Research Progress on the Protective Effect of Green Tea Polyphenol (-)-Epigallocatechin-3-Gallate (EGCG) on the Liver
Abstract
1. Introduction
2. EGCG: An Overview
2.1. Physicochemical Characteristics of EGCG and Its Bioavailability
2.2. Safety of EGCG
3. Effect of EGCG in Liver Diseases
3.1. Viral Hepatitis
3.2. Autoimmune Hepatitis
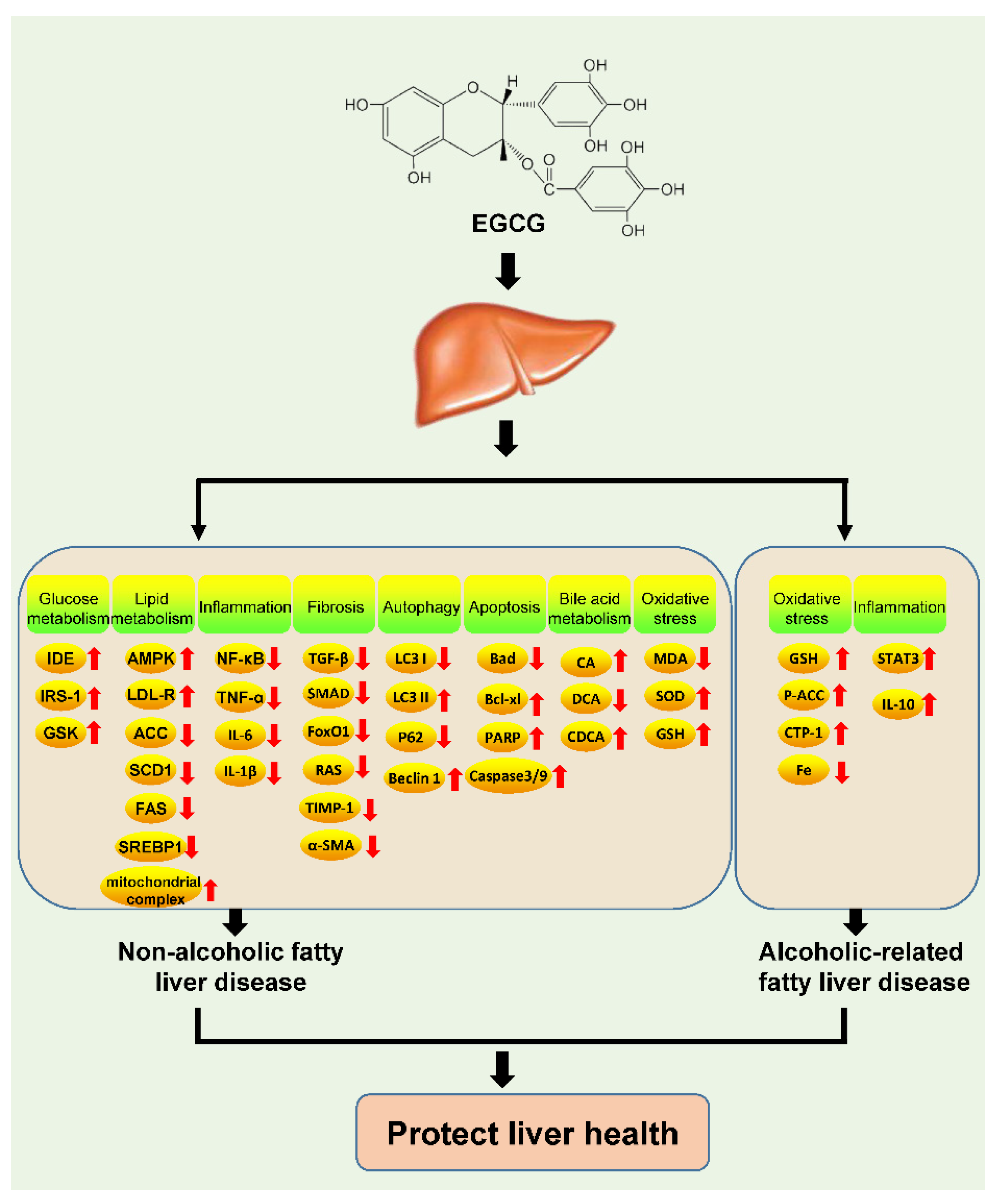
3.3. Fatty Liver Disease
3.4. Non-Alcoholic Fatty Liver Disease
3.5. Alcohol-Related Fatty Liver Disease
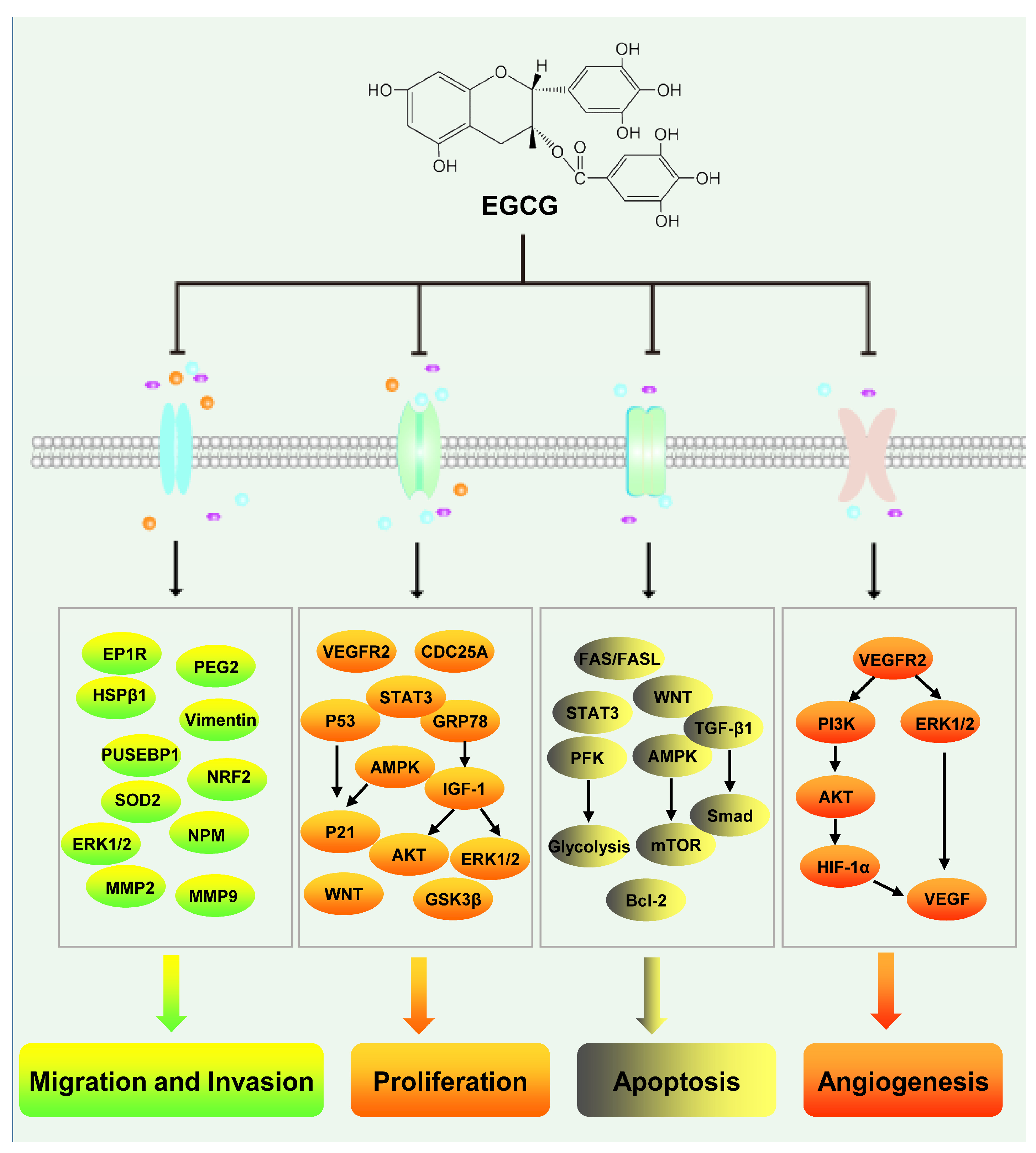
3.6. Hepatocellular Carcinoma
4. Applications of EGCG and Its Potential in the Fight Against Liver Diseases
5. Discussion and Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Lanini, S.; Ustianowski, A.; Pisapia, R.; Zumla, A.; Ippolito, G. Viral Hepatitis: Etiology, Epidemiology, Transmission, Diagnostics, Treatment, and Prevention. Infect. Dis. Clin. N. Am. 2019, 33, 1045–1062. [Google Scholar]
- Cotter, T.G.; Rinella, M. Nonalcoholic Fatty Liver Disease 2020: The State of the Disease. Gastroenterology 2020, 158, 1851–1864. [Google Scholar]
- Collaborators, G.B.D.O.; Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar]
- Heneghan, M.A.; Yeoman, A.D.; Verma, S.; Smith, A.D.; Longhi, M.S. Autoimmune hepatitis. Lancet 2013, 382, 1433–1444. [Google Scholar] [PubMed]
- Kulik, L.; El-Serag, H.B. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology 2019, 156, 477–491.e1. [Google Scholar]
- Brody, H. Tea. Nature 2019, 566, S1. [Google Scholar]
- Drew, L. The growth of tea. Nature 2019, 566, S2–S4. [Google Scholar]
- Zhou, J.; Ho, C.T.; Long, P.; Meng, Q.; Zhang, L.; Wan, X. Preventive Efficiency of Green Tea and Its Components on Nonalcoholic Fatty Liver Disease. J. Agric. Food Chem. 2019, 67, 5306–5317. [Google Scholar]
- Li, C.F.; Zhu, Y.; Yu, Y.; Zhao, Q.Y.; Wang, S.J.; Wang, X.C.; Yao, M.Z.; Luo, D.; Li, X.; Chen, L.; et al. Global transcriptome and gene regulation network for secondary metabolite biosynthesis of tea plant (Camellia sinensis). BMC Genom. 2015, 16, 560. [Google Scholar]
- Li, P.; Liu, A.; Xiong, W.; Lin, H.; Xiao, W.; Huang, J.; Zhang, S.; Liu, Z. Catechins enhance skeletal muscle performance. Crit. Rev. Food Sci. Nutr. 2020, 60, 515–528. [Google Scholar]
- James, A.; Wang, K.; Wang, Y. Therapeutic Activity of Green Tea Epigallocatechin-3-Gallate on Metabolic Diseases and Non-Alcoholic Fatty Liver Diseases: The Current Updates. Nutrients 2023, 15, 3022. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Gulzar, M.; Akhtar, M.S.; Rashid, S.; Zulfareen; Tanuja; Shamsi, A.; Hassan, M.I. Epigallocatechin-3-gallate therapeutic potential in human diseases: Molecular mechanisms and clinical studies. Mol. Biomed. 2024, 5, 73. [Google Scholar]
- Mokra, D.; Joskova, M.; Mokry, J. Therapeutic Effects of Green Tea Polyphenol (−)-Epigallocatechin-3-Gallate (EGCG) in Relation to Molecular Pathways Controlling Inflammation, Oxidative Stress, and Apoptosis. Int. J. Mol. Sci. 2023, 24, 340. [Google Scholar]
- Qu, Z.; Liu, A.; Li, P.; Liu, C.; Xiao, W.; Huang, J.; Liu, Z.; Zhang, S. Advances in physiological functions and mechanisms of (−)-epicatechin. Crit. Rev. Food Sci. Nutr. 2021, 61, 211–233. [Google Scholar]
- Bakun, P.; Mlynarczyk, D.T.; Koczorowski, T.; Cerbin-Koczorowska, M.; Piwowarczyk, L.; Kolasinski, E.; Stawny, M.; Kuzminska, J.; Jelinska, A.; Goslinski, T. Tea-break with epigallocatechin gallate derivatives—Powerful polyphenols of great potential for medicine. Eur. J. Med. Chem. 2023, 261, 115820. [Google Scholar] [PubMed]
- Zhang, S.; Mao, B.; Shumao, C.; Zhang, Q.; Zhao, J.; Tang, X.; Chen, W. Absorption, metabolism, bioactivity, and biotransformation of epigallocatechin gallate. Crit. Rev. Food Sci. Nutr. 2024, 64, 6546–6566. [Google Scholar]
- Chow, H.H.S.; Cai, Y.; Hakim, I.A.; Crowell, J.A.; Shahi, F.; Brooks, C.A.; Dorr, R.T.; Hara, Y.; Alberts, D.S. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenon E in healthy individuals. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2003, 9, 3312–3319. [Google Scholar]
- Misaka, S.; Kawabe, K.; Onoue, S.; Werba, J.P.; Giroli, M.; Kimura, J.; Watanabe, H.; Yamada, S. Development of rapid and simultaneous quantitative method for green tea catechins on the bioanalytical study using UPLC/ESI-MS. Biomed. Chromatogr. 2013, 27, 1–6. [Google Scholar]
- Zhu, M.; Chen, Y.; Li, R.C. Oral absorption and bioavailability of tea catechins. Planta Medica 2000, 66, 444–447. [Google Scholar]
- Lin, L.-C.; Wang, M.-N.; Tseng, T.-Y.; Sung, J.-S.; Tsai, T.-H. Pharmacokinetics of (−)-epigallocatechin-3-gallate in conscious and freely moving rats and its brain regional distribution. J. Agric. Food Chem. 2007, 55, 1517–1524. [Google Scholar]
- Nakagawa, K.; Miyazawa, T. Absorption and distribution of tea catechin, (−)-epigallocatechin-3-gallate, in the rat. J. Nutr. Sci. Vitaminol. 1997, 43, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Zagury, Y.; Kazir, M.; Livney, Y.D. Improved antioxidant activity, bioaccessibility and bioavailability of EGCG by delivery in β-lactoglobulin particles. J. Funct. Foods 2019, 52, 121–130. [Google Scholar] [CrossRef]
- Yang, C.S.; Sang, S.; Lambert, J.D.; Lee, M.-J. Bioavailability issues in studying the health effects of plant polyphenolic compounds. Mol. Nutr. Food Res. 2008, 52, S139–S151. [Google Scholar] [CrossRef]
- Sun, W.; Yang, Y.; Wang, C.; Liu, M.; Wang, J.; Qiao, S.; Jiang, P.; Sun, C.; Jiang, S. Epigallocatechin-3-gallate at the nanoscale: A new strategy for cancer treatment. Pharm. Biol. 2024, 62, 676–690. [Google Scholar] [CrossRef]
- Liu, J.B.; Li, J.L.; Zhuang, K.; Liu, H.; Wang, X.; Xiao, Q.H.; Li, X.D.; Zhou, R.H.; Zhou, L.; Ma, T.C.; et al. Epigallocatechin-3-gallate local pre-exposure application prevents SHIV rectal infection of macaques. Mucosal Immunol. 2018, 11, 1230–1238. [Google Scholar] [CrossRef] [PubMed]
- Isbrucker, R.A.; Edwards, J.A.; Wolz, E.; Davidovich, A.; Bausch, J. Safety studies on epigallocatechin gallate (EGCG) preparations. Part 2: Dermal, acute and short-term toxicity studies. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2006, 44, 636–650. [Google Scholar] [CrossRef]
- Yates, A.A.; Erdman, J.W., Jr.; Shao, A.; Dolan, L.C.; Griffiths, J.C. Bioactive nutrients—Time for tolerable upper intake levels to address safety. Regul. Toxicol. Pharmacol. 2017, 84, 94–101. [Google Scholar] [CrossRef]
- Galati, G.; Lin, A.; Sultan, A.M.; O’Brien, P.J. Cellular and in vivo hepatotoxicity caused by green tea phenolic acids and catechins. Free. Radic. Biol. Med. 2006, 40, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Samavat, H.; Newman, A.R.; Wang, R.; Yuan, J.M.; Wu, A.H.; Kurzer, M.S. Effects of green tea catechin extract on serum lipids in postmenopausal women: A randomized, placebo-controlled clinical trial. Am. J. Clin. Nutr. 2016, 104, 1671–1682. [Google Scholar] [CrossRef]
- Hu, J.; Webster, D.; Cao, J.; Shao, A. The safety of green tea and green tea extract consumption in adults—Results of a systematic review. Regul. Toxicol. Pharmacol. 2018, 95, 412–433. [Google Scholar] [CrossRef]
- Wang, L.; Li, P.; Feng, K. EGCG adjuvant chemotherapy: Current status and future perspectives. Eur. J. Med. Chem. 2023, 250, 115197. [Google Scholar]
- Wang, Y.-Q.; Lu, J.-L.; Liang, Y.-R.; Li, Q.-S. Suppressive Effects of EGCG on Cervical Cancer. Molecules 2018, 23, 2334. [Google Scholar] [CrossRef]
- Lambert, J.D.; Kennett, M.J.; Sang, S.; Reuhl, K.R.; Ju, J.; Yang, C.S. Hepatotoxicity of high oral dose (−)-epigallocatechin-3-gallate in mice. Food Chem. Toxicol. 2010, 48, 409–416. [Google Scholar] [PubMed]
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipic, M.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; et al. Scientific opinion on the safety of green tea catechins. EFSA J. 2018, 16, e05239. [Google Scholar] [PubMed]
- Sanlier, N.; Gokcen, B.B.; Altug, M. Tea consumption and disease correlations. Trends Food Sci. Technol. 2018, 78, 95–106. [Google Scholar]
- Xu, J.; Wang, J.; Deng, F.; Hu, Z.; Wang, H. Green tea extract and its major component epigallocatechin gallate inhibits hepatitis B virus in vitro. Antivir. Res. 2008, 78, 242–249. [Google Scholar] [PubMed]
- Huang, H.C.; Tao, M.H.; Hung, T.M.; Chen, J.C.; Lin, Z.J.; Huang, C. (−)-Epigallocatechin-3-gallate inhibits entry of hepatitis B virus into hepatocytes. Antivir. Res. 2014, 111, 100–111. [Google Scholar]
- Lai, Y.H.; Sun, C.P.; Huang, H.C.; Chen, J.C.; Liu, H.K.; Huang, C. Epigallocatechin gallate inhibits hepatitis B virus infection in human liver chimeric mice. BMC Complement. Altern. Med. 2018, 18, 248. [Google Scholar]
- He, W.; Li, L.X.; Liao, Q.J.; Liu, C.L.; Chen, X.L. Epigallocatechin gallate inhibits HBV DNA synthesis in a viral replication—Inducible cell line. World J. Gastroenterol. 2011, 17, 1507–1514. [Google Scholar]
- Karamese, M.; Aydogdu, S.; Karamese, S.A.; Altoparlak, U.; Gundogdu, C. Preventive effects of a major component of green tea, epigallocathechin-3-gallate, on hepatitis-B virus DNA replication. Asian Pac. J. Cancer Prev. 2015, 16, 4199–4202. [Google Scholar]
- Pang, J.Y.; Zhao, K.J.; Wang, J.B.; Ma, Z.J.; Xiao, X.H. Green tea polyphenol, epigallocatechin-3-gallate, possesses the antiviral activity necessary to fight against the hepatitis B virus replication in vitro. J. Zhejiang Univ. Sci. B 2014, 15, 533–539. [Google Scholar] [PubMed]
- Wang, Z.Y.; Li, Y.Q.; Guo, Z.W.; Zhou, X.H.; Lu, M.D.; Xue, T.C.; Gao, B. ERK1/2-HNF4alpha axis is involved in epigallocatechin-3-gallate inhibition of HBV replication. Acta Pharmacol. Sin. 2020, 41, 278–285. [Google Scholar]
- Xu, J.; Gu, W.; Li, C.; Li, X.; Xing, G.; Li, Y.; Song, Y.; Zheng, W. Epigallocatechin gallate inhibits hepatitis B virus via farnesoid X receptor alpha. J. Nat. Med. 2016, 70, 584–591. [Google Scholar] [PubMed]
- Mekky, R.Y.; El-Ekiaby, N.; El Sobky, S.A.; Elemam, N.M.; Youness, R.A.; El-Sayed, M.; Hamza, M.T.; Esmat, G.; Abdelaziz, A.I. Epigallocatechin gallate (EGCG) and miR-548m reduce HCV entry through repression of CD81 receptor in HCV cell models. Arch. Virol. 2019, 164, 1587–1595. [Google Scholar]
- Calland, N.; Sahuc, M.E.; Belouzard, S.; Pene, V.; Bonnafous, P.; Mesalam, A.A.; Deloison, G.; Descamps, V.; Sahpaz, S.; Wychowski, C.; et al. Polyphenols Inhibit Hepatitis C Virus Entry by a New Mechanism of Action. J. Virol. 2015, 89, 10053–10063. [Google Scholar]
- Ciesek, S.; von Hahn, T.; Colpitts, C.C.; Schang, L.M.; Friesland, M.; Steinmann, J.; Manns, M.P.; Ott, M.; Wedemeyer, H.; Meuleman, P.; et al. The green tea polyphenol, epigallocatechin-3-gallate, inhibits hepatitis C virus entry. Hepatology 2011, 54, 1947–1955. [Google Scholar]
- Wang, Y.; Li, J.; Wang, X.; Pena, J.C.; Li, K.; Zhang, T.; Ho, W. (−)-Epigallocatechin-3-Gallate Enhances Hepatitis C Virus Double-Stranded RNA Intermediates-Triggered Innate Immune Responses in Hepatocytes. Sci. Rep. 2016, 6, 21595. [Google Scholar]
- Xu, T.; Liu, R.; Zhu, H.; Zhou, Y.; Pei, T.; Yang, Z. The Inhibition of LPS-Induced Oxidative Stress and Inflammatory Responses Is Associated with the Protective Effect of (−)-Epigallocatechin-3-Gallate on Bovine Hepatocytes and Murine Liver. Antioxidants 2022, 11, 914. [Google Scholar] [CrossRef]
- Li, S.; Xia, Y.; Chen, K.; Li, J.; Liu, T.; Wang, F.; Lu, J.; Zhou, Y.; Guo, C. Epigallocatechin-3-gallate attenuates apoptosis and autophagy in concanavalin A-induced hepatitis by inhibiting BNIP3. Drug Des. Dev. Ther. 2016, 10, 631–647. [Google Scholar]
- Gan, L.; Meng, Z.J.; Xiong, R.B.; Guo, J.Q.; Lu, X.C.; Zheng, Z.W.; Deng, Y.P.; Luo, B.D.; Zou, F.; Li, H. Green tea polyphenol epigallocatechin-3-gallate ameliorates insulin resistance in non-alcoholic fatty liver disease mice. Acta Pharmacol. Sin. 2015, 36, 597–605. [Google Scholar]
- Kim, J.J.; Tan, Y.; Xiao, L.; Sun, Y.L.; Qu, X. Green tea polyphenol epigallocatechin-3-gallate enhance glycogen synthesis and inhibit lipogenesis in hepatocytes. Biomed. Res. Int. 2013, 2013, 920128. [Google Scholar]
- Liu, Z.; Li, Q.; Huang, J.; Liang, Q.; Yan, Y.; Lin, H.; Xiao, W.; Lin, Y.; Zhang, S.; Tan, B.; et al. Proteomic analysis of the inhibitory effect of epigallocatechin gallate on lipid accumulation in human HepG2 cells. Proteome Sci. 2013, 11, 32. [Google Scholar] [PubMed]
- Santamarina, A.B.; Oliveira, J.L.; Silva, F.P.; Carnier, J.; Mennitti, L.V.; Santana, A.A.; de Souza, G.H.; Ribeiro, E.B.; Oller do Nascimento, C.M.; Lira, F.S.; et al. Green Tea Extract Rich in Epigallocatechin-3-Gallate Prevents Fatty Liver by AMPK Activation via LKB1 in Mice Fed a High-Fat Diet. PLoS ONE 2015, 10, e0141227. [Google Scholar]
- Friedrich, M.; Petzke, K.J.; Raederstorff, D.; Wolfram, S.; Klaus, S. Acute effects of epigallocatechin gallate from green tea on oxidation and tissue incorporation of dietary lipids in mice fed a high-fat diet. Int. J. Obes. 2012, 36, 735–743. [Google Scholar]
- Santamarina, A.B.; Carvalho-Silva, M.; Gomes, L.M.; Okuda, M.H.; Santana, A.A.; Streck, E.L.; Seelaender, M.; do Nascimento, C.M.; Ribeiro, E.B.; Lira, F.S.; et al. Decaffeinated green tea extract rich in epigallocatechin-3-gallate prevents fatty liver disease by increased activities of mitochondrial respiratory chain complexes in diet-induced obesity mice. J. Nutr. Biochem. 2015, 26, 1348–1356. [Google Scholar] [PubMed]
- Ding, Y.; Sun, X.; Chen, Y.; Deng, Y.; Qian, K. Epigallocatechin gallate attenuated non-alcoholic steatohepatitis induced by methionine- and choline-deficient diet. Eur. J. Pharmacol. 2015, 761, 405–412. [Google Scholar]
- Xiao, J.; Ho, C.T.; Liong, E.C.; Nanji, A.A.; Leung, T.M.; Lau, T.Y.; Fung, M.L.; Tipoe, G.L. Epigallocatechin gallate attenuates fibrosis, oxidative stress, and inflammation in non-alcoholic fatty liver disease rat model through TGF/SMAD, PI3 K/Akt/FoxO1, and NF-kappa B pathways. Eur. J. Nutr. 2014, 53, 187–199. [Google Scholar]
- Kochi, T.; Shimizu, M.; Terakura, D.; Baba, A.; Ohno, T.; Kubota, M.; Shirakami, Y.; Tsurumi, H.; Tanaka, T.; Moriwaki, H. Non-alcoholic steatohepatitis and preneoplastic lesions develop in the liver of obese and hypertensive rats: Suppressing effects of EGCG on the development of liver lesions. Cancer Lett. 2014, 342, 60–69. [Google Scholar]
- Du, Y.; Paglicawan, L.; Soomro, S.; Abunofal, O.; Baig, S.; Vanarsa, K.; Hicks, J.; Mohan, C. Epigallocatechin-3-Gallate Dampens Non-Alcoholic Fatty Liver by Modulating Liver Function, Lipid Profile and Macrophage Polarization. Nutrients 2021, 13, 599. [Google Scholar] [CrossRef]
- Wu, D.; Liu, Z.; Wang, Y.; Zhang, Q.; Li, J.; Zhong, P.; Xie, Z.; Ji, A.; Li, Y. Epigallocatechin-3-Gallate Alleviates High-Fat Diet-Induced Nonalcoholic Fatty Liver Disease via Inhibition of Apoptosis and Promotion of Autophagy through the ROS/MAPK Signaling Pathway. Oxidative Med. Cell. Longev. 2021, 2021, 5599997. [Google Scholar]
- Ushiroda, C.; Naito, Y.; Takagi, T.; Uchiyama, K.; Mizushima, K.; Higashimura, Y.; Yasukawa, Z.; Okubo, T.; Inoue, R.; Honda, A.; et al. Green tea polyphenol (epigallocatechin-3-gallate) improves gut dysbiosis and serum bile acids dysregulation in high-fat diet-fed mice. J. Clin. Biochem. Nutr. 2019, 65, 34–46. [Google Scholar] [PubMed]
- Xu, K.-H.; Yang, D.-F.; Liu, M.-Y.; Xu, W.; Li, Y.-H.; Xiao, W.-J. Hepatoprotective effects and mechanisms of l-theanine and epigallocatechin gallate combined intervention in alcoholic fatty liver rats. J. Sci. Food Agric. 2024, 104, 8230–8239. [Google Scholar] [PubMed]
- Yun, J.W.; Kim, Y.K.; Lee, B.S.; Kim, C.W.; Hyun, J.S.; Baik, J.H.; Kim, J.J.; Kim, B.H. Effect of dietary epigallocatechin-3-gallate on cytochrome P450 2E1-dependent alcoholic liver damage: Enhancement of fatty acid oxidation. Biosci. Biotechnol. Biochem. 2007, 71, 2999–3006. [Google Scholar]
- Ren, Y.; Deng, F.; Zhu, H.; Wan, W.; Ye, J.; Luo, B. Effect of epigallocatechin-3-gallate on iron overload in mice with alcoholic liver disease. Mol. Biol. Rep. 2011, 38, 879–886. [Google Scholar]
- Megahed, F.A.K.; Zhou, X.; Sun, P. The Interactions Between HBV and the Innate Immunity of Hepatocytes. Viruses 2020, 12, 285. [Google Scholar] [CrossRef] [PubMed]
- Salpini, R.; D’Anna, S.; Benedetti, L.; Piermatteo, L.; Gill, U.; Svicher, V.; Kennedy, P.T.F. Hepatitis B virus DNA integration as a novel biomarker of hepatitis B virus-mediated pathogenetic properties and a barrier to the current strategies for hepatitis B virus cure. Front. Microbiol. 2022, 13, 972687. [Google Scholar]
- Nassal, M. HBV cccDNA: Viral persistence reservoir and key obstacle for a cure of chronic hepatitis B. Gut 2015, 64, 1972–1984. [Google Scholar]
- Wu, C.-C.; Chen, Y.-S.; Cao, L.; Chen, X.-W.; Lu, M.-J. Hepatitis B virus infection: Defective surface antigen expression and pathogenesis. World J. Gastroenterol. 2018, 24, 3488–3499. [Google Scholar]
- Mehmankhah, M.; Bhat, R.; Anvar, M.S.; Ali, S.; Alam, A.; Farooqui, A.; Amir, F.; Anwer, A.; Khan, S.; Azmi, I.; et al. Structure-Guided Approach to Identify Potential Inhibitors of Large Envelope Protein to Prevent Hepatitis B Virus Infection. Oxidative Med. Cell. Longev. 2019, 2019, 1297484. [Google Scholar]
- Cooper, A.; Paran, N.; Shaul, Y. The earliest steps in hepatitis B virus infection. Biochim. Biophys. Acta (BBA)-Biomembr. 2003, 1614, 89–96. [Google Scholar]
- Al-Bari, A.A.; Ito, Y.; Thomes, P.G.; Menon, M.B.; Garcia-Macia, M.; Fadel, R.; Stadlin, A.; Peake, N.; Faris, M.E.; Eid, N.; et al. Emerging mechanistic insights of selective autophagy in hepatic diseases. Front. Pharmacol. 2023, 14, 1149809. [Google Scholar] [CrossRef]
- Tian, Y.; Sir, D.; Kuo, C.F.; Ann, D.K.; Ou, J.H. Autophagy required for hepatitis B virus replication in transgenic mice. J. Virol. 2011, 85, 13453–13456. [Google Scholar] [CrossRef] [PubMed]
- Sir, D.; Tian, Y.; Chen, W.L.; Ann, D.K.; Yen, T.S.; Ou, J.H. The early autophagic pathway is activated by hepatitis B virus and required for viral DNA replication. Proc. Natl. Acad. Sci. USA 2010, 107, 4383–4388. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, Y.; Wang, Z.; Liu, K.; Wang, Y.; Liu, J.; Ding, H.; Yuan, Z. Subversion of cellular autophagy machinery by hepatitis B virus for viral envelopment. J. Virol. 2011, 85, 6319–6333. [Google Scholar] [CrossRef]
- Zhong, L.; Hu, J.; Shu, W.; Gao, B.; Xiong, S. Epigallocatechin-3-gallate opposes HBV-induced incomplete autophagy by enhancing lysosomal acidification, which is unfavorable for HBV replication. Cell Death Dis. 2015, 6, e1770. [Google Scholar] [CrossRef] [PubMed]
- Petruzziello, A.; Marigliano, S.; Loquercio, G.; Cozzolino, A.; Cacciapuoti, C. Global epidemiology of hepatitis C virus infection: An up-date of the distribution and circulation of hepatitis C virus genotypes. World J. Gastroenterol. 2016, 22, 7824–7840. [Google Scholar] [CrossRef]
- Lingala, S.; Ghany, M.G. Natural History of Hepatitis C. Gastroenterol. Clin. N. Am. 2015, 44, 717–734. [Google Scholar] [CrossRef]
- Gemma, S.; Brogi, S.; Novellino, E.; Campiani, G.; Maga, G.; Brindisi, M.; Butini, S. HCV-targeted Antivirals: Current Status and Future Challenges. Curr. Pharm. Des. 2014, 20, 3445–3464. [Google Scholar] [CrossRef]
- Song, J.M. Anti-infective potential of catechins and their derivatives against viral hepatitis. Clin. Exp. Vaccine Res. 2018, 7, 37–42. [Google Scholar] [CrossRef]
- Fukazawa, H.; Suzuki, T.; Wakita, T.; Murakami, Y. A cell-based, microplate colorimetric screen identifies 7,8-benzoflavone and green tea gallate catechins as inhibitors of the hepatitis C virus. Biol. Pharm. Bull. 2012, 35, 1320–1327. [Google Scholar] [CrossRef]
- Mekky, R.Y.; El-Ekiaby, N.M.; Hamza, M.T.; Elemam, N.M.; El-Sayed, M.; Esmat, G.; Abdelaziz, A.I. Mir-194 is a hepatocyte gate keeper hindering HCV entry through targeting CD81 receptor. J. Infect. 2015, 70, 78–87. [Google Scholar] [PubMed]
- Manns, M.P.; Lohse, A.W.; Vergani, D. Autoimmune hepatitis—Update 2015. J. Hepatol. 2015, 62 (Suppl. S1), S100–S111. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Gu, J.; Lin, J.; Wang, Y.; Yang, F.; Yin, J.; Yu, Z.; Wu, S.; Lv, H.; Ji, X.; et al. (−)-Epigallocatechin-3-gallate (EGCG) modulates polarized macrophages to suppress M1 phenotype and promote M2 polarization in vitro and in vivo. J. Funct. Foods 2021, 87, 104743. [Google Scholar]
- Akhtar, N.; Haqqi, T.M. Epigallocatechin-3-gallate suppresses the global interleukin-1beta-induced inflammatory response in human chondrocytes. Arthritis Res. Ther. 2011, 13, R93. [Google Scholar]
- Liu, D.; Zhang, X.; Jiang, L.; Guo, Y.; Zheng, C. Epigallocatechin-3-gallate (EGCG) attenuates concanavalin A-induced hepatic injury in mice. Acta Histochem. 2014, 116, 654–662. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- Nikolova-Karakashian, M. Alcoholic and non-alcoholic fatty liver disease: Focus on ceramide. Adv. Biol. Regul. 2018, 70, 40–50. [Google Scholar]
- Chen, C.; Liu, Q.; Liu, L.; Hu, Y.Y.; Feng, Q. Potential Biological Effects of (−)-Epigallocatechin-3-gallate on the Treatment of Nonalcoholic Fatty Liver Disease. Mol. Nutr. Food Res. 2018, 62, 1700483. [Google Scholar]
- Neuschwander-Tetri, B.A. Non-alcoholic fatty liver disease. BMC Med. 2017, 15, 45. [Google Scholar] [CrossRef]
- Younossi, Z.M. Non-alcoholic fatty liver disease—A global public health perspective. J. Hepatol. 2019, 70, 531–544. [Google Scholar]
- Pan, M.H.; Lai, C.S.; Tsai, M.L.; Ho, C.T. Chemoprevention of nonalcoholic fatty liver disease by dietary natural compounds. Mol. Nutr. Food Res. 2014, 58, 147–171. [Google Scholar]
- Marin-Juez, R.; Jong-Raadsen, S.; Yang, S.; Spaink, H.P. Hyperinsulinemia induces insulin resistance and immune suppression via Ptpn6/Shp1 in zebrafish. J. Endocrinol. 2014, 222, 229–241. [Google Scholar]
- Mu, W.; Cheng, X.F.; Liu, Y.; Lv, Q.Z.; Liu, G.L.; Zhang, J.G.; Li, X.Y. Potential Nexus of Non-alcoholic Fatty Liver Disease and Type 2 Diabetes Mellitus: Insulin Resistance Between Hepatic and Peripheral Tissues. Front. Pharmacol. 2018, 9, 1566. [Google Scholar]
- Yamamoto, K.; Ikeya, T.; Okuyama, S.; Fukuda, K.; Kobayashi, D. The association between non-alcoholic fatty liver disease (with or without metabolic syndrome) and extrahepatic cancer development. J. Gastroenterol. Hepatol. 2021, 36, 1971–1978. [Google Scholar]
- Li, X.; Zhang, Y.; Zhao, C.; Zhang, B.; Peng, B.; Zhang, Y.; Wang, J.; Wang, S. Positive effects of Epigallocatechin-3-gallate (EGCG) intervention on insulin resistance and gut microbial dysbiosis induced by bisphenol A. J. Funct. Foods 2022, 93, 105083. [Google Scholar]
- Luo, K.; Ma, C.; Xing, S.; An, Y.; Feng, J.; Dang, H.; Huang, W.; Qiao, L.; Cheng, J.; Xie, L. White tea and its active polyphenols lower cholesterol through reduction of very-low-density lipoprotein production and induction of LDLR expression. Biomed. Pharmacother. 2020, 127, 110146. [Google Scholar] [CrossRef] [PubMed]
- Naito, Y.; Ushiroda, C.; Mizushima, K.; Inoue, R.; Yasukawa, Z.; Abe, A.; Takagi, T. Epigallocatechin-3-gallate (EGCG) attenuates non-alcoholic fatty liver disease via modulating the interaction between gut microbiota and bile acids. J. Clin. Biochem. Nutr. 2020, 67, 2–9. [Google Scholar] [PubMed]
- Yu, J.; Marsh, S.; Hu, J.; Feng, W.; Wu, C. Gut Microbiota and Metagenomic Advancement in Digestive Disease. Gastroenterol. Res. Pract. 2016, 2016, 4703406. [Google Scholar]
- Takaki, A.; Kawai, D.; Yamamoto, K. Multiple hits, including oxidative stress, as pathogenesis and treatment target in non-alcoholic steatohepatitis (NASH). Int. J. Mol. Sci. 2013, 14, 20704–20728. [Google Scholar] [CrossRef]
- Yang, X.H.; Zhang, B.L.; Zhang, X.M.; Tong, J.D.; Gu, Y.H.; Guo, L.L.; Jin, H.M. EGCG Attenuates Renal Damage via Reversing Klotho Hypermethylation in Diabetic db/db Mice and HK-2 Cells. Oxidative Med. Cell. Longev. 2020, 2020, 6092715. [Google Scholar]
- Han, J.; Wang, M.; Jing, X.; Shi, H.; Ren, M.; Lou, H. (−)-Epigallocatechin gallate protects against cerebral ischemia-induced oxidative stress via Nrf2/ARE signaling. Neurochem. Res. 2014, 39, 1292–1299. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, F.; Adler, E.; Garcia-Pavia, P. Alcoholic cardiomyopathy: An update. Eur. Heart J. 2024, 45, 2294–2305. [Google Scholar] [CrossRef]
- Thoudam, T.; Gao, H.; Jiang, Y.; Huda, N.; Yang, Z.; Ma, J.; Liangpunsakul, S. Mitochondrial quality control in alcohol-associated liver disease. Hepatol. Commun. 2024, 8, 771. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Wang, F.; Wong, N.K.; Lv, Y.; Li, X.; Li, M.; Tipoe, G.L.; So, K.F.; Xu, A.; Chen, S.; et al. Divergent Roles of Kupffer Cell TLR2/3 Signaling in Alcoholic Liver Disease and the Protective Role of EGCG. Cell. Mol. Gastroenterol. Hepatol. 2020, 9, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, A. Hepatocellular Carcinoma. N. Engl. J. Med. 2019, 15, 1450–1462. [Google Scholar] [CrossRef]
- Xu, J. Trends in Liver Cancer Mortality Among Adults Aged 25 and Over in the United States, 2000–2016. NCHS Data Brief 2018, 314, 1–8. [Google Scholar]
- Zheng, R.; Qu, C.; Zhang, S.; Zeng, H.; Sun, K.; Gu, X.; Xia, C.; Yang, Z.; Li, H.; Wei, W.; et al. Liver cancer incidence and mortality in China: Temporal trends and projections to 2030. Chin. J. Cancer Res. 2018, 30, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.J.; von Felden, J.; Garcia-Lezana, T.; Sarcognato, S.; Villanueva, A. Tumour evolution in hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 139–152. [Google Scholar] [CrossRef]
- Balogh, J.; Victor, D., 3rd; Asham, E.H.; Burroughs, S.G.; Boktour, M.; Saharia, A.; Li, X.; Ghobrial, R.M.; Monsour, H.P., Jr. Hepatocellular carcinoma: A review. J. Hepatocell. Carcinoma 2016, 3, 41–53. [Google Scholar] [CrossRef]
- Niu, L.; Liu, L.; Yang, S.; Ren, J.; Lai, P.B.S.; Chen, G.G. New insights into sorafenib resistance in hepatocellular carcinoma: Responsible mechanisms and promising strategies. Biochim. Biophys. Acta Rev. Cancer 2017, 1868, 564–570. [Google Scholar] [CrossRef]
- Khiewkamrop, P.; Phunsomboon, P.; Richert, L.; Pekthong, D.; Srisawang, P. Epistructured catechins, EGCG and EC facilitate apoptosis induction through targeting de novo lipogenesis pathway in HepG2 cells. Cancer Cell Int. 2018, 18, 46. [Google Scholar] [PubMed]
- Roomi, M.W.; Monterrey, J.C.; Kalinovsky, T.; Rath, M.; Niedzwiecki, A. Comparative effects of EGCG, green tea and a nutrient mixture on the patterns of MMP-2 and MMP-9 expression in cancer cell lines. Oncol. Rep. 2010, 24, 747–757. [Google Scholar]
- Roomi, M.W.; Kalinovsky, T.; Bhanap, B.; Niedzwiecki, A.; Rath, M. In Vitro Effect of Cytokines, Inducers, and Inhibitors on the Secretion of MMP-2 and MMP-9 in Hepatocarcinoma Cell Line SK-Hep-1. Integr. Cancer Ther. 2019, 18, 1534735419889155. [Google Scholar]
- Zhang, Y.; Owusu, L.; Duan, W.; Jiang, T.; Zang, S.; Ahmed, A.; Xin, Y. Anti-metastatic and differential effects on protein expression of epigallocatechin-3-gallate in HCCLM6 hepatocellular carcinoma cells. Int. J. Mol. Med. 2013, 32, 959–964. [Google Scholar] [CrossRef]
- Zapf, M.A.; Kothari, A.N.; Weber, C.E.; Arffa, M.L.; Wai, P.Y.; Driver, J.; Gupta, G.N.; Kuo, P.C.; Mi, Z. Green tea component epigallocatechin-3-gallate decreases expression of osteopontin via a decrease in mRNA half-life in cell lines of metastatic hepatocellular carcinoma. Surgery 2015, 158, 1039–1047; discussion 1047–1048. [Google Scholar]
- Zhang, G.; Miura, Y.; Yagasaki, K. Suppression of adhesion and invasion of hepatoma cells in culture by tea compounds through antioxidative activity. Cancer Lett. 2000, 159, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Chang, Y.; Wei, W.; He, Y.F.; Hu, S.S.; Wang, D.; Wu, Y.J. Prostanoid EP1 receptor as the target of (−)-epigallocatechin-3-gallate in suppressing hepatocellular carcinoma cells in vitro. Acta Pharmacol. Sin. 2012, 33, 701–709. [Google Scholar]
- Kang, Q.; Tong, Y.; Gowd, V.; Wang, M.; Chen, F.; Cheng, K.W. Oral administration of EGCG solution equivalent to daily achievable dosages of regular tea drinkers effectively suppresses miR483-3p induced metastasis of hepatocellular carcinoma cells in mice. Food Funct. 2021, 12, 3381–3392. [Google Scholar]
- Ren, T.; Zhang, H.; Wang, J.; Zhu, J.; Jin, M.; Wu, Y.; Guo, X.; Ji, L.; Huang, Q.; Zhang, H.; et al. MCU-dependent mitochondrial Ca2+ inhibits NAD+/SIRT3/SOD2 pathway to promote ROS production and metastasis of HCC cells. Oncogene 2017, 36, 5897–5909. [Google Scholar] [CrossRef]
- Kuo, P.L.; Lin, C.C. Green tea constituent (−)-epigallocatechin-3-gallate inhibits Hep G2 cell proliferation and induces apoptosis through p53-dependent and Fas-mediated pathways. J. Biomed. Sci. 2003, 10, 219–227. [Google Scholar]
- Cho, A.-R.; Park, W.-Y.; Lee, H.-J.; Sim, D.-Y.; Im, E.; Park, J.-E.; Ahn, C.-H.; Shim, B.-S.; Kim, S.-H. Antitumor Effect of Morusin via G1 Arrest and Antiglycolysis by AMPK Activation in Hepatocellular Cancer. Int. J. Mol. Sci. 2021, 22, 10619. [Google Scholar] [CrossRef]
- Shirakami, Y.; Shimizu, M.; Adachi, S.; Sakai, H.; Nakagawa, T.; Yasuda, Y.; Tsurumi, H.; Hara, Y.; Moriwaki, H. (−)-Epigallocatechin gallate suppresses the growth of human hepatocellular carcinoma cells by inhibiting activation of the vascular endothelial growth factor-vascular endothelial growth factor receptor axis. Cancer Sci. 2009, 100, 1957–1962. [Google Scholar] [PubMed]
- Tang, Y.; Cao, J.; Cai, Z.; An, H.; Li, Y.; Peng, Y.; Chen, N.; Luo, A.; Tao, H.; Li, K. Epigallocatechin gallate induces chemopreventive effects on rats with diethylnitrosamineinduced liver cancer via inhibition of cell division cycle 25A. Mol. Med. Rep. 2020, 22, 3873–3885. [Google Scholar] [PubMed]
- Caban, M.; Owczarek, K.; Chojnacka, K.; Lewandowska, U. Overview of polyphenols and polyphenol-rich extracts as modulators of IGF-1, IGF-1R, and IGFBP expression in cancer diseases. J. Funct. Foods 2019, 52, 389–407. [Google Scholar] [CrossRef]
- Yin, Y.; Chen, C.; Chen, J.; Zhan, R.; Zhang, Q.; Xu, X.; Li, D.; Li, M. Cell surface GRP78 facilitates hepatoma cells proliferation and migration by activating IGF-IR. Cell. Signal. 2017, 35, 154–162. [Google Scholar] [CrossRef]
- Sur, S.; Pal, D.; Mandal, S.; Roy, A.; Panda, C.K. Tea polyphenols epigallocatechin gallete and theaflavin restrict mouse liver carcinogenesis through modulation of self-renewal Wnt and hedgehog pathways. J. Nutr. Biochem. 2016, 27, 32–42. [Google Scholar] [CrossRef]
- Gao, F.; Li, M.; Liu, W.B.; Zhou, Z.S.; Zhang, R.; Li, J.L.; Zhou, K.C. Epigallocatechin gallate inhibits human tongue carcinoma cells via HK2-mediated glycolysis. Oncol. Rep. 2015, 33, 1533–1539. [Google Scholar] [CrossRef]
- Li, S.; Wu, L.; Feng, J.; Li, J.; Liu, T.; Zhang, R.; Xu, S.; Cheng, K.; Zhou, Y.; Zhou, S.; et al. In vitro and in vivo study of epigallocatechin-3-gallate-induced apoptosis in aerobic glycolytic hepatocellular carcinoma cells involving inhibition of phosphofructokinase activity. Sci. Rep. 2016, 6, 28479. [Google Scholar]
- Tong, J.L.; Nie, F.; Ran, Z.H.; Pan, C.Q.; Xu, X.T.; Zhu, M.M.; Xiao, S.D. Epigallocatechin gallate induces apoptosis in human hepatocellular carcinoma HepG2 cells via TGF/Smad signaling pathway. Zhonghua Zhong Liu Za Zhi 2009, 31, 646–650. [Google Scholar]
- Shen, X.; Zhang, Y.; Feng, Y.; Zhang, L.; Li, J.; Xie, Y.A.; Luo, X. Epigallocatechin-3-gallate inhibits cell growth, induces apoptosis and causes S phase arrest in hepatocellular carcinoma by suppressing the AKT pathway. Int. J. Oncol. 2014, 44, 791–796. [Google Scholar] [CrossRef]
- Tsang, W.P.; Kwok, T.T. Epigallocatechin gallate up-regulation of miR-16 and induction of apoptosis in human cancer cells. J. Nutr. Biochem. 2010, 21, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wang, J.; Deng, X.; Xiong, F.; Zhang, S.; Gong, Z.; Li, X.; Cao, K.; Deng, H.; He, Y.; et al. The role of microenvironment in tumor angiogenesis. J. Exp. Clin. Cancer Res. 2020, 39, 204. [Google Scholar]
- Liao, Z.-H.; Zhu, H.-Q.; Chen, Y.-Y.; Chen, R.-L.; Fu, L.-X.; Li, L.; Zhou, H.; Zhou, J.-L.; Liang, G. The epigallocatechin gallate derivative Y 6 inhibits human hepatocellular carcinoma by inhibiting angiogenesis in MAPK/ERK1/2 and PI3K/AKT/HIF-1α/VEGF dependent pathways. J. Ethnopharmacol. 2020, 259, 112852. [Google Scholar]
- Hashimoto, O.; Nakamura, A.; Nakamura, T.; Iwamoto, H.; Hiroshi, M.; Inoue, K.; Torimura, T.; Ueno, T.; Sata, M. Methylated-(3″)-epigallocatechin gallate analog suppresses tumor growth in Huh7 hepatoma cells via inhibition of angiogenesis. Nutr. Cancer 2014, 66, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Chen, T.; Ho, C.-T. Redox and Other Biological Activities of Tea Catechins That May Affect Health: Mechanisms and Unresolved Issues. J. Agric. Food Chem. 2022, 70, 7887–7899. [Google Scholar]
- Nikoo, M.; Regenstein, J.M.; Ahmadi Gavlighi, H. Antioxidant and Antimicrobial Activities of (−)-Epigallocatechin-3-gallate (EGCG) and its Potential to Preserve the Quality and Safety of Foods. Compr. Rev. Food Sci. Food Saf. 2018, 17, 732–753. [Google Scholar] [PubMed]
- Dekant, W.; Fujii, K.; Shibata, E.; Morita, O.; Shimotoyodome, A. Safety assessment of green tea based beverages and dried green tea extracts as nutritional supplements. Toxicol. Lett. 2017, 277, 104–108. [Google Scholar]
- Shiha, G.; Soliman, R.; Elbasiony, M.; Darwish, N.H.E.; Mousa, S.A. Addition of Epigallocatechin Gallate 400 mg to Sofosbuvir 400 mg + Daclatisvir 60 mg with or Without Ribavirin in Treatment of Patients with Chronic Hepatitis C Improves the Safety Profile: A Pilot Study. Sci. Rep. 2019, 9, 13593. [Google Scholar] [CrossRef] [PubMed]
- Shiha, G.; Soliman, R.; Elbasiony, M.; Darwish, N.H.E.; Mousa, S.A. Novel combined single dose anti-hepatitis C therapy: A pilot study. Sci. Rep. 2021, 11, 4623. [Google Scholar]
- Zhu, Z.; Wang, Y.; Liu, Z.; Wang, F.; Zhao, Q. Inhibitory effects of epigallocatechin-3-gallate on cell proliferation and the expression of HIF-1α and P-gp in the human pancreatic carcinoma cell line PANC-1. Oncol. Rep. 2012, 27, 1567–1572. [Google Scholar]
- Chen, L.; Ye, H.L.; Zhang, G.; Yao, W.M.; Chen, X.Z.; Zhang, F.C.; Liang, G. Autophagy inhibition contributes to the synergistic interaction between EGCG and doxorubicin to kill the hepatoma Hep3B cells. PLoS ONE 2014, 9, e85771. [Google Scholar]
- Wang, H.; Jiang, H.; Zhou, M.; Xu, Z.; Liu, S.; Shi, B.; Yao, X.; Yao, M.; Gu, J.; Li, Z. Epidermal growth factor receptor vIII enhances tumorigenicity and resistance to 5-fluorouracil in human hepatocellular carcinoma. Cancer Lett. 2009, 279, 30–38. [Google Scholar]
- Yang, X.W.; Wang, X.L.; Cao, L.Q.; Jiang, X.F.; Peng, H.P.; Lin, S.M.; Xue, P.; Chen, D. Green tea polyphenol epigallocatechin-3-gallate enhances 5-fluorouracil-induced cell growth inhibition of hepatocellular carcinoma cells. Hepatol. Res. 2012, 42, 494–501. [Google Scholar]
- Sabry, D.; Abdelaleem, O.O.; El Amin Ali, A.M.; Mohammed, R.A.; Abdel-Hameed, N.D.; Hassouna, A.; Khalifa, W.A. Anti-proliferative and anti-apoptotic potential effects of epigallocatechin-3-gallate and/or metformin on hepatocellular carcinoma cells: In vitro study. Mol. Biol. Rep. 2019, 46, 2039–2047. [Google Scholar]
- Yang, H.; Wang, M.; Sun, H.; Zhu, S.; Jin, J. Synergetic Effect of EP1 Receptor Antagonist and (−)-Epigallocatechin-3-gallate in Hepatocellular Carcinoma. Pharmacology 2019, 104, 267–275. [Google Scholar]
- Abou El Naga, R.N.; Azab, S.S.; El-Demerdash, E.; Shaarawy, S.; El-Merzabani, M.; Ammar, E.M. Sensitization of TRAIL-induced apoptosis in human hepatocellular carcinoma HepG2 cells by phytochemicals. Life Sci. 2013, 92, 555–561. [Google Scholar]
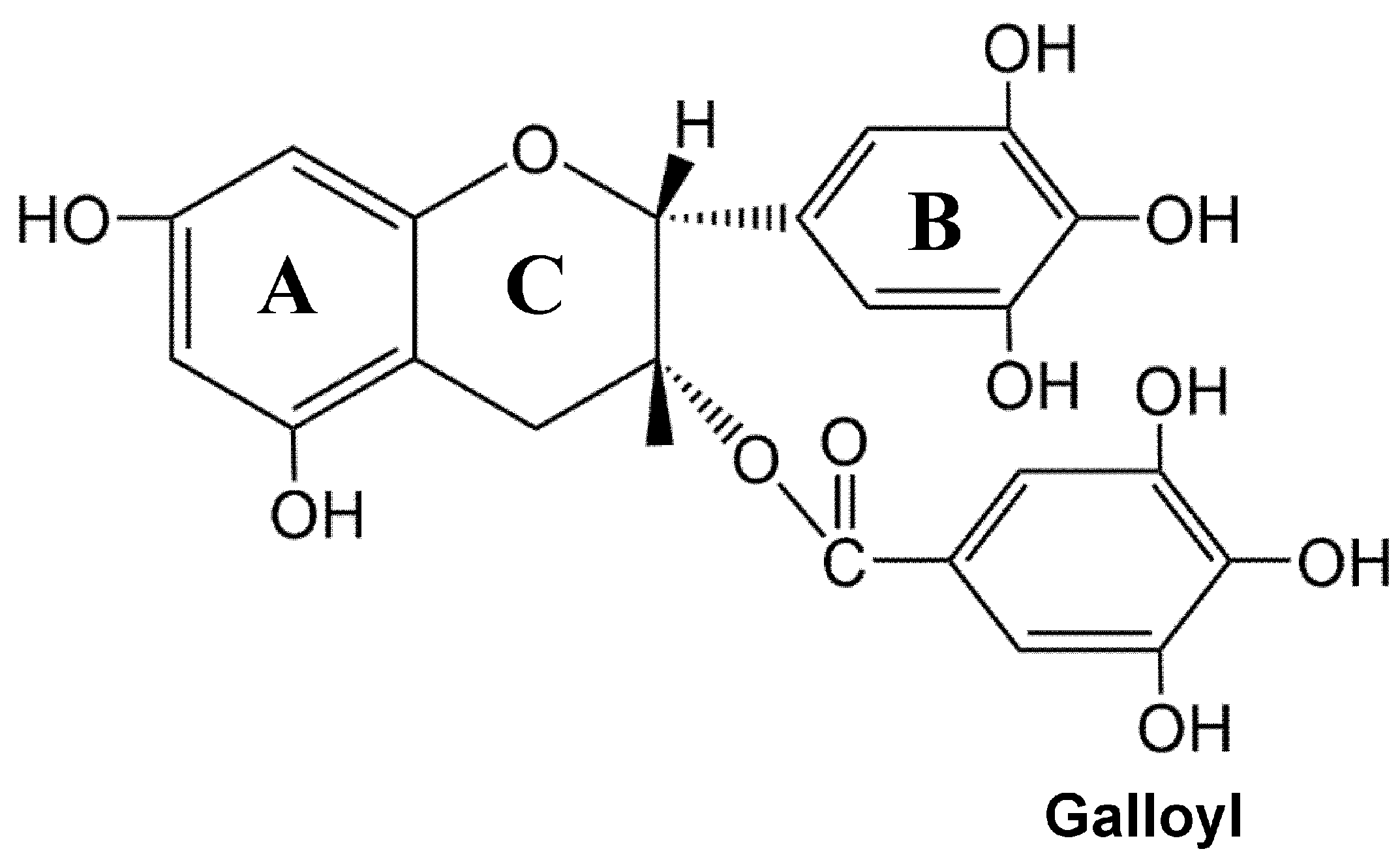
| No. | Disease Type | Model/Species | Test Compound | Dose | Administration Method | Effects | References |
|---|---|---|---|---|---|---|---|
| 1 | Hepatitis B virus | HepG2-N10 cells | GTE (contains EGCG) | - | - | The 50% effective concentrations of GTE on HBsAg, HBeAg, extracellular HBV DNA, and intracellular HBV DNA were 5.02, 5.681, 19.81, and 10.76 mu g/mL, respectively. | [36] |
| 2 | Hepatitis B virus | HuS-E/2 and Huh7 cells | EGCG | 50 μM | - | EGCG has the potential to inhibit HBV entry into host cells, with an inhibition rate of up to 80% at a concentration of 50 μM; EGCG induced clathrin-dependent endocytosis of NTCP from the plasma membrane followed by protein degradation; EGCG inhibited the clathrin-mediated endocytosis of transferrin. | [37] |
| 3 | Hepatitis B virus | HuS-E/2 cells | EGCG | 0, 10, and 20 μM | - | EGCG treatment during infection led to a dose-dependent reduction in HBV rcDNA and HBsAg mRNA in HuS-E/2 cells, with HBV mRNA levels being decreased by 80% compared to control cells when treated with 10 μM of EGCG. The half-maximal inhibitory concentration was estimated to be below 10 μM. | [38] |
| 4 | Hepatitis B virus | Hu-FRG mice | EGCG | 50 mg/kg | Injected intraperitoneally | EGCG inhibited HBV infection, the expression of FAH and HBcAg. | [38] |
| 5 | Hepatitis B virus | HepG2.117 cells | EGCG | 0, 50, 100, 200, and 400 μM | - | EGCG inhibited HBV replication by disrupting the synthesis of HBV replicative intermediates, leading to a decrease in the production of HBV covalently closed circular DNA. | [39] |
| 6 | Hepatitis B virus | Hep3B2.1-7 cells | EGCG | 100 μM | - | EGCG could have strong effects on HBsAg and HBeAg levels and prevent HBV DNA replication. | [40] |
| 7 | Hepatitis B virus | HepG2 2.2.15 cells | EGCG | 0.11–0.44 μM | - | EGCG effectively suppressed the secretion of HBsAg and HBeAg in a dose- and time-dependent manner. | [41] |
| 8 | Hepatitis B virus | HepG2.2.15 cells | EGCG | 12.5–50 μM | - | EGCG dose-dependently inhibited HBV gene expression and replication; EGCG significantly activated ERK1/2 MAPK signaling, and slightly activated p38 MAPK and JAK2/STAT3 signaling | [42] |
| 9 | Hepatitis B virus | HBV infection mice | EGCG | 25 mg/kg | Injected intraperitoneally | EGCG inhibited HBV gene expression and replication, which involves ERK1/2-mediated downregulation of HNF4α. | [42] |
| 10 | Hepatitis B virus | HepG2-N10 cells | EGCG | 0–100 μM | - | EGCG inhibited the regulation of HBV antigens by interacting with FXRα, which in turn regulates HBV antigens and activates Enhll core promoter activity through the FXRα/RXRα axis. | [43] |
| 11 | Hepatitis C virus | Huh7 HCVcc cells | EGCG | 10 μg/mL | - | EGCG enhanced miR-548m expression and repressing CD81 receptor to reduce cellular infectivity. | [44] |
| 12 | Hepatitis C virus | Huh-7 cells | EGCG | 50 μM | - | EGCG altered the viral particle structure and impaired its attachment to the cell surface. | [45] |
| 13 | Hepatitis C virus | Huh-7.5 cells and Primary human hepatocytes | EGCG | 0–100 μM | - | EGCG inhibited cell-culture-derived HCV entry into hepatoma cell lines as well as primary human hepatocytes. | [46] |
| 14 | Hepatitis C virus | Huh-7 cells | EGCG | 0–10 μM | - | EGCG significantly enhanced HCV dsRNAs-induced expression of IFN-lambda 1, TLR3, RIG-I, and antiviral ISGs in hepatocytes. | [47] |
| 15 | Autoimmune hepatitis | Bovine hepatocytes | EGCG | 50 μM | - | GCG significantly attenuates inflammatory reactions and oxidative stress under the control of the NF-κB and MAPK cascades and the Nrf2 complex. | [48] |
| 16 | Autoimmune hepatitis | Balb/C mice received intraperitoneal injection with GalN (700 mg/kg) and LPS (10 μg/kg) | EGCG | 10, 25, and 50 mg/kg | Oral gavage | EGCG was hepatoprotective via inhibition of MAPK/NF-κB signaling and activation of the Nrf2 cascade. | [48] |
| 17 | Autoimmune hepatitis | Balb/C mice were injected with ConA (25 mg/kg) | EGCG | 10 and 30 mg/kg | Oral gavage | EGCG attenuated liver injury in ConA-induced hepatitis by downregulating IL-6/JAKs/STAT3/BNIP3-mediated apoptosis and autophagy. | [49] |
| 18 | Non-alcoholic fatty liver disease | HFD-fed mice | EGCG | 10, 20, and 40 mg/kg | Injected intraperitoneally | EGCG demonstrated dose-dependent improvement in hepatic morphology and function, reduction in body weight, and alleviation of hyperlipidemia, hyperglycemia, hyperinsulinemia, and insulin resistance in NAFLD mice. Additionally, EGCG dose-dependently enhanced insulin clearance and upregulated IDE protein expression and enzyme activity in the liver of NAFLD mice. | [50] |
| 19 | Non-alcoholic fatty liver disease | HepG2 cells | EGCG | 10 μM | - | EGCG was capable of enhancing insulin-mediated glucose and lipid metabolism by regulating enzymes involved in glycogen synthesis and lipogenesis. | [51] |
| 20 | Non-alcoholic fatty liver disease | HepG2 cells | EGCG | 50 μM | EGCG reduced cellular lipid accumulation in FFA-induced HepG2 cells through the activation of AMP-activated protein kinase resulting from the generation of reactive oxygen species | [52] | |
| 21 | Non-alcoholic fatty liver disease | HFD-fed mice | GTE (contain EGCG) | 50 mg/kg | Oral gavage | The effects of decaffeinated green tea extract may be related to the activation of AMPK via LKB1 in the liver of HFD-fed mice. | [53] |
| 22 | Non-alcoholic fatty liver disease | HFD-C57BL/6 mice | GTE (contain EGCG) | 1% (w/w) | Additive feed | EGCG decreased post-prandial triglyceride and glycogen content in liver, increased oxidation of dietary lipids, and decreased incorporation of dietary 13C-enriched lipids into fat tissues, liver, and skeletal muscle. EGCG dose-dependently reversed high-fat diet-induced effects on intestinal substrate transporters (CD36, FATP4, and SGLT1) and downregulated lipogenesis-related genes (ACC, FAS, and SCD1) in the liver. | [54] |
| 23 | Non-alcoholic fatty liver disease | HFD-fed mice | EGCG | 50 mg/kg | Oral gavage | EGCG also increases the oxidation of long-chain fatty acids by increasing the activity of the mitochondrial complex, thus halting NAFLD progression. | [55] |
| 24 | Non-alcoholic steatohepatitis | MCD diet mice | EGCG | 25, 50, and 100 mg/kg | Oral gavage | EGCG attenuated NASH induced by MCD diet associated with ameliorating fibrosis, oxidative stress, and hepatic inflammation. | [56] |
| 25 | Non-alcoholic fatty liver disease | HFD-fed rats | EGCG | 50 mg/kg | Injected intraperitoneally | EGCG reduced the severity of liver injury in an experimental model of NAFLD associated with lower concentration of pro-fibrogenic, oxidative stress, and pro-inflammatory mediators partly through modulating the activities of the TGF/SMAD, PI3 K/Akt/FoxO1, and NF-kappa B pathways. | [57] |
| 26 | Non-alcoholic steatohepatitis | CCL4-induced rats | EGCG | 0.1% (w/w) | Injected intraperitoneally | EGCG inhibited the development of hepatic premalignant lesions by improving liver fibrosis, inhibiting RAS activation, and attenuating inflammation and oxidative stress. | [58] |
| 27 | Non-alcoholic fatty liver disease | HFD-induced mice | EGCG | 25 and 50 mg/kg | Oral gavage | EGCG impacted M1/M2 macrophage polarization. | [59] |
| 28 | Non-alcoholic fatty liver disease | HFD-induced mice | EGCG | 50 mg/kg | Oral gavage | EGCG alleviated HFD-induced NAFLD possibly by decreasing apoptosis and increasing autophagy via the ROS/MAPK pathway. | [60] |
| 29 | Non-alcoholic fatty liver disease | HFD-induced mice | EGCG | 0.32% (w/w) | Additive feed | EGCG could alter bile acid metabolism, especially taurine deconjugation, and suppress fatty liver disease by improving the intestinal luminal environment. | [61] |
| 30 | Alcohol-related fatty liver disease | Alcohol-fed rats | EGCG | 200 mg/kg | Oral gavage | EGCG inhibited fatty acid synthesis and the alleviation of lipid peroxidation through the downregulation of the mRNA and protein expression of TNF-alpha, SREBP1c, and CYP2E1 and the upregulation of the mRNA and protein expression of ADH1, ALDH2, Lipin-1, PPAR α, AMPK, and PGC-1 α, thereby promoting the oxidative decomposition of fatty acids and reducing the synthesis of cholesterol and glucose. | [62] |
| 31 | Alcohol-related fatty liver disease | Alcohol-fed rats | EGCG | 3 g/L | Additive feed | EGCG markedly reversed the effect of ethanol on hepatic p-ACC and CPT-1 levels, prevented ethanol-induced hepatotoxicity, and inhibits the development of a fatty liver. | [63] |
| 32 | Alcohol-related fatty liver disease | Alcohol-fed mice | EGCG | 10, 20, and 30 mg/kg | Injected intraperitoneally | EGCG ameliorated liver injuries; decreased serum iron level, hepatic iron levels, and liver MDA contents; increased hepcidin mRNA level; and decreased Tf and TfR1 protein expression in the liver. | [64] |
| No. | Compounds | Effects on HCC | Proposed Mechanisms of Anti-Hepatocarcinogenesis | References |
|---|---|---|---|---|
| 1 | EGCG + doxorubicin | Inhibit proliferation, enhance cell sensitivity to doxorubicin | Downregulate expression of MDR1 and p-glycoprotein, inhibit autophagy | [141] |
| 2 | EGCG + 5-FU | Inhibit proliferation | Activate AMPK, decrease COX2 expression and reduce PEG2 secretion, inhibit AKT signaling | [143] |
| 3 | EGCG + Metformin | Cell cycle arrest, promote apoptosis, inhibit angiogenesis | Downregulate expression of cyclinD1, survivin and VEGFA, upregulate caspase3 | [144] |
| 4 | EGCG + EP1 inhibitor | Inhibit migration and survival | Suppress EP1 receptor expression and PGE2 production | [145] |
| 5 | EGCG + TRAIL | Enhanced cell sensitivity to TRAIL, promote apoptosis | Enhance caspase3 activity, induce DR4/DR5 expression, downregulate Bcl-2 expression | [146] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, F.; Deng, S.; Luo, Y.; Liu, Z.; Liu, C. Research Progress on the Protective Effect of Green Tea Polyphenol (-)-Epigallocatechin-3-Gallate (EGCG) on the Liver. Nutrients 2025, 17, 1101. https://doi.org/10.3390/nu17071101
Zhou F, Deng S, Luo Y, Liu Z, Liu C. Research Progress on the Protective Effect of Green Tea Polyphenol (-)-Epigallocatechin-3-Gallate (EGCG) on the Liver. Nutrients. 2025; 17(7):1101. https://doi.org/10.3390/nu17071101
Chicago/Turabian StyleZhou, Fang, Sengwen Deng, Yong Luo, Zhonghua Liu, and Changwei Liu. 2025. "Research Progress on the Protective Effect of Green Tea Polyphenol (-)-Epigallocatechin-3-Gallate (EGCG) on the Liver" Nutrients 17, no. 7: 1101. https://doi.org/10.3390/nu17071101
APA StyleZhou, F., Deng, S., Luo, Y., Liu, Z., & Liu, C. (2025). Research Progress on the Protective Effect of Green Tea Polyphenol (-)-Epigallocatechin-3-Gallate (EGCG) on the Liver. Nutrients, 17(7), 1101. https://doi.org/10.3390/nu17071101







