Programming the Brain: How Maternal Overnutrition Shapes Cognitive Aging in Offspring
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Housing
2.2. Maternal High-Fat Diet Exposure
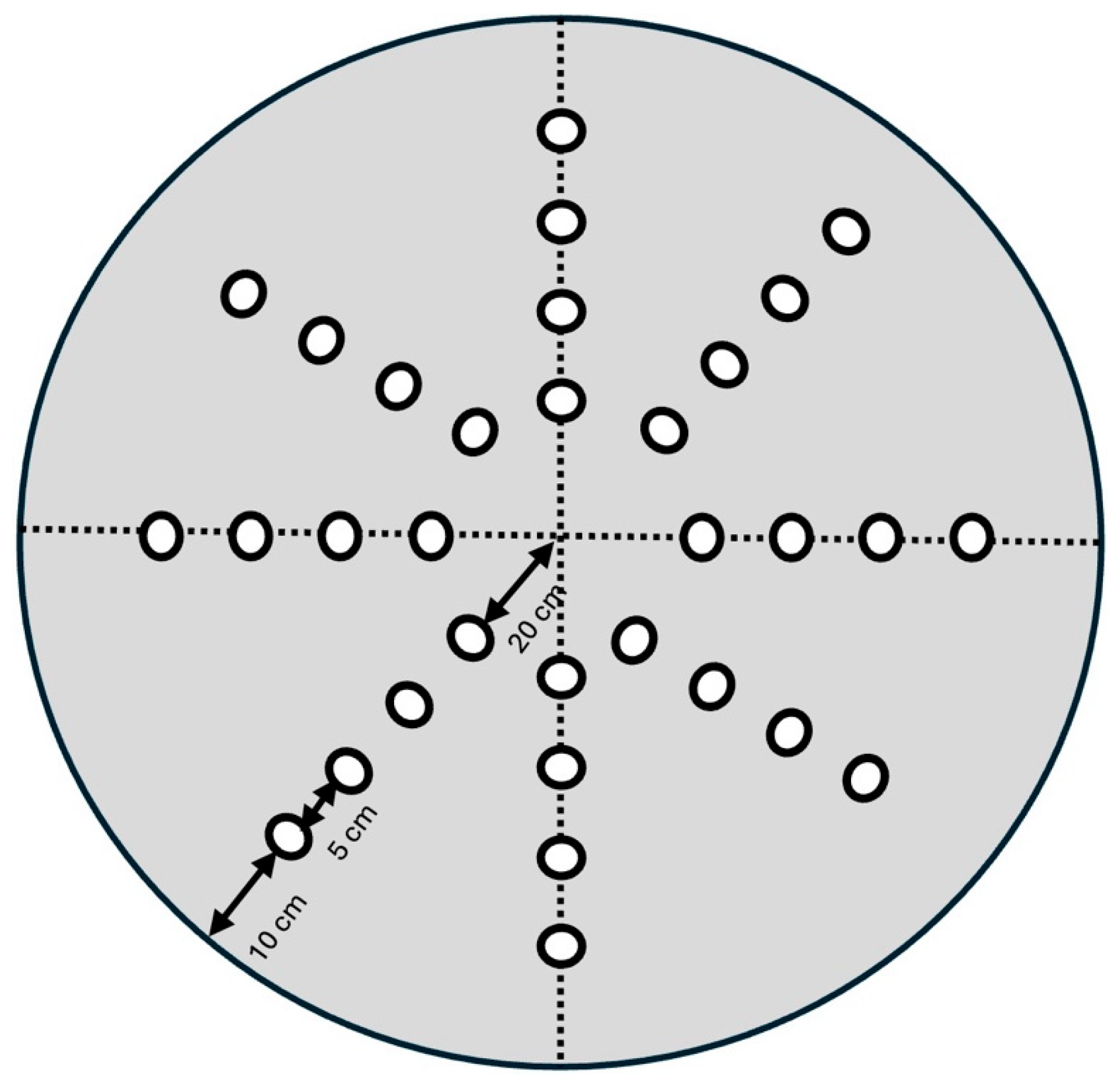
2.3. Reference Memory Tested in the Dry Maze
2.4. Statistical Analysis
3. Results
3.1. Body Weight
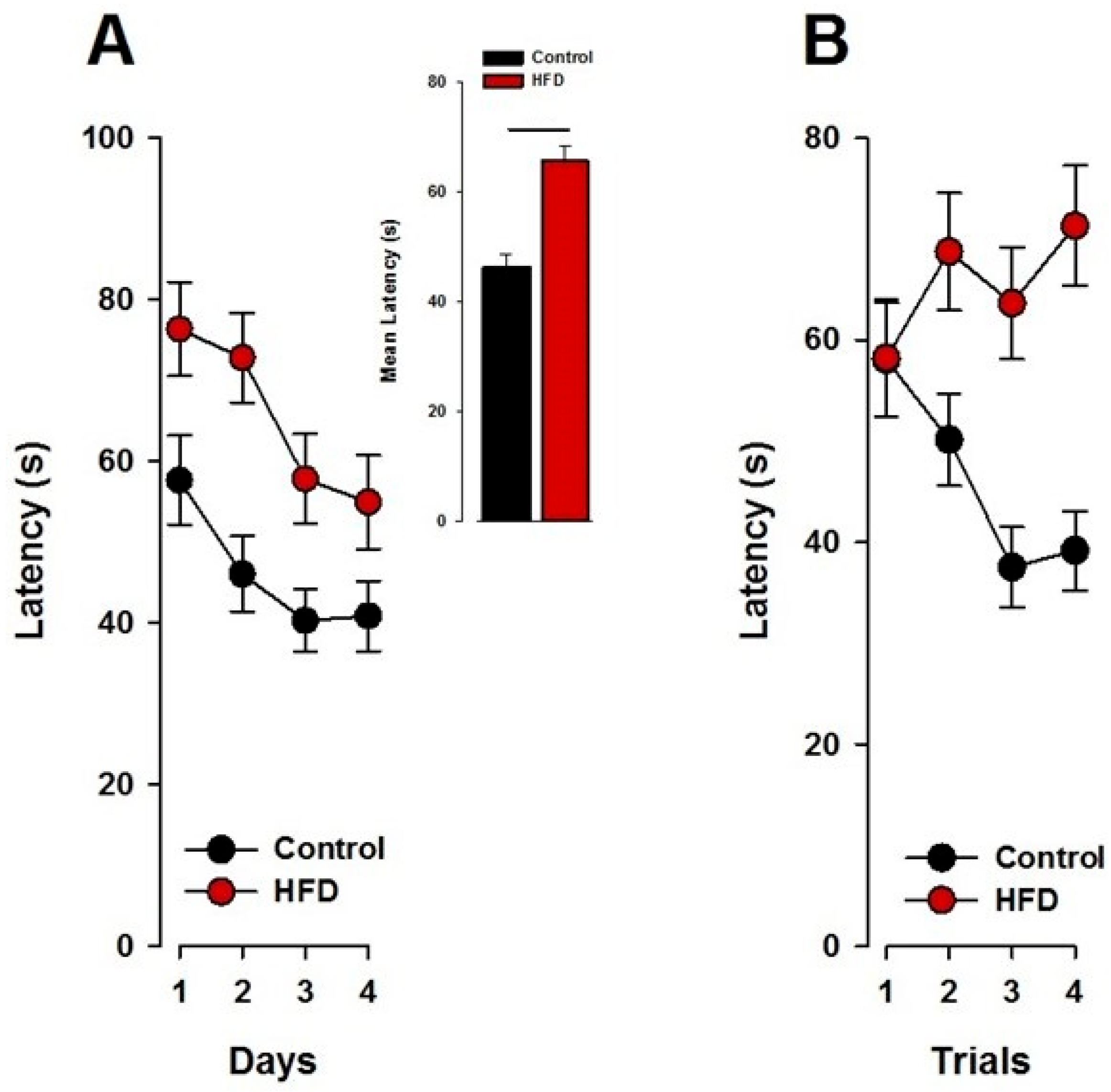
3.2. Spatial Reference Memory in the Dry Maze
3.2.1. Visual Cue Task (Pretraining)
3.2.2. Reference Memory
3.2.3. The Probe Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization (WHO). 1 March 2024. Obesity and Overweight Fact Sheet. 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight#:~:text=Key%20facts,years%20and%20older)%20were%20overweight (accessed on 4 March 2025). (In English).
- Hruby, A.; Hu, F.B. The Epidemiology of Obesity: A Big Picture. Pharmacoeconomics 2015, 33, 673–689. (In English) [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J. The origins of the developmental origins theory. J. Intern. Med. 2007, 261, 412–417. (In English) [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J.; Bull, A.R.; Osmond, C.; Simmonds, S.J. Fetal and placental size and risk of hypertension in adult life. BMJ 1990, 301, 259–262. (In English) [Google Scholar] [CrossRef] [PubMed]
- McMillen, I.C.; Adam, C.L.; Muhlhausler, B.S. Early origins of obesity: Programming the appetite regulatory system. J. Physiol. 2005, 565, 9–17. (In English) [Google Scholar] [CrossRef]
- Kominiarek, M.A.; Chauhan, S.P. Obesity Before, During, and After Pregnancy: A Review and Comparison of Five National Guidelines. Am. J. Perinatol. 2016, 33, 433–441. (In English) [Google Scholar] [CrossRef]
- Lawlor, D.A.; Smith, G.D.; O’Callaghan, M.; Alati, R.; Mamun, A.A.; Williams, G.M.; Najman, J.M. Epidemiologic evidence for the fetal overnutrition hypothesis: Findings from the mater-university study of pregnancy and its outcomes. Am. J. Epidemiol. 2007, 165, 418–424. (In English) [Google Scholar] [CrossRef]
- Whitaker, R.C. Predicting preschooler obesity at birth: The role of maternal obesity in early pregnancy. Pediatrics 2004, 114, e29–e36. (In English) [Google Scholar] [CrossRef]
- Van Lieshout, R.J. Role of maternal adiposity prior to and during pregnancy in cognitive and psychiatric problems in offspring. Nutr. Rev. 2013, 71 (Suppl. S1), S95–S101. (In English) [Google Scholar] [CrossRef]
- Neggers, Y.H.; Goldenberg, R.L.; Ramey, S.L.; Cliver, S.P. Maternal prepregnancy body mass index and psychomotor development in children. Acta Obstet. Gynecol. Scand. 2003, 82, 235–240. (In English) [Google Scholar] [CrossRef]
- Tanda, R.; Salsberry, P.J.; Reagan, P.B.; Fang, M.Z. The impact of prepregnancy obesity on children’s cognitive test scores. Matern. Child. Health J. 2013, 17, 222–229. (In English) [Google Scholar] [CrossRef]
- Brion, M.J.; Zeegers, M.; Jaddoe, V.; Verhulst, F.; Tiemeier, H.; Lawlor, D.A.; Smith, G.D. Intrauterine effects of maternal prepregnancy overweight on child cognition and behavior in 2 cohorts. Pediatrics 2011, 127, e202–e211. (In English) [Google Scholar] [CrossRef] [PubMed]
- Buss, C.; Entringer, S.; Davis, E.P.; Hobel, C.J.; Swanson, J.M.; Wadhwa, P.D.; Sandman, C.A. Impaired executive function mediates the association between maternal pre-pregnancy body mass index and child ADHD symptoms. PLoS ONE 2012, 7, e37758. (In English) [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A. Maternal pre-pregnancy obesity and risk for inattention and negative emotionality in children. J. Child. Psychol. Psychiatry 2010, 51, 134–143. (In English) [Google Scholar] [CrossRef]
- Rodriguez, A.; Miettunen, J.; Henriksen, T.B.; Olsen, J.; Obel, C.; Taanila, A.; Ebeling, H.; Linnet, K.M.; Moilanen, I.; Jarvelin, M.R. Maternal adiposity prior to pregnancy is associated with ADHD symptoms in offspring: Evidence from three prospective pregnancy cohorts. Int. J. Obes. 2008, 32, 550–557. (In English) [Google Scholar] [CrossRef]
- Stranahan, A.M.; Mattson, M.P. Impact of energy intake and expenditure on neuronal plasticity. Neuromol. Med. 2008, 10, 209–218. (In English) [Google Scholar] [CrossRef]
- Sellbom, K.S.; Gunstad, J. Cognitive function and decline in obesity. J. Alzheimer’s Dis. 2012, 30 (Suppl. S2), S89–S95. (In English) [Google Scholar] [CrossRef]
- WHO. Nutrition Counselling During Pregnancy. Available online: https://www.who.int/tools/elena/interventions/nutrition-counselling-pregnancy (accessed on 4 March 2025).
- Peleg-Raibstein, D.; Luca, E.; Wolfrum, C. Maternal high-fat diet in mice programs emotional behavior in adulthood. Behav. Brain Res. 2012, 233, 398–404. (In English) [Google Scholar] [CrossRef]
- Whishaw, I.Q. A comparison of rats and mice in a swimming pool place task and matching to place task: Some surprising differences. Physiol. Behav. 1995, 58, 687–693. (In English) [Google Scholar] [CrossRef]
- Llano Lopez, L.; Hauser, J.; Feldon, J.; Gargiulo, P.A.; Yee, B.K. Evaluating spatial memory function in mice: A within-subjects comparison between the water maze test and its adaptation to dry land. Behav. Brain Res. 2010, 209, 85–92. (In English) [Google Scholar] [CrossRef]
- Wolfrum, C.; Peleg-Raibstein, D. Maternal overnutrition leads to cognitive and neurochemical abnormalities in C57BL/6 mice. Nutr. Neurosci. 2018, 22, 688–699. (In English) [Google Scholar] [CrossRef]
- Peleg-Raibstein, D.; Sarker, G.; Litwan, K.; Kramer, S.D.; Ametamey, S.M.; Schibli, R.; Wolfrum, C. Enhanced sensitivity to drugs of abuse and palatable foods following maternal overnutrition. Transl. Psychiatry 2016, 6, e911. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P. The impact of dietary energy intake on cognitive aging. Front. Aging Neurosci. 2010, 2, 5. (In English) [Google Scholar] [CrossRef] [PubMed]
- Yeung, E.H.; Sundaram, R.; Ghassabian, A.; Xie, Y.; Buck Louis, G. Parental Obesity and Early Childhood Development. Pediatrics 2017, 139, e20161459. (In English) [Google Scholar] [CrossRef]
- Edlow, A.G. Maternal obesity and neurodevelopmental and psychiatric disorders in offspring. Prenat. Diagn. 2017, 37, 95–110. (In English) [Google Scholar] [CrossRef]
- Connolly, N.; Anixt, J.; Manning, P.; Ping, I.L.D.; Marsolo, K.A.; Bowers, K. Maternal metabolic risk factors for autism spectrum disorder-An analysis of electronic medical records and linked birth data. Autism Res. 2016, 9, 829–837. (In English) [Google Scholar] [CrossRef]
- Campoy, C.; Escolano-Margarit, M.V.; Ramos, R.; Parrilla-Roure, M.; Csábi, G.; Beyer, J.; Ramirez-Tortosa, M.C.; Molloy, A.M.; Decsi, T.; Koletzko, B.V. Effects of prenatal fish-oil and 5-methyltetrahydrofolate supplementation on cognitive development of children at 6.5 y of age. Am. J. Clin. Nutr. 2011, 94, S1880–S1888. (In English) [Google Scholar] [CrossRef]
- Cusick, S.E.; Georgieff, M.K. The Role of Nutrition in Brain Development: The Golden Opportunity of the “First 1000 Days”. J. Pediatr. 2016, 175, 16–21. (In English) [Google Scholar] [CrossRef]
- Bordeleau, M.; Fernández de Cossío, L.; Lacabanne, C.; Savage, J.C.; Vernoux, N.; Chakravarty, M.; Tremblay, M. Maternal high-fat diet modifies myelin organization, microglial interactions, and results in social memory and sensorimotor gating deficits in adolescent mouse offspring. Brain Behav. Immun. Health 2021, 15, 100281. (In English) [Google Scholar] [CrossRef]
- Bordeleau, M.; Comin, C.H.; Fernández de Cossío, L.; Lacabanne, C.; Freitas-Andrade, M.; González Ibáñez, F.; Raman-Nair, J.; Wakem, M.; Chakravarty, M.; Costa, L.d.F.; et al. Maternal high-fat diet in mice induces cerebrovascular, microglial and long-term behavioural alterations in offspring. Commun. Biol. 2022, 5, 26. [Google Scholar] [CrossRef]
- Gawlińska, K.; Gawliński, D.; Filip, M.; Przegaliński, E. Relationship of maternal high-fat diet during pregnancy and lactation to offspring health. Nutr. Rev. 2021, 79, 709–725. (In English) [Google Scholar] [CrossRef]
- Hawkes, C.A.; Gentleman, S.M.; Nicoll, J.A.; Carare, R.O. Prenatal high-fat diet alters the cerebrovasculature and clearance of β-amyloid in adult offspring. J. Pathol. 2015, 235, 619–631. (In English) [Google Scholar] [CrossRef] [PubMed]
- Peleg-Raibstein, D. Understanding the Link Between Maternal Overnutrition, Cardio-Metabolic Dysfunction and Cognitive Aging. Front. Neurosci. 2021, 15, 645569. (In English) [Google Scholar] [CrossRef] [PubMed]
- Shiadeh, S.M.J.; Goretta, F.; Svedin, P.; Jansson, T.; Mallard, C.; Ardalan, M. Long-term impact of maternal obesity on the gliovascular unit and ephrin signaling in the hippocampus of adult offspring. J. Neuroinflamm. 2024, 21, 39. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Lin, Y.; Luo, J.; Yu, J.; Cheng, Y.; Wu, X.; Lin, L.; Lin, Y. Maternal High-Fat Diet Multigenerationally Impairs Hippocampal Synaptic Plasticity and Memory in Male Rat Offspring. Endocrinology 2021, 162, bqaa214. (In English) [Google Scholar] [CrossRef]
- Boitard, C.; Cavaroc, A.; Sauvant, J.; Aubert, A.; Castanon, N.; Laye, S.; Ferreira, G. Impairment of hippocampal-dependent memory induced by juvenile high-fat diet intake is associated with enhanced hippocampal inflammation in rats. Brain Behav. Immun. 2014, 40, 9–17. (In English) [Google Scholar] [CrossRef]
- Niculescu, M.D.; Lupu, D.S. High fat diet-induced maternal obesity alters fetal hippocampal development. Int. J. Dev. Neurosci. 2009, 27, 627–633. (In English) [Google Scholar] [CrossRef]
- Hu, X.; An, J.; Ge, Q.; Sun, M.; Zhang, Z.; Cai, Z.; Tan, R.; Ma, T.; Lu, H. Maternal High-Fat Diet Reduces Type-2 Neural Stem Cells and Promotes Premature Neuronal Differentiation during Early Postnatal Development. Nutrients 2022, 14, 2813. (In English) [Google Scholar] [CrossRef]
- Fabianová, K.; Babeľová, J.; Fabian, D.; Popovičová, A.; Martončíková, M.; Raček, A.; Račeková, E. Maternal High-Energy Diet during Pregnancy and Lactation Impairs Neurogenesis and Alters the Behavior of Adult Offspring in a Phenotype-Dependent Manner. Int. J. Mol. Sci. 2022, 23, 5564. [Google Scholar] [CrossRef]
- Bilbo, S.D.; Tsang, V. Enduring consequences of maternal obesity for brain inflammation and behavior of offspring. FASEB 2010, 24, 2104–2115. (In English) [Google Scholar] [CrossRef]
- White, C.L.; Pistell, P.J.; Purpera, M.N.; Gupta, S.; Fernandez-Kim, S.O.; Hise, T.L.; Keller, J.N.; Ingram, D.K.; Morrison, C.D.; Bruce-Keller, A.J. Effects of high fat diet on Morris maze performance, oxidative stress, and inflammation in rats: Contributions of maternal diet. Neurobiol. Dis. 2009, 35, 3–13. (In English) [Google Scholar] [CrossRef]
- Tozuka, Y.; Kumon, M.; Wada, E.; Onodera, M.; Mochizuki, H.; Wada, K. Maternal obesity impairs hippocampal BDNF production and spatial learning performance in young mouse offspring. Neurochem. Int. 2010, 57, 235–247. (In English) [Google Scholar] [CrossRef] [PubMed]
- Urbonaite, G.; Knyzeliene, A.; Bunn, F.S.; Smalskys, A.; Neniskyte, U. The impact of maternal high-fat diet on offspring neurodevelopment. Front. Neurosci. 2022, 16, 909762. [Google Scholar] [CrossRef]
- Sarker, G.; Peleg-Raibstein, D. Maternal Overnutrition Induces Long-Term Cognitive Deficits Across Several Generations. Nutrients 2018, 11, 7. (In English) [Google Scholar] [CrossRef]
- Tozuka, Y.; Wada, E.; Wada, K. Diet-induced obesity in female mice leads to peroxidized lipid accumulations and impairment of hippocampal neurogenesis during the early life of their offspring. FASEB 2009, 23, 1920–1934. (In English) [Google Scholar] [CrossRef]
- Frankowska, M.; Surówka, P.; Gawlińska, K.; Borczyk, M.; Korostyński, M.; Filip, M.; Smaga, I. A maternal high-fat diet during pregnancy and lactation induced depression-like behavior in offspring and myelin-related changes in the rat prefrontal cortex. Front. Mol. Neurosci. 2024, 16, 1303718. [Google Scholar] [CrossRef]
- Grissom, N.M.; Herdt, C.T.; Desilets, J.; Lidsky-Everson, J.; Reyes, T.M. Dissociable deficits of executive function caused by gestational adversity are linked to specific transcriptional changes in the prefrontal cortex. Neuropsychopharmacology 2015, 40, 1353–1363. [Google Scholar] [CrossRef]
- Wu, T.; Deng, S.; Li, W.-G.; Yu, Y.; Li, F.; Mao, M. Maternal obesity caused by overnutrition exposure leads to reversal learning deficits and striatal disturbance in rats. PLoS ONE 2013, 8, e78876. [Google Scholar] [CrossRef]
- Naef, L.; Srivastava, L.; Gratton, A.; Hendrickson, H.; Owens, S.M.; Walker, C.D. Maternal high fat diet during the perinatal period alters mesocorticolimbic dopamine in the adult rat offspring: Reduction in the behavioral responses to repeated amphetamine administration. Psychopharmacology 2008, 197, 83–94. (In English) [Google Scholar] [CrossRef]
- Volqvartz, T.; Andersen, H.H.B.; Pedersen, L.H.; Larsen, A. Obesity in pregnancy-Long-term effects on offspring hypothalamic-pituitary-adrenal axis and associations with placental cortisol metabolism: A systematic review. Eur. J. Neurosci. 2023, 58, 4393–4422. (In English) [Google Scholar] [CrossRef]
- Lin, C.; Lei, Q.; Yu, M.; Lin, Y.; Lu, H.; Huang, Y.; Wang, H.; Xu, H.; Lin, Y. Maternal High-Fat Diet Multigenerationally Programs HPA Function and Behaviors in Male Rat Offspring. Endocrinology 2023, 164, bqad090. [Google Scholar] [CrossRef]
- Niu, X.; Wu, X.; Ying, A.; Shao, B.; Li, X.; Zhang, W.; Lin, C.; Lin, Y. Maternal high fat diet programs hypothalamic-pituitary-adrenal function in adult rat offspring. Psychoneuroendocrinology 2019, 102, 128–138. (In English) [Google Scholar] [CrossRef] [PubMed]
- Glendining, K.A.; Fisher, L.C.; Jasoni, C.L. Maternal high fat diet alters offspring epigenetic regulators, amygdala glutamatergic profile and anxiety. Psychoneuroendocrinology 2018, 96, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, A.; de Vega, W.; Sivanathan, S.; St-Cyr, S.; McGowan, P.O. Maternal high-fat diet alters anxiety behavior and glucocorticoid signaling in adolescent offspring. Neuroscience 2014, 272, 92–101. (In English) [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Simar, D.; Morris, M.J. Hypothalamic neuroendocrine circuitry is programmed by maternal obesity: Interaction with postnatal nutritional environment. PLoS ONE 2009, 4, e6259. (In English) [Google Scholar] [CrossRef]
- Ferezou-Viala, J.; Roy, A.F.; Serougne, C.; Gripois, D.; Parquet, M.; Bailleux, V.; Gertler, A.; Delplanque, B.; Djiane, J.; Riottot, M.; et al. Long-term consequences of maternal high-fat feeding on hypothalamic leptin sensitivity and diet-induced obesity in the offspring. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R1056–R1062. (In English) [Google Scholar] [CrossRef]
- Vogt, M.C.; Paeger, L.; Hess, S.; Steculorum, S.M.; Awazawa, M.; Hampel, B.; Neupert, S.; Nicholls, H.T.; Mauer, J.; Hausen, A.C.; et al. Neonatal insulin action impairs hypothalamic neurocircuit formation in response to maternal high-fat feeding. Cell 2014, 156, 495–509. (In English) [Google Scholar] [CrossRef]
- Cordner, Z.A.; Tamashiro, K.L. Effects of high-fat diet exposure on learning & memory. Physiol. Behav. 2015, 152, 363–371. (In English) [Google Scholar] [CrossRef]
- Kelly, A.C.; Powell, T.L.; Jansson, T. Placental function in maternal obesity. Clin. Sci. 2020, 134, 961–984. (In English) [Google Scholar] [CrossRef]
- Godfrey, K.M. The role of the placenta in fetal programming—A review. Placenta 2002, 23 (Suppl. A), S20–S27. (In English) [Google Scholar] [CrossRef]
- Bellisario, V.; Panetta, P.; Balsevich, G.; Baumann, V.; Noble, J.; Raggi, C.; Nathan, O.; Berry, A.; Seckl, J.; Schmidt, M.; et al. Maternal high-fat diet acts as a stressor increasing maternal glucocorticoids’ signaling to the fetus and disrupting maternal behavior and brain activation in C57BL/6J mice. Psychoneuroendocrinology 2015, 60, 138–150. (In English) [Google Scholar] [CrossRef]
- Gallagher, M.; Nicolle, M.M. Animal models of normal aging: Relationship between cognitive decline and markers in hippocampal circuitry. Behav. Brain Res. 1993, 57, 155–162. (In English) [Google Scholar] [CrossRef] [PubMed]
- Geinisman, Y.; Ganeshina, O.; Yoshida, R.; Berry, R.W.; Disterhoft, J.F.; Gallagher, M. Aging, spatial learning, and total synapse number in the rat CA1 stratum radiatum. Neurobiol. Aging 2004, 25, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, D.J.; Gray, A.; Simon, A.; Taylor, A.M.; Deacon, R.M.; Seeburg, P.H.; Sprengel, R.; Good, M.A.; Rawlins, J.N.; Bannerman, D.M. Deletion of glutamate receptor-A (GluR-A) AMPA receptor subunits impairs one-trial spatial memory. Behav. Neurosci. 2007, 121, 559–569. (In English) [Google Scholar] [CrossRef] [PubMed]
- Morris, R.G.; Garrud, P.; Rawlins, J.N.; O’Keefe, J. Place navigation impaired in rats with hippocampal lesions. Nature 1982, 297, 681–683. (In English) [Google Scholar] [CrossRef]
- DeToledo-Morrell, L.; Geinisman, Y.; Morrell, F. Age-dependent alterations in hippocampal synaptic plasticity: Relation to memory disorders. Neurobiol. Aging 1988, 9, 581–590. (In English) [Google Scholar] [CrossRef]
- Bach, M.E.; Barad, M.; Son, H.; Zhuo, M.; Lu, Y.F.; Shih, R.; Mansuy, I.; Hawkins, R.D.; Kandel, E.R. Age-related defects in spatial memory are correlated with defects in the late phase of hippocampal long-term potentiation in vitro and are attenuated by drugs that enhance the cAMP signaling pathway. Proc. Natl. Acad. Sci. USA 1999, 96, 5280–5285. (In English) [Google Scholar] [CrossRef]
- Leuner, B.; Gould, E. Structural Plasticity and Hippocampal Function. Annu. Rev. Psychol. 2010, 61, 111–140. [Google Scholar] [CrossRef]
- Nyberg, L.; Lövdén, M.; Riklund, K.; Lindenberger, U.; Bäckman, L. Memory aging and brain maintenance. Trends Cogn. Sci. 2012, 16, 292–305. (In English) [Google Scholar] [CrossRef]
- Nithianantharajah, J.; Hannan, A.J. The neurobiology of brain and cognitive reserve: Mental and physical activity as modulators of brain disorders. Progress. Neurobiol. 2009, 89, 369–382. [Google Scholar] [CrossRef]
- Lindsay, K.L.; Buss, C.; Wadhwa, P.D.; Entringer, S. The interplay between maternal nutrition and stress during pregnancy: Issues and considerations. Ann. Nutr. Metab. 2017, 70, 191–200. [Google Scholar] [CrossRef]
- Lépinay, A.L.; Larrieu, T.; Joffre, C.; Acar, N.; Gárate, I.; Castanon, N.; Ferreira, G.; Langelier, B.; Guesnet, P.; Brétillon, L.; et al. Perinatal high-fat diet increases hippocampal vulnerability to the adverse effects of subsequent high-fat feeding. Psychoneuroendocrinology 2015, 53, 82–93. (In English) [Google Scholar] [CrossRef] [PubMed]
- Berk, M.; Malhi, G.S.; Gray, L.J.; Dean, O.M. The promise of N-acetylcysteine in neuropsychiatry. Trends Pharmacol. Sci. 2013, 34, 167–177. [Google Scholar] [CrossRef] [PubMed]
- McKee, S.E.; Reyes, T.M. Effect of supplementation with methyl-donor nutrients on neurodevelopment and cognition: Considerations for future research. Nutr. Rev. 2018, 76, 497–511. [Google Scholar] [CrossRef] [PubMed]
- Sarker, G.; Litwan, K.; Kastli, R.; Peleg-Raibstein, D. Maternal overnutrition during critical developmental periods leads to different health adversities in the offspring: Relevance of obesity, addiction and schizophrenia. Sci. Rep. 2019, 9, 17322. (In English) [Google Scholar] [CrossRef]
- Morris, R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 1984, 11, 47–60. [Google Scholar] [CrossRef]
- Vorhees, C.V.; Williams, M.T. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006, 1, 848–858. (In English) [Google Scholar] [CrossRef]
- D’Hooge, R.; De Deyn, P.P. Applications of the Morris water maze in the study of learning and memory. Brain Research. Brain Res. Rev. 2001, 36, 60–90. (In English) [Google Scholar] [CrossRef]
- Bizon, J.L.; Foster, T.C.; Alexander, G.E.; Glisky, E.L. Characterizing cognitive aging of working memory and executive function in animal models. Front. Aging Neurosci. 2012, 4, 19. (In English) [Google Scholar] [CrossRef]
- Foster, T.C. Dissecting the age-related decline on spatial learning and memory tasks in rodent models: N-methyl-D-aspartate receptors and voltage-dependent Ca2+ channels in senescent synaptic plasticity. Prog. Neurobiol. 2012, 96, 283–303. (In English) [Google Scholar] [CrossRef]
- Davis, S.; Butcher, S.P.; Morris, R.G. The NMDA receptor antagonist D-2-amino-5-phosphonopentanoate (D-AP5) impairs spatial learning and LTP in vivo at intracerebral concentrations comparable to those that block LTP in vitro. J. Neurosci. 1992, 12, 21–34. (In English) [Google Scholar] [CrossRef]
- McEwen, B.S. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiol. Rev. 2007, 87, 873–904. (In English) [Google Scholar] [CrossRef] [PubMed]
- Lupien, S.J.; McEwen, B.S.; Gunnar, M.R.; Heim, C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 2009, 10, 434–445. (In English) [Google Scholar] [CrossRef] [PubMed]
- Naef, L.; Moquin, L.; Dal Bo, G.; Giros, B.; Gratton, A.; Walker, C.D. Maternal high-fat intake alters presynaptic regulation of dopamine in the nucleus accumbens and increases motivation for fat rewards in the offspring. Neuroscience 2011, 176, 225–236. (In English) [Google Scholar] [CrossRef]
- Rivera, H.M.; Kievit, P.; Kirigiti, M.A.; Bauman, L.A.; Baquero, K.; Blundell, P.; Dean, T.A.; Valleau, J.C.; Takahashi, D.L.; Frazee, T.; et al. Maternal high-fat diet and obesity impact palatable food intake and dopamine signaling in nonhuman primate offspring. Obesity 2015, 23, 2157–2164. (In English) [Google Scholar] [CrossRef]
- Guillaumin, M.C.; Peleg-Raibstein, D.J.N. Maternal over-and malnutrition and increased risk for addictive and eating disorders in the offspring. Nutrients 2023, 15, 1095. [Google Scholar] [CrossRef]
- Sarker, G.; Berrens, R.; von Arx, J.; Pelczar, P.; Reik, W.; Wolfrum, C.; Peleg-Raibstein, D. Transgenerational transmission of hedonic behaviors and metabolic phenotypes induced by maternal overnutrition. Transl. Psychiatry 2018, 8, 195. [Google Scholar] [CrossRef]
- Vucetic, Z.; Kimmel, J.; Totoki, K.; Hollenbeck, E.; Reyes, T.M. Maternal high-fat diet alters methylation and gene expression of dopamine and opioid-related genes. Endocrinology 2010, 151, 4756–4764. (In English) [Google Scholar] [CrossRef]
- Ong, Z.Y.; Muhlhausler, B.S. Maternal “junk-food” feeding of rat dams alters food choices and development of the mesolimbic reward pathway in the offspring. FASEB 2011, 25, 2167–2179. (In English) [Google Scholar] [CrossRef]
- Teegarden, S.L.; Nestler, E.J.; Bale, T.L. Delta FosB-mediated alterations in dopamine signaling are normalized by a palatable high-fat diet. Biol. Psychiatry 2008, 64, 941–950. (In English) [Google Scholar] [CrossRef]
- Reichelt, A.C.; Rank, M.M. The impact of junk foods on the adolescent brain. Birth Defects Res. 2017, 109, 1649–1658. (In English) [Google Scholar] [CrossRef]
- Stice, E.; Spoor, S.; Bohon, C.; Veldhuizen, M.G.; Small, D.M. Relation of reward from food intake and anticipated food intake to obesity: A functional magnetic resonance imaging study. J. Abnorm. Psychol. 2008, 117, 924–935. (In English) [Google Scholar] [CrossRef] [PubMed]
- Patterson, Z.R.; Abizaid, A.B. Stress induced obesity: Lessons from rodent models of stress. Front. Neurosci. 2013, 7, 130. [Google Scholar] [CrossRef] [PubMed]
- Sarker, G.; Sun, W.; Rosenkranz, D.; Pelczar, P.; Opitz, L.; Efthymiou, V.; Wolfrum, C.; Peleg-Raibstein, D. Maternal overnutrition programs hedonic and metabolic phenotypes across generations through sperm tsRNAs. Proc. Natl. Acad. Sci. USA 2019, 116, 10547–10556. (In English) [Google Scholar] [CrossRef] [PubMed]
- Cirulli, F.; Musillo, C.; Berry, A. Maternal Obesity as a Risk Factor for Brain Development and Mental Health in the Offspring. Neuroscience 2020, 447, 122–135. [Google Scholar] [CrossRef]
- Diamond, A.; Lee, K. Interventions shown to aid executive function development in children 4 to 12 years old. Science 2011, 333, 959–964. (In English) [Google Scholar] [CrossRef]
- Diamond, A.; Ling, D.S. 143Review of the Evidence on, and Fundamental Questions About, Efforts to Improve Executive Functions, Including Working Memory. In Cognitive and Working Memory Training: Perspectives from Psychology, Neuroscience, and Human Development; Novick, J.M., Bunting, M.F., Dougherty, M.R., Engle, R.W., Eds.; Oxford University Press: Oxford, UK, 2019. [Google Scholar]
- Howard, S.L.; Beaudin, S.A.; Strupp, B.J.; Smith, D.R. Maternal choline supplementation lessens the behavioral dysfunction produced by developmental manganese exposure in a rodent model of ADHD. Neurotoxicol. Teratol. 2024, 102, 107337. [Google Scholar] [CrossRef]
- Glenn, M.J.; Gibson, E.M.; Kirby, E.D.; Mellott, T.J.; Blusztajn, J.K.; Williams, C.L. Prenatal choline availability modulates hippocampal neurogenesis and neurogenic responses to enriching experiences in adult female rats. Eur. J. Neurosci. 2007, 25, 2473–2482. (In English) [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kandasamey, P.; Peleg-Raibstein, D. Programming the Brain: How Maternal Overnutrition Shapes Cognitive Aging in Offspring. Nutrients 2025, 17, 988. https://doi.org/10.3390/nu17060988
Kandasamey P, Peleg-Raibstein D. Programming the Brain: How Maternal Overnutrition Shapes Cognitive Aging in Offspring. Nutrients. 2025; 17(6):988. https://doi.org/10.3390/nu17060988
Chicago/Turabian StyleKandasamey, Pratheba, and Daria Peleg-Raibstein. 2025. "Programming the Brain: How Maternal Overnutrition Shapes Cognitive Aging in Offspring" Nutrients 17, no. 6: 988. https://doi.org/10.3390/nu17060988
APA StyleKandasamey, P., & Peleg-Raibstein, D. (2025). Programming the Brain: How Maternal Overnutrition Shapes Cognitive Aging in Offspring. Nutrients, 17(6), 988. https://doi.org/10.3390/nu17060988





