Exercise Mimetics in Aging: Suggestions from a Systematic Review
Abstract
1. Introduction
2. Materials and Methods
- Participants: humans, animal models, in vitro cell systems;
- Interventions: application of exercise mimetics (both active compounds and physical methods);
- Comparison: the study’s outcome parameters must have been measured pre-treatment and post-treatment or with and without treatment;
- Outcomes: analysis of physical exercise capacity, systemic parameters, metabolic pathways, and molecular mechanisms;
- Study design: randomized and non-randomized clinical trials, animal and in vitro studies, longitudinal and cross-sectional protocols.
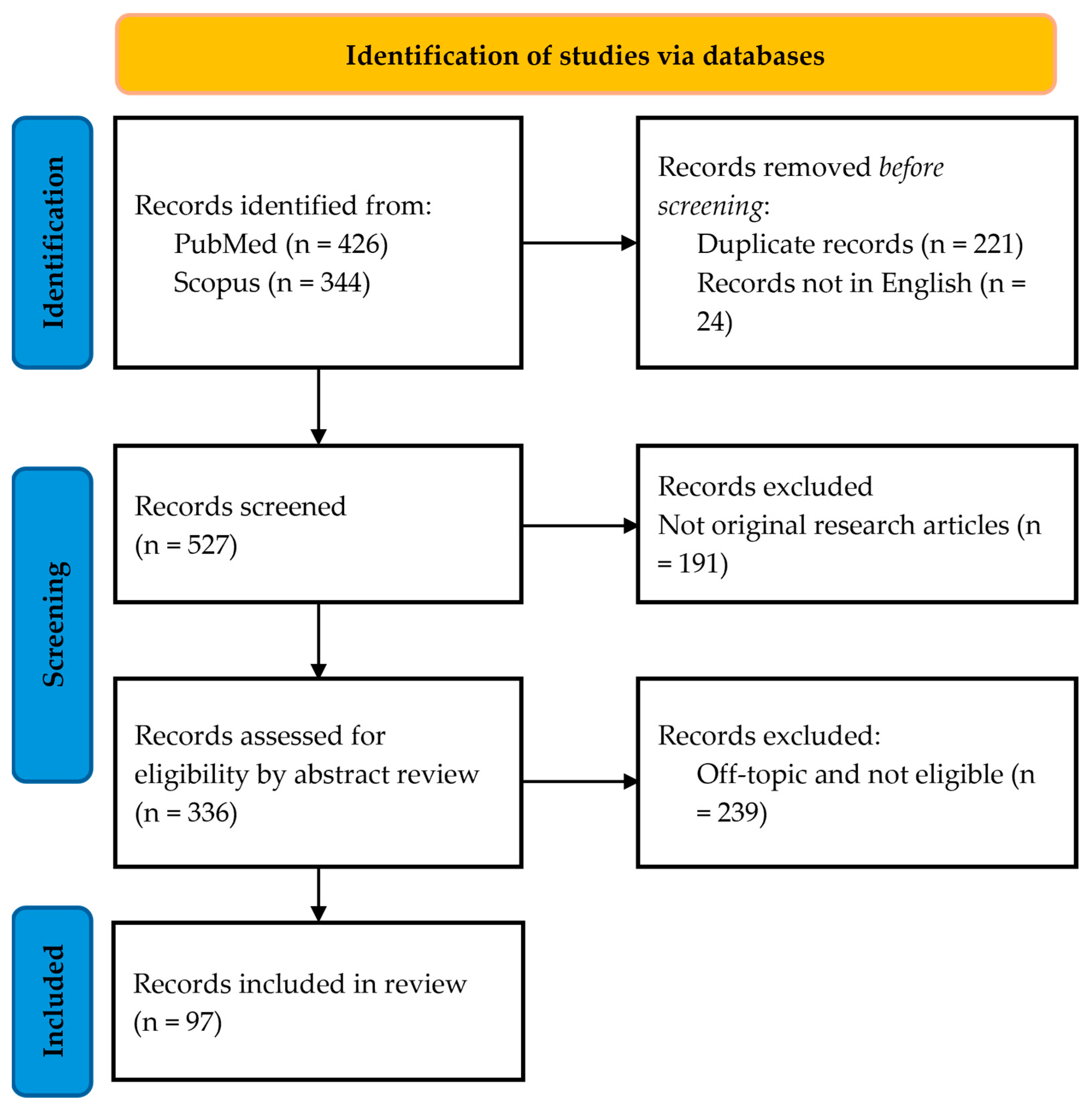
3. Results and Discussion
3.1. Metabolic Pathways
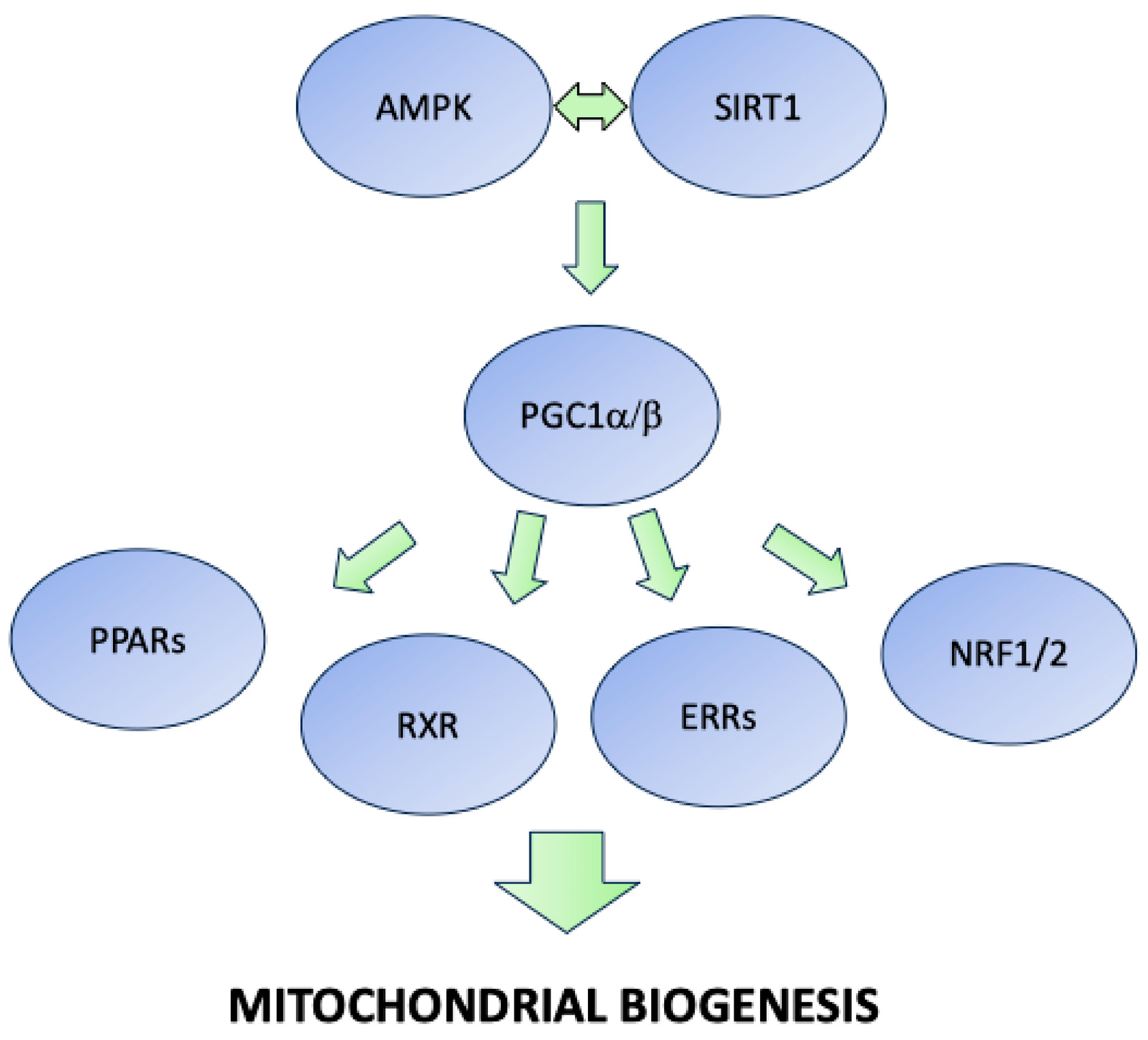
3.1.1. Sirtuins, AMPK, PGC1, and PPAR Agonists
| Active Principle | Tissue/Organ | Main Effects | Proposed Targets | First Author, Year |
|---|---|---|---|---|
| Carbon monoxide | Skeletal muscle | Improvement in skeletal muscle loss, increase in mitochondrial biogenesis factors | Metabolism, PGC-1 alpha | Noguchi, 2024 [38] |
| Nicotinamide mononucleotide | Gut | Restored predicted microbial functions | Metabolism | Yu, 2024 [46] |
| Sulforaphane, urolithin A, and ZLN005 | Skeletal muscle | Improved mitochondrial respiration | Mitochondrial metabolism, AMPK, Nrf-2 | Moradi, 2024 [37] |
| MDL-801 | Skeletal muscle | Enhanced endurance performance, increased oxidative fibers and mitochondrial oxidative capacity | Mitochondrial metabolism, Sirt6 | Song, 2022 [43] |
| O304, pan-AMPK activator | Cardiac system, systemic | Prevention of insulin resistance, improved cardiac function, | Metabolism, AMPK | Ericsson, 2021 [33] |
| Indoprofen | Skeletal muscle | Activation of oxidative metabolism, increased muscle mass | Metabolism, AMPK | Kim, 2020 [36] |
| GW0742 | Lymphoid tissue, skeletal muscle, systemic | Weight loss, visceral fat mass reduction, better insulin sensitivity, reduced inflammation | Metabolism, AMPK | Garf, 2019 [41] |
| Small molecule activators of AMPK | Skeletal muscle, heart, liver, adipose tissue | Better glucose tolerance, improved glucose accumulation and glycogen mobilization, better fatty acid oxidation | Metabolism, AMPK | Muise, 2019 [35] |
| AICAR | Liver, systemic | Improved hepatic metabolism | Metabolism, AMPK | Linecker, 2020 [28] |
| Nicotinamide mononucleotide | Skeletal muscle, vessels | Angiogenesis promotion | Metabolism, Sirt-1 | Das, 2018 [45] |
| AICAR | Skeletal muscle, brain | Improved muscle phenotype | Metabolism, AMPK | Paré, 2017 [27] |
| AICAR | Skeletal muscle, nervous tissue | Improved skeletal muscle atrophy and neuromuscular junctions, no effects on motoneuron glutamatergic synapse or on microglial and astroglial reaction | Metabolism, PGC-1 alpha | Cerveró, 2016 [26] |
| R419 | Skeletal muscle, systemic | Improved insulin sensitivity, improved exercise capacity | Metabolism, AMPK | Marcinko, 2015 [31] |
| AICAR | Skeletal muscle, brain | Better synaptic plasticity, cell proliferation, gene expression, oxidative stress | Metabolism, AMPK, Myokines | Guerrieri, 2015 [29] |
| CNX-013-B2 | Skeletal muscle, adipose tissue, liver | Improved insulin sensitivity and glucose tolerance, better body weight, alteration in gene expression | Metabolism, PPAR alpha, beta, delta | Sadasivuni, 2014 [42] |
| Free fatty acids, adrenaline, AICAR | Skeletal muscle | Modulation of Il-15 and Il-6 expression | Metabolism, myokines | Sánchez, 2013 [30] |
| AICAR, GW501516 | Skeletal muscle | Influence on body weight and animal activity, increased oxidative capacity, satellite cell activation, better muscle fibrosis | Metabolism, PGC-1 alpha | Jahnke, 2012 [40] |
| GW501516, PF-879 | Skeletal muscle, adipose tissue, liver, systemic | Changes in body weight, fat mass and lean mass, better mitochondrial activity and fiber size, better lipid profiles, improved physical activity | Metabolism, PPAR-gamma, myokines, myostatin | Bernardo, 2010 [39] |
| GW501516, AICAR | Skeletal muscle, systemic | Better muscle gene expression, muscle remodeling, increased running endurance | Metabolism, AMPK-alpha, PPAR-delta | Narkar, 2008 [25] |
3.1.2. Estrogen Receptors (ERs) and Estrogen-Related Receptor (ERR) Ligands
| Natural Product/Compound | Tissue/Organ | Main Effects | Proposed Targets | First Author, Year |
|---|---|---|---|---|
| Eugenol | Skeletal muscle, adipose tissue | Increased exercise endurance, fiber-type switch, white fat browning, lipolysis | Metabolism, myokines, TPRV1 | Huang, 2024 [57] |
| Eicosapentaenoic acid | Skeletal muscle, systemic | Increased oxidative metabolism, increased body fat oxidation, better muscle performance | Metabolism, PPR-delta | Komiya, 2024 [58] |
| Chrysanthemum zawadskii, linarin | Skeletal muscle | Prevention of sarcopenia and muscle loss, better mitochondrial function and proteostasis | Metabolism, PPR-delta, ERR-gamma | Nirmala, 2024 [50] |
| Sulforaphane, urolithin A, and ZLN005 | Skeletal muscle | Improved mitochondrial respiration | Mitochondrial metabolism, AMPK, Nrf-2 | Moradi, 2024 [37] |
| Resveratrol | Vessels | Prevention of endothelial dysfunction | Oxidative stress, SIRT-1 | Kim, 2023 [59] |
| Zynamite(®), quercetin | Skeletal muscle | Enhanced physical performance | GSK3beta, stress kinases | Martinez-Canton, 2023 [60] |
| Essential amino acids | Brain, primary cortical neurons | Improved mitochondrial biogenesis, antioxidant response | Mitochondrial metabolism, eNOS/mTOR | Ragni, 2023 [61] |
| 7,8-DHF@ZIF-8, 7,8-Dihydroxyflavone | Bone, vessels | Improved osteogenesis and angiogenesis | BDNF | Sun, 2023 [62] |
| Limonium tetragonum | Skeletal muscle | Enhanced exercise endurance, increased oxidative fibers, increased mitochondrial content | Mitochondrial metabolism, PKA–CREB–PGC1 alpha | Lee, 2022 [63] |
| (-)-Epicatechin | Skeletal muscle | Increased fiber size | MyomiRs | Palma-Flores, 2023 [64] |
| Multi-ingredient supplement | Skin | Upregulation of proteins involved in mitochondrial function and oxidative phosphorylation, improvement in antioxidant activity | Oxidative stress, PPAR-gamma, Il-15 | Rebalka, 2022 [65] |
| d-Allulose | Skeletal muscle, systemic | Improved performance, better insulin sensivity | Metabolism, AMPK, PGC-1 alpha | Liu, 2022 [66] |
| Trehalose | Brain | Improved learning and memory | AMPK, TOR, autophagy | Pan, 2022 [67] |
| Epicatechin | Central nervous system, skeletal muscle | Resilience to depression | Kynurenine aminotransferases, PGC-1 alpha-PPAR-delta/alpha | Martínez-Damas, 2021 [68] |
| Olive oil | Skeletal muscle | Improved running endurance, increased muscle triacylglycerol | Metabolism, DGAT1 | Komiya, 2021 [69] |
| Resveratrol | Brain, skeletal muscle | Better capillary density in the ipsilesional hemisphere, mitigation of stroke-induced muscle fiber changes | Sirtuins | McDonald, 2021 [70] |
| Lycium barbarum extract | Skeletal muscle | Increase in muscle mass and endurance, switch from glycolytic to oxidative metabolism | Metabolism, ERR-gamma, sirtuins, PGC-1 alpha/beta | Meng, 2020 [52] |
| cis-Banglene | Skeletal muscle | Improved glucose uptake, improve mitochondrial biogenesis | Myokines, metabolisms, IL-6, AMPK | Norikura, 2020 [71] |
| Epicatechin | Skeletal muscle | Modulation of skeletal muscle protein expression, better mitochondrial morphology | Regeneration | McDonald, 2021 [72] |
| Estradiol, resveratrol | Vessels | Enhanced basal endothelial function | Estrogen receptors | Ozemek, 2020 [73] |
| Ursolic acid | Skeletal muscle, bone | Improved muscle mass and bone density | No suggestion | Kang, 2019 [74] |
| Multi-ingredient supplement | Locomotor system | Improved mean survivorship, improved morphological properties, improved jumping | No suggestion | Tran, 2018 [75] |
| Ursolic acid | Skeletal muscle | Improvement in atrophied muscle mass, reduction in atrophic genes expression | Atrophy, Murf-1, Atrogin-1 | Kim, 2018 [76] |
| 7,8-dihydroxyflavone (BDNF-mimetic) | Brain | Improved brain plasticity, associative learning | BDNF | Parrini, 2017 [77] |
| Resveratrol, metformin | Skeletal muscle | Better skeletal musle morphology and neuromuscular junction structure | No suggestions | Stockinger, 2017 [78] |
| Cocoa procyanidins | Skeletal muscle | Improved glucose uptake and glycogen synthesis | Metabolism, AKT | Bowser, 2017 [79] |
| Hypericum perforatum L. | Bone, systemic | Better testosterone levels, better bone specific weight and mass density | No suggestions | Seferos, 2016 [53] |
| Fenugreek | Skeletal muscle | Increased total creatine, modulation of protein expression | Metabolism, insulin | Tomcik, 2017 [80] |
| Linoleic acid | Skeletal muscle | Body weight reduction, better voluntary movement, better mitochondrial biogenesis | Metabolism, AMPK-alpha, PPAR-gamma | Kim, 2016 [81] |
| Dihydromyricetin | Skeletal muscle, systemic | Higher irisin levels | Myokines, PGC1-alpha | Zhou, 2015 [82] |
| Resveratrol | Lung endothelium | Attenuation of oxidative damage, better endothelial permeability and lung histomorphology | Oxidative stress, Nfr-2 | Dong, 2015 [83] |
| Resveratrol | Skeletal muscle | No effect | Metabolism | Olesen, 2014 [84] |
| Ginsenoside Rg3 | Cardiac system | improved cardiac adaptations and mitochondrial homeostasis | Metabolism, PGC-1alpha, Nrf-2 | Sun, 2013 [85] |
| Resveratrol | Skeletal muscle, systemic | Variation in protein expression, better energy expenditure | Metabolism, Sirt-1 | Goh, 2014 [86] |
| Chitooligosaccharide | Skeletal muscle | Increased mitochondrial content, improved exercise endurance | Metabolism, AMPK, PGC-1 alpha, Sirt1 | Jeong, 2012 [87] |
| (-)-Epicatechin | Skeletal muscle, cardiac tissue | Better physical performance, regulation of oxidative phosphorylation complexes, improved mitochondrial quantity and morphology | Metabolism, oxidative stress | Nogueira, 2011 [88] |
| Resveratrol | Skeletal muscle, adipose tissue, bone, cardiovascular system, systemic | Prevention of muscle atrophy and loss of function, oxidative capacity maintenance and improved oxidative stress, prevention of bone demineralization | Metabolism, PGC-1 alpha, Sirt1 | Momken, 2011 [89] |
| Cordyceps sinensis | Skeletal muscle, systemic | Improvement in endurance capacity, better glucose transport, better angiogenic and antioxidant response | Metabolism, AMPK, PGC-1 alpha | Kumar, 2011 [90] |
| Trichopus zeylanicus | Skeletal muscle, systemic | Anti-fatigue effect | No suggestions | Tharakan, 2006 [91] |
3.1.3. Antioxidants
3.1.4. Products of Natural Origin
3.1.5. Products with Miscellaneous Targets
3.2. Myokines
| Therapeutic Agent | Tissue/Organ | Main Effects | Proposed Targets | First Author, Year |
|---|---|---|---|---|
| Eugenol | Skeletal muscle, adipose tissue | Increased exercise endurance, fiber-type switch, white fat browning, lipolysis | Metabolism, myokines, TPRV1 | Huang, 2024 [57] |
| Irisin | Cartilage, bone | Improved extracellular matrix synthesis, improved chondrogenic differentiation | ERK phosphorylation, irisin | Posa, 2023 [141] |
| 7,8-DHF@ZIF-8, 7,8-Dihydroxyflavone | Bone, vessels | Improved osteogenesis and angiogenesis | BDNF | Sun, 2023 [62] |
| Multi-ingredient supplement | Skin | Upregulation of proteins involved in mitochondrial function and oxidative phosphorylation, improvement in antioxidant activity | Oxidative stress, PPAR-gamma, Il-15 | Rebalka, 2022 [65] |
| Irisin | Reproductive organs | Better sexual performance, improved sperm morphology and motility, reduced testicular damage | Myokines, irisin | Yardimci, 2022 [142] |
| Irisin | Skeletal muscle | Differential expression of muscle proteins | Myokines | Momenzadeh, 2021 [137] |
| cis-Banglene | Skeletal muscle | Improved glucose uptake, improved mitochondrial biogenesis | Myokines, metabolisms, IL-6, AMPK | Norikura, 2020 [71] |
| Irisin | Skeletal muscle | Attenuation of dexamethasone-induced atrophy | Myokines, irisin | Chang, 2020 [138] |
| Irisin | Bone | Inhibition of apoptosis | Myokines, apoptosis, Erk1/Erk2, caspase 9/3 | Storlino, 2020 [140] |
| 7,8-dihydroxyflavone | Brain | Improved brain plasticity, associative learning | BDNF | Parrini, 2017 [77] |
| Dihydromyricetin | Skeletal muscle, systemic | Higher irisin levels | Myokines, PGC1-alpha | Zhou, 2015 [82] |
| Irisin | Bone | Enhanced differentiation | Myokines | Colaianni, 2014 [139] |
3.3. Physical Approaches
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ageing and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 24 February 2025).
- Westerterp, K.R. Changes in Physical Activity over the Lifespan: Impact on Body Composition and Sarcopenic Obesity. Obes. Rev. 2018, 19, 8–13. [Google Scholar] [CrossRef]
- Chia, C.W.; Egan, J.M.; Ferrucci, L. Age-Related Changes in Glucose Metabolism, Hyperglycemia, and Cardiovascular Risk. Circ. Res. 2018, 123, 886–904. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.-L. The Frailty Syndrome: Definition and Natural History. Clin. Geriatr. Med. 2011, 27, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Searle, S.D.; Mitnitski, A.; Gahbauer, E.A.; Gill, T.M.; Rockwood, K. A Standard Procedure for Creating a Frailty Index. BMC Geriatr. 2008, 8, 24. [Google Scholar] [CrossRef]
- Martinez, R.; Morsch, P.; Soliz, P.; Hommes, C.; Ordunez, P.; Vega, E. Life Expectancy, Healthy Life Expectancy, and Burden of Disease in Older People in the Americas, 1990–2019: A Population-Based Study. Rev. Panam. Salud Publica 2021, 45, e114. [Google Scholar] [CrossRef]
- Giacomello, E.; Toniolo, L. Nutrition, Diet and Healthy Aging. Nutrients 2021, 14, 190. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.J.; Cyr-campbell, D. Nutrition, Exercise, and Healthy Aging. J. Am. Diet. Assoc. 1997, 97, 632–638. [Google Scholar] [CrossRef]
- Warburton, D.E.R.; Nicol, C.W.; Bredin, S.S.D. Health Benefits of Physical Activity: The Evidence. CMAJ 2006, 174, 801–809. [Google Scholar] [CrossRef]
- Chan, J.S.Y.; Liu, G.; Liang, D.; Deng, K.; Wu, J.; Yan, J.H. Special Issue—Therapeutic Benefits of Physical Activity for Mood: A Systematic Review on the Effects of Exercise Intensity, Duration, and Modality. J. Psychol. 2019, 153, 102–125. [Google Scholar] [CrossRef]
- Severinsen, M.C.K.; Pedersen, B.K. Muscle–Organ Crosstalk: The Emerging Roles of Myokines. Endocr. Rev. 2020, 41, 594–609. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Åkerström, T.C.A.; Nielsen, A.R.; Fischer, C.P. Role of Myokines in Exercise and Metabolism. J. Appl. Physiol. 2007, 103, 1093–1098. [Google Scholar] [CrossRef]
- Heinonen, I.; Kalliokoski, K.K.; Hannukainen, J.C.; Duncker, D.J.; Nuutila, P.; Knuuti, J. Organ-Specific Physiological Responses to Acute Physical Exercise and Long-Term Training in Humans. Physiology 2014, 29, 421–436. [Google Scholar] [CrossRef] [PubMed]
- Gubert, C.; Hannan, A.J. Exercise Mimetics: Harnessing the Therapeutic Effects of Physical Activity. Nat. Rev. Drug Discov. 2021, 20, 862–879. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Demontis, F. Systemic Nutrient and Stress Signaling via Myokines and Myometabolites. Annu. Rev. Physiol. 2016, 78, 85–107. [Google Scholar] [CrossRef]
- Aiello, A.; Accardi, G.; Candore, G.; Gambino, C.M.; Mirisola, M.; Taormina, G.; Virruso, C.; Caruso, C. Nutrient Sensing Pathways as Therapeutic Targets for Healthy Ageing. Expert Opin. Ther. Targets 2017, 21, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.S.; Brachmann, C.B.; Celic, I.; Kenna, M.A.; Muhammad, S.; Starai, V.J.; Avalos, J.L.; Escalante-Semerena, J.C.; Grubmeyer, C.; Wolberger, C.; et al. A Phylogenetically Conserved NAD+-Dependent Protein Deacetylase Activity in the Sir2 Protein Family. Proc. Natl. Acad. Sci. USA 2000, 97, 6658–6663. [Google Scholar] [CrossRef]
- Riera, C.E.; Merkwirth, C.; De Magalhaes Filho, C.D.; Dillin, A. Signaling Networks Determining Life Span. Annu. Rev. Biochem. 2016, 85, 35–64. [Google Scholar] [CrossRef]
- Giacomello, E.; Toniolo, L. The Potential of Calorie Restriction and Calorie Restriction Mimetics in Delaying Aging: Focus on Experimental Models. Nutrients 2021, 13, 2346. [Google Scholar] [CrossRef]
- Zhu, Y.; Song, G. Molecular Origin and Biological Effects of Exercise Mimetics. J. Exerc. Sci. Fit. 2024, 22, 73–85. [Google Scholar] [CrossRef]
- Lenaz, G. Role of Mitochondria in Oxidative Stress and Ageing. Biochim. Et Biophys. Acta (BBA)—Bioenerg. 1998, 1366, 53–67. [Google Scholar] [CrossRef]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of Metabolism and Mitochondrial Homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef]
- Frøsig, C.; Jørgensen, S.B.; Hardie, D.G.; Richter, E.A.; Wojtaszewski, J.F.P. 5′-AMP-Activated Protein Kinase Activity and Protein Expression Are Regulated by Endurance Training in Human Skeletal Muscle. Am. J. Physiol.-Endocrinol. Metab. 2004, 286, E411–E417. [Google Scholar] [CrossRef] [PubMed]
- Narkar, V.A.; Downes, M.; Yu, R.T.; Embler, E.; Wang, Y.-X.; Banayo, E.; Mihaylova, M.M.; Nelson, M.C.; Zou, Y.; Juguilon, H.; et al. AMPK and PPARδ Agonists Are Exercise Mimetics. Cell 2008, 134, 405. [Google Scholar] [CrossRef]
- Cerveró, C.; Montull, N.; Tarabal, O.; Piedrafita, L.; Esquerda, J.E.; Calderó, J. Chronic Treatment with the AMPK Agonist AICAR Prevents Skeletal Muscle Pathology but Fails to Improve Clinical Outcome in a Mouse Model of Severe Spinal Muscular Atrophy. Neurotherapeutics 2016, 13, 198–216. [Google Scholar] [CrossRef]
- Paré, M.-F.; Jasmin, B.J. Chronic 5-Aminoimidazole-4-Carboxamide-1-β-d-Ribofuranoside Treatment Induces Phenotypic Changes in Skeletal Muscle, but Does Not Improve Disease Outcomes in the R6/2 Mouse Model of Huntington’s Disease. Front. Neurol. 2017, 8, 516. [Google Scholar] [CrossRef]
- Linecker, M.; Frick, L.; Kron, P.; Limani, P.; Kambakamba, P.; Tschuor, C.; Langiewicz, M.; Kachaylo, E.; Tian, Y.; Schneider, M.A.; et al. Exercise Improves Outcomes of Surgery on Fatty Liver in Mice: A Novel Effect Mediated by the AMPK Pathway. Ann. Surg. 2020, 271, 347. [Google Scholar] [CrossRef] [PubMed]
- Guerrieri, D.; Praag, H. van Exercise-Mimetic AICAR Transiently Benefits Brain Function. Oncotarget 2015, 6, 18293–18313. [Google Scholar] [CrossRef]
- Sánchez, J.; Nozhenko, Y.; Palou, A.; Rodríguez, A.M. Free Fatty Acid Effects on Myokine Production in Combination with Exercise Mimetics. Mol. Nutr. Food Res. 2013, 57, 1456–1467. [Google Scholar] [CrossRef]
- Marcinko, K.; Bujak, A.L.; Lally, J.S.V.; Ford, R.J.; Wong, T.H.; Smith, B.K.; Kemp, B.E.; Jenkins, Y.; Li, W.; Kinsella, T.M.; et al. The AMPK Activator R419 Improves Exercise Capacity and Skeletal Muscle Insulin Sensitivity in Obese Mice. Mol. Metab. 2015, 4, 643–651. [Google Scholar] [CrossRef]
- Steneberg, P.; Lindahl, E.; Dahl, U.; Lidh, E.; Straseviciene, J.; Backlund, F.; Kjellkvist, E.; Berggren, E.; Lundberg, I.; Bergqvist, I.; et al. PAN-AMPK Activator O304 Improves Glucose Homeostasis and Microvascular Perfusion in Mice and Type 2 Diabetes Patients. JCI Insight 2018, 3, e99114. [Google Scholar] [CrossRef] [PubMed]
- Ericsson, M.; Steneberg, P.; Nyrén, R.; Edlund, H. AMPK Activator O304 Improves Metabolic and Cardiac Function, and Exercise Capacity in Aged Mice. Commun. Biol. 2021, 4, 1306. [Google Scholar] [CrossRef]
- Hunter, R.W.; Treebak, J.T.; Wojtaszewski, J.F.P.; Sakamoto, K. Molecular Mechanism by Which AMP-Activated Protein Kinase Activation Promotes Glycogen Accumulation in Muscle. Diabetes 2011, 60, 766–774. [Google Scholar] [CrossRef]
- Muise, E.S.; Guan, H.-P.; Liu, J.; Nawrocki, A.R.; Yang, X.; Wang, C.; Rodríguez, C.G.; Zhou, D.; Gorski, J.N.; Kurtz, M.M.; et al. Pharmacological AMPK Activation Induces Transcriptional Responses Congruent to Exercise in Skeletal and Cardiac Muscle, Adipose Tissues and Liver. PLoS ONE 2019, 14, e0211568. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Cho, S.C.; Jeong, H.; Lee, H.; Jeong, M.; Pyun, J.; Ryu, D.; Kim, M.; Lee, Y.; Kim, M.S.; et al. Indoprofen Prevents Muscle Wasting in Aged Mice through Activation of PDK1/AKT Pathway. J. Cachexia Sarcopenia Muscle 2020, 11, 1070–1088. [Google Scholar] [CrossRef]
- Moradi, N.; Champsi, S.; Hood, D.A. Sulforaphane, Urolithin A, and ZLN005 Induce Time-Dependent Alterations in Antioxidant Capacity, Mitophagy, and Mitochondrial Biogenesis in Muscle Cells. Sports Med. Health Sci. 2025, 7, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, I.; Maeda, H.; Kobayashi, K.; Nagasaki, T.; Kato, H.; Yanagisawa, H.; Wada, N.; Kanazawa, G.; Kaji, T.; Sakai, H.; et al. Carbon Monoxide-Loaded Cell Therapy as an Exercise Mimetic for Sarcopenia Treatment. Free Radic. Biol. Med. 2024, 220, 67–77. [Google Scholar] [CrossRef]
- Bernardo, B.L.; Wachtmann, T.S.; Cosgrove, P.G.; Kuhn, M.; Opsahl, A.C.; Judkins, K.M.; Freeman, T.B.; Hadcock, J.R.; LeBrasseur, N.K. Postnatal PPARδ Activation and Myostatin Inhibition Exert Distinct yet Complimentary Effects on the Metabolic Profile of Obese Insulin-Resistant Mice. PLoS ONE 2010, 5, e11307. [Google Scholar] [CrossRef]
- Jahnke, V.E.; Meulen, J.H.V.D.; Johnston, H.K.; Ghimbovschi, S.; Partridge, T.; Hoffman, E.P.; Nagaraju, K. Metabolic Remodeling Agents Show Beneficial Effects in the Dystrophin-Deficient Mdx Mouse Model. Skelet. Muscle 2012, 2, 16. [Google Scholar] [CrossRef]
- Garf, S.L.; Murdaca, J.; Mothe-Satney, I.; Sibille, B.; Menn, G.L.; Chinetti, G.; Neels, J.G.; Rousseau, A.-S. Complementary Immunometabolic Effects of Exercise and PPARβ/δ Agonist in the Context of Diet-Induced Weight Loss in Obese Female Mice. Int. J. Mol. Sci. 2019, 20, 5182. [Google Scholar] [CrossRef]
- Sadasivuni, M.K.; Reddy, B.M.; Singh, J.; Anup, M.O.; Sunil, V.; Lakshmi, M.N.; Yogeshwari, S.; Chacko, S.K.; Pooja, T.L.; Dandu, A.; et al. CNX-013-B2, a Unique Pan Tissue Acting Rexinoid, Modulates Several Nuclear Receptors and Controls Multiple Risk Factors of the Metabolic Syndrome without Risk of Hypertriglyceridemia, Hepatomegaly and Body Weight Gain in Animal Models. Diabetol. Metab. Syndr. 2014, 6, 83. [Google Scholar] [CrossRef] [PubMed]
- Song, M.-Y.; Han, C.Y.; Moon, Y.J.; Lee, J.H.; Bae, E.J.; Park, B.-H. Sirt6 Reprograms Myofibers to Oxidative Type through CREB-Dependent Sox6 Suppression. Nat. Commun. 2022, 13, 1808. [Google Scholar] [CrossRef]
- Bonkowski, M.S.; Sinclair, D.A. Slowing Ageing by Design: The Rise of NAD+ and Sirtuin-Activating Compounds. Nat. Rev. Mol. Cell Biol. 2016, 17, 679–690. [Google Scholar] [CrossRef]
- Das, A.; Huang, G.X.; Bonkowski, M.S.; Longchamp, A.; Li, C.; Schultz, M.B.; Kim, L.-J.; Osborne, B.; Joshi, S.; Lu, Y.; et al. Impairment of an Endothelial NAD+-H2S Signaling Network Is a Reversible Cause of Vascular Aging. Cell 2018, 173, 74–89.e20. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Youngson, N.A.; Laybutt, D.R.; Morris, M.J.; Leigh, S.-J. Complementary yet Divergent Effects of Exercise and an Exercise Mimetic on Microbiome in High-Fat Diet-Induced Obesity. Physiol. Genom. 2024, 56, 136–144. [Google Scholar] [CrossRef]
- Yoh, K.; Ikeda, K.; Horie, K.; Inoue, S. Roles of Estrogen, Estrogen Receptors, and Estrogen-Related Receptors in Skeletal Muscle: Regulation of Mitochondrial Function. Int. J. Mol. Sci. 2023, 24, 1853. [Google Scholar] [CrossRef] [PubMed]
- Mauvais-Jarvis, F. Estrogen and Androgen Receptors: Regulators of Fuel Homeostasis and Emerging Targets for Diabetes and Obesity. Trends Endocrinol. Metab. 2011, 22, 24–33. [Google Scholar] [CrossRef]
- Transcriptional Control of Energy Homeostasis by the Estrogen-Related Receptors|Endocrine Reviews|Oxford Academic. Available online: https://academic.oup.com/edrv/article/29/6/677/2355000 (accessed on 15 November 2024).
- Nirmala, F.S.; Lee, H.; Kim, Y.-I.; Hahm, J.; Seo, H.-D.; Kim, M.; Jung, C.H.; Ahn, J. Exercise-Induced Signaling Activation by Chrysanthemum zawadskii and Its Active Compound, Linarin, Ameliorates Age-Related Sarcopenia through Sestrin 1 Regulation. Phytomedicine 2024, 129, 155695. [Google Scholar] [CrossRef]
- Kim, M.; Sujkowski, A.; Namkoong, S.; Gu, B.; Cobb, T.; Kim, B.; Kowalsky, A.H.; Cho, C.-S.; Semple, I.; Ro, S.-H.; et al. Sestrins Are Evolutionarily Conserved Mediators of Exercise Benefits. Nat Commun 2020, 11, 190. [Google Scholar] [CrossRef]
- Meng, J.; Lv, Z.; Sun, C.; Qiao, X.; Chen, C. An Extract of Lycium Barbarum Mimics Exercise to Improve Muscle Endurance through Increasing Type IIa Oxidative Muscle Fibers by Activating ERRγ. FASEB J. 2020, 34, 11460–11473. [Google Scholar] [CrossRef]
- Seferos, N.; Petrokokkinos, L.; Kotsiou, A.; Rallis, G.; Tesseromatis, C. Hypericum Perforatum L. Treatment Restored Bone Mass Changes in Swimming Stressed Rats. Stomatologija 2016, 18, 9–13. [Google Scholar] [PubMed]
- Ponnusamy, S.; Tran, Q.T.; Harvey, I.; Smallwood, H.S.; Thiyagarajan, T.; Banerjee, S.; Johnson, D.L.; Dalton, J.T.; Sullivan, R.D.; Miller, D.D.; et al. Pharmacologic Activation of Estrogen Receptor β Increases Mitochondrial Function, Energy Expenditure, and Brown Adipose Tissue. FASEB J. 2016, 31, 266. [Google Scholar] [CrossRef] [PubMed]
- Billon, C.; Sitaula, S.; Banerjee, S.; Welch, R.; Elgendy, B.; Hegazy, L.; Oh, T.G.; Kazantzis, M.; Chatterjee, A.; Chrivia, J.; et al. Synthetic ERRα/β/γ Agonist Induces an ERRα-Dependent Acute Aerobic Exercise Response and Enhances Exercise Capacity. ACS Chem. Biol. 2023, 18, 756–771. [Google Scholar] [CrossRef] [PubMed]
- Billon, C.; Schoepke, E.; Avdagic, A.; Chatterjee, A.; Butler, A.A.; Elgendy, B.; Walker, J.K.; Burris, T.P. A Synthetic ERR Agonist Alleviates Metabolic Syndrome. J. Pharmacol. Exp. Ther. 2024, 388, 232–240. [Google Scholar] [CrossRef]
- Huang, T.; Chen, X.; He, J.; Zheng, P.; Luo, Y.; Wu, A.; Yan, H.; Yu, B.; Chen, D.; Huang, Z. Eugenol Mimics Exercise to Promote Skeletal Muscle Fiber Remodeling and Myokine IL-15 Expression by Activating TRPV1 Channel. eLife 2024, 12, RP90724. [Google Scholar] [CrossRef]
- Komiya, Y.; Sakazaki, Y.; Goto, T.; Kawabata, F.; Suzuki, T.; Sato, Y.; Sawano, S.; Nakamura, M.; Tatsumi, R.; Ikeuchi, Y.; et al. Eicosapentaenoic Acid Increases Proportion of Type 1 Muscle Fibers through PPARδ and AMPK Pathways in Rats. iScience 2024, 27, 109816. [Google Scholar] [CrossRef]
- Kim, J.-S.; Kim, J.-S. Aerobic Exercise-Mimetic Effects of Resveratrol on the Prevention of Vascular Endothelial Senescence. Exerc. Sci. 2023, 32, 168–174. [Google Scholar] [CrossRef]
- Martinez-Canton, M.; Galvan-Alvarez, V.; Garcia-Gonzalez, E.; Gallego-Selles, A.; Gelabert-Rebato, M.; Garcia-Perez, G.; Santana, A.; Lopez-Rios, L.; Vega-Morales, T.; Martin-Rincon, M.; et al. A Mango Leaf Extract (Zynamite®) Combined with Quercetin Has Exercise-Mimetic Properties in Human Skeletal Muscle. Nutrients 2023, 15, 2848. [Google Scholar] [CrossRef]
- Ragni, M.; Fenaroli, F.; Ruocco, C.; Segala, A.; D’Antona, G.; Nisoli, E.; Valerio, A. A Balanced Formula of Essential Amino Acids Promotes Brain Mitochondrial Biogenesis and Protects Neurons from Ischemic Insult. Front. Neurosci 2023, 17, 1197208. [Google Scholar] [CrossRef]
- Sun, Q.; Liu, Z.; Xie, C.; Hu, L.; Li, H.; Ge, Y.; Lin, L.; Tang, B. The Development of Novel Multifunctional Drug System 7,8-DHF@ZIF-8 and Its Potential Application in Bone Defect Healing. Colloids Surf. B Biointerfaces 2023, 222, 113102. [Google Scholar] [CrossRef]
- Lee, Y.G.; Song, M.-Y.; Cho, H.; Jin, J.S.; Park, B.-H.; Bae, E.J. Limonium Tetragonum Promotes Running Endurance in Mice through Mitochondrial Biogenesis and Oxidative Fiber Formation. Nutrients 2022, 14, 3904. [Google Scholar] [CrossRef] [PubMed]
- Palma-Flores, C.; Zárate-Segura, P.B.; Hernández-Hernández, J.M.; de los Santos, S.; Tejeda-Gómez, A.S.; Cano-Martínez, L.J.; Canto, P.; Garcia-Rebollar, J.O.; Coral-Vázquez, R.M. (−)-Epicatechin Modulates the Expression of myomiRs Implicated in Exercise Response in Mouse Skeletal Muscle. Gene 2023, 849, 146907. [Google Scholar] [CrossRef]
- Rebalka, I.A.; May, L.; Nederveen, J.P.; Tarnopolsky, M.A. Multi-Ingredient Supplement Supports Mitochondrial Health through Interleukin-15 Signaling in Older Adult Human Dermal Fibroblasts. Cosmetics 2022, 9, 47. [Google Scholar] [CrossRef]
- Liu, B.; Gou, Y.; Tsuzuki, T.; Yamada, T.; Iida, T.; Wang, S.; Banno, R.; Toyoda, Y.; Koike, T. D-Allulose Improves Endurance and Recovery from Exhaustion in Male C57BL/6J Mice. Nutrients 2022, 14, 404. [Google Scholar] [CrossRef]
- Pan, S.; Guo, S.; Dai, J.; Gu, Y.; Wang, G.; Wang, Y.; Qin, Z.; Luo, L. Trehalose Ameliorates Autophagy Dysregulation in Aged Cortex and Acts as an Exercise Mimetic to Delay Brain Aging in Elderly Mice. Food Sci. Hum. Wellness 2022, 11, 1036–1044. [Google Scholar] [CrossRef]
- Martínez-Damas, M.G.; Genis-Mendoza, A.D.; la Cruz, V.P.; Canela-Tellez, G.D.; Jiménez-Estrada, I.; Sanchez, J.H.N.; Ramos-Chávez, L.A.; García, S.; Ramírez-Ramírez, M.; Coral-Vázquez, R.M. Epicatechin Treatment Generates Resilience to Chronic Mild Stress-Induced Depression in a Murine Model through a Modulatory Effect on KAT. Physiol. Behav. 2021, 238, 113466. [Google Scholar] [CrossRef] [PubMed]
- Komiya, Y.; Sugiyama, M.; Ochiai, M.; Osawa, N.; Adachi, Y.; Iseki, S.; Arihara, K. Dietary Olive Oil Intake Improves Running Endurance with Intramuscular Triacylglycerol Accumulation in Mice. Nutrients 2021, 13, 1164. [Google Scholar] [CrossRef]
- McDonald, M.W.; Jeffers, M.S.; Issa, L.; Carter, A.; Ripley, A.; Kuhl, L.M.; Morse, C.; Comin, C.H.; Jasmin, B.J.; Lacoste, B.; et al. An Exercise Mimetic Approach to Reduce Poststroke Deconditioning and Enhance Stroke Recovery. Neurorehabilit. Neural Repair 2021, 35, 471. [Google Scholar] [CrossRef]
- Norikura, T.; Kajiya, S.; Sugawara, M.; Kubo, M.; Fukuyama, Y.; Sato, S. Cis-Banglene, a Bangle (Zingiber purpureum)-Derived Bioactive Compound, Promotes Mitochondrial Biogenesis and Glucose Uptake by Activating the IL-6/AMPK Signaling Pathway in C2C12 Skeletal Muscle Cells. J. Funct. Foods 2020, 64, 103632. [Google Scholar] [CrossRef]
- McDonald, C.M.; Ramirez-Sanchez, I.; Oskarsson, B.; Joyce, N.; Aguilar, C.; Nicorici, A.; Dayan, J.; Goude, E.; Abresch, R.T.; Villarreal, F.; et al. (−)-Epicatechin Induces Mitochondrial Biogenesis and Markers of Muscle Regeneration in Adults with Becker Muscular Dystrophy. Muscle Nerve 2020, 63, 239. [Google Scholar] [CrossRef]
- Ozemek, C.; Hildreth, K.L.; Blatchford, P.J.; Hurt, K.J.; Bok, R.; Seals, D.R.; Kohrt, W.M.; Moreau, K.L. Effects of Resveratrol or Estradiol on Postexercise Endothelial Function in Estrogen-Deficient Postmenopausal Women. J. Appl. Physiol. 2020, 128, 739. [Google Scholar] [CrossRef]
- Kang, Y.S.; Noh, E.B.; Kim, S.H. Effects of Ursolic Acid on Muscle Mass and Bone Microstructure in Rats with Casting-Induced Muscle Atrophy. J. Exerc. Nutr. Biochem. 2019, 23, 45–49. [Google Scholar] [CrossRef]
- Tran, J.; Aksenov, V.; Rollo, C.D. A Multi-Ingredient Athletic Supplement Disproportionately Enhances Hind Leg Musculature, Jumping Performance, and Spontaneous Locomotion in Crickets (Cheta Domesticus). Entomol. Exp. Et Appl. 2018, 166, 63–73. [Google Scholar] [CrossRef]
- Kim, J.C.; Kang, Y.S.; Noh, E.B.; Seo, B.W.; Seo, D.Y.; Park, G.D.; Kim, S.H. Concurrent Treatment with Ursolic Acid and Low-Intensity Treadmill Exercise Improves Muscle Atrophy and Related Outcomes in Rats. Korean J. Physiol. Pharmacol. Off. J. Korean Physiol. Soc. Korean Soc. Pharmacol. 2018, 22, 427. [Google Scholar] [CrossRef] [PubMed]
- Parrini, M.; Ghezzi, D.; Deidda, G.; Medrihan, L.; Castroflorio, E.; Alberti, M.; Baldelli, P.; Cancedda, L.; Contestabile, A. Aerobic Exercise and a BDNF-Mimetic Therapy Rescue Learning and Memory in a Mouse Model of Down Syndrome. Sci. Rep. 2017, 7, 16825. [Google Scholar] [CrossRef]
- Stockinger, J.; Maxwell, N.; Shapiro, D.; deCabo, R.; Valdez, G. Caloric Restriction Mimetics Slow Aging of Neuromuscular Synapses and Muscle Fibers. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Bowser, S.M.; Moore, W.T.; McMillan, R.P.; Dorenkott, M.R.; Goodrich, K.M.; Ye, L.; O’Keefe, S.F.; Hulver, M.W.; Neilson, A.P. High-Molecular-Weight Cocoa Procyanidins Possess Enhanced Insulin-Enhancing and Insulin Mimetic Activities in Human Primary Skeletal Muscle Cells Compared to Smaller Procyanidins. J. Nutr. Biochem. 2017, 39, 48–58. [Google Scholar] [CrossRef]
- Tomcik, K.A.; Smiles, W.J.; Camera, D.M.; Hügel, H.M.; Hawley, J.A.; Watts, R. Fenugreek Increases Insulin-Stimulated Creatine Content in L6C11 Muscle Myotubes. Eur. J. Nutr. 2017, 56, 973–979. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, D.; Good, D.J.; Park, Y. Conjugated Linoleic Acid (CLA) Influences Muscle Metabolism via Stimulating Mitochondrial Biogenesis Signaling in Adult-Onset Inactivity Induced Obese Mice. Eur. J. Lipid Sci. Technol. 2016, 118, 1305–1316. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, K.; Liu, P.; Gao, Y.; Zou, D.; Deng, H.; Huang, Y.; Zhang, Q.; Zhu, J.; Mi, M. Dihydromyricetin Stimulates Irisin Secretion Partially via the PGC-1α Pathway. Mol. Cell. Endocrinol. 2015, 412, 349–357. [Google Scholar] [CrossRef]
- Dong, W.-W.; Liu, Y.-J.; Lv, Z.; Mao, Y.-F.; Wang, Y.-W.; Zhu, X.-Y.; Jiang, L. Lung Endothelial Barrier Protection by Resveratrol Involves Inhibition of HMGB1 Release and HMGB1-Induced Mitochondrial Oxidative Damage via an Nrf2-Dependent Mechanism. Free Radic. Biol. Med. 2015, 88, 404–416. [Google Scholar] [CrossRef] [PubMed]
- Olesen, J.; Gliemann, L.; Biensø, R.; Schmidt, J.; Hellsten, Y.; Pilegaard, H. Exercise Training, but Not Resveratrol, Improves Metabolic and Inflammatory Status in Skeletal Muscle of Aged Men. J. Physiol. 2014, 592, 1873–1886. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Huang, C.; Wang, C.; Zheng, J.; Zhang, P.; Xu, Y.; Chen, H.; Shen, W. Ginsenoside Rg3 Improves Cardiac Mitochondrial Population Quality: Mimetic Exercise Training. Biochem. Biophys. Res. Commun. 2013, 441, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Goh, K.P.; Lee, H.Y.; Lau, D.P.; Supaat, W.; Chan, Y.H.; Koh, A.F.Y. Effects of Resveratrol in Patients with Type 2 Diabetes Mellitus on Skeletal Muscle SIRT1 Expression and Energy Expenditure. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 2–13. [Google Scholar] [CrossRef]
- Jeong, H.W.; Cho, S.Y.; Kim, S.; Shin, E.S.; Kim, J.M.; Song, M.J.; Park, P.J.; Sohn, J.H.; Park, H.; Seo, D.-B.; et al. Chitooligosaccharide Induces Mitochondrial Biogenesis and Increases Exercise Endurance through the Activation of Sirt1 and AMPK in Rats. PLoS ONE 2012, 7, e40073. [Google Scholar] [CrossRef]
- Nogueira, L.; Ramirez-Sanchez, I.; Perkins, G.A.; Murphy, A.; Taub, P.R.; Ceballos, G.; Villarreal, F.J.; Hogan, M.C.; Malek, M.H. (–)-Epicatechin Enhances Fatigue Resistance and Oxidative Capacity in Mouse Muscle. J. Physiol. 2011, 589, 4615. [Google Scholar] [CrossRef]
- Momken, I.; Stevens, L.; Bergouignan, A.; Desplanches, D.; Rudwill, F.; Chery, I.; Zahariev, A.; Zahn, S.; Stein, T.P.; Sebedio, J.L.; et al. Resveratrol Prevents the Wasting Disorders of Mechanical Unloading by Acting as a Physical Exercise Mimetic in the Rat. FASEB J. 2011, 25, 3646–3660. [Google Scholar] [CrossRef]
- Kumar, R.; Negi, P.S.; Singh, B.; Ilavazhagan, G.; Bhargava, K.; Sethy, N.K. Cordyceps Sinensis Promotes Exercise Endurance Capacity of Rats by Activating Skeletal Muscle Metabolic Regulators. J. Ethnopharmacol. 2011, 136, 260–266. [Google Scholar] [CrossRef]
- Tharakan, B.; Dhanasekaran, M.; Brown-Borg, H.M.; Manyam, B.V. Trichopus Zeylanicus Combats Fatigue without Amphetamine-Mimetic Activity. Phytother. Res. 2006, 20, 165–168. [Google Scholar] [CrossRef]
- Reid, M.B. Redox Interventions to Increase Exercise Performance. J. Physiol. 2016, 594, 5125–5133. [Google Scholar] [CrossRef]
- Powers, S.K.; Deminice, R.; Ozdemir, M.; Yoshihara, T.; Bomkamp, M.P.; Hyatt, H. Exercise-Induced Oxidative Stress: Friend or Foe? J. Sport Health Sci. 2020, 9, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.; Ito, O.; Ito, D.; Rong, R.; Zheng, Y.; Kohzuki, M. Combination of Exercise Training and SOD Mimetic Tempol Enhances Upregulation of Nitric Oxide Synthase in the Kidney of Spontaneously Hypertensive Rats. Int. J. Hypertens. 2020, 2020, 2142740. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Lu, J.; Liu, J.; Li, J. Local Injections of Superoxide Dismutase Attenuate the Exercise Pressor Reflex in Rats with Femoral Artery Occlusion. Front. Physiol. 2018, 9, 39. [Google Scholar] [CrossRef] [PubMed]
- Koba, S.; Hisatome, I.; Watanabe, T. Central Command Dysfunction in Rats with Heart Failure Is Mediated by Brain Oxidative Stress and Normalized by Exercise Training. J. Physiol. 2014, 592, 3917–3931. [Google Scholar] [CrossRef]
- McCord, J.L.; Tsuchimochi, H.; Yamauchi, K.; Leal, A.; Kaufman, M.P. Tempol Attenuates the Exercise Pressor Reflex Independently of Neutralizing Reactive Oxygen Species in Femoral Artery Ligated Rats. J. Appl. Physiol. 2011, 111, 971–979. [Google Scholar] [CrossRef]
- Brestoff, J.R.; Brodsky, T.; Sosinsky, A.Z.; McLoughlin, R.; Stansky, E.; Fussell, L.; Sheppard, A.; DiSanto-Rose, M.; Kershaw, E.E.; Thomas H Reynolds, I.V. Manganese [III] Tetrakis [5,10,15,20]-Benzoic Acid Porphyrin Reduces Adiposity and Improves Insulin Action in Mice with Pre-Existing Obesity. PLoS ONE 2015, 10, e0137388. [Google Scholar] [CrossRef]
- Zhang, H.J.; Doctrow, S.R.; Xu, L.; Oberley, L.W.; Beecher, B.; Morrison, J.; Oberley, T.D.; Kregel, K.C. Redox Modulation of the Liver with Chronic Antioxidant Enzyme Mimetic Treatment Prevents Age-Related Oxidative Damage Associated with Environmental Stress. FASEB J. 2004, 18, 1547–1549. [Google Scholar] [CrossRef]
- Molinari, F.; Pin, F.; Gorini, S.; Chiandotto, S.; Pontecorvo, L.; Penna, F.; Rizzuto, E.; Pisu, S.; Musarò, A.; Costelli, P.; et al. The Mitochondrial Metabolic Reprogramming Agent Trimetazidine as an ‘Exercise Mimetic’ in Cachectic C26-Bearing Mice. J. Cachexia Sarcopenia Muscle 2017, 8, 954–973. [Google Scholar] [CrossRef]
- Casso, A.G.; VanDongen, N.S.; Gioscia-Ryan, R.A.; Clayton, Z.S.; Greenberg, N.T.; Ziemba, B.P.; Hutton, D.A.; Neilson, A.P.; Davy, K.P.; Seals, D.R.; et al. Initiation of 3,3-Dimethyl-1-Butanol at Midlife Prevents Endothelial Dysfunction and Attenuates in Vivo Aortic Stiffening with Ageing in Mice. J. Physiol. 2022, 600, 4633–4651. [Google Scholar] [CrossRef]
- Brunt, V.E.; Gioscia-Ryan, R.A.; Casso, A.G.; VanDongen, N.S.; Ziemba, B.P.; Sapinsley, Z.J.; Richey, J.J.; Zigler, M.C.; Neilson, A.P.; Davy, K.P.; et al. Trimethylamine-N-Oxide Promotes Age-Related Vascular Oxidative Stress and Endothelial Dysfunction in Mice and Healthy Humans. Hypertension 2020, 76, 101–112. [Google Scholar] [CrossRef]
- Saxena, S.; Shukla, D.; Bansal, A. Augmentation of Aerobic Respiration and Mitochondrial Biogenesis in Skeletal Muscle by Hypoxia Preconditioning with Cobalt Chloride. Toxicol. Appl. Pharmacol. 2012, 264, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.; Shukla, D.; Saxena, S.; Khan, Y.A.; Singh, M.; Bansal, A.; Sairam, M.; Jain, S.K. Hypoxia Preconditioning by Cobalt Chloride Enhances Endurance Performance and Protects Skeletal Muscles from Exercise-Induced Oxidative Damage in Rats. Acta Physiol. 2010, 200, 249–263. [Google Scholar] [CrossRef]
- Déry, M.-A.C.; Michaud, M.D.; Richard, D.E. Hypoxia-Inducible Factor 1: Regulation by Hypoxic and Non-Hypoxic Activators. Int. J. Biochem. Cell Biol. 2005, 37, 535–540. [Google Scholar] [CrossRef]
- Xu, D.-P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.-J.; Li, H.-B. Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef]
- Craig, D.M.; Ashcroft, S.P.; Belew, M.Y.; Stocks, B.; Currell, K.; Baar, K.; Philp, A. Utilizing Small Nutrient Compounds as Enhancers of Exercise-Induced Mitochondrial Biogenesis. Front. Physiol. 2015, 6, 296. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Liu, X.; Luo, X.; Lou, X.; Li, P.; Li, X.; Liu, X. Antiaging Effects of Dietary Supplements and Natural Products. Front. Pharmacol. 2023, 14, 1192714. [Google Scholar] [CrossRef]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K.; et al. Resveratrol Improves Health and Survival of Mice on a High-Calorie Diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef]
- Pearson, K.J.; Baur, J.A.; Lewis, K.N.; Peshkin, L.; Price, N.L.; Labinskyy, N.; Swindell, W.R.; Kamara, D.; Minor, R.K.; Perez, E.; et al. Resveratrol Delays Age-Related Deterioration and Mimics Transcriptional Aspects of Dietary Restriction without Extending Life Span. Cell Metab. 2008, 8, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Toniolo, L.; Concato, M.; Giacomello, E. Resveratrol, a Multitasking Molecule That Improves Skeletal Muscle Health. Nutrients 2023, 15, 3413. [Google Scholar] [CrossRef]
- Toniolo, L.; Formoso, L.; Torelli, L.; Crea, E.; Bergamo, A.; Sava, G.; Giacomello, E. Long-Term Resveratrol Treatment Improves the Capillarization in the Skeletal Muscles of Ageing C57BL/6J Mice. Int. J. Food Sci. Nutr. 2021, 72, 37–44. [Google Scholar] [CrossRef]
- Sirago, G.; Toniolo, L.; Crea, E.; Giacomello, E. A Short-Term Treatment with Resveratrol Improves the Inflammatory Conditions of Middle-Aged Mice Skeletal Muscles. Int. J. Food Sci. Nutr. 2022, 73, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Toniolo, L.; Fusco, P.; Formoso, L.; Mazzi, A.; Canato, M.; Reggiani, C.; Giacomello, E. Resveratrol Treatment Reduces the Appearance of Tubular Aggregates and Improves the Resistance to Fatigue in Aging Mice Skeletal Muscles. Exp. Gerontol. 2018, 111, 170–179. [Google Scholar] [CrossRef]
- Prakash, M.; Basavaraj, B.V.; Chidambara Murthy, K.N. Biological Functions of Epicatechin: Plant Cell to Human Cell Health. J. Funct. Foods 2019, 52, 14–24. [Google Scholar] [CrossRef]
- Imran, M.; Arshad, M.S.; Butt, M.S.; Kwon, J.-H.; Arshad, M.U.; Sultan, M.T. Mangiferin: A Natural Miracle Bioactive Compound against Lifestyle Related Disorders. Lipids Health Dis. 2017, 16, 84. [Google Scholar] [CrossRef]
- Öz, M.; Ucak, İ.; Nayik, G.A. Chapter 10—PUFA and MUFA. In Nutraceuticals and Health Care; Kour, J., Nayik, G.A., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 199–215. ISBN 978-0-323-89779-2. [Google Scholar]
- Chen, X.; Li, L.; Liu, X.; Luo, R.; Liao, G.; Li, L.; Liu, J.; Cheng, J.; Lu, Y.; Chen, Y. Oleic Acid Protects Saturated Fatty Acid Mediated Lipotoxicity in Hepatocytes and Rat of Non-Alcoholic Steatohepatitis. Life Sci. 2018, 203, 291–304. [Google Scholar] [CrossRef]
- Wang, J.; Song, M.-Y.; Bae, U.-J.; Lim, J.M.; Kwon, K.S.; Park, B.-H. N-3 Polyunsaturated Fatty Acids Protect against Pancreatic β-Cell Damage Due to ER Stress and Prevent Diabetes Development. Mol. Nutr. Food Res. 2015, 59, 1791–1802. [Google Scholar] [CrossRef] [PubMed]
- Fürstova, V.; Kopska, T.; James, R.F.L.; Kovar, J. Comparison of the Effect of Individual Saturated and Unsaturated Fatty Acids on Cell Growth and Death Induction in the Human Pancreatic β-Cell Line NES2Y. Life Sci. 2008, 82, 684–691. [Google Scholar] [CrossRef]
- Posa, F.; Zerlotin, R.; Ariano, A.; Cosola, M.D.; Colaianni, G.; Fazio, A.D.; Colucci, S.; Grano, M.; Mori, G. Irisin Role in Chondrocyte 3D Culture Differentiation and Its Possible Applications. Pharmaceutics 2023, 15, 585. [Google Scholar] [CrossRef]
- Yardimci, A.; Ulker, N.; Bulmus, O.; Sahin, E.; Alver, A.; Gungor, I.H.; Turk, G.; Artas, G.; Kaya Tektemur, N.; Ozcan, M.; et al. Irisin Improves High-Fat Diet-Induced Sexual Dysfunction in Obese Male Rats. Neuroendocrinology 2022, 112, 1087–1103. [Google Scholar] [CrossRef]
- Momenzadeh, S.; Zamani, S.; Pourteymourfard-Tabrizi, Z.; Barreiro, C.; Jami, M.-S. Muscles Proteome Analysis; Irisin Administration Mimics Some Molecular Effects of Exercise in Quadriceps Muscle. Biochimie 2021, 189, 144–157. [Google Scholar] [CrossRef]
- Chang, J.S.; Kong, I.D. Irisin Prevents Dexamethasone-Induced Atrophy in C2C12 Myotubes. Pflug. Archiv. 2020, 472, 495. [Google Scholar] [CrossRef]
- Storlino, G.; Colaianni, G.; Sanesi, L.; Lippo, L.; Brunetti, G.; Errede, M.; Colucci, S.; Passeri, G.; Grano, M. Irisin Prevents Disuse-Induced Osteocyte Apoptosis. J. Bone Miner. Res. 2020, 35, 766–775. [Google Scholar] [CrossRef]
- Colaianni, G.; Cuscito, C.; Mongelli, T.; Oranger, A.; Mori, G.; Brunetti, G.; Colucci, S.; Cinti, S.; Grano, M. Irisin Enhances Osteoblast Differentiation In Vitro. Int. J. Endocrinol. 2014, 2014, 902186. [Google Scholar] [CrossRef]
- Drucker, D.J. The Biology of Incretin Hormones. Cell Metab. 2006, 3, 153–165. [Google Scholar] [CrossRef]
- Fehse, F.; Trautmann, M.; Holst, J.J.; Halseth, A.E.; Nanayakkara, N.; Nielsen, L.L.; Fineman, M.S.; Kim, D.D.; Nauck, M.A. Exenatide Augments First- and Second-Phase Insulin Secretion in Response to Intravenous Glucose in Subjects with Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2005, 90, 5991–5997. [Google Scholar] [CrossRef]
- Poon, T.; Nelson, P.; Shen, L.; Mihm, M.; Taylor, K.; Fineman, M.; Kim, D. Exenatide Improves Glycemic Control and Reduces Body Weight in Subjects with Type 2 Diabetes: A Dose-Ranging Study. Diabetes Technol. Ther. 2005, 7, 467–477. [Google Scholar] [CrossRef]
- Nelson, P.; Poon, T.; Guan, X.; Schnabel, C.; Wintle, M.; Fineman, M. The Incretin Mimetic Exenatide as a Monotherapy in Patients with Type 2 Diabetes. Diabetes Technol. Ther. 2007, 9, 317–326. [Google Scholar] [CrossRef]
- Carmina, E.; Longo, R.A. Semaglutide Treatment of Excessive Body Weight in Obese PCOS Patients Unresponsive to Lifestyle Programs. J. Clin. Med. 2023, 12, 5921. [Google Scholar] [CrossRef]
- Lee, T.H.; Ahadullah; Christie, B.R.; Lin, K.; Siu, P.M.; Zhang, L.; Yuan, T.; Komal, P.; Xu, A.; So, K.; et al. Chronic AdipoRon Treatment Mimics the Effects of Physical Exercise on Restoring Hippocampal Neuroplasticity in Diabetic Mice. Mol. Neurobiol. 2021, 58, 4666. [Google Scholar] [CrossRef]
- Kadowaki, T.; Yamauchi, T. Adiponectin and Adiponectin Receptors. Endocr. Rev. 2005, 26, 439–451. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Li, L.; Hölscher, C. Neuroprotective Effects of (Val8)GLP-1-Glu-PAL in the MPTP Parkinson’s Disease Mouse Model. Behav. Brain Res. 2015, 293, 107–113. [Google Scholar] [CrossRef][Green Version]
- Liu, W.; Li, Y.; Jalewa, J.; Saunders-Wood, T.; Li, L.; Hölscher, C. Neuroprotective Effects of an Oxyntomodulin Analogue in the MPTP Mouse Model of Parkinson’s Disease. Eur. J. Pharmacol. 2015, 765, 284–290. [Google Scholar] [CrossRef]
- Lee, C.; Zeng, J.; Drew, B.G.; Sallam, T.; Martin-Montalvo, A.; Wan, J.; Kim, S.-J.; Mehta, H.; Hevener, A.L.; de Cabo, R.; et al. The Mitochondrial-Derived Peptide MOTS-c Promotes Metabolic Homeostasis and Reduces Obesity and Insulin Resistance. Cell Metab. 2015, 21, 443–454. [Google Scholar] [CrossRef]
- Kim, S.-J.; Miller, B.; Mehta, H.H.; Xiao, J.; Wan, J.; Arpawong, T.E.; Yen, K.; Cohen, P. The Mitochondrial-Derived Peptide MOTS-c Is a Regulator of Plasma Metabolites and Enhances Insulin Sensitivity. Physiol. Rep. 2019, 7, e14171. [Google Scholar] [CrossRef]
- Kharitonenkov, A.; Wroblewski, V.J.; Koester, A.; Chen, Y.-F.; Clutinger, C.K.; Tigno, X.T.; Hansen, B.C.; Shanafelt, A.B.; Etgen, G.J. The Metabolic State of Diabetic Monkeys Is Regulated by Fibroblast Growth Factor-21. Endocrinology 2007, 148, 774–781. [Google Scholar] [CrossRef]
- Dong, J.Q.; Rossulek, M.; Somayaji, V.R.; Baltrukonis, D.; Liang, Y.; Hudson, K.; Hernandez-Illas, M.; Calle, R.A. Pharmacokinetics and Pharmacodynamics of PF-05231023, a Novel Long-Acting FGF21 Mimetic, in a First-in-Human Study. Br. J. Clin. Pharmacol. 2015, 80, 1051–1063. [Google Scholar] [CrossRef]
- Lezi, E.; Lu, J.; Selfridge, J.E.; Burns, J.M.; Swerdlow, R.H. Lactate Administration Reproduces Specific Brain and Liver Exercise-Related Changes. J. Neurochem. 2013, 127, 91–100. [Google Scholar] [CrossRef]
- Truel, A.F.; Boix, F.; Escorihuela, R.M.; Yáñez, P.; Tobeña, A. Sodium Valporate Reduces Immobility in the Behavioral ‘Depair’ Test in Rats. Eur. J. Pharmacol. 1988, 152, 1–7. [Google Scholar] [CrossRef]
- Dambrova, M.; Zvejniece, L.; Liepinsh, E.; Cirule, H.; Zharkova, O.; Veinberg, G.; Kalvinsh, I. Comparative Pharmacological Activity of Optical Isomers of Phenibut. Eur. J. Pharmacol. 2008, 583, 128–134. [Google Scholar] [CrossRef]
- BINDER, D.K.; SCHARFMAN, H.E. Brain-Derived Neurotrophic Factor. Growth Factors 2004, 22, 123–131. [Google Scholar] [CrossRef]
- Rasmussen, P.; Brassard, P.; Adser, H.; Pedersen, M.V.; Leick, L.; Hart, E.; Secher, N.H.; Pedersen, B.K.; Pilegaard, H. Evidence for a Release of Brain-Derived Neurotrophic Factor from the Brain during Exercise. Exp. Physiol. 2009, 94, 1062–1069. [Google Scholar] [CrossRef]
- Koliatsos, V.E.; Clatterbuck, R.E.; Winslow, J.W.; Cayouette, M.H.; Prices, D.L. Evidence That Brain-Derived Neurotrophic Factor Is a Trophic Factor for Motor Neurons in Vivo. Neuron 1993, 10, 359–367. [Google Scholar] [CrossRef]
- Matthews, V.B.; Åström, M.-B.; Chan, M.H.S.; Bruce, C.R.; Krabbe, K.S.; Prelovsek, O.; Åkerström, T.; Yfanti, C.; Broholm, C.; Mortensen, O.H.; et al. Brain-Derived Neurotrophic Factor Is Produced by Skeletal Muscle Cells in Response to Contraction and Enhances Fat Oxidation via Activation of AMP-Activated Protein Kinase. Diabetologia 2009, 52, 1409–1418. [Google Scholar] [CrossRef]
- Magalhães, C.B.; Casquilho, N.V.; Machado, M.N.; Riva, D.R.; Travassos, L.H.; Leal-Cardoso, J.H.; Fortunato, R.S.; Faffe, D.S.; Zin, W.A. The Anti-Inflammatory and Anti-Oxidative Actions of Eugenol Improve Lipopolysaccharide-Induced Lung Injury. Respir. Physiol. Neurobiol. 2019, 259, 30–36. [Google Scholar] [CrossRef]
- Dolny, D.G.; Reyes, G.F.C. Whole Body Vibration Exercise: Training and Benefits. Curr. Sports Med. Rep. 2008, 7, 152. [Google Scholar] [CrossRef]
- McGee-Lawrence, M.E.; Wenger, K.H.; Misra, S.; Davis, C.L.; Pollock, N.K.; Elsalanty, M.; Ding, K.; Isales, C.M.; Hamrick, M.W.; Wosiski-Kuhn, M.; et al. Whole-Body Vibration Mimics the Metabolic Effects of Exercise in Male Leptin Receptor–Deficient Mice. Endocrinology 2017, 158, 1160. [Google Scholar] [CrossRef]
- Yu, J.C.; Hale, V.L.; Khodadadi, H.; Baban, B. Whole Body Vibration-Induced Omental Macrophage Polarization and Fecal Microbiome Modification in a Murine Model. Int. J. Mol. Sci. 2019, 20, 3125. [Google Scholar] [CrossRef]
- Sanni, A.A.; Blanks, A.M.; Derella, C.C.; Horsager, C.; Crandall, R.H.; Looney, J.; Sanchez, S.; Norland, K.; Ye, B.; Thomas, J.; et al. The Effects of Whole-Body Vibration Amplitude on Glucose Metabolism, Inflammation, and Skeletal Muscle Oxygenation. Physiol. Rep. 2022, 10, e15208. [Google Scholar] [CrossRef]
- Betik, A.C.; Parker, L.; Kaur, G.; Wadley, G.D.; Keske, M.A. Whole-Body Vibration Stimulates Microvascular Blood Flow in Skeletal Muscle. Med. Sci. Sports Exerc. 2021, 53, 375. [Google Scholar] [CrossRef]
- Broniec, M.N.; Norland, K.; Thomas, J.; Wang, X.; Harris, R.A. The Decorin and Myostatin Response to Acute Whole Body Vibration: Impact of Adiposity, Sex, and Race. Int. J. Obes. 2024, 48, 1803–1808. [Google Scholar] [CrossRef]
- John, K. Hot Pants: The Emerging Field of Exercise Mimetics, from Hospital Beds to the International Space Station. Physiol. Rep. 2024, 12, e70108. [Google Scholar] [CrossRef]
- James, T.J.; Corbett, J.; Cummings, M.; Allard, S.; Young, J.S.; Towse, J.; Carey-Jones, K.; Eglin, C.; Hopkins, B.; Morgan, C.; et al. Timing of Acute Passive Heating on Glucose Tolerance and Blood Pressure in People with Type 2 Diabetes: A Randomized, Balanced Crossover, Control Trial. J. Appl. Physiol. 2021, 130, 1093–1105. [Google Scholar] [CrossRef]
- Li, S.; Miao, X.; Zhang, J.; Wei, D.; Dong, H.; Xue, R.; Li, J.; Zhang, Y.; Feng, X.; Li, J.; et al. Far-Infrared Therapy Promotes Exercise Capacity and Glucose Metabolism in Mice by Modulating Microbiota Homeostasis and Activating AMPK. Sci. Rep. 2024, 14, 16314. [Google Scholar] [CrossRef]
- Hussain, J.N.; Cohen, M.M.; Mantri, N.; O’Malley, C.J.; Greaves, R.F. Infrared Sauna as Exercise-Mimetic? Physiological Responses to Infrared Sauna vs Exercise in Healthy Women: A Randomized Controlled Crossover Trial. Complement. Ther. Med. 2022, 64, 102798. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giacomello, E.; Nicoletti, C.; Canato, M.; Toniolo, L. Exercise Mimetics in Aging: Suggestions from a Systematic Review. Nutrients 2025, 17, 969. https://doi.org/10.3390/nu17060969
Giacomello E, Nicoletti C, Canato M, Toniolo L. Exercise Mimetics in Aging: Suggestions from a Systematic Review. Nutrients. 2025; 17(6):969. https://doi.org/10.3390/nu17060969
Chicago/Turabian StyleGiacomello, Emiliana, Claudio Nicoletti, Marta Canato, and Luana Toniolo. 2025. "Exercise Mimetics in Aging: Suggestions from a Systematic Review" Nutrients 17, no. 6: 969. https://doi.org/10.3390/nu17060969
APA StyleGiacomello, E., Nicoletti, C., Canato, M., & Toniolo, L. (2025). Exercise Mimetics in Aging: Suggestions from a Systematic Review. Nutrients, 17(6), 969. https://doi.org/10.3390/nu17060969





