Effects of Different Proportions of DHA and ARA on Cognitive Development in Infants: A Meta-Analysis
Abstract
:1. Introduction
2. Methods
2.1. Literature Search
2.2. Inclusion and Exclusion Criteria
2.3. Cognitive Performance Measures
2.4. Data Extraction and Quality Assessment
2.5. Statistical Analysis
3. Results
3.1. Study Selection
3.2. Study Characteristics
3.3. Risk of Bias Assessment
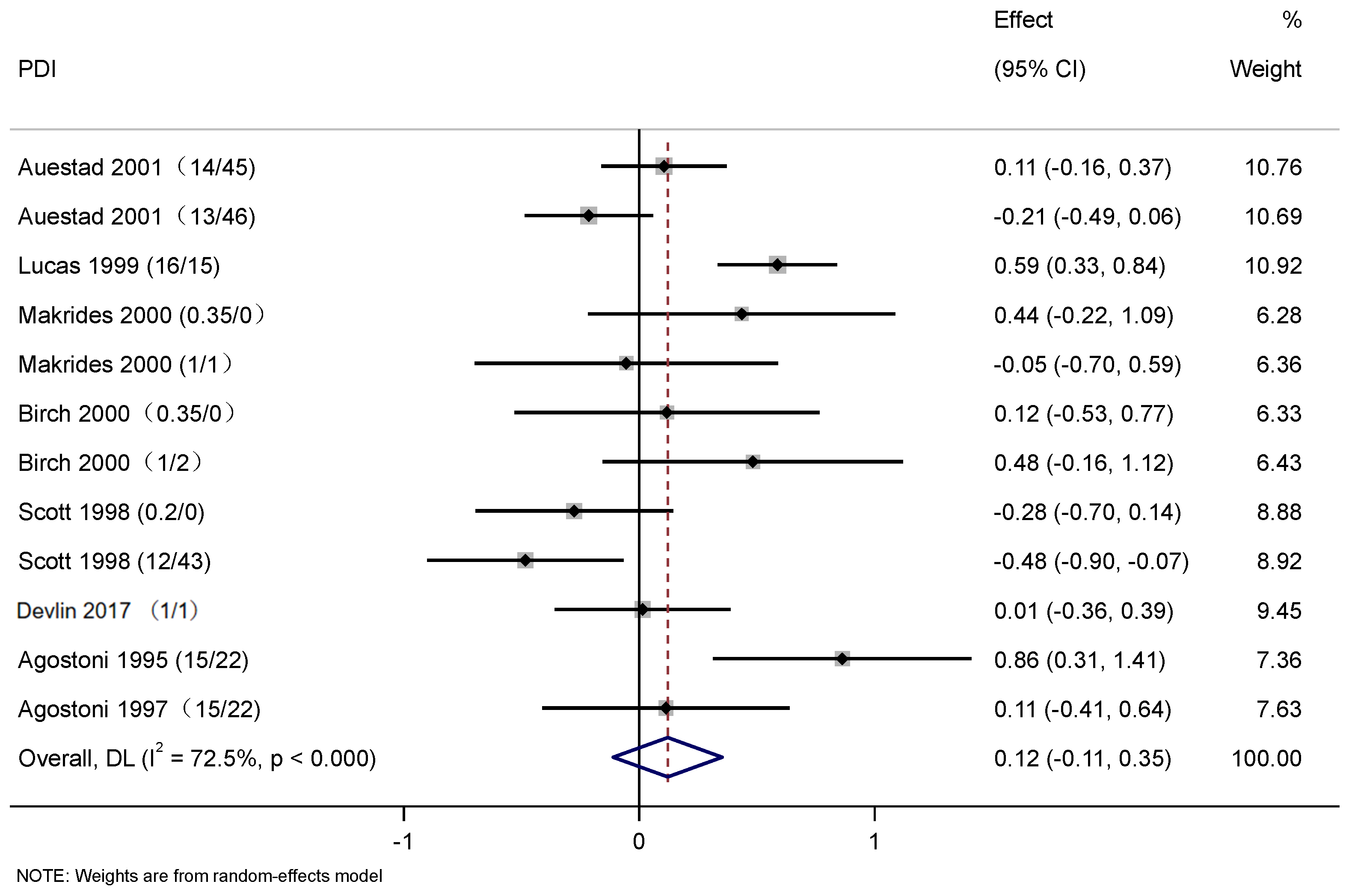
3.4. The Effect of DHA and ARA Supplementation on Cognitive Development in Infants

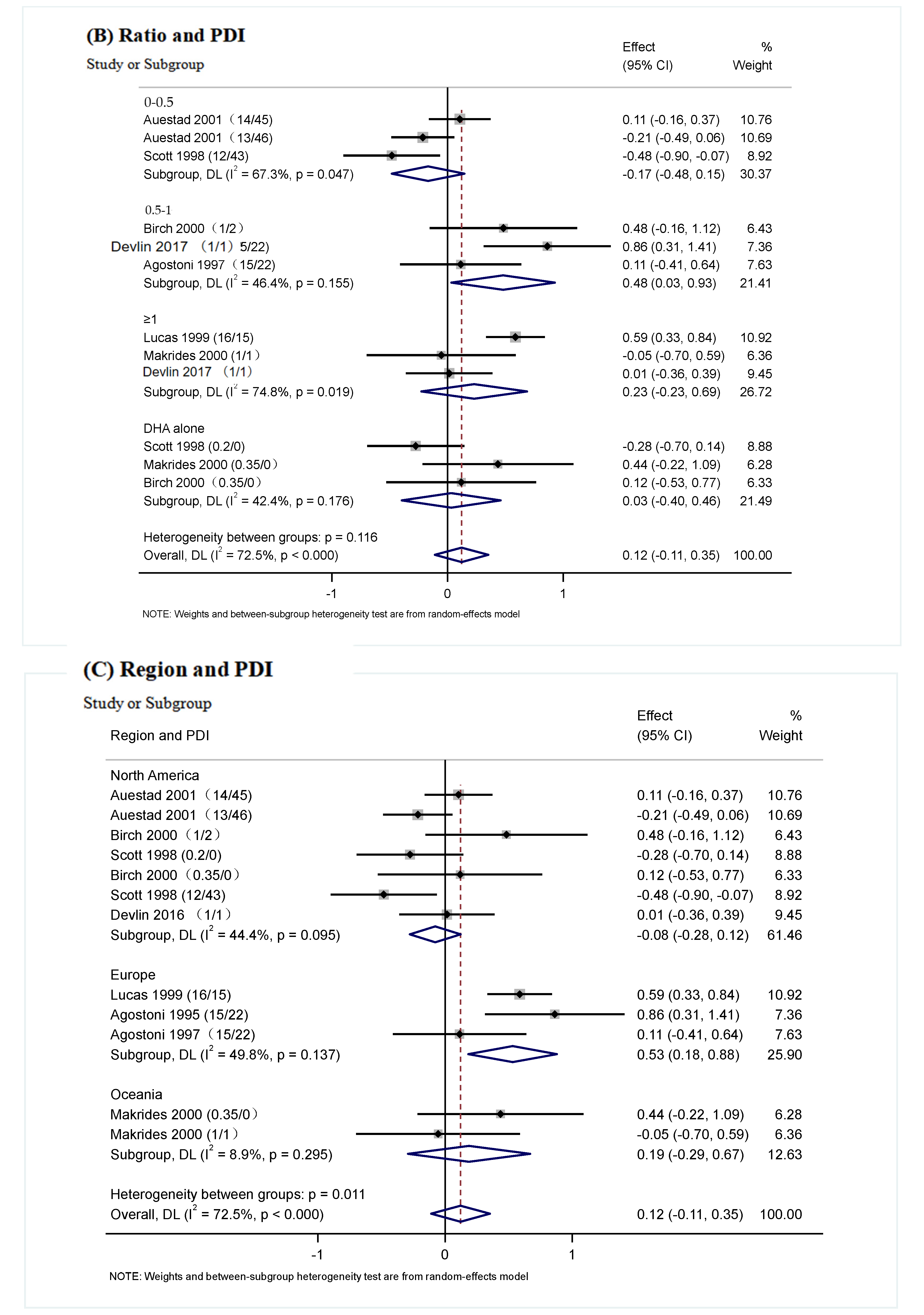
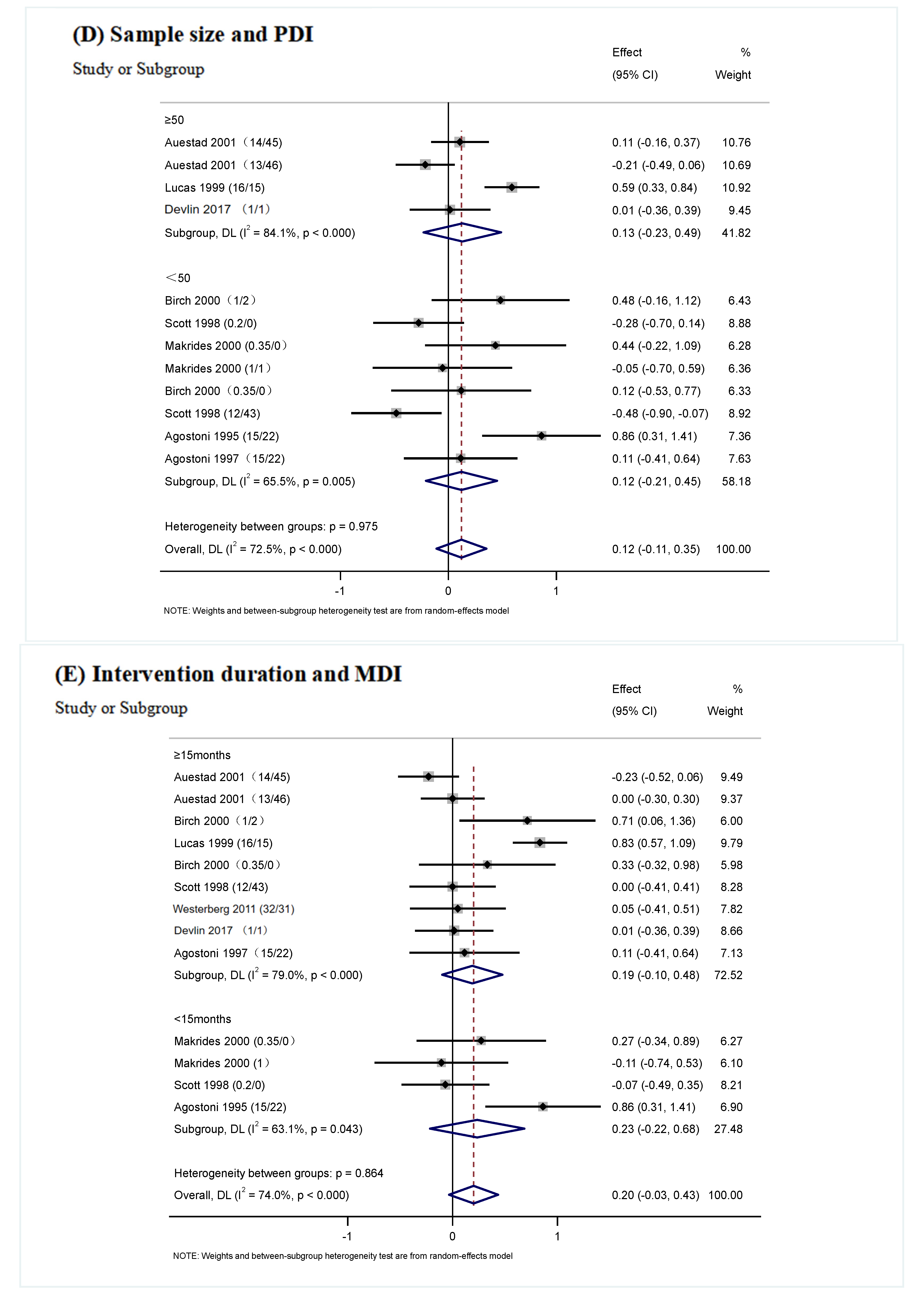
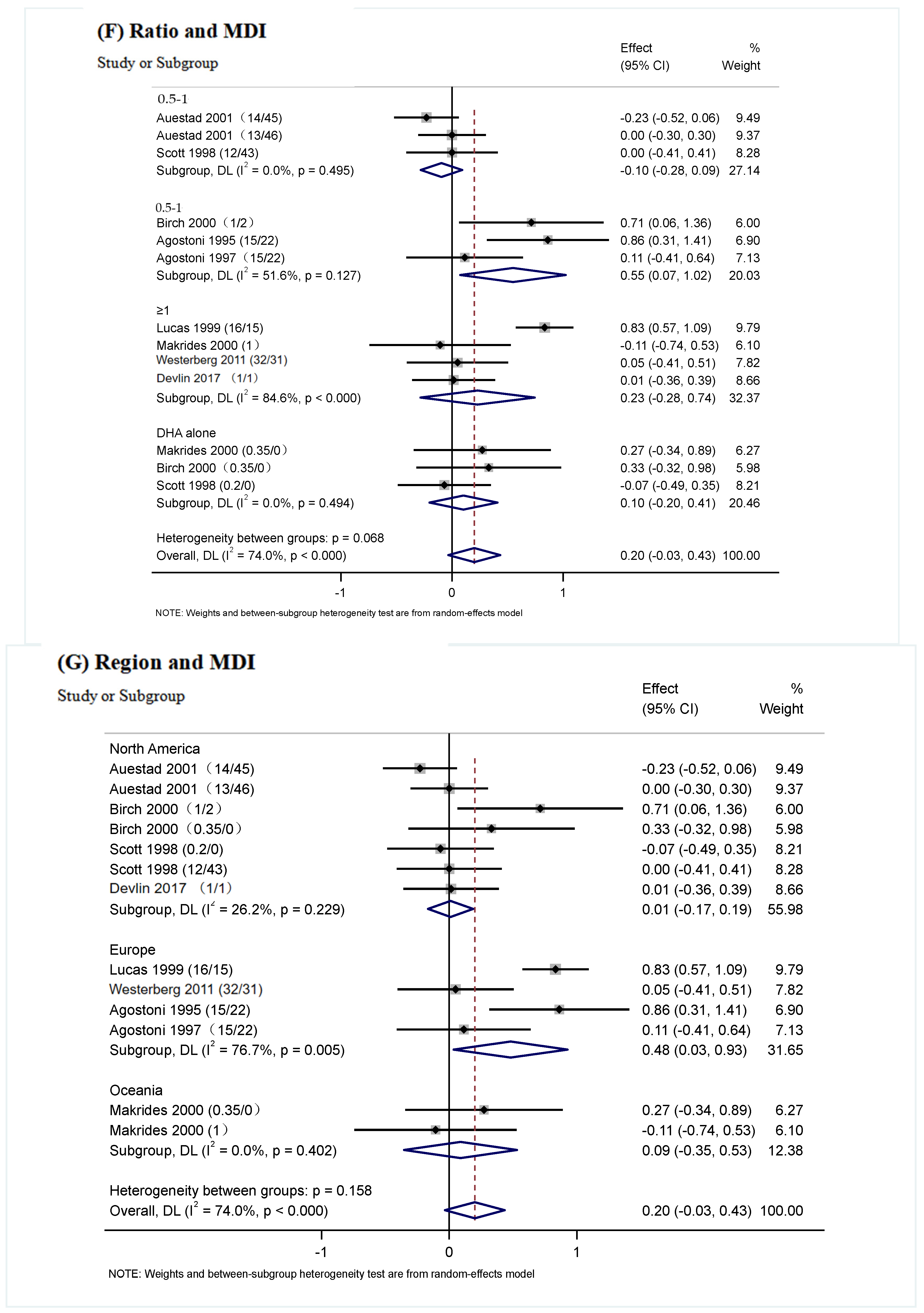
3.5. Subgroup Analysis
3.6. Sensitivity Analysis
3.7. Publication Bias
4. Discussion
Strengths and Weakness
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DHA | Docosahexaenoic acid |
| ARA | Arachidonic acid |
| BSID | Bayley Scales of Infant and Toddler Development |
| MDI | Mental Development Index |
| PDI | Psychomotor Development Index |
| DQ | Developmental quotient |
| RCT | Randomized clinical trial |
| CI | Confidence interval |
| WMD | Weighted mean difference |
| SMD | Standardized mean difference |
| LCPUFAs | Long-chain polyunsaturated fatty acids |
References
- Tan, K.; Zhang, H.; Zheng, H. Climate change and n-3 LC-PUFA availability. Prog. Lipid Res. 2022, 86, 101161. [Google Scholar] [CrossRef] [PubMed]
- Miles, E.A.; Childs, C.E.; Calder, P.C. Long-Chain Polyunsaturated Fatty Acids (LCPUFAs) and the Developing Immune System: A Narrative Review. Nutrients 2021, 13, 247. [Google Scholar] [CrossRef]
- Sun, G.Y.; Simonyi, A.; Fritsche, K.L.; Chuang, D.Y.; Hannink, M.; Gu, Z.; Greenlief, C.M.; Yao, J.K.; Lee, J.C.; Beversdorf, D.Q. Docosahexaenoic acid (DHA): An essential nutrient and a nutraceutical for brain health and diseases. Prostaglandins Leukot. Essent. Fat. Acids 2018, 136, 3–13. [Google Scholar] [CrossRef]
- Innes, J.K.; Calder, P.C. Omega-6 fatty acids and inflammation. Prostaglandins Leukot. Essent. Fat. Acids 2018, 132, 41–48. [Google Scholar] [CrossRef]
- Brenna, J.T. Efficiency of conversion of alpha-linolenic acid to long chain n-3 fatty acids in man. Curr. Opin. Clin. Nutr. Metab. Care 2002, 5, 127–132. [Google Scholar] [CrossRef]
- Emken, E.A.; Adlof, R.O.; Gulley, R.M. Dietary linoleic acid influences desaturation and acylation of deuterium-labeled linoleic and linolenic acids in young adult males. Biochim. Biophys. Acta (BBA) Lipids Lipid Metab. 1994, 1213, 277–288. [Google Scholar] [CrossRef]
- Lien, E.L.; Richard, C.; Hoffman, D.R. DHA and ARA addition to infant formula: Current status and future research directions. Prostaglandins Leukot. Essent. Fat. Acids 2018, 128, 26–40. [Google Scholar] [CrossRef]
- Tounian, P.; Bellaïche, M.; Legrand, P. ARA or no ARA in infant formulae, that is the question. Arch. Pédiatr. 2021, 28, 69–74. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.S. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance—A review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef]
- Weiser, M.J.; Butt, C.M.; Mohajeri, M.H. Docosahexaenoic Acid and Cognition throughout the Lifespan. Nutrients 2016, 8, 99. [Google Scholar] [CrossRef]
- Dyall, S.C.; Balas, L.; Bazan, N.G.; Brenna, J.T.; Chiang, N.; da Costa Souza, F.; Dalli, J.; Durand, T.; Galano, J.M.; Lein, P.J.; et al. Polyunsaturated fatty acids and fatty acid-derived lipid mediators: Recent advances in the understanding of their biosynthesis, structures, and functions. Prog. Lipid Res. 2022, 86, 101165. [Google Scholar] [CrossRef] [PubMed]
- Sambra, V.; Echeverria, F.; Valenzuela, A.; Chouinard-Watkins, R.; Valenzuela, R. Docosahexaenoic and Arachidonic Acids as Neuroprotective Nutrients throughout the Life Cycle. Nutrients 2021, 13, 986. [Google Scholar] [CrossRef] [PubMed]
- Oboh, A.; Kabeya, N.; Carmona-Antoñanzas, G.; Castro, L.F.C.; Dick, J.R.; Tocher, D.R.; Monroig, O. Two alternative pathways for docosahexaenoic acid (DHA, 22:6n-3) biosynthesis are widespread among teleost fish. Sci. Rep. 2017, 7, 3889. [Google Scholar] [CrossRef]
- Koletzko, B.; Boey, C.C.; Campoy, C.; Carlson, S.E.; Chang, N.; Guillermo-Tuazon, M.A.; Joshi, S.; Prell, C.; Quak, S.H.; Sjarif, D.R.; et al. Current information and Asian perspectives on long-chain polyunsaturated fatty acids in pregnancy, lactation, and infancy: Systematic review and practice recommendations from an early nutrition academy workshop. Ann. Nutr. Metab. 2014, 65, 49–80. [Google Scholar] [CrossRef]
- Li, D. Omega-3 polyunsaturated fatty acids and non-communicable diseases: Meta-analysis based systematic review. Asia Pac. J. Clin. Nutr. 2015, 24, 10–15. [Google Scholar] [CrossRef]
- Abedi, E.; Sahari, M.A. Long-chain polyunsaturated fatty acid sources and evaluation of their nutritional and functional properties. Food Sci. Nutr. 2014, 2, 443–463. [Google Scholar] [CrossRef]
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 fatty acids EPA and DHA: Health benefits throughout life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M. Tissue levels of polyunsaturated fatty acids during early human development. J. Pediatr. 1992, 120 Pt 2, S129–S138. [Google Scholar] [CrossRef]
- Fan, L.; Wang, X.; Szeto, I.M.; Liu, B.; Sinclair, A.J.; Li, D. Dietary intake of different ratios of ARA/DHA in early stages and its impact on infant development. Food Funct. 2024, 15, 3259–3273. [Google Scholar] [CrossRef]
- Zárate, R.; El Jaber-Vazdekis, N.; Tejera, N.; Pérez, J.A.; Rodríguez, C. Significance of long chain polyunsaturated fatty acids in human health. Clin. Transl. Med. 2017, 6, 25. [Google Scholar] [CrossRef]
- Dyall, S.C. Interplay Between n-3 and n-6 Long-Chain Polyunsaturated Fatty Acids and the Endocannabinoid System in Brain Protection and Repair. Lipids 2017, 52, 885–900. [Google Scholar] [CrossRef] [PubMed]
- Arellanes, I.C.; Choe, N.; Solomon, V.; He, X.; Kavin, B.; Martinez, A.E.; Kono, N.; Buennagel, D.P.; Hazra, N.; Kim, G.; et al. Brain delivery of supplemental docosahexaenoic acid (DHA): A randomized placebo-controlled clinical trial. eBioMedicine 2020, 59, 102883. [Google Scholar] [CrossRef] [PubMed]
- Stonehouse, W.; Conlon, C.A.; Podd, J.; Hill, S.R.; Minihane, A.M.; Haskell, C.; Kennedy, D. DHA supplementation improved both memory and reaction time in healthy young adults: A randomized controlled trial123. Am. J. Clin. Nutr. 2013, 97, 1134–1143. [Google Scholar] [CrossRef]
- Moltu, S.J.; Nordvik, T.; Rossholt, M.E.; Wendel, K.; Chawla, M.; Server, A.; Gunnarsdottir, G.; Pripp, A.H.; Domellöf, M.; Bratlie, M.; et al. Arachidonic and docosahexaenoic acid supplementation and brain maturation in preterm infants; a double blind RCT. Clin. Nutr. 2024, 43, 176–186. [Google Scholar] [CrossRef] [PubMed]
- E, Z.; Chen, C.; Yang, J.; Tong, H.; Li, T.; Wang, L.; Chen, H. Genome-wide analysis of fatty acid desaturase genes in rice (Oryza sativa L.). Sci. Rep. 2019, 9, 19445. [Google Scholar] [CrossRef]
- Castillo Salinas, F.; Montaner Ramón, A.; Castillo Ferrer, F.J.; Domingo-Carnice, A.; Cordobilla, B.; Domingo, J.C. Erythrocyte Membrane Docosahexaenoic Acid (DHA) and Lipid Profile in Preterm Infants at Birth and Over the First Month of Life: A Comparative Study with Infants at Term. Nutrients 2022, 14, 4956. [Google Scholar] [CrossRef]
- Alshweki, A.; Muñuzuri, A.P.; Baña, A.M.; de Castro, M.J.; Andrade, F.; Aldamiz-Echevarría, L.; de Pipaón, M.S.; Fraga, J.M.; Couce, M.L. Effects of different arachidonic acid supplementation on psychomotor development in very preterm infants; a randomized controlled trial. Nutr. J. 2015, 14, 101. [Google Scholar] [CrossRef] [PubMed]
- Westerberg, A.C.; Schei, R.; Henriksen, C.; Smith, L.; Veierod, M.B.; Drevon, C.A.; Iversen, P.O. Attention among very low birth weight infants following early supplementation with docosahexaenoic and arachidonic acid. Acta Paediatr. 2011, 100, 47–52. [Google Scholar] [CrossRef]
- Devlin, A.M.; Chau, C.M.Y.; Dyer, R.; Matheson, J.; McCarthy, D.; Yurko-Mauro, K.; Innis, S.M.; Grunau, R.E. Developmental Outcomes at 24 Months of Age in Toddlers Supplemented with Arachidonic Acid and Docosahexaenoic Acid: Results of a Double Blind Randomized, Controlled Trial. Nutrients 2017, 9, 975. [Google Scholar] [CrossRef]
- Birch, E.E.; Garfield, S.; Hoffman, D.R.; Uauy, R.; Birch, D.G. A randomized controlled trial of early dietary supply of long-chain polyunsaturated fatty acids and mental development in term infants. Dev. Med. Child. Neurol. 2000, 42, 174–181. [Google Scholar] [CrossRef]
- Baker, P.; Smith, J.; Salmon, L.; Friel, S.; Kent, G.; Iellamo, A.; Dadhich, J.P.; Renfrew, M.J. Global trends and patterns of commercial milk-based formula sales: Is an unprecedented infant and young child feeding transition underway? Public Health Nutr. 2016, 19, 2540–2550. [Google Scholar] [CrossRef] [PubMed]
- Brenna, J.T.; Diau, G.Y. The influence of dietary docosahexaenoic acid and arachidonic acid on central nervous system polyunsaturated fatty acid composition. Prostaglandins Leukot. Essent. Fat. Acids 2007, 77, 247–250. [Google Scholar] [CrossRef]
- Burdge, G.C.; Calder, P.C. Conversion of alpha-linolenic acid to longer-chain polyunsaturated fatty acids in human adults. Reprod. Nutr. Dev. 2005, 45, 581–597. [Google Scholar] [CrossRef]
- Koletzko, B.; Baker, S.; Cleghorn, G.; Neto, U.F.; Gopalan, S.; Hernell, O.; Hock, Q.S.; Jirapinyo, P.; Lonnerdal, B.; Pencharz, P.; et al. Global standard for the composition of infant formula: Recommendations of an ESPGHAN coordinated international expert group. J. Pediatr. Gastroenterol. Nutr. 2005, 41, 584–599. [Google Scholar] [CrossRef]
- Schulzke, S.M.; Patole, S.K.; Simmer, K. Long-chain polyunsaturated fatty acid supplementation in preterm infants. Cochrane Database Syst. Rev. 2011, 16, CD000375. [Google Scholar] [CrossRef]
- Lefebvre, C.; Glanville, J.; Briscoe, S.; Littlewood, A.; Marshall, C.; Metzendorf, M.-I.; Noel-Storr, A.; Rader, T.; Shokraneh, F.; Thomas, J.; et al. Searching for and selecting studies. In Cochrane Handbook for Systematic Reviews of Interventions; The Cochrane Collaboration: London, UK, 2019; pp. 67–107. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/9781119536604.ch4 (accessed on 25 June 2024).
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Agostoni, C.; Trojan, S.; Bellù, R.; Riva, E.; Giovannini, M. Neurodevelopmental quotient of healthy term infants at 4 months and feeding practice: The role of long-chain polyunsaturated fatty acids. Pediatr. Res. 1995, 38, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Agostoni, C.; Trojan, S.; Bellù, R.; Riva, E.; Bruzzese, M.G.; Giovannini, M. Developmental quotient at 24 months and fatty acid composition of diet in early infancy: A follow up study. Arch. Dis. Child. 1997, 76, 421–424. [Google Scholar] [CrossRef]
- Scott, D.T.; Janowsky, J.S.; Carroll, R.E.; Taylor, J.A.; Auestad, N.; Montalto, M.B. Formula supplementation with long-chain polyunsaturated fatty acids: Are there developmental benefits? Pediatrics 1998, 102, E59. [Google Scholar] [CrossRef]
- Lucas, A.; Stafford, M.; Morley, R.; Abbott, R.; Stephenson, T.; MacFadyen, U.; Elias-Jones, A.; Clements, H. Efficacy and safety of long-chain polyunsaturated fatty acid supplementation of infant-formula milk: A randomised trial. Lancet 1999, 354, 1948–1954. [Google Scholar] [CrossRef]
- Makrides, M.; Neumann, M.A.; Simmer, K.; Gibson, R.A. A critical appraisal of the role of dietary long-chain polyunsaturated fatty acids on neural indices of term infants: A randomized, controlled trial. Pediatrics 2000, 105 Pt 1, 32–38. [Google Scholar] [CrossRef]
- Auestad, N.; Halter, R.; Hall, R.T.; Blatter, M.; Bogle, M.L.; Burks, W.; Erickson, J.R.; Fitzgerald, K.M.; Dobson, V.; Innis, S.M.; et al. Growth and development in term infants fed long-chain polyunsaturated fatty acids: A double-masked, randomized, parallel, prospective, multivariate study. Pediatrics 2001, 108, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Jasani, B.; Simmer, K.; Patole, S.K.; Rao, S.C. Long chain polyunsaturated fatty acid supplementation in infants born at term. Cochrane Database Syst. Rev. 2017, 3, Cd000376. [Google Scholar] [CrossRef] [PubMed]
- Simmer, K.; Patole, S.K.; Rao, S.C. Long-chain polyunsaturated fatty acid supplementation in infants born at term. Cochrane Database Syst. Rev. 2011, 10, Cd000376. [Google Scholar] [CrossRef]
- Colombo, J.; Cheatham, C.L. The emergence and basis of endogenous attention in infancy and early childhood. Adv. Child. Dev. Behav. 2006, 34, 283–322. [Google Scholar] [CrossRef]
- Sugasini, D.; Thomas, R.; Yalagala, P.C.R.; Tai, L.M.; Subbaiah, P.V. Dietary docosahexaenoic acid (DHA) as lysophosphatidylcholine, but not as free acid, enriches brain DHA and improves memory in adult mice. Sci. Rep. 2017, 7, 11263. [Google Scholar] [CrossRef]
- Carlson, S.E.; Colombo, J. Docosahexaenoic Acid and Arachidonic Acid Nutrition in Early Development. Adv. Pediatr. 2016, 63, 453–471. [Google Scholar] [CrossRef]
- Friedman, N.P.; Robbins, T.W. The role of prefrontal cortex in cognitive control and executive function. Neuropsychopharmacology 2022, 47, 72–89. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Como, P.G.; Downey, L.C.; Murphy, D.; Ariagno, R.L.; Rodriguez, W. Infant formula and neurocognitive outcomes: Impact of study end-point selection. J. Perinatol. 2015, 35, 867–874. [Google Scholar] [CrossRef]
- Saldanha, I.J.; Skelly, A.C.; Ley, K.V.; Wang, Z.; Berliner, E.; Bass, E.B.; Devine, B.; Hammarlund, N.; Adam, G.P.; Duan-Porter, D.; et al. AHRQ Methods for Effective Health Care. In Inclusion of Nonrandomized Studies of Interventions in Systematic Reviews of Intervention Effectiveness: An Update; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2022. [Google Scholar] [CrossRef]
- Burr, G.O.; Burr, M.M. On the Nature and RÔle of the Fatty Acids Essential in Nutrition. J. Biol. Chem. 1930, 86, 587–621. [Google Scholar] [CrossRef]
- Hadley, K.B.; Ryan, A.S.; Forsyth, S.; Gautier, S.; Salem, N., Jr. The Essentiality of Arachidonic Acid in Infant Development. Nutrients 2016, 8, 216. [Google Scholar] [CrossRef] [PubMed]
- Doepfner, M.; Dose, C.; Breuer, D.; Heintz, S.; Schiffhauer, S.; Banaschewski, T. Efficacy of Omega-3/Omega-6 Fatty Acids in Preschool Children at Risk of ADHD: A Randomized Placebo-Controlled Trial. J. Atten. Disord. 2021, 25, 1096–1106. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, D.R.; Harris, C.L.; Wampler, J.L.; Patterson, A.C.; Berseth, C.L. Growth, tolerance, and DHA and ARA status of healthy term infants receiving formula with two different ARA concentrations: Double-blind, randomized, controlled trial. Prostaglandins Leukot. Essent. Fat. Acids 2019, 146, 19–27. [Google Scholar] [CrossRef]
- Innis, S.M.; Adamkin, D.H.; Hall, R.T.; Kalhan, S.C.; Lair, C.; Lim, M.; Stevens, D.C.; Twist, P.F.; Dieresen-Schade, D.A.; Harris, C.L.; et al. Docosahexaenoic acid and arachidonic acid enhance growth with no adverse effects in preterm infants fed formula. J. Pediatr. 2002, 140, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Authority, E.F.S. Guidance on the assessment of exposure of operators, workers, residents and bystanders in risk assessment for plant protection products. EFSA J. 2014, 12, 3874. [Google Scholar] [CrossRef]
- Donnelly, J.E.; Sharp, T.; Houmard, J.; Carlson, M.G.; Hill, J.O.; Whatley, J.E.; Israel, R.G. Muscle hypertrophy with large-scale weight loss and resistance training. Am. J. Clin. Nutr. 1993, 58, 561–565. [Google Scholar] [CrossRef]
- Fewtrell, M.; Bronsky, J.; Campoy, C.; Domellöf, M.; Embleton, N.; Fidler Mis, N.; Hojsak, I.; Hulst, J.M.; Indrio, F.; Lapillonne, A.; et al. Complementary Feeding: A Position Paper by the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 119–132. [Google Scholar] [CrossRef]
- Colombo, J.; Carlson, S.E.; Cheatham, C.L.; Shaddy, D.J.; Kerling, E.H.; Thodosoff, J.M.; Gustafson, K.M.; Brez, C. Long-term effects of LCPUFA supplementation on childhood cognitive outcomes. Am. J. Clin. Nutr. 2013, 98, 403–412. [Google Scholar] [CrossRef]
- Basak, S.; Mallick, R.; Banerjee, A.; Pathak, S.; Duttaroy, A.K. Maternal Supply of Both Arachidonic and Docosahexaenoic Acids Is Required for Optimal Neurodevelopment. Nutrients 2021, 13, 2061. [Google Scholar] [CrossRef]
- Colombo, J.; Jill Shaddy, D.; Kerling, E.H.; Gustafson, K.M.; Carlson, S.E. Docosahexaenoic acid (DHA) and arachidonic acid (ARA) balance in developmental outcomes. Prostaglandins Leukot Essent Fat. Acids 2017, 121, 52–56. [Google Scholar] [CrossRef]
- Colombo, J.; Carlson, S.E. Is the measure the message: The BSID and nutritional interventions. Pediatrics 2012, 129, 1166–1167. [Google Scholar] [CrossRef] [PubMed]




| Inclusion Criteria | Exclusion Criteria | |
|---|---|---|
| Participants | Infants | Mothers or infants with defined diseases or disorders |
| Interventions | DHA and ARA Long-chain unsaturated fatty acids containing DHA and ARA into the intervention, DHA and ARA as the primary compositional distinctions. | In combination with drugs, nutrients, or other interventions The principal distinction between the interventions was not the inclusion of DHA and ARA |
| Comparators | Placebo or no intervention | |
| Outcomes of interest | Cognition development | Uncorrelated results |
| Study design | Randomized controlled study | Non-randomized/uncontrolled/observational studies (cross-sectional, case-control, and cohort) Animal models, in vitro, in vivo, ex-vivo trials, or quasi-experimental studies |
| Publications | English articles and original research | Non-original research (commentaries, editorials, or reviews), duplicated studies, unpublished studies, abstracts Published as conference proceedings Languages other than English |
| Author (Year) | Study Groups | DHA Percentage of Total Fatty Acid | ARA Percentage of Total Fatty Acid | DHA/ARA | Evaluations, Outcome | Region | Intervention Duration | |
|---|---|---|---|---|---|---|---|---|
| Westerberg 2011 [28] | G1 N = 44 | 0 | 0 | 0 | BSID (including MDI) | - | Europe | 20 months |
| G2 N = 48 | 0.32% | 0.31% | 32/31 | |||||
| Devlin 2017 [29] | G1 N = 58 | 0 | 0 | 0 | BSID (including MDI) | BSID (including PDI) | North America | 24 months |
| G2 N = 52 | 0.12% | 0.12% | 1/1 | |||||
| Birch 2000 [30] | G1 N = 20 | 0 | 0 | 0 | BSID (including MDI) | BSID (including PDI) | North America | 18 months |
| G2 N = 17 | 0.35% | 0 | 0.35/0 | |||||
| G3 N = 19 | 0.36% | 0.72% | 1/2 | |||||
| Agostoni 1995 [38] | G1 N = 27 | 0.30% | 0.44% | 15/22 | Brunet–Lezine (including MDI) | Brunet–Lezine (including PDI) | Europe | 4 months |
| G2 N = 29 | 0 | 0 | 0 | |||||
| Agostoni 1997 [39] | G1 N = 26 | 0.30% | 0.44% | 15/22 | Brunet–Lezine (including MDI) | Brunet–Lezine (including PDI) | Europe | 24 months |
| G2 N = 29 | 0 | 0 | 0 | |||||
| Scott 1998 [40] | G1 N = 42 | 0 | 0 | 0 | BSID (including MDI) | BSID (including PDI) | North America | 12 months |
| G2 N = 33 | 0.20% | 0 | 0.2/0 | |||||
| G3 N = 38 | 0.12% | 0.43% | 12/43 | |||||
| Lucas 1999 [41] | G1 N = 125 | 0 | 0 | 0/0 | BSID (including MDI) | BSID (including PDI) | Europe | 18 months |
| G2 N = 125 | 0.32% | 0.30% | 16/15 | |||||
| Makrides 2000 [42] | G1 N = 21 | 0 | 0 | 0 | BSID (including MDI) | BSID (including PDI) | Oceania | 18 months |
| G2 N = 23 | 0.35% | 0 | 0.35/0 | |||||
| G3 N = 24 | 0.34% | 0.34% | 1/1 | |||||
| Auestad 2001 [43] | G1 N = 77 | 0 | 0 | 0 | BSID (including MDI) | BSID (including PDI) | North America | 6, 12 months |
| G2 N = 80 | 0.14% | 0.45% | 14/45 | |||||
| G3 N = 82 | 0.13% | 0.46% | 13/46 | |||||
| Variables | Subgroups | Number of Effect Sizes | WMD (95% CI) | p a | Test for Subgroup Difference p | I2 | p b |
|---|---|---|---|---|---|---|---|
| Intervention duration | >15 months | 8 | 0.08 (−0.19, 0.35) | p < 0.001 | p = 0.22 | 75.3% | p = 0.636 |
| ≤15 months | 4 | 0.23 (−0.32, 0.77) | p = 0.009 | p = 0.46 | 74.0% | ||
| The ratio of Intervention (DHA/ARA) | 0–0.5 | 3 | −0.17 (−0.48, 0.15) | p = 0.047 | p = 0.16 | 67.3% | p = 0.116 |
| 0.5–1 | 3 | 0.48 (0.03, 0.93) | p = 0.155 | p = 0.04 | 46.4% | ||
| ≥1 | 3 | 0.23 (−0.23, 0.69) | p = 0.019 | p = 0.32 | 74.8% | ||
| DHA alone | 3 | 0.03 (−0.40, 0.46) | p = 0.176 | p = 0.90 | 42.4% | ||
| Region | North America | 7 | −0.08 (−0.28, 0.12) | p = 0.095 | p = 0.44 | 44.4% | p = 0.011 |
| Europe | 3 | 0.53 (0.18, 0.88) | p = 0.137 | p =0.003 | 49.8% | ||
| Oceania | 2 | 0.19 (−0.29, 0.67) | p = 0.295 | p = 0.44 | 8.9% | ||
| Sample size | ≥50 persons | 4 | 0.13 (−0.23, 0.49) | p < 0.000 | p = 0.48 | 84.1% | p = 0.975 |
| <50 persons | 8 | 0.12 (−0.21, 0.45) | p = 0.005 | p = 0.48 | 65.5% |
| Variables | Subgroups | Number of Effect Sizes | WMD (95% CI) | p a | Test for Subgroup Difference p | I2 | p b |
|---|---|---|---|---|---|---|---|
| Intervention duration | >15 months | 9 | 0.19 (−0.10, 0.48) | p < 0.000 | p = 0.15 | 79.0% | p = 0.864 |
| ≤15 months | 4 | 0.23 (−0.22, 0.68) | p = 0.043 | p = 0.32 | 63.1% | ||
| The ratio of Intervention (DHA/ARA) | 0–0.5 | 3 | −0.10 (−0.28, 0.09) | p = 0.495 | p = 0.32 | 0.0% | p = 0.068 |
| 0.5–1 | 3 | 0.55 (0.07, 1.02) | p = 0.127 | p = 0.02 | 51.6% | ||
| ≥1 | 4 | 0.23 (−0.28, 0.74) | p < 0.000 | p = 0.38 | 84.6% | ||
| DHA alone | 3 | 0.10 (−0.20, 0.41) | p = 0494 | p = 0.52 | 0.0% | ||
| Region | North America | 7 | 0.01 (−0.17, 0.19) | p = 0.229 | p = 0.46 | 26.2% | p = 0.158 |
| Europe | 4 | 0.48 (0.03, 0.93) | p = 0.005 | p = 0.04 | 76.7% | ||
| Oceania | 2 | 0.09 (−0.35, 0.53) | p = 0.402 | p = 0.70 | 0.0% | ||
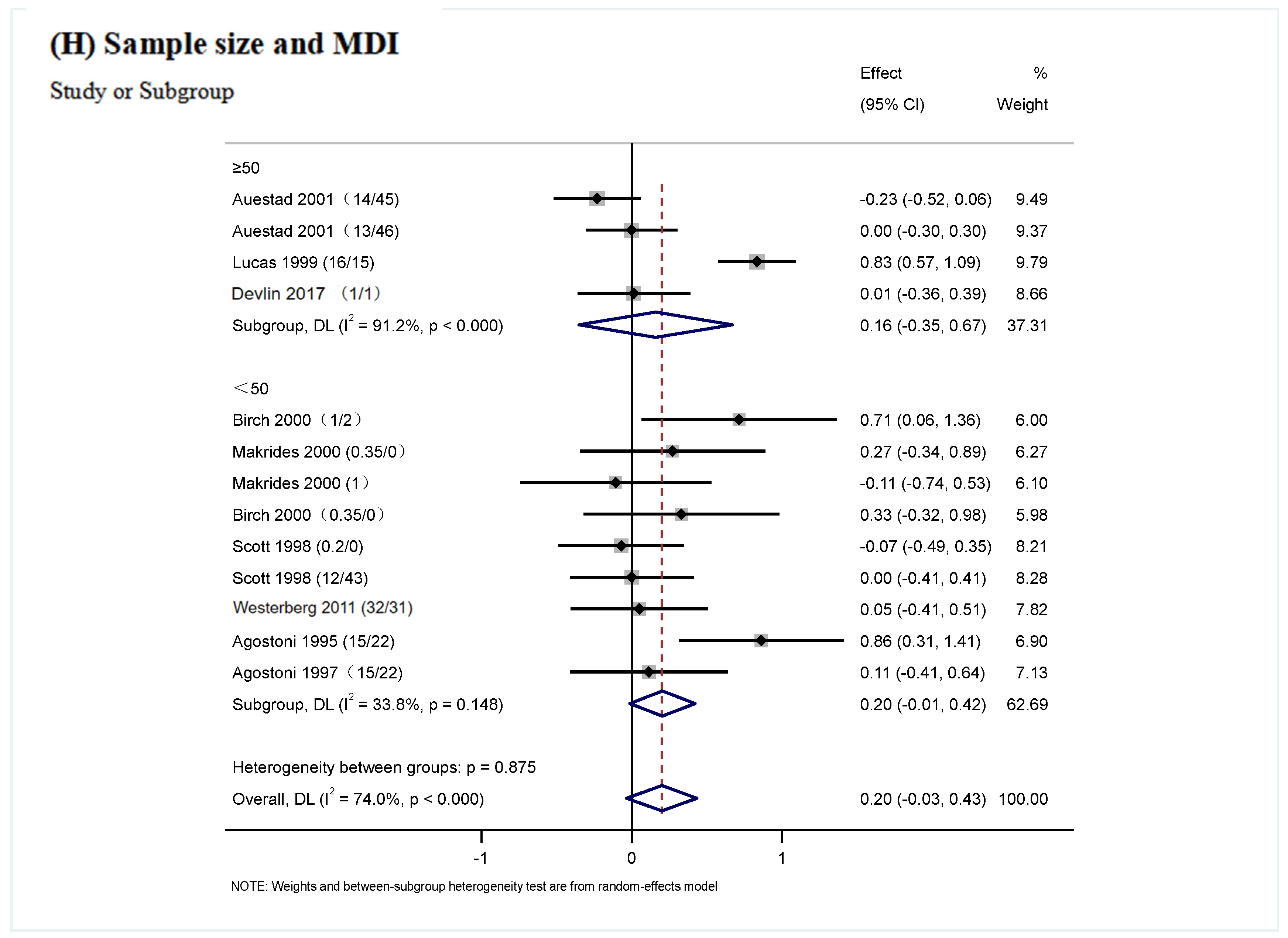
| Sample size | ≥50 persons | 4 | 0.16 (−0.35, 0.67) | p < 0.000 | p = 0.54 | 91.2% | p = 0.875 |
| <50 persons | 9 | 0.20 (−0.01, 0.42) | p = 0.148 | p = 0.07 | 33.8% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, A.; Xu, L.; Szeto, I.M.-Y.; Wang, X.; Li, D. Effects of Different Proportions of DHA and ARA on Cognitive Development in Infants: A Meta-Analysis. Nutrients 2025, 17, 1091. https://doi.org/10.3390/nu17061091
Tian A, Xu L, Szeto IM-Y, Wang X, Li D. Effects of Different Proportions of DHA and ARA on Cognitive Development in Infants: A Meta-Analysis. Nutrients. 2025; 17(6):1091. https://doi.org/10.3390/nu17061091
Chicago/Turabian StyleTian, Ailing, Lirong Xu, Ignatius Man-Yau Szeto, Xuemin Wang, and Duo Li. 2025. "Effects of Different Proportions of DHA and ARA on Cognitive Development in Infants: A Meta-Analysis" Nutrients 17, no. 6: 1091. https://doi.org/10.3390/nu17061091
APA StyleTian, A., Xu, L., Szeto, I. M.-Y., Wang, X., & Li, D. (2025). Effects of Different Proportions of DHA and ARA on Cognitive Development in Infants: A Meta-Analysis. Nutrients, 17(6), 1091. https://doi.org/10.3390/nu17061091






