Hydrogen and Methane Detection in Breath in Response to Two Different Types of Dietary Fiber and Its Relationship to Postprandial Glucose Concentration in Obese Patients with Type 2 Diabetes and Normoglycemic Subjects
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Subjects
2.2. Supplementation Protocol
2.3. Intervention
2.4. Anthropometric and Metabolic Assessments
2.4.1. HbA1c Assessments
2.4.2. Glucose Detection
2.4.3. Exhaled Gases Detection
2.5. Statistical Analysis
3. Results
3.1. Subject Characteristics
3.2. Gas Production Analysis
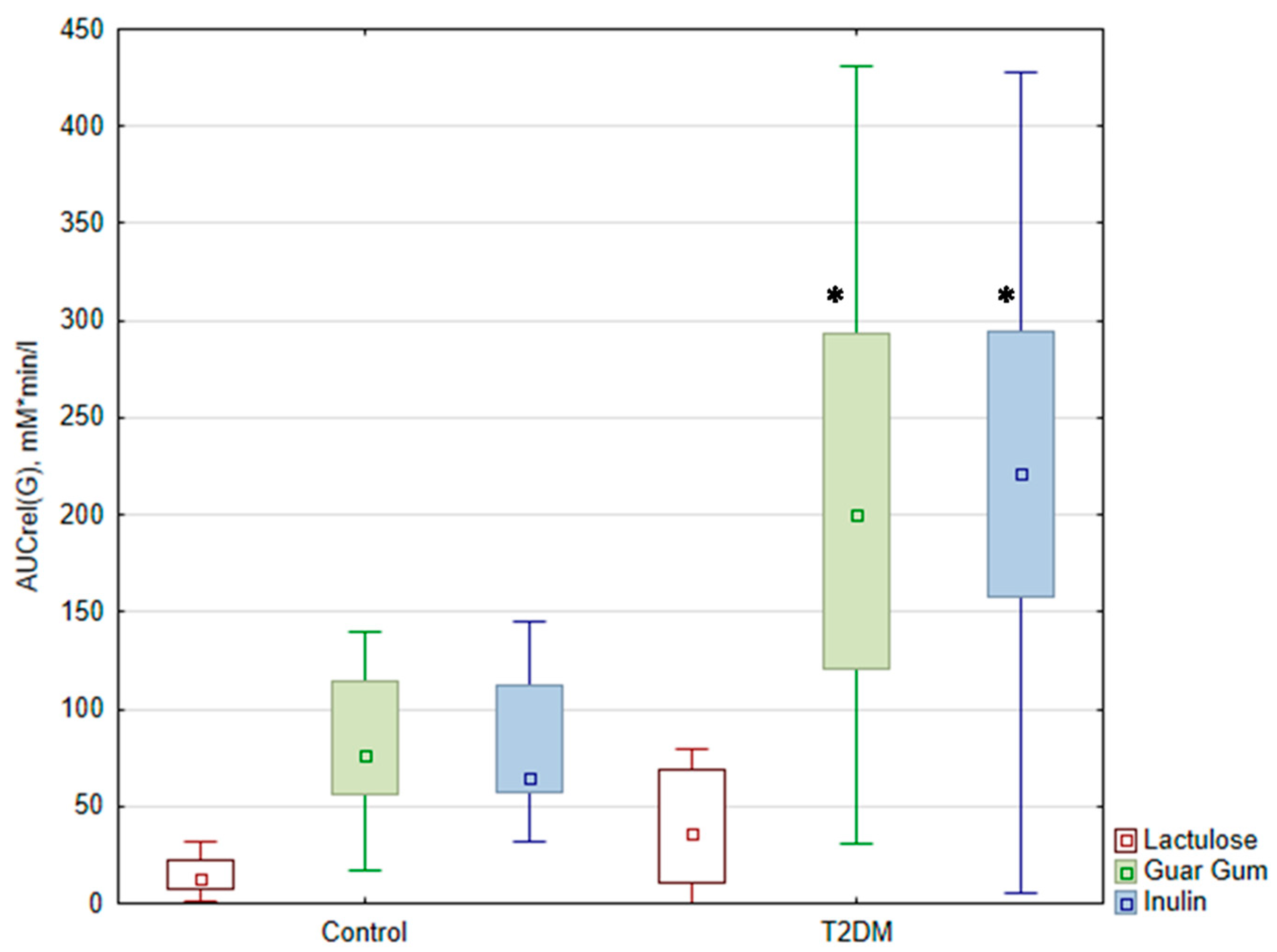
3.3. Glucose Level Analysis
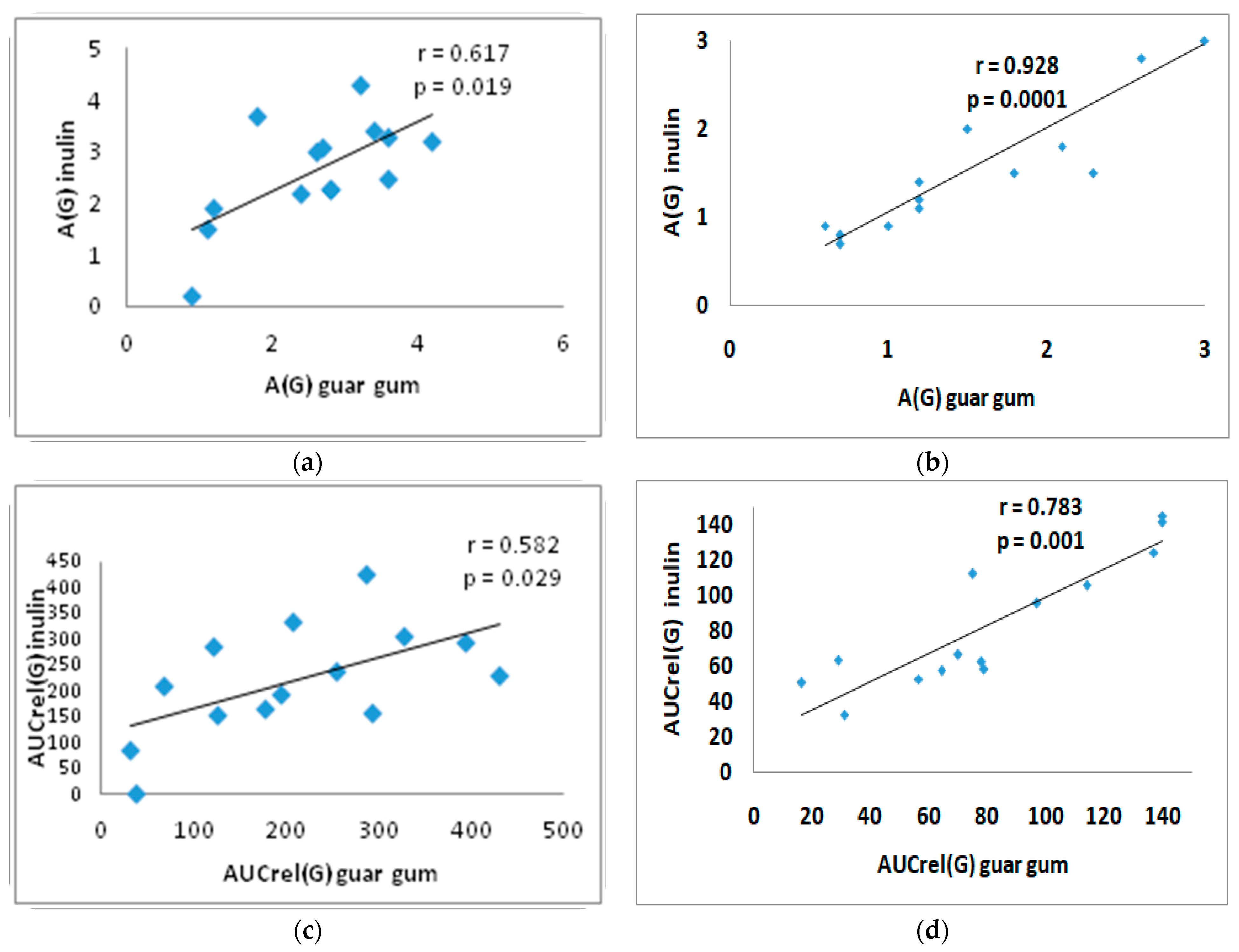
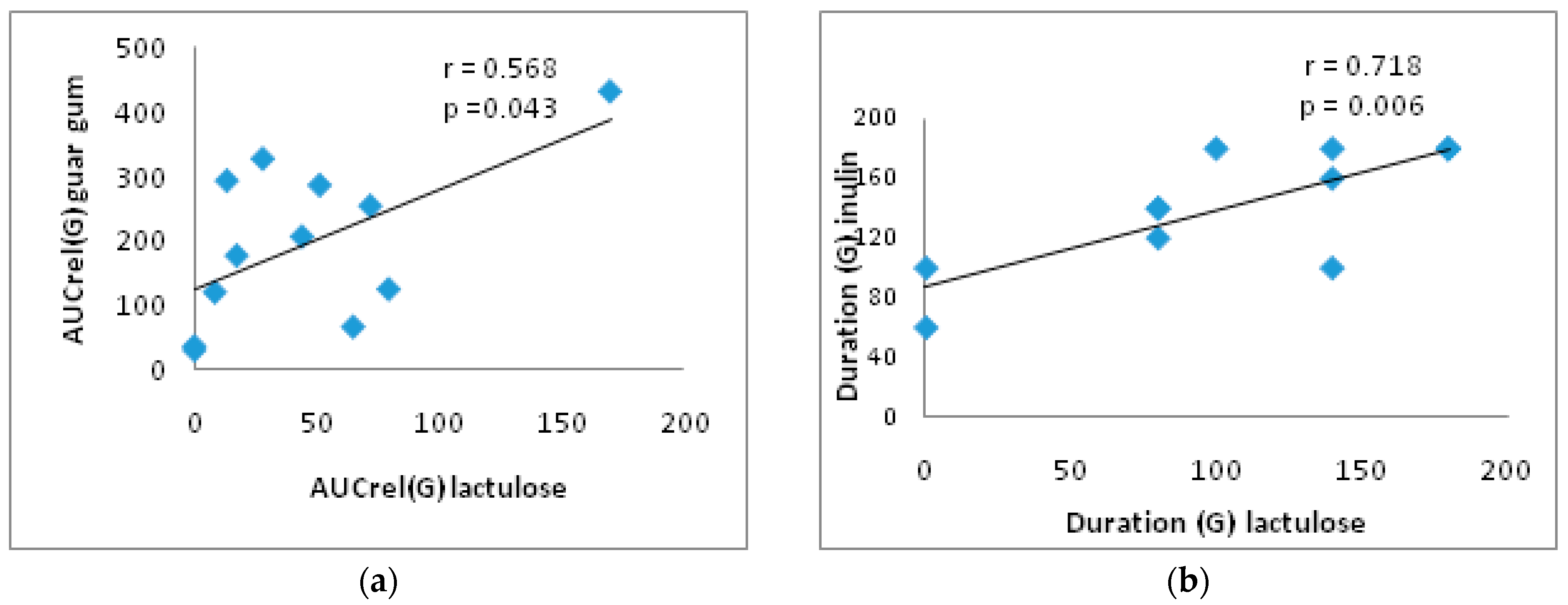
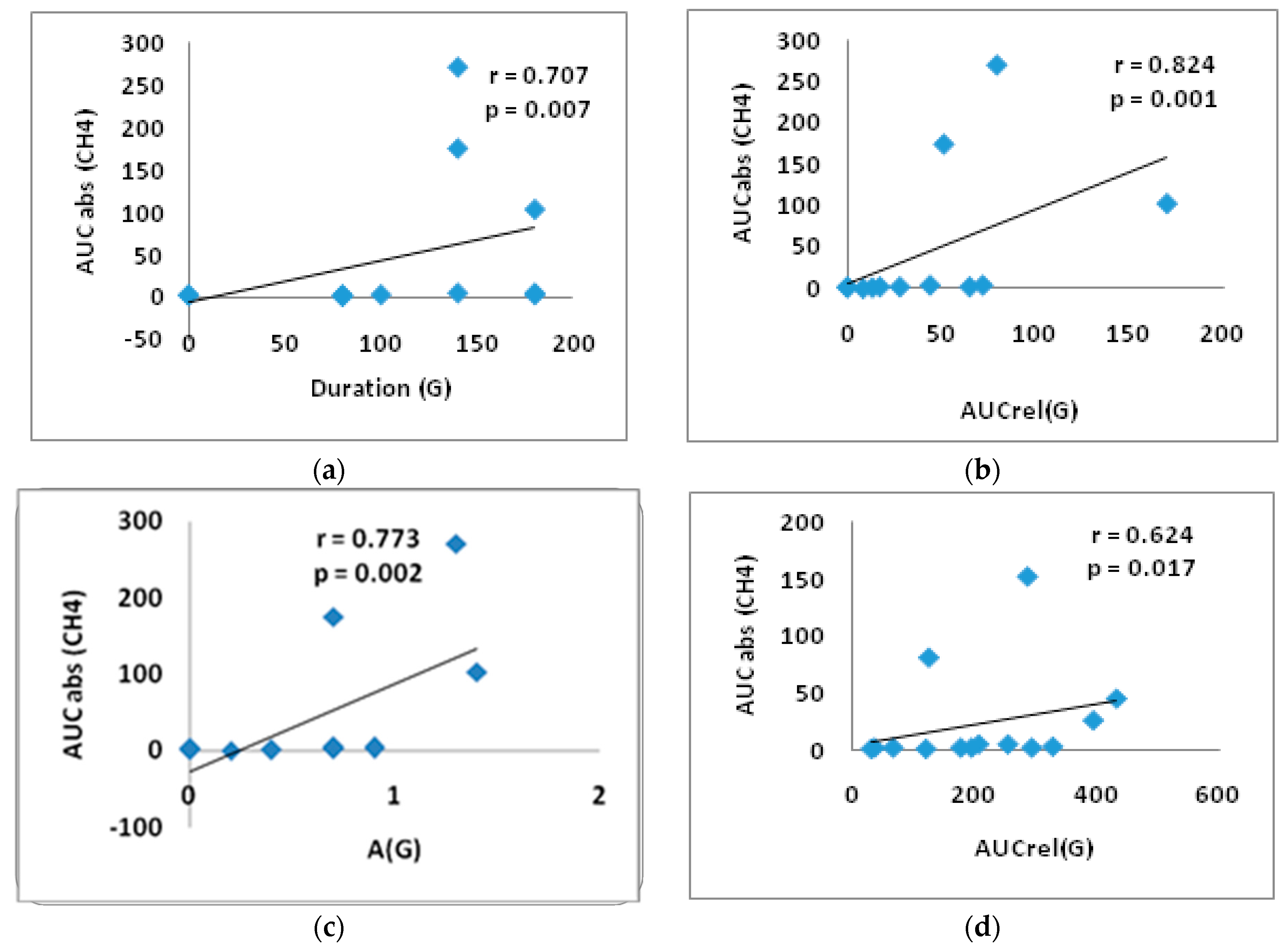
3.4. Correlation Analysis
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organisation. WHO Updates Guidelines on Fats and Carbohydrates. Available online: https://www.who.int/news/item/17-07-2023-who-updates-guidelines-on-fats-and-carbohydrates (accessed on 22 December 2024).
- Dahl, W.J.; Stewart, M.L. Position of the Academy of Nutrition and Dietetics: Health Implications of Dietary Fiber. J. Acad. Nutr. Diet. 2015, 115, 1861–1870. [Google Scholar] [CrossRef]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 5. Facilitating Positive Health Behaviors and Well-being to Improve Health Outcomes: Standards of Care in Diabetes-2023. Diabetes Care 2023, 46, 68–96. [Google Scholar] [CrossRef] [PubMed]
- Soliman, G.A. Dietary Fiber, Atherosclerosis, and Cardiovascular Disease. Nutrients 2019, 11, 1155. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- O’Keefe, S.J. Diet, microorganisms and their metabolites, and colon cancer. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 691–706. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, E.; Rifkin, S. Colorectal Cancer and Diet: Risk Versus Prevention, Is Diet an Intervention? Gastroenterol. Clin. N. Am. 2021, 50, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Jia, X.; Wang, N.; Kang, J.; Hu, X.; Goff, H.D.; Cui, S.W.; Ding, H.; Guo, Q. Therapeutic potential of non-starch polysaccharides on type 2 diabetes: From hypoglycemic mechanism to clinical trials. Crit. Rev. Food Sci. Nutr. 2024, 64, 1177–1210. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Chu, Q.; Ma, S.; Ma, H.; Song, H. Dietary Fiber and Its Potential Role in Obesity: A Focus on Modulating the Gut Microbiota. J. Agric. Food Chem. 2023, 71, 14853–14869. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Tuck, C.; Gibson, P.R.; Chey, W.D. The Role of Food in the Treatment of Bowel Disorders: Focus on Irritable Bowel Syndrome and Functional Constipation. Am. J. Gastroenterol. 2022, 117, 947–957. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Karppinen, S.; Liukkonen, K.; Aura, A.-M.; Forssell, P.; Poutanen, K. In Vitro fermentation of polysaccharides of rye, wheat and oat brans and inulin by human faecal bacteria. J. Sci. Food Agric. 2000, 80, 1469–1476. [Google Scholar] [CrossRef]
- Vernia, P.; Di Camillo, M.; Marinaro, V.; Caprilli, R. Effect of predominant methanogenic flora on the outcome of lactose breath test in irritable bowel syndrome patients. Eur. J. Clin. Nutr. 2003, 57, 1116–1119. [Google Scholar] [CrossRef]
- Perman, J.A.; Modler, S.; Olson, A.C. Role of pH in production of hydrogen from carbohydrates by colonic bacterial flora. Studies in vivo and in vitro. J. Clin. Investig. 1981, 67, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Lium, C.; Kurokawa, R.; Fujino, M.; Hirano, S.; Sato, B.; Li, X.-K. Estimation of the hydrogen concentration in rat tissue using an airtigt tube following the administration of hydrogen via various routes. Sci. Rep. 2014, 4, 5485. [Google Scholar] [CrossRef]
- Sano, M.; Ichihara, G.; Katsumata, Y.; Hiraide, T.; Hirai, A.; Momoi, M.; Tamura, T.; Ohata, S.; Kobayashi, E. Pharmacokinetics of a single inhalation of hydrogen gas in pigs. PLoS ONE 2020, 15, e0234626. [Google Scholar] [CrossRef]
- Hammer, H.F.; Fox, M.R.; Keller, J.; Salvatore, S.; Basilisco, G.; Hammer, J.; Lopetuso, L.; Benninga, M.; Borrelli, O.; Dumitrascu, D.; et al. European H2-CH4-breath test group. European guideline on indications, performance, and clinical impact of hydrogen and methane breath tests in adult and pediatric patients: European Association for Gastroenterology, Endoscopy and Nutrition, European Society of Neurogastroenterology and Motility, and European Society for Peadiatric Gastroenterology Hepatology and Nutrition consensus. United Eur. Gastroenterol. J. 2022, 10, 15–40. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rezaie, A.; Buresi, M.; Lembo, A.; Lin, H.; McCallum, R.; Rao, S.; Schmulson, M.; Valdovinos, M.; Zakko, S.; Pimentel, M. Hydrogen and methane-based breath testing in gastrointestinal disorders: The North American Consensus. Am. J. Gastroenterol. 2017, 112, 775–784. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Levitt, M.D. Production and excretion of hydrogen gas in man. N. Engl. J. Med. 1969, 281, 122–127. [Google Scholar] [CrossRef]
- Strocchi, A.; Levitt, M.D. Maintaining intestinal H2 balance: Credit the colonic bacteria. Gastroenterology 1992, 102, 1424–1426. [Google Scholar] [CrossRef]
- Pimentel, M.; Saad, R.J.; Long, M.D.; Rao, S.S.C. ACG Clinical Guideline: Small Intestinal Bacterial Overgrowth. Am. J. Gastroenterol. 2020, 115, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Costa, K.C.; Leigh, J.A. Metabolic versatility in methanogens. Curr. Opin. Biotechnol. 2014, 29, 70–75. [Google Scholar] [CrossRef]
- Barancik, M.; Kura, B.; LeBaron, T.W.; Bolli, R.; Buday, J.; Slezak, J. Molecular and cellular mechanisms associated with effects of molecular hydrogen in cardiovascular and central nervous systems. Antioxidants 2020, 9, 1281. [Google Scholar] [CrossRef]
- Medvedev, O.S. Role of human and animal microbiome’s hydrogen and methane in an antioxidant organism defense. Uspekhi Sovremennoybiologii 2022, 142, 349–364. [Google Scholar] [CrossRef]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.-I.; Katayama, Y.; Asoh, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Boros, M.; Keppler, F. Methane Production and Bioactivity-A Link to Oxido-Reductive Stress. Front. Physiol. 2019, 10, 1244. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ernst, L.; Steinfeld, B.; Barayeu, U.; Klintzsch, T.; Kurth, M.; Grimm, D.; Dick, T.P.; Rebelein, J.G.; Bischofs, I.B.; Keppler, F. Methane formation driven by reactive oxygen species across all living organisms. Nature 2022, 603, 482–487. [Google Scholar] [CrossRef]
- Keppler, F.; Boros, M.; Polag, D. Radical-Driven Methane Formation in Humans Evidenced by Exogenous Isotope-Labeled DMSO and Methionine. Antioxidants 2023, 12, 1381. [Google Scholar] [CrossRef]
- Ivanova, A.Y.; Shirokov, I.V.; Toshchakov, S.V.; Kozlova, A.D.; Obolenskaya, O.N.; Mariasina, S.S.; Ivlev, V.A.; Gartseev, I.B.; Medvedev, O.S. Effects of Coenzyme Q10 on the Biomarkers (Hydrogen, Methane, SCFA and TMA) and Composition of the Gut Microbiome in Rats. Pharmaceuticals 2023, 16, 686. [Google Scholar] [CrossRef]
- World Health Organization. Carbohydrate Intake for Adults and Children: WHO Guideline Summary. 2023. Available online: https://www.who.int/publications/i/item/9789240073593 (accessed on 22 December 2024).
- Qin, Y.Q.; Wang, L.Y.; Yang, X.Y.; Xu, Y.J.; Fan, G.; Fan, Y.G.; Ren, J.N.; An, Q.; Li, X. Inulin: Properties and health benefits. Food Funct. 2023, 14, 2948–2968. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Meng, G.; Zhang, Q.; Liu, L.; Yao, Z.; Wu, H.; Gu, Y.; Wang, Y.; Zhang, T.; Wang, X.; et al. Dietary fibre intake and risk of prediabetes in China: Results from the Tianjin Chronic Low-grade Systemic Inflammation and Health (TCLSIH) Cohort Study. Br. J. Nutr. 2022, 128, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Gurry, T.; Nguyen, L.T.T.; Richardson, H.S.; Alm, E.J. Prebiotics and Community Composition Influence Gas Production of the Human Gut Microbiota. mBio 2020, 11, e00217-20. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Müller, M.; Canfora, E.E.; Blaak, E.E. Gastrointestinal Transit Time, Glucose Homeostasis and Metabolic Health: Modulation by Dietary Fibers. Nutrients 2018, 10, 275. [Google Scholar] [CrossRef]
- Hughes, R.L.; Horn, W.H.; Finnegan, P.; Newman, J.W.; Marco, M.L.; Keim, N.L.; Kable, M.E. Resistant Starch Type 2 from Wheat Reduces Postprandial Glycemic Response with Concurrent Alterations in Gut Microbiota Composition. Nutrients 2021, 13, 645. [Google Scholar] [CrossRef] [PubMed]
- Ranaivo, H.; Zhang, Z.; Alligier, M.; Van Den Berghe, L.; Sothier, M.; Lambert-Porcheron, S.; Feugier, N.; Cuerq, C.; Machon, C.; Neyrinck, A.M.; et al. Chitin-glucan supplementation improved postprandial metabolism and altered gut microbiota in subjects at cardiometabolic risk in a randomized trial. Sci. Rep. 2022, 12, 8830. [Google Scholar] [CrossRef] [PubMed]
- Mathur, R.; Amichai, M.; Chua, K.S.; Mirocha, J.; Barlow, G.M.; Pimentel, M. Methane and hydrogen positivity on breath test is associated with greater body mass index and body fat. J. Clin. Endocrinol. Metab. 2013, 98, E698–E702. [Google Scholar] [CrossRef]
- Feng, X.; Li, X.Q. The prevalence of small intestinal bacterial overgrowth in diabetes mellitus: A systematic review and meta-analysis. Aging 2022, 14, 975–988. [Google Scholar] [CrossRef]
- Scarpellini, E.; Abenavoli, L.; Balsano, C.; Gabrielli, M.; Luzza, F.; Tack, J. Breath tests for the assessment of the orocecal transit time. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 39–44. [Google Scholar]
- Cuoco, L.; Montalto, M.; Jorizzo, R.A.; Santarelli, L.; Arancio, F.; Cammarota, G.; Gasbarrini, G. Eradication of small intestinal bacterial overgrowth and oro-cecal transit in diabetics. Hepatogastroenterology 2002, 49, 1582–1586. [Google Scholar]
- Gottlieb, K.; Le, C.; Wacher, V.; Sliman, J.; Cruz, C.; Porter, T.; Carter, S. Selection of a cut-off for high- and low-methane producers using a spot-methane breath test: Results from a large north American dataset of hydrogen, methane and carbon dioxide measurements in breath. Gastroenterol. Rep. 2017, 5, 193–199. [Google Scholar] [CrossRef]
- Takakura, W.; Pimentel, M.; Rao, S.; Villanueva-Millan, M.J.; Chang, C.; Morales, W.; Sanchez, M.; Torosyan, J.; Rashid, M.; Hosseini, A.; et al. A Single Fasting Exhaled Methane Level Correlates with Fecal Methanogen Load, Clinical Symptoms and Accurately Detects Intestinal Methanogen Overgrowth. Am. J. Gastroenterol. 2022, 117, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Meiller, L.; Sauvinet, V.; Breyton, A.E.; Ranaivo, H.; Machon, C.; Mialon, A.; Meynier, A.; Bischoff, S.C.; Walter, J.; Neyrinck, A.M.; et al. Metabolic signature of 13C-labeled wheat bran consumption related to gut fermentation in humans: A pilot study. Eur. J. Nutr. 2023, 62, 2633–2648. [Google Scholar] [CrossRef] [PubMed]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Heindel, J.J.; Lustig, R.H.; Howard, S.; Corkey, B.E. Obesogens: A unifying theory for the global rise in obesity. Int. J. Obes. 2024, 48, 449–460. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cesario, V.; Di Rienzo, T.A.; Campanale, M.; D’angelo, G.; Barbaro, F.; Gigante, G.; Vitale, G.; Scavone, G.; Pitocco, D.; Gasbarrini, A.; et al. Methane intestinal production and poor metabolic control in type I diabetes complicated by autonomic neuropathy. Minerva Endocrinol. 2014, 39, 201–207. [Google Scholar] [PubMed]
- Zhang, M.; Xu, Y.; Zhang, J.; Sun, Z.; Ban, Y.; Wang, B.; Hou, X.; Cai, Y.; Li, J.; Wang, M.; et al. Application of methane and hydrogen-based breath test in the study of gestational diabetes mellitus and intestinal microbes. Diabetes Res. Clin. Pract. 2021, 176, 108818. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhang, Y.; Wang, Y.; Chen, Y.; Fan, W.; Zhou, J.; Qiao, J.; Wei, Y. Hydrogen, a Novel Therapeutic Molecule, Regulates Oxidative Stress, Inflammation, and Apoptosis. Front. Physiol. 2021, 12, 789507. [Google Scholar] [CrossRef]
- Giuntini, E.B.; Sardá, F.A.H.; de Menezes, E.W. The Effects of Soluble Dietary Fibers on Glycemic Response: An Overview and Futures Perspectives. Foods 2022, 11, 3934. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pathan, S.; Glover, M.; Ryan, J.; Quan, S.D. The Role of Inulin in Human Health and Sustainable Food Applications [Internet]. In Probiotics, Prebiotics, and Postbiotics in Human Health and Sustainable Food Systems [Working Title]; IntechOpen: London, UK, 2024. [Google Scholar] [CrossRef]
- Chu, N.; Ling, J.; Jie, H.; Leung, K.; Poon, E. The potential role of lactulose pharmacotherapy in the treatment and prevention of diabetes. Front. Endocrinol. 2022, 13, 956203. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tendulkar, P.; Kant, R.; Rana, S.; Yadav, P.; Mirza, A.A.; Agarwal, D. Efficacy of Pro-Kinetic Agents in Type 2 Diabetes Mellitus Patients With Gastroparesis Using Lactulose Hydrogen Breath Testing: A Randomized Trial. Cureus 2022, 14, e20990. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yuan, H.L.; Zhang, X.; Peng, D.Z.; Lin, G.B.; Li, H.H.; Li, F.X.; Lu, J.J.; Chu, W.W. Development and Validation of a Risk Nomogram Model for Predicting Constipation in Patients with Type 2 Diabetes Mellitus. Diabetes Metab. Syndr. Obes. 2023, 16, 1109–1120. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Paudel, D.; Nair, D.V.T.; Tian, S.; Hao, F.; Goand, U.K.; Joseph, G.; Prodes, E.; Chai, Z.; Robert, C.E.M.; Chassaing, B.; et al. Dietary fiber guar gum-induced shift in gut microbiota metabolism and intestinal immune activity enhances susceptibility to colonic inflammation. Gut Microbes 2024, 16, 2341457. [Google Scholar] [CrossRef]
- Song, Y.; Liu, Y.; Qi, B.; Cui, X.; Dong, X.; Wang, Y.; Han, X.; Li, F.; Shen, D.; Zhang, X.; et al. Association of Small Intestinal Bacterial Overgrowth With Heart Failure and Its Prediction for Short-Term Outcomes. J. Am. Heart Assoc. 2021, 10, e015292. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Boros, M.; Ghyczy, M.; Érces, D.; Varga, G.; Tőkés, T.; Kupai, K.; Torday, C.; Kaszaki, J. The anti-inflammatory effects of methane. Crit. Care Med. 2012, 40, 1269–1278. [Google Scholar] [CrossRef]
- Zaorskaa, E.; Gawryś-Kopczyńska, M.; Ostaszewski, R.; Koszelewski, D.; Ufnal, M. Methane, a gut bacteria-produced gas, does not affect arterial blood pressure in normotensive anaesthetized rats. bioRxiv 2021. [Google Scholar] [CrossRef]
- Benke, K.; Jász, D.K.; Szilágyi, Á.L.; Baráth, B.; Tuboly, E.; Márton, A.R.; Varga, P.; Mohácsi, Á.; Szabó, A.; Széll, Z.; et al. Methane supplementation improves graft function in experimental heart transplantation. J. Heart Lung Transplant. 2021, 40, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Juhász, L.; Tallósy, S.P.; Nászai, A.; Varga, G.; Érces, D.; Boros, M. Bioactivity of Inhaled Methane and Interactions with Other Biological Gases. Front. Cell Dev. Biol. 2022, 9, 824749. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Djemai, K.; Drancourt, M.; Tidjani Alou, M. Bacteria and Methanogens in the Human Microbiome: A Review of Syntrophic Interactions. Microb. Ecol. 2022, 83, 536–554. [Google Scholar] [CrossRef]
- Kim, J.W.; Park, S.Y.; Chung, J.O.; Cho, H.A.; Kim, D.H.; Yoon, J.H.; Park, C.H.; Kim, H.S.; Choi, S.K.; Rew, J.S. Influencing Factors on Lactulose Breath Test Results. Korean J. Gastroenterol. 2020, 75, 23–28. (In Korean) [Google Scholar] [CrossRef] [PubMed]
- Santos, A.N.D.R.; Soares, A.C.F.; Oliveira, R.P.; Morais, M.B. The impact of small intestinal bacterial overgrowth on the growth of children and adolescents. Rev. Paul. Pediatr. 2020, 38, e2018164. [Google Scholar] [CrossRef]
- Le Marchand, L.; Wilkens, L.R.; Harwood, P.; Cooney, R.V. Breath hydrogen and methane in populations at different risk for colon cancer. Int. J. Cancer 1993, 55, 887–890. [Google Scholar] [CrossRef] [PubMed]
- Kwiecień, J.; Hajzler, W.; Kosek, K.; Balcerowicz, S.; Grzanka, D.; Gościniak, W.; Górowska-Kowolik, K. No Correlation between Positive Fructose Hydrogen Breath Test and Clinical Symptoms in Children with Functional Gastrointestinal Disorders: A Retrospective Single-Centre Study. Nutrients 2021, 13, 2891. [Google Scholar] [CrossRef]
- Plauzolles, A.; Uras, S.; Pénaranda, G.; Bonnet, M.; Dukan, P.; Retornaz, F.; Halfon, P. Small Intestinal Bacterial Overgrowths and Intestinal Methanogen Overgrowths Breath Testing in a Real-Life French Cohort. Clin. Transl. Gastroenterol. 2023, 14, e00556. [Google Scholar] [CrossRef]
- Newberry, C.; Tierney, A.; Pickett-Blakely, O. Lactulose Hydrogen Breath Test Result Is Associated with Age and Gender. Biomed. Res. Int. 2016, 2016, 1064029. [Google Scholar] [CrossRef]
- Kiow, L.C.J.; Bellila, R.; Therrien, A.; Sidani, S.; Bouin, M. Predictors of Small Intestinal Bacterial Overgrowth in Symptomatic Patients Referred for Breath Testing. J. Clin. Med. Res. 2020, 12, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Basseri, R.J.; Basseri, B.; Pimentel, M.; Chong, K.; Youdim, A.; Low, K.; Hwang, L.; Soffer, E.; Chang, C.; Mathur, R. Intestinal methane production in obese individuals is associated with a higher body mass index. Gastroenterol. Hepatol. 2012, 8, 22–28. [Google Scholar] [PubMed] [PubMed Central]
- Wielgosz-Grochowska, J.P.; Domanski, N.; Drywień, M.E. Influence of Body Composition and Specific Anthropometric Parameters on SIBO Type. Nutrients 2023, 15, 4035. [Google Scholar] [CrossRef]
- Cortez, A.P.B.; Fisberg, M.; de Morais, M.B. Intestinal permeability and small intestine bacterial overgrowth in excess weight adolescents. Pediatr. Obes. 2021, 16, e12741. [Google Scholar] [CrossRef]


| Participant Characteristics | T2DM | Control |
|---|---|---|
| Number of subjects, (f 1) | 14 (11) | 14(13) |
| Age (years) | 58 [54; 62] | 46 (28; 56) |
| Weight (kg) | 111.5 [92.0; 123.3] | 63.5 (57.2; 68.2) |
| BMI 2 (kg/m2) | 42.3 [35.6; 47.7] | 22.6 (20.6; 25.8) |
| HbA1c 3 (%) | 8.4 [6.9; 9.9] | - |
| Subject Characteristics | T2DM(n) | Control(n) |
|---|---|---|
| up to 90 min of the test | ||
| PCH4+/H2+ | 1 | 7 |
| PCH4+ | 2 | 1 |
| PH2+ | 4 | 4 |
| PCH4−/H2− | 6 | 2 |
| up to 180 min of the test | ||
| PCH4+/H2+ | 2 | 8 |
| PCH4+ | 1 | 0 |
| PH2+ | 9 | 6 |
| PCH4−/H2− | 1 | 0 |
| Groups | Me (1)[Q1; Q3] (2) Mean ± SD | Lactulose | Guar Gum | Inulin | |||
|---|---|---|---|---|---|---|---|
| H2 | CH4 | H2 | CH4 | H2 | CH4 | ||
| T2DM | AUCabs (ppm*h) | (1) 96 [65; 118] (2)93 ± 41 | (1) 2 [1; 4] (2) 44 ± 87 | (1) 43 [34; 70] (2) 50 ± 28 | (1) 3 [2; 21] (2) 23 ± 44 | (1) 105 [70; 151] (2) 117 ± 62 | (1) 4 [1; 44] (2) 38 ± 69 |
| A (ppm) | (1) 61 [30; 66] (2) 58 ± 36 | (1) 1 [1; 2] (2) 11 ± 20 | (1) 17 [8; 33] (2) 23 ± 19 | (1) 1 [−1; 2] (2)2 ± 5 | (1) 71 [44; 81] (2) 69 ± 31 | (1) 1 [−1; 2] (2)2 ± 5 | |
| tmax (min) | (1) 160 [140; 180] (2) 149 ± 36 | (1) 60 [20; 140] (2) 75 ± 65 | (1) 140 [80; 160] (2) 120 ± 60 | (1) 30 [0; 75] (2) 41 ± 45 | (1) 170 [120; 180] (2) 148 ± 37 | (1) 40 [0; 110] (2) 54 ± 58 | |
| Control group | AUCabs (ppm*h) | (1) 126 (98; 203) * (2) 148 ± 68 | (1) 5 (1; 25) (2) 62 ± 111 | (1) 51 (32; 74) (2) 56 ± 34 | (1) 14 (1; 41) (2) 52 ± 89 | (1) 123 (86; 212) (2) 140 ± 80 | (1) 8 (1; 30) (2) 65 ± 118 |
| A (ppm) | (1) 75 (60; 111) (2) 81 ± 35 | (1) 2 (0; 4) (2) 20 ± 41 | (1) 16 (12; 26) (2)20 ± 15 | (1) 2 (1; 6) (2)9 ± 17 | (1) 74 (41; 84) (2)68 ± 37 | (1) 1 (−1; 9) (2)9 ± 22 | |
| tmax (min) | (1) 120 (85; 140) * (2) 116 ± 38 | (1) 30 (5; 60) (2)49 ± 56 | (1) 160 (125; 160) (2) 146 ± 35 | (1) 70 (20; 115) (2)70 ± 54 | (1) 120 (100; 155) (2) 116 ± 50 | (1) 50 (0; 75) (2) 53 ± 52 | |
| (1) Me [Q1; Q3] (2) Mean ± SD | Lactulose (a) | Guar Gum (b) | Inulin (c) | |||
|---|---|---|---|---|---|---|
| T2DM | Control | T2DM | Control | T2DM | Control | |
| A (G) (mM/L) | (1) 0.7 [0.3; 0.9] (2) 0.7 ± 0.5 | (1) 0.3 (0.2; 0.5) (2) 0.6 ± 0.7 | (1) 2.8 † [1.7; 3.5] (2) 2.6 ± 1.02 | (1) 1.4 (1.1; 2.2) * (2) 1.6 ± 0.8 | (1) 2.8 [2.1; 3.3] † (2) 2.6 ± 1.0 | (1) 1.5 (1.0; 2.0) * (2) 1.6 ± 0.8 |
| Duration (min) | (1) 80 [120; 170] (2) 108 ± 63 | (1) 80 (80; 95) (2) 90 ± 44 | (1) 180 [140; 180] (2) 154 ± 37 | (1) 100 (100; 175) (2) 123 ± 44 | (1) 140 [115; 180] (2) 143 ± 37 | (1) 100 (85; 150) (2) 117.1 ± 41.4 |
| AUCrel(G) (mM*min/L) | (1) 36 [9.6; 70.3] (2) 45.7 ± 48.1 | (1) 13 (8.5; 21) (2) 30 ± 47 | (1) 200.7 † [107.4; 301.5] (2) 210.3 ± 126.9 | (1) 76.6 (58.4; 109.8) * (2) 81 ± 41 | (1) 221.6 † [157.1; 297.0] (2) 221.8 ± 107.6 | (1) 64.8 (57.3; 110.4) * (2) 83 ± 37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Misnikova, I.; Kovaleva, Y.; Shokur, S.; LeBaron, T.W.; Povarova, O.; Medvedev, O. Hydrogen and Methane Detection in Breath in Response to Two Different Types of Dietary Fiber and Its Relationship to Postprandial Glucose Concentration in Obese Patients with Type 2 Diabetes and Normoglycemic Subjects. Nutrients 2025, 17, 917. https://doi.org/10.3390/nu17050917
Misnikova I, Kovaleva Y, Shokur S, LeBaron TW, Povarova O, Medvedev O. Hydrogen and Methane Detection in Breath in Response to Two Different Types of Dietary Fiber and Its Relationship to Postprandial Glucose Concentration in Obese Patients with Type 2 Diabetes and Normoglycemic Subjects. Nutrients. 2025; 17(5):917. https://doi.org/10.3390/nu17050917
Chicago/Turabian StyleMisnikova, Inna, Yulia Kovaleva, Svetlana Shokur, Tyler W. LeBaron, Oxana Povarova, and Oleg Medvedev. 2025. "Hydrogen and Methane Detection in Breath in Response to Two Different Types of Dietary Fiber and Its Relationship to Postprandial Glucose Concentration in Obese Patients with Type 2 Diabetes and Normoglycemic Subjects" Nutrients 17, no. 5: 917. https://doi.org/10.3390/nu17050917
APA StyleMisnikova, I., Kovaleva, Y., Shokur, S., LeBaron, T. W., Povarova, O., & Medvedev, O. (2025). Hydrogen and Methane Detection in Breath in Response to Two Different Types of Dietary Fiber and Its Relationship to Postprandial Glucose Concentration in Obese Patients with Type 2 Diabetes and Normoglycemic Subjects. Nutrients, 17(5), 917. https://doi.org/10.3390/nu17050917








