Diabetes Therapeutics of Prebiotic Soluble Dietary Fibre and Antioxidant Anthocyanin Supplement in Patients with Type 2 Diabetes: Randomised Placebo-Controlled Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.2.1. Sample Size Calculation
2.2.2. Inclusion and Exclusion Criteria
2.2.3. Randomisation, Allocation, and Blinding
2.2.4. Supplements Safety, Storage, and Preparation
Safety Testing
Preparation, Content, and Storage
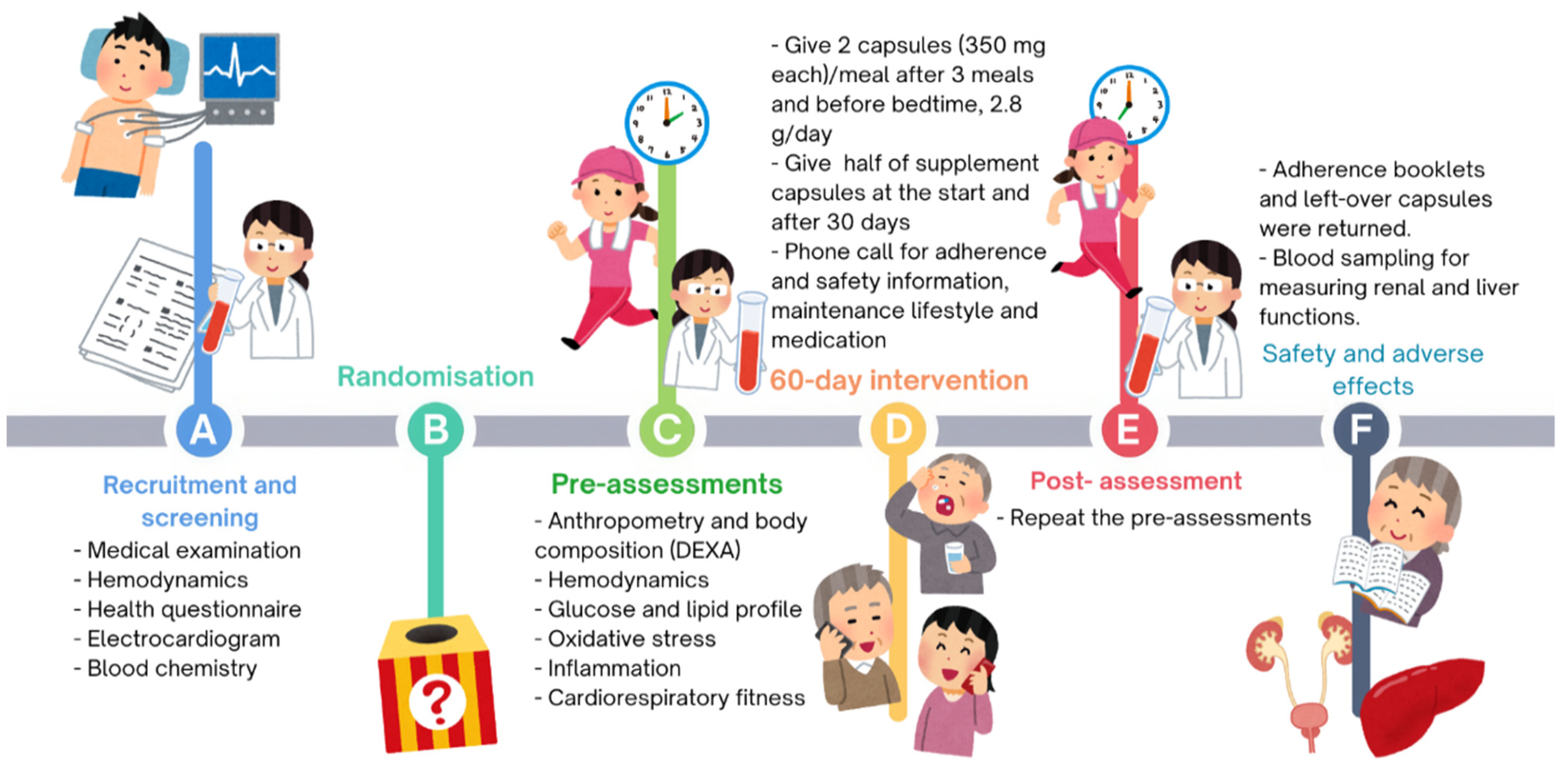
2.3. Experimental Procedures
2.3.1. Pre-Intervention Assessments
Anthropometry and Body Composition Measurements
Heart Rate and Blood Pressure Measurements
Supplementation Protocol
Blood Sampling and Analysis Protocol
- -
- Blood Sampling
- -
- Analysis of Blood Glucose and Lipid Profile
- -
- Analysis of Markers of Oxidative Stress and Inflammation
- -
- Analysis of Markers of Renal and Liver Functions
Cardiorespiratory Fitness Assessment
2.3.2. 60-Day Intervention
2.3.3. Post-Intervention Assessments
2.4. Data Analysis and Statistics
3. Results
3.1. Diabetes Biomarkers
3.1.1. Glucose and Lipid Profiles
3.1.2. Oxidative Stress and Inflammation Blood Biomarkers
3.1.3. Cardiorespiratory Fitness
3.1.4. Adverse Effects
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| T2DM | Type 2 Diabetes Mellitus |
| SG | Supplement group |
| CG | Control group |
| FPG | Fasting plasma glucose |
| HbA1c | Glycated haemoglobin A1c |
| LDL-C | Low-density lipoprotein cholesterol |
| TG | Triglycerides |
| HOMA-IR | Homeostasis model assessment-estimated insulin resistance |
| TC | Total cholesterol |
| HDL-C | High-density lipoprotein cholesterol |
| MDA | Malondialdehyde |
| hsCRP | High sensitivity C-reactive protein |
| WBC | White blood cell |
| 6MWT | 6-minute walk test |
| 6MWD | 6-minute walk distance |
| Cr | Creatinine |
| eGFR | Estimated glomerular filtration rate |
| SGPT | Serum glutamic-pyruvic transaminase |
| SBP | Systolic blood pressure |
| DBP | Diastolic blood pressure |
| MET | Metabolic equivalent of task |
| VO2peak | Peak oxygen uptake |
References
- International Diabetes Federation. IDF Diabetes Atlas-9th Edition. Diabetes Atlas [Serial Online]. 2019. Available online: https://diabetesatlas.org/upload/resources/material/20200302_133351_IDFATLAS9e-final-web.pdf (accessed on 12 June 2023).
- Einarson, T.R.; Acs, A.; Ludwig, C.; Panton, U.H. Prevalence of cardiovascular disease in type 2 diabetes: A systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc. Diabetol. 2018, 17, 83. [Google Scholar] [CrossRef]
- Alkhatib, A. Optimizing Lifestyle Behaviors in Preventing Multiple Long-Term Conditions. Encyclopedia 2023, 3, 468–477. [Google Scholar] [CrossRef]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar]
- Tuomilehto, J.; Lindström, J.; Eriksson, J.G.; Valle, T.T.; Hämäläinen, H.; Ilanne-Parikka, P.; Keinänen-Kiukaanniemi, S.; Laakso, M.; Louheranta, A.; Rastas, M.; et al. Prevention of Type 2 Diabetes Mellitus by Changes in Lifestyle among Subjects with Impaired Glucose Tolerance. N. Engl. J. Med. 2001, 344, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Alkhatib, A. Personalising Exercise and Nutrition Behaviours in Diabetes Lifestyle Prevention. Eur. Med. J. 2020, 5, 67–77. [Google Scholar] [CrossRef]
- Alkhatib, A.; Tsang, C.; Tiss, A.; Bahorun, T.; Arefanian, H.; Barake, R.; Khadir, A.; Tuomilehto, J. Functional Foods and Lifestyle Approaches for Diabetes Prevention and Management. Nutrients 2017, 9, 1310. [Google Scholar] [CrossRef] [PubMed]
- Sapian, S.; Taib, I.S.; Katas, H.; Latip, J.; Zainalabidin, S.; Hamid, Z.A.; Anuar, N.N.M.; Budin, S.B. The Role of Anthocyanin in Modulating Diabetic Cardiovascular Disease and Its Potential to Be Developed as a Nutraceutical. Pharmaceuticals 2022, 15, 1344. [Google Scholar] [CrossRef]
- Tian, L.; Tan, Y.; Chen, G.; Wang, G.; Sun, J.; Ou, S.; Chen, W.; Bai, W. Metabolism of anthocyanins and consequent effects on the gut microbiota. Crit. Rev. Food Sci. Nutr. 2019, 59, 982–991. [Google Scholar] [CrossRef]
- Mao, T.; Akshit, F.N.U.; Mohan, M.S. Effects of anthocyanin supplementation in diet on glycemic and related cardiovascular biomarkers in patients with type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. Front. Nutr. 2023, 10, 1199815. [Google Scholar] [CrossRef]
- Kozłowska, A.; Nitsch-Osuch, A. Anthocyanins and Type 2 Diabetes: An Update of Human Study and Clinical Trial. Nutrients 2024, 16, 1674. [Google Scholar] [CrossRef]
- Dhingra, D.; Michael, M.; Rajput, H.; Patil, R.T. Dietary fibre in foods: A review. J. Food Sci. Technol. 2012, 49, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.A.; Sami, S.A.; Khan, F.A. Effects of stabilized rice bran, its soluble and fiber fractions on blood glucose levels and serum lipid parameters in humans with diabetes mellitus types I and II. J. Nutr. Biochem. 2002, 13, 175–187. [Google Scholar]
- Ogawa, Y.; Shobako, N.; Fukuhara, I.; Satoh, H.; Kobayashi, E.; Kusakari, T.; Suwa, M.; Matsumoto, M.; Ishikado, A. Rice Bran Supplement Containing A Functional Substance, the Novel Peptide Leu-Arg-Ala, has Anti-Hypertensive Effects: A Double-Blind, Randomized, Placebo-Controlled Study. Nutrients 2019, 11, 726. [Google Scholar] [CrossRef]
- Muangchan, N.; Khiewvan, B.; Chatree, S.; Pongwattanapakin, K.; Kunlaket, N.; Dokmai, T.; Chaikomin, R. Riceberry rice (Oryza sativa L.) slows gastric emptying and improves the postprandial glycaemic response. Br. J. Nutr. 2022, 128, 424–432. [Google Scholar] [CrossRef]
- Takahashi, H.; Nakajima, A.; Matsumoto, Y.; Mori, H.; Inoue, K.; Yamanouchi, H.; Tanaka, K.; Tomiga, Y.; Miyahara, M.; Yada, T.; et al. Administration of Jerusalem artichoke reduces the postprandial plasma glucose and glucose-dependent insulinotropic polypeptide (GIP) concentrations in humans. Food Nutr. Res. 2022, 66, 7870. [Google Scholar]
- Rao, M.; Gao, C.; Xu, L.; Jiang, L.; Zhu, J.; Chen, G.; Law, B.Y.K.; Xu, Y. Effect of Inulin-Type Carbohydrates on Insulin Resistance in Patients with Type 2 Diabetes and Obesity: A Systematic Review and Meta-Analysis. J. Diabetes Res. 2019, 2019, 5101423. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Tang, Y.; Huang, J.; Yang, Y.; Yang, Q.; Hu, H. Efficacy of inulin supplementation in improving insulin control, HbA1c and HOMA-IR in patients with type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. J. Clin. Biochem. Nutr. 2020, 66, 176–183. [Google Scholar] [CrossRef]
- Dehghan, P.; Gargari, B.P.; Jafar-Abadi, M.A.; Aliasgharzadeh, A. Inulin controls inflammation and metabolic endotoxemia in women with type 2 diabetes mellitus: A randomized-controlled clinical trial. Int. J. Food Sci. Nutr. 2014, 65, 117–123. [Google Scholar] [CrossRef]
- Sawangwan, T.; Kajadman, D.; Kulchananimit, R. Determination of prebiotic properties of rice bran extract. Biosci. Microbiota Food Health 2024, 43, 222–226. [Google Scholar]
- Shao, T.; Yu, Q.; Zhu, T.; Liu, A.; Gao, X.; Long, X.; Liu, Z. Inulin from Jerusalem artichoke tubers alleviates hyperglycaemia in high fat diet-induced diabetes mice through the intestinal microflora improvement. Br. J. Nutr. 2020, 23, 308–318. [Google Scholar] [CrossRef]
- Pongnaratorn, P.; Kanpetta, Y.; Aneknan, P.; Uriyapongson, J.; Wannanon, P.; Panthongviriyakul, C.; Leelayuwat, N. Effects of Anthocyanin, Inulin, and Rice Bran Extracts on Adiposity, Fat Utilization, and Cardiorespiratory Functions in Obese Subjects. J. Exerc. Physiol. 2019, 22, 22–37. [Google Scholar]
- Schulz, K.F.; Altman, D.G.; Moher, D.; CONSORT Group. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c332. [Google Scholar] [CrossRef] [PubMed]
- Gargari, B.P.; Dehghan, P.; Aliasgharzadeh, A.; Jafar-Abadi, M.A. Effects of high performance inulin supplementation on glycemic control and antioxidant status in women with type 2 diabetes. Diabetes Metab. J. 2013, 37, 140–148. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Erlbaum: Hillsdale, NJ, USA, 1988. [Google Scholar]
- American Diabetes Association. Standards of medical care in diabetes—2011. Diabetes Care 2011, 34, S11–S61. [Google Scholar] [CrossRef]
- Urbaniak, G.C.; Plous, S.; Research Randomizer (Version 4.0) [Computer Software]. Retrieved on 22 June 2013. 2013. Available online: http://www.randomizer.org/ (accessed on 13 November 2018).
- Available online: http://bqsf.dmsc.moph.go.th/bqsfWeb/index.php/afrl/ (accessed on 16 July 2018).
- Association of Official Analytical Chemists. Official Method of Analysis, 19th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2012. [Google Scholar]
- Puyanda, I.R.; Uriyapongson, S.; Uriyapongson, J. Influence of drying method on qualities of Jerusalem artichoke (Helianthus tuberosus L.) tuber harvested in Northeastern Thailand. Songklanakarin J. Sci. Technol. 2020, 42, 1279–1285. [Google Scholar]
- Kapcum, C.; Uriyapongson, S.; Uriyapongson, J. Phenolics, anthocyanins and antioxidant activities in waste products from different parts of purple waxy corn (Zea mays L.). Songklanakarin J. Sci. Technol. 2021, 43, 398–405. [Google Scholar]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef]
- Stratil, P.; Klejdus, B.; Kuban, V. Determination of total content of phenolic compounds and their antioxidant activity in vegetables evaluation of spectrophotometric methods. J. Agric. Food Chem. 2006, 54, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Nirdnoy, N.; Sranacharoenpong, K.; Mayurasakorn, K.; Surawit, A.; Pinsawas, B.; Mongkolsucharitkul, P.; Pongkunakorn, T.; Suta, S. Development of the Thai semiquantitative food frequency questionnaire (semi-FFQ) for people at risk for metabolic syndrome. J. Public Health 2023, 31, 1–8. [Google Scholar] [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelundu, A.; Yngve, J.F.; James, F.S.; et al. International Physical Activity Questionnaire: 12-Country Reliability and Validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- Ainsworth, B.E.; Haskell, W.L.; Herrmann, S.D.; Meckes, N.; Bassett, D.R., Jr.; Tudor-Locke, C.; Greer, J.L.; Vezina, J.; Whitt-Glover, M.C.; Leon, A.S. Compendium of physical activities: A second update of codes and MET values. Med. Sci. Sports Exerc. 2011, 43, 1575–1581. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.Y.; Yang, C.Y.; Shih, S.R.; Hsieh, H.J.; Hung, C.S.; Chiu, F.C.; Lin, M.S.; Liu, P.H.; Hua, C.H.; Hsein, Y.C.; et al. Measurement of Waist Circumference: Midabdominal or iliac crest? Diabetes Care 2013, 36, 1660–1666. [Google Scholar]
- Matthews, M.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Draper, H.H.; Squires, E.J.; Mahmoodi, H.; Wu, J.; Agarwal, S.; Hadley, M. A comparative evaluation of thiobarbituric acid methods for the determination of malondialdehyde in biological materials. Free Radic. Biol. Med. 1993, 15, 353–363. [Google Scholar]
- Zhang, H.; Li, J.; Wang, K.; Du, X.; Li, Q. A simple and sensitive assay for ascorbate using potassium ferricyanide as spectroscopic probe reagent. Anal. Biochem. 2009, 388, 40–46. [Google Scholar]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [PubMed]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117, Erratum in Am. J. Respir. Crit. Care Med. 2016, 193, 1185. [Google Scholar]
- Lee, M.C. Validity of the 6-minute walk test and step test for evaluation of cardiorespiratory fitness in patients with type 2 diabetes mellitus. J. Exerc. Nutrition Biochem. 2018, 22, 49–55. [Google Scholar]
- Li, D.; Zhang, Y.; Liu, Y.; Sun, R.; Xia, M. Purified anthocyanin supplementation reduces dyslipidemia, enhances antioxidant capacity, and prevents insulin resistance in diabetic patients. J. Nutr. 2015, 145, 742–748. [Google Scholar]
- Cheng, H.H.; Huang, H.Y.; Chen, Y.Y.; Huang, C.L.; Chang, C.J.; Chen, H.L.; Lai, M.H. Ameliorative effects of stabilized rice bran on type 2 diabetes patients. Ann. Nutr. Metab. 2010, 56, 45–51. [Google Scholar] [PubMed]
- Li, L.; Li, P.; Xu, L. Assessing the effects of inulin-type fructan intake on body weight, blood glucose, and lipid profile: A systematic review and meta-analysis of randomized controlled trials. Food Sci. Nutr. 2021, 9, 4598–4616. [Google Scholar] [PubMed]
- Alkhatib, A.; Tuomilehto, J. Lifestyle Diabetes Prevention. In Encyclopedia of Endocrine Diseases, 2nd ed.; Huhtaniemi, I., Martini, L., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2019; pp. 148–159. ISBN 9780128122006. [Google Scholar]
- Davidson, M.H.; Maki, K.C. Effects of dietary inulin on serum lipids. J. Nutr. 1999, 129, 1474S–1477S. [Google Scholar]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar]
- Pongnaratorn, P.; Kanpetta, Y.; Aneknan, P.; Uriyapongson, J.; Wannanon, P.; Panthongviriyakul, C.; Leelayuwat, N. Prebiotic capsules containing anthocyanin, inulin and rice bran extracts increased plasma ascorbate of overweight or obese subjects. Arch. Allied Health Sci. 2021, 33, 19–33. [Google Scholar]
- Ebenuwa, I.; Violet, P.C.; Padayatty, S.; Wang, Y.; Wang, Y.; Sun, H.; Adhikari, P.; Smith, S.; Tu, H.; Niyyati, M.; et al. Abnormal urinary loss of vitamin C in diabetes: Prevalence and clinical characteristics of a vitamin C renal leak. Am. J. Clin. Nutr. 2022, 116, 274–284. [Google Scholar]
- Palencia-Argel, M.; Rodríguez-Villamil, H.; Bernal-Castro, C.; Díaz-Moreno, C.; Fuenmayor, C.A. Probiotics in anthocyanin-rich fruit beverages: Research and development for novel synbiotic products. Crit. Rev. Food Sci. Nutr. 2024, 64, 110–126. [Google Scholar] [PubMed]
- Akkarachiyasit, S.; Charoenlertkul, P.; Yibchok-anun, S.; Adisakwattana, S. Inhibitory activities of cyanidin and its glycosides and synergistic effect with acarbose against intestinal glucosidase and pancreatic amylase. Int. J. Mol. Sci. 2010, 11, 3387–3396. [Google Scholar]
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X.; et al. Short-chain fatty acids and their association with signaling pathways in inflammation, glucose and lipid metabolism. Int. J. Mol. Sci. 2020, 21, 6356. [Google Scholar] [CrossRef]
- El, K.; Campbell, J.E. The role of GIP in α-cells and glucagon secretion. Peptides 2020, 125, 170213. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, L.; Jia, X.; Liu, L.; Chi, J.; Huang, F.; Ma, Q.; Zhang, M.; Zhang, R. Bound Phenolics Ensure the Antihyperglycemic Effect of Rice Bran Dietary Fiber in db/db Mice via Activating the Insulin Signaling Pathway in Skeletal Muscle and Altering Gut Microbiota. J. Agric. Food Chem. 2020, 68, 4387–4398. [Google Scholar] [PubMed]
- Birkeland, E.; Gharagozlian, S.; Birkeland, K.I.; Valeur, J.; Måge, I.; Rud, I.; Aas, A.-M. Prebiotic effect of inulin-type fructans on faecal microbiota and short-chain fatty acids in type 2 diabetes: A randomised controlled trial. Eur. J. Nut. 2020, 59, 3325–3338. [Google Scholar]
- Li, J.; Jia, S.; Yuan, C.; Yu, B.; Zhang, Z.; Zhao, M.; Liu, P.; Li, X.; Cui, B. Jerusalem artichoke inulin supplementation ameliorates hepatic lipid metabolism in type 2 diabetes mellitus mice by modulating the gut microbiota and fecal metabolome. Food Funct. 2022, 13, 11503–11517. [Google Scholar]
- Qin, Y.; Xia, M.; Ma, J.; Hao, Y.; Liu, J.; Mou, H.; Cao, L.; Ling, W. Anthocyanin supplementation improves serum LDL- and HDL-cholesterol concentrations associated with the inhibition of cholesteryl ester transfer protein in dyslipidemic subjects. Am. J. Clin. Nutr. 2009, 90, 485–492. [Google Scholar]
- Daou, C.; Zhang, H. Functional and physiological properties of total, soluble, and insoluble dietary fibres derived from defatted rice bran. J. Food Sci. Technol. 2014, 51, 3878–3885. [Google Scholar]
- Alkhatib, A. Sedentary Lifestyle: Predictive Factors, Health Risks and Physiological Implications; Nova Science Publishers, Inc.: New York, NY, USA, 2016; ISBN 9781634846837. [Google Scholar]
- Alkhatib, A. High prevalence of sedentary risk factors amongst university employees and potential health benefits of campus workplace exercise intervention. Work 2015, 52, 589–595. [Google Scholar] [PubMed]
- Aya, V.; Flórez, A.; Perez, L.; Ramírez, J.D. Association between physical activity and changes in intestinal microbiota composition: A systematic review. PLoS ONE 2021, 16, e0247039. [Google Scholar]
- Verhagen, A.P.; de Vet, H.C.; de Bie, R.A.; Boers, M.; van den Brandt, P.A. The art of quality assessment of RCTs included in systematic reviews. J. Clin. Epidemiol. 2001, 54, 651–654. [Google Scholar]
- Jayasvasti, I.; Jayasvasti, M.; Pengpid, S.; Peltzer, K.; Nanthananate, P. Participants’ Views on Lifestyle Intervention Program Enhancing Long-Term Health-Related Behaviors Improvement among Prehypertension and/or Prediabetes Mellitus Older Adults: A Qualitative Study Examined in Thailand at Health Promoting Hospitals. Inquiry 2021, 58, 469580211025209. [Google Scholar]
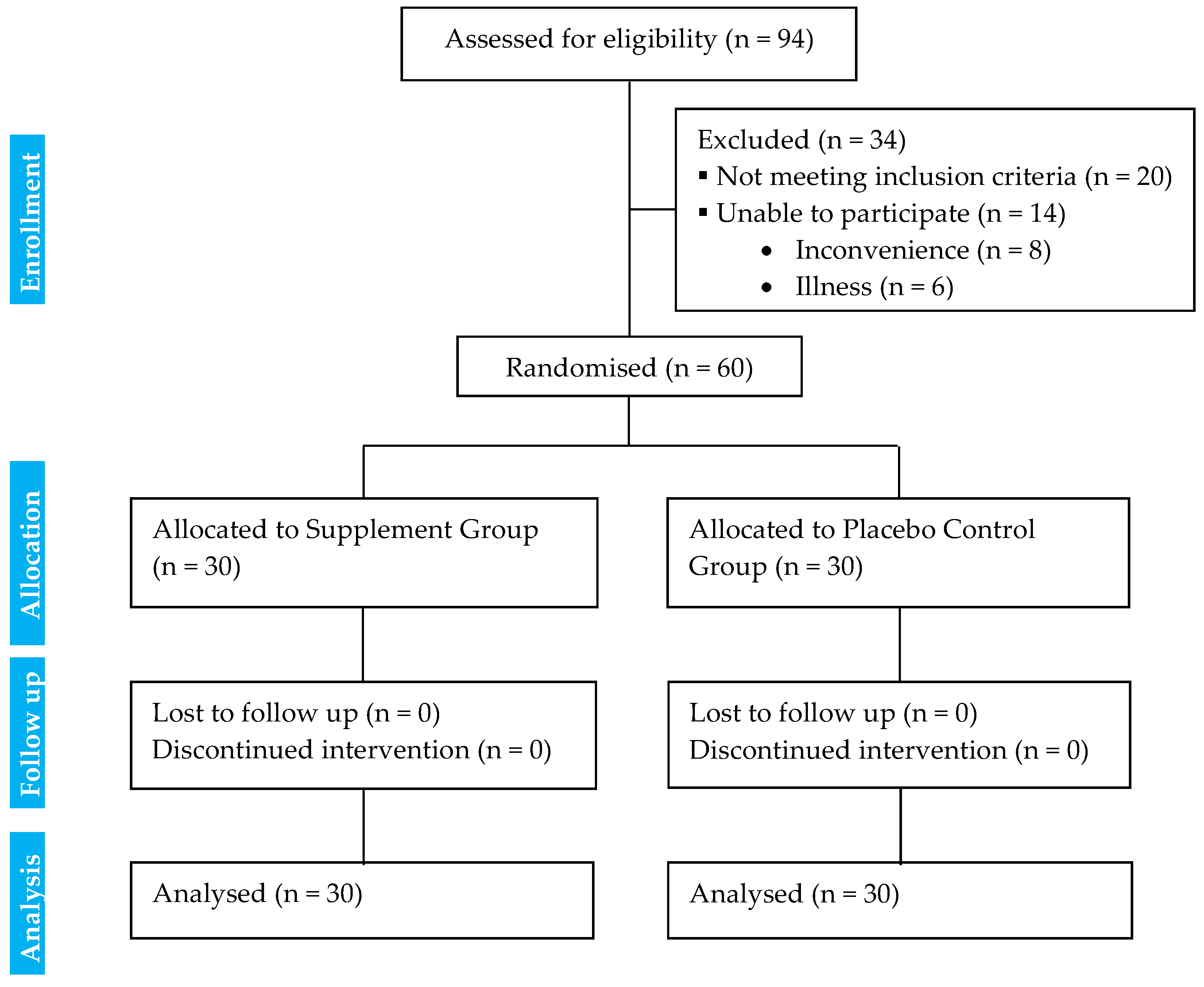
| CG | SG | Mean Difference [95% CI] | Between Groups at Baseline p-Value | Interaction Effects (Duration × Supplement) p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| Before (n = 30) | After (n = 30) | Duration Effects (Within Group) p-Value | Before (n = 30) | After (n = 30) | Duration Effects (Within Group) p-Value | ||||
| Protein (g/day) | 64 ± 3.8 | 64 ± 3.5 | 0.98 | 62 ± 3.0 | 72 ± 5.0 | 0.05 | 10 [−3.9, 20.8] | 0.64 | 0.18 |
| Carbohydrate (g/day) | 169 ± 10.6 | 163 ± 10.9 | 0.59 | 170 ± 9.9 | 184 ± 8.2 | 0.16 | 20 [−3.5, 44.4] | 0.93 | 0.09 |
| Fat (g/day) | 31 ± 2.8 | 31 ± 2.2 | 0.88 | 31 ± 2.4 | 36 ± 4.0 | 0.14 | 5 [−3.1, 14.0] | 0.99 | 0.21 |
| Vitamin C (mg/day) | 68 ± 7.1 | 66 ± 6.2 | 0.77 | 67 ± 7.2 | 70 ± 6.4 | 0.70 | 5 [−12.5, 21.2] | 0.91 | 0.61 |
| Energy intake (kcal/day) | 1211 ± 66.4 | 1185 ± 68.3 | 0.70 | 1298 ± 55.7 | 1294 ± 56.3 | 0.93 | 22 [−90.0, 206.4] | 0.32 | 0.44 |
| Energy expenditure (kcal/day) | 1284 ± 62.4 | 1292 ± 61.6 | 0.85 | 1350 ± 59.2 | 1326 ± 60.2 | 0.57 | −32 [−123.9, 88.9] | 0.45 | 0.74 |
| CG | SG | Mean Difference [95% CI] | Between Groups at Baseline p-Value | Interaction Effects (Duration × Supplement) p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| Before (n = 30) | After (n = 30) | Duration Effects (Within Group) p-Value | Before (n = 30) | After (n = 30) | Duration Effects (Within Group) p-Value | ||||
| Age $ (yr) | 53 ± 0.9 | 54 ± 0.9 | 1 [−3.4, 1.7] | 0.5 | |||||
| F/M (n) | 28/2 | 28/2 | |||||||
| Diabetes duration $ (yr) | 7 ± 0.7 | 6 ± 0.8 | −1 [−1.6, 2.5] | 0.65 | |||||
| Diabetes duration @ (n (%)) | 0.49 | ||||||||
| >2 years | 24 (80.0) | 26 (86.7) | |||||||
| <2 years | 6 (20.0) | 4 (13.3) | |||||||
| Menstruation @ (n (%)) | 0.76 | ||||||||
| Menstruation | 8 (28.6) | 7 (25.0) | |||||||
| Menopause | 20 (71.4) | 21 (75.0) | |||||||
| Marital status @ (n (%)) | 0.50 | ||||||||
| Married | 29 (96.7) | 28 (93.3) | |||||||
| Single | 1 (3.3) | 1 (3.3) | |||||||
| Widowed | 0 (0.0) | 1 (3.3) | |||||||
| Occupation @ (n (%)) | 0.65 | ||||||||
| Merchant | 7 (23.3) | 4 (13.3) | |||||||
| Farmer | 9 (30.0) | 9 (30.0) | |||||||
| Employee | 11 (36.7) | 15 (50.0) | |||||||
| Unemployed | 3 (10.0) | 2 (6.7) | |||||||
| Education level @ (n (%)) | 1.00 | ||||||||
| <high school | 28 (93.3) | 28 (93.3) | |||||||
| ≥high school | 2 (6.7) | 2 (6.7) | |||||||
| BM $ (kg) | 63 ± 2.1 | 62 ± 2.0 | 0.09 | 62 ± 2.0 | 62 ± 2.0 | 0.76 | 1 [−0.3, 1.8] | 0.71 | 0.15 |
| BMI $ (kg/m2) | 27 ± 0.8 | 27 ± 0.8 | 0.11 | 26 ± 0.8 | 26 ± 0.8 | 0.73 | 0 [−0.2, 0.7] | 0.44 | 0.24 |
| Nutritional status @ (n (%)) | 1.00 | 0.62 | 0.72 | ||||||
| Normal # | 5 (16.7) | 5 (16.7) | 6 (20.0) | 4 (13.3) | |||||
| Overweight # | 5 (16.7) | 5 (16.7) | 7 (23.3) | 10 (33.3) | |||||
| Obese # | 20 (66.7) | 20 (66.7) | 17 (56.7) | 16 (53.3) | |||||
| W $ (cm) | 91 ± 1.8 | 90 ± 2.0 | 0.11 | 91 ± 2.0 | 90 ± 2.1 | 0.44 | 0 [−1.4, 2.7] | 0.96 | 0.55 |
| H $ (cm) | 99 ± 1.5 | 99 ± 1.7 | 0.64 | 99 ± 1.5 | 98 ± 1.5 | 0.04 | −1 [−2.6, 0.1] | 0.90 | 0.07 |
| W/H $ ratio | 0.90 ± 0.0 | 0.90 ± 0.0 | 0.05 | 0.90 ± 0.0 | 0.90 ± 0.0 | 0.55 | 0 [−0.0, 0.0] | 0.87 | 0.08 |
| BF $ (%) | 34 ± 1.0 | 34 ± 1.0 | 0.08 | 33 ± 1.0 | 34 ± 1.0 | 0.50 | 1 [−0.2, 1.8] | 0.51 | 0.11 |
| FM $ (kg) | 22 ± 1.1 | 22 ± 1.1 | 0.08 | 21 ± 1.2 | 22 ± 1.1 | 0.50 | 1 [−0.1, 1.3] | 0.61 | 0.10 |
| LBM $ (kg) | 40 ± 1.3 | 40 ± 1.4 | 0.47 | 40 ± 1.1 | 40 ± 1.1 | 0.95 | 0 [−0.9, 0.5] | 0.83 | 0.58 |
| CG | SG | Mean Difference [95% CI] | Between Groups at Baseline p-Value | Interaction Effects (Duration × Supplement) p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| Before (n = 30) | After (n = 30) | Duration Effects (Within Group) p-Value | Before (n = 30) | After (n = 30) | Duration Effects (Within Group) p-Value | ||||
| FPG $ (mg/dL) | 217.9 ± 13.1 | 220.7 ± 18.0 | 0.82 | 230.1 ± 16.3 | 187.8 ± 12.3 | 0.01 | −45.1 [−76.1, −4.6] | 0.56 | 0.03 |
| HbA1c $ (%) | 8.6 ± 0.3 | 8.8 ± 0.3 | 0.35 | 8.6 ± 0.5 | 7.9 ± 0.4 | 0.004 | −0.9 [−1.5, −0.3] | 0.92 | 0.002 |
| Insulin γ (µIU/mL) | 5.5 (0.0–10.8) | 6.0 (0.0–10.0) | 0.43 | 4.0 (0.8–7.8) | 4.5 (0.0–8.3) | 0.53 | 0.4 [−1.5, 0.7] | 0.81 | 0.46 |
| HOMA-IR γ | 3.0 (0.2–6.2) | 3.1 (0.0–5.9) | 0.60 | 2.3 (0.4–5.0) | 1.8 (0.0–4.5) | 0.52 | −0.6 [−1.3, 0.3] | 0.70 | 0.20 |
| LDL-C $ (mg/dL) | 101 ± 7.3 | 102 ± 6.6 | 0.86 | 96 ± 7.4 | 82 ± 7.0 | 0.006 | −15 [−29.1, −2.8] | 0.63 | 0.02 |
| TC $ (mg/dL) | 187 ± 7.6 | 180 ± 8.1 | 0.12 | 174 ± 9.5 | 160 ± 8.5 | 0.002 | −7 [−21.0, 3.6] | 0.30 | 0.16 |
| TG $ (mg/dL) | 179 ± 21.1 | 171 ± 8.1 | 0.66 | 164 ± 16.1 | 171 ± 16.2 | 0.69 | 15 [−35.7, 50.6] | 0.58 | 0.73 |
| HDL-C $ (mg/dL) | 43 ± 2.0 | 44 ± 1.9 | 0.45 | 44 ± 2.1 | 44 ± 2.6 | 0.70 | −0.4 [−4.9, 4.1] | 0.75 | 0.85 |
| SGPT $ (U/L) | 18.11 ± 1.3 | 17.56 ± 1.3 | 0.48 | 18.91 ± 1.1 | 18.93 ± 1.4 | 0.98 | 0.57 [−1.6, 2.8] | 0.64 | 0.58 |
| Cr $ (mg/dL) | 0.77 ± 0.0 | 0.76 ± 0.0 | 0.35 | 0.79 ± 0.0 | 0.76 ± 0.0 | 0.10 | −0.02 [−0.1, 0.0] | 0.68 | 0.70 |
| eGFR $ (mL/min/1.73 m2) | 92.7 ± 3.2 | 94.3 ± 2.7 | 0.32 | 90.8 ± 3.2 | 95.1 ± 3.1 | 0.01 | 2.7 [−1.9, 6.5] | 0.67 | 0.28 |
| CG | SG | Mean Difference [95% CI] | Between Groups at Baseline p-Value | Interaction Effects (Duration × Supplement) p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| Before (n = 30) | After (n = 30) | Duration Effects (Within Group) p-Value | Before (n = 30) | After (n = 30) | Duration Effects (Within Group) p-Value | ||||
| Plasma vitamin C $ (µmol/L) | 38.8 ± 2.2 | 33.6 ± 2.6 | 0.07 | 38.2 ± 3.5 | 36.6 ± 3.7 | 0.56 | 3.6 [−4.0, 10.6] | 0.88 | 0.37 |
| Plasma MDA $ (µmol/mL) | 7.2 ± 0.8 | 6.3 ± 0.6 | 0.14 | 8.2 ± 0.9 | 7.1 ± 0.9 | 0.08 | −0.2 [−1.3, 1.7] | 0.46 | 0.82 |
| Serum hsCRP γ (mg/L) | 1.5 (1.0–4.3) | 2.0 (1.0–5.3) | 0.28 | 2.0 (1.0–3.0) | 1.5 (1.0–3.0) | 0.14 | −0.3 [−0.8, 0.2] | 0.90 | 0.07 |
| WBC in plasma $ (×109/L) | 9.8 ± 0.5 | 10.7 ± 0.6 | 0.12 | 10.4 ± 0.7 | 9.7 ± 0.4 | 0.38 | −1.6 [−2.4, 0.2] | 0.52 | 0.07 |
| Neutrophil $ (%) | 55.7 ± 1.9 | 56.3 ± 1.5 | 0.74 | 54.9 ± 2.0 | 53.4 ± 1.6 | 0.39 | −2.1 [−6.2, 1.2] | 0.77 | 0.19 |
| Lymphocyte $ (%) | 33.5 ± 1.7 | 33.0 ± 1.2 | 0.76 | 34.5 ± 1.4 | 36.0 ± 1.3 | 0.28 | 2 [−0.5, 5.7] | 0.66 | 0.10 |
| Monocyte $ (%) | 5.4 ± 0.2 | 5.4 ± 0.2 | 0.98 | 5.3 ± 0.2 | 5.4 ± 0.2 | 0.64 | 0.1 [−0.4, 0.5] | 0.79 | 0.81 |
| Eosinophil $ (%) | 5.2 ± 0.6 | 5.1 ± 0.7 | 0.93 | 5.1 ± 0.8 | 5.1 ± 0.8 | 0.94 | 0.1 [−0.7, 0.8] | 0.91 | 0.92 |
| Basophil $ (%) | 0.3 ± 0.1 | 0.2 ± 0.1 | 0.26 | 0.3 ± 0.1 | 0.2 ± 0.1 | 0.63 | 0.0 [−0.2, 0.2] | 0.50 | 0.97 |
| CG | SG | Mean Difference [95% CI] | Between Groups at Baseline p-Value | Interaction Effects (Duration × Supplement) p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| Before (n = 30) | After (n = 30) | Duration Effects (Within Group) p-Value | Before (n = 30) | After (n = 30) | Duration Effects (Within Group) p-Value | ||||
| 6MWD $ (m) | 433.2 ± 10.2 | 432.2 ± 10.7 | 0.84 | 452.6 ± 11.6 | 443.7 ± 12.9 | 0.26 | −7.9 [−25.1, 12.6] | 0.21 | 0.51 |
| VO2peak $ (mL/kg/min) | 15.6 ± 0.4 | 15.6 ± 0.4 | 0.91 | 16.3 ± 0.5 | 16.0 ± 0.5 | 0.18 | −0.3 [−0.7, 0.3] | 0.27 | 0.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teparak, C.; Uriyapongson, J.; Phoemsapthawee, J.; Tunkamnerdthai, O.; Aneknan, P.; Tong-un, T.; Panthongviriyakul, C.; Leelayuwat, N.; Alkhatib, A. Diabetes Therapeutics of Prebiotic Soluble Dietary Fibre and Antioxidant Anthocyanin Supplement in Patients with Type 2 Diabetes: Randomised Placebo-Controlled Clinical Trial. Nutrients 2025, 17, 1098. https://doi.org/10.3390/nu17071098
Teparak C, Uriyapongson J, Phoemsapthawee J, Tunkamnerdthai O, Aneknan P, Tong-un T, Panthongviriyakul C, Leelayuwat N, Alkhatib A. Diabetes Therapeutics of Prebiotic Soluble Dietary Fibre and Antioxidant Anthocyanin Supplement in Patients with Type 2 Diabetes: Randomised Placebo-Controlled Clinical Trial. Nutrients. 2025; 17(7):1098. https://doi.org/10.3390/nu17071098
Chicago/Turabian StyleTeparak, Chompoonut, Juntanee Uriyapongson, Jatuporn Phoemsapthawee, Orathai Tunkamnerdthai, Ploypailin Aneknan, Terdthai Tong-un, Charnchai Panthongviriyakul, Naruemon Leelayuwat, and Ahmad Alkhatib. 2025. "Diabetes Therapeutics of Prebiotic Soluble Dietary Fibre and Antioxidant Anthocyanin Supplement in Patients with Type 2 Diabetes: Randomised Placebo-Controlled Clinical Trial" Nutrients 17, no. 7: 1098. https://doi.org/10.3390/nu17071098
APA StyleTeparak, C., Uriyapongson, J., Phoemsapthawee, J., Tunkamnerdthai, O., Aneknan, P., Tong-un, T., Panthongviriyakul, C., Leelayuwat, N., & Alkhatib, A. (2025). Diabetes Therapeutics of Prebiotic Soluble Dietary Fibre and Antioxidant Anthocyanin Supplement in Patients with Type 2 Diabetes: Randomised Placebo-Controlled Clinical Trial. Nutrients, 17(7), 1098. https://doi.org/10.3390/nu17071098








