Glutamine Peptides: Preparation, Analysis, Applications, and Their Role in Intestinal Barrier Protection
Abstract
1. Introduction
2. Methodology
3. Overview of Glutamine Peptides
3.1. Preparation Method of Glutamine Peptides
3.1.1. Chemical Synthesis
3.1.2. Enzymatic Hydrolysis
3.2. Method for Determination of Glutamine in Glutamine Peptides
3.3. Application of Glutamine Peptides
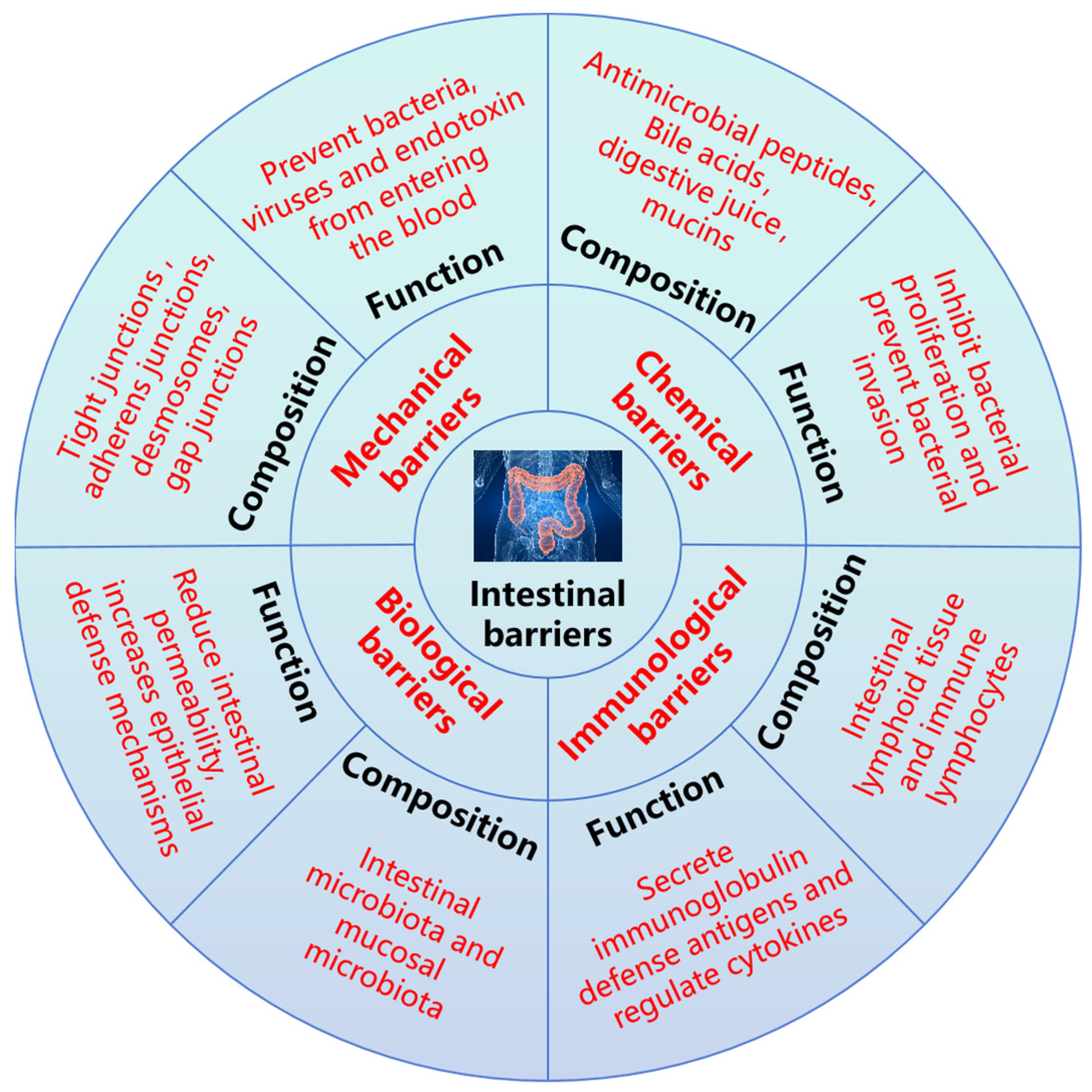
4. Protective Effect of Glutamine Peptides on Intestinal Barrier Function
4.1. Protective Effect of Glutamine Peptides on Mechanical Barrier
4.2. Protective Effect of Glutamine Peptides on Chemical Barrier
4.3. Protective Effect of Glutamine Peptides on Immunological Barrier
4.4. Protective Effect of Glutamine Peptides on the Biological Barrier
5. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, Z.H.; Xu, C.F.; Zheng, T.H.; Li, Q.H.; Yang, S.W.; Shao, J.Y.; Guan, W.T.; Zhang, S.A. A critical role of AMP-activated protein kinase in regulating intestinal nutrient absorption, barrier function, and intestinal diseases. J. Cell. Physiol. 2022, 237, 3705–3716. [Google Scholar] [CrossRef]
- Yin, S.J.; Yang, H.F.; Tao, Y.; Wei, S.M.; Li, L.H.; Liu, M.J.; Li, J.G. Artesunate ameliorates DSS-induced ulcerative colitis by protecting intestinal barrier and inhibiting inflammatory response. Inflammation 2020, 43, 765–776. [Google Scholar] [CrossRef]
- Chen, J.; Wan, J.H.; Ye, J.F.; Xia, L.; Lu, N.H. Emerging role of lncRNAs in the normal and diseased intestinal barrier. Inflamm. Res. 2018, 67, 757–764. [Google Scholar] [CrossRef]
- Yu, H.B.; Gao, Q.F.; Dong, S.L.; Lan, Y.; Ye, Z.; Wen, B. Regulation of dietary glutamine on the growth, intestinal function, immunity and antioxidant capacity of sea cucumber Apostichopus japonicus (Selenka). Fish Shellfish Immun. 2016, 50, 56–65. [Google Scholar] [CrossRef]
- Wang, J.; Ji, H.F. Tight Junction Proteins in the Weaned Piglet Intestine: Roles and Regulation. Curr. Protein Pept. Sc. 2019, 20, e13357. [Google Scholar] [CrossRef]
- Yu, J.; Song, Y.Y.; Yu, B.; He, J.; Zheng, P.; Mao, X.B.; Huang, Z.Q.; Luo, Y.H.; Luo, J.Q.; Yan, H.; et al. Tannic acid prevents post-weaning diarrhea by improving intestinal barrier integrity and function in weaned piglets. J. Anim. Sci. Biotechno. 2020, 11, 87. [Google Scholar] [CrossRef]
- Zhang, H.L.; Ran, C.; Teame, T.; Ding, Q.W.; Hoseinifar, S.H.; Xie, M.X.; Zhang, Z.; Yang, Y.L.; Olsen, R.E.; Gatlin, D.M.; et al. Research progress on gut health of farmers teleost fish: A viewpoint concerning the intestinal mucosal barrier and the impact of its damage. Rev. Fish Biol. Fisher. 2020, 30, 569–586. [Google Scholar] [CrossRef]
- Bhat, Z.F.; Kumar, S.; Bhat, H.F. Bioactive peptides of animal origin: A review. J. Food Sci. Tech. 2015, 52, 5377–5392. [Google Scholar] [CrossRef]
- He, Y.; Qu, J.; Yang, Q.; Wu, Z.L.; Liu, M.; Tso, P. Effect of L-Glutamine on Chylomicron Formation and Fat-Induced Activation of Intestinal Mucosal Mast Cells in Sprague-Dawley Rats. Nutrients 2022, 14, 1777. [Google Scholar] [CrossRef]
- Browne, N.; Horgan, K. The Impact of a Proprietary Blend of Yeast Cell Wall, Short-Chain Fatty Acids, and Zinc Proteinate on Growth, Nutrient Utilisation, and Endocrine Hormone Secretion in Intestinal Cell Models. Animals 2024, 14, 238. [Google Scholar] [CrossRef]
- Chen, Y.; Tsai, Y.H.; Tseng, B.J.; Tseng, S.H. Influence of Growth Hormone and Glutamine on Intestinal Stem Cells: A Narrative Review. Nutrients 2019, 11, 1941. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, Y.; Wei, Y.; Fan, S.; Xia, L.; Chen, Q.; Lu, Y.; Wu, D.; Liu, X.; Peng, X. Glutamine alleviates intestinal injury in a murine burn sepsis model by maintaining intestinal intraepithelial lymphocyte homeostasis. Eur. J. Pharmacol. 2023, 940, 175480. [Google Scholar] [CrossRef]
- Halama, A.; Suhre, K. Advancing Cancer Treatment by Targeting Glutamine Metabolism-A Roadmap. Cancers 2022, 14, 553. [Google Scholar] [CrossRef]
- Kim, M.H.; Kim, H. The Roles of Glutamine in the Intestine and Its Implication in Intestinal Diseases. Int. J. Mol. Sci. 2017, 18, 1051. [Google Scholar] [CrossRef]
- Yue, H.; Tian, Y.; Feng, X.; Bo, Y.; Leng, Z.; Dong, P.; Xue, C.; Wang, J. Novel peptides from sea cucumber intestinal hydrolysates promote longitudinal bone growth in adolescent mice through accelerating cell cycle progress by regulating glutamine metabolism. Food Funct. 2022, 13, 7730–7739. [Google Scholar] [CrossRef]
- Wernerman, J. Glutamine supplementation. Ann. Intensive Care 2011, 1, 25. [Google Scholar] [CrossRef]
- Jane, C.; Rosalie, M.; Hall, J. Glutamine: Metabolism and application in nutrition support. Asia Pac. J. Clin. Nutr. 2004, 13, 25–31. [Google Scholar] [CrossRef]
- Bao, X.Y.; Wu, J.P. Impact of food-derived bioactive peptides on gut function and health. Food Res. Int. 2021, 147, 110485. [Google Scholar] [CrossRef]
- Abumrad, N.N.; Morse, E.L.; Lochs, H.; Williams, P.E.; Adibi, S.A. Possible sources of glutamine for parenteral nutrition: Impact on glutamine metabolism. Am. J. Physiol. Endocrinol. Metab. 1989, 257, E228–E234. [Google Scholar] [CrossRef]
- Furukawa, T.; Hara, T. Potential of γ-L-glutamyl-L-glutamine as an L-glutamine-containing dipeptide for parenteral nutrition. Amino Acids 1989, 11, 1114–1118. [Google Scholar] [CrossRef]
- Lu, Y.J.; Wang, J.; Soladoye, O.P.; Aluko, R.E.; Fu, Y.; Zhang, Y.H. Preparation, receptors, bioactivity and bioavailability of γ-glutamyl peptides: A comprehensive review. Trends Food Sci. Tech. 2021, 113, 301–314. [Google Scholar] [CrossRef]
- Alzaydi, A.; Barbhuiya, R.I.; Routray, W.; Elsayed, A.; Singh, A. Bioactive peptides: Synthesis, applications, and associated challenges. Food Bioeng. 2023, 2, 273–290. [Google Scholar] [CrossRef]
- Li, Y.; Yu, J.M. Research Progress in Structure-Activity Relationship of Bioactive Peptides. J. Med. Food 2015, 18, 147–156. [Google Scholar] [CrossRef]
- Akbarian, M.; Khani, A.; Eghbalpour, S.; Uversky, V.N. Bioactive Peptides: Synthesis, Sources, Applications, and Proposed Mechanisms of Action. Int. J. Mol. Sci. 2022, 23, 1445. [Google Scholar] [CrossRef]
- Liu, W.Y.; Fang, L.; Feng, X.W.; Li, G.M.; Gu, R.Z. In vitro antioxidant and angiotensin I-converting enzyme inhibitory properties of peptides derived from corn gluten meal. Eur. Food Res. Technol. 2020, 246, 2017–2027. [Google Scholar] [CrossRef]
- Shimonishi, Y.; Sakakibara, S.; Akabori, S. Studies on the Synthesis of Peptides Containing Glutamine as the C-Terminal. I. Protection of Amide-nitrogen with Xanthyl Group during Peptide Synthesis. B. Chem. Soc. Jpn. 1962, 35, 1966–1970. [Google Scholar] [CrossRef]
- Merrifield, R. Solid Phase Peptide Synthesis. I. The Synthesis of a Tetrapeptide. J. Am. Chem. Soc. 1963, 85, 2149–2154. [Google Scholar] [CrossRef]
- Furst, P.; Pogan, K.; Stehle, P. Glutamine dipeptides in clinical nutrition. Nutrition 1997, 13, 731–737. [Google Scholar] [CrossRef]
- Yagasaki, M.; Hashimoto, S. Synthesis and application of dipeptides; current status and perspectives. Appl. Microbiol. Biot. 2008, 81, 13–22. [Google Scholar] [CrossRef]
- Yu, T.F.; Hu, T.S.; Na, k.; Zhang, L.; Lu, S.; Guo, X.H. Glutamine-derived peptides: Current progress and future directions. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13386. [Google Scholar] [CrossRef]
- Xie, Y.L.; Liang, X.H.; Wei, M.; Zhao, W.H.; He, B.S.; Lu, Q.Y.; Huo, Q.G.; Ma, C.Y. Optimization of Glutamine Peptide Production from Soybean Meal and Analysis of Molecular Weight Distribution of Hydrolysates. Int. J. Mol. Sci. 2012, 13, 7483–7495. [Google Scholar] [CrossRef]
- Higaki-Sato, N.; Sato, K.; Esumi, Y.; Okumura, T.; Yoshikawa, H.; Tanaka-Kuwajima, C.; Kurata, A.; Ohtsuki, K. Isolation and Identification of Indigestible Pyroglutamyl Peptides in an Enzymatic Hydrolysate of Wheat Gluten Prepared on an Industrial Scale. J. Agric. Food Chem. 2003, 51, 8–13. [Google Scholar] [CrossRef]
- Soichi, T.; Michiko, W.; Soichi, A. Production of a High-Glutamine Oligopeptide Fraction from Gluten by Enzymatic Treatment and Evaluation of its nutritional effect on the small Intestine of rats. J. Food Biochem. 1992, 16, 235–248. [Google Scholar] [CrossRef]
- Tanabe, S.; Arai, S.; Watanabe, M. Large-scale production of a high-glutamine peptide. J. Jpn. Soc. Food Sci. 1999, 52, 103–106. [Google Scholar] [CrossRef]
- Singh, B.P.; Bangar, S.P.; Alblooshi, M.; Ajayi, F.F.; Mudgil, P.; Maqsood, S. Plant-derived proteins as a sustainable source of bioactive peptides: Recent research updates on emerging production methods, bioactivities, and potential application. Crit. Rev. Food Sci. 2023, 63, 9539–9560. [Google Scholar] [CrossRef]
- Liu, W.Y.; Miyakawa, T.; Lu, J.; Hsieh, Y.H.; Gu, R.; Miyauchi, Y.; Katsuno, K.; Cai, M.Y.; Tanokura, M. Isolation and characterization of oligopeptides with vascular disease suppression effects derived from wheat gluten. J. Food Sci. Technol. 2021, 58, 3504–3513. [Google Scholar] [CrossRef]
- Mannheim, A.; Cheryan, M. Water soluble zein by enzymatic modification in organic solvents. Cereal Chem. 1993, 70, 115–121. [Google Scholar] [CrossRef]
- Chen, M.; Ma, A.; Sun, Z.; Xie, B.; Shi, L.; Chen, S.; Chen, L.; Xiong, G.; Wang, L.; Wu, W. Enhancing activity of food protein-derived peptides: An overview of pretreatment, preparation, and modification methods. Compr. Rev. Food Sci. Food Saf. 2023, 22, 4698–4733. [Google Scholar] [CrossRef]
- Swails, W.S.; Bell, S.J.; Borlase, B.C.; Forse, R.A.; Blackburn, G.L. Glutamine content of whole proteins: Implications for enteral formulas. Nutr. Clin. Pract. 1992, 7, 77–80. [Google Scholar] [CrossRef]
- Jochum, F.; Colling, S.; Meinardus, P.; Alteheld, B.; Stehle, P.; Fusch, C. Total glutamine content in human milk is not influenced by gestational age. Acta Paediatr. 2006, 95, 985–990. [Google Scholar] [CrossRef]
- Baxter, J.H.; Phillips, R.R.; Dowlati, L.; Johns, P.W. Glutamine in Commercial Liquid Nutritional Products. J. Agric. Food Chem. 2004, 52, 4963–4968. [Google Scholar] [CrossRef]
- Kuhn, S.K.; Stehle, P.; Furst, P. Quantitative Analyses of Glutamine in Peptides and Proteins. J. Agric. Food Chem. 1996, 44, 1808–1811. [Google Scholar] [CrossRef]
- Xiong, Y.L.; Peng, C.; Deng, Y.J.; Li, W.; Huang, Y.; Tian, Y. Amoxicillin-associated hemorrhagic colitis: A case report and literature review. Medicine 2024, 103, e40800. [Google Scholar] [CrossRef]
- Yu, R.H.; Xiao, Y.M.; Xu, W.H.; Zhang, T.; Wang, Y.Z.; Hu, H. Case Report: Eosinophilic gastritis with pyloric stenosis in immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome. Front. Pediatr. 2022, 10, 1039341. [Google Scholar] [CrossRef]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic peptides: Current applications and future directions. Signal Transduct. Tar. 2022, 7, 48. [Google Scholar] [CrossRef]
- Liu, L.; Li, S.; Zheng, J.; Bu, T.; He, G.; Wu, J. Safety considerations on food protein-derived bioactive peptides. Trends Food Sci. Tech. 2020, 96, 199–207. [Google Scholar] [CrossRef]
- Ji, F.J.; Wang, L.X.; Yang, H.S.; Hu, A.; Yin, Y.L. All About the RNA: Interferon-Stimulated Genes That Interfere with Viral RNA Processes. Front. Immunol. 2019, 13, 2727–2735. [Google Scholar] [CrossRef]
- Salavati, M.E.; Razaeipour, V.; Abdullahpour, R.; Mousavi, N. Effects of Graded Inclusion of Bioactive Peptides Derived from Sesame Meal on the Growth Performance, Internal Organs, Gut Microbiota and Intestinal Morphology of Broiler Chickens. Int. J. Pept. Res. Ther. 2020, 26, 1541–1548. [Google Scholar] [CrossRef]
- Li, C.Q.; Zhang, M.Z.; Li, M.; Zhang, Q.; Qian, Y.X.; Wang, R.X. Effect of dietary alanyl-glutamine dipeptide against chronic ammonia stress induced hyperammonemia in the juvenile yellow catfish (Pelteobagrus fulvidraco). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2018, 213, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Sawaki, K.; Takaoka, I.; Sakuraba, K.; Suzuki, Y. Effects of distance running and subsequent intake of glutamine rich peptide on biomedical parameters of male Japanese athletes. Nutr. Res. 2004, 24, 59–71. [Google Scholar] [CrossRef]
- Gao, Y.A.; Meng, L.; Liu, H.M.; Wang, J.Q.; Zhang, N. The Compromised Intestinal Barrier Induced by Mycotoxins. Toxins 2020, 12, 0619. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Madsen, K.; Spiller, R.; Van Meerveld, B.G.; Verne, G.N. Intestinal barrier function in health and gastrointestinal disease. Neurogastroent. Motil. 2012, 24, 503–512. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Liu, Y.Q.; Wang, Y.Q.; Fan, R.; Hu, X.R.; Zhang, F.; Yang, J.H.; Chen, J.W. The Role of Intestinal Mucosal Barrier in Autoimmune Disease: A Potential Target. Front. Immunol. 2022, 13, 871713. [Google Scholar] [CrossRef]
- Xi, P.B.; Jiang, Z.Y.; Zheng, C.T.; Lin, Y.C.; Wu, G.Y. Regulation of protein metabolism by glutamine: Implications for nutrition and health. Front. Biosci. 2011, 16, 578–597. [Google Scholar] [CrossRef] [PubMed]
- Haertel, F.; Nuding, S.; Reisberg, D.; Peters, M.; Werdan, K.; Schulze, P.C.; Ebelt, H. The Prognostic Value of a Liver Function Test Using Indocyanine Green (ICG) Clearance in Patients with Multiple Organ Dysfunction Syndrome (MODS). J. Clin. Med. 2024, 13, 1039. [Google Scholar] [CrossRef]
- Song, W.K.; Chen, Q.R.; Wang, Y.; Han, Y.; Zhang, H.W.; Li, B.; Yu, G.L. Identification and Structure-Activity Relationship of Intestinal Epithelial Barrier Function Protective Collagen Peptides from Alaska Pollock Skin. Mar. Drugs 2019, 17, 450. [Google Scholar] [CrossRef]
- Jing, Y.; Liu, X.L.; Wang, J.Y.; Ma, Y.Q.; Zheng, X.Q. Production of Corn Protein Hydrolysate with Glutamine-Rich Peptides and Its Antagonistic Function in Ulcerative Colitis in Vivo. Foods 2022, 11, 3359. [Google Scholar] [CrossRef]
- Jing, Y.; Liu, X.L.; Wang, J.Y.; Zheng, X.Q. Corn protein hydrolysate with glutamine-rich peptides protects intestinal barrier in Caco-2 cells: Insights into structural characteristics of identified glutamine peptides. J. Funct. Foods 2024, 117, 106232. [Google Scholar] [CrossRef]
- Marcela, G.M.; Blanca, H.L.; Jose, M.S.; Rosalva, M.E.; Cristina, M.V. Peptides derived from in vitro gastrointestinal digestion of germinated soybean proteins inhibit human colon cancer cells proliferation and inflammation. Food Chem. 2018, 242, 75–82. [Google Scholar] [CrossRef]
- Xu, Q.B.; Hu, M.Y.; Li, M.; Hou, J.X.; Zhang, X.H.; Gao, Y.; Chachar, B.; Li, X. Dietary Bioactive Peptide Alanyl-Glutamine Attenuates Dextran Sodium Sulfate-Induced Colitis by Modulating Gut Microbiota. Oxid. Med. Cell. Longev. 2021, 2021, 5543003. [Google Scholar] [CrossRef]
- Liu, J.; Zong, C.G.; Yu, X.; Ding, Y.; Chang, B.; Wang, R.Y.; Sang, L.X. Alanyl-glutamine (Ala-Gln) ameliorates dextran sulfate sodium (DSS)-induced acute colitis by regulating the gut microbiota, PI3K-Akt/NF-κB/STAT3 signaling, and associated pulmonary injury. ACS Infect. Dis. 2023, 9, 979–992. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.Q.; Xu, B.Y.; Yin, B.Q.; Xu, X.F.; Niu, Y.R.; Tang, Y.M.; Wang, X.K.; Xie, C.L.; Yang, T.; Zhou, S.Y.; et al. Modulation of gut microbial community and metabolism by dietary Glycyl-glutamine supplementation may favor weaning transition in piglets. Front. Microbiol. 2020, 10, 3125. [Google Scholar] [CrossRef] [PubMed]
- Majumder, K.; Chakrabarti, S.; Davidge, S.T.; Wu, J.P. Structure and Activity Study of Egg Protein Ovotransferrin Derived Peptides (IRW and IQW) on Endothelial Inflammatory Response and Oxidative Stress. J. Agric. Food Chem. 2013, 61, 2120–2129. [Google Scholar] [CrossRef]
- Hsieh, L.S.; Lu, M.S.; Chiang, W.D. Identification and characterization of immunomodulatory peptides from pepsin-soy protein hydrolysates. Bioresour. Bioprocess. 2022, 9, 39. [Google Scholar] [CrossRef]
- Zhang, H.; Kovacs, N.J.; Kodera, T.; Eto, Y.; Mine, Y. γ-Glutamyl cysteine and γ-glutamyl valine inhibit TNF-α signaling in intestinal epithelial cells and reduce inflammation in a mouse model of colitis via allosteric activation of the calcium-sensing receptor. BBA-Mol. Basis Dis. 2015, 1852, 792–804. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.W.; Lee, S.J.; Kim, Y.S.; Kim, E.K.; Ahn, C.B.; Jeon, Y.J.; Moon, S.H.; Jeon, B.T.; Park, P.J. Purification and characterization of a novel peptide with inhibitory effects on colitis induced mice by dextran sulfate sodium from enzymatic hydrolysates of Crassostrea gigas. Fish Shellfish Immun. 2012, 33, 993–999. [Google Scholar] [CrossRef]
- Buckley, A.; Turner, J.R. Cell biology of tight junction barrier regulation and mucosal disease. CSH. Perspect. Biol. 2018, 10, a029314. [Google Scholar] [CrossRef]
- Freitas, A.K.L.; Silva, M.T.B.; Silva, C.M.S.; Prata, M.M.G.; Rodrigues, F.A.P.; Siqueira, R.J.B.; Lima, A.A.M.; Santos, A.A.; Havt, A. Alanyl-glutamine protects the intestinal barrier function in trained rats against the impact of acute exhaustive exercise. Braz. J. Med. Biol. Res. 2020, 53, e9211. [Google Scholar] [CrossRef]
- Xing, S.; Zhang, B.L.; Lin, M.; Zhou, P.; Li, J.L.; Zhang, L.; Gao, F.; Zhou, G.H. Effects of alanyl-glutamine supplementation on the small intestinal mucosa barrier in weaned piglets. Asian-Austral. J. Anim. Sci. 2017, 30, 236–245. [Google Scholar] [CrossRef]
- Yasumatsu, H.; Tanabe, S. The casein peptide Asn-Pro-Trp-Asp-Gln enforces the intestinal tight junction partly by increasing occludin expression in Caco-2 cells. Brit. J. Nutr. 2010, 104, 951–956. [Google Scholar] [CrossRef]
- Tanabe, S. Short peptide modules for enhancing intestinal barrier function. Curr. Pharm. Des. 2012, 18, 776–781. [Google Scholar] [CrossRef]
- Chen, S.W.; Zhu, J.; Chen, G.W.; Zuo, S.; Zhang, J.L.; Chen, Z.Y.; Wang, X.; Li, J.X.; Liu, Y.C.; Wang, P.Y. 1,25-dihydroxyvitamin D3 preserves intestinal epithelial barrier function from TNF-alpha induced injury via suppression of NF-κBp65 mediated MLCK-P-MLC signaling pathway. Biochem. Biophys. Res. Commun. 2015, 460, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.R.; Gao, X.; Zhang, H.W.; Li, B.F.; Yu, G.L.; Li, B. Collagen peptides administration in early enteral nutrition intervention attenuates burn-induced intestinal barrier disruption: Effects on tight junction structure. J. Funct. Foods 2019, 55, 167–174. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, H.; Lv, K.; Zhao, W.; Zhang, N.; Yang, F.; Wen, X.; Jiang, X.H.; Tian, J.R.; Liu, X.J.; et al. Pterostilbene Ameliorates DSS-Induced Intestinal Epithelial Barrier Loss in Mice via Suppression of the NF-κB-Mediated MLCK-MLC Signaling Pathway. J. Agric. Food Chem. 2021, 69, 3871–3878. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Z.C.; Dai, J.H.; Yang, P.; Hu, H.B.; Ai, Q.H.; Zhang, W.B.; Zhang, Y.G.; Zhang, Y.J.; Mai, K.S. The protective role of glutamine on enteropathy induced by high dose of soybean meal in turbot, Scophthalmus maximus L. Aquaculture 2018, 497, 510–519. [Google Scholar] [CrossRef]
- Li, W.J.; Li, H.M.; Song, J.Q.; Xing, Y.H.; Fang, L.; Wang, X.Y.; Wu, D.; Min, W.H. Mechanism of Intestinal Epithelial Absorption and Electrophysiological Regulation of the Shrimp Peptide QMDDQ. J. Agric. Food Chem. 2023, 72, 326–338. [Google Scholar] [CrossRef]
- Li, X.; Tan, C.P.; Liu, Y.F.; Xu, Y.J. Interactions between Food Hazards and Intestinal Barrier: Impact on Foodborne Diseases. J. Agric. Food Chem. 2020, 68, 14728–14738. [Google Scholar] [CrossRef]
- Sonnenburg, E.D.; Smits, S.A.; Tikhonov, M.; Higginbottom, S.K.; Wingreen, N.S.; Sonnenburg, J.L. Diet-induced extinctions in the gut microbiota compound over generations. Nature 2016, 529, 212–215. [Google Scholar] [CrossRef]
- Wu, C.Q.; Gao, Y.N.; Li, S.L.; Huang, X.; Bao, X.Y.; Wang, J.Q.; Zheng, N. Modulation of Intestinal Epithelial Permeability and Mucin mRNA (MUC2, MUC5AC, and MUC5B) Expression and Protein Secretion in Caco-2/HT29-MTX Co-culturesExposed to Aflatoxin M1, Ochratoxin A, and Zearalenone Individually or Collectively. Toxicol. Lett. 2019, 309, 1–9. [Google Scholar] [CrossRef]
- Gu, A.X.; Yang, L.G.; Wang, J.J.; Li, J.P.; Shan, A.S. Protective effect of glutamine and alanyl-glutamine against zearalenone-induced intestinal epithelial barrier dysfunction in IPEC-J2 cells. Res. Vet. Sci. 2021, 137, 48–55. [Google Scholar] [CrossRef]
- Hou, Y.C.; Chu, C.C.; Ko, T.L.; Yeh, C.L.; Yeh, S.L. Effects of alanyl-glutamine dipeptide on the expression of colon-inflammatory mediators during the recovery phase of colitis induced by dextran sulfate sodium. Eur. J. Nutr. 2013, 52, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.E.; Hansson, G.C. Immunological aspects of intestinal mucus and mucins. Nat. Rev. Immunol. 2016, 16, 639–649. [Google Scholar] [CrossRef]
- Hao, W.D.; Hao, C.Y.; Wu, C.R.; Xu, Y.Q.; Jin, C.H. Aluminum induced intestinal dysfunction via mechanical, immune, chemical and biological barriers. Chemosphere 2022, 288, 132556. [Google Scholar] [CrossRef]
- Goll, R.; Granlund, A.V. Intestinal barrier homeostasis in inflammatory bowel disease. Scand. J. Gastroentero. 2015, 50, 3–12. [Google Scholar] [CrossRef]
- Kong, C.L.; Faas, M.M.; de Vos, P.; Akkerman, R. Impact of dietary fibers in infant formulas on gut microbiota and the intestinal immune barrier. Food Funct. 2020, 11, 9445–9467. [Google Scholar] [CrossRef] [PubMed]
- Ogata, H.; Hibi, T. Cytokine and anti-cytokine therapies for inflammatory bowel disease. Curr. Pharm. Des. 2003, 9, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Wang, L.; Zhang, M.N.; Lai, Z.J. Glabridin attenuates atopic dermatitis progression through downregulating the TLR4/MyD88/NF-κB signaling pathway. Genes Genom. 2021, 43, 847–855. [Google Scholar] [CrossRef]
- Zhou, M.X.; Xu, W.M.; Wang, J.Z.; Yan, J.K.; Shi, Y.Y.; Zhang, C.; Ge, W.S.; Wu, J.; Du, P.; Chen, Y.W. Boosting mTOR-dependent autophagy via upstream TLR4-MyD88-MAPK signalling and downstream NF-κB pathway quenches intestinal inflammation and oxidative stress injury. EBioMedicine 2018, 35, 345–360. [Google Scholar] [CrossRef]
- Li, Y.L.; Tian, X.D.; Li, S.C.; Chang, L.J.; Sun, P.; Lu, Y.B.; Yu, X.Y.; Chen, S.W.; Wu, Z.Q.; Xu, Z.; et al. Total polysaccharides of adlay bran (Coix lachryma-jobi L.) improve TNF-α induced epithelial barrier dysfunction in Caco-2 cells via inhibition of the inflammatory response. Food Funct. 2019, 10, 2906–2913. [Google Scholar] [CrossRef]
- He, Y.F.; Liang, J.F.; Dong, X.H.; Yang, Q.H.; Liu, H.Y.; Zhang, S.; Chi, S.Y.; Tan, B.P. Glutamine alleviates β-conglycinin-induced enteritis in juvenile hybrid groupers Epinephelus fuscoguttatus♀ × E. lanceolatus♂ by suppressing the MyD88/NF-κB pathway. Aquaculture 2022, 549, 737735. [Google Scholar] [CrossRef]
- Malinowski, J.; Klempt, M.; Clawin-Radecker, I.; Lorenzen, P.C.; Meisel, H. Identification of a NF-κB inhibitory peptide from tryptic β-casein hydrolysate. Food Chem. 2014, 165, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Tanida, S.; Mizoshita, T.; Mizushima, T.; Sasaki, M.; Shimura, T.; Kamiya, T.; Kataoka, H.; Joh, T. Involvement of oxidative stress and mucosal address in cell adhesion molecule-1 (MAdCAM-1) in inflammatory bowel disease. J. Clin. Biochem. Nutr. 2011, 48, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.X.; Keane, R.; Sheng, L.L.; Wan, Y.J.Y. Implications of microbiota and bile acid in liver injury and regeneration. J. Hepatol. 2015, 63, 1502–1510. [Google Scholar] [CrossRef]
- Ma, Y.; Jiang, H.M.; Fang, J.; Liu, G. IRW and IQW Reduce Colitis-Associated Cancer Risk by Alleviating DSS-Induced Colonic Inflammation. Biomed. Res. Int. 2019, 2019, 6429845. [Google Scholar] [CrossRef]
- Pepe, G.; Sommella, E.; Ventre, G.; Scala, M.C.; Adesso, S.; Ostacolo, C.; Marzocco, S.; Novellino, E.; Campiglia, P. Antioxidant peptides released from gastrointestinal digestion of “Stracchino” soft cheese: Characterization, in vitro intestinal protection and bioavailability. J. Funct. Foods 2016, 26, 494–505. [Google Scholar] [CrossRef]
- Camilleri, M. Human Intestinal Barrier: Effects of Stressors, Diet, Prebiotics, and Probiotics. Clin. Transl. Gastroen. 2021, 12, e00308. [Google Scholar] [CrossRef]
- Natividad, J.M.; Verdu, E.F. Modulation of intestinal barrier by intestinal microbiota: Pathological and therapeutic implications. Pharmacol. Res. 2013, 69, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Yu, S.Y.; Sun, Y.B.; Shao, X.Y.; Zhou, Y.Q.; Yu, Y.; Kuai, X.Y.; Zhou, C.L. Leaky Gut in IBD: Intestinal Barrier-Gut Microbiota Interaction. J. Microbiol. Biotechn. 2022, 32, 825–834. [Google Scholar] [CrossRef]
- Liu, J.H.; He, Z.Y.; Ma, N.; Chen, Z.Y. Beneficial Effects of Dietary Polyphenols on High-Fat Diet-Induced Obesity Linking with Modulation of Gut Microbiota. J. Agric. Food Chem. 2020, 68, 33–47. [Google Scholar] [CrossRef]
- Hao, S.Q.; Ming, L.; Li, Y.F.; Lv, H.D.; Li, L.; Jambal, T.; Ji, R. Modulatory effect of camel milk on intestinal microbiota of mice with non-alcoholic fatty liver disease. Front. Nutr. 2022, 9, 1072133. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, M.X.; Li, W.; Ma, L.N.; Liu, X.L.; Ding, Q.T.; Yu, W.M.; Yu, T.J.; Ding, C.B.; Liu, W.C. Research progress of natural plant polysaccharides inhibiting inflammatory signaling pathways and regulating intestinal flora and metabolism to protect inflammatory bowel disease. Int. J. Biol. Macromol. 2023, 253, 126799. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Liu, X.L.; Deng, Y.P.; Zheng, X.Q. Preparation of Glutamine-Enriched Fermented Feed from Corn Gluten Meal and Its Functionality Evaluation. Foods 2023, 12, 4336. [Google Scholar] [CrossRef]
- Huo, J.Y.; Wu, Z.Y.; Sun, W.Z.; Wang, Z.H.; Wu, J.H.; Huang, M.Q.; Wang, B.W.; Sun, B.G. Protective Effects of Natural Polysaccharides on Intestinal Barrier Injury: A Review. J. Agric. Food Chem. 2022, 70, 711–735. [Google Scholar] [CrossRef] [PubMed]
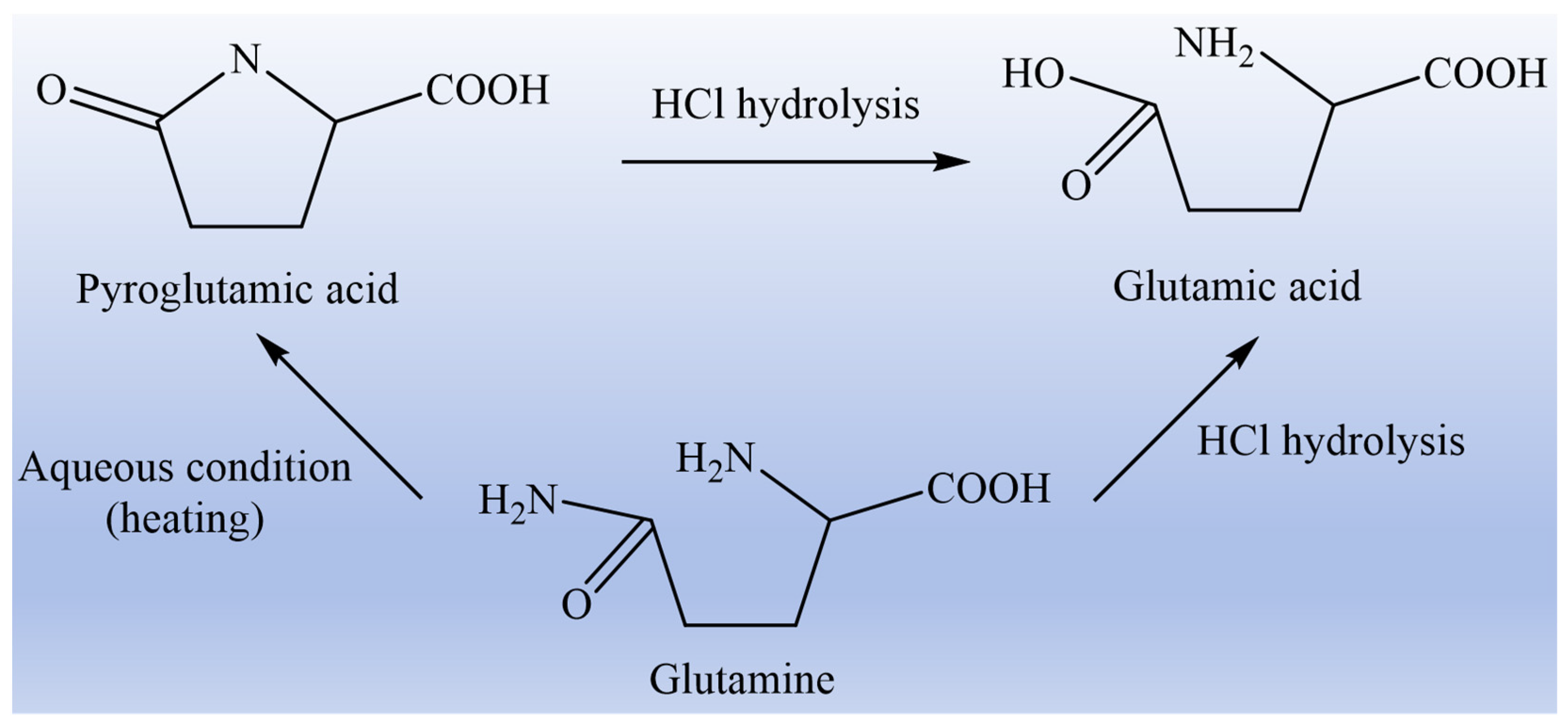


| Protease | Pepsin | Trypsin | Papain | Flavourzyme | Alcalase | Neutrase | Protamex |
|---|---|---|---|---|---|---|---|
| Property | Endopeptidase | Endopeptidase | Endopeptidase | Endopeptidase/Exopeptidase | Endopeptidase | Endopeptidase | Endopeptidase |
| Action site | Leu-Phe- | Lys-Arg- | Asn-Gln, Glu-Ala, Leu-Val | — | Ala-Leu- | — | — |
| Temperature | 37 | 37 | 45–75 | 50 | 55–70 | 45–55 | 35–60 |
| pH | 1.8–3.0 | 6.0–9.0 | 5.0–7.0 | 5.0–7.0 | 6.5–8.5 | 5.5–7.5 | 5.5–7.5 |
| Method | Advantage | Limitation |
|---|---|---|
| Chemical synthesis | High efficiency, high product purity, and excellent stability | High cost, complex operation, and pollute the environment |
| Enzymatic hydrolysis | Simple operation and mild reaction conditions | Low efficiency, non-unique product, and low purity |
| Method | Advantage | Limitation |
|---|---|---|
| Enzymatic Hydrolysis | Simple operation and mild reaction conditions | An indirect method for determination and only suitable for determination of short peptides |
| Edman Degradation | A direct determination method with accurate results | High cost, complex operation, and only suitable for single peptide samples |
| BTI Derivative | Simple operation and no special restrictions on the samples | An indirect method for determination and only suitable for determination of non-N-terminal glutamine |
| Peptides/Hydrolysates | Sequence | Model/Method | Main Mechanism | References |
|---|---|---|---|---|
| Collagen peptide | GPSGPQGSR | In vitro model of TNF-α treated Caco-2 cell monolayers | Increased TEER of Caco-2 cell monolayer and decreased its permeability | (Song et al., 2019) [56] |
| Corn protein hydrolysate with glutamine-rich peptides | A total of 374 corn glutamine peptides | A mouse model of DSS-induced colitis; In vitro model of LPS-treated Caco-2 cell | Reduced the permeability of the colonic mucosa in mice, regulated the abundance and diversity of the intestinal microbiota; Up-regulated the gene expression of tight junction proteins, and regulated levels of inflammatory cytokines | (Jing et al., 2022; Jing et al., 2024) [57,58] |
| Peptides derived from in vitro gastrointestinal digestion of germinated soybean proteins | QQQQQGGSQSQ, QEPQESQQ, QQQQQGGSQSQSQKG, PETMQQQQQQ | In vitro model of LPS-treated RAW267.4 | Reduced inflammatory response | (Marcela et al., 2018) [59] |
| Peptide | AQ | A mouse model of DSS-induced colitis | Decreased levels of inflammatory cytokines and increased the expression of occludin; Modulated gut microbiota and microflora metabolites | (Xu et al., 2021; Liu et al., 2023) [60,61] |
| Peptide | GQ | A Piglet model | Modulated the gut microbiota and microflora metabolites | (Yan et al., 2020) [62] |
| Peptide derived from egg white protein ovotransferrin | IQW | In vitro model of TNF-induced endothelial cells | Inhibited the up-regulation of intercellular cell adhesion molecule-I | (Majumder et al. 2013) [63] |
| Peptide derived from pepsin–soy protein hydrolysates | EKPQQQSSRRGS | In vitro model of LPS-treated RAW267.4 | Enhance immune regulation | (Hsieh et al. 2022) [64] |
| Peptide | CQ, VQ | A mouse model of DSS-induced colitis; In vitro model of TNF-α treated Caco-2 cell | Inhibit the expression of inflammatory cytokines | (Zhang et al. 2015) [65] |
| Peptide derived from Crassostrea gigas | QCQCAVEGGL | A mouse model of DSS-induced colitis | Decreased serum IgE levels and increased spleen CD4+/ CD8+ levels | (Hwang et al. 2012) [66] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; He, Y.; Liu, Z.; Liu, X.; Jing, Y. Glutamine Peptides: Preparation, Analysis, Applications, and Their Role in Intestinal Barrier Protection. Nutrients 2025, 17, 1017. https://doi.org/10.3390/nu17061017
Wang J, He Y, Liu Z, Liu X, Jing Y. Glutamine Peptides: Preparation, Analysis, Applications, and Their Role in Intestinal Barrier Protection. Nutrients. 2025; 17(6):1017. https://doi.org/10.3390/nu17061017
Chicago/Turabian StyleWang, Jinyu, Yating He, Zedan Liu, Xiaolan Liu, and Yan Jing. 2025. "Glutamine Peptides: Preparation, Analysis, Applications, and Their Role in Intestinal Barrier Protection" Nutrients 17, no. 6: 1017. https://doi.org/10.3390/nu17061017
APA StyleWang, J., He, Y., Liu, Z., Liu, X., & Jing, Y. (2025). Glutamine Peptides: Preparation, Analysis, Applications, and Their Role in Intestinal Barrier Protection. Nutrients, 17(6), 1017. https://doi.org/10.3390/nu17061017





