Can Iron Absorption in Molasses Be Increased with Probiotic Additives? “Molasses with Increased Bioavailability”
Abstract
1. Introduction
2. Method
3. Procedures
4. Results
5. Quantitative Analysis
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Özbey, A.; Öncül, N.; Erdoğan, K.; Yıldırım, Z.; Yıldırım, M. Some Physical, Chemical and Microbiological Properties of Çalma Pekmez Produced in Tokat Region, Turkey. Akad. Gıda. 2013, 11, 46–52. Available online: https://dergipark.org.tr/tr/pub/akademik-gida/issue/55794/763795 (accessed on 23 December 2023).
- Akbulut, M.; Özcan, M. Some Physical, Chemical, and Rheological Properties of Sweet Sorghum (Sorghum bicolor (L) Moench) Pekmez (Molasses). Int. J. Food Prop. 2008, 11, 79–91. [Google Scholar] [CrossRef]
- Salık, M.A.; Arslaner, A.; Çakmakçı, S. Determination of Some Physical, Chemical and Antioxidant Properties of Erzincan Traditional Mulberry Pekmez (Molasses). Turk. J. Agric. Food Sci. Technol. 2021, 9, 181–190. [Google Scholar] [CrossRef]
- Akbulut, M.; Özcan, M.M. Comparison of mineral contents of mulberry (Morus spp.) fruits and their pekmez (boiled mulberry juice) samples. Int. J. Food Sci. Nutr. 2009, 60, 231–239. [Google Scholar] [CrossRef]
- Cifci, A.; Özkan, M. Iron physiopathology and approach to iron deficiency anemia: New treatment strategies. J. Health Sci. Med. 2018, 1, 40–44. [Google Scholar] [CrossRef][Green Version]
- Hoppe, M.; Önning, G.; Berggren, A.; Hulthén, L. Probiotic strain Lactobacillus plantarum 299v increases iron absorption from an iron-supplemented fruit drink: A double-isotope cross-over single-blind study in women of reproductive age. Br. J. Nutr. 2015, 114, 1195–1202. [Google Scholar] [CrossRef]
- Rosen, G.M.; Morrissette, S.; Larson, A.; Stading, P.; Griffin, K.H.; Barnes, T.L. Use of a Probiotic to Enhance Iron Absorption in a Randomized Trial of Pediatric Patients Presenting with Iron Deficiency. J. Pediatr. 2019, 207, 192–197. [Google Scholar] [CrossRef]
- Chaudhari, A.S.; Raghuvanshi, R.; Kumar, G.N. Genetically engineered Escherichia coli Nissle 1917 synbiotic counters fructose-induced metabolic syndrome and iron deficiency. Appl. Microbiol. Biotechnol. 2017, 101, 4713–4723. [Google Scholar] [CrossRef]
- Vonderheid, S.C.; Tussing-Humphreys, L.; Park, C.; Pauls, H.; Hemphill, N.O.; LaBomascus, B.; McLeod, A.; Koenig, M.D. A Systematic Review and Meta-Analysis on the Effects of Probiotic Species on Iron Absorption and Iron Status. Nutrients 2019, 11, 2938. [Google Scholar] [CrossRef]
- Kasaoka, S.; Yamagishi, H.; Kitano, T. Differences in the effect of iron-deficient diet on tissue weight, hemoglobin concentration and serum triglycerides in Fischer-344, Sprague-Dawley and Wistar rats. J. Nutr. Sci. Vitaminol. 1999, 45, 359–366. [Google Scholar] [CrossRef]
- Kortman, G.A.M.; Mulder, M.L.M.; Richters, T.J.W.; Shanmugam, N.K.N.; Trebicka, E.; Boekhorst, J.; Timmerman, H.M.; Roelofs, R.; Wiegerinck, E.T.; Laarakkers, C.M.; et al. Low dietary iron intake restrains the intestinal inflammatory response and pathology of enteric infection by food-borne bacterial pathogens. Eur. J. Immunol. 2015, 45, 2553–2567. [Google Scholar] [CrossRef] [PubMed]
- Skrypnik, K.; Bogdański, P.; Schmidt, M.; Suliburska, J. The Effect of Multispecies Probiotic Supplementation on Iron Status in Rats. Biol. Trace Element Res. 2019, 192, 234–243. [Google Scholar] [CrossRef]
- Deschemin, J.; Noordine, M.; Remot, A.; Willemetz, A.; Afif, C.; Canonne-Hergaux, F.; Langella, P.; Karim, Z.; Vaulont, S.; Thomas, M.; et al. The microbiota shifts the iron sensing of intestinal cells. FASEB J. 2015, 30, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Karababa, E.; Isikli, N.D. Pekmez: A Traditional Concentrated Fruit Product. Food Rev. Int. 2005, 21, 357–366. [Google Scholar] [CrossRef]
- Softic, S.; Stanhope, K.L.; Boucher, J.; Divanovic, S.; Lanaspa, M.A.; Johnson, R.J.; Kahn, C.R. Fructose and hepatic insulin resistance. Crit. Rev. Clin. Lab. Sci. 2020, 57, 308–322. [Google Scholar] [CrossRef]
- Lowndes, J.; Sinnett, S.; Yu, Z.; Rippe, J. The Effects of Fructose-Containing Sugars on Weight, Body Composition and Cardiometabolic Risk Factors When Consumed at up to the 90th Percentile Population Consumption Level for Fructose. Nutrients 2014, 6, 3153–3168. [Google Scholar] [CrossRef]
- Cerdó, T.; García-Santos, J.A.; Bermúdez, M.G.; Campoy, C. The Role of Probiotics and Prebiotics in the Prevention and Treatment of Obesity. Nutrients 2019, 11, 635. [Google Scholar] [CrossRef]
- Crovesy, L.; Ostrowski, M.; Ferreira, D.M.T.P.; Rosado, E.L.; Soares-Mota, M. Effect of Lactobacillus on body weight and body fat in overweight subjects: A systematic review of randomized controlled clinical trials. Int. J. Obes. 2017, 41, 1607–1614. [Google Scholar] [CrossRef]
- Mo, R.; Zhang, X.; Yang, Y. Effect of probiotics on lipid profiles in hypercholesterolaemic adults: A meta-analysis of randomized controlled trials. Med. Clin. 2018, 152, 473–481. [Google Scholar] [CrossRef]
- Khalili, H.; de Silva, P.S.; Ananthakrishnan, A.N.; Lochhead, P.; Joshi, A.; Garber, J.J.; Richter, J.R.; Sauk, J.; Chan, A.T. Dietary Iron and Heme Iron Consumption, Genetic Susceptibility, and Risk of Crohn’s Disease and Ulcerative Colitis. Inflamm. Bowel Dis. 2017, 23, 1088–1095. [Google Scholar] [CrossRef]
- Ng, O. Iron, microbiota and colorectal cancer. Wien. Med. Wochenschr. 2016, 166, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Batra, J.; Seth, P.K. Effect of iron deficiency on developing rat brain. Indian J. Clin. Biochem. 2002, 17, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Gunshin, H.; Fujiwara, Y.; Custodio, A.O.; DiRenzo, C.; Robine, S.; Andrews, N.C. Slc11a2 is required for intestinal iron absorption and erythropoiesis but dispensable in placenta and liver. J. Clin. Investig. 2005, 115, 1258–1266. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Xing, Y.; Liu, Y. Emerging roles for the ER stress sensor IRE1α in metabolic regulation and disease. J. Biol. Chem. 2019, 294, 18726–18741. [Google Scholar] [CrossRef]
- Shi, F.; Wang, Z.; Wu, Q.; Zhong, X.; Zhang, M.; Li, B.; Ren, W.; Yuan, S.; Chen, Y. Iron deficiency promotes aortic media degeneration by activating endoplasmic reticulum stress-mediated IRE1 signaling pathway. Pharmacol. Res. 2022, 183, 106366. [Google Scholar] [CrossRef]
- Song, S.; Christova, T.; Perusini, S.; Alizadeh, S.; Bao, R.-Y.; Miller, B.W.; Hurren, R.; Jitkova, Y.; Gronda, M.; Isaac, M.; et al. Wnt Inhibitor Screen Reveals Iron Dependence of β-Catenin Signaling in Cancers. Cancer Res. 2011, 71, 7628–7639. [Google Scholar] [CrossRef]
- Luo, C.; Xu, W.; Tang, X.; Liu, X.; Cheng, Y.; Wu, Y.; Xie, Z.; Wu, X.; He, X.; Wang, Q.; et al. Canonical Wnt signaling works downstream of iron overload to prevent ferroptosis from damaging osteoblast differentiation. Free. Radic. Biol. Med. 2022, 188, 337–350. [Google Scholar] [CrossRef]
- Scheers, N.; Rossander-Hulthen, L.; Torsdottir, I.; Sandberg, A.-S. Increased iron bioavailability from lactic-fermented vegetables is likely an effect of promoting the formation of ferric iron (Fe3+). Eur. J. Nutr. 2015, 55, 373–382. [Google Scholar] [CrossRef]
- Manoppo, J.; Tasiringan, H.; Wahani, A.; Umboh, A.; Mantik, M. The role of Lactobacillus reuteri DSM 17938 for the absorption of iron preparations in children with iron deficiency anemia. Korean J. Pediatr. 2019, 62, 173–178. [Google Scholar] [CrossRef]
- Khan, W.U.; Shafique, S.; Shikder, H.; Shakur, Y.A.; Sellen, D.W.; Chowdhury, J.S.; Zlotkin, S.H. Home fortification with calcium reduces Hb response to iron among anaemic Bangladeshi infants consuming a new multi-micronutrient powder formulation. Public Health Nutr. 2013, 17, 1578–1586. [Google Scholar] [CrossRef]
- Hoppe, M.; Önning, G.; Hulthén, L. Freeze-dried Lactobacillus plantarum 299v increases iron absorption in young females—Double isotope sequential single-blind studies in menstruating women. PLoS ONE 2017, 12, e0189141. [Google Scholar] [CrossRef] [PubMed]
- Ciont, C.; Mesaroș, A.; Pop, O.L.; Vodnar, D.C. Iron oxide nanoparticles carried by probiotics for iron absorption: A systematic review. J. Nanobiotechnol. 2023, 21, 1–19. [Google Scholar] [CrossRef] [PubMed]
- González, A.; Gálvez, N.; Martín, J.; Reyes, F.; Pérez-Victoria, I.; Dominguez-Vera, J.M. Identification of the key excreted molecule by Lactobacillus fermentum related to host iron absorption. Food Chem. 2017, 228, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Garcés, V.; Rodríguez-Nogales, A.; González, A.; Gálvez, N.; Rodríguez-Cabezas, M.E.; García-Martín, M.-L.; Gutiérrez, L.; Rondón, D.; Olivares, M.; Gálvez, J.; et al. Bacteria-Carried Iron Oxide Nanoparticles for Treatment of Anemia. Bioconj. Chem. 2018, 29, 1785–1791. [Google Scholar] [CrossRef]
- Novin, D.; Seifan, M.; Ebrahiminezhad, A.; Berenjian, A. The effect of iron oxide nanoparticles on Lactobacillus acidophilus growth at pH 4. Bioprocess Biosyst. Eng. 2021, 44, 39–45. [Google Scholar] [CrossRef]
- Rusu, I.G.; Suharoschi, R.; Vodnar, D.C.; Pop, C.R.; Socaci, S.A.; Vulturar, R.; Istrati, M.; Moroșan, I.; Fărcaș, A.C.; Kerezsi, A.D.; et al. Iron Supplementation Influence on the Gut Microbiota and Probiotic Intake Effect in Iron Deficiency—A Literature-Based Review. Nutrients 2020, 12, 1993. [Google Scholar] [CrossRef]

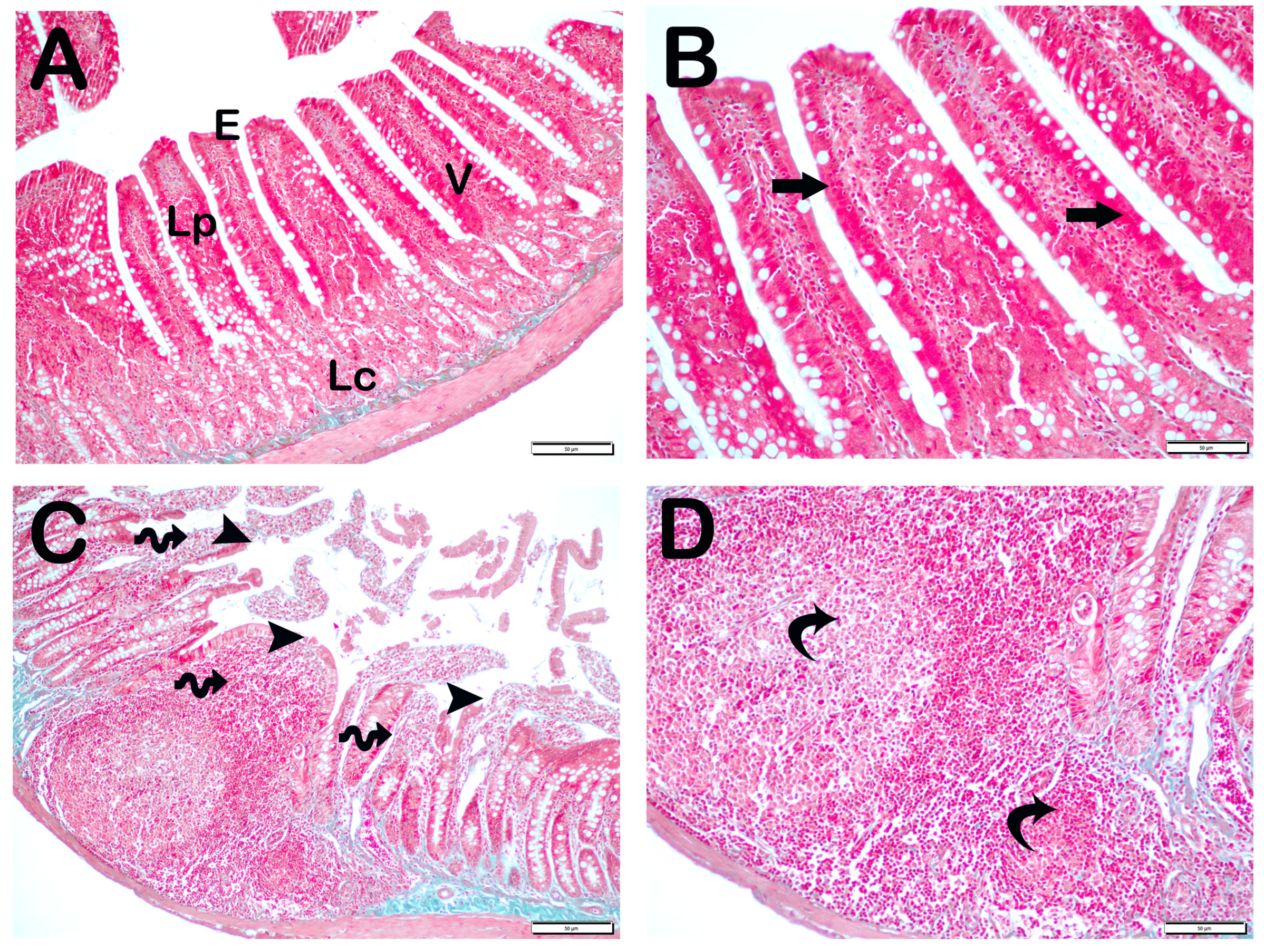

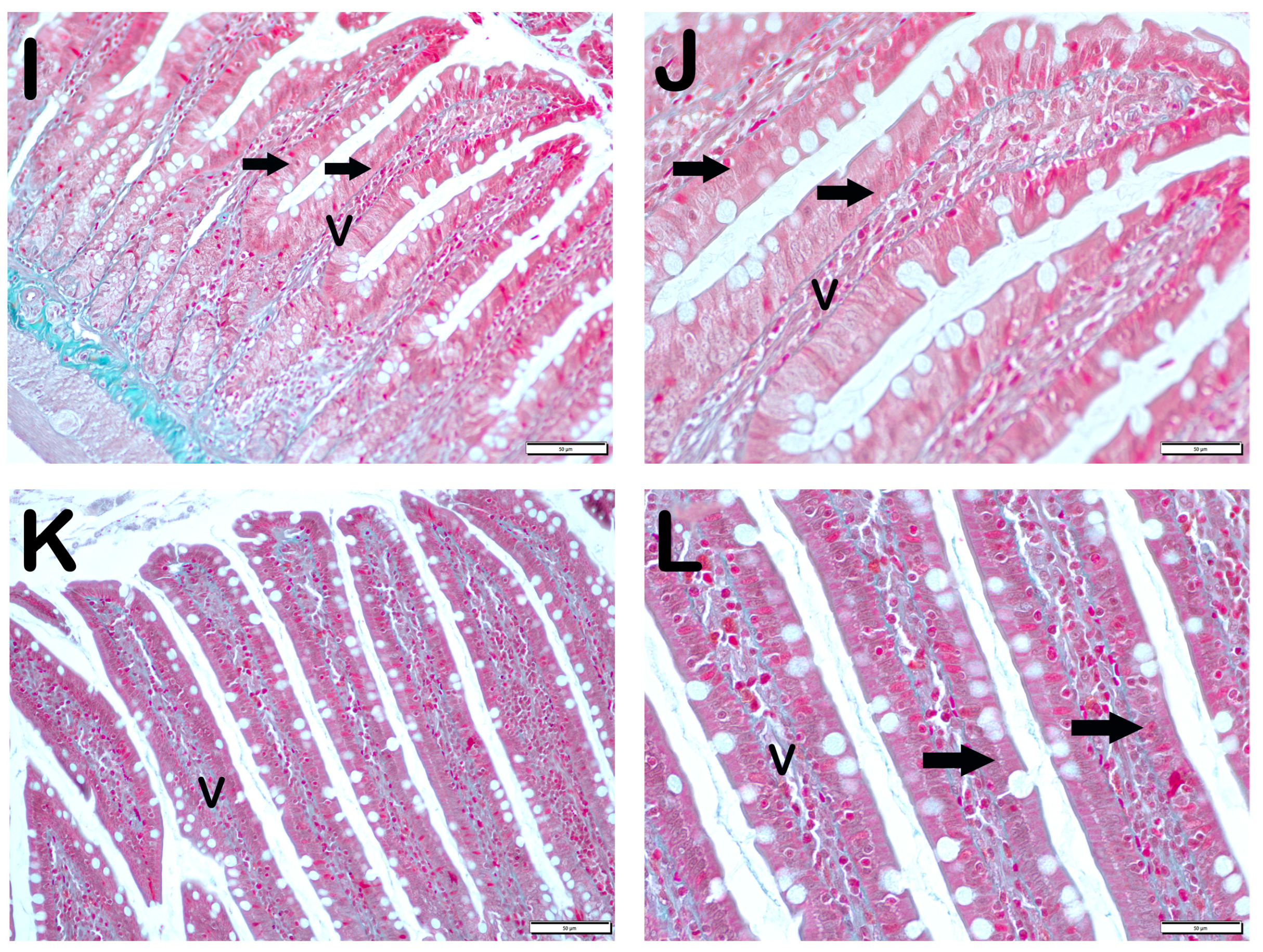


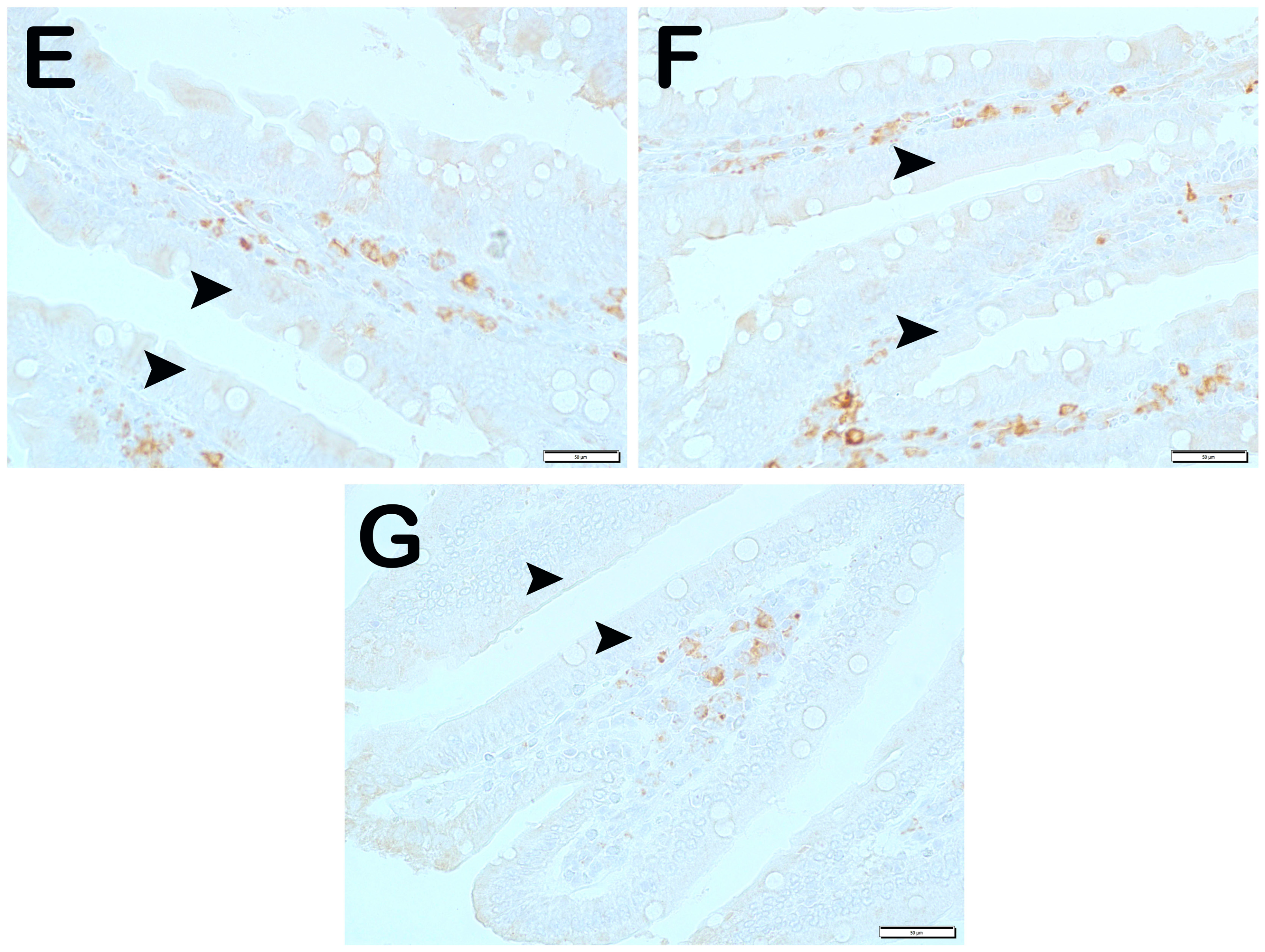
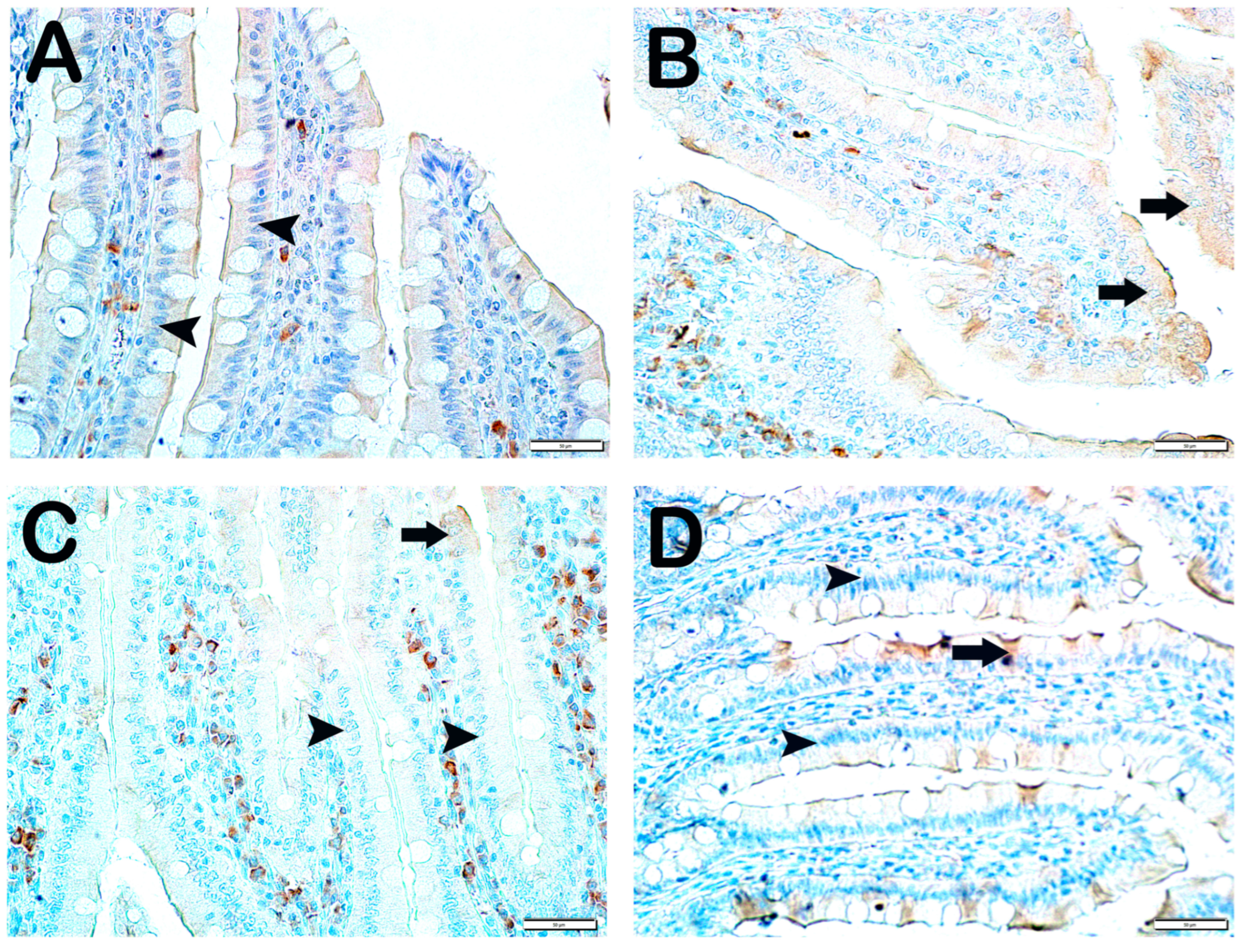


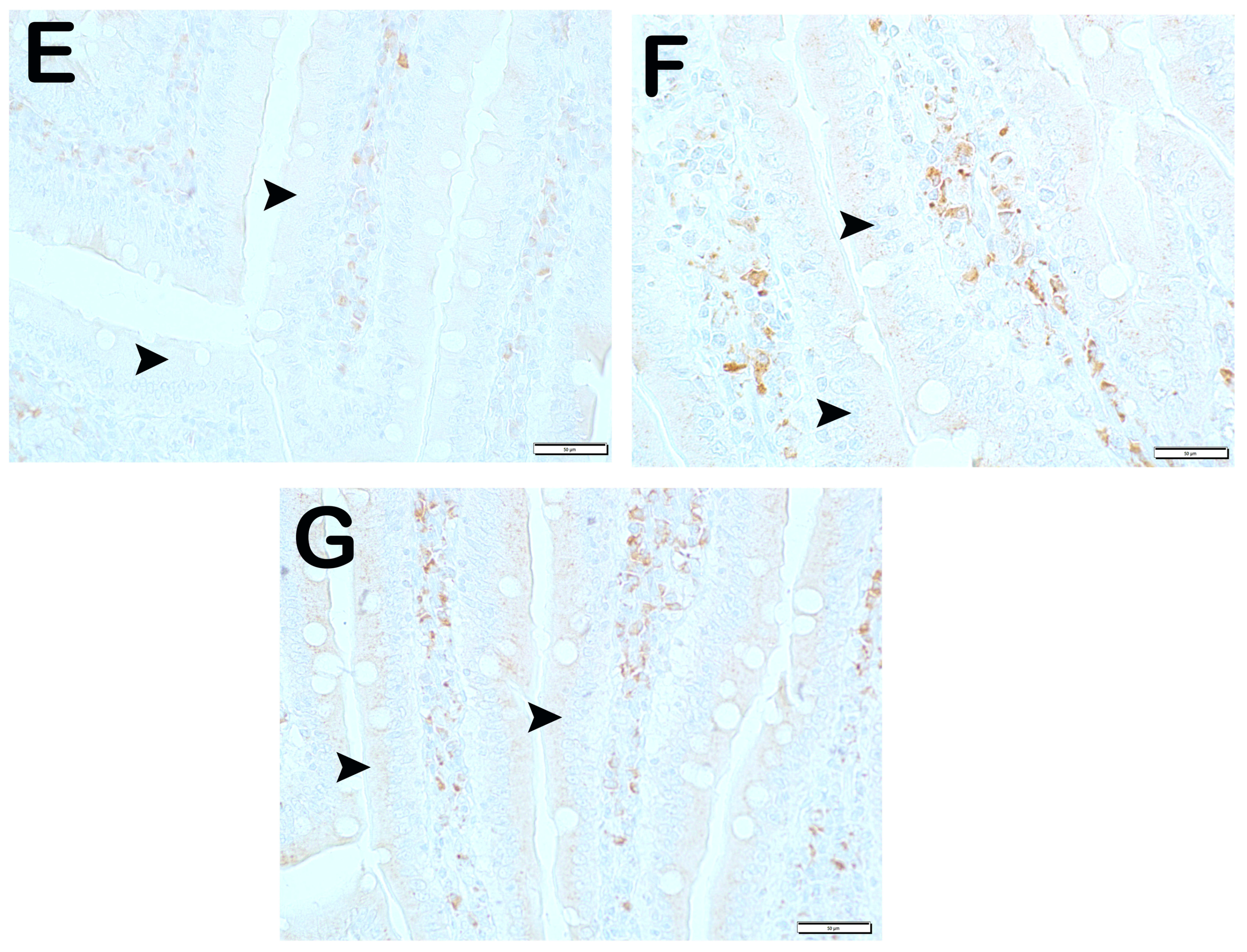
| Group 1 (Classic) (n = 6) | Group 2 (Iron Purified) (n = 6) | Group 3 (Molasses) (n = 7) | Group 4 (Fe3+) (n = 6) | Group 5 (LP299v.) (n = 7) | Group 6 (Polyprobiotic) (n = 7) | Group 7 (Polyprobiotic + vit) (n = 7) | |
|---|---|---|---|---|---|---|---|
| Classic feed | + | − | − | − | − | − | − |
| Iron purified feed | − | + | + | + | + | + | + |
| Molasses | − | − | − | + | + | + | + |
| Fe3+ | − | − | + | − | − | − | − |
| Lactobacillus plantarum 299v. | − | − | − | − | + | − | − |
| Polyprobiotic | − | − | − | − | − | + | − |
| Polyprobiotic + vitamin complex | − | − | − | − | − | − | + |
| a | b | |
|---|---|---|
| Grade | Sequence of Morphological Changes | Results |
| 0 | Normal mucosal villi | Less Than 5% |
| 1 | Development of subepithelial Gruenhagen’s space, usually at the apex of the villus; often with capillary congestion. | Between 5–25% |
| 2 | Extension of the subepithelial space with moderate liftingof epithelial layer from the lamina propria. | Between 25–50% |
| 3 | Mild crypt loss (four crypts in each villus) accompanying villar fusion | Between 51–75% |
| 4 | Moderate crypt loss (fewer than four crypts in each villus) accompanying villar fusion | More than 75% |
| 5 | Severe crypt loss (fewer than three crypts in each villus) |
| Group | Mean | SS | Min | Max | p | Group | Mean | SS | Min | Max | p |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Body Weight (g) | Glucose (mg/dL) | ||||||||||
| Classic | 321.7 | 52.9 | 260 | 382 | 0.83 | Classic | 157.7 | 22 | 129 | 191 | 0.23 |
| Iron purified | 309.8 | 70.2 | 244 | 382 | Iron purified | 164.2 | 25.7 | 126 | 199 | ||
| Molasses | 305.1 | 69.4 | 226 | 386 | Molasses | 203 | 51.7 | 143 | 295 | ||
| Fe3+ | 316 | 80.7 | 230 | 403 | Fe3+ | 170.8 | 29.6 | 133 | 201 | ||
| LP299v. | 286.6 | 69.5 | 228 | 399 | LP299v. | 150.3 | 40.8 | 97 | 221 | ||
| Polyprobiotic | 304.4 | 78.6 | 246 | 424 | Polyprobiotic | 144.1 | 28.1 | 97 | 181 | ||
| Polyprobiotic + vit | 325.7 | 43.2 | 281 | 380 | Polyprobiotic + vit | 162 | 38.9 | 102 | 202 | ||
| Triglyceride (mg/dL) | Cholesterol (mg/dL) | ||||||||||
| Classic | 78.5 | 11.5 | 64 | 93 | 0.02 | Classic | 69.8 | 18.4 | 45 | 101 | 0.14 |
| Iron purified | 33 | 1.8 | 27 | 39 | Iron purified | 71.5 | 4.4 | 57 | 88 | ||
| Molasses | 44.3 | 3.7 | 32 | 59 | Molasses | 92.3 | 10.7 | 65 | 142 | ||
| Fe3+ | 45.8 | 5.7 | 29 | 66 | Fe3+ | 74.2 | 7.8 | 55 | 99 | ||
| LP299v. | 47.4 | 5.1 | 34 | 76 | LP299v. | 85.6 | 8.8 | 57 | 115 | ||
| Polyprobiotic | 42.9 | 5.5 | 25 | 65 | Polyprobiotic | 74.4 | 2.8 | 65 | 87 | ||
| Polyprobiotic + vit | 38.3 | 2.9 | 29 | 51 | Polyprobiotic + vit | 63.1 | 4.1 | 54 | 84 | ||
| LDL Cholesterol (mg/dL) | HDL Cholesterol (mg/dL) | ||||||||||
| Classic | 16.7 | 5.2 | 11 | 24 | 0.36 | Classic | 43 | 11.5 | 33.6 | 64 | 0.08 |
| Iron purified | 18.5 | 3.4 | 15 | 24 | Iron purified | 46.5 | 7.8 | 35.9 | 58.1 | ||
| Molasses | 25.6 | 10.6 | 15 | 43 | Molasses | 57.8 | 17.9 | 39.9 | 89.4 | ||
| Fe3+ | 17.2 | 6.3 | 11 | 25 | Fe3+ | 47.8 | 11.5 | 37 | 63.3 | ||
| LP299v. | 22.7 | 10.3 | 9 | 36 | LP299v. | 53.5 | 12.1 | 38.3 | 67.5 | ||
| Polyprobiotic | 18.3 | 6.1 | 8 | 27 | Polyprobiotic | 47.6 | 4.4 | 43 | 56.5 | ||
| Polyprobiotic + vit | 15.7 | 3.7 | 11 | 22 | Polyprobiotic + vit | 39.9 | 7.3 | 33.7 | 54.7 | ||
| ALT (IU/L) | AST (IU/L) | ||||||||||
| Classic | 39.5 | 9.7 | 31 | 55 | 0.06 | Classic | 146.8 | 39 | 102 | 215 | 0.94 |
| Iron purified | 25.2 | 7.2 | 19 | 37 | Iron purified | 159.3 | 54.4 | 110 | 258 | ||
| Molasses | 29.1 | 8.6 | 22 | 44 | Molasses | 150.4 | 34 | 102 | 187 | ||
| Fe3+ | 28.2 | 2.4 | 26 | 31 | Fe3+ | 148.7 | 29.2 | 116 | 192 | ||
| LP299v. | 28 | 8.4 | 15 | 39 | LP299v. | 177 | 115.2 | 101 | 425 | ||
| Polyprobiotic | 30.4 | 5 | 21 | 37 | Polyprobiotic | 155 | 18.2 | 130 | 188 | ||
| Polyprobiotic + vit | 36.6 | 10.1 | 29 | 57 | Polyprobiotic + vit | 178.3 | 69.3 | 110 | 314 | ||
| Group | Mean | SS | Min | Max | p | Group | Mean | SS | Min | Max | p |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Iron (mg/dL) | UIBC (mg/dL) | ||||||||||
| Classic | 245.7 | 98.3 | 120 | 346 | 0.41 | Classic | 316.83 | 52.121 | 214 | 348 | 0.12 |
| Iron purified | 182.0 | 66.8 | 112 | 265 | Iron purified | 325.67 | 25.264 | 275 | 341 | ||
| Molasses | 276.7 | 84.0 | 168 | 355 | Molasses | 305.14 | 62.715 | 172 | 346 | ||
| Fe3+ | 211.7 | 89.6 | 117 | 317 | Fe3+ | 312.83 | 45.146 | 228 | 344 | ||
| LP299v. | 234.7 | 54.7 | 136 | 291 | LP299v. | 310.43 | 37.411 | 252 | 343 | ||
| Polyprobiotic | 224.7 | 68.3 | 151 | 322 | Polyprobiotic | 323.43 | 30.794 | 260 | 345 | ||
| Polyprobiotic + vit | 221.1 | 67.3 | 138 | 319 | Polyprobiotic + vit | 343.14 | 1676 | 342 | 346 | ||
| RBC (103/µL) | Hb (g/dL) | ||||||||||
| Classic | 7.7 | 0.7 | 6.7 | 8.7 | 0.34 | Classic | 14.8 | 1.2 | 13.4 | 16.6 | 0.4 |
| Iron purified | 7.0 | 0.4 | 6.6 | 7.6 | Iron purified | 13.0 | 0.7 | 12.2 | 14.2 | ||
| Molasses | 7.2 | 0.4 | 6.4 | 7.6 | Molasses | 13.5 | 0.8 | 12.5 | 14.6 | ||
| Fe3+ | 7.2 | 0.7 | 6.6 | 7.8 | Fe3+ | 13.2 | 0.4 | 12.7 | 13.7 | ||
| LP299v. | 7.4 | 0.7 | 6.5 | 8.0 | LP299v. | 14.2 | 1.2 | 11.8 | 15.2 | ||
| Polyprobiotic | 7.3 | 0.8 | 5.8 | 8.3 | Polyprobiotic | 13.6 | 1.7 | 10.1 | 15.1 | ||
| Polyprobiotic + vit | 7.3 | 0.5 | 6.4 | 7.9 | Polyprobiotic + vit | 13.8 | 0.6 | 13.1 | 14.6 | ||
| Ferritin (ng/mL) | Hepcidin (ng/mL) | ||||||||||
| Classic | 21.9 | 1.28 | 20.3 | 23.4 | 0.03 | Classic | 302.7 | 34.4 | 275.8 | 366.5 | 0.02 |
| Iron purified | 17.7 | 1.1 | 15.8 | 18.7 | Iron purified | 285.0 | 35.9 | 253.0 | 338.5 | ||
| Molasses | 20.2 | 2.2 | 16.9 | 23.8 | Molasses | 301.7 | 43.1 | 244.8 | 371.2 | ||
| Fe3+ | 16.5 | 2.6 | 14.1 | 20.6 | Fe3+ | 239.5 | 35.3 | 210.4 | 295.5 | ||
| LP299v. | 19.3 | 1.7 | 16.7 | 21.4 | LP299v. | 336.2 | 116.6 | 250.9 | 592.9 | ||
| Polyprobiotic | 19.8 | 1.8 | 16.9 | 22.1 | Polyprobiotic | 341.7 | 34.6 | 310.9 | 404.7 | ||
| Polyprobiotic + vit | 19.2 | 2.1 | 15.6 | 21.5 | Polyprobiotic + vit | 301.9 | 37.9 | 250.2 | 358.7 | ||
| Group | MHDS |
|---|---|
| Classic | 0 (0–0) |
| Iron purified | 3.5 (3–4) a |
| Molasses | 2 (2–2) a,b |
| Fe3+ | 2 (1–2) a,b |
| LP299v. | 2 (1–2) a,b |
| Polyprobiotic | 1 (1–2) a,b |
| Polyprobiotic + vit | 1 (1–1) a,b,c,d |
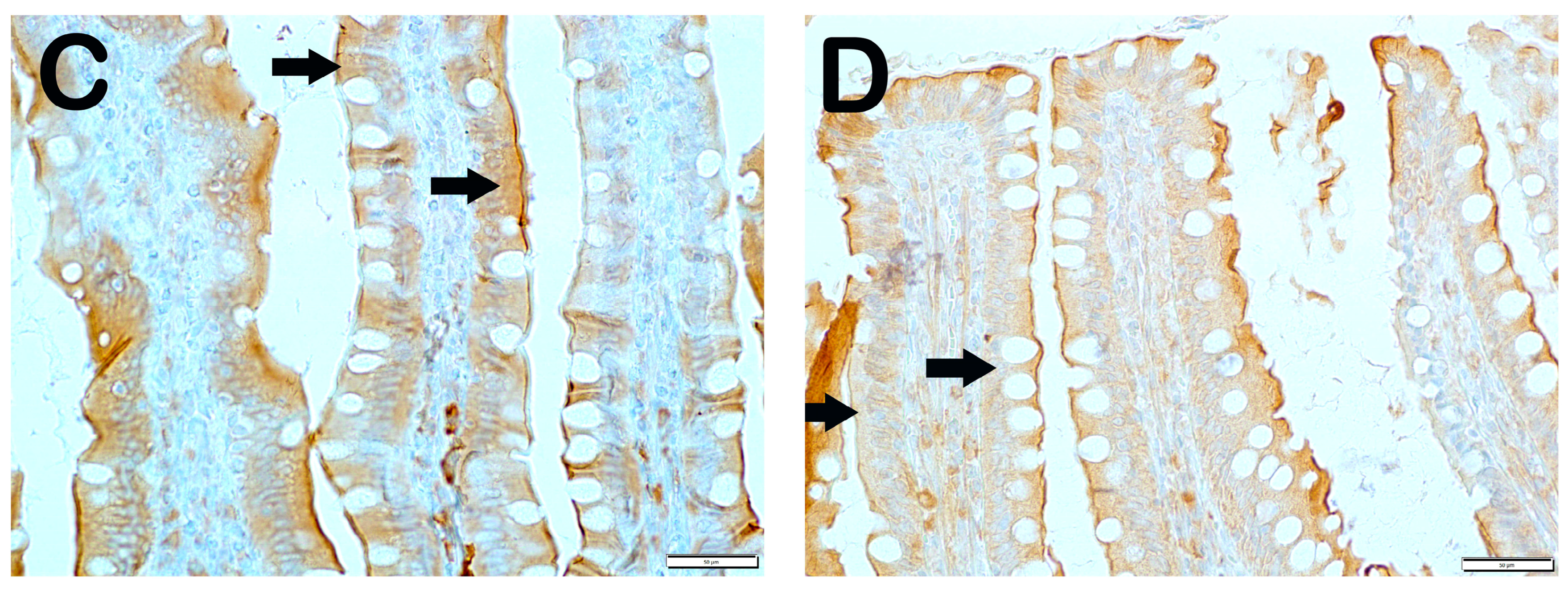
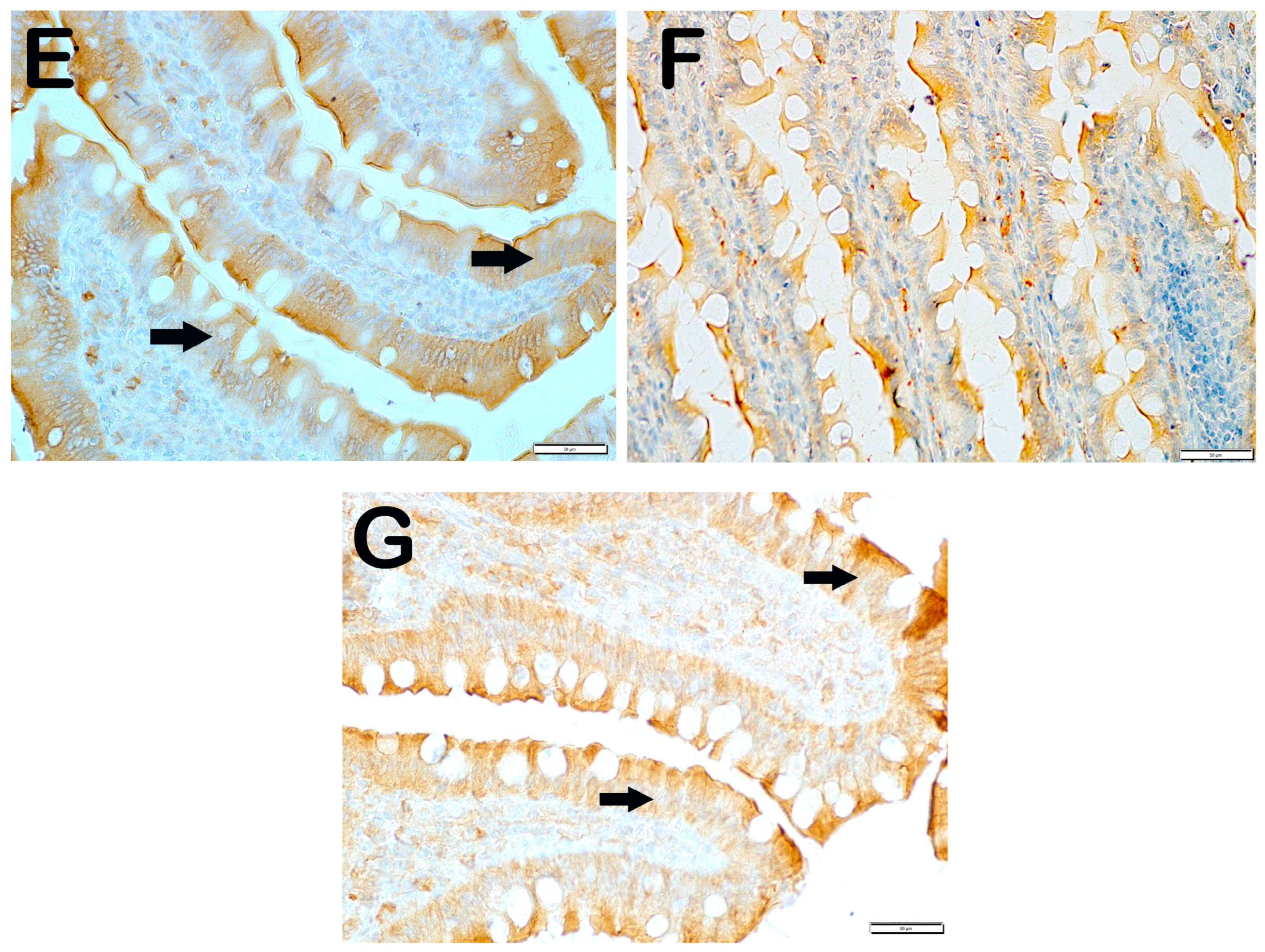
| Group | Slc11a | IRE1 | WNT2 | CD71 |
|---|---|---|---|---|
| Classic | 62.6 ± 4.02 | 11.7 ± 2.68 | 6.9 ± 2.75 | 4.7 ± 0.65 |
| Iron purified | 16.8 ± 4.19 a | 66.5 ± 92.26 a | 28.4 ± 6.26 a | 22.95 ± 8.89 a |
| Molasses | 38.3 ± 3.46 a,b | 20.4 ± 3.81 b | 12.7 ± 2.34 a,b | 14.6 ± 1.78 a,b |
| Fe3+ | 41.65 ± 3.99 a,b | 21.30 ± 4.81 b,g | 12.95 ± 1.57 a,b,g | 15.45 ± 1.79 i,b,d |
| LP299v. | 44.15 ± 5.73 a,b,c | 17.25 ± 1.83 b | 13.45 ± 1.67 a,b | 9.5 ± 2.21 a,b,d,e |
| Polyprobiotic | 52.2 ± 4.2 a,b,e,f | 10.4 ± 2.98 b | 10.55 ± 1.87 h,b | 10.35 ± 2.27 h,b,c,e |
| Polyprobiotic + vit | 55.85 ± 3.44 a,b,e,f | 9.15 ± 2.13 b | 9.3 ± 2.15 b,j,k,f | 9.2 ± 2.91 l,b,d,e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yıldız, Y.; Topçu, A.; Mercantepe, T.; Arpa, M.; Yıldız, İ.E.; Tümkaya, L. Can Iron Absorption in Molasses Be Increased with Probiotic Additives? “Molasses with Increased Bioavailability”. Nutrients 2025, 17, 1150. https://doi.org/10.3390/nu17071150
Yıldız Y, Topçu A, Mercantepe T, Arpa M, Yıldız İE, Tümkaya L. Can Iron Absorption in Molasses Be Increased with Probiotic Additives? “Molasses with Increased Bioavailability”. Nutrients. 2025; 17(7):1150. https://doi.org/10.3390/nu17071150
Chicago/Turabian StyleYıldız, Yasin, Atilla Topçu, Tolga Mercantepe, Medeni Arpa, İlknur Esen Yıldız, and Levent Tümkaya. 2025. "Can Iron Absorption in Molasses Be Increased with Probiotic Additives? “Molasses with Increased Bioavailability”" Nutrients 17, no. 7: 1150. https://doi.org/10.3390/nu17071150
APA StyleYıldız, Y., Topçu, A., Mercantepe, T., Arpa, M., Yıldız, İ. E., & Tümkaya, L. (2025). Can Iron Absorption in Molasses Be Increased with Probiotic Additives? “Molasses with Increased Bioavailability”. Nutrients, 17(7), 1150. https://doi.org/10.3390/nu17071150








