Red Meat Amino Acids for Beginners: A Narrative Review
Abstract
:1. Introduction
1.1. What Is Meat?
1.2. Processed Meat
2. Red Meat Composition
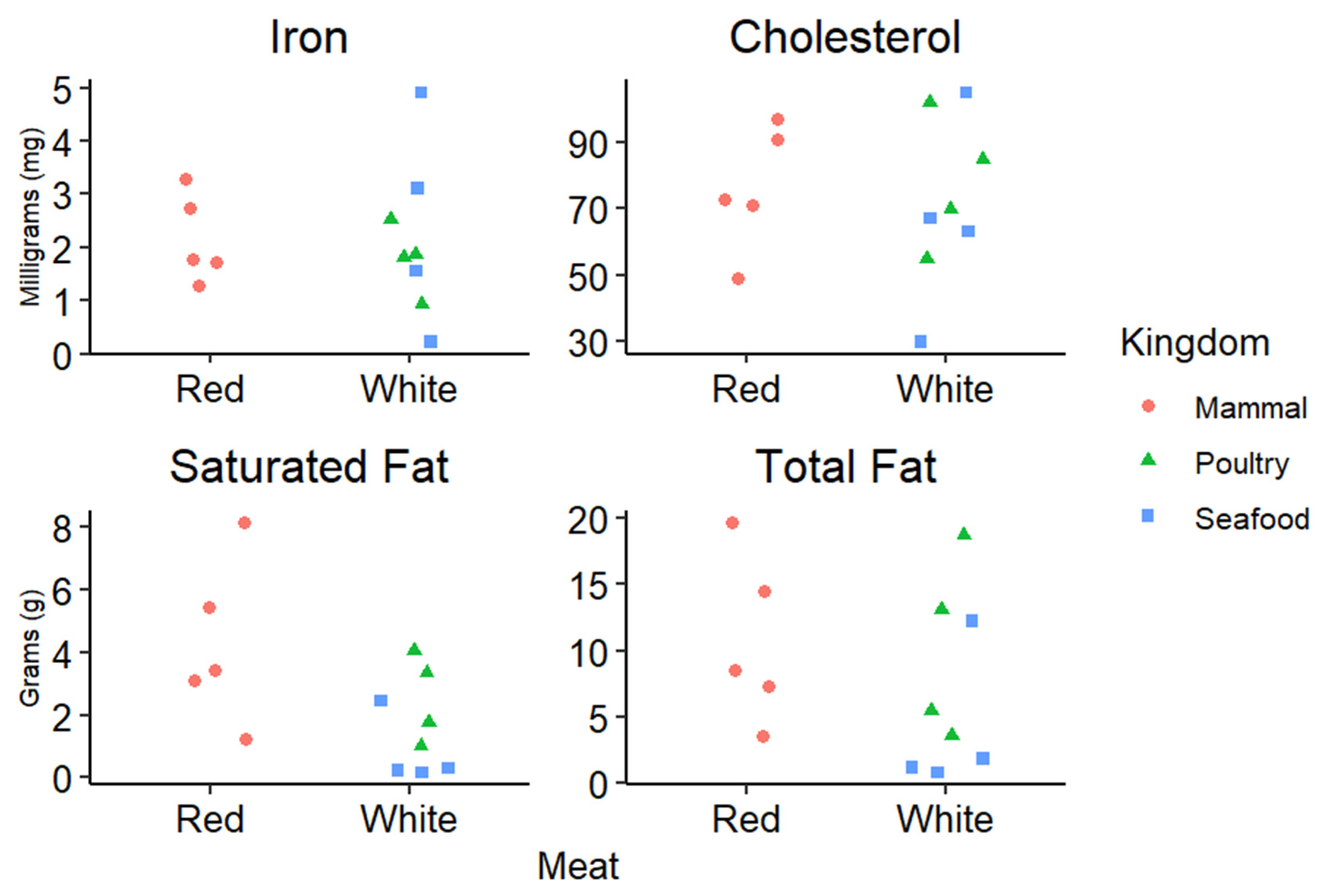
2.1. Fat
2.2. Vitamins and Minerals
2.3. Protein and Amino Acids
3. Discussions
3.1. Amino Acids in Sports Nutrition
3.2. Cardiovascular Disease
3.3. Red Meat, Obesity and Type 2 Diabetes
3.4. The Gut Microbiome
3.5. Colorectal Cancer
3.6. Metabolic-Disorder Associated Steatotic Liver Disease (MASLD)
3.7. Red Meat and Biological Aging
3.8. Red Meat and Sex
3.9. The Future of Red Meat
4. Limitations and Strengths
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dennis, L.S.; Dustin, D.B.; Carr, C.C.; Michael, E.D.; Casey, M.O.; Jimmy, T.K.; Pringle, T.D.; Jeffrey, J.S.; Dale, R.W.; Amilton, S.d.M.; et al. Meat Science Lexicon. Meat Muscle Biol. 2018, 2, 127–141. [Google Scholar] [CrossRef]
- Keeton, J.T.; Dikeman, M.E. ‘Red’ and ‘white’ meats—Terms that lead to confusion. Anim. Front. 2017, 7, 29–33. [Google Scholar] [CrossRef]
- WHO. IARC Monographs Evaluate Consumption of Red and Processed Meat. World Food Regul. Rev. 2015, 25, 30. [Google Scholar]
- Vahmani, P.; Mapiye, C.; Prieto, N.; Rolland, D.C.; McAllister, T.A.; Aalhus, J.L.; Dugan, M.E.R. The scope for manipulating the polyunsaturated fatty acid content of beef: A review. J. Anim. Sci. Biotechnol. 2015, 6, 29. [Google Scholar] [CrossRef]
- Perna, M.; Hewlings, S. Saturated Fatty Acid Chain Length and Risk of Cardiovascular Disease: A Systematic Review. Nutrients 2023, 15, 30. [Google Scholar] [CrossRef]
- Lawrence, G.D. Dietary fats and health: Dietary recommendations in the context of scientific evidence. Adv. Nutr. 2013, 4, 294–302. [Google Scholar] [CrossRef]
- Juárez, M.; Lam, S.; Bohrer, B.M.; Dugan, M.E.R.; Vahmani, P.; Aalhus, J.; Juárez, A.; López-Campos, O.; Prieto, N.; Segura, J. Enhancing the Nutritional Value of Red Meat through Genetic and Feeding Strategies. Foods 2021, 10, 872. [Google Scholar] [CrossRef]
- Momot, M.; Nogalski, Z.; Pogorzelska-Przybyłek, P.; Sobczuk-Szul, M. Influence of Genotype and Slaughter Age on the Content of Selected Minerals and Fatty Acids in the Longissimus Thoracis Muscle of Crossbred Bulls. Animals 2020, 10, 2004. [Google Scholar] [CrossRef]
- Nogoy, K.M.C.; Sun, B.; Shin, S.; Lee, Y.; Zi Li, X.; Choi, S.H.; Park, S. Fatty Acid Composition of Grain- and Grass-Fed Beef and Their Nutritional Value and Health Implication. Food Sci. Anim. Resour. 2022, 42, 18–33. [Google Scholar] [CrossRef]
- Burnett, D.D.; Legako, J.F.; Phelps, K.J.; Gonzalez, J.M. Biology, strategies, and fresh meat consequences of manipulating the fatty acid composition of meat. J. Anim. Sci. 2020, 98, skaa033. [Google Scholar] [CrossRef]
- Douny, C.; El Khoury, R.; Delmelle, J.; Brose, F.; Degand, G.; Moula, N.; Farnir, F.; Clinquart, A.; Maghuin-Rogister, G.; Scippo, M.L. Effect of storage and cooking on the fatty acid profile of omega-3 enriched eggs and pork meat marketed in Belgium. Food Sci. Nutr. 2015, 3, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, E.G.; Fuerniss, H.F.; Legako, J.F.; Thompson, L.D.; Woerner, D.R. Nutrient Analysis of Raw and Cooked USDA Prime Beef Cuts. Nutrients 2024, 16, 2912. [Google Scholar] [CrossRef] [PubMed]
- Hanna, M.; Jaqua, E.; Nguyen, V.; Clay, J. B Vitamins: Functions and Uses in Medicine. Perm. J. 2022, 26, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Daley, C.A.; Abbott, A.; Doyle, P.S.; Nader, G.A.; Larson, S. A review of fatty acid profiles and antioxidant content in grass-fed and grain-fed beef. Nutr. J. 2010, 9, 10. [Google Scholar] [CrossRef]
- Weyh, C.; Krüger, K.; Peeling, P.; Castell, L. The Role of Minerals in the Optimal Functioning of the Immune System. Nutrients 2022, 14, 644. [Google Scholar] [CrossRef]
- Rederstorff, M.; Krol, A.; Lescure, A. Understanding the importance of selenium and selenoproteins in muscle function. Cell. Mol. Life Sci. 2006, 63, 52–59. [Google Scholar] [CrossRef]
- Geiker, N.R.W.; Bertram, H.C.; Mejborn, H.; Dragsted, L.O.; Kristensen, L.; Carrascal, J.R.; Bügel, S.; Astrup, A. Meat and Human Health-Current Knowledge and Research Gaps. Foods 2021, 10, 1556. [Google Scholar] [CrossRef]
- Aglago, E.K.; Cross, A.J.; Riboli, E.; Fedirko, V.; Hughes, D.J.; Fournier, A.; Jakszyn, P.; Freisling, H.; Gunter, M.J.; Dahm, C.C.; et al. Dietary intake of total, heme and non-heme iron and the risk of colorectal cancer in a European prospective cohort study. Br. J. Cancer 2023, 128, 1529–1540. [Google Scholar] [CrossRef]
- Wu, G. Dietary protein intake and human health. Food Funct. 2016, 7, 1251–1265. [Google Scholar] [CrossRef]
- Patricia, J.J.; Dhamoon, A.S. Physiology, Digestion. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Lopez, M.J.; Mohiuddin, S.S. Biochemistry, Essential Amino Acids. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Klurfeld, D.M. What is the role of meat in a healthy diet? Anim. Front. 2018, 8, 5–10. [Google Scholar] [CrossRef]
- Alagawany, M.; Elnesr, S.S.; Farag, M.R.; Tiwari, R.; Yatoo, M.I.; Karthik, K.; Michalak, I.; Dhama, K. Nutritional significance of amino acids, vitamins and minerals as nutraceuticals in poultry production and health—A comprehensive review. Vet Q. 2020, 41, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Yin, Y.; Wu, G. Dietary essentiality of “nutritionally non-essential amino acids” for animals and humans. Exp. Biol. Med. 2015, 240, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Hodgkinson, S.M. Protein and amino acid digestibility: Definitions and conventional oro-ileal determination in humans. Front. Nutr. 2024, 11, 1407604. [Google Scholar] [CrossRef] [PubMed]
- Moughan, P.J.; Wolfe, R.R. Determination of Dietary Amino Acid Digestibility in Humans. J. Nutr. 2019, 149, 2101–2109. [Google Scholar] [CrossRef]
- Moughan, P.J.; Lim, W.X.J. Digestible indispensable amino acid score (DIAAS): 10 years on. Front. Nutr. 2024, 11, 1389719. [Google Scholar] [CrossRef]
- Ferrari, L.; Panaite, S.A.; Bertazzo, A.; Visioli, F. Animal- and Plant-Based Protein Sources: A Scoping Review of Human Health Outcomes and Environmental Impact. Nutrients 2022, 14, 5115. [Google Scholar] [CrossRef]
- Marinangeli, C.P.F.; House, J.D. Potential impact of the digestible indispensable amino acid score as a measure of protein quality on dietary regulations and health. Nutr. Rev. 2017, 75, 658–667. [Google Scholar] [CrossRef]
- Williams, P. Nutritional composition of red meat. Nutr. Diet. 2007, 64, S113–S119. [Google Scholar] [CrossRef]
- Connolly, G.; Campbell, W.W. Poultry Consumption and Human Cardiometabolic Health-Related Outcomes: A Narrative Review. Nutrients 2023, 15, 3550. [Google Scholar] [CrossRef]
- Ford, S.; Ilgaz, F.; Hawker, S.; Cochrane, B.; Hill, M.; Ellerton, C.; MacDonald, A. Amino Acid Analyses of Plant Foods Used in the Dietary Management of Inherited Amino Acid Disorders. Nutrients 2023, 15, 2387. [Google Scholar] [CrossRef]
- Dara, P.K.; Geetha, A.; Mohanty, U.; Raghavankutty, M.; Mathew, S.; Nagarajarao, R.C.; Rangasamy, A. Extraction and Characterization of Myofibrillar Proteins from Different Meat Sources: A Comparative Study. J. Bioresour. Bioprod. 2021, 6, 367–378. [Google Scholar] [CrossRef]
- Listrat, A.; Lebret, B.; Louveau, I.; Astruc, T.; Bonnet, M.; Lefaucheur, L.; Picard, B.; Bugeon, J. How Muscle Structure and Composition Influence Meat and Flesh Quality. Sci. World J. 2016, 2016, 3182746. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Zheng, W.; Locasale, J.W. Amino acid variability, tradeoffs and optimality in human diet. Nat. Commun. 2022, 13, 6683. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarska, K.; Taylor, M.; Piyasiri, U.; Frank, D. Flavor and Metabolite Profiles of Meat, Meat Substitutes, and Traditional Plant-Based High-Protein Food Products Available in Australia. Foods 2021, 10, 801. [Google Scholar] [CrossRef]
- Fanelli, N.S.; Bailey, H.M.; Thompson, T.W.; Delmore, R.; Nair, M.N.; Stein, H.H. Digestible indispensable amino acid score (DIAAS) is greater in animal-based burgers than in plant-based burgers if determined in pigs. Eur. J. Nutr. 2022, 61, 461–475. [Google Scholar] [CrossRef]
- Wu, G.; Cross, H.R.; Gehring, K.B.; Savell, J.W.; Arnold, A.N.; McNeill, S.H. Composition of free and peptide-bound amino acids in beef chuck, loin, and round cuts. J. Anim. Sci. 2016, 94, 2603–2613. [Google Scholar] [CrossRef]
- Hodgkinson, S.M.; Montoya, C.A.; Scholten, P.T.; Rutherfurd, S.M.; Moughan, P.J. Cooking Conditions Affect the True Ileal Digestible Amino Acid Content and Digestible Indispensable Amino Acid Score (DIAAS) of Bovine Meat as Determined in Pigs. J. Nutr. 2018, 148, 1564–1569. [Google Scholar] [CrossRef]
- Ling, Z.-N.; Jiang, Y.-F.; Ru, J.-N.; Lu, J.-H.; Ding, B.; Wu, J. Amino acid metabolism in health and disease. Signal Transduct. Target. Ther. 2023, 8, 345. [Google Scholar] [CrossRef]
- López-Pedrouso, M.; Zaky, A.A.; Lorenzo, J.M.; Camiña, M.; Franco, D. A review on bioactive peptides derived from meat and by-products: Extraction methods, biological activities, applications and limitations. Meat Sci. 2023, 204, 109278. [Google Scholar] [CrossRef]
- Di Corcia, M.; Tartaglia, N.; Polito, R.; Ambrosi, A.; Messina, G.; Francavilla, V.C.; Cincione, R.I.; Della Malva, A.; Ciliberti, M.G.; Sevi, A.; et al. Functional Properties of Meat in Athletes’ Performance and Recovery. Int. J. Environ. Res. Public Health 2022, 19, 5145. [Google Scholar] [CrossRef]
- Luckose, F.; Pandey, M.C.; Radhakrishna, K. Effects of Amino Acid Derivativeson Physical, Mental, and Physiological Activities. Crit. Rev. Food Sci. Nutr. 2015, 55, 1793–1807. [Google Scholar] [CrossRef] [PubMed]
- Kreider, R.B.; Stout, J.R. Creatine in Health and Disease. Nutrients 2021, 13, 447. [Google Scholar] [CrossRef] [PubMed]
- Jukić, I.; Kolobarić, N.; Stupin, A.; Matić, A.; Kozina, N.; Mihaljević, Z.; Mihalj, M.; Šušnjara, P.; Stupin, M.; Ćurić, Ž.B.; et al. Carnosine, Small but Mighty-Prospect of Use as Functional Ingredient for Functional Food Formulation. Antioxidants 2021, 10, 1037. [Google Scholar] [CrossRef] [PubMed]
- Pizzorno, J. Glutathione! Integr. Med. 2014, 13, 8–12. [Google Scholar]
- Liu, Q.; Yu, X.; Jia, F.; Wen, R.; Sun, C.; Yu, Q. Comprehensive analyses of meat quality and metabolome alterations with aging under different aging methods in beef. Food Chem. 2025, 472, 142936. [Google Scholar] [CrossRef]
- Costantino, A.; Maiese, A.; Lazzari, J.; Casula, C.; Turillazzi, E.; Frati, P.; Fineschi, V. The Dark Side of Energy Drinks: A Comprehensive Review of Their Impact on the Human Body. Nutrients 2023, 15, 3922. [Google Scholar] [CrossRef]
- Leiper, J.B. Fate of ingested fluids: Factors affecting gastric emptying and intestinal absorption of beverages in humans. Nutr. Rev. 2015, 73, 57–72. [Google Scholar] [CrossRef]
- Lu, C.-C.; Ke, C.-Y.; Wu, W.-T.; Lee, R.-P. L-Glutamine is better for treatment than prevention in exhaustive exercise. Front. Physiol. 2023, 14, 1172342. [Google Scholar] [CrossRef]
- Whillier, S.; Garcia, B.; Chapman, B.E.; Kuchel, P.W.; Raftos, J.E. Glutamine and α-ketoglutarate as glutamate sources for glutathione synthesis in human erythrocytes. FEBS J. 2011, 278, 3152–3163. [Google Scholar] [CrossRef]
- Garlick, P.J. The Role of Leucine in the Regulation of Protein Metabolism11. J. Nutr. 2005, 135, 1553S–1556S. [Google Scholar] [CrossRef]
- Shi, W.; Huang, X.; Schooling, C.M.; Zhao, J.V. Red meat consumption, cardiovascular diseases, and diabetes: A systematic review and meta-analysis. Eur. Heart J. 2023, 44, 2626–2635. [Google Scholar] [CrossRef] [PubMed]
- Canyelles, M.; Borràs, C.; Rotllan, N.; Tondo, M.; Escolà-Gil, J.C.; Blanco-Vaca, F. Gut Microbiota-Derived TMAO: A Causal Factor Promoting Atherosclerotic Cardiovascular Disease? Int. J. Mol. Sci. 2023, 24, 1940. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.E.; Taesuwan, S.; Malysheva, O.V.; Bender, E.; Tulchinsky, N.F.; Yan, J.; Sutter, J.L.; Caudill, M.A. Trimethylamine-N-oxide (TMAO) response to animal source foods varies among healthy young men and is influenced by their gut microbiota composition: A randomized controlled trial. Mol. Nutr. Food Res. 2017, 61, 1600324. [Google Scholar] [CrossRef] [PubMed]
- McGarrah, R.W.; White, P.J. Branched-chain amino acids in cardiovascular disease. Nat. Rev. Cardiol. 2023, 20, 77–89. [Google Scholar] [CrossRef]
- Wang, Y.; Beydoun, M.A. Meat consumption is associated with obesity and central obesity among US adults. Int. J. Obes. 2009, 33, 621–628. [Google Scholar] [CrossRef]
- Khodayari, S.; Sadeghi, O.; Safabakhsh, M.; Mozaffari-Khosravi, H. Meat consumption and the risk of general and central obesity: The Shahedieh study. BMC Res. Notes 2022, 15, 339. [Google Scholar] [CrossRef]
- Magkos, F.; Rasmussen, S.I.; Hjorth, M.F.; Asping, S.; Rosenkrans, M.I.; Sjödin, A.M.; Astrup, A.V.; Geiker, N.R.W. Unprocessed red meat in the dietary treatment of obesity: A randomized controlled trial of beef supplementation during weight maintenance after successful weight loss. Am. J. Clin. Nutr. 2022, 116, 1820–1830. [Google Scholar] [CrossRef]
- Cai, J.; Chen, Z.; Wu, W.; Lin, Q.; Liang, Y. High animal protein diet and gut microbiota in human health. Crit. Rev. Food Sci. Nutr. 2022, 62, 6225–6237. [Google Scholar] [CrossRef]
- Pan, A.; Sun, Q.; Bernstein, A.M.; Schulze, M.B.; Manson, J.E.; Willett, W.C.; Hu, F.B. Red meat consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis123. Am. J. Clin. Nutr. 2011, 94, 1088–1096. [Google Scholar] [CrossRef]
- Petrelli, F.; Cangelosi, G.; Scuri, S.; Thu, N.C.T.; Debernardi, G.; Benni, A.; Vesprini, A.; Rocchi, R.; De Carolis, C.; Pantanetti, P.; et al. Food knowledge of patients at the first access to a Diabetology center. Acta Biomed. 2020, 91, 160–164. [Google Scholar] [CrossRef]
- Truman, E.; Bischoff, M.; Elliott, C. Which literacy for health promotion: Health, food, nutrition or media? Health Promot. Int. 2019, 35, 432–444. [Google Scholar] [CrossRef] [PubMed]
- Ardoin, T.W.; Hamer, D.; Mason, N.; Reine, A.; Barleycorn, L.; Francis, D.; Johnson, A. Effectiveness of a Patient-Centered Dietary Educational Intervention. Ochsner J. 2022, 22, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.; Araújo, R.; Lopes, F.; Ray, S. Nutrition and Food Literacy: Framing the Challenges to Health Communication. Nutrients 2023, 15, 4708. [Google Scholar] [CrossRef]
- Chai, W.; Morimoto, Y.; Cooney, R.V.; Franke, A.A.; Shvetsov, Y.B.; Le Marchand, L.; Haiman, C.A.; Kolonel, L.N.; Goodman, M.T.; Maskarinec, G. Dietary Red and Processed Meat Intake and Markers of Adiposity and Inflammation: The Multiethnic Cohort Study. J. Am. Coll. Nutr. 2017, 36, 378–385. [Google Scholar] [CrossRef]
- Shiraseb, F.; Hosseininasab, D.; Mirzababaei, A.; Bagheri, R.; Wong, A.; Suzuki, K.; Mirzaei, K. Red, white, and processed meat consumption related to inflammatory and metabolic biomarkers among overweight and obese women. Front. Nutr. 2022, 9, 1015566. [Google Scholar] [CrossRef]
- Rakha, A.; Mehak, F.; Shabbir, M.A.; Arslan, M.; Ranjha, M.; Ahmed, W.; Socol, C.T.; Rusu, A.V.; Hassoun, A.; Aadil, R.M. Insights into the constellating drivers of satiety impacting dietary patterns and lifestyle. Front. Nutr. 2022, 9, 1002619. [Google Scholar] [CrossRef]
- Mendoza-Herrera, K.; Florio, A.A.; Moore, M.; Marrero, A.; Tamez, M.; Bhupathiraju, S.N.; Mattei, J. The Leptin System and Diet: A Mini Review of the Current Evidence. Front. Endocrinol. 2021, 12, 749050. [Google Scholar] [CrossRef]
- Xia, M.; Zhang, F. Amino Acids Metabolism in Retinopathy: From Clinical and Basic Research Perspective. Metabolites 2022, 12, 1244. [Google Scholar] [CrossRef]
- Mizukami, H. Serine supplementation: Is it a new option for the treatment of diabetic polyneuropathy? J. Diabetes Investig. 2023, 14, 1157–1159. [Google Scholar] [CrossRef]
- Liu, L.; Xu, J.; Zhang, Z.; Ren, D.; Wu, Y.; Wang, D.; Zhang, Y.; Zhao, S.; Chen, Q.; Wang, T. Metabolic Homeostasis of Amino Acids and Diabetic Kidney Disease. Nutrients 2022, 15, 184. [Google Scholar] [CrossRef]
- Lee, C.; Lee, J.; Eor, J.Y.; Kwak, M.J.; Huh, C.S.; Kim, Y. Effect of Consumption of Animal Products on the Gut Microbiome Composition and Gut Health. Food Sci. Anim. Resour. 2023, 43, 723–750. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Bhat, Z.F.; Gounder, R.S.; Mohamed Ahmed, I.A.; Al-Juhaimi, F.Y.; Ding, Y.; Bekhit, A.E.A. Effect of Dietary Protein and Processing on Gut Microbiota-A Systematic Review. Nutrients 2022, 14, 453. [Google Scholar] [CrossRef] [PubMed]
- Starke, S.; Harris, D.M.M.; Zimmermann, J.; Schuchardt, S.; Oumari, M.; Frank, D.; Bang, C.; Rosenstiel, P.; Schreiber, S.; Frey, N.; et al. Amino acid auxotrophies in human gut bacteria are linked to higher microbiome diversity and long-term stability. ISME J. 2023, 17, 2370–2380. [Google Scholar] [CrossRef] [PubMed]
- Diakité, M.T.; Diakité, B.; Koné, A.; Balam, S.; Fofana, D.; Diallo, D.; Kassogué, Y.; Traoré, C.B.; Kamaté, B.; Ba, D.; et al. Relationships between gut microbiota, red meat consumption and colorectal cancer. J. Carcinog. Mutagen. 2022, 13, 1000385. [Google Scholar]
- Li, T.-T.; Chen, X.; Huo, D.; Arifuzzaman, M.; Qiao, S.; Jin, W.-B.; Shi, H.; Li, X.V.; Iliev, I.D.; Artis, D.; et al. Microbiota metabolism of intestinal amino acids impacts host nutrient homeostasis and physiology. Cell Host Microbe 2024, 32, 661–675.e610. [Google Scholar] [CrossRef]
- Farvid, M.S.; Sidahmed, E.; Spence, N.D.; Mante Angua, K.; Rosner, B.A.; Barnett, J.B. Consumption of red meat and processed meat and cancer incidence: A systematic review and meta-analysis of prospective studies. Eur. J. Epidemiol. 2021, 36, 937–951. [Google Scholar] [CrossRef]
- Zhang, H.; Lv, X.; Su, W.; Chen, B.-H.; Lai, Y.-W.; Xie, R.; Lin, Q.; Chen, L.; Cao, H. Exploring the roles of excess amino acids, creatine, creatinine, and glucose in the formation of heterocyclic aromatic amines by UPLC-MS/MS. Food Chem. 2024, 446, 138760. [Google Scholar] [CrossRef]
- Gofton, C.; Upendran, Y.; Zheng, M.H.; George, J. MAFLD: How is it different from NAFLD? Clin. Mol. Hepatol. 2023, 29, S17–S31. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): A systematic review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef]
- Hashemian, M.; Merat, S.; Poustchi, H.; Jafari, E.; Radmard, A.R.; Kamangar, F.; Freedman, N.; Hekmatdoost, A.; Sheikh, M.; Boffetta, P.; et al. Red Meat Consumption and Risk of Nonalcoholic Fatty Liver Disease in a Population with Low Meat Consumption: The Golestan Cohort Study. Am. J. Gastroenterol. 2021, 116, 1667–1675. [Google Scholar] [CrossRef]
- Zelber-Sagi, S.; Ivancovsky-Wajcman, D.; Isakov, N.F.; Webb, M.; Orenstein, D.; Shibolet, O.; Kariv, R. High red and processed meat consumption is associated with non-alcoholic fatty liver disease and insulin resistance. J. Hepatol. 2018, 68, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.; Wu, L.Z.; Yun, L.; Cai, G.; Huan, Z.; Juan, S.L.; Yu, H.C.; Ming, L. The Prevalence of Nonalcoholic Fatty Liver Disease and its Association with Lifestyle/dietary Habits among University Faculty and Staff in Chengdu. Biomed. Environ. Sci. 2012, 25, 383. [Google Scholar] [CrossRef]
- Liao, Y.; Chen, Q.; Liu, L.; Huang, H.; Sun, J.; Bai, X.; Jin, C.; Li, H.; Sun, F.; Xiao, X.; et al. Amino acid is a major carbon source for hepatic lipogenesis. Cell Metab. 2024, 36, 2437–2448.e8. [Google Scholar] [CrossRef]
- Kim, M.N.; Lo, C.-H.; Corey, K.E.; Luo, X.; Long, L.; Zhang, X.; Chan, A.T.; Simon, T.G. Red meat consumption, obesity, and the risk of nonalcoholic fatty liver disease among women: Evidence from mediation analysis. Clin. Nutr. 2022, 41, 356–364. [Google Scholar] [CrossRef]
- Yasutake, K.; Nakamuta, M.; Shima, Y.; Ohyama, A.; Masuda, K.; Haruta, N.; Fujino, T.; Aoyagi, Y.; Fukuizumi, K.; Yoshimoto, T.; et al. Nutritional investigation of non-obese patients with non-alcoholic fatty liver disease: The significance of dietary cholesterol. Scand. J. Gastroenterol. 2009, 44, 471–477. [Google Scholar] [CrossRef]
- Recaredo, G.; Marin-Alejandre, B.A.; Cantero, I.; Monreal, J.I.; Herrero, J.I.; Benito-Boillos, A.; Elorz, M.; Tur, J.A.; Martínez, J.A.; Zulet, M.A.; et al. Association between Different Animal Protein Sources and Liver Status in Obese Subjects with Non-Alcoholic Fatty Liver Disease: Fatty Liver in Obesity (FLiO) Study. Nutrients 2019, 11, 2359. [Google Scholar] [CrossRef]
- Chen, H. Iron metabolism in non-alcoholic fatty liver disease: A promising therapeutic target. Liver Res. 2022, 6, 203–213. [Google Scholar] [CrossRef]
- Leitão, C.; Mignano, A.; Estrela, M.; Fardilha, M.; Figueiras, A.; Roque, F.; Herdeiro, M.T. The Effect of Nutrition on Aging-A Systematic Review Focusing on Aging-Related Biomarkers. Nutrients 2022, 14, 554. [Google Scholar] [CrossRef]
- Rallis, C.; Mülleder, M.; Smith, G.; Au, Y.Z.; Ralser, M.; Bähler, J. Amino Acids Whose Intracellular Levels Change Most During Aging Alter Chronological Life Span of Fission Yeast. J. Gerontol. Ser. A 2020, 76, 205–210. [Google Scholar] [CrossRef]
- Babygirija, R.; Lamming, D.W. The regulation of healthspan and lifespan by dietary amino acids. Transl. Med. Aging 2021, 5, 17–30. [Google Scholar] [CrossRef]
- Macho-González, A.; Garcimartín, A.; López-Oliva, M.E.; Bastida, S.; Benedí, J.; Ros, G.; Nieto, G.; Sánchez-Muniz, F.J. Can Meat and Meat-Products Induce Oxidative Stress? Antioxidants 2020, 9, 638. [Google Scholar] [CrossRef] [PubMed]
- Fretts, A.M.; Howard, B.V.; Siscovick, D.S.; Best, L.G.; Beresford, S.A.; Mete, M.; Eilat-Adar, S.; Sotoodehnia, N.; Zhao, J. Processed Meat, but Not Unprocessed Red Meat, Is Inversely Associated with Leukocyte Telomere Length in the Strong Heart Family Study. J. Nutr. 2016, 146, 2013–2018. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Y.; Gu, X.; Liu, Y.; Dong, D.; Kang, J.H.; Wang, M.; Eliassen, H.; Willett, W.C.; Stampfer, M.J.; et al. Long-Term Intake of Red Meat in Relation to Dementia Risk and Cognitive Function in US Adults. Neurology 2025, 104, e210286. [Google Scholar] [CrossRef] [PubMed]
- Kitada, M.; Ogura, Y.; Monno, I.; Koya, D. The impact of dietary protein intake on longevity and metabolic health. EBioMedicine 2019, 43, 632–640. [Google Scholar] [CrossRef]
- Struijk, E.A.; Fung, T.T.; Sotos-Prieto, M.; Rodriguez-Artalejo, F.; Willett, W.C.; Hu, F.B.; Lopez-Garcia, E. Red meat consumption and risk of frailty in older women. J. Cachexia Sarcopenia Muscle 2022, 13, 210–219. [Google Scholar] [CrossRef]
- Ardisson Korat, A.V.; Shea, M.K.; Jacques, P.F.; Sebastiani, P.; Wang, M.; Eliassen, A.H.; Willett, W.C.; Sun, Q. Dietary protein intake in midlife in relation to healthy aging–results from the prospective Nurses’ Health Study cohort. Am. J. Clin. Nutr. 2024, 119, 271–282. [Google Scholar] [CrossRef]
- Merz, B.; Frommherz, L.; Rist, M.J.; Kulling, S.E.; Bub, A.; Watzl, B. Dietary Pattern and Plasma BCAA-Variations in Healthy Men and Women-Results from the KarMeN Study. Nutrients 2018, 10, 623. [Google Scholar] [CrossRef]
- Garcia-Sifuentes, Y.; Maney, D.L. Reporting and misreporting of sex differences in the biological sciences. Elife 2021, 10, 70817. [Google Scholar] [CrossRef]
- Imamura, F.; Micha, R.; Khatibzadeh, S.; Fahimi, S.; Shi, P.; Powles, J.; Mozaffarian, D. Dietary quality among men and women in 187 countries in 1990 and 2010: A systematic assessment. Lancet Glob. Health 2015, 3, e132–e142. [Google Scholar] [CrossRef]
- Song, S.; Kim, J.; Kim, J. Gender Differences in the Association between Dietary Pattern and the Incidence of Hypertension in Middle-Aged and Older Adults. Nutrients 2018, 10, 252. [Google Scholar] [CrossRef]
- Burra, P.; Bizzaro, D.; Gonta, A.; Shalaby, S.; Gambato, M.; Morelli, M.C.; Trapani, S.; Floreani, A.; Marra, F.; Brunetto, M.R.; et al. Clinical impact of sexual dimorphism in non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH). Liver Int. 2021, 41, 1713–1733. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Zhao, J.V. Sex-Specific Associations of Red Meat and Processed Meat Consumption with Serum Metabolites in the UK Biobank. Nutrients 2022, 14, 5306. [Google Scholar] [CrossRef]
- Janssen, I.; Heymsfield, S.B.; Wang, Z.; Ross, R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J. Appl. Physiol. 2000, 89, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Ma, X.; Zhao, A.; Wang, C.; Zhang, Y.; Nieman, D.; Nicholson, J.K.; Jia, W.; Bao, Y.; Jia, W. The metabolite profiles of the obese population are gender-dependent. J. Proteome Res. 2014, 13, 4062–4073. [Google Scholar] [CrossRef] [PubMed]
- Chhimwal, J.; Patial, V.; Padwad, Y. Beverages and Non-alcoholic fatty liver disease (NAFLD): Think before you drink. Clin. Nutr. 2021, 40, 2508–2519. [Google Scholar] [CrossRef]
- Grzych, G.; Vonghia, L.; Bout, M.-A.; Weyler, J.; Verrijken, A.; Dirinck, E.; Chevalier Curt, M.J.; Van Gaal, L.; Paumelle, R.; Francque, S.; et al. Plasma BCAA Changes in Patients with NAFLD Are Sex Dependent. J. Clin. Endocrinol. Metab. 2020, 105, 2311–2321. [Google Scholar] [CrossRef]
- Severinsen, M.C.K.; Pedersen, B.K. Muscle–Organ Crosstalk: The Emerging Roles of Myokines. Endocr. Rev. 2020, 41, 594–609. [Google Scholar] [CrossRef]
- Velez, L.M.; Van, C.; Moore, T.; Zhou, Z.; Johnson, C.; Hevener, A.L.; Seldin, M.M. Genetic variation of putative myokine signaling is dominated by biological sex and sex hormones. eLife 2022, 11, e76887. [Google Scholar] [CrossRef]
- Barr, B.; Gollahon, L. The Modification of Dietary Protein with Ammonium Hydroxide Enhancement Improves Longevity and Metabolic Outcomes in a Sex-Dependent Manner. Nutrients 2024, 16, 2787. [Google Scholar] [CrossRef]
- Menikdiwela, K.R.; Guimarães, J.P.T.; Scoggin, S.; Gollahon, L.S.; Moustaid-Moussa, N. Dietary pH Enhancement Improves Metabolic Outcomes in Diet-Induced Obese Male and Female Mice: Effects of Beef vs. Casein Proteins. Nutrients 2022, 14, 2583. [Google Scholar] [CrossRef]
| Essential | Non-Essential |
|---|---|
| Histidine * | Alanine |
| Isoleucine | Arginine * |
| Leucine | Asparagine |
| Lysine | Aspartic acid |
| Methionine | Cysteine * |
| Phenylalanine | Glutamic acid |
| Threonine | Glutamine * |
| Tryptophan | Glycine |
| Valine | Proline |
| Serine | |
| Tyrosine * |
| EAA | NEAA | ||||||
|---|---|---|---|---|---|---|---|
| AA | Beef | Pork | % Difference | AA | Beef | Pork | % Difference |
| Histidine * | 129 | 93 | 32.43243 | Aspartic acid | 5 | 18 | 113.0435 |
| Leucine | 1059 | 799 | 27.98708 | Tyrosine * | 15 | 49 | 106.25 |
| Isoleucine | 560 | 453 | 21.12537 | Glutamine * | 1375 | 674 | 68.42362 |
| Phenylalanine | 491 | 400 | 20.42649 | Asparagine | 28 | 21 | 28.57143 |
| Methionine | 251 | 296 | 16.45338 | Serine | 98 | 76 | 25.28736 |
| Lysine | 366 | 311 | 16.24815 | Proline | 966 | 1148 | 17.21854 |
| Tryptophan | 38 | 38 | 0 | Glutamic acid | 405 | 460 | 12.71676 |
| Alanine | 319 | 334 | 4.594181 | ||||
| Glycine | 25 | 24 | 4.081633 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barr, B.; Levitt, D.E.; Gollahon, L. Red Meat Amino Acids for Beginners: A Narrative Review. Nutrients 2025, 17, 939. https://doi.org/10.3390/nu17060939
Barr B, Levitt DE, Gollahon L. Red Meat Amino Acids for Beginners: A Narrative Review. Nutrients. 2025; 17(6):939. https://doi.org/10.3390/nu17060939
Chicago/Turabian StyleBarr, Benjamin, Danielle E. Levitt, and Lauren Gollahon. 2025. "Red Meat Amino Acids for Beginners: A Narrative Review" Nutrients 17, no. 6: 939. https://doi.org/10.3390/nu17060939
APA StyleBarr, B., Levitt, D. E., & Gollahon, L. (2025). Red Meat Amino Acids for Beginners: A Narrative Review. Nutrients, 17(6), 939. https://doi.org/10.3390/nu17060939







