Alterations of Exercise-Induced Carbohydrate and Fat Oxidation by Anthocyanin-Rich New Zealand Blackcurrant Are Associated with the Pre-Intervention Metabolic Function: A Secondary Analysis of Randomized Crossover Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Statistical Analysis
3. Results
3.1. Exercise-Induced Respiratory Exchange Ratio Relationships for Females and Males
3.2. Exercise-Induced Respiratory Exchange Ratio Relationships in the Placebo/Control Condition and Changes in Exercise-Induced Fat Oxidation with the Intake of New Zealand Blackcurrants in Females and Males
3.3. Exercise-Induced Respiratory Exchange Ratio Relationships in the Placebo/Control Condition and Changes in Exercise-Induced Carbohydrate Oxidation with the Intake of New Zealand Blackcurrant in Females and Males
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MET | Metabolic equivalent; |
| NZBC | New Zealand blackcurrant; |
| RER | Respiratory exchange ratio. |
References
- Chasiotis, D.; Bergstrom, M.; Hultman, E. ATP utilization and force during intermittent and continuous muscle contractions. J. Appl. Physiol. 1987, 63, 167–174. [Google Scholar] [CrossRef]
- Jones, D.A.; Turner, D.L.; McIntyre, D.B.; Newham, D.J. Energy turnover in relation to slowing of contractile properties during fatiguing contractions of the human anterior tibialis muscle. J. Physiol. 2009, 587, 4329–4338. [Google Scholar] [CrossRef]
- Rui, H.; Das, A.; Nakamoto, R.; Roux, B. Proton countertransport and coupled gating in the sarcoplasmic reticulum calcium pump. J. Mol. Biol. 2018, 430, 5050–5065. [Google Scholar] [CrossRef]
- Hargreaves, M.; Spriet, L.L. Skeletal muscle energy metabolism during exercise. Nat. Metabol. 2020, 2, 817–828. [Google Scholar] [CrossRef]
- Muscella, A.; Stefàno, E.; Lunetti, P.; Capobianco, L.; Marsigliante, S. The regulation of fat metabolism during aerobic exercise. Biomolecules 2020, 10, 1699. [Google Scholar] [CrossRef]
- Cermak, N.M.; van Loon, L.J. The use of carbohydrates during exercise as an ergogenic aid. Sports Med. 2013, 43, 1139–1155. [Google Scholar] [CrossRef]
- Matarese, L.E. Indirect calorimetry: Technical aspects. J. Am. Diet Assoc. 1997, 97, S154–S160. [Google Scholar] [CrossRef]
- Péronnet, F.; Massicotte, D. Table of nonprotein respiratory quotient: An update. Can. J. Sport Sci. 1991, 16, 23–29. [Google Scholar]
- Goedecke, J.H.; Gibson, A.S.C.; Grobler, L.; Collins, M.; Noakes, T.D.; Lambert, E.V. Determinants of the variability in respiratory exchange ratio at rest and during exercise in trained athletes. Am. J. Physiol. Endocrinol. Metabol. 2000, 279, E1325–E1334. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, J.A.; Kilding, A.E.; Stewart, T.; Plews, D.J. Factors influencing substrate oxidation during submaximal cycling: A modelling analysis. Sports Med. 2022, 52, 2775–2795. [Google Scholar] [CrossRef]
- Carter, S.L.; Rennie, C.; Tarnopolsky, M.A. Substrate utilization during endurance exercise in men and women after endurance training. Am. J. Physiol. Endocrinol. Metabol. 2001, 280, E898–E907. [Google Scholar] [CrossRef]
- Astorino, T.A.; Schubert, M.M. Changes in fat oxidation in response to various regimes of high intensity interval training (HIIT). Eur. J. Appl. Physiol. 2018, 118, 51–63. [Google Scholar] [CrossRef]
- Hawkins, K.R.; Hansen, K.C.; Schoeller, D.A.; Cooper, J.A. Effect of exercise on the diurnal variation in energy substrate use during a high-fat diet. Eur. J. Appl. Physiol. 2012, 112, 3775–3785. [Google Scholar] [CrossRef]
- Schrauwen, P.; van Marken Lichtenbelt, W.D.; Saris, W.H.; Westerterp, K.R. Changes in fat oxidation in response to a high-fat diet. Am. J. Clin. Nutr. 1997, 66, 276–282. [Google Scholar] [CrossRef]
- Conger, S.A.; Tuthill, L.M.; Millard-Stafford, M.L. Does caffeine increase fat metabolism? A systematic review and meta-analysis. Int. J. Sport Nutr. Exerc. Metabol. 2022, 33, 112–120. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, Z.; Wang, X.; Wang, G.; Wang, Y.; Tang, K.; Gao, B. Influence of chronic nitrate-rich beetroot juice supplementation on the endurance performance of active winter triathletes: A randomized controlled trial. J. Am. Nutr. Assoc. 2023, 42, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Lyall, K.A.; Hurst, S.M.; Cooney, J.; Jensen, D.; Lo, K.; Hurst, R.D.; Stevenson, L.M. Short-term blackcurrant extract consumption modulates exercise-induced oxidative stress and lipopolysaccharide-stimulated inflammatory responses. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, R70–R81. [Google Scholar] [CrossRef]
- Šimerdová, B.; Bobríková, M.; Lhotská, I.; Kaplan, J.; Křenová, A.; Šatínský, D. Evaluation of anthocyanin profiles in various blackcurrant cultivars over a three-year period using a fast HPLC-DAD method. Foods 2021, 10, 1745. [Google Scholar] [CrossRef]
- Rodriguez-Mateos, A.; Rendeiro, C.; Bergillos-Meca, T.; Tabatabaee, S.; George, T.W.; Heiss, C.; Spencer, J.P. Intake and time dependence of blueberry flavonoid–induced improvements in vascular function: A randomized, controlled, double-blind, crossover intervention study with mechanistic insights into biological activity. Am. J. Clin. Nutr. 2013, 98, 1179–1191. [Google Scholar] [CrossRef]
- Cook, M.D.; Myers, S.D.; Blacker, S.D.; Willems, M.E.T. New Zealand blackcurrant extract improves cycling performance and fat oxidation in cyclists. Eur. J. Appl. Physiol. 2015, 115, 2357–2365. [Google Scholar] [CrossRef]
- Cook, M.D.; Myers, S.D.; Gault, M.L.; Edwards, V.C.; Willems, M.E.T. Dose effects of New Zealand blackcurrant on substrate oxidation and physiological responses during prolonged cycling. Eur. J. Appl. Physiol. 2017, 117, 1207–1216. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.D.; Shan, Y.; Willems, M.E.T. Effects of New Zealand Black Currant Extract on Exercising Substrate Utilization and Postexercise Blood Pressure in Men and Women. Int. J. Sport Nutr. Exerc. Metab. 2025, 35, 150–161. [Google Scholar] [CrossRef]
- Şahin, M.A.; Bilgiç, P.; Montanari, S.; Willems, M.E.T. Intake duration of anthocyanin-rich New Zealand blackcurrant extract affects metabolic responses during moderate intensity walking exercise in adult males. J. Diet. Suppl. 2021, 18, 406–417. [Google Scholar] [CrossRef]
- Strauss, J.A.; Willems, M.E.T.; Shepherd, S.O. New Zealand blackcurrant extract enhances fat oxidation during prolonged cycling in endurance-trained females. Eur. J. Appl. Physiol. 2018, 118, 1265–1272. [Google Scholar] [CrossRef]
- Hiles, A.M.; Flood, T.R.; Lee, B.J.; Wheeler, L.E.; Costello, R.; Walker, E.F.; Ashdown, K.M.; Kuennen, M.R.; Willems, M.E.T. Dietary supplementation with New Zealand blackcurrant extract enhances fat oxidation during submaximal exercise in the heat. J. Sci. Med. Sport 2020, 23, 908–912. [Google Scholar] [CrossRef]
- Willems, M.E.T.; Banic, M.; Cadden, R.; Barnett, L. Enhanced walking-induced fat oxidation by New Zealand blackcurrant extract is body composition-dependent in recreationally active adult females. Nutrients 2022, 14, 1475. [Google Scholar] [CrossRef]
- Willems, M.E.T.; Briggs, A.R. Running-induced metabolic and physiological responses using New Zealand blackcurrant extract in a male ultra-endurance runner: A case study. J. Funct. Morphol. Kinesiol. 2022, 7, 104. [Google Scholar] [CrossRef]
- Willems, M.E.T.; Bray, P.W.; Bassett, H.M.; Spurr, T.J.; West, A.T. Effects of CurraNZ, a New Zealand blackcurrant extract during 1 hour of treadmill running in female and male Marathon des Sables Athletes in hot conditions: Two case studies. J. Funct. Morphol. Kinesiol. 2024, 9, 76. [Google Scholar] [CrossRef]
- Willems, M.E.T.; Spurr, T.J.; Lacey, J.; Briggs, A.R. Beneficial Physiological and Metabolic Effects with Acute Intake of New Zealand Blackcurrant Extract during 4 h of Indoor Cycling in a Male Ironman Athlete: A Case Study. J. Funct. Morphol. Kinesiol. 2024, 9, 141. [Google Scholar] [CrossRef]
- Cano, A.; Ventura, L.; Martinez, G.; Cugusi, L.; Caria, M.; Deriu, F.; Manca, A. Analysis of sex-based differences in energy substrate utilization during moderate-intensity aerobic exercise. Eur. J. Appl. Physiol. 2022, 122, 29–70. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.D.; Myers, S.D.; Gault, M.L.; Willems, M.E.T. Blackcurrant alters physiological responses and femoral artery diameter during sustained isometric contraction. Nutrients 2017, 9, 556. [Google Scholar] [CrossRef]
- Pilolla, K.D.; Armendariz, J.; Burrus, B.M.; Baston, D.S.; McCarthy, K.A.; Bloedon, T.K. Effects of wild blueberries on fat oxidation rates in aerobically trained males. Nutrients 2023, 15, 1339. [Google Scholar] [CrossRef]
- Teets, C.; Ghanem, N.; Ma, G.; Minj, J.; Perkins-Veazie, P.; Johnson, S.A.; Etter, A.J.; Carbonero, F.G.; Solverson, P.M. A One-Week Elderberry Juice Intervention Augments the Fecal Microbiota and Suggests Improvement in Glucose Tolerance and Fat Oxidation in a Randomized Controlled Trial. Nutrients 2024, 16, 3555. [Google Scholar] [CrossRef]
- Jardon, K.M.; Goossens, G.H.; Most, J.; Galazzo, G.; Venema, K.; Penders, J.; Blaak, E.E. Examination of sex-specific interactions between gut microbiota and host metabolism after 12-week combined polyphenol supplementation in individuals with overweight or obesity. Gut Microbes 2024, 16, 2392875. [Google Scholar] [CrossRef] [PubMed]
- Most, J.; Timmers, S.; Warnke, I.; Jocken, J.W.; van Boekschoten, M.; de Groot, P.; Bendik, I.; Schrauwen, P.; Goossens, G.H.; Blaak, E.E. Combined epigallocatechin-3-gallate and resveratrol supplementation for 12 wk increases mitochondrial capacity and fat oxidation, but not insulin sensitivity, in obese humans: A randomized controlled trial. Am. J. Clin. Nutr. 2016, 104, 215–227. [Google Scholar] [CrossRef]
- Kenig, S.; Kramberger, K.; Petelin, A.; Bandelj, D.; Baruca Arbeiter, A.; Miklavčič Višnjevec, A.; Peeters, K.; Mohorko, N.; Šik Novak, K.; Jenko Pražnikar, Z. Helichrysum italicum ssp. italicum infusion promotes fat oxidation in hepatocytes and stimulates energy expenditure and fat oxidation after acute ingestion in humans: A pilot study. Plants 2021, 10, 1516. [Google Scholar] [CrossRef]
- Zhang, S.; Takano, J.; Murayama, N.; Tominaga, M.; Abe, T.; Park, I.; Seol, J.; Ishihara, A.; Tanaka, Y.; Yajima, K.; et al. Subacute ingestion of caffeine and oolong tea increases fat oxidation without affecting energy expenditure and sleep architecture: A randomized, placebo-controlled, double-blinded cross-over trial. Nutrients 2020, 12, 3671. [Google Scholar] [CrossRef]
- Nieman, D.C.; Simonson, A.; Sakaguchi, C.A.; Sha, W.; Blevins, T.; Hattabaugh, J.; Kohlmeier, M. Acute ingestion of a mixed flavonoid and caffeine supplement increases energy expenditure and fat oxidation in adult women: A randomized, crossover clinical trial. Nutrients 2019, 11, 2665. [Google Scholar] [CrossRef]
- Solverson, P.M.; Rumpler, W.V.; Leger, J.L.; Redan, B.W.; Ferruzzi, M.G.; Baer, D.J.; Castonguay, T.W.; Novotny, J.A. Blackberry feeding increases fat oxidation and improves insulin sensitivity in overweight and obese males. Nutrients 2018, 10, 1048. [Google Scholar] [CrossRef]
- Park, I.; Ochiai, R.; Ogata, H.; Kayaba, M.; Hari, S.; Hibi, M.; Katsuragi, Y.; Satoh, M.; Tokuyama, K. Effects of subacute ingestion of chlorogenic acids on sleep architecture and energy metabolism through activity of the autonomic nervous system: A randomised, placebo-controlled, double-blinded cross-over trial. Br. J. Nutr. 2017, 117, 979–984. [Google Scholar] [CrossRef]
- Roberts, J.D.; Roberts, M.G.; Tarpey, M.D.; Weekes, J.C.; Thomas, C.H. The effect of a decaffeinated green tea extract formula on fat oxidation, body composition and exercise performance. J. Int. Soc. Sports Nutr. 2015, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Mori, N.; Nakanishi, S.; Shiomi, S.; Kiyokawa, S.; Kakimoto, S.; Nakagawa, K.; Hosoe, K.; Minami, K.; Nadamoto, T. Enhancement of fat oxidation by licorice flavonoid oil in healthy humans during light exercise. J. Nutr. Sci. Vitaminol. 2015, 61, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, A.B.; Randell, R.K.; Jeukendrup, A.E. The effect of green tea extract on fat oxidation at rest and during exercise: Evidence of efficacy and proposed mechanisms. Adv. Nutr. 2013, 4, 129–140. [Google Scholar] [CrossRef]
- Venables, M.C.; Hulston, C.J.; Cox, H.R.; Jeukendrup, A.E. Green tea extract ingestion, fat oxidation, and glucose tolerance in healthy humans. Am. J. Clin. Nutr. 2008, 87, 778–784. [Google Scholar] [CrossRef]
- Dulloo, A.G.; Duret, C.; Rohrer, D.; Girardier, L.; Mensi, N.; Fathi, M.; Chantre, P.; Vandermander, J. Efficacy of a green tea extract rich in catechin polyphenols and caffeine in increasing 24-h energy expenditure and fat oxidation in humans. Am. J. Clin. Nutr. 1999, 70, 1040–1045. [Google Scholar] [CrossRef]
- Willems, M.E.T.; Fry, H.L.; Belding, M.A.; Kaviani, M. Three weeks daily intake of Matcha green tea powder affects substrate oxidation during moderate-intensity exercise in females. J. Diet. Suppl. 2021, 18, 566–576. [Google Scholar] [CrossRef]
- Willems, M.E.T.; Şahin, M.A.; Cook, M.D. Matcha green tea drinks enhance fat oxidation during brisk walking in females. Int. J. Sport Nutr. Exerc. Metabol. 2018, 28, 536–541. [Google Scholar] [CrossRef]
- Froio de Araujo Dias, G.; da Eira Silva, V.; de Salles Painelli, V.; Sale, C.; Giannini Artioli, G.; Gualano, B.; Saunders, B. (In) consistencies in responses to sodium bicarbonate supplementation: A randomised, repeated measures, counterbalanced and double-blind study. PLoS ONE 2015, 10, e0143086. [Google Scholar]
- Perkins, I.C.; Blacker, S.D.; Willems, M.E.T. Individual responses to repeated dosing with anthocyanin-rich New Zealand blackcurrant extract during high-intensity intermittent treadmill running in active males. Nutrients 2024, 16, 4253. [Google Scholar] [CrossRef]
- Shaw, C.S.; Swinton, C.; Morales-Scholz, M.G.; McRae, N.; Erftemeyer, T.; Aldous, A.; Murphy, R.M.; Howlett, K.F. Impact of exercise training status on the fiber type-specific abundance of proteins regulating intramuscular lipid metabolism. J. Appl. Physiol. 2020, 128, 379–389. [Google Scholar] [CrossRef]
- Schmitt, B.; Fluck, M.; Décombaz, J.; Kreis, R.; Boesch, C.; Wittwer, M.; Graber, F.; Vogt, M.; Howald, H.; Hoppeler, H. Transcriptional adaptations of lipid metabolism in tibialis anterior muscle of endurance-trained athletes. Physiol. Genom. 2003, 15, 148–157. [Google Scholar] [CrossRef] [PubMed]
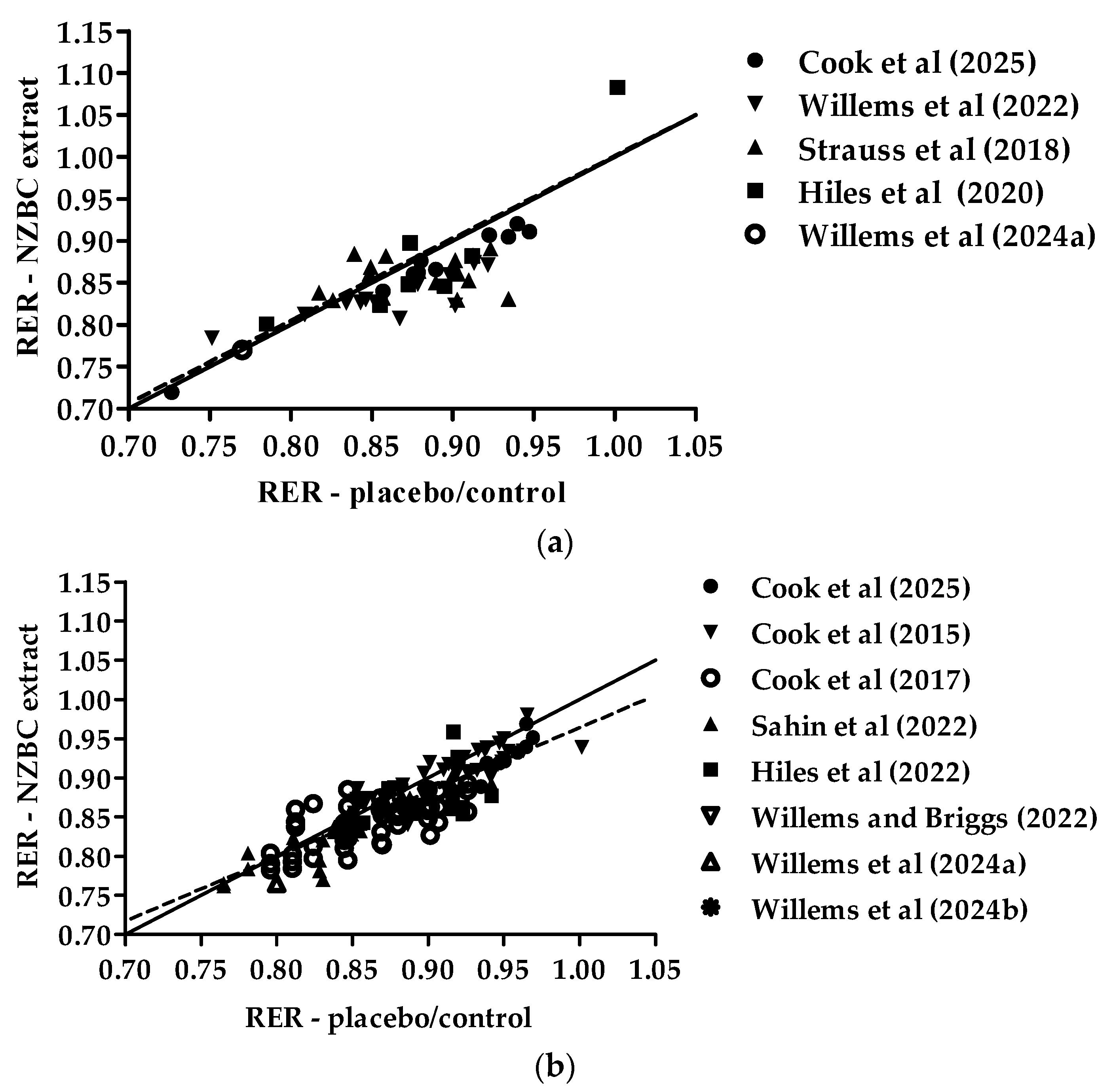
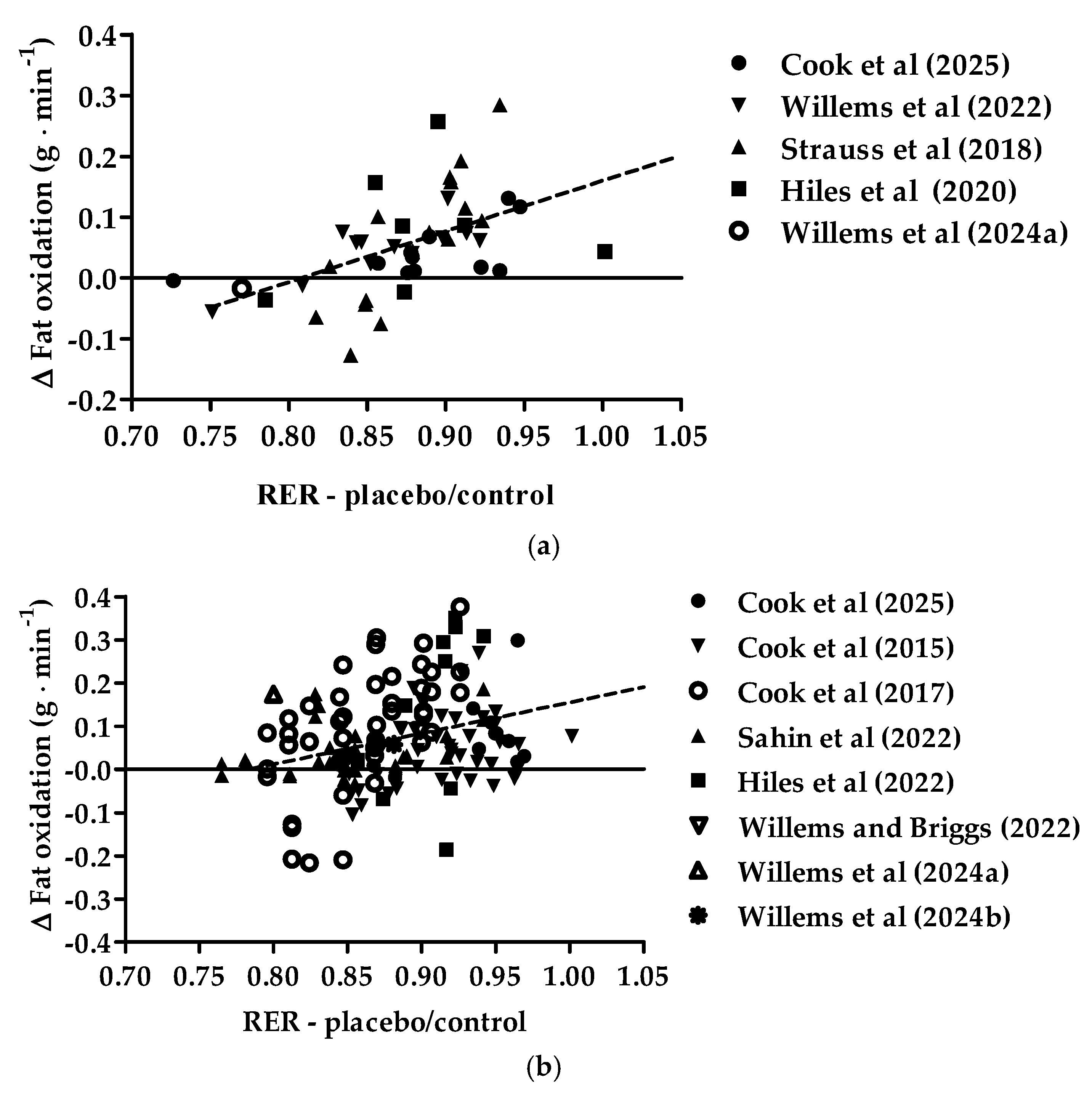
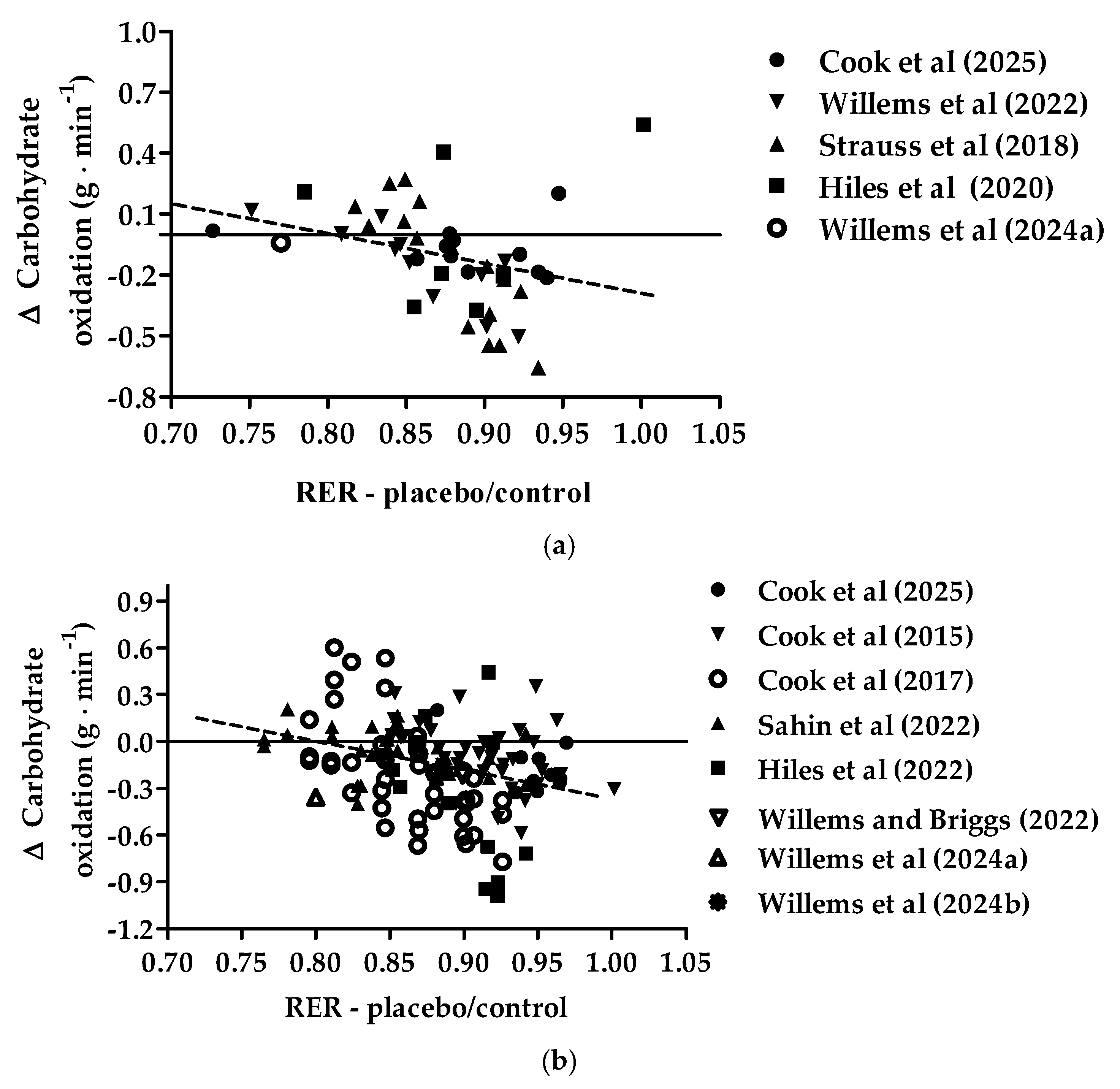
| Source | Participant(s) | Design | Dosing Strategy | Exercise Task | RER Outcome |
|---|---|---|---|---|---|
| Cook et al. [20] | 14 males, cycling 8–10 h per week, age: 38 ± 13 years | double blind, placebo-controlled, randomized, cross-over | 105 mg NZBC anthocyanins in extract form for 7 days | O2max | 45%: ↓0.01 (p = 0.006) 55%: ↓0.02 (p = 0.102) 65%: ↓0.01 (p = 0.043) |
| Cook et al. [21] | 15 males, cycling 6–10 h per week, age: 38 ± 15 years | randomized, counterbalanced, latin-square | control (no dose), 105, 210, and 315 mg NZBC anthocyanins in extract form for 7 days | O2max | 210 mg: ↓0.03 (p < 0.05) 315 mg: ↓0.02 (p < 0.05) |
| Cook et al. [22] | recreationally active, 11 females, age 27 ± 7 years; 11 males, age: 32 ± 9 years | double blind, placebo-controlled, randomized, cross-over | 210 mg NZBC anthocyanins in extract form for 7 days | 60 min of treadmill walking at 50% O2max | males: ↓0.02 (p < 0.05) females: ↓0.02 (p < 0.05) |
| Willems et al. [26] | recreationally active, 12 females, age 21 ± 2 years | double blind, placebo-controlled, randomized, cross-over | 210 mg NZBC anthocyanins in extract form for 7 days | 30 min of treadmill walking at moderate intensity (4.7 ± 0.4 MET) | ↓0.03 (p = 0.009) |
| Strauss et al. [24] | endurance trained, 16 females, age: 28 ± 8 years | double blind, placebo-controlled, randomized, cross-over | 210 mg NZBC anthocyanins in extract form for 7 days | O2max | ↓0.02 (p = 0.063) |
| Şahin et al. [23] | 16 males, age 24 ± 6 years | randomized, cross-over | control (no dose) and 210 mg NZBC anthocyanins in extract form for 7 and 14 days | 30 min of treadmill walking at moderate-intensity (n = 3: 4-MET, n = 13: 5-MET) | 7 days: ↓0.009 (p = 0.122) 14 days: ↓0.016 (p = 0.004) |
| Hiles et al. [25] | recreationally active; 6 females, 12 males: age 27 ± 6 years | double blind, placebo-controlled, randomized, cross-over | 210 mg NZBC anthocyanins in extract form for 7 days | 60 min of fasted running at 65% O2max (34 °C and 40% relative humidity) | ↓0.02 (p = 0.04) |
| Willems and Briggs [27] | male amateur ultra-endurance runner, age: 40 years | allocated, cross-over | 210 mg NZBC anthocyanins in extract form for 7 days | O2max (26 °C and relative humidity of 70%) | ↓0.02 (p < 0.01) |
| Willems et al. [28] | female (age: 23 years) and male (age: 38 years) amateur Marathon des Sables athletes | randomized, cross-over | control (no dose) and 210 mg NZBC anthocyanins in extract form for 7 days | O2max (34 °C and relative humidity of 30%) | male: ↓0.03 (p = 0.04) female: no change |
| Willems et al. [29] | male amateur Ironman athlete | single blind, placebo-controlled, randomized, cross-over | acute intake of 420 mg NZBC anthocyanins in extract form | 240 min of indoor cycling at 165 Watts | ↓0.02 (p = 0.042) |
| Source | Fat Oxidation | Carbohydrate Oxidation |
|---|---|---|
| Cook et al. [20] | 1.695 × CO2 − 1.701 × CO2 | Low intensity: 4.344 × CO2 − 3.061 × O2 |
| Moderate intensity: 4.210 × CO2 − 2.962 × O2 | ||
| Cook et al. [21], Willems et al. [26], Strauss et al. [24], Şahin et al. [23], Hiles et al. [25], Willems and Briggs [27], Willems et al. [28] | O2 − 1.701 × CO2 | CO2 − 2.962 × O2 |
| Cook et al. [22] | O2 − 1.701 × CO2 | CO2 − 3.061 × O2 |
| Willems et al. [29] | O2 − 1.67 × CO2 | CO2 − 3.21 × O2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Willems, M.E.T.; Cook, M.D. Alterations of Exercise-Induced Carbohydrate and Fat Oxidation by Anthocyanin-Rich New Zealand Blackcurrant Are Associated with the Pre-Intervention Metabolic Function: A Secondary Analysis of Randomized Crossover Trials. Nutrients 2025, 17, 997. https://doi.org/10.3390/nu17060997
Willems MET, Cook MD. Alterations of Exercise-Induced Carbohydrate and Fat Oxidation by Anthocyanin-Rich New Zealand Blackcurrant Are Associated with the Pre-Intervention Metabolic Function: A Secondary Analysis of Randomized Crossover Trials. Nutrients. 2025; 17(6):997. https://doi.org/10.3390/nu17060997
Chicago/Turabian StyleWillems, Mark E. T., and Matthew D. Cook. 2025. "Alterations of Exercise-Induced Carbohydrate and Fat Oxidation by Anthocyanin-Rich New Zealand Blackcurrant Are Associated with the Pre-Intervention Metabolic Function: A Secondary Analysis of Randomized Crossover Trials" Nutrients 17, no. 6: 997. https://doi.org/10.3390/nu17060997
APA StyleWillems, M. E. T., & Cook, M. D. (2025). Alterations of Exercise-Induced Carbohydrate and Fat Oxidation by Anthocyanin-Rich New Zealand Blackcurrant Are Associated with the Pre-Intervention Metabolic Function: A Secondary Analysis of Randomized Crossover Trials. Nutrients, 17(6), 997. https://doi.org/10.3390/nu17060997








