Is There an Association Between Hydration Status, Beverage Consumption Frequency, Blood Pressure, Anthropometric Characteristics, and Urinary Biomarkers in Adults?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
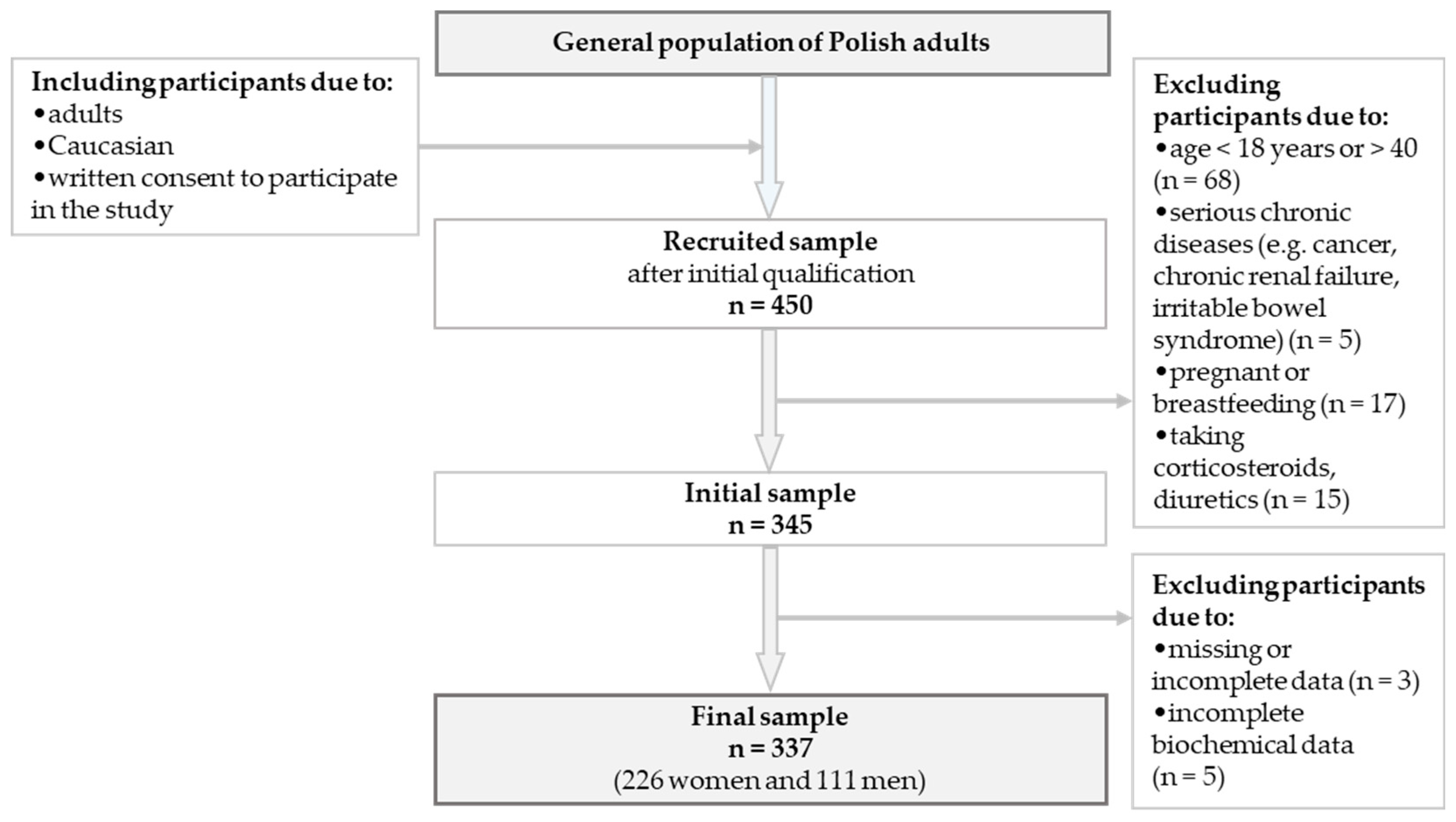
2.2. Study Population and Data Collection
2.3. Sociodemographic and Lifestyle Data
2.4. Beverage Consumption Data and Dietary Patterns (DPs)
2.5. Blood Pressure (BP) and Anthropometric Measurements
2.6. BIA Measurements
2.7. Biochemical Analysis in Urine
2.8. Hydration Status
2.9. Statistical Analysis
3. Results
3.1. Participants Characteristic
3.2. Frequency of Consumption and Symptoms of Dehydration Based on the WUT Components
3.3. Factors Influencing Dehydration Risk Based on WUT Components
4. Discussion
4.1. Fatigue, Headaches, and Dehydration
4.2. Beverage Consumption Frequency and Hydration Status
4.3. Anthropometric and Biochemical Analyses and Hydration Status
4.4. Strength and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheuvront, S.N.; Kenefick, R.W. Am I Drinking Enough? Yes, No, and Maybe. J. Am. Coll. Nutr. 2016, 35, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US). Panel on Dietary Reference Intakes for Water, Potassium, Sodium, Chloride, and Sulfate; National Academies Press (US): Washington, DC, USA, 2004; ISBN 0-309-53049-0. [Google Scholar]
- Kostelnik, S.B.; Davy, K.P.; Hedrick, V.E.; Thomas, D.T.; Davy, B.M. The Validity of Urine Color as a Hydration Biomarker within the General Adult Population and Athletes: A Systematic Review. J. Am. Coll. Nutr. 2021, 40, 172–179. [Google Scholar] [CrossRef]
- Liska, D.; Mah, E.; Brisbois, T.; Barrios, P.L.; Baker, L.B.; Spriet, L.L. Narrative Review of Hydration and Selected Health Outcomes in the General Population. Nutrients 2019, 11, 70. [Google Scholar] [CrossRef]
- Rychlik, E.; Stoś, K.; Woźniak, A.; Mojska, H. Nutrition Standards for the Polish Population; National Institute of Public Health—National Research Institute: Warsaw, Poland, 2024; ISBN 978-83-65870-78-0.
- Lacey, J.; Corbett, J.; Forni, L.; Hooper, L.; Hughes, F.; Minto, G.; Moss, C.; Price, S.; Whyte, G.; Woodcock, T.; et al. A Multidisciplinary Consensus on Dehydration: Definitions, Diagnostic Methods and Clinical Implications. Ann. Med. 2019, 51, 232–251. [Google Scholar] [CrossRef]
- Ekingen, T.; Sob, C.; Hartmann, C.; Rühli, F.J.; Matthes, K.L.; Staub, K.; Bender, N. Associations between Hydration Status, Body Composition, Sociodemographic and Lifestyle Factors in the General Population: A Cross-Sectional Study. BMC Public Health 2022, 22, 900. [Google Scholar] [CrossRef] [PubMed]
- Keefe, M.S.; Luk, H.-Y.; Rolloque, J.-J.S.; Jiwan, N.C.; McCollum, T.B.; Sekiguchi, Y. The Weight, Urine Colour and Thirst Venn Diagram Is an Accurate Tool Compared with Urinary and Blood Markers for Hydration Assessment at Morning and Afternoon Timepoints in Euhydrated and Free-Living Individuals. Br. J. Nutr. 2024, 131, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Keefe, M.S.; Luk, H.-Y.; Rolloque, J.-J.; Jiwan, N.C.; Sekiguchi, Y. Hydration Assessment in Males and Females Using the WUT (Weight, Urine Color, and Thirst) Venn Diagram Compared to Blood and Urinary Indices. Nutrients 2025, 17, 689. [Google Scholar] [CrossRef]
- Kundzewicz, Z.W.; Piniewski, M.; Mezghani, A.; Okruszko, T.; Pińskwar, I.; Kardel, I.; Hov, Ø.; Szcześniak, M.; Szwed, M.; Benestad, R.E.; et al. Assessment of Climate Change and Associated Impact on Selected Sectors in Poland. Acta Geophys. 2018, 66, 1509–1523. [Google Scholar] [CrossRef]
- Widłak, P.; Malara, M.; Tomczyk, Ł.; Dania, A.; Panagiotakou, G.; Papoulia, G. Evaluation of the Dietary Habits of Polish and Greek University Students in the Context of the Health Benefits of Their Diets. Nutrition 2024, 16, 3819. [Google Scholar] [CrossRef]
- Millard-Stafford, M.; Wendland, D.M.; O’Dea, N.K.; Norman, T.L. Thirst and Hydration Status in Everyday Life. Nutr. Rev. 2012, 70, S147–S151. [Google Scholar] [CrossRef]
- Pachucka, P. Assessment of Beverage Consumption in a Selected Group of Adults—Comparison of Two Methods. Master’s Thesis, Institute of Human Nutrition Sciences, Warsaw, Poland, 2017. [Google Scholar]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
- Stergiou, G.S.; Palatini, P.; Parati, G.; O’Brien, E.; Januszewicz, A.; Lurbe, E.; Persu, A.; Mancia, G.; Kreutz, R. 2021 European Society of Hypertension Practice Guidelines for Office and Out-of-Office Blood Pressure Measurement. J. Hypertens. 2021, 39, 1293–1302. [Google Scholar] [CrossRef]
- Unda Villafuerte, F.; Llobera Cànaves, J.; Lorente Montalvo, P.; Moreno Sancho, M.L.; Oliver Oliver, B.; Bassante Flores, P.; Estela Mantolan, A.; Pou Bordoy, J.; Rodríguez Ruiz, T.; Requena Hernández, A.; et al. Effectiveness of a Multifactorial Intervention, Consisting of Self-Management of Antihypertensive Medication, Self-Measurement of Blood Pressure, Hypocaloric and Low Sodium Diet, and Physical Exercise, in Patients with Uncontrolled Hypertension Taking 2 or more antihypertensive drugs: The MEDICHY study. Medicine 2020, 99, e19769. [Google Scholar] [CrossRef] [PubMed]
- Norton, K.; Eston, R. Kinanthropometry and Exercise Physiology, 4th ed.; Routledge: London, UK, 2018. [Google Scholar] [CrossRef]
- Batsis, J.A.; Mackenzie, T.A.; Bartels, S.J.; Sahakyan, K.R.; Somers, V.K.; Lopez-Jimenez, F. Diagnostic Accuracy of Body Mass Index to Identify Obesity in Older Adults: NHANES 1999–2004. Int. J. Obes. 2015, 40, 761. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European Consensus on Definition and Diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Holmes, C.J.; Racette, S.B. The Utility of Body Composition Assessment in Nutrition and Clinical Practice: An Overview of Current Methodology. Nutrients 2021, 13, 2493. [Google Scholar] [CrossRef]
- Wood, A.D.; Edward, G.D.; Cumming, K.; Kafri, M.W.; Soiza, R.L.; Hooper, L.; Potter, J.F.; Myint, P.K. Bioelectrical Impedance Versus Biochemical Analysis of Hydration Status: Predictive Value for Prolonged Hospitalisation and Poor Discharge Destination for Older Patients. Healthcare 2021, 9, 154. [Google Scholar] [CrossRef]
- Schoutteten, M.K.; Lindeboom, L.; Brys, A.; Lanssens, D.; Smeets, C.J.P.; De Cannière, H.; De Moor, B.; Peeters, J.; Heylen, L.; Van Hoof, C.; et al. Comparison of Whole Body versus Thoracic Bioimpedance in Relation to Ultrafiltration Volume and Systolic Blood Pressure during Hemodialysis. J. Appl. Physiol. 2023, 135, 1330–1338. [Google Scholar] [CrossRef]
- Cleymaet, R.; D’Hondt, M.; Scheinok, T.; Malbrain, L.; De Laet, I.; Schoonheydt, K.; Dits, H.; Van Regenmortel, N.; Mekeirele, M.; Cordemans, C.; et al. Comparison of Bioelectrical Impedance Analysis (BIA)-Derived Parameters in Healthy Volunteers and Critically Ill Patients. Life 2024, 14, 27. [Google Scholar] [CrossRef]
- Nishi, S.K.; Babio, N.; Paz-Graniel, I.; Serra-Majem, L.; Vioque, J.; Fitó, M.; Corella, D.; Pintó, X.; Bueno-Cavanillas, A.; Tur, J.A.; et al. Water Intake, Hydration Status and 2-Year Changes in Cognitive Performance: A Prospective Cohort Study. BMC Med. 2023, 21, 82. [Google Scholar] [CrossRef]
- Fernández-Elías, V.E.; Martinez-Abellán, A.; López-Gullón, J.M.; Morán-Navarro, R.; Pallarés, J.G.; De La Cruz-Sánchez, E.; Mora-Rodriguez, R. Validity of Hydration Non-Invasive Indices during the Weightcutting and Official Weigh-in for Olympic Combat Sports. PLoS ONE 2014, 9, e95336. [Google Scholar] [CrossRef]
- Dmitrieva, N.I.; Boehm, M.; Yancey, P.H.; Enhörning, S. Long-Term Health Outcomes Associated with Hydration Status. Nat. Rev. Nephrol. 2024, 20, 275–294. [Google Scholar] [CrossRef]
- Serra-Prat, M.; Lorenzo, I.; Martínez, J.; Palomera, E.; Pleguezuelos, E.; Ferrer, P. Relationship between Hydration Status and Muscle Catabolism in the Aged Population: A Cross-Sectional Study. Nutrients 2023, 15, 4718. [Google Scholar] [CrossRef] [PubMed]
- Critzer, S.S.; Bosch, T.J.; Fercho, K.A.; Scholl, J.L.; Baugh, L.A. Water and Brain Function: Effects of Hydration Status on Neurostimulation with Transcranial Magnetic Stimulation. J. Neurophysiol. 2024, 132, 791–807. [Google Scholar] [CrossRef] [PubMed]
- Braun, H.; von Andrian-Werburg, J.; Malisova, O.; Athanasatou, A.; Kapsokefalou, M.; Ortega, J.; Mora-Rodriguez, R.; Thevis, M. Differing Water Intake and Hydration Status in Three European Countries—A Day-to-Day Analysis. Nutrients 2019, 11, 773. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhang, J.; Wang, X.; Li, Y.; Yan, Y.; Ma, G. Behaviors of Water Intake, Hydration Status, and Related Hydration Biomarkers among Physically Active Male Young Adults in Beijing, China: A Cross-Sectional Study. Int. J. Clin. Pract. 2022, 2022, 9436186. [Google Scholar] [CrossRef]
- Maffeis, C.; Tommasi, M.; Tomasselli, F.; Spinelli, J.; Fornari, E.; Scattolo, N.; Marigliano, M.; Morandi, A. Fluid Intake and Hydration Status in Obese vs Normal Weight Children. Eur. J. Clin. Nutr. 2016, 70, 560–565. [Google Scholar] [CrossRef]
- Chang, T.; Ravi, N.; Plegue, M.A.; Sonneville, K.R.; Davis, M.M. Inadequate Hydration, BMI, and Obesity Among US Adults: NHANES 2009-2012. Ann. Fam. Med. 2016, 14, 320–324. [Google Scholar] [CrossRef]
- Rosinger, A.Y.; Lawman, H.G.; Akinbami, L.J.; Ogden, C.L. The Role of Obesity in the Relation between Total Water Intake and Urine Osmolality in US Adults, 2009–2012. Am. J. Clin. Nutr. 2016, 104, 1554–1561. [Google Scholar] [CrossRef]
- Padrão, P.; Sousa, A.; Guerra, R.; Álvares, L.; Santos, A.; Borges, N.; Afonso, C.; Amaral, T.; Moreira, P. A Cross-Sectional Study on the Association between 24-h Urine Osmolality and Weight Status in Older Adults. Nutrients 2017, 9, 1272. [Google Scholar] [CrossRef]
- Abdulsalam, R.; Alsadah, A.; Alkhuboli, M.; Muala, D.; Hussein, A.; Elmoselhi, A.B. Hydration Status Assessment and Impinging Factors among University Students in the UAE. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 6451–6458. [Google Scholar] [CrossRef]
- Lafontan, M.; Visscher, T.L.S.; Farpour-Lambert, N.; Yumuk, V. Opportunities for Intervention Strategies for Weight Management: Global Actions on Fluid Intake Patterns. Obes. Facts 2015, 8, 54–76. [Google Scholar] [CrossRef] [PubMed]
- Thornton, S.N. Increased Hydration Can Be Associated with Weight Loss. Front. Nutr. 2016, 3, 18. [Google Scholar] [CrossRef] [PubMed]
- Buschmann, M.; Steiniger, J.; Hille, U.; Tank, J.; Adams, F.; Sharma, A.M.; Klaus, S.; Luft, F.C.; Jordan, J. Water-Induced Thermogenesis. J. Clin. Endocrinol. Metab. 2003, 88, 6015–6019. [Google Scholar] [CrossRef] [PubMed]
- Rolls, B.J. Dietary Energy Density: Applying Behavioural Science to Weight Management. Nutr. Bull. 2017, 42, 246–253. [Google Scholar] [CrossRef]
- Pehlivan, M.; Saleki, N.; Sezer, F.E.; Özyürek, F.; Delice, B.; Hızlı Güldemir, H. Water and Beverage Consumption Habits of Adults in Turkey by Gender and BMI: A Cross-Sectional Survey. Int. J. Environ. Health Res. 2024, 34, 1910–1925. [Google Scholar] [CrossRef]
- He, F.J.; Marrero, N.M.; MacGregor, G.A. Salt Intake Is Related to Soft Drink Consumption in Children and Adolescents: A Link to Obesity? Hypertension 2008, 51, 629–634. [Google Scholar] [CrossRef]
- Cheuvront, S.N.; Kenefick, R.W. Dehydration: Physiology, Assessment, and Performance Effects. Compr. Physiol. 2014, 4, 257–285. [Google Scholar] [CrossRef]
- Perrier, E.T.; Buendia-Jimenez, I.; Vecchio, M.; Armstrong, L.E.; Tack, I.; Klein, A. Twenty-Four-Hour Urine Osmolality as a Physiological Index of Adequate Water Intake. Dis. Markers 2015, 2015, 231063. [Google Scholar] [CrossRef]
- Yeh, H.-C.; Lin, Y.-S.; Kuo, C.-C.; Weidemann, D.; Weaver, V.; Fadrowski, J.; Neu, A.; Navas-Acien, A. Urine Osmolality in the US Population: Implications for Environmental Biomonitoring. Environ. Res. 2015, 136, 482–490. [Google Scholar] [CrossRef]
- Perinpam, M.; Ware, E.B.; Smith, J.A.; Turner, S.T.; Kardia, S.L.R.; Lieske, J.C. Key Influence of Sex on Urine Volume and Osmolality. Biol. Sex Differ. 2016, 7, 12. [Google Scholar] [CrossRef]
- Willis-Gray, M.; Wu, J.M.; Markland, A. Urinary Incontinence and Hydration: A Population-based Analysis. Neurourol. Urodyn. 2018, 37, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Suh, H.; Summers, L.G.; Seal, A.D.; Colburn, A.T.; Mauromoustakos, A.; Perrier, E.T.; Bottin, J.H.; Kavouras, S.A. Afternoon Urine Osmolality Is Equivalent to 24 h for Hydration Assessment in Healthy Children. Eur. J. Clin. Nutr. 2020, 74, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Baron, S.; Courbebaisse, M.; Lepicard, E.M.; Friedlander, G. Assessment of Hydration Status in a Large Population. Br. J. Nutr. 2015, 113, 147–158. [Google Scholar] [CrossRef]
- Volkert, D.; Beck, A.M.; Cederholm, T.; Cruz-Jentoft, A.; Hooper, L.; Kiesswetter, E.; Maggio, M.; Raynaud-Simon, A.; Sieber, C.; Sobotka, L.; et al. ESPEN Practical Guideline: Clinical Nutrition and Hydration in Geriatrics. Clin. Nutr. 2022, 41, 958–989. [Google Scholar] [CrossRef] [PubMed]
- Zubac, D.; Reale, R.; Karnincic, H.; Sivric, A.; Jelaska, I. Urine Specific Gravity as an Indicator of Dehydration in Olympic Combat Sport Athletes; Considerations for Research and Practice. Eur. J. Sport Sci. 2018, 18, 920–929. [Google Scholar] [CrossRef]
- Mendes, J.; Padrão, P.; Moreira, P.; Santos, A.; Borges, N.; Afonso, C.; Negrão, R.; Amaral, T.F. Handgrip Strength and Its Association with Hydration Status and Urinary Sodium-to-Potassium Ratio in Older Adults. J. Am. Coll. Nutr. 2020, 39, 192–199. [Google Scholar] [CrossRef]
| Variables | 0–1 WUT (n = 172) | 2–3 WUT (n = 165) | p–Value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Age (years) | 24.3 ± 4.5 * 23.0 ** | 23.9 ± 4.5 * 23.0 ** | 0.565 | ||
| Gender | |||||
| Male | 52 | 30.2 | 59 | 35.8 | 0.281 |
| Female | 120 | 69.8 | 106 | 64.2 | |
| Education | |||||
| Secondary/‘I study’ | 112 | 65.1 | 121 | 73.3 | 0.103 |
| Higher | 60 | 34.9 | 44 | 26.7 | |
| Place of residence | |||||
| Village | 32 | 18.6 | 32 | 19.4 | 0.841 |
| Town | 40 | 23.3 | 34 | 20.6 | |
| City | 100 | 58.1 | 99 | 60.0 | |
| Economic status | |||||
| Very poor/poor | 44 | 25.6 | 48 | 29.1 | 0.469 |
| Average/very good | 128 | 74.4 | 117 | 70.9 | |
| Health status | |||||
| Very poor/poor | 32 | 18.6 | 61 | 37.0 | 0.048 |
| Average/very good | 140 | 81.4 | 104 | 63.0 | |
| Cigarette smoking | |||||
| Yes | 21 | 12.2 | 17 | 10.4 | 0.594 |
| No | 151 | 87.8 | 148 | 89.6 | |
| Physical activity | |||||
| No/low | 47 | 27.3 | 71 | 43.0 | 0.046 |
| Moderate | 41 | 23.9 | 51 | 30.9 | |
| High | 84 | 48.8 | 43 | 26.1 | |
| BMI | |||||
| <18.5 (kg/m2) | 10 | 5.8 | 5 | 3.0 | 0.031 |
| 18.5–24.9 (kg/m2) | 125 | 72.7 | 102 | 61.8 | |
| ≥25.0 (kg/m2) | 37 | 21.5 | 58 | 35.2 | |
| Body mass change | |||||
| Decreased by more than 1 kg | 17 | 9.9 | 21 | 12.7 | 0.001 |
| Increased by more than 1 kg | 44 | 25.6 | 128 | 77.6 | |
| No change | 91 | 52.9 | 13 | 7.9 | |
| ‘I don’t know’ | 20 | 11.6 | 3 | 1.8 | |
| Thirst level | 2.77 ± 1.27 * 3.00 ** | 4.12 ± 1.22 * 5.0 ** | 0.001 | ||
| Ucol | 2.11 ± 0.71 * 3.0 ** | 4.55 ± 1.35 * 5.0 ** | 0.001 | ||
| Variables | 0–1 WUT (n = 172) | 2–3 WUT (n = 165) | p-Value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Fatigue during the day | 36 | 20.7 | 67 | 40.4 | 0.009 |
| Headaches | 84 | 48.8 | 106 | 64.2 | 0.024 |
| Excessive sweating | 24 | 14.0 | 36 | 21.8 | 0.079 |
| Feeling of fullness and an expanding abdomen | 61 | 35.7 | 64 | 38.9 | 0.997 |
| Heaviness in the legs | 20 | 11.6 | 46 | 27.9 | 0.002 |
| Swelling-related symptoms | 45 | 26.1 | 49 | 30.0 | 0.244 |
| Lower urinary tract infections | 6 | 3.5 | 7 | 4.2 | 0.719 |
| Oliguria | 1 | 0.6 | 2 | 1.2 | 0.537 |
| Very frequent urination | 25 | 14.5 | 33 | 20.0 | 0.183 |
| Diarrhea | 18 | 10.5 | 34 | 20.6 | 0.049 |
| Variables | 0–1 WUT (n = 172) | 2–3 WUT (n = 165) | p-Value |
|---|---|---|---|
| Mean ± SD; Median | |||
| Tea | 5.9 ± 1.4; 6.0 | 4.9 ± 1.3; 5.0 | 0.047 |
| Coffee | 4.7 ± 1.5; 6.0 | 3.8 ± 1.5; 4.0 | 0.037 |
| Herbal infusions | 3.3 ± 0.8; 3.0 | 2.2 ± 0.6; 2.0 | 0.003 |
| Milk | 4.0 ± 1.5; 4.0 | 3.8 ± 1.3; 3.0 | 0.148 |
| Natural fermented milk drinks | 3.8 ± 1.4; 3.0 | 2.8 ± 1.1; 2.0 | 0.036 |
| Flavored fermented milk drinks | 2.1 ± 0.6; 2.0 | 1.9 ± 0.5; 2.0 | 0.938 |
| Carbonated mineral water | 2.6 ± 0.7; 1.0 | 2.4 ± 0.6; 2.0 | 0.975 |
| Non-carbonated mineral water | 6.2 ± 1.3; 7.0 | 5.2 ± 1.1; 6.0 | 0.045 |
| Fruit juices | 4.5 ± 1.3; 4.0 | 2.9 ± 0.4; 3.0 | 0.023 |
| Vegetable juices | 1.7 ± 0.3; 1.0 | 1.7 ± 0.4; 1.0 | 0.278 |
| Fruit and vegetable juice | 1.7 ± 0.5; 1.0 | 1.9 ± 0.6; 1.0 | 0.385 |
| Non-carbonated fruit drinks | 1.5 ± 0.5; 1.0 | 1.7 ± 0.7; 1.0 | 0.253 |
| Sweetened carbonated drinks | 1.8 ± 0.4; 1.0 | 4.9 ± 1.1; 1.0 | 0.415 |
| Tea drinks | 1.7 ± 0.5; 1.0 | 1.6 ± 0.5; 1.0 | 0.765 |
| Cola drinks | 1.9 ± 0.6; 2.0 | 2.6 ± 1.2; 2.0 | 0.801 |
| Energy drinks | 1.6 ± 0.5; 1.0 | 1.4 ± 0.3; 1.0 | 0.113 |
| Isotonic drinks | 1.2 ± 0.2; 1.0 | 1.1 ± 0.4; 1.0 | 0.507 |
| Non-alcoholic beer | 1.8 ± 0.4; 1.0 | 1.9 ± 0.5; 1.0 | 0.854 |
| Beer | 2.1 ± 0.4; 2.0 | 2.1 ± 0.3; 2.0 | 0.433 |
| Wine | 2.1 ± 0.6; 2.0 | 2.1 ± 0.4; 2.0 | 0.795 |
| Vodka | 1.8 ± 0.7; 1.0 | 1.5 ± 0.5; 1.0 | 0.327 |
| Alcoholic drinks | 1.7 ± 0.7; 1.0 | 1.5 ± 0.7; 1.0 | 0.395 |
| Dietary patterns (%) | |||
| Reasonable | 30.7 | 22.3 | 0.643 |
| Unhealthy | 25.1 | 29.7 | |
| Minimalist | 24.7 | 23.8 | |
| Loving Sweet Beverages | 19.5 | 24.2 | |
| Variables | 0–1 WUT (n = 172) | 2–3 WUT (n = 165) | p-Value |
|---|---|---|---|
| Mean ± SD; Median | |||
| Blood pressure | |||
| SBP (mmHg) | 121 ± 15.54; 120 | 120 ± 15.21; 118.5 | 0.560 |
| DBP (mmHg) | 77.3 ± 8.40; 77.0 | 77.6 ± 8.21; 78.0 | 0.762 |
| Anthropometric measurements | |||
| BMI (kg/m2) | 22.7 ± 3.2; 22.3 | 26.1 ± 3.6; 25.9 | 0.047 |
| HC (cm) | 96.6 ± 8.88; 95.0 | 99.6 ± 8.57; 99.0 | 0.039 |
| WC (cm) | 73.5 ± 9.51; 72.2 | 77.9 ± 10.68; 78.0 | 0.036 |
| HGS—right hand (kg) | 35.0 ± 19.3; 36.0 | 30.6 ±19.7; 29.8 | 0.032 |
| HGS—left hand (kg) | 23.1 ± 7.00; 23.0 | 21.6 ± 8.53; 20.0 | 0.105 |
| Body composition—BIA | |||
| MM (kg) | 23.6 ± 5.09; 21.7 | 23.8 ± 5.56; 21.8 | 0.931 |
| MM (%) | 9.50 ± 2.48; 10.4 | 10.3 ± 3.45; 10.5 | 0.714 |
| FFM (kg) | 50.9 ± 10.1; 47.1 | 52.4 ± 11.4; 48.7 | 0.282 |
| FFM (%) | 76.9 ± 8.20; 77.4 | 76.5 ± 8.33; 76.9 | 0.601 |
| TBW (kg) | 37.2 ± 7.61; 34.4 | 40.11 ± 8.32; 37.4 | 0.039 |
| TBW (%) | 55.9 ± 5.76; 55.9 | 50.7 ± 5.83; 50.8 | 0.041 |
| ECW (kg) | 16.7 ± 3.64; 16.1 | 17.2 ± 3.77; 16.7 | 0.192 |
| ECW (%) | 44.7 ± 3.31; 44.4 | 44.9 ± 3.71; 44.7 | 0.391 |
| ICW (kg) | 20.8 ± 4.74; 19.0 | 21.0 ± 4.60; 19.5 | 0.278 |
| ICW (%) | 55.3 ± 3.31; 55.7 | 55.0 ± 3.70; 55.3 | 0.391 |
| FM (kg) | 14.7 ± 7.80; 13.5 | 19.5 ± 8.28; 19.8 | 0.026 |
| FM (%) | 23.1 ± 8.23; 22.5 | 26.4 ± 8.37; 26.6 | 0.038 |
| Biochemical analysis—urine | |||
| USG | 1.014 ± 0.006; 1.017 | 1.026 ± 0.007; 1.024 | 0.001 |
| Uosm | 558 ± 166; 442 | 767 ± 171; 698 | 0.001 |
| pH | 6.13 ± 0.61; 6.00 | 6.10 ± 0.53; 5.90 | 0.375 |
| Variables | 2–3 WUT | ||||
|---|---|---|---|---|---|
| β a | eβ b | 95% CI c | p-Value d | ||
| Physical activity | |||||
| No/low | 1 | 1 | 1 | 1 | |
| Moderate | 0.051 | 1.07 | 0.84 | 1.32 | 0.625 |
| High | 0.478 | 1.61 | 0.95 | 2.12 | 0.112 |
| Health status | |||||
| Very poor/poor | 1 | 1 | 1 | 1 | |
| Good/very good | 0.318 | 1.27 | 0.95 | 1.42 | 0.212 |
| Fatigue during the day | 0.173 | 1.33 | 1.11 | 1.59 | 0.025 |
| Headaches | 0.457 | 1.46 | 1.15 | 1.78 | 0.031 |
| Tea e | 0.421 | 0.52 | 0.38 | 0.70 | 0.009 |
| Non-carbonated mineral water e | −0.357 | 0.45 | 0.29 | 0.73 | 0.011 |
| SBP (mmHg) | 0.078 | 1.01 | 0.85 | 1.18 | 0.435 |
| BMI (kg/m2) | 1.116 | 1.49 | 2.53 | 3.60 | 0.036 |
| WC (cm) | −0.413 | 1.79 | 1.26 | 2.42 | 0.021 |
| HGS—right hand (kg) | 0.367 | 0.81 | 0.68 | 0.97 | 0.047 |
| TBW (%) | −0.717 | 0.49 | 0.32 | 0.76 | 0.004 |
| USG | 0.849 | 2.29 | 1.62 | 4.12 | 0.001 |
| Uosm | 0.555 | 1.75 | 1.27 | 2.48 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frąckiewicz, J.; Szewczyk, K. Is There an Association Between Hydration Status, Beverage Consumption Frequency, Blood Pressure, Anthropometric Characteristics, and Urinary Biomarkers in Adults? Nutrients 2025, 17, 952. https://doi.org/10.3390/nu17060952
Frąckiewicz J, Szewczyk K. Is There an Association Between Hydration Status, Beverage Consumption Frequency, Blood Pressure, Anthropometric Characteristics, and Urinary Biomarkers in Adults? Nutrients. 2025; 17(6):952. https://doi.org/10.3390/nu17060952
Chicago/Turabian StyleFrąckiewicz, Joanna, and Kacper Szewczyk. 2025. "Is There an Association Between Hydration Status, Beverage Consumption Frequency, Blood Pressure, Anthropometric Characteristics, and Urinary Biomarkers in Adults?" Nutrients 17, no. 6: 952. https://doi.org/10.3390/nu17060952
APA StyleFrąckiewicz, J., & Szewczyk, K. (2025). Is There an Association Between Hydration Status, Beverage Consumption Frequency, Blood Pressure, Anthropometric Characteristics, and Urinary Biomarkers in Adults? Nutrients, 17(6), 952. https://doi.org/10.3390/nu17060952







