Effects of Supplementation with Microalgae Extract from Tetradesmus obliquus Strain Mi175.B1.a on Gastrointestinal Symptoms and Mental Health in Healthy Adults: A Pilot Randomized, Double-Blind, Placebo-Controlled, Parallel-Arm Trial
Abstract
:1. Introduction
2. Methods
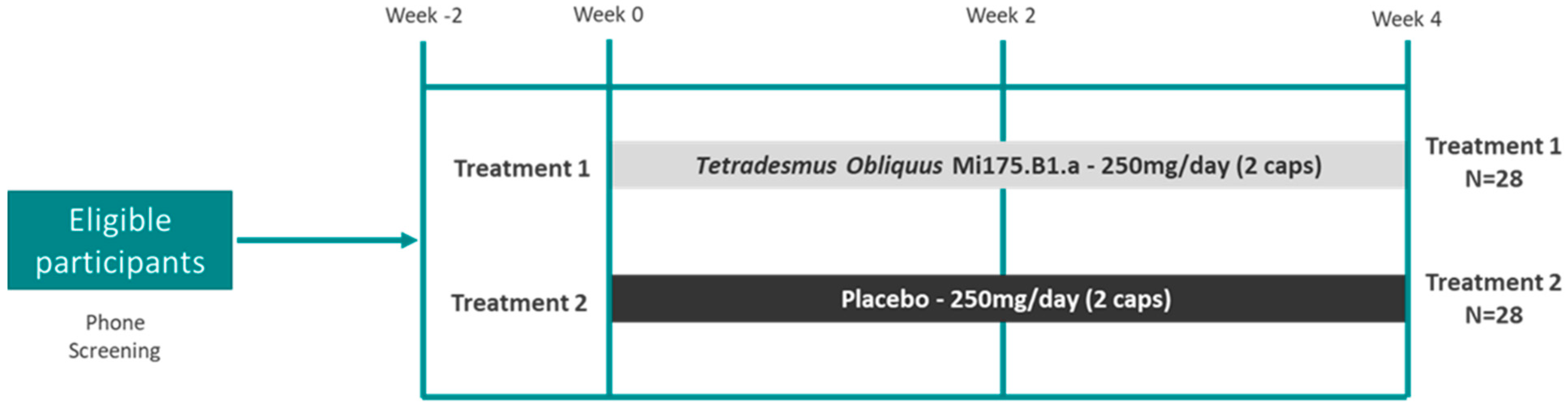
2.1. Research Design
2.2. Intervention and Compliance
2.3. Anthropometrics
2.4. GI Disorder and Bowel Movement Assessments
2.5. Mental Health Assessments
2.6. Hemodynamics and Pulse Wave Analysis at Rest and During CPT
2.7. Biological Specimen Collection
2.8. Microbiome 16s rRNA Gene Sequencing and Processing
2.9. Short-Chain Fatty Acid (SCFA) Extraction and Analysis
2.10. Blood, Saliva, and Stool ELISA Assays
2.11. PBMC Culturing and Stimulation
2.12. Lipid and Comprehensive Metabolic Panels
2.13. Statistical Analyses
2.13.1. Analysis of Biomarkers and Participant Outcomes
2.13.2. Microbiome Analyses
3. Results
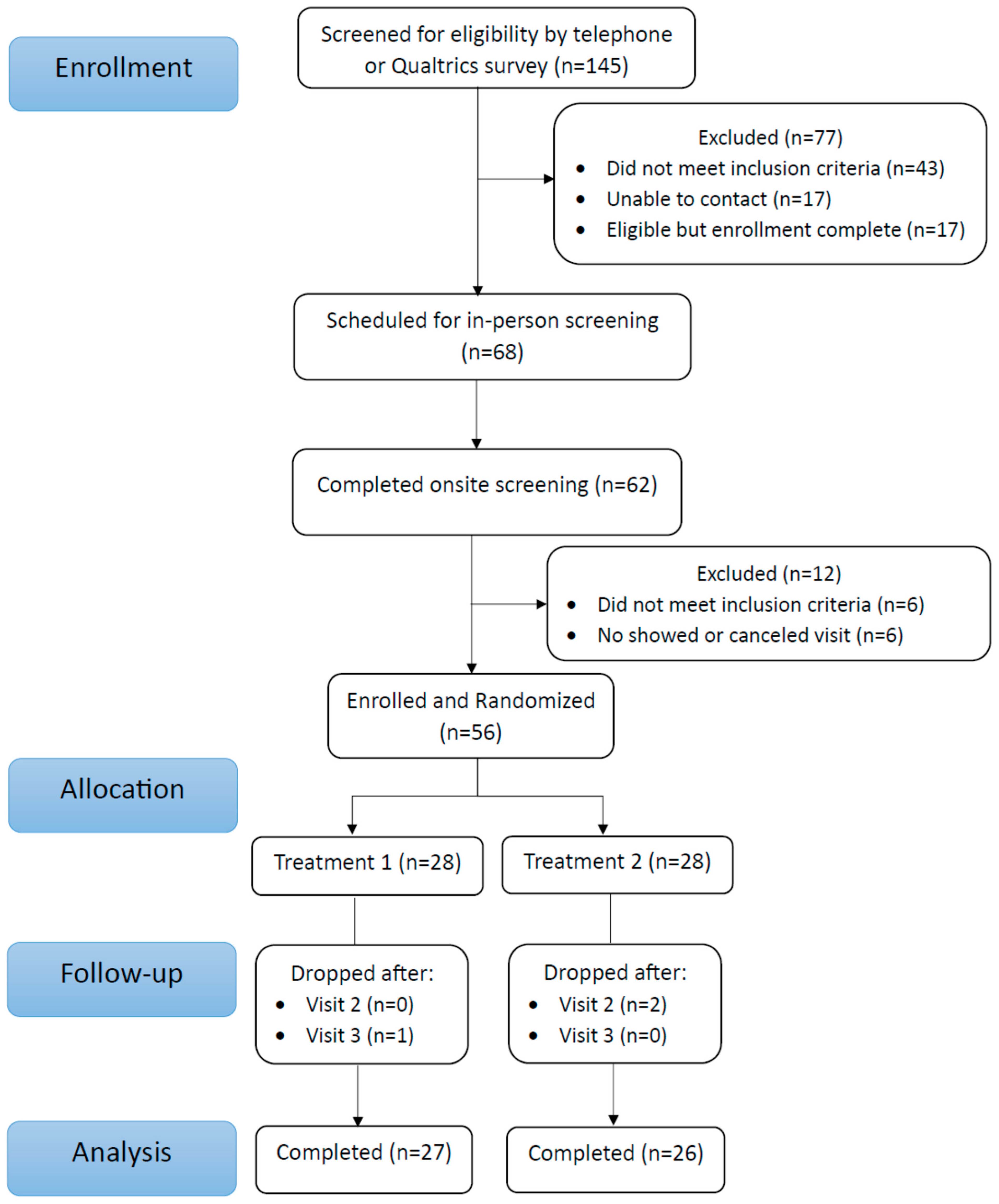
3.1. Participant Characteristics
Compliance, Safety, Tolerability, and Diet
3.2. Gut Function
3.2.1. GI Symptom Severity and Bowel Movement Assessments
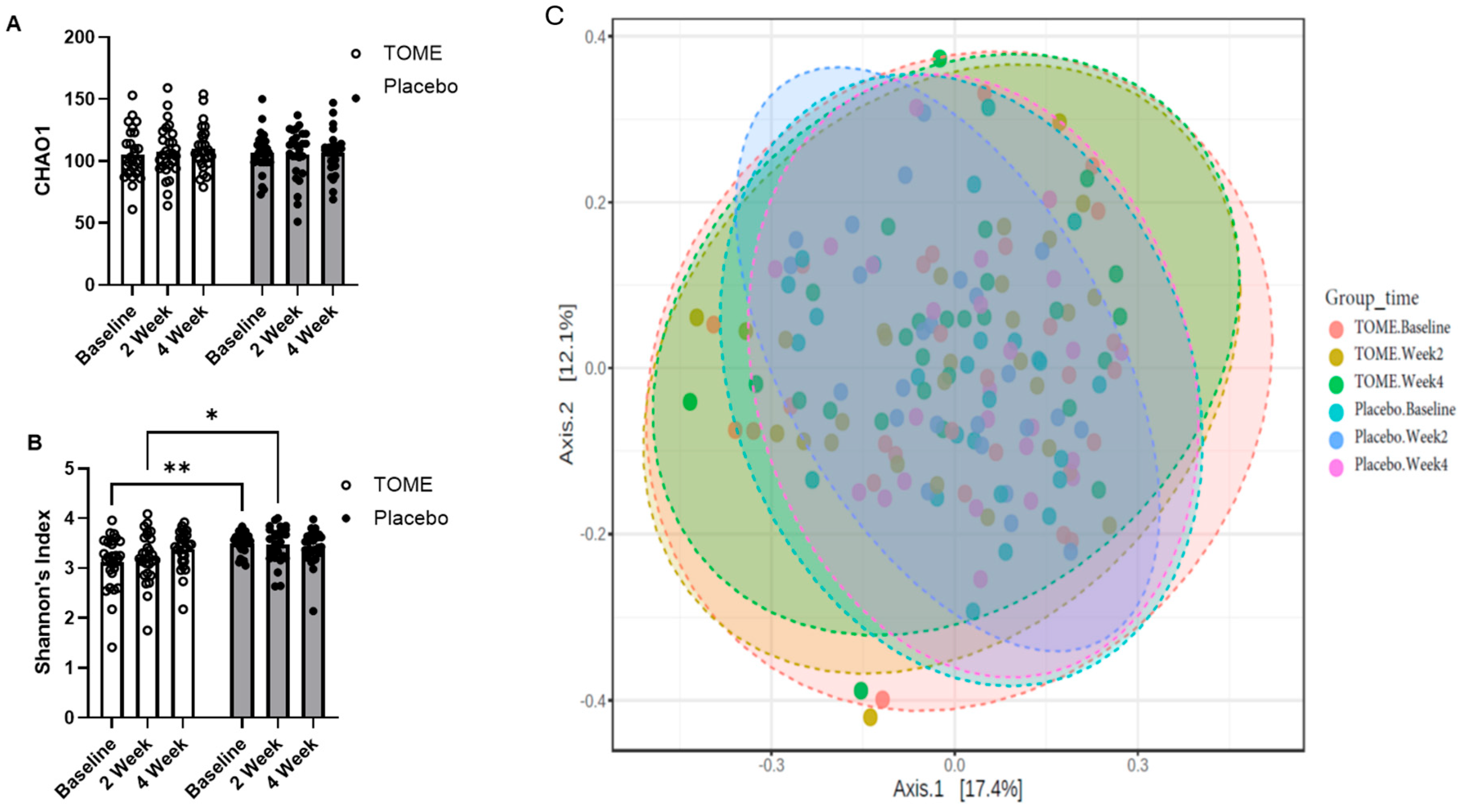
3.2.2. Gut Microbiota Assessment
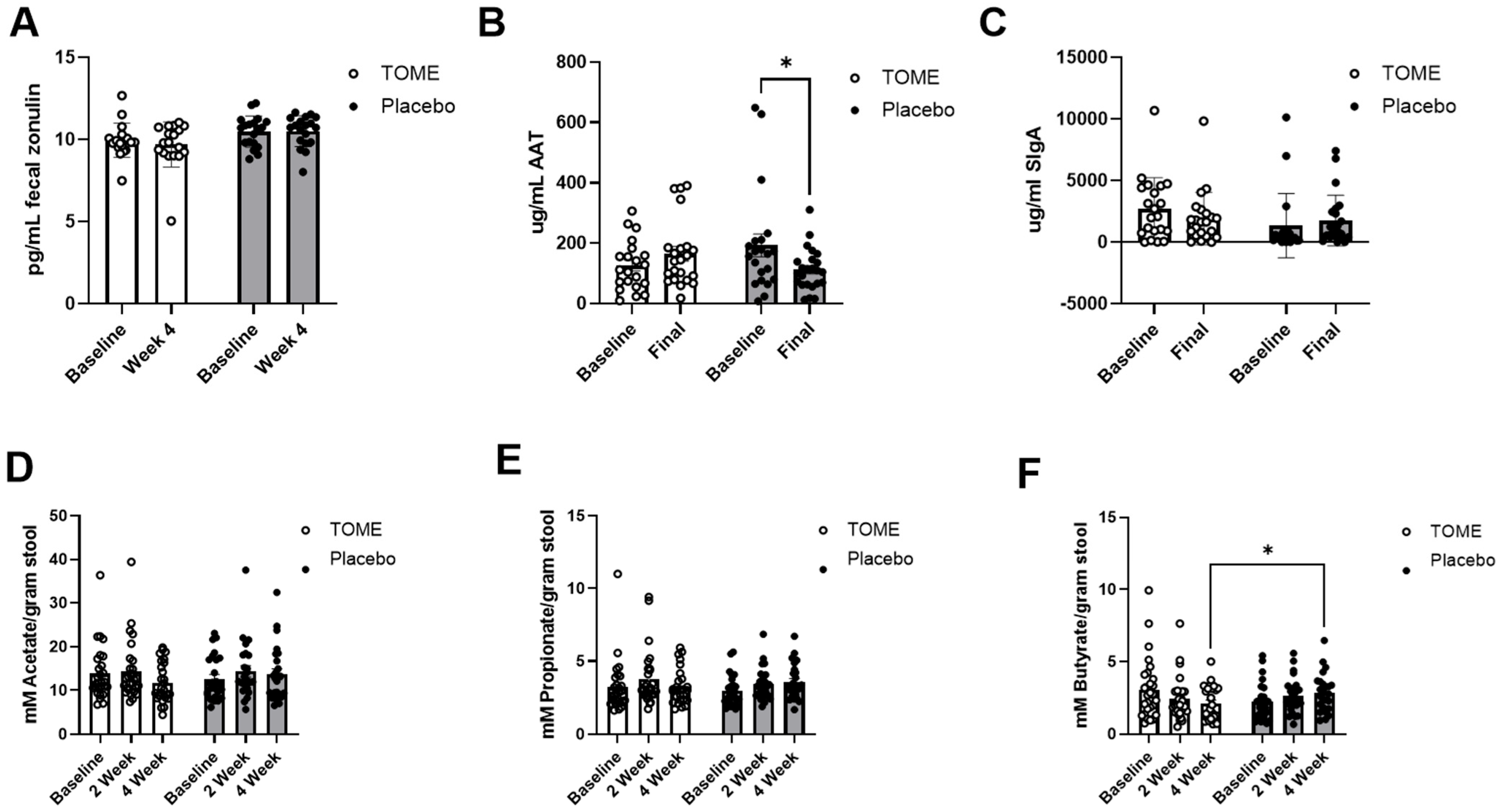
3.2.3. Markers of Gut Barrier Integrity and Microbial Activity
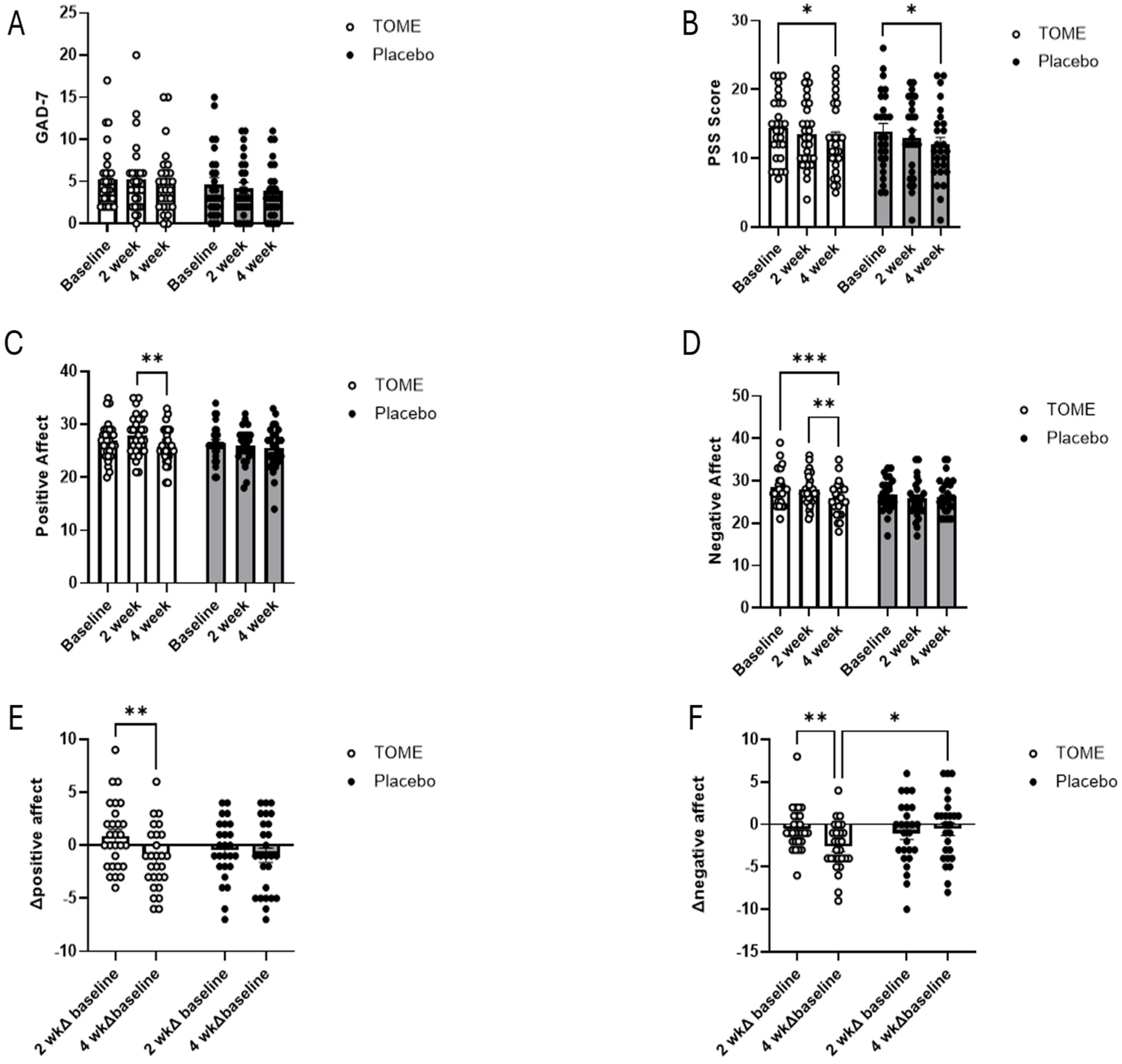
3.3. Effects on Mental Health Parameters
3.3.1. Self-Reported Assessments of Affect, Anxiety, and Stress
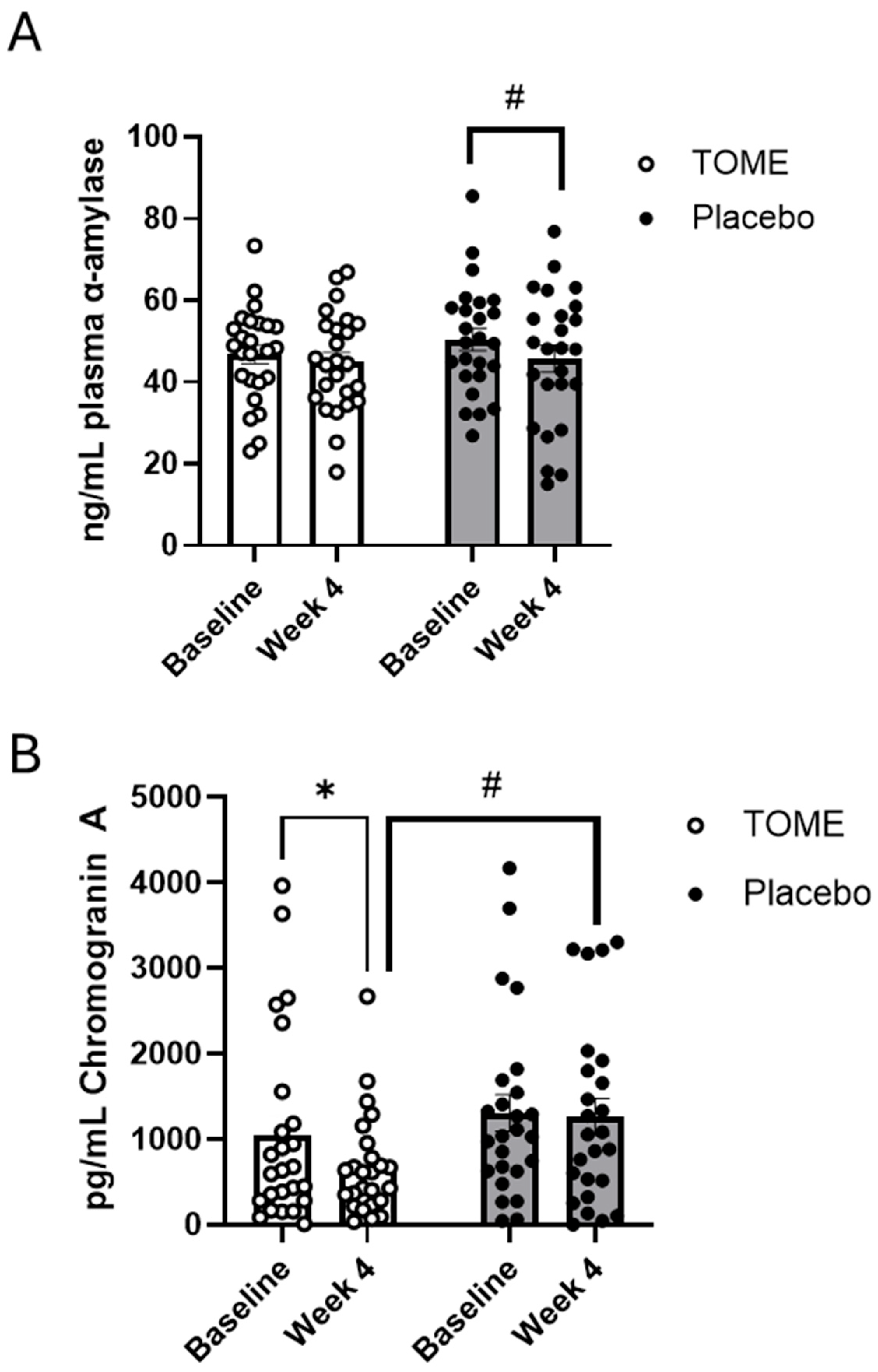
3.3.2. Blood and Saliva Markers of Stress
3.4. Cardiovascular-Related Outcomes
3.5. Markers of Systemic Inflammation and Immune Function
4. Discussion
4.1. Gut Health, Microbiota, and Intestinal Function
4.2. Mental Health and Gut–Brain Axis
4.3. Study Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sperber, A.D.; Bangdiwala, S.I.; Drossman, D.A.; Ghoshal, U.C.; Simren, M.; Tack, J.; Whitehead, W.E.; Dumitrascu, D.L.; Fang, X.; Fukudo, S.; et al. Worldwide Prevalence and Burden of Functional Gastrointestinal Disorders, Results of Rome Foundation Global Study. Gastroenterology 2021, 160, 99–114.e3. [Google Scholar] [CrossRef] [PubMed]
- Fekete, M.; Lehoczki, A.; Tarantini, S.; Fazekas-Pongor, V.; Csípő, T.; Csizmadia, Z.; Varga, J.T. Improving Cognitive Function with Nutritional Supplements in Aging: A Comprehensive Narrative Review of Clinical Studies Investigating the Effects of Vitamins, Minerals, Antioxidants, and Other Dietary Supplements. Nutrients 2023, 15, 5116. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Dinan, T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef]
- Clapp, M.; Aurora, N.; Herrera, L.; Bhatia, M.; Wilen, E.; Wakefield, S. Gut Microbiota’s Effect on Mental Health: The Gut-Brain Axis. Clin. Pract. 2017, 7, 987. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Zhao, S.; Yin, Y.; Zhang, H.; Needham, D.M.; Evans, E.D.; Dai, C.L.; Lu, P.J.; Alm, E.J.; Weitz, D.A. High-throughput, single-microbe genomics with strain resolution, applied to a human gut microbiome. Science 2022, 376, eabm1483. [Google Scholar] [CrossRef]
- Bistas, K.G.; Tabet, J.P. The Benefits of Prebiotics and Probiotics on Mental Health. Cureus 2023, 15, e43217. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Toral, M.; Robles-Vera, I.; De La Visitación, N.; Romero, M.; Yang, T.; Sánchez, M.; Gómez-Guzmán, M.; Jiménez, R.; Raizada, M.K.; Duarte, J. Critical Role of the Interaction Gut Microbiota—Sympathetic Nervous System in the Regulation of Blood Pressure. Front. Physiol. 2019, 10, 231. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Z.; Geng, B.; Cai, J. The Bidirectional Signal Communication of Microbiota-Gut-Brain Axis in Hypertension. Int. J. Hypertens. 2021, 2021, 8174789. [Google Scholar] [CrossRef]
- Robles-Vera, I.; Toral, M.; Duarte, J. Microbiota and Hypertension: Role of the Sympathetic Nervous System and the Immune System. Am. J. Hypertens. 2020, 33, 890–901. [Google Scholar] [CrossRef]
- O’Connor, K.M.; Lucking, E.F.; Cryan, J.F.; O’Halloran, K.D. Bugs, breathing and blood pressure: Microbiota–gut–brain axis signalling in cardiorespiratory control in health and disease. J. Physiol. 2020, 598, 4159–4179. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.A.; Rinaman, L.; Cryan, J.F. Stress & the gut-brain axis: Regulation by the microbiome. Neurobiol. Stress 2017, 7, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Randeni, N.; Bordiga, M.; Xu, B. A Comprehensive Review of the Triangular Relationship among Diet–Gut Microbiota–Inflammation. Int. J. Mol. Sci. 2024, 25, 9366. [Google Scholar] [CrossRef]
- Lu, T.; Huang, C.; Weng, R.; Wang, Z.; Sun, H.; Ma, X. Enteric glial cells contribute to chronic stress-induced alterations in the intestinal microbiota and barrier in rats. Heliyon 2024, 10, e24899. [Google Scholar] [CrossRef] [PubMed]
- Bruun, C.F.; Haldor Hansen, T.; Vinberg, M.; Kessing, L.V.; Coello, K. Associations between short-chain fatty acid levels and mood disorder symptoms: A systematic review. Nutr. Neurosci. 2024, 27, 899–912. [Google Scholar] [CrossRef]
- Xiong, R.-G.; Li, J.; Cheng, J.; Zhou, D.-D.; Wu, S.-X.; Huang, S.-Y.; Saimaiti, A.; Yang, Z.-J.; Gan, R.-Y.; Li, H.-B. The Role of Gut Microbiota in Anxiety, Depression, and Other Mental Disorders as Well as the Protective Effects of Dietary Components. Nutrients 2023, 15, 3258. [Google Scholar] [CrossRef]
- Kennedy, P.J.; Murphy, A.B.; Cryan, J.F.; Ross, P.R.; Dinan, T.G.; Stanton, C. Microbiome in brain function and mental health. Trends Food Sci. Technol. 2016, 57, 289–301. [Google Scholar] [CrossRef]
- Yang, T.; Zubcevic, J. Gut–Brain Axis in Regulation of Blood Pressure. Front. Physiol. 2017, 8, 845. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Jaberi, K.R.; Alamdari-palangi, V.; Savardashtaki, A.; Vatankhah, P.; Jamialahmadi, T.; Tajbakhsh, A.; Sahebkar, A. Modulatory Effects of Phytochemicals on Gut–Brain Axis: Therapeutic Implication. Curr. Dev. Nutr. 2024, 8, 103785. [Google Scholar] [CrossRef]
- Stringham, N.T.; Holmes, P.V.; Stringham, J.M. Supplementation with macular carotenoids reduces psychological stress, serum cortisol, and sub-optimal symptoms of physical and emotional health in young adults. Nutr. Neurosci. 2018, 21, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Repici, A.; Hasan, A.; Capra, A.P.; Scuderi, S.A.; Paterniti, I.; Campolo, M.; Ardizzone, A.; Esposito, E. Marine Algae and Deriving Biomolecules for the Management of Inflammatory Bowel Diseases: Potential Clinical Therapeutics to Decrease Gut Inflammatory and Oxidative Stress Markers? Mar. Drugs 2024, 22, 336. [Google Scholar] [CrossRef] [PubMed]
- Ou, J.; Wang, Z.; Liu, X.; Song, B.; Chen, J.; Li, R.; Jia, X.; Huang, R.; Xiang, W.; Zhong, S. Regulatory effects of marine polysaccharides on gut microbiota dysbiosis: A review. Food Chem. X 2022, 15, 100444. [Google Scholar] [CrossRef]
- Patel, A.K.; Singhania, R.R.; Awasthi, M.K.; Varjani, S.; Bhatia, S.K.; Tsai, M.-L.; Hsieh, S.-L.; Chen, C.-W.; Dong, C.-D. Emerging prospects of macro- and microalgae as prebiotic. Microb. Cell Fact. 2021, 20, 112. [Google Scholar] [CrossRef] [PubMed]
- De Medeiros, V.P.B.; De Souza, E.L.; De Albuquerque, T.M.R.; Da Costa Sassi, C.F.; Dos Santos Lima, M.; Sivieri, K.; Pimentel, T.C.; Magnani, M. Freshwater microalgae biomasses exert a prebiotic effect on human colonic microbiota. Algal Res. 2021, 60, 102547. [Google Scholar] [CrossRef]
- Kiani, H.; Aznar, R.; Poojary, M.M.; Tiwari, B.K.; Halim, R. Chromatographic Techniques to Separate and Identify Bioactive Compounds in Microalgae. Front. Energy Res. 2022, 10, 904014. [Google Scholar] [CrossRef]
- Leonard, M.; Maury, J.; Dickerson, B.; Gonzalez, D.E.; Kendra, J.; Jenkins, V.; Nottingham, K.; Yoo, C.; Xing, D.; Ko, J.; et al. Effects of Dietary Supplementation of a Microalgae Extract Containing Fucoxanthin Combined with Guarana on Cognitive Function and Gaming Performance. Nutrients 2023, 15, 1918. [Google Scholar] [CrossRef]
- Dickerson, B.; Maury, J.; Jenkins, V.; Nottingham, K.; Xing, D.; Gonzalez, D.E.; Leonard, M.; Kendra, J.; Ko, J.; Yoo, C.; et al. Effects of Supplementation with Microalgae Extract from Phaeodactylum tricornutum (Mi136) to Support Benefits from a Weight Management Intervention in Overweight Women. Nutrients 2024, 16, 990. [Google Scholar] [CrossRef]
- Yoo, C.; Maury, J.; Gonzalez, D.E.; Ko, J.; Xing, D.; Jenkins, V.; Dickerson, B.; Leonard, M.; Estes, L.; Johnson, S.; et al. Effects of Supplementation with a Microalgae Extract from Phaeodactylum tricornutum Containing Fucoxanthin on Cognition and Markers of Health in Older Individuals with Perceptions of Cognitive Decline. Nutrients 2024, 16, 2999. [Google Scholar] [CrossRef]
- Velankanni, P.; Go, S.-H.; Jin, J.B.; Park, J.-S.; Park, S.; Lee, S.-B.; Kwon, H.-K.; Pan, C.-H.; Cha, K.H.; Lee, C.-G. Chlorella vulgaris Modulates Gut Microbiota and Induces Regulatory T Cells to Alleviate Colitis in Mice. Nutrients 2023, 15, 3293. [Google Scholar] [CrossRef]
- Chandrarathna, H.P.S.U.; Liyanage, T.D.; Edirisinghe, S.L.; Dananjaya, S.H.S.; Thulshan, E.H.T.; Nikapitiya, C.; Oh, C.; Kang, D.-H.; De Zoysa, M. Marine Microalgae, Spirulina maxima-Derived Modified Pectin and Modified Pectin Nanoparticles Modulate the Gut Microbiota and Trigger Immune Responses in Mice. Mar. Drugs 2020, 18, 175. [Google Scholar] [CrossRef]
- Wang, M.; Ma, H.; Guan, S.; Luo, T.; Zhao, C.; Cai, G.; Zheng, Y.; Jia, X.; Di, J.; Li, R.; et al. Astaxanthin from Haematococcus pluvialis alleviates obesity by modulating lipid metabolism and gut microbiota in mice fed a high-fat diet. Food Funct. 2021, 12, 9719–9738. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.Y.B.; Oliveira, C.D.L.; Prasad, R.; Ong, H.C.; Araujo, E.S.; Shabnam, N.; Gálvez, A.O. A multidisciplinary review of Tetradesmus obliquus: A microalga suitable for large-scale biomass production and emerging environmental applications. Rev. Aquac. 2021, 13, 1594–1618. [Google Scholar] [CrossRef]
- Cabrita, A.R.J.; Guilherme-Fernandes, J.; Spínola, M.; Maia, M.R.G.; Yergaliyev, T.; Camarinha-Silva, A.; Fonseca, A.J.M. Effects of microalgae as dietary supplement on palatability, digestibility, fecal metabolites, and microbiota in healthy dogs. Front. Vet. Sci. 2023, 10, 1245790. [Google Scholar] [CrossRef] [PubMed]
- Piasecka, A.; Nawrocka, A.; Wiącek, D.; Krzemińska, I. Agro-industrial by-product in photoheterotrophic and mixotrophic culture of Tetradesmus obliquus: Production of ω3 and ω6 essential fatty acids with biotechnological importance. Sci. Rep. 2020, 10, 6411. [Google Scholar] [CrossRef]
- Kumar, V.; Rohilla, A.; Ahire, J.J. Omega-3 fatty acids and the gut microbiome: A new frontier in cardiovascular disease prevention. Discov. Med. 2025, 2, 53. [Google Scholar] [CrossRef]
- Mora-Flores, L.P.; Moreno-Terrazas Casildo, R.M.-T.; Fuentes-Cabrera, J.; Pérez-Vicente, H.A.; De Anda-Jáuregui, G.; Neri-Torres, E.E. The Role of Carbohydrate Intake on the Gut Microbiome: A Weight of Evidence Systematic Review. Microorganisms 2023, 11, 1728. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Kaden-Volynets, V.; Filipe Rosa, L.; Guseva, D.; Seethaler, B. Regulation of the gut barrier by carbohydrates from diet—Underlying mechanisms and possible clinical implications. Int. J. Med. Microbiol. 2021, 311, 151499. [Google Scholar] [CrossRef]
- Woolf, E.K.; Terwoord, J.D.; Litwin, N.S.; Vazquez, A.R.; Lee, S.Y.; Ghanem, N.; Michell, K.A.; Smith, B.T.; Grabos, L.E.; Ketelhut, N.B.; et al. Daily blueberry consumption for 12 weeks improves endothelial function in postmenopausal women with above-normal blood pressure through reductions in oxidative stress: A randomized controlled trial. Food Funct. 2023, 14, 2621–2641. [Google Scholar] [CrossRef]
- ISO 12228-1; Determination of Individual and Total Sterols Contents—Gas Chromatographic Method, Part 1: Animal and Vegetable Fats and Oils. ISO: Geneva, Switzerland, 2014.
- World Health Organization. Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation, Geneva, 8–11 December 2008; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Kreutzer, J.S.; DeLuca, J.; Caplan, B. (Eds.) Encyclopedia of Clinical Neuropsychology; Springer Nature Living Reference Behavioral Science and Psychology; Springer: Cham, Switzerland, 2019; ISBN 978-3-319-56782-2. [Google Scholar]
- Spitzer, R.L.; Kroenke, K.; Williams, J.B.W.; Löwe, B. A Brief Measure for Assessing Generalized Anxiety Disorder: The GAD-7. Arch. Intern. Med. 2006, 166, 1092. [Google Scholar] [CrossRef]
- Lee, E.-H. Review of the Psychometric Evidence of the Perceived Stress Scale. Asian Nurs. Res. 2012, 6, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Litwin, N.S.; Van Ark, H.J.; Hartley, S.C.; Michell, K.A.; Vazquez, A.R.; Fischer, E.K.; Melby, C.L.; Weir, T.L.; Wei, Y.; Rao, S.; et al. Impact of Red Beetroot Juice on Vascular Endothelial Function and Cardiometabolic Responses to a High-Fat Meal in Middle-Aged/Older Adults with Overweight and Obesity: A Randomized, Double-Blind, Placebo-Controlled, Crossover Trial. Curr. Dev. Nutr. 2019, 3, nzz113. [Google Scholar] [CrossRef]
- Freedman, K.E.; Hill, J.L.; Wei, Y.; Vazquez, A.R.; Grubb, D.S.; Trotter, R.E.; Wrigley, S.D.; Johnson, S.A.; Foster, M.T.; Weir, T.L. Examining the Gastrointestinal and Immunomodulatory Effects of the Novel Probiotic Bacillus subtilis DE111. Int. J. Mol. Sci. 2021, 22, 2453. [Google Scholar] [CrossRef] [PubMed]
- Litwin, N.; Wrigley, S.; Van Ark, H.; Hartley, S.; Michell, K.; Vazquez, A.; Fischer, E.; Melby, C.; Wei, Y.; Rao, S.; et al. Effects of Red Beetroot Juice and Inorganic Nitrate Supplementation on Oral Bacteria and Nitric Oxide Metabolites in Middle-Aged/Older Adults with Overweight and Obesity. Curr. Dev. Nutr. 2020, 4, nzaa045_061. [Google Scholar] [CrossRef]
- Thompson, L.R.; Sanders, J.G.; McDonald, D.; Amir, A.; Ladau, J.; Locey, K.J.; Prill, R.J.; Tripathi, A.; Gibbons, S.M.; Ackermann, G.; et al. A communal catalogue reveals Earth’s multiscale microbial diversity. Nature 2017, 551, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Chong, J.; Liu, P.; Zhou, G.; Xia, J. Using MicrobiomeAnalyst for comprehensive statistical, functional, and meta-analysis of microbiome data. Nat. Protoc. 2020, 15, 799–821. [Google Scholar] [CrossRef]
- Febvre, H.P.; Rao, S.; Gindin, M.; Goodwin, N.D.M.; Finer, E.; Vivanco, J.S.; Lu, S.; Manter, D.K.; Wallace, T.C.; Weir, T.L. PHAGE Study: Effects of Supplemental Bacteriophage Intake on Inflammation and Gut Microbiota in Healthy Adults. Nutrients 2019, 11, 666. [Google Scholar] [CrossRef]
- Fields, F.J.; Lejzerowicz, F.; Schroeder, D.; Ngoi, S.M.; Tran, M.; McDonald, D.; Jiang, L.; Chang, J.T.; Knight, R.; Mayfield, S. Effects of the microalgae Chlamydomonas on gastrointestinal health. J. Funct. Foods 2020, 65, 103738. [Google Scholar] [CrossRef]
- Singh, G.; Dixit, I.; Kalman, D.; Gogineni, N.T. A Novel Herbal Composition Alleviates Functional Constipation, Reduces Gastrointestinal Transit Time, and Improves Bowel Function in Adults: A Double-Blind, Randomized Clinical Study. J. Am. Nutr. Assoc. 2024, 43, 553–566. [Google Scholar] [CrossRef]
- Lin, H.; Peddada, S.D. Analysis of compositions of microbiomes with bias correction. Nat. Commun. 2020, 11, 3514. [Google Scholar] [CrossRef]
- Mallick, H.; Rahnavard, A.; McIver, L.J.; Ma, S.; Zhang, Y.; Nguyen, L.H.; Tickle, T.L.; Weingart, G.; Ren, B.; Schwager, E.H.; et al. Multivariable association discovery in population-scale meta-omics studies. PLoS Comput. Biol. 2021, 17, e1009442. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Torres-Tiji, Y.; Fields, F.J.; Mayfield, S.P. Microalgae as a future food source. Biotechnol. Adv. 2020, 41, 107536. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Y.; Tso, R.; Teo, H.S.; Haldar, S. The utility of algae as sources of high value nutritional ingredients, particularly for alternative/complementary proteins to improve human health. Front. Nutr. 2023, 10, 1277343. [Google Scholar] [CrossRef]
- Costantini, L.; Molinari, R.; Farinon, B.; Merendino, N. Impact of Omega-3 Fatty Acids on the Gut Microbiota. Int. J. Mol. Sci. 2017, 18, 2645. [Google Scholar] [CrossRef]
- Alves-Santos, A.M.; Sugizaki, C.S.A.; Lima, G.C.; Naves, M.M.V. Prebiotic effect of dietary polyphenols: A systematic review. J. Funct. Foods 2020, 74, 104169. [Google Scholar] [CrossRef]
- Lee, S.-H.; Ku, X.; Oh, H.-S.; Jung, Y.; Chun, J.; Choi, I. Longitudinal associations between gut microbiome diversity and emotional well-being. Health Psychol. 2024, 43, 323–327. [Google Scholar] [CrossRef]
- Fasano, A. Zonulin and Its Regulation of Intestinal Barrier Function: The Biological Door to Inflammation, Autoimmunity, and Cancer. Physiol. Rev. 2011, 91, 151–175. [Google Scholar] [CrossRef]
- Janciauskiene, S.; Wrenger, S.; Immenschuh, S.; Olejnicka, B.; Greulich, T.; Welte, T.; Chorostowska-Wynimko, J. The Multifaceted Effects of Alpha1-Antitrypsin on Neutrophil Functions. Front. Pharmacol. 2018, 9, 341. [Google Scholar] [CrossRef]
- Strygler, B.; Nicar, M.J.; Santangelo, W.C.; Porter, J.L.; Fordtran, J.S. α1-antitrypsin excretion in stool in normal subjects and in patients with gastrointestinal disorders. Gastroenterology 1990, 99, 1380–1387. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Lee, G.; Son, H.; Koh, H.; Kim, E.S.; Unno, T.; Shin, J.-H. Butyrate producers, “The Sentinel of Gut”: Their intestinal significance with and beyond butyrate, and prospective use as microbial therapeutics. Front. Microbiol. 2023, 13, 1103836. [Google Scholar] [CrossRef]
- Kelly, C.J.; Zheng, L.; Campbell, E.L.; Saeedi, B.; Scholz, C.C.; Bayless, A.J.; Wilson, K.E.; Glover, L.E.; Kominsky, D.J.; Magnuson, A.; et al. Crosstalk between Microbiota-Derived Short-Chain Fatty Acids and Intestinal Epithelial HIF Augments Tissue Barrier Function. Cell Host Microbe 2015, 17, 662–671. [Google Scholar] [CrossRef] [PubMed]
- McOrist, A.L.; Miller, R.B.; Bird, A.R.; Keogh, J.B.; Noakes, M.; Topping, D.L.; Conlon, M.A. Fecal Butyrate Levels Vary Widely among Individuals but Are Usually Increased by a Diet High in Resistant Starch1,2. J. Nutr. 2011, 141, 883–889. [Google Scholar] [CrossRef]
- Medawar, E.; Haange, S.-B.; Rolle-Kampczyk, U.; Engelmann, B.; Dietrich, A.; Thieleking, R.; Wiegank, C.; Fries, C.; Horstmann, A.; Villringer, A.; et al. Gut microbiota link dietary fiber intake and short-chain fatty acid metabolism with eating behavior. Transl. Psychiatry 2021, 11, 500. [Google Scholar] [CrossRef] [PubMed]
- Talbott, S.M.; Hantla, D.; Capelli, B.; Ding, L.; Li, Y.; Artaria, C. Effect of Astaxanthin Supplementation on Psychophysiological Heart-Brain Axis Dynamics in Healthy Subjects. Funct. Foods Health Dis. 2019, 9, 521. [Google Scholar] [CrossRef]
- Muntjewerff, E.M.; Tang, K.; Lutter, L.; Christoffersson, G.; Nicolasen, M.J.T.; Gao, H.; Katkar, G.D.; Das, S.; Ter Beest, M.; Ying, W.; et al. Chromogranin A regulates gut permeability via the antagonistic actions of its proteolytic peptides. Acta Physiol. 2021, 232, e13655. [Google Scholar] [CrossRef]
- Li, Y.; Song, Y.; Dang, W.; Guo, L.; Xu, W. The associations between anxiety/depression and plasma chromogranin A among healthy workers: Results from EHOP study. J. Occup. Health 2020, 62, e12113. [Google Scholar] [CrossRef]
- Helle, K.B.; Corti, A. Chromogranin A: A paradoxical player in angiogenesis and vascular biology. Cell. Mol. Life Sci. 2015, 72, 339–348. [Google Scholar] [CrossRef]
- Merino Del Portillo, M.; Clemente-Suárez, V.J.; Ruisoto, P.; Jimenez, M.; Ramos-Campo, D.J.; Beltran-Velasco, A.I.; Martínez-Guardado, I.; Rubio-Zarapuz, A.; Navarro-Jiménez, E.; Tornero-Aguilera, J.F. Nutritional Modulation of the Gut–Brain Axis: A Comprehensive Review of Dietary Interventions in Depression and Anxiety Management. Metabolites 2024, 14, 549. [Google Scholar] [CrossRef]
- Ralph, A.F.; Grenier, C.; Costello, H.M.; Stewart, K.; Ivy, J.R.; Dhaun, N.; Bailey, M.A. Activation of the Sympathetic Nervous System Promotes Blood Pressure Salt-Sensitivity in C57BL6/J Mice. Hypertension 2021, 77, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Rau, S.; Gregg, A.; Yaceczko, S.; Limketkai, B. Prebiotics and Probiotics for Gastrointestinal Disorders. Nutrients 2024, 16, 778. [Google Scholar] [CrossRef] [PubMed]

| Composition | Methods | |
|---|---|---|
| Tetradesmus obliquus Mi175.B1.a | 20–35% (w/w) | |
| MCT oil based on coconut oil | 65–80% (w/w) | |
| Mix of non-GMO tocopherols (antioxidant)—E 306 | 0.45–0.55% (w/w) | |
| Total lipids | ≥80% (w/w) | Gravimetry |
| Proteins | ≤5% (w/w) | Kjeldahl (Titrimetry) |
| Humidity | <5% (w/w) | Thermogravimetry |
| Total PUFAs | 3.5–11.5% (w/w) | GC-FID |
| Carbohydrates | ≤20% (w/w) | Calculation |
| Total MUFAs | 1–5.5% (w/w) | GC-FID |
| Lutein | 0.3%(w/w) | HPLC |
| Sterol content (g/100 g product) | 0.3–0.9 | NF en ISO 12228-1 [40] |
| Variable | N | Mean | p-Value | |||
|---|---|---|---|---|---|---|
| Sex F(M) | Placebo | 28 | 16 (12) | 0.64 | ||
| TOME | 28 | 18 (10) | ||||
| Age | Placebo | 28 | 30.1 | ± | 6.25 | 0.11 |
| TOME | 28 | 33.7 | ± | 8.68 | ||
| Total | 56 | 31.9 | ± | 7.72 | ||
| Height | Placebo | 28 | 170.7 | ± | 9.97 | 0.94 |
| (cm) | TOME | 28 | 170.5 | ± | 10.03 | |
| Total | 56 | 170.6 | ± | 10.00 | ||
| Body Mass Index (kg/m2) | Placebo | 28 | 24.9 | ± | 2.82 | 0.35 |
| TOME | 28 | 24.2 | ± | 2.73 | ||
| Total | 56 | 24.6 | ± | 2.77 | ||
| Weight | Placebo | 28 | 72.9 | ± | 12.43 | 0.53 |
| (kg) | TOME | 28 | 70.8 | ± | 12.92 | |
| Total | 56 | 71.8 | ± | 12.61 | ||
| Waist Circumference | Placebo | 28 | 81.5 | ± | 8.43 | 0.50 |
| (cm) | TOME | 28 | 79.9 | ± | 9.08 | |
| Total | 56 | 80.7 | ± | 8.72 | ||
| Hip Circumference | Placebo | 28 | 101.6 | ± | 7.31 | 0.16 |
| (cm) | TOME | 28 | 99.1 | ± | 5.63 | |
| Total | 56 | 100.3 | ± | 6.58 | ||
| Resting Heart Rate | Placebo | 28 | 61.4 | ± | 8.75 | 0.84 |
| (beats/min) | TOME | 28 | 60.9 | ± | 8.66 | |
| Total | 56 | 61.2 | ± | 8.63 | ||
| Systolic Blood Pressure (mmHg) | Placebo | 28 | 113.1 | ± | 10.13 | 0.34 |
| TOME | 28 | 110.8 | ± | 7.10 | ||
| Total | 56 | 112.0 | ± | 8.72 | ||
| Diastolic Blood Pressure | Placebo | 28 | 67.8 | ± | 8.05 | 0.85 |
| (mmHg) | TOME | 28 | 67.4 | ± | 6.27 | |
| Total | 56 | 67.6 | ± | 7.13 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maltz, S.; Nacey, A.T.; Maury, J.; Ghanem, N.; Lee, S.Y.; Aquilino, T.M.; Graham, E.L.; Wrigley, S.D.; Whittington, J.M.; Khandaker, A.M.; et al. Effects of Supplementation with Microalgae Extract from Tetradesmus obliquus Strain Mi175.B1.a on Gastrointestinal Symptoms and Mental Health in Healthy Adults: A Pilot Randomized, Double-Blind, Placebo-Controlled, Parallel-Arm Trial. Nutrients 2025, 17, 960. https://doi.org/10.3390/nu17060960
Maltz S, Nacey AT, Maury J, Ghanem N, Lee SY, Aquilino TM, Graham EL, Wrigley SD, Whittington JM, Khandaker AM, et al. Effects of Supplementation with Microalgae Extract from Tetradesmus obliquus Strain Mi175.B1.a on Gastrointestinal Symptoms and Mental Health in Healthy Adults: A Pilot Randomized, Double-Blind, Placebo-Controlled, Parallel-Arm Trial. Nutrients. 2025; 17(6):960. https://doi.org/10.3390/nu17060960
Chicago/Turabian StyleMaltz, Sydnie, Aaron T. Nacey, Jonathan Maury, Nancy Ghanem, Sylvia Y. Lee, Thomas M. Aquilino, Elliot L. Graham, Scott D. Wrigley, Jennifer M. Whittington, Afsana M. Khandaker, and et al. 2025. "Effects of Supplementation with Microalgae Extract from Tetradesmus obliquus Strain Mi175.B1.a on Gastrointestinal Symptoms and Mental Health in Healthy Adults: A Pilot Randomized, Double-Blind, Placebo-Controlled, Parallel-Arm Trial" Nutrients 17, no. 6: 960. https://doi.org/10.3390/nu17060960
APA StyleMaltz, S., Nacey, A. T., Maury, J., Ghanem, N., Lee, S. Y., Aquilino, T. M., Graham, E. L., Wrigley, S. D., Whittington, J. M., Khandaker, A. M., Hart, R. A., Byrne, L., Wei, Y., Pradelles, R., Johnson, S. A., & Weir, T. L. (2025). Effects of Supplementation with Microalgae Extract from Tetradesmus obliquus Strain Mi175.B1.a on Gastrointestinal Symptoms and Mental Health in Healthy Adults: A Pilot Randomized, Double-Blind, Placebo-Controlled, Parallel-Arm Trial. Nutrients, 17(6), 960. https://doi.org/10.3390/nu17060960










