Early Time-Restricted Eating Improves Weight Loss While Preserving Muscle: An 8-Week Trial in Young Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval and Participants
- (1)
- No regular resistance training experience in the previous six months;
- (2)
- No serious musculoskeletal or cardiovascular disorders;
- (3)
- Non-smokers and non-heavy drinkers.
2.2. Interventions
- The eTRE group (early time-restricted eating): 8:00 AM–2:00 PM, permitting ±30 min flexibility for the first and last meal;
- The dTRE group (delayed time-restricted eating): 12:00 PM–6:00 PM, also permitting ±30 min flexibility in their meal timing;
- The control group: 8:00 AM–8:00 PM, with no additional time restrictions.
2.3. Outcome Measurements
- Body weight: This was measured using a calibrated digital scale with 0.1 kg precision. The participants wore light clothing and were instructed to empty their bladder prior to measurement.
- Thickness of the long head of the triceps brachii: This was assessed using an ultrasound device (LV8-4L65S-3, Telemed, Vilnius, Lithuania). The participants were seated, with their forearms resting on a table with their palms facing downward. The midpoint between the acromion and the olecranon was marked, and a water-soluble ultrasound gel was evenly applied. The transducer was placed vertically to measure muscle thickness. Each participant underwent three measurements, and the mean value was used for analysis. All measurements were performed by the same experienced technician to minimize inter-rater variability. The triceps brachii was selected as the primary muscle for analysis for two main reasons. First, push-ups effectively stimulate the triceps brachii, making it a relevant muscle to assess in this study. Second, compared to other muscle groups, the triceps brachii is more accessible for measurement, allowing for greater accuracy and reproducibility in ultrasound assessments.
- Muscular endurance: This was defined as the maximum number of knee-supported push-ups the participant could complete in a single test session, summing the repetitions from up to four sets. The first test was performed at baseline, and the second was scheduled at least 48 h after the final training session to minimize the effects of fatigue. Participants were instructed to maintain proper form; once they could no longer meet the standard, testing stopped, and the last valid repetition was recorded.
2.4. Statistical Analysis
3. Results
3.1. Physical Characteristics
3.2. Adherence to Eating Time and Sleep Duration
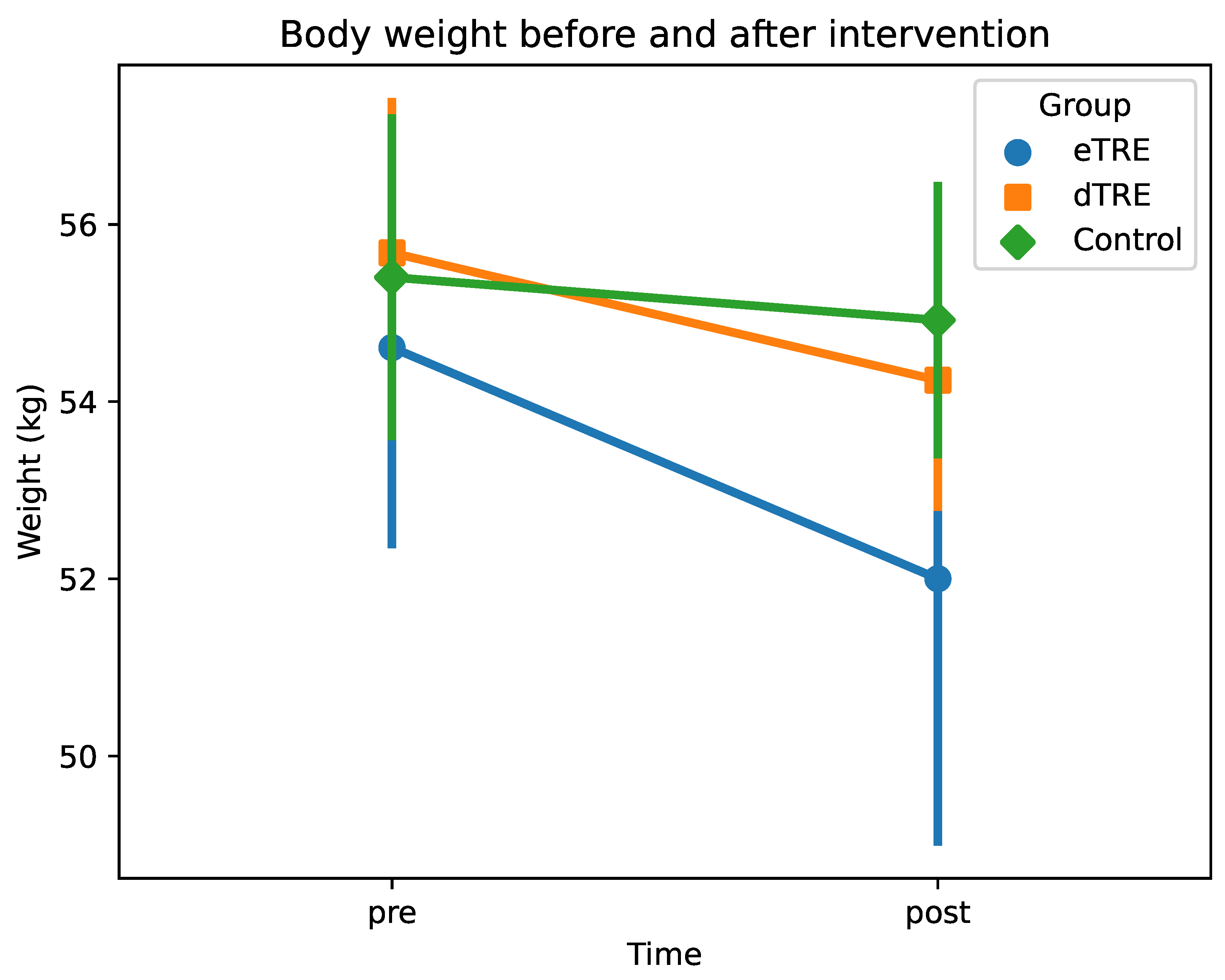
3.3. Changes in Body Weight
3.4. Changes in the Thickness of the Long Head of the Triceps Brachii
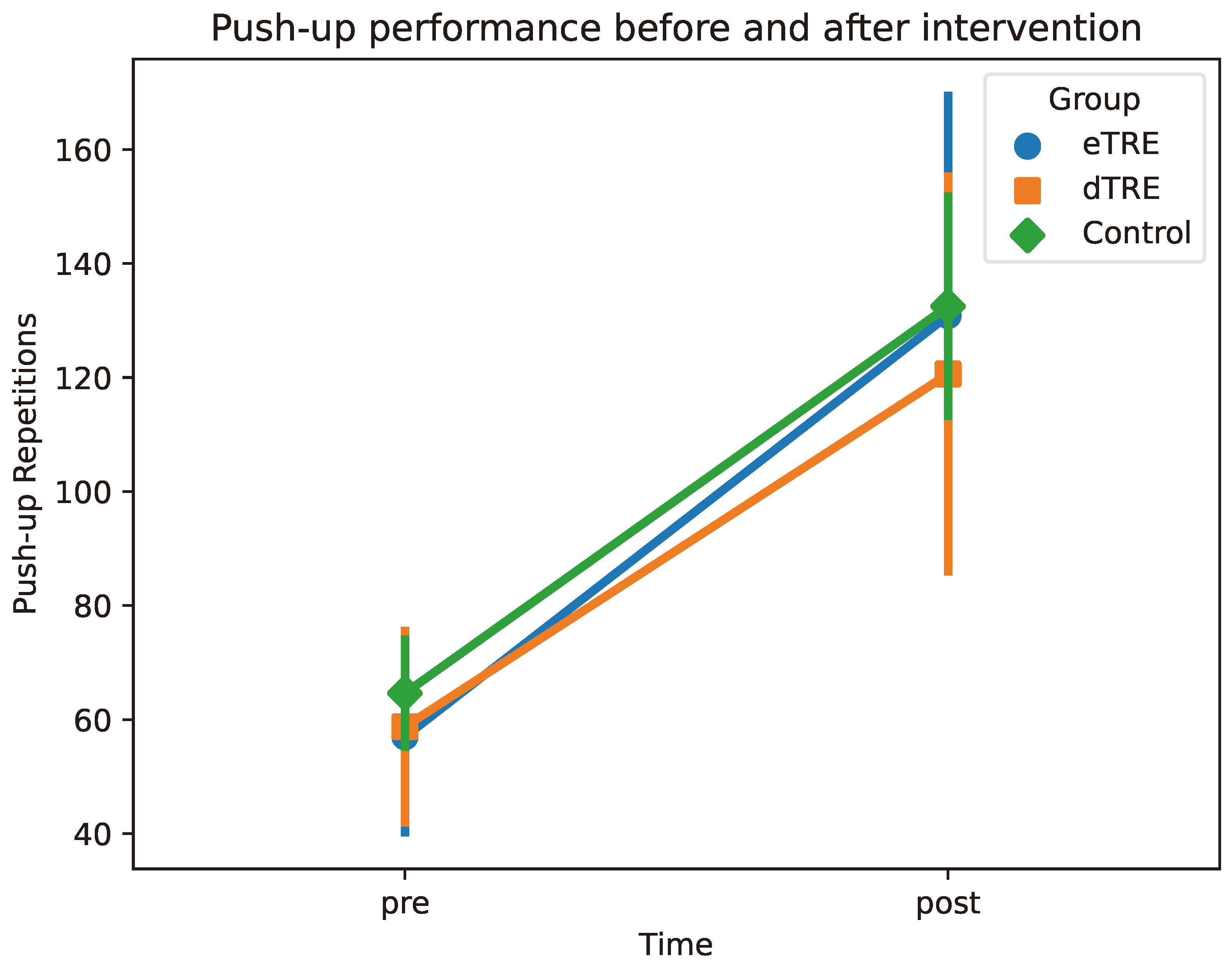
3.5. Changes in Push-Up Performance
4. Discussion
4.1. Significant Weight Loss Effects of eTRE
4.2. The Limited Influence of TRE on Muscle Hypertrophy and Endurance
4.3. Hormonal Responses to TRE
4.4. Secondary Findings: Sleep Quality and Adherence
4.5. Limitations and Directions for Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TRE | time-restricted eating |
| eTRE | early time-restricted eating |
| dTRE | delayed time-restricted eating |
| SCN | suprachiasmatic nucleus |
| MyoPS | myofibrillar protein synthesis |
| RT | resistance training |
| GH | growth hormone |
References
- Evans, W.J. Skeletal muscle loss: Cachexia, sarcopenia, and inactivity. Am. J. Clin. Nutr. 2010, 91, 1123S–1127S. [Google Scholar] [CrossRef] [PubMed]
- Zurlo, F.; Larson, K.; Bogardus, C.; Ravussin, E. Skeletal muscle metabolism is a major determinant of resting energy expenditure. J. Clin. Investig. 1990, 86, 1423–1427. [Google Scholar] [CrossRef]
- Choi, K.M. Sarcopenia and sarcopenic obesity. Korean J. Intern. Med. 2016, 31, 1054. [Google Scholar] [CrossRef]
- Wannamethee, S.G.; Atkins, J.L. Muscle loss and obesity: The health implications of sarcopenia and sarcopenic obesity. Proc. Nutr. Soc. 2015, 74, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Mesinovic, J.; Zengin, A.; De Courten, B.; Ebeling, P.R.; Scott, D. Sarcopenia and type 2 diabetes mellitus: A bidirectional relationship. Diabetes Metab. Syndr. Obes. 2019, 12, 1057–1072. [Google Scholar] [CrossRef]
- Schoenfeld, B.J. The mechanisms of muscle hypertrophy and their application to resistance training. J. Strength Cond. Res. 2010, 24, 2857–2872. [Google Scholar] [CrossRef] [PubMed]
- Willis, L.H.; Slentz, C.A.; Bateman, L.A.; Shields, A.T.; Piner, L.W.; Bales, C.W.; Houmard, J.A.; Kraus, W.E. Effects of aerobic and/or resistance training on body mass and fat mass in overweight or obese adults. J. Appl. Physiol. 2012, 113, 1831–1837. [Google Scholar] [CrossRef]
- Garthe, I.; Raastad, T.; Refsnes, P.E.; Sundgot-Borgen, J. Effect of nutritional intervention on body composition and performance in elite athletes. Eur. J. Sport Sci. 2013, 13, 295–303. [Google Scholar] [CrossRef]
- Funderburk, L.; Heileson, J.; Peterson, M.; Willoughby, D.S. Efficacy of L-Leucine supplementation coupled with a calorie-restricted diet to promote weight loss in mid-life women. J. Am. Coll. Nutr. 2021, 40, 699–707. [Google Scholar] [CrossRef]
- Weiss, E.P.; Racette, S.B.; Villareal, D.T.; Fontana, L.; Steger-May, K.; Schechtman, K.B.; Klein, S.; Ehsani, A.A.; Holloszy, J.O.; Washington University School of Medicine CALERIE Group. Lower extremity muscle size and strength and aerobic capacity decrease with caloric restriction but not with exercise-induced weight loss. J. Appl. Physiol. 2007, 102, 634–640. [Google Scholar] [CrossRef]
- Regmi, P.; Heilbronn, L.K. Time-restricted eating: Benefits, mechanisms, and challenges in translation. iScience 2020, 23, 101161. [Google Scholar] [CrossRef] [PubMed]
- Manoogian, E.N.; Chow, L.S.; Taub, P.R.; Laferrère, B.; Panda, S. Time-restricted eating for the prevention and management of metabolic diseases. Endocr. Rev. 2022, 43, 405–436. [Google Scholar] [CrossRef]
- Longo, V.D.; Panda, S. Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metab. 2016, 23, 1048–1059. [Google Scholar] [CrossRef]
- Panda, S. Circadian physiology of metabolism. Science 2016, 354, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Ulgherait, M.; Midoun, A.M.; Park, S.J.; Gatto, J.A.; Tener, S.J.; Siewert, J.; Klickstein, N.; Canman, J.C.; Ja, W.W.; Shirasu-Hiza, M. Circadian autophagy drives iTRF-mediated longevity. Nature 2021, 598, 353–358. [Google Scholar] [CrossRef]
- Froy, O.; Miskin, R. Effect of feeding regimens on circadian rhythms: Implications for aging and longevity. Aging 2010, 2, 7. [Google Scholar] [CrossRef]
- Vitaterna, M.H.; Takahashi, J.S.; Turek, F.W. Overview of circadian rhythms. Alcohol Res. Health 2001, 25, 85. [Google Scholar] [PubMed]
- Chaix, A.; Manoogian, E.N.; Melkani, G.C.; Panda, S. Time-restricted eating to prevent and manage chronic metabolic diseases. Annu. Rev. Nutr. 2019, 39, 291–315. [Google Scholar] [CrossRef]
- Sutton, E.F.; Beyl, R.; Early, K.S.; Cefalu, W.T.; Ravussin, E.; Peterson, C.M. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 2018, 27, 1212–1221. [Google Scholar] [CrossRef]
- Mishra, S.; Persons, P.A.; Lorenzo, A.M.; Chaliki, S.S.; Bersoux, S. Time-restricted eating and its metabolic benefits. J. Clin. Med. 2023, 12, 7007. [Google Scholar] [CrossRef]
- Adafer, R.; Messaadi, W.; Meddahi, M.; Patey, A.; Haderbache, A.; Bayen, S.; Messaadi, N. Food timing, circadian rhythm and chrononutrition: A systematic review of time-restricted eating’s effects on human health. Nutrients 2020, 12, 3770. [Google Scholar] [CrossRef] [PubMed]
- Che, T.; Yan, C.; Tian, D.; Zhang, X.; Liu, X.; Wu, Z. Time-restricted feeding improves blood glucose and insulin sensitivity in overweight patients with type 2 diabetes: A randomised controlled trial. Nutr. Metab. 2021, 18, 88. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Longo, V.D.; Harvie, M. Impact of intermittent fasting on health and disease processes. Ageing Res. Rev. 2017, 39, 46–58. [Google Scholar] [CrossRef]
- Bass, J. Circadian topology of metabolism. Nature 2012, 491, 348–356. [Google Scholar] [CrossRef]
- Lundell, L.S.; Parr, E.B.; Devlin, B.L.; Ingerslev, L.R.; Altıntaş, A.; Sato, S.; Sassone-Corsi, P.; Barrès, R.; Zierath, J.R.; Hawley, J.A. Time-restricted feeding alters lipid and amino acid metabolite rhythmicity without perturbing clock gene expression. Nat. Commun. 2020, 11, 4643. [Google Scholar] [CrossRef] [PubMed]
- Cienfuegos, S.; Gabel, K.; Kalam, F.; Ezpeleta, M.; Wiseman, E.; Pavlou, V.; Lin, S.; Oliveira, M.L.; Varady, K.A. Effects of 4-and 6-h time-restricted feeding on weight and cardiometabolic health: A randomized controlled trial in adults with obesity. Cell Metab. 2020, 32, 366–378. [Google Scholar] [CrossRef]
- Wilkinson, M.J.; Manoogian, E.N.; Zadourian, A.; Lo, H.; Fakhouri, S.; Shoghi, A.; Wang, X.; Fleischer, J.G.; Navlakha, S.; Panda, S.; et al. Ten-hour time-restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome. Cell Metab. 2020, 31, 92–104. [Google Scholar] [CrossRef]
- Chaix, A.; Zarrinpar, A.; Miu, P.; Panda, S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 2014, 20, 991–1005. [Google Scholar] [CrossRef]
- Pellegrini, M.; Cioffi, I.; Evangelista, A.; Ponzo, V.; Goitre, I.; Ciccone, G.; Ghigo, E.; Bo, S. Effects of time-restricted feeding on body weight and metabolism. A systematic review and meta-analysis. Rev. Endocr. Metab. Disord. 2020, 21, 17–33. [Google Scholar] [CrossRef]
- Tinsley, G.M.; Moore, M.L.; Graybeal, A.J.; Paoli, A.; Kim, Y.; Gonzales, J.U.; Harry, J.R.; VanDusseldorp, T.A.; Kennedy, D.N.; Cruz, M.R. Time-restricted feeding plus resistance training in active females: A randomized trial. Am. J. Clin. Nutr. 2019, 110, 628–640. [Google Scholar] [CrossRef]
- Kotarsky, C.J.; Johnson, N.R.; Mahoney, S.J.; Mitchell, S.L.; Schimek, R.L.; Stastny, S.N.; Hackney, K.J. Time-restricted eating and concurrent exercise training reduces fat mass and increases lean mass in overweight and obese adults. Physiol. Rep. 2021, 9, e14868. [Google Scholar] [CrossRef] [PubMed]
- Stote, K.S.; Baer, D.J.; Spears, K.; Paul, D.R.; Harris, G.K.; Rumpler, W.V.; Strycula, P.; Najjar, S.S.; Ferrucci, L.; Ingram, D.K.; et al. A controlled trial of reduced meal frequency without caloric restriction in healthy, normal-weight, middle-aged adults. Am. J. Clin. Nutr. 2007, 85, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Tinsley, G.M.; La Bounty, P.M. Effects of intermittent fasting on body composition and clinical health markers in humans. Nutr. Rev. 2015, 73, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Moro, T.; Tinsley, G.; Bianco, A.; Marcolin, G.; Pacelli, Q.F.; Battaglia, G.; Palma, A.; Gentil, P.; Neri, M.; Paoli, A. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J. Transl. Med. 2016, 14, 290. [Google Scholar] [CrossRef]
- Tinsley, G.M.; Forsse, J.S.; Butler, N.K.; Paoli, A.; Bane, A.A.; La Bounty, P.M.; Morgan, G.B.; Grandjean, P.W. Time-restricted feeding in young men performing resistance training: A randomized controlled trial. Eur. J. Sport Sci. 2017, 17, 200–207. [Google Scholar] [CrossRef]
- Hutchison, A.T.; Regmi, P.; Manoogian, E.N.; Fleischer, J.G.; Wittert, G.A.; Panda, S.; Heilbronn, L.K. Time-restricted feeding improves glucose tolerance in men at risk for type 2 diabetes: A randomized crossover trial. Obesity 2019, 27, 724–732. [Google Scholar] [CrossRef]
- Xie, Z.; Sun, Y.; Ye, Y.; Hu, D.; Zhang, H.; He, Z.; Zhao, H.; Yang, H.; Mao, Y. Randomized controlled trial for time-restricted eating in healthy volunteers without obesity. Nat. Commun. 2022, 13, 1003. [Google Scholar] [CrossRef]
- Liu, J.; Yi, P.; Liu, F. The effect of early time-restricted eating vs later time-restricted eating on weight loss and metabolic health. J. Clin. Endocrinol. Metab. 2023, 108, 1824–1834. [Google Scholar] [CrossRef]
- Petridi, F.; Geurts, J.M.; Nyakayiru, J.; Schaafsma, A.; Schaafsma, D.; Meex, R.C.; Singh-Povel, C.M. Effects of Early and Late Time-Restricted Feeding on Parameters of Metabolic Health: An Explorative Literature Assessment. Nutrients 2024, 16, 1721. [Google Scholar] [CrossRef]
- Shimizu, H.; Hanzawa, F.; Kim, D.; Sun, S.; Laurent, T.; Umeki, M.; Ikeda, S.; Mochizuki, S.; Oda, H. Delayed first active-phase meal, a breakfast-skipping model, led to increased body weight and shifted the circadian oscillation of the hepatic clock and lipid metabolism-related genes in rats fed a high-fat diet. PLoS ONE 2018, 13, e0206669. [Google Scholar] [CrossRef]
- Regmi, P.; Chaudhary, R.; Page, A.J.; Hutchison, A.T.; Vincent, A.D.; Liu, B.; Heilbronn, L. Early or delayed time-restricted feeding prevents metabolic impact of obesity in mice. J. Endocrinol. 2021, 248, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Jamshed, H.; Beyl, R.A.; Della Manna, D.L.; Yang, E.S.; Ravussin, E.; Peterson, C.M. Early time-restricted feeding improves 24-hour glucose levels and affects markers of the circadian clock, aging, and autophagy in humans. Nutrients 2019, 11, 1234. [Google Scholar] [CrossRef]
- Schoenfeld, B.J. Potential mechanisms for a role of metabolic stress in hypertrophic adaptations to resistance training. Sports Med. 2013, 43, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.W.; Jeong, J.H.; Hong, S.C. The impact of sleep and circadian disturbance on hormones and metabolism. Int. J. Endocrinol. 2015, 2015, 591729. [Google Scholar] [CrossRef]
- Brambilla, D.J.; Matsumoto, A.M.; Araujo, A.B.; McKinlay, J.B. The effect of diurnal variation on clinical measurement of serum testosterone and other sex hormone levels in men. J. Clin. Endocrinol. Metab. 2009, 94, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Saad, A.; Dalla Man, C.; Nandy, D.K.; Levine, J.A.; Bharucha, A.E.; Rizza, R.A.; Basu, R.; Carter, R.E.; Cobelli, C.; Kudva, Y.C.; et al. Diurnal pattern to insulin secretion and insulin action in healthy individuals. Diabetes 2012, 61, 2691–2700. [Google Scholar] [CrossRef]
- Schoenfeld, B.J.; Ratamess, N.A.; Peterson, M.D.; Contreras, B.; Sonmez, G.; Alvar, B.A. Effects of different volume-equated resistance training loading strategies on muscular adaptations in well-trained men. J. Strength Cond. Res. 2014, 28, 2909–2918. [Google Scholar] [CrossRef]
- Richter, J.; Herzog, N.; Janka, S.; Baumann, T.; Kistenmacher, A.; Oltmanns, K.M. Twice as high diet-induced thermogenesis after breakfast vs dinner on high-calorie as well as low-calorie meals. J. Clin. Endocrinol. Metab. 2020, 105, e211–e221. [Google Scholar] [CrossRef]
- Paoli, A.; Tinsley, G.; Bianco, A.; Moro, T. The influence of meal frequency and timing on health in humans: The role of fasting. Nutrients 2019, 11, 719. [Google Scholar] [CrossRef]
- Garaulet, M.; Gómez-Abellán, P. Timing of food intake and obesity: A novel association. Physiol. Behav. 2014, 134, 44–50. [Google Scholar] [CrossRef]
- Wall, B.T.; Dirks, M.L.; Snijders, T.; van Dijk, J.W.; Fritsch, M.; Verdijk, L.B.; van Loon, L.J. Short-term muscle disuse lowers myofibrillar protein synthesis rates and induces anabolic resistance to protein ingestion. Am. J. Physiol.-Endocrinol. Metab. 2016, 310, E137–E147. [Google Scholar] [CrossRef] [PubMed]
- Parr, E.B.; Kouw, I.W.; Wheeler, M.J.; Radford, B.E.; Hall, R.C.; Senden, J.M.; Goessens, J.P.; Van Loon, L.J.; Hawley, J.A. Eight-hour time-restricted eating does not lower daily myofibrillar protein synthesis rates: A randomized control trial. Obesity 2023, 31, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Ascenzi, F.; Barberi, L.; Dobrowolny, G.; Villa Nova Bacurau, A.; Nicoletti, C.; Rizzuto, E.; Rosenthal, N.; Scicchitano, B.M.; Musarò, A. Effects of IGF-1 isoforms on muscle growth and sarcopenia. Aging Cell 2019, 18, e12954. [Google Scholar] [CrossRef] [PubMed]
- Herbst, K.L.; Bhasin, S. Testosterone action on skeletal muscle. Curr. Opin. Clin. Nutr. Metab. Care 2004, 7, 271–277. [Google Scholar] [CrossRef]
- Karastergiou, K.; Smith, S.R.; Greenberg, A.S.; Fried, S.K. Sex differences in human adipose tissues—The biology of pear shape. Biol. Sex Differ. 2012, 3, 13. [Google Scholar] [CrossRef]
- Power, M.L.; Schulkin, J. Sex differences in fat storage, fat metabolism, and the health risks from obesity: Possible evolutionary origins. Br. J. Nutr. 2008, 99, 931–940. [Google Scholar] [CrossRef]
- Kesztyüs, D.; Fuchs, M.; Cermak, P.; Kesztyüs, T. Associations of time-restricted eating with health-related quality of life and sleep in adults: A secondary analysis of two pre-post pilot studies. BMC Nutr. 2020, 6, 76. [Google Scholar] [CrossRef]

| eTRE | dTRE | Control | |
|---|---|---|---|
| Age (year) | 24.1 ± 2.10 | 23.3 ± 0.89 | 22.1 ± 2.53 |
| Body Weight (kg) | 54.6 ± 2.21 | 55.7 ± 1.70 | 55.4 ± 1.79 |
| Height (cm) | 163.4 ± 4.74 | 162.6 ± 3.64 | 163.2 ± 2.98 |
| eTRE (n = 8) | dTRE (n = 8) | Control (n = 8) | Time | Interaction | |
|---|---|---|---|---|---|
| Body weight (kg) pre | 54.61 ± 2.22 | 55.68 ± 1.70 | 55.40 ± 1.79 | 0.157 | 0.001 (eTRE) |
| Body weight (kg) post | 52.00 ± 2.96 | 54.24 ± 1.43 | 54.92 ± 1.51 | 0.047 (dTRE) | |
| Muscle thickness (mm) pre | 20.61 ± 1.73 | 20.10 ± 1.25 | 19.00 ± 1.58 | 0.001 | 0.780 (eTRE) |
| Muscle thickness (mm) post | 22.08 ± 1.62 | 21.46 ± 1.30 | 20.55 ± 1.49 | 0.506 (dTRE) | |
| Push-up performance (reps) pre | 57.00 ± 16.73 | 58.75 ± 16.78 | 64.62 ± 9.40 | 0.001 | 0.486 (eTRE) |
| Push-up performance (reps) post | 130.88 ± 38.54 | 120.62 ± 34.63 | 132.50 ± 19.23 | 0.486 (dTRE) |
| eTRE | dTRE | Control | |
|---|---|---|---|
| Average sleep duration (h) | 7.66 ± 0.52 | 7.49 ± 0.48 | 7.58 ± 0.45 |
| Dietary program adherence (%) | 85.6 | 89.5 | 95.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Z.; Ueda, T. Early Time-Restricted Eating Improves Weight Loss While Preserving Muscle: An 8-Week Trial in Young Women. Nutrients 2025, 17, 1022. https://doi.org/10.3390/nu17061022
Yu Z, Ueda T. Early Time-Restricted Eating Improves Weight Loss While Preserving Muscle: An 8-Week Trial in Young Women. Nutrients. 2025; 17(6):1022. https://doi.org/10.3390/nu17061022
Chicago/Turabian StyleYu, Zifu, and Takeshi Ueda. 2025. "Early Time-Restricted Eating Improves Weight Loss While Preserving Muscle: An 8-Week Trial in Young Women" Nutrients 17, no. 6: 1022. https://doi.org/10.3390/nu17061022
APA StyleYu, Z., & Ueda, T. (2025). Early Time-Restricted Eating Improves Weight Loss While Preserving Muscle: An 8-Week Trial in Young Women. Nutrients, 17(6), 1022. https://doi.org/10.3390/nu17061022





