l-Carnitine and Acetyl-l-Carnitine Induce Metabolism Alteration and Mitophagy-Related Cell Death in Colorectal Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Growth and Treatments
2.2. Cell Viability Assay
2.3. Cell Cycle Distribution
2.4. Cellular Bioenergetics Analysis
2.5. Mitochondrial State
2.6. Autophagy and Cell Death Mechanism Evaluation
2.7. Immunoblotting
2.8. Statistical Analysis
3. Results
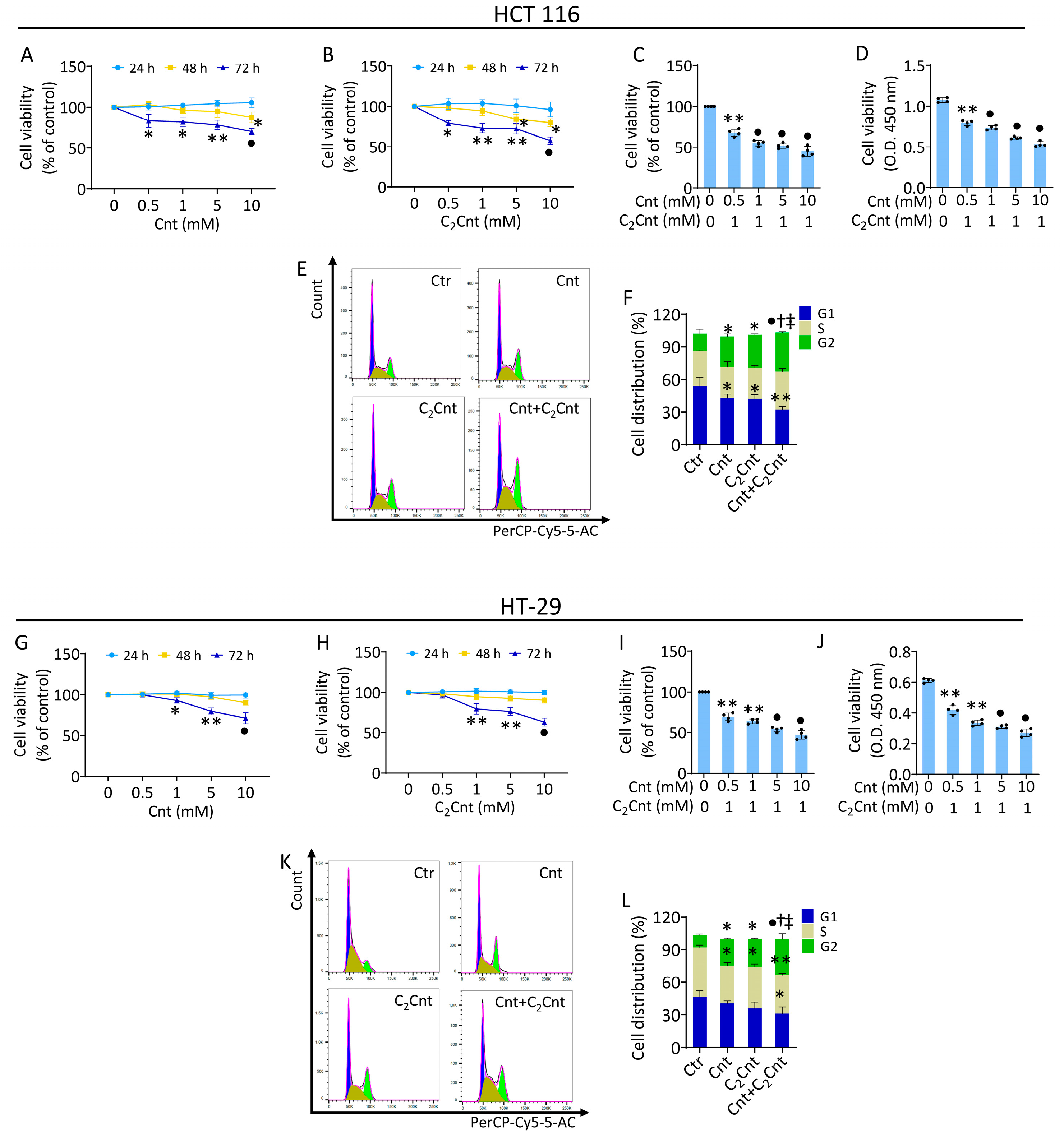
3.1. Effect of Cnt and C2Cnt on CRC Viability and Cell Cycle Progression
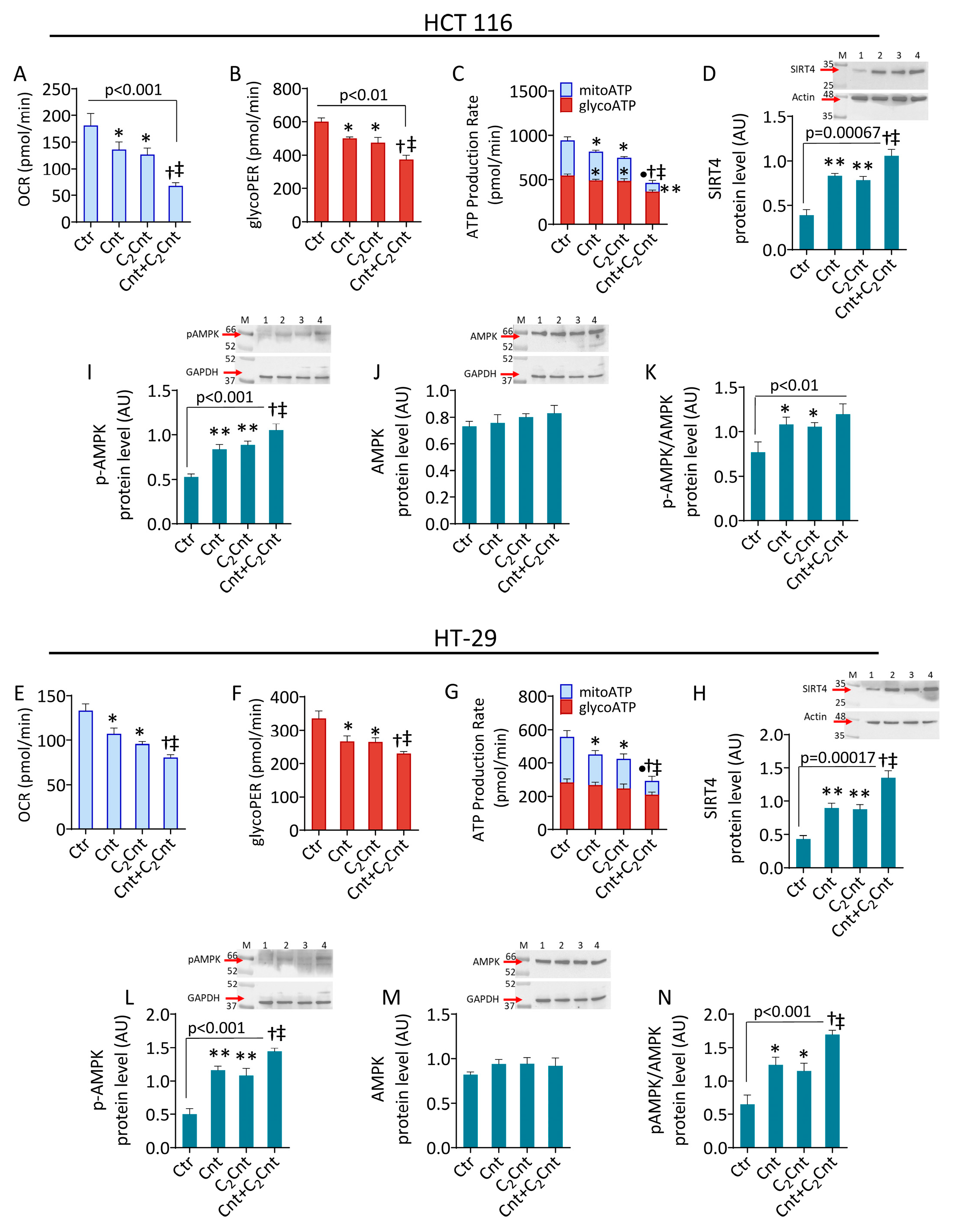
3.2. Cnt and C2Cnt Impaired the Bioenergetics of CRC Cells and Upregulated SIRT4 and AMPK
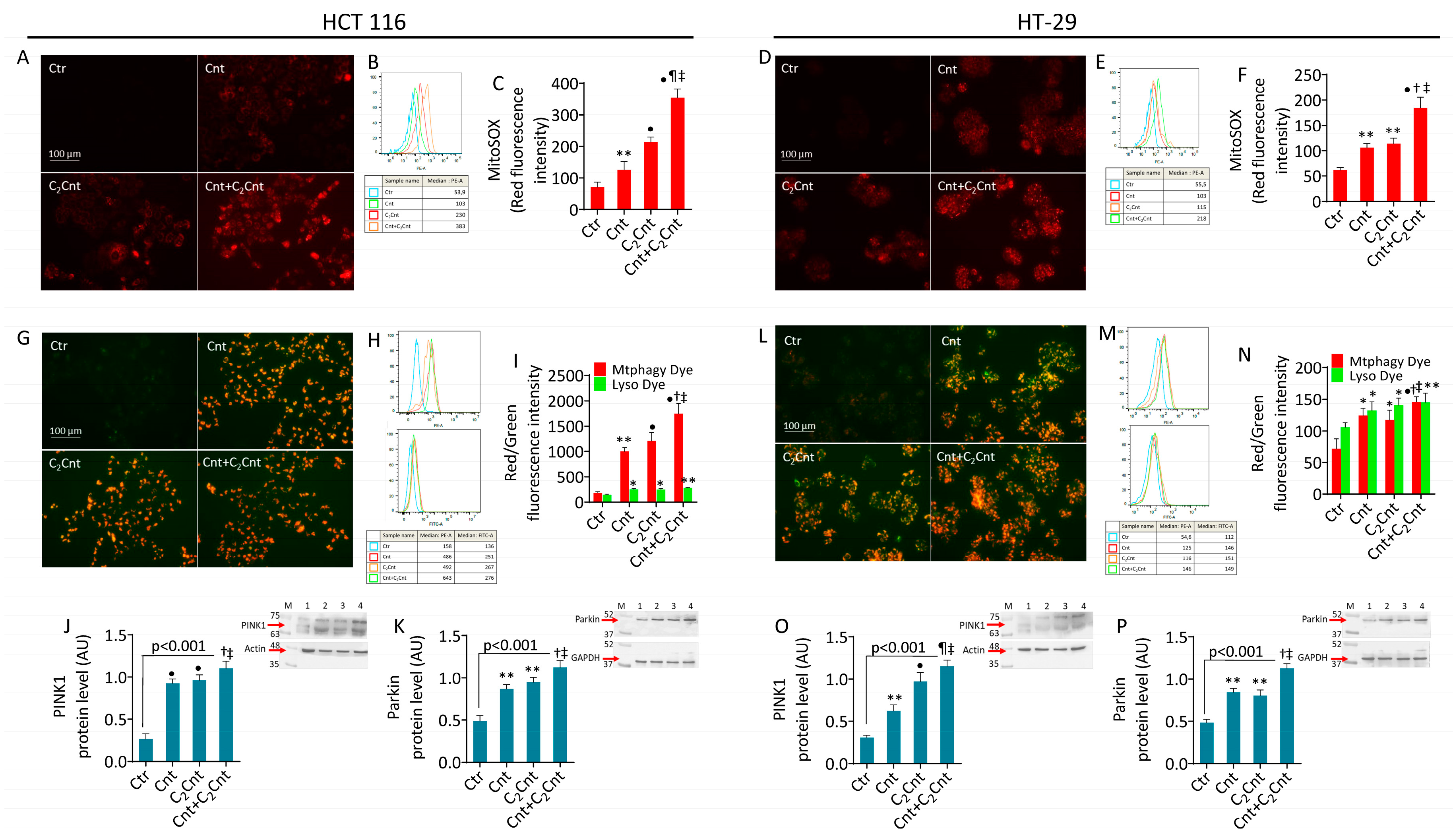
3.3. Cnt and C2Cnt Promoted Mitochondrial Oxidative Stress and Mitophagy
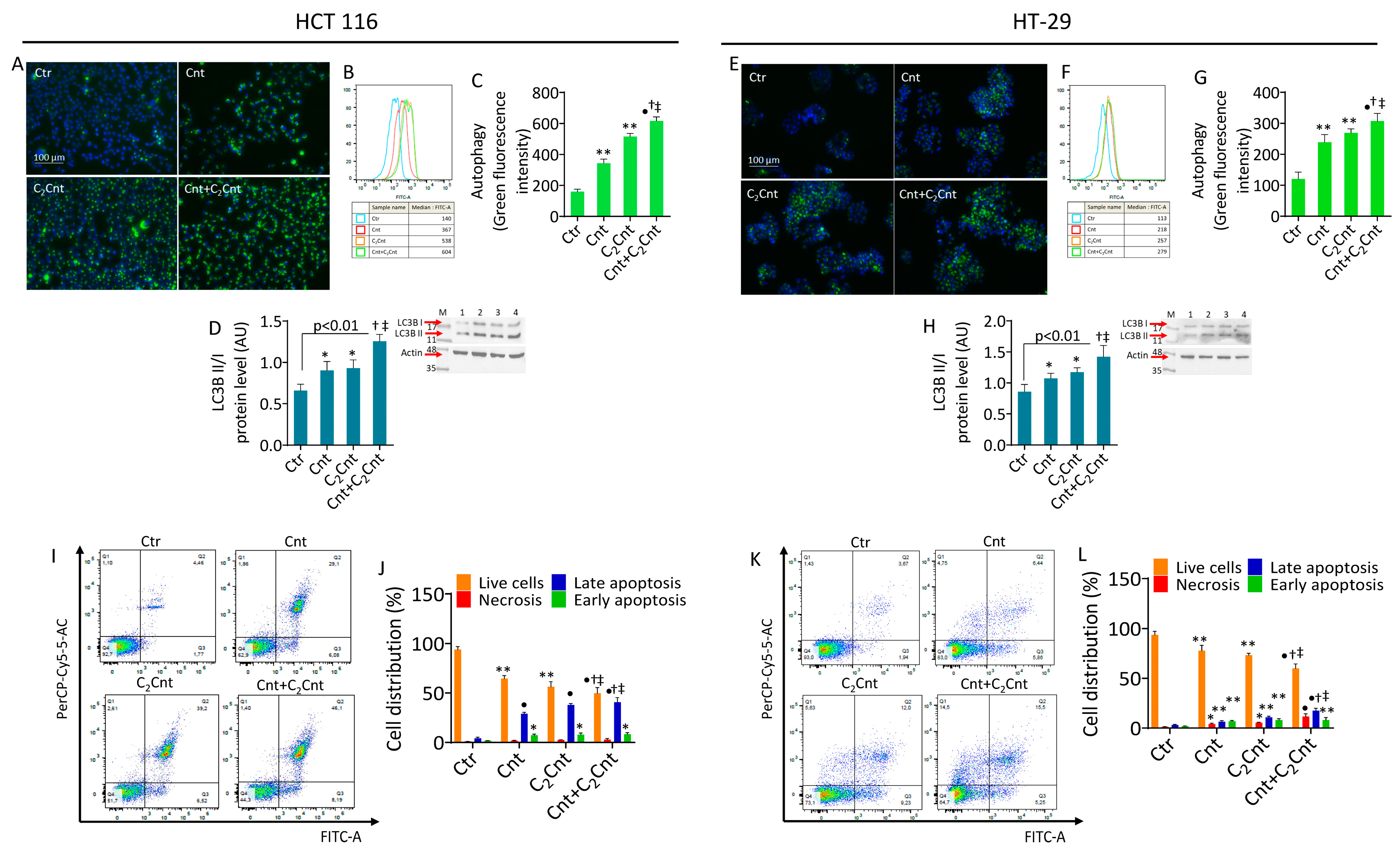
3.4. Cnt and C2Cnt Induced Autophagy and Apoptotic Death of CRC Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Fadlallah, H.; El Masri, J.; Fakhereddine, H.; Youssef, J.; Chemaly, C.; Doughan, S.; Abou-Kheir, W. Colorectal cancer: Recent advances in management and treatment. World J. Clin. Oncol. 2024, 15, 1136–1156. [Google Scholar] [CrossRef]
- Saha, B.; Rithi, A.T.; Adhikary, S.; Banerjee, A.; Radhakrishnan, A.K.; Duttaroy, A.K.; Pathak, S. Exploring the Relationship Between Diet, Lifestyle and Gut Microbiome in Colorectal Cancer Development: A Recent Update. Nutr. Cancer 2024, 76, 789–814. [Google Scholar] [CrossRef]
- Murphy, N.; Moreno, V.; Hughes, D.J.; Vodicka, L.; Vodicka, P.; Aglago, E.K.; Gunter, M.J.; Jenab, M. Lifestyle and dietary environmental factors in colorectal cancer susceptibility. Mol. Asp. Med. 2019, 69, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Colloca, A.; Donisi, I.; Anastasio, C.; Balestrieri, M.L.; D’Onofrio, N. Metabolic Alteration Bridging the Prediabetic State and Colorectal Cancer. Cells 2024, 13, 663. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, D.; Weissbrod, O.; Barkan, E.; Kurilshikov, A.; Korem, T.; Zeevi, D.; Costea, P.I.; Godneva, A.; Kalka, I.N.; Bar, N.; et al. Environment dominates over host genetics in shaping human gut microbiota. Nature 2018, 555, 210–215. [Google Scholar] [CrossRef]
- Pascale, R.M.; Calvisi, D.F.; Simile, M.M.; Feo, C.F.; Feo, F. The Warburg Effect 97 Years after Its Discovery. Cancers 2020, 12, 2819. [Google Scholar] [CrossRef] [PubMed]
- Colloca, A.; Balestrieri, A.; Anastasio, C.; Balestrieri, M.L.; D’Onofrio, N. Mitochondrial Sirtuins in Chronic Degenerative Diseases: New Metabolic Targets in Colorectal Cancer. Int. J. Mol. Sci. 2022, 23, 3212. [Google Scholar] [CrossRef]
- Thanikachalam, K.; Khan, G. Colorectal Cancer and Nutrition. Nutrients 2019, 11, 164. [Google Scholar] [CrossRef]
- Wang, K.; Song, M. Personalized nutrition for colorectal cancer. Adv. Cancer Res. 2021, 151, 109–136. [Google Scholar] [CrossRef]
- Adeva-Andany, M.M.; Calvo-Castro, I.; Fernández-Fernández, C.; Donapetry-García, C.; Pedre-Piñeiro, A.M. Significance of l-carnitine for human health. IUBMB Life 2017, 69, 578–594. [Google Scholar] [CrossRef] [PubMed]
- Almannai, M.; Alfadhel, M.; El-Hattab, A.W. Carnitine Inborn Errors of Metabolism. Molecules 2019, 24, 3251. [Google Scholar] [CrossRef]
- Servillo, L.; D’Onofrio, N.; Neglia, G.; Casale, R.; Cautela, D.; Marrelli, M.; Limone, A.; Campanile, G.; Balestrieri, M.L. Carnitine Precursors and Short-Chain Acylcarnitines in Water Buffalo Milk. J. Agric. Food Chem. 2018, 66, 8142–8149. [Google Scholar] [CrossRef]
- Li, X.S.; Wang, Z.; Cajka, T.; Buffa, J.A.; Nemet, I.; Hurd, A.G.; Gu, X.; Skye, S.M.; Roberts, A.B.; Wu, Y.; et al. Untargeted metabolomics identifies trimethyllysine, a TMAO-producing nutrient precursor, as a predictor of incident cardiovascular disease risk. JCI Insight 2018, 3, e99096. [Google Scholar] [CrossRef] [PubMed]
- Byrd, D.A.; Zouiouich, S.; Karwa, S.; Li, X.S.; Wang, Z.; Sampson, J.N.; Loftfield, E.; Huang, W.Y.; Hazen, S.L.; Sinha, R. Associations of serum trimethylamine N-oxide and its precursors with colorectal cancer risk in the Prostate, Lung, Colorectal, Ovarian Cancer Screening Trial Cohort. Cancer 2024, 130, 1982–1990. [Google Scholar] [CrossRef]
- D’Onofrio, N.; Cacciola, N.A.; Martino, E.; Borrelli, F.; Fiorino, F.; Lombardi, A.; Neglia, G.; Balestrieri, M.L.; Campanile, G. ROS-Mediated Apoptotic Cell Death of Human Colon Cancer LoVo Cells by Milk δ-Valerobetaine. Sci. Rep. 2020, 10, 8978. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, N.; Martino, E.; Balestrieri, A.; Mele, L.; Neglia, G.; Balestrieri, M.L.; Campanile, G. SIRT3 and Metabolic Reprogramming Mediate the Antiproliferative Effects of Whey in Human Colon Cancer Cells. Cancers 2021, 13, 5196. [Google Scholar] [CrossRef]
- D’Onofrio, N.; Martino, E.; Mele, L.; Colloca, A.; Maione, M.; Cautela, D.; Castaldo, D.; Balestrieri, M.L. Colorectal Cancer Apoptosis Induced by Dietary δ-Valerobetaine Involves PINK1/Parkin Dependent-Mitophagy and SIRT3. Int. J. Mol. Sci. 2021, 22, 8117. [Google Scholar] [CrossRef] [PubMed]
- Alhasaniah, A.H. l-carnitine: Nutrition, pathology, and health benefits. Saudi J. Biol. Sci. 2023, 30, 103555. [Google Scholar] [CrossRef]
- Farahzadi, R.; Hejazi, M.S.; Molavi, O.; Pishgahzadeh, E.; Montazersaheb, S.; Jafari, S. Clinical Significance of Carnitine in the Treatment of Cancer: From Traffic to the Regulation. Oxid. Med. Cell Longev. 2023, 2023, 9328344. [Google Scholar] [CrossRef]
- Wang, S.; Xu, J.; Zheng, J.; Zhang, X.; Shao, J.; Zhao, L.; Hao, J. Anti-Inflammatory and Antioxidant Effects of Acetyl-L-Carnitine on Atherosclerotic Rats. Med. Sci. Monit. 2020, 26, e920250. [Google Scholar] [CrossRef] [PubMed]
- Fathi, E.; Farahzadi, R.; Javanmardi, S.; Vietor, I. L-carnitine Extends the Telomere Length of the Cardiac Differentiated CD117+- Expressing Stem Cells. Tissue Cell 2020, 67, 101429. [Google Scholar] [CrossRef] [PubMed]
- Nicassio, L.; Fracasso, F.; Sirago, G.; Musicco, C.; Picca, A.; Marzetti, E.; Calvani, R.; Cantatore, P.; Gadaleta, M.N.; Pesce, V. Dietary supplementation with acetyl-l-carnitine counteracts age-related alterations of mitochondrial biogenesis, dynamics and antioxidant defenses in brain of old rats. Exp. Gerontol. 2017, 98, 99–109. [Google Scholar] [CrossRef]
- Ferreira, G.C.; McKenna, M.C. L-Carnitine and Acetyl-L-carnitine Roles and Neuroprotection in Developing Brain. Neurochem. Res. 2017, 42, 1661–1675. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhu, M.; Lu, Z.; Zhang, Y.; Li, L.; Li, N.; Yin, L.; Wang, H.; Song, W.; Xu, H. L-carnitine ameliorates the muscle wasting of cancer cachexia through the AKT/FOXO3a/MaFbx axis. Nutr. Metab. 2021, 18, 98. [Google Scholar] [CrossRef]
- Matsui, H.; Einama, T.; Shichi, S.; Kanazawa, R.; Shibuya, K.; Suzuki, T.; Matsuzawa, F.; Hashimoto, T.; Homma, S.; Yamamoto, J.; et al. L-Carnitine supplementation reduces the general fatigue of cancer patients during chemotherapy. Mol. Clin. Oncol. 2018, 8, 413–416. [Google Scholar] [CrossRef]
- Baci, D.; Bruno, A.; Cascini, C.; Gallazzi, M.; Mortara, L.; Sessa, F.; Pelosi, G.; Albini, A.; Noonan, D.M. Acetyl-L-Carnitine downregulates invasion (CXCR4/CXCL12, MMP-9) and angiogenesis (VEGF, CXCL8) pathways in prostate cancer cells: Rationale for prevention and interception strategies. J. Exp. Clin. Cancer Res. 2019, 38, 464. [Google Scholar] [CrossRef]
- Albogami, S. The Potential Inhibitory Role of Acetyl-L-Carnitine on Proliferation, Migration, and Gene Expression in HepG2 and HT29 Human Adenocarcinoma Cell Lines. Curr. Issues Mol. Biol. 2023, 45, 2393–2408. [Google Scholar] [CrossRef]
- Wu, Q.J.; Zhang, T.N.; Chen, H.H.; Yu, X.F.; Lv, J.L.; Liu, Y.Y.; Liu, Y.S.; Zheng, G.; Zhao, J.Q.; Wei, Y.F.; et al. The sirtuin family in health and disease. Signal Transduct. Target. Ther. 2022, 7, 402. [Google Scholar] [CrossRef]
- Betsinger, C.N.; Cristea, I.M. Mitochondrial Function, Metabolic Regulation, and Human Disease Viewed through the Prism of Sirtuin 4 (SIRT4) Functions. J. Proteome Res. 2019, 18, 1929–1938. [Google Scholar] [CrossRef]
- Shi, Q.; Liu, T.; Zhang, X.; Geng, J.; He, X.; Nu, M.; Pang, D. Decreased sirtuin 4 expression is associated with poor prognosis in patients with invasive breast cancer. Oncol. Lett. 2016, 12, 2606–2612. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Cheng, J.; Yu, F.; Liu, X.; Yuan, C.; Liu, C.; Chen, X.; Peng, Z. Clinical and therapeutic significance of sirtuin-4 expression in colorectal cancer. Oncol. Rep. 2016, 35, 2801–2810. [Google Scholar] [CrossRef][Green Version]
- Huang, G.; Cui, F.; Yu, F.; Lu, H.; Zhang, M.; Tang, H.; Peng, Z. Sirtuin-4 (SIRT4) is downregulated and associated with some clinicopathological features in gastric adenocarcinoma. Biomed. Pharmacother. 2015, 72, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, G.; Li, X.; Wang, T.; Weng, M.; Zhang, Y. Knockout of SIRT4 decreases chemosensitivity to 5-FU in colorectal cancer cells. Oncol. Lett. 2018, 16, 1675–1681. [Google Scholar] [CrossRef]
- Cai, G.; Ge, Z.; Xu, Y.; Cai, L.; Sun, P.; Huang, G. SIRT4 functions as a tumor suppressor during prostate cancer by inducing apoptosis and inhibiting glutamine metabolism. Sci. Rep. 2022, 12, 12208. [Google Scholar] [CrossRef] [PubMed]
- Miyo, M.; Yamamoto, H.; Konno, M.; Colvin, H.; Nishida, N.; Koseki, J.; Kawamoto, K.; Ogawa, H.; Hamabe, A.; Uemura, M.; et al. Tumour-suppressive function of SIRT4 in human colorectal cancer. Br. J. Cancer 2015, 113, 492–499. [Google Scholar] [CrossRef]
- Cui, Y.; Bai, Y.; Yang, J.; Yao, Y.; Zhang, C.; Liu, C.; Shi, J.; Li, Q.; Zhang, J.; Lu, X.; et al. SIRT4 is the molecular switch mediating cellular proliferation in colorectal cancer through GLS mediated activation of AKT/GSK3β/CyclinD1 pathway. Carcinogenesis 2021, 42, 481–492. [Google Scholar] [CrossRef]
- De Gaetano, A.; Gibellini, L.; Zanini, G.; Nasi, M.; Cossarizza, A.; Pinti, M. Mitophagy and Oxidative Stress: The Role of Aging. Antioxidants 2021, 10, 794. [Google Scholar] [CrossRef]
- Parsons, H.M.; Forte, M.L.; Abdi, H.I.; Brandt, S.; Claussen, A.M.; Wilt, T.; Klein, M.; Ester, E.; Landsteiner, A.; Shaukut, A.; et al. Nutrition as prevention for improved cancer health outcomes: A systematic literature review. JNCI Cancer Spectr. 2023, 7, pkad035. [Google Scholar] [CrossRef]
- Chen, Z.; Xie, Y.; Luo, J.; Chen, T.; Xi, Q.; Zhang, Y.; Sun, J. Milk exosome-derived miRNAs from water buffalo are implicated in immune response and metabolism process. BMC Vet. Res. 2020, 16, 123. [Google Scholar] [CrossRef]
- Cacciola, N.A.; Salzano, A.; D’Onofrio, N.; Venneri, T.; Cicco, P.; Vinale, F.; Petillo, O.; Martano, M.; Maiolino, P.; Neglia, G.; et al. Buffalo Milk Whey Activates Necroptosis and Apoptosis in a Xenograft Model of Colorectal Cancer. Int. J. Mol. Sci. 2022, 23, 8464. [Google Scholar] [CrossRef] [PubMed]
- Salzano, A.; Licitra, F.; D’Onofrio, N.; Balestrieri, M.L.; Limone, A.; Campanile, G.; D’Occhio, M.J.; Neglia, G. Short communication: Space allocation in intensive Mediterranean buffalo production influences the profile of functional biomolecules in milk and dairy products. J. Dairy. Sci. 2019, 102, 7717–7722. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Li, Q.; Zhong, W.; Dong, J.; Wang, Z.; Wang, C. L-carnitine ameliorated fatty liver in high-calorie diet/STZ-induced type 2 diabetic mice by improving mitochondrial function. Diabetol. Metab. Syndr. 2011, 3, 31. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Guo, A.; Shu, Q.; Qi, Y.; Kong, Y.; Sun, Z.; Sun, S.; Fu, Z. L-Carnitine intake prevents irregular feeding-induced obesity and lipid metabolism disorder. Gene 2015, 554, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Casariego, A.; Burgos-Peláez, R.; Martínez-Faedo, C.; Calvo-Gracia, F.; Valero-Zanuy, M.Á.; Luengo-Pérez, L.M.; Cuerda-Compés, C. Metabolic effects of L-carnitine on type 2 diabetes mellitus: Systematic review and meta-analysis. Exp. Clin. Endocrinol. Diabetes 2013, 121, 234–238. [Google Scholar] [CrossRef]
- Mirrafiei, A.; Jayedi, A.; Shab-Bidar, S. The Effects of L-Carnitine Supplementation on Weight Loss, Glycemic Control, and Cardiovascular Risk Factors in Patients With Type 2 Diabetes: A Systematic Review and Dose-response Meta-Analysis of Randomized Controlled Trials. Clin. Ther. 2024, 46, 404–410. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; Lavie, C.J.; Fares, H.; Menezes, A.R.; O’Keefe, J.H. L-carnitine in the secondary prevention of cardiovascular disease: Systematic review and meta-analysis. Mayo Clin. Proc. 2013, 88, 544–551. [Google Scholar] [CrossRef]
- Maldonado, C.; Vázquez, M.; Fagiolino, P. Potential Therapeutic Role of Carnitine and Acetylcarnitine in Neurological Disorders. Curr. Pharm. Des. 2020, 26, 1277–1285. [Google Scholar] [CrossRef]
- Arioz, D.T.; Kanat-Pektas, M.; Tuncer, N.; Koken, T.; Unlu, B.S.; Koken, G.; Yilmazer, M. L-Carnitine: A new insight into the pathogenesis of endometrial cancer. Arch. Gynecol. Obstet. 2015, 291, 1147–1152. [Google Scholar] [CrossRef]
- Kang, C.; Zhang, J.; Xue, M.; Li, X.; Ding, D.; Wang, Y.; Jiang, S.; Chu, F.F.; Gao, Q.; Zhang, M. Metabolomics analyses of cancer tissue from patients with colorectal cancer. Mol. Med. Rep. 2023, 28, 219. [Google Scholar] [CrossRef]
- Lai, J.S.; Haertling, T.; Weinstein, J.; Rademaker, A.W.; Goldman, S. A cross-sectional study of carnitine deficiency and fatigue in pediatric cancer patients. Childs Nerv. Syst. 2016, 32, 475–483. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, S.; Wu, H.J.; Zhang, Z.Q.; Chen, Q.; Liu, B.; Wu, J.P.; Zhu, L. L-carnitine ameliorates cancer cachexia in mice by regulating the expression and activity of carnitine palmityl transferase. Cancer Biol. Ther. 2011, 12, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Zhang, Z.; Zhang, Y.; Pan, X.; Yu, L.; Liu, S. L-Carnitine Ameliorates Cancer Cachexia in Mice Partly via the Carnitine Palmitoyltransferase-Associated PPAR-γ Signaling Pathway. Oncol. Res. Treat. 2015, 38, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Zhang, Z.; Zhang, Y.; Wu, J.; Yu, L.; Liu, S. L-carnitine ameliorates the liver inflammatory response by regulating carnitine palmitoyltransferase I-dependent PPARγ signaling. Mol. Med. Rep. 2016, 13, 1320–1328. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, L.; Huo, Z.; Li, N.; Tong, N.; Chong, F.; Liu, J.; Zhang, Y.; Xu, H. l-Carnitine relieves cachexia-related skeletal muscle fibrosis by inducing deltex E3 ubiquitin ligase 3L to negatively regulate the Runx2/COL1A1 axis. J. Cachexia Sarcopenia Muscle 2024, 15, 1953–1964. [Google Scholar] [CrossRef]
- Chang, B.; Nishikawa, M.; Nishiguchi, S.; Inoue, M. L-carnitine inhibits hepatocarcinogenesis via protection of mitochondria. Int. J. Cancer 2005, 113, 719–729. [Google Scholar] [CrossRef]
- Wenzel, U.; Nickel, A.; Daniel, H. Increased carnitine-dependent fatty acid uptake into mitochondria of human colon cancer cells induces apoptosis. J. Nutr. 2005, 135, 1510–1514. [Google Scholar] [CrossRef]
- Fan, J.P.; Kim, H.S.; Han, G.D. Induction of apoptosis by L-carnitine through regulation of two main pathways in Hepa1c1c 7 cells. Amino Acids 2009, 36, 365–372. [Google Scholar] [CrossRef]
- Wang, X.; Yang, C.; Huang, C.; Wang, W. Dysfunction of the carnitine cycle in tumor progression. Heliyon 2024, 10, e35961. [Google Scholar] [CrossRef]
- Han, A.; Bennett, N.; MacDonald, A.; Johnstone, M.; Whelan, J.; Donohoe, D.R. Cellular Metabolism and Dose Reveal Carnitine-Dependent and -Independent Mechanisms of Butyrate Oxidation in Colorectal Cancer Cells. J. Cell Physiol. 2016, 231, 1804–1813. [Google Scholar] [CrossRef]
- Sarzi-Puttini, P.; Giorgi, V.; Di Lascio, S.; Fornasari, D. Acetyl-L-carnitine in chronic pain: A narrative review. Pharmacol. Res. 2021, 173, 105874. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhan, H.; Ren, Y.; Feng, M.; Wang, Q.; Jiao, Q.; Wang, Y.; Liu, X.; Zhang, S.; Du, L.; et al. Sirtuin 4 activates autophagy and inhibits tumorigenesis by upregulating the p53 signaling pathway. Cell Death Differ. 2023, 30, 313–326. [Google Scholar] [CrossRef]
- Sun, Q.; Tian, Q.; Bravo Iniguez, A.; Sun, X.; Zhang, H.; Deavila, J.; Du, M.; Zhu, M.J. AMPK Deficiency Increases DNA Methylation and Aggravates Colorectal Tumorigenesis in AOM/DSS Mice. Genes 2024, 15, 835. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Cao, P.; Wang, J.H.; Feng, W.; Zhang, Y.; Yang, H. Sulforaphane triggers Sirtuin 3-mediated ferroptosis in colorectal cancer cells via activating the adenosine 5′-monophosphate (AMP)-activated protein kinase/ mechanistic target of rapamycin signaling pathway. Hum. Exp. Toxicol. 2024, 43, 9603271241266106. [Google Scholar] [CrossRef]
- Bai, Y.; Yang, J.; Cui, Y.; Yao, Y.; Wu, F.; Liu, C.; Fan, X.; Zhang, Y. Research Progress of Sirtuin4 in Cancer. Front. Oncol. 2021, 10, 562950. [Google Scholar] [CrossRef]
- Ni, X.; Shang, F.S.; Wang, T.F.; Wu, D.J.; Chen, D.G.; Zhuang, B. Ellagic acid induces apoptosis and autophagy in colon cancer through the AMPK/mTOR pathway. Tissue Cell 2023, 81, 102032. [Google Scholar] [CrossRef] [PubMed]
- Lauzier, A.; Normandeau-Guimond, J.; Vaillancourt-Lavigueur, V.; Boivin, V.; Charbonneau, M.; Rivard, N.; Scott, M.S.; Dubois, C.M.; Jean, S. Colorectal cancer cells respond differentially to autophagy inhibition in vivo. Sci. Rep. 2019, 9, 11316. [Google Scholar] [CrossRef]
- Abu El Maaty, M.A.; Strassburger, W.; Qaiser, T.; Dabiri, Y.; Wölfl, S. Differences in p53 status significantly influence the cellular response and cell survival to 1,25-dihydroxyvitamin D3-metformin cotreatment in colorectal cancer cells. Mol. Carcinog. 2017, 56, 2486–2498. [Google Scholar] [CrossRef]
- Servillo, L.; Giovane, A.; Cautela, D.; Castaldo, D.; Balestrieri, M.L. Where does N(ε)-trimethyllysine for the carnitine biosynthesis in mammals come from? PLoS ONE 2014, 9, e84589. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donisi, I.; Balestrieri, A.; Del Vecchio, V.; Bifulco, G.; Balestrieri, M.L.; Campanile, G.; D’Onofrio, N. l-Carnitine and Acetyl-l-Carnitine Induce Metabolism Alteration and Mitophagy-Related Cell Death in Colorectal Cancer Cells. Nutrients 2025, 17, 1010. https://doi.org/10.3390/nu17061010
Donisi I, Balestrieri A, Del Vecchio V, Bifulco G, Balestrieri ML, Campanile G, D’Onofrio N. l-Carnitine and Acetyl-l-Carnitine Induce Metabolism Alteration and Mitophagy-Related Cell Death in Colorectal Cancer Cells. Nutrients. 2025; 17(6):1010. https://doi.org/10.3390/nu17061010
Chicago/Turabian StyleDonisi, Isabella, Anna Balestrieri, Vitale Del Vecchio, Giovanna Bifulco, Maria Luisa Balestrieri, Giuseppe Campanile, and Nunzia D’Onofrio. 2025. "l-Carnitine and Acetyl-l-Carnitine Induce Metabolism Alteration and Mitophagy-Related Cell Death in Colorectal Cancer Cells" Nutrients 17, no. 6: 1010. https://doi.org/10.3390/nu17061010
APA StyleDonisi, I., Balestrieri, A., Del Vecchio, V., Bifulco, G., Balestrieri, M. L., Campanile, G., & D’Onofrio, N. (2025). l-Carnitine and Acetyl-l-Carnitine Induce Metabolism Alteration and Mitophagy-Related Cell Death in Colorectal Cancer Cells. Nutrients, 17(6), 1010. https://doi.org/10.3390/nu17061010







