High-Density Lipoprotein Particles, Inflammation, and Coronary Heart Disease Risk
Abstract
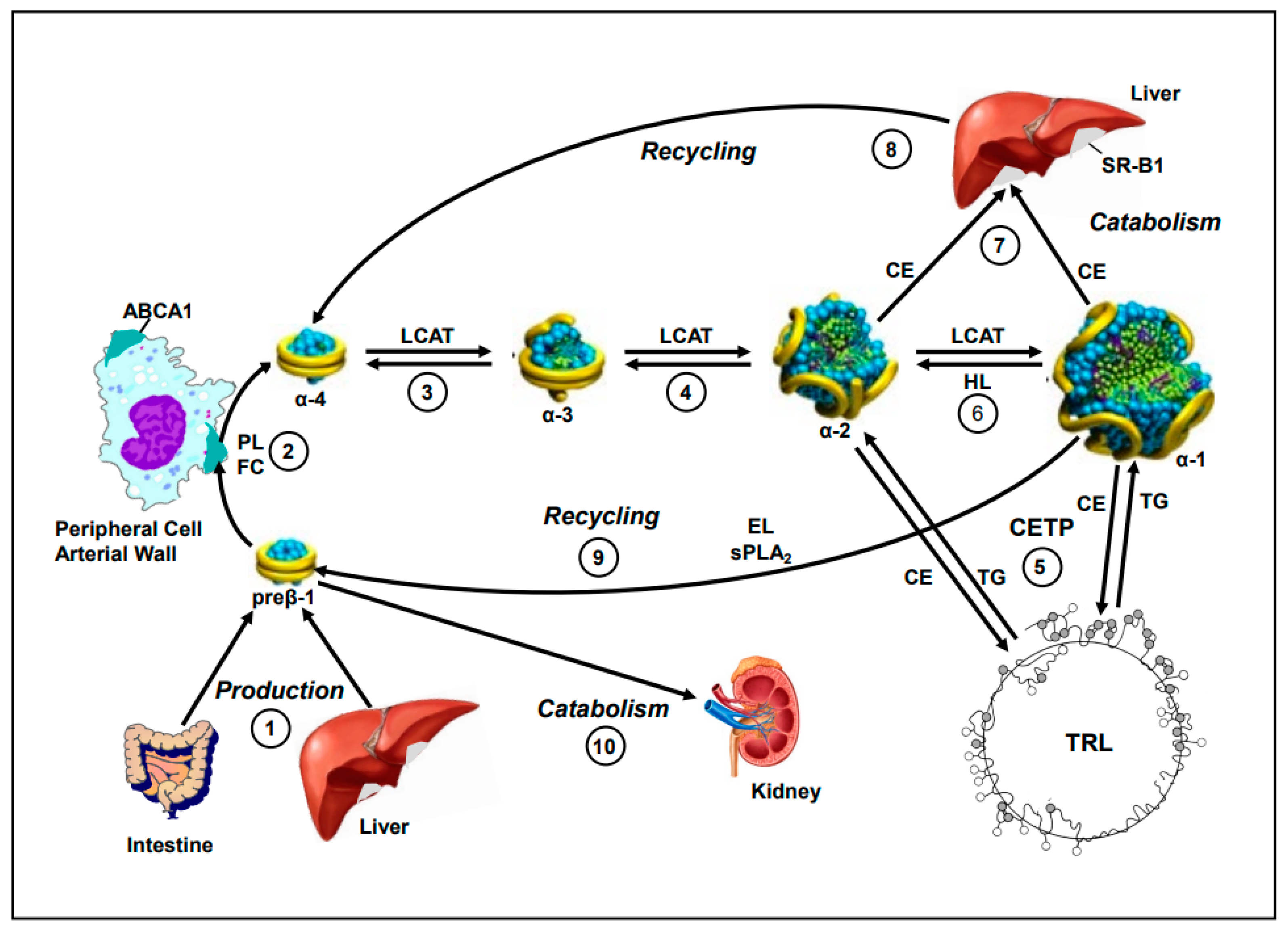
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Laboratory Measurements
2.3. Statistical Analysis
2.4. Machine-Learning Analysis
3. Results
3.1. Standard Risk Factors
3.2. Advanced Biochemical Risk Factors
3.3. Multivariate Analysis
3.4. Machine Learning Analysis
4. Discussion
Sex Differences in Biomarker Profiles and Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, S.S.; Matsushita, K.; Sang, Y.; Ballew, S.H.; Grams, M.E.; Surapaneni, A.; Blaha, M.J.; Carson, A.P.; Chang, A.R.; Ciemins, E.; et al. Development and Validation of the American Heart Association’s PREVENT Equations. Circulation 2024, 149, 430–449, Erratum in Circulation 2024, 149, e956. https://doi.org/10.1161/CIR.0000000000001230. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khan, S.S.; Coresh, J.; Pencina, M.J.; Ndumele, C.E.; Rangaswami, J.; Chow, S.L.; Palaniappan, L.P.; Sperling, L.S.; Virani, S.S.; Ho, J.E.; et al. Novel Prediction Equations for Absolute Risk Assessment of Total Cardiovascular Disease Incorporating Cardiovascular-Kidney-Metabolic Health: A Scientific Statement from the American Heart Association. Circulation 2023, 148, 1982–2004. [Google Scholar] [CrossRef] [PubMed]
- GGrundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, e1082–e1143, Erratum in Circulation 2019, 139, e1182–e1186. https://doi.org/10.1161/CIR.0000000000000698. Erratum in Circulation 2023, 148, e5. https://doi.org/10.1161/CIR.0000000000001172. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ridker, P.M.; Buring, J.E.; Rifai, N.; Cook, N.R. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: The Reynolds Risk Score. JAMA 2007, 297, 611–619. [Google Scholar] [CrossRef]
- Ridker, P.M.; Paynter, N.P.; Rifai, N.; Gaziano, J.M.; Cook, N.R. C-reactive protein and parental history improve global cardiovascular risk prediction: The Reynolds Risk Score for men. Circulation 2008, 118, 2243–2251. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ridker, P.M.; Danielson, E.; Fonseca, F.A.; Genest, J.; Gotto, A.M.; Kastelein, J.J.; Koenig, W.; Libby, P.; Lorenzatti, A.J.; MacFadyen, J.G.; et al. Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: A prospective study of the JUPITER trial. Lancet 2009, 373, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.J.; Hazen, S.L. Myeloperoxidase and cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1102–1111. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.J.; Hazen, S.L. Myeloperoxidase, modified lipoproteins, and atherogenesis. J. Lipid Res. 2009, 50, S346–S351. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.-L.; Penn, M.S.; Van Lente, F.; Nambi, V.; Shishehbor, M.H.; Aviles, R.J.; Goormastic, M.; Pepoy, M.L.; McErlean, E.S.; Topol, E.J.; et al. Prognostic value of myeloperoxidase in patients with chest pain. N. Engl. J. Med. 2003, 349, 1595–1604. [Google Scholar] [CrossRef] [PubMed]
- Meuwese, M.C.; Stroes, E.S.; Hazen, S.L.; van Miert, J.N.; Kuivenhoven, J.A.; Schaub, R.G.; Wareham, N.J.; Luben, R.; Kastelein, J.J.; Khaw, K.-T.; et al. Serum myeloperoxidase levels are associated with the future risk of coronary artery disease in apparently healthy individuals: The EPIC-Norfolk Prospective Population Study. J. Am. Coll. Cardiol. 2007, 50, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Silverman, M.G.; Ference, B.A.; Im, K.; Wiviott, S.D.; Giugliano, R.P.; Grundy, S.M.; Braunwald, E.; Sabatine, M.S. Association Between Lowering LDL-C and Cardiovascular Risk Reduction Among Different Therapeutic Interventions: A Systematic Review and Meta-analysis. JAMA 2016, 316, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Ikezaki, H.; A Fisher, V.; Lim, E.; Ai, M.; Liu, C.-T.; Cupples, L.A.; Nakajima, K.; Asztalos, B.F.; Furusyo, N.; Schaefer, E.J. Direct Versus Calculated LDL Cholesterol and C-Reactive Protein in Cardiovascular Disease Risk Assessment in the Framingham Offspring Study. Clin. Chem. 2019, 65, 1102–1114. [Google Scholar] [CrossRef] [PubMed]
- Austin, M.A.; Breslow, J.L.; Hennekens, C.H.; Buring, J.E.; Willett, W.C.; Krauss, R.M. Low-Density Lipoprotein Subclass Patterns and Risk of Myocardial Infarction. JAMA 1988, 260, 1917–1921. [Google Scholar] [CrossRef]
- Campos, H.; Genest, J.J., Jr.; Blijlevens, E.; McNamara, J.R.; Jenner, J.L.; Ordovas, J.M.; Wilson, P.W.; Schaefer, E.J. Low density lipoprotein particle size and coronary artery disease. Arterioscler. Thromb. 1992, 12, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Ikezaki, H.; Lim, E.; Cupples, L.A.; Liu, C.; Asztalos, B.F.; Schaefer, E.J. Small Dense Low-Density Lipoprotein Cholesterol Is the Most Atherogenic Lipoprotein Parameter in the Prospective Framingham Offspring Study. J. Am. Heart Assoc. 2021, 10, e019140. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schaefer, E.J.; Ikezaki, H.; Diffenderfer, M.R.; Lim, E.; Liu, C.-T.; Hoogeveen, R.C.; Guan, W.; Tsai, M.Y.; Ballantyne, C.M. Atherosclerotic cardiovascular disease risk and small dense low-density lipoprotein cholesterol in men, women, African Americans and non-African Americans: The pooling project. Atherosclerosis 2023, 367, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Ai, M.; Otokozawa, S.; Asztalos, B.F.; Nakajima, K.; Stein, E.; Jones, P.H.; Schaefer, E.J. Effects of maximal doses of atorvastatin versus rosuvastatin on small dense low-density lipoprotein cholesterol levels. Am. J. Cardiol. 2008, 101, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, E.J.; Anthanont, P.; Diffenderfer, M.R.; Polisecki, E.; Asztalos, B.F. Diagnosis and treatment of high-density lipoprotein deficiency. Prog. Cardiovasc. Dis. 2016, 59, 97–106. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kane, J.P.; Malloy, M.J. Prebeta-1 HDL and coronary heart disease. Curr. Opin. Lipidol. 2012, 23, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Asztalos, B.F.; de la Llera-Moya, M.; Dallal, G.E.; Horvath, K.V.; Schaefer, E.J.; Rothblat, G.H. Differential effects of HDL subpopulations on cellular ABCA1 and SR-B1-mediated cholesterol efflux. J. Lipid Res. 2005, 46, 2246–2253. [Google Scholar] [CrossRef] [PubMed]
- Khera, A.V.; Cuchel, M.; de la Llera-Moya, M.; Rodrigues, A.; Burke, M.F.; Jafri, K.; French, B.C.; Phillips, J.A.; Mucksavage, M.L.; Wilensky, R.L.; et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N. Engl. J. Med. 2011, 364, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Rohatgi, A.; Khera, A.; Berry, J.D.; Givens, E.G.; Ayers, C.R.; Wedin, K.E.; Neeland, I.J.; Yuhanna, I.S.; Rader, D.R.; de Lemos, J.A.; et al. HDL cholesterol efflux capacity and incident cardiovascular events. N. Engl. J. Med. 2014, 371, 2383–2393. [Google Scholar] [CrossRef]
- Khera, A.V.; Demler, O.V.; Adelman, S.J.; Collins, H.L.; Glynn, R.J.; Ridker, P.M.; Rader, D.J.; Mora, S. Cholesterol Efflux Capacity, High-Density Lipoprotein Particle Number, and Incident Cardiovascular Events: An Analysis From the JUPITER Trial (Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin). Circulation 2017, 135, 2494–2504. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Asztalos, B.F.; Hauser, T.H.; Goldfine, A.B.; Welty, F.K.; Horvath, K.V.; Schaefer, E.J. The role of HDL- and non-HDL-related parameters in cell-cholesterol efflux capacity. Atherosclerosis 2022, 345, 1–6. [Google Scholar] [CrossRef]
- Vaisar, T.; Babenko, I.; Horvath, K.V.; Niisuke, K.; Asztalos, B.F. Relationships between HDL subpopulation proteome and HDL function in overweight/obese people with and without coronary heart disease. Atherosclerosis 2024, 397, 118565. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schaefer, E.J.; Tsunoda, F.; Diffenderfer, M.; Polisecki, E.; Thai, N.; Asztalos, B. The Measurement of Lipids, Lipoproteins, Apolipoproteins, Fatty Acids, and Sterols, and Next Generation Sequencing for the Diagnosis and Treatment of Lipid Disorders; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Eds.; MDText. com, Inc.: South Dartmouth, MA, USA, 2016. [Google Scholar]
- Niisuke, K.; Kuklenyik, Z.; Horvath, K.V.; Gardner, M.S.; Toth, C.A.; Asztalos, B.F. Composition-function analysis of HDL subpopulations: Influence of lipid composition on particle functionality. J. Lipid Res. 2020, 61, 306–315. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saharan, S.S.; Nagar, P.; Creasy, K.T.; Stock, E.O.; Feng, J.; Malloy, M.J.; Kane, J.P. Application of Machine Learning Ensemble Super Learner for analysis of the cytokines transported by high density lipoproteins (HDL) of smokers and nonsmokers. In Proceedings of the 2021 International Conference on Computational Science and Computational Intelligence (CSCI), Las Vegas, NV, USA, 15–17 December 2021; Volume 2021, pp. 370–375. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saharan, S.S.; Nagar, P.; Creasy, K.T.; Stock, E.O.; James, F.; Malloy, M.J.; Kane, J.P. Logistic Regression and Statistical Regularization Techniques for Risk Classification of Coronary Artery Disease using Cytokines transported by high density lipoproteins. In Proceedings of the 2023 International Conference on Computational Science and Computational Intelligence (CSCI), Las Vegas, NV, USA, 13–15 December 2023; Volume 2023, pp. 652–660. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Asztalos, B.F.; Sloop, C.H.; Wong, L.; Roheim, P.S. Two-dimensional electrophoresis of plasma lipoproteins: Recognition of new apo A-I-containing subpopulations. Biochim. Biophys. Acta 1993, 1169, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Geller, A.S.; Polisecki, E.Y.; Diffenderfer, M.R.; Asztalos, B.F.; Karathanasis, S.K.; Hegele, R.A.; Schaefer, E.J. Genetic and secondary causes of severe HDL deficiency and cardiovascular disease. J. Lipid Res. 2018, 59, 2421–2435. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stock, E.O.; Ferrara, C.T.; O’Connor, P.M.; Naya-Vigne, J.M.; Frost, P.H.; Malloy, M.J.; Kane, J.P.; Pullinger, C.R. Levels of prebeta-1 high-density lipoprotein are elevated in 3 phenotypes of dyslipidemia. J. Clin. Lipidol. 2018, 12, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Creasy, K.T.; Kane, J.P.; Malloy, M.J. Emerging roles of HDL in immune function. Curr. Opin. Lipidol. 2018, 29, 486–487. [Google Scholar] [CrossRef] [PubMed]
- Asztalos, B.F.; Roheim, P.S.; Milani, R.L.; Lefevre, M.; McNamara, J.R.; Horvath, K.V.; Schaefer, E.J. Distribution of ApoA-I-containing HDL subpopulations in patients with coronary heart disease. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2670–2676. [Google Scholar] [CrossRef] [PubMed]
- Asztalos, B.F.; Batista, M.; Horvath, K.V.; Cox, C.E.; Dallal, G.E.; Morse, J.S.; Brown, G.B.; Schaefer, E.J. Change in alpha1 HDL concentration predicts progression in coronary artery stenosis. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 847–852. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Asztalos, B.F.; Cupples, L.A.; Demissie, S.; Horvath, K.V.; Cox, C.E.; Batista, M.C.; Schaefer, E.J. High-density lipoprotein subpopulation profile and coronary heart disease prevalence in male participants of the Framingham Offspring Study. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 2181–2187. [Google Scholar] [CrossRef] [PubMed]
- Asztalos, B.F.; Le Maulf, F.; Dallal, G.E.; Stein, E.; Jones, P.H.; Horvath, K.V.; McTaggart, F.; Schaefer, E.J. Comparison of the effects of high doses of rosuvastatin versus atorvastatin on the subpopulations of high-density lipoproteins. Am. J. Cardiol. 2007, 99, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Asztalos, B.F.; Swarbrick, M.M.; Schaefer, E.J.; Dallal, G.E.; Horvath, K.V.; Ai, M.; Stanhope, K.L.; Austrheim-Smith, I.; Wolfe, B.M.; Ali, M.; et al. Effects of weight loss, induced by gastric bypass surgery, on HDL remodeling in obese women. J. Lipid Res. 2010, 51, 2405–2412. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Dansinger, M.; Williams, P.T.; Superko, H.R.; Asztalos, B.F.; Schaefer, E.J. Effects of weight change on HDL-cholesterol and its subfractions in over 28,000 men and women. J. Clin. Lipidol. 2019, 13, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Guey, L.T.; Pullinger, C.R.; Ishida, B.Y.; O’Connor, P.M.; Zellner, C.; Francone, O.L.; Laramie, J.M.; Naya-Vigne, J.M.; Siradze, K.A.; Deedwania, P.; et al. Relation of increased prebeta-1 high-density lipoprotein levels to risk of coronary heart disease. Am. J. Cardiol. 2011, 108, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Pullinger, C.R.; O’connor, P.M.; Naya-Vigne, J.M.; Kunitake, S.T.; Movsesyan, I.; Frost, P.H.; Malloy, M.J.; Kane, J.P. Levels of Prebeta-1 High-Density Lipoprotein Are a Strong Independent Positive Risk Factor for Coronary Heart Disease and Myocardial Infarction: A Meta-Analysis. J. Am. Heart Assoc. 2021, 10, e018381. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Asztalos, B.F.; Collins, D.; Horvath, K.V.; Bloomfield, H.E.; Robins, S.J.; Schaefer, E.J. Relation of gemfibrozil treatment and high-density lipoprotein subpopulation profile with cardiovascular events in the Veterans Affairs High-Density Lipoprotein Intervention Trial. Metabolism 2008, 57, 77–83. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lamon-Fava, S.; Herrington, D.M.; Reboussin, D.M.; Sherman, M.; Horvath, K.V.; Cupples, L.A.; White, C.; Demissie, S.; Schaefer, E.J.; Asztalos, B.F. Plasma levels of HDL subpopulations and remnant lipoproteins predict the extent of angiographically-defined coronary artery disease in postmenopausal women. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Asztalos, B.F.; Collins, D.; Cupples, L.A.; Demissie, S.; Horvath, K.V.; Bloomfield, H.E.; Robins, S.J.; Schaefer, E.J. Value of high-density lipoprotein (HDL) subpopulations in predicting recurrent cardiovascular events in the Veterans Affairs HDL Intervention Trial. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2185–2191. [Google Scholar] [CrossRef] [PubMed]
- Duchnowski, P.; Śmigielski, W. Usefulness of myocardial damage biomarkers in predicting cardiogenic shock in patients undergoing heart valve surgery. Pol. Heart J. 2024, 82, 423–426. [Google Scholar] [CrossRef]
- Nguyen, K.; Fan, W.; Bertoni, A.; Budoff, M.J.; Defilippi, C.; Lombardo, D.; Maisel, A.; Szklo, M.; Wong, N.D. N-terminal Pro B-type Natriuretic Peptide and High-sensitivity Cardiac Troponin as Markers for Heart Failure and Cardiovascular Disease Risks According to Glucose Status (from the Multi-Ethnic Study of Atherosclerosis [MESA]). Am. J. Cardiol. 2020, 125, 1194–1201. [Google Scholar] [CrossRef] [PubMed]
| Variable | Controls (n = 285) | CHD Cases (n = 120) | Percent Difference † | p ‡ |
|---|---|---|---|---|
| Standard Risk Factors | ||||
| Age, years | 68.8 (11.0) | 61.0 (18.3) | −11.3% | <0.001 |
| BMI, kg/m2 | 25.7 (4.7) | 27.1 (5.5) | +5.4% | 0.002 |
| Hypertension, % | 24.9% | 60.0% | +141.0% | <0.001 |
| Diabetes, % | 0.4% | 30.0% | +7400% | <0.001 |
| Current smoker, % | 1.8% | 4.2% | +133.3% | 0.281 |
| Lipids, mg/dL | ||||
| Total cholesterol | 194.0 (45.0) | 196.5 (67.0) | +1.3% | 0.616 |
| Triglycerides | 124.0 (89.0) | 148.5 (157.5) | +19.8% | <0.001 |
| HDL-C | 50.0 (20.0) | 38.0 (19.0) | −24.0% | <0.001 |
| Non-HDL-C | 142.0 (43.0) | 158.5 (76.5) | +11.6% | 0.002 |
| Calculated LDL-C | 115.8 (42.6) | 117.6 (67.0) | +1.6% | 0.733 |
| Advanced Risk Factors | ||||
| Hs-C-reactive protein, mg/L | 0.9 (1.7) | 1.6 (3.2) | +77.8% | <0.001 |
| Serum amyloid A, mg/L | 2.5 (2.8) | 4.6 (6.4) | +84.0% | <0.001 |
| Myeloperoxidase, pmol/L | 197 (61) | 411 (251) | +109% | <0.001 |
| Direct LDL-C, mg/dL | 116.0 (40.0) | 119.0 (63.5) | +2.6% | 0.683 |
| sdLDL-C, mg/dL | 28.0 (18.0) | 41.5 (35.0) | +48.2% | <0.001 |
| sdLDL-C, % of total LDL-C | 24.0 (10.3) | 33.1 (19.0) | +37.9% | <0.001 |
| VLDL-C, mg/dL | 25.0 (13.0) | 31.0 (21.2) | +24.0% | <0.001 |
| ApoB, mg/dL | 96.0 (28.0) | 102.5 (42.0) | +6.8% | 0.007 |
| Lp(a), mg/dL | 10.6 (16.4) | 15.5 (32.9) | +46.2% | 0.031 |
| Total apoA-I, mg/dL | 148.1 (32.9) | 133.6 (34.2) | −9.8% | <0.001 |
| ApoA-I in preβ-1 HDL, mg/dL | 11.4 (5.2) | 13.3 (7.1) | +16.7% | 0.003 |
| ApoA-I in α-4 HDL, mg/dL | 17.8 (5.1) | 15.5 (4.6) | −12.9% | <0.001 |
| ApoA-I in α-3 HDL, mg/dL | 23.6 (5.5) | 22.6 (6.5) | −4.2% | 0.059 |
| ApoA-I in α-2 HDL, mg/dL | 55.7 (15.5) | 50.8 (16.5) | −8.8% | <0.001 |
| ApoA-I in α-1 HDL, mg/dL | 26.9 (16.7) | 17.7 (14.3) | −34.2% | <0.001 |
| Variable | Controls (n = 243) | CHD Cases (n = 107) | Percent Difference † | p ‡ |
|---|---|---|---|---|
| Standard Risk Factors | ||||
| Age, years | 65.3 (12.2) | 60.5 (17.2) | +7.4% | 0.004 |
| BMI, kg/m2 | 24.3 (5.4) | 26.3 (7.9) | +8.2% | <0.001 |
| Hypertension, % | 16.5% | 59.8% | +262.4% | <0.001 |
| Diabetes, % | 0.4% | 25.2% | +6200% | <0.001 |
| Current smoker, % | 2.1% | 10.3% | +390.5% | 0.002 |
| Lipids | ||||
| Total cholesterol, mg/dL | 208.0 (47.0) | 211.0 (72.5) | +1.4% | 0.375 |
| Triglycerides, mg/dL | 111.0 (72.0) | 144.0 (86.5) | +29.7% | <0.001 |
| HDL-C, mg/dL | 67.0 (23.0) | 49.0 (23.5) | −26.9% | <0.001 |
| Non-HDL-C, mg/dL | 140.0 (48.5) | 164.0 (88.5) | +17.1% | <0.001 |
| Calculated LDL-C, mg/dL | 116.0 (44.8) | 129.6 (62.9) | +11.7% | 0.017 |
| Advanced Risk Factors | ||||
| Hs-C-reactive protein, mg/L | 0.9 (2.1) | 2.7 (4.4) | +200.0% | <0.001 |
| Serum amyloid A, mg/L | 3.9 (4.7) | 5.2 (8.1) | +33.3% | 0.007 |
| Myeloperoxidase, pmol/L | 183 (99) | 377 (222) | +106% | <0.001 |
| Direct LDL-C, mg/dL | 116.0 (40.5) | 135.0 (60.0) | +16.4% | 0.003 |
| sdLDL-C, mg/dL | 26.0 (13.0) | 37.0 (32.0) | +42.3% | <0.001 |
| sdLDL-C, % of total LDL-C | 23.0 (6.2) | 24.8 (17.5) | +7.8% | <0.001 |
| VLDL-C, mg/dL | 23.0 (11.0) | 25.0 (17.0) | +8.7% | 0.079 |
| ApoB, mg/dL | 92.0 (29.5) | 107.0 (45.0) | +16.3% | <0.001 |
| Lp(a), mg/dL | 10.6 (17.9) | 18.0 (44.4) | +69.8% | 0.009 |
| Total apoA-I, mg/dL | 175.4 (36.6) | 160.8 (42.0) | −8.3% | <0.001 |
| ApoA-I in preβ-1 HDL, mg/dL | 13.1 (6.3) | 15.2 (7.4) | +16.0% | 0.005 |
| ApoA-I in α-4 HDL, mg/dL | 17.7 (5.6) | 16.7 (4.5) | −5.6% | 0.010 |
| ApoA-I in α-3 HDL, mg/dL | 24.3 (5.4) | 23.3 (6.5) | −4.1% | 0.094 |
| ApoA-I in α-2 HDL, mg/dL | 68.5 (15.3) | 62.5 (19.6) | −8.8% | <0.001 |
| ApoA-I in α-1 HDL, mg/dL | 38.3 (18.0) | 28.2 (20.2) | −26.4% | <0.001 |
| Odds Ratio * | SE | 95% CI | |
|---|---|---|---|
| Standard Lipid Model † | |||
| HDL-C | 0.932 | 0.012 | 0.908, 0.954 |
| Non-HDL-C | 1.008 | 0.004 | 1.001, 1.015 |
| Diabetes | 126.242 | 137.504 | 22.211, 2422.739 |
| Hypertension | 3.922 | 1.135 | 2.236, 6.976 |
| Smoking | 3.659 | 2.632 | 0.858, 15.343 |
| Standard Lipid Model Plus hsCRP ‡ | |||
| HDL-C | 0.934 | 0.012 | 0.909, 0.956 |
| Non-HDL-C | 1.009 | 0.004 | 1.002, 1.016 |
| Diabetes | 107.950 | 117.569 | 19.062, 2073.068 |
| Hypertension | 3.826 | 1.115 | 2.172, 6.829 |
| Smoking | 3.726 | 2.680 | 0.874, 15.614 |
| hsCRP | 1.018 | 0.019 | 0.988, 1.070 |
| Standard Model Plus hsCRP Plus Atherogenic Particles § | |||
| HDL-C | 0.939 | 0.012 | 0.915, 0.962 |
| Non-HDL-C | 0.998 | 0.007 | 0.984, 1.012 |
| Diabetes | 122.297 | 134.677 | 21.078, 2383.910 |
| Hypertension | 3.622 | 1.071 | 2.038, 6.517 |
| Smoking | 3.953 | 2.877 | 0.918, 16.880 |
| hsCRP | 1.020 | 0.019 | 0.990, 1.072 |
| Log sdLDL | 2.558 | 1.485 | 0.823, 8.073 |
| Log Lp(a) | 1.488 | 0.249 | 1.070, 2.068 |
| Standard Model Plus hsCRP Plus Atherogenic Particles Plus HDL Particles ¶ | |||
| HDL-C | 0.837 | 0.031 | 0.775, 0.899 |
| Non-HDL-C | 1.005 | 0.008 | 0.989, 1.021 |
| Diabetes | 555.141 | 949.233 | 40.137, 36,420.222 |
| Hypertension | 4.550 | 1.505 | 2.404, 8.836 |
| Smoking | 5.575 | 4.679 | 1.045, 29.939 |
| hsCRP | 0.983 | 0.019 | 0.950, 1.032 |
| Log sdLDL | 1.213 | 0.886 | 0.288, 5.108 |
| Log Lp(a) | 1.446 | 0.268 | 1.004, 2.085 |
| ApoA-I in preβ-1 | 1.137 | 0.049 | 1.046, 1.239 |
| ApoA-I in α-1 | 1.043 | 0.036 | 0.974, 1.116 |
| ApoA-I in α-2 | 1.099 | 0.030 | 1.044, 1.161 |
| ApoA-I in α-3 | 0.898 | 0.044 | 0.814, 0.987 |
| ApoA-I in α-4 | 0.899 | 0.043 | 0.817, 0.986 |
| Odds Ratio * | SE | 95% CI | |
|---|---|---|---|
| Standard Lipid Model † | |||
| HDL-C | 0.962 | 0.009 | 0.944, 0.980 |
| Non-HDL-C | 1.006 | 0.003 | 0.999, 1.013 |
| Diabetes | 27.323 | 28.708 | 5.263, 502.902 |
| Hypertension | 4.872 | 1.462 | 2.719, 8.842 |
| Smoking | 2.510 | 1.770 | 0.629, 10.415 |
| Standard Lipid Model Plus hsCRP ‡ | |||
| HDL-C | 0.965 | 0.009 | 0.946, 0.983 |
| Non-HDL-C | 1.006 | 0.003 | 0.999, 1.013 |
| Diabetes | 22.151 | 23.266 | 4.272, 407.569 |
| Hypertension | 4.818 | 1.458 | 2.675, 8.790 |
| Smoking | 2.261 | 1.597 | 0.570, 9.454 |
| hsCRP | 1.059 | 0.030 | 1.002, 1.122 |
| Standard Model Plus hsCRP Plus Atherogenic Particles § | |||
| HDL-C | 0.965 | 0.010 | 0.945, 0.983 |
| Non-HDL-C | 1.003 | 0.007 | 0.989, 1.016 |
| Diabetes | 21.355 | 22.514 | 4.078, 394.563 |
| Hypertension | 4.798 | 1.471 | 2.644, 8.826 |
| Smoking | 2.089 | 1.502 | 0.512, 8.941 |
| hsCRP | 1.064 | 0.031 | 1.005, 1.128 |
| Log sdLDL | 1.297 | 0.790 | 0.393, 4.321 |
| Log Lp(a) | 1.396 | 0.261 | 0.966, 2.018 |
| Standard Model Plus hsCRP Plus Atherogenic Particles Plus HDL Particles ¶ | |||
| HDL-C | 0.857 | 0.026 | 0.805, 0.907 |
| Non-HDL-C | 1.002 | 0.007 | 0.988, 1.017 |
| Diabetes | 20.673 | 23.305 | 3.347, 415.223 |
| Hypertension | 5.365 | 1.875 | 2.732, 10.805 |
| Smoking | 2.581 | 2.308 | 0.463, 15.194 |
| Log hsCRP | 1.078 | 0.034 | 1.014, 1.147 |
| Log sdLDL | 1.450 | 1.085 | 0.335, 6.371 |
| Log Lp(a) | 1.420 | 0.298 | 0.941, 2.150 |
| ApoA-I in preβ-1 | 1.116 | 0.043 | 1.036, 1.205 |
| ApoA-I in α-1 | 1.142 | 0.036 | 1.075, 1.218 |
| ApoA-I in α-2 | 1.028 | 0.023 | 0.985, 1.074 |
| ApoA-I in α-3 | 0.889 | 0.045 | 0.803, 0.980 |
| ApoA-I in α-4 | 0.915 | 0.048 | 0.825, 1.013 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stock, E.O.; Asztalos, B.F.; Miller, J.M.; He, L.; Creasy, K.T.; Schwemberger, R.; Quinn, A.; Pullinger, C.R.; Malloy, M.J.; Diffenderfer, M.R.; et al. High-Density Lipoprotein Particles, Inflammation, and Coronary Heart Disease Risk. Nutrients 2025, 17, 1182. https://doi.org/10.3390/nu17071182
Stock EO, Asztalos BF, Miller JM, He L, Creasy KT, Schwemberger R, Quinn A, Pullinger CR, Malloy MJ, Diffenderfer MR, et al. High-Density Lipoprotein Particles, Inflammation, and Coronary Heart Disease Risk. Nutrients. 2025; 17(7):1182. https://doi.org/10.3390/nu17071182
Chicago/Turabian StyleStock, Eveline O., Bela F. Asztalos, John M. Miller, Lihong He, Kate Townsend Creasy, Rachel Schwemberger, Alexander Quinn, Clive R. Pullinger, Mary J. Malloy, Margaret R. Diffenderfer, and et al. 2025. "High-Density Lipoprotein Particles, Inflammation, and Coronary Heart Disease Risk" Nutrients 17, no. 7: 1182. https://doi.org/10.3390/nu17071182
APA StyleStock, E. O., Asztalos, B. F., Miller, J. M., He, L., Creasy, K. T., Schwemberger, R., Quinn, A., Pullinger, C. R., Malloy, M. J., Diffenderfer, M. R., & Kane, J. P. (2025). High-Density Lipoprotein Particles, Inflammation, and Coronary Heart Disease Risk. Nutrients, 17(7), 1182. https://doi.org/10.3390/nu17071182








