M., Szabó, I., Gerencsér, G., Molnár, Á., Raposa, B. L., & Varjas, T. (2025). The Impact of Tartrazine on DNA Methylation, Histone Deacetylation, and Genomic Stability in Human Cell Lines. Nutrients, 17(5), 913. https://doi.org/10.3390/nu17050913










