Synergistic Antiviral Activity of European Black Elderberry Fruit Extract and Quinine Against SARS-CoV-2 and Influenza A Virus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inhibitors
2.2. Viruses
2.3. Infection Experiments
2.4. Cell Culture
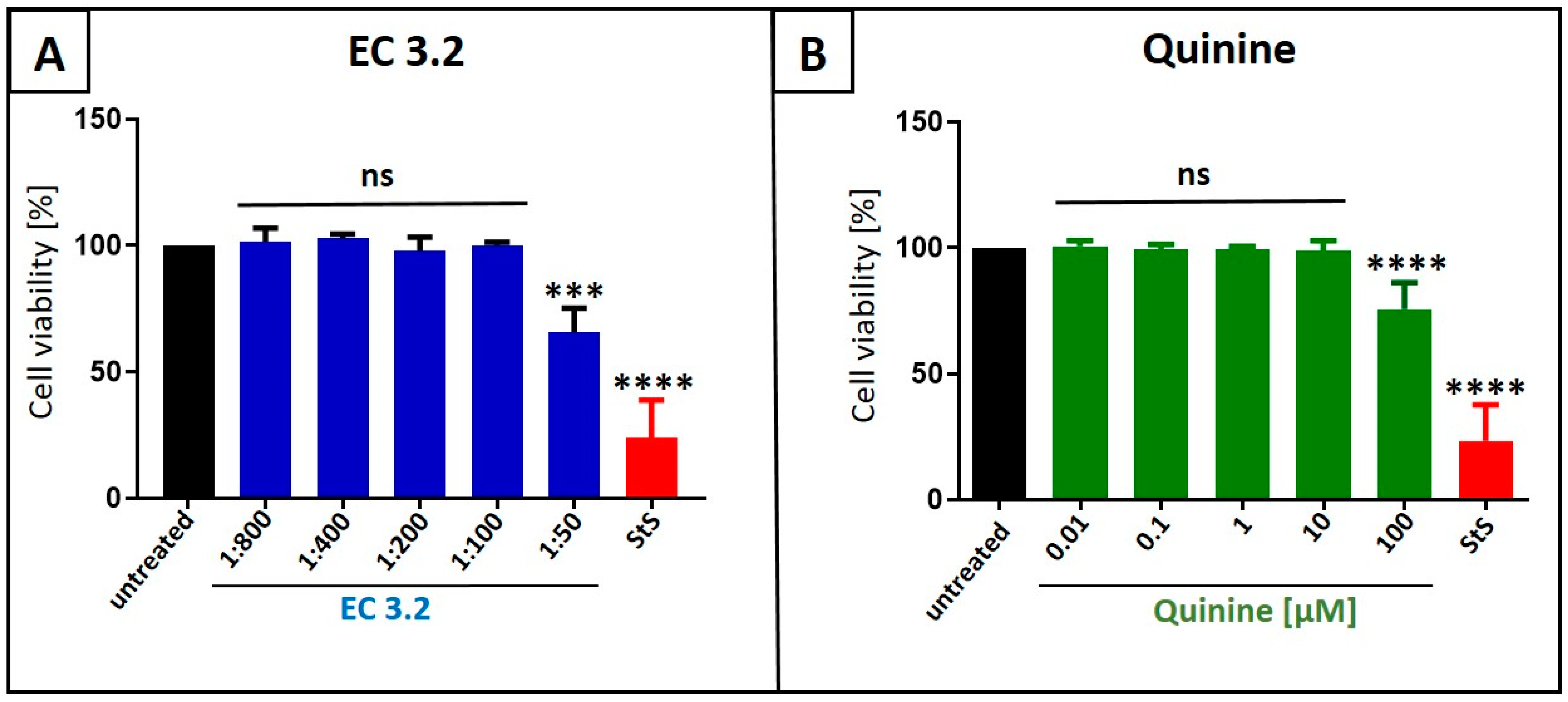
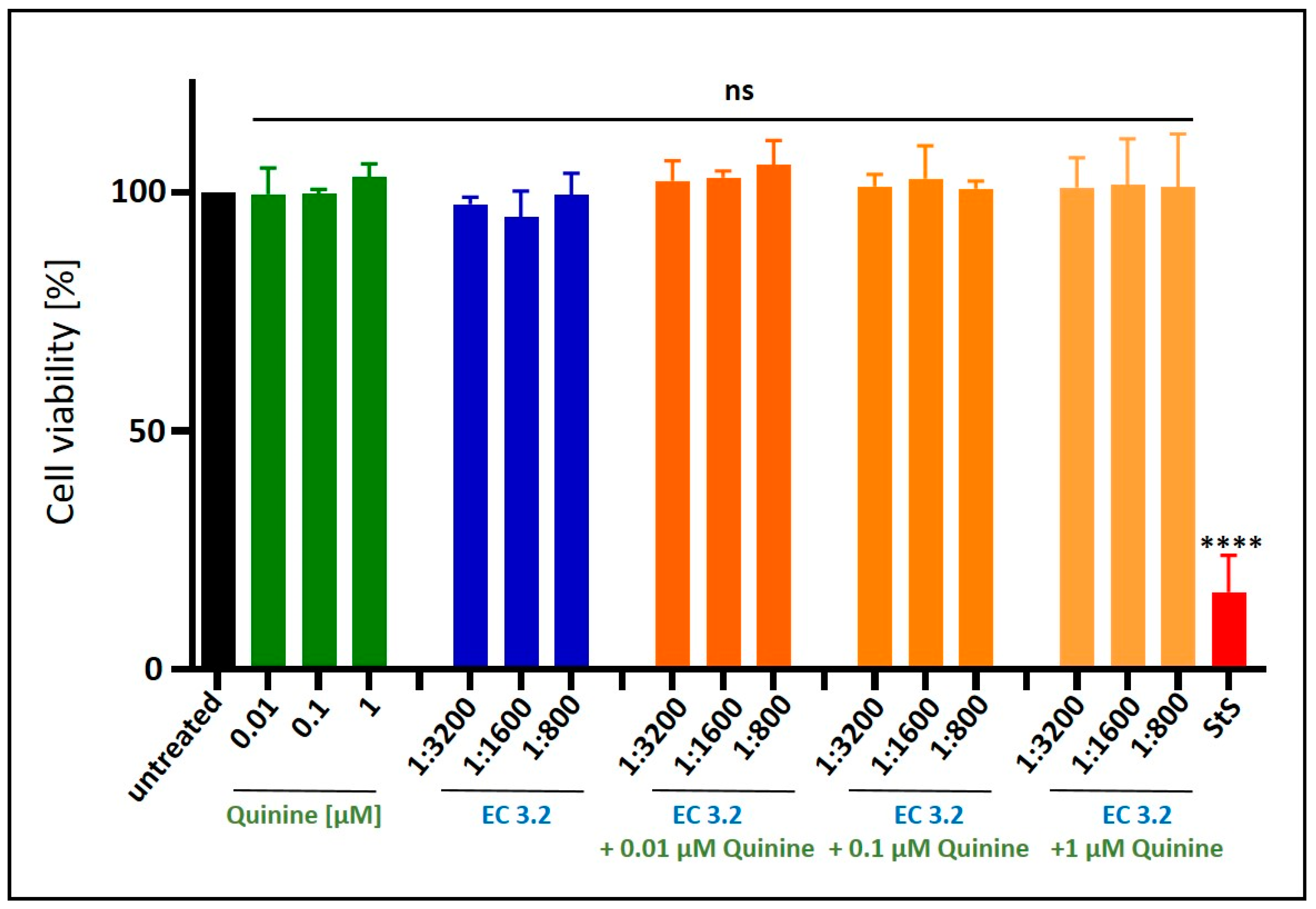
2.5. Assessment of Cell Viability
2.6. Determination of the Amount of Viral RNA Copies from Released Viruses via qRT-PCR
2.7. Software and Statistics
3. Results
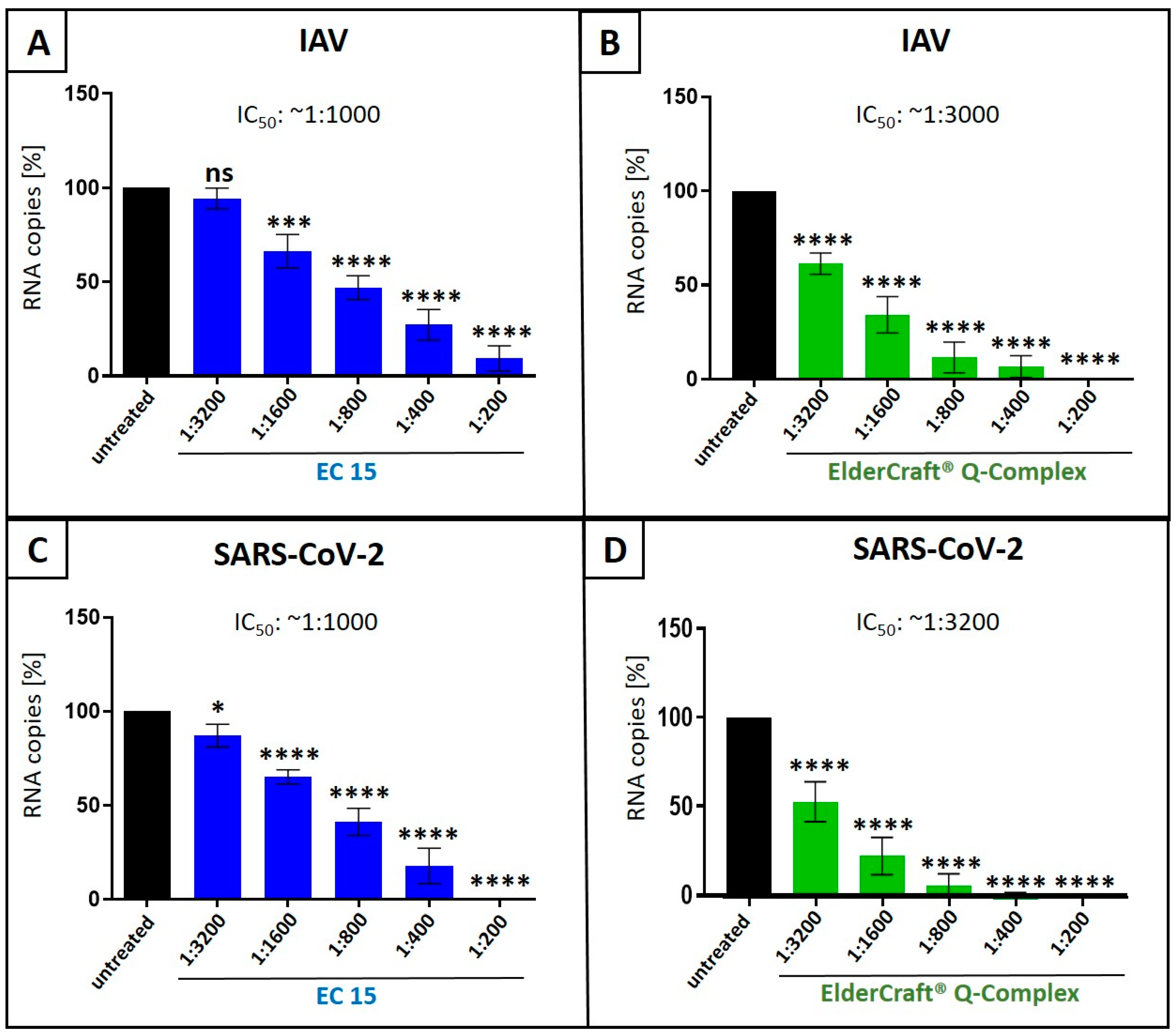
3.1. European Black Elderberry Fruit Extract and Quinine Exhibit Antiviral Activity Against Influenza A Virus in MDCKII Cells
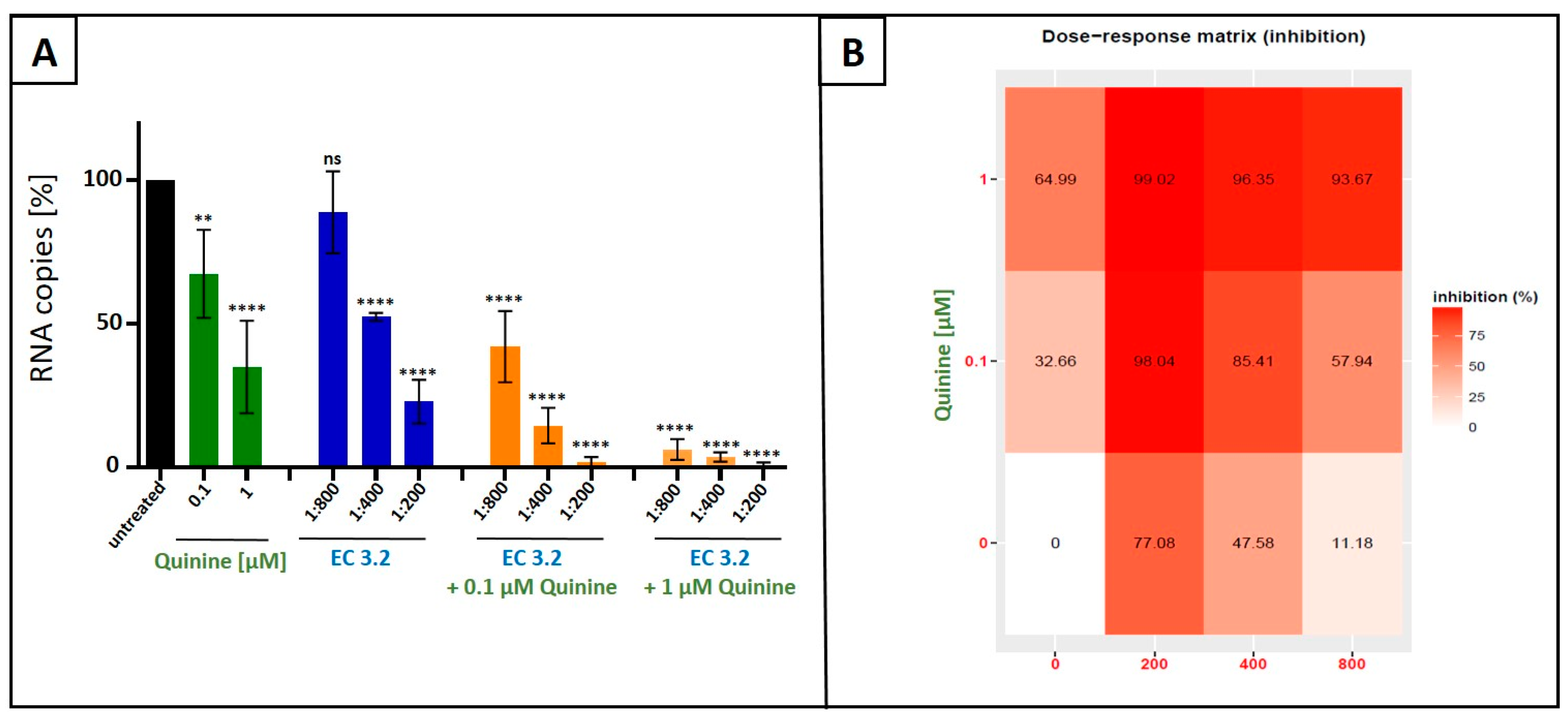
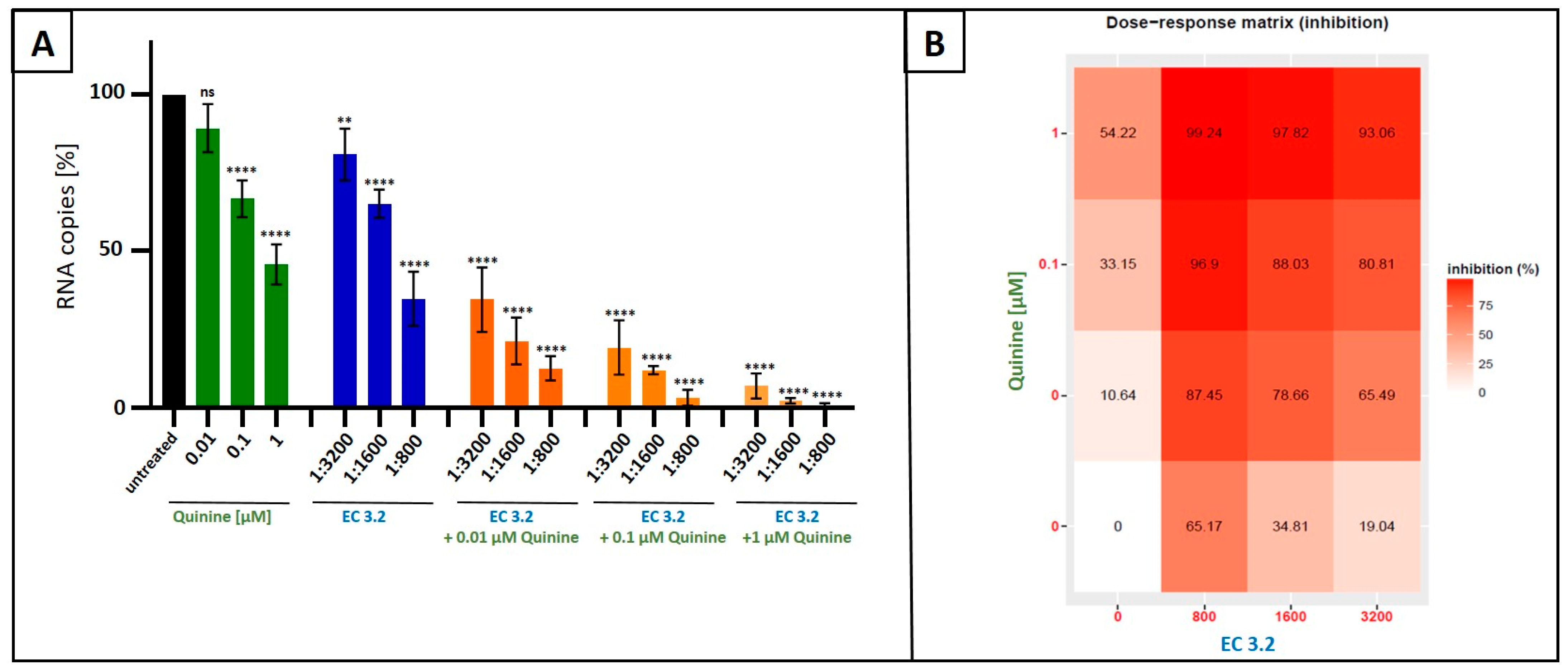
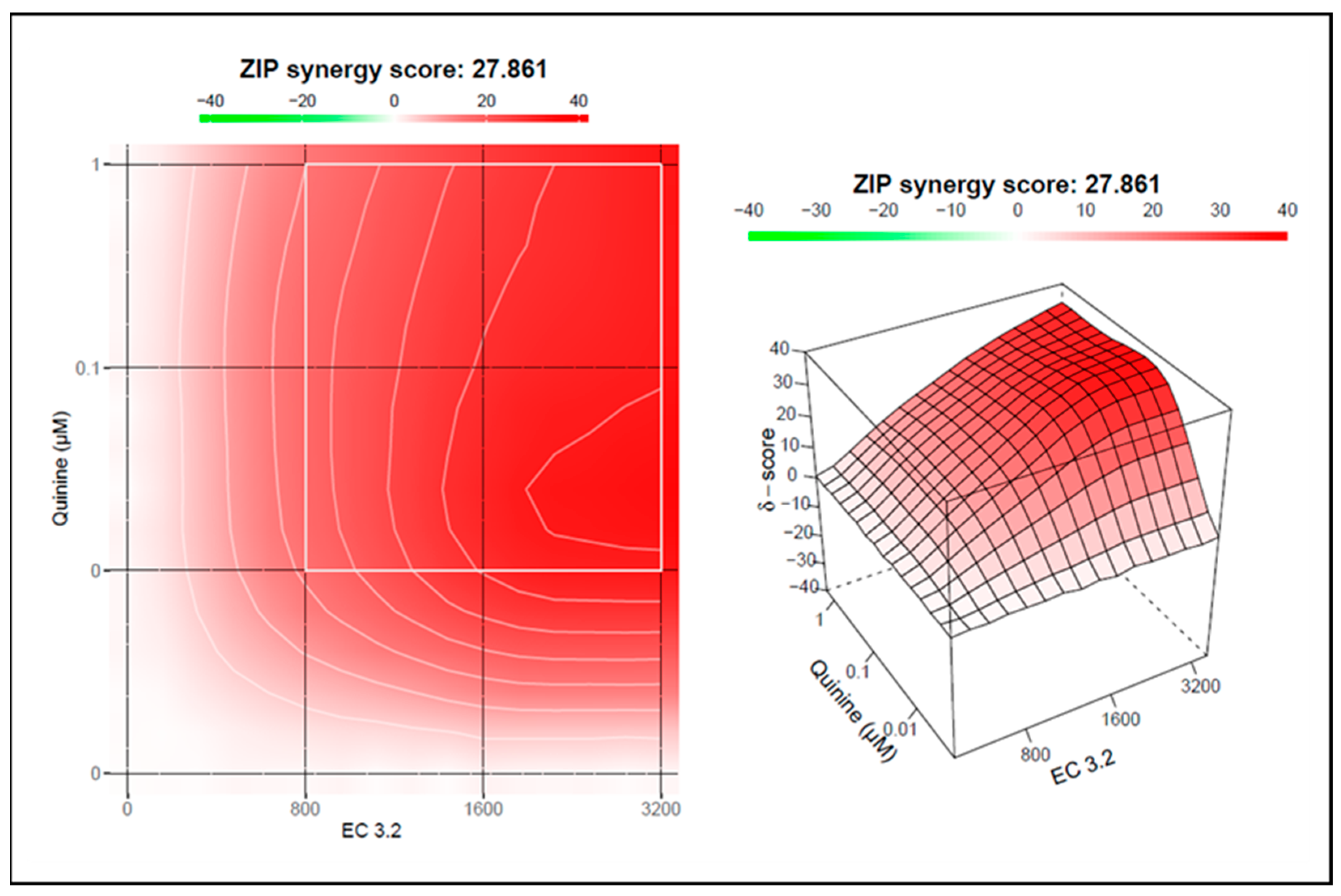
3.2. Combination Treatment with Black Elderberry Fruit Extract and Quinine Exhibits Synergistic Antiviral Activity Against IAV
3.3. Treatment with a Combination of European Black Elderberry Fruit Extract and Quinine Exhibits Synergistic Antiviral Activity Against SARS-CoV-2
3.4. Antiviral Activity of ElderCraft® Q-Complex Against IAV and SARS-CoV-2 in Comparison to ElderCraft® Without Quinine
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Williams, B.A.; Jones, C.H.; Welch, V.; True, J.M. Outlook of pandemic preparedness in a post-COVID-19 world. npj Vaccines 2023, 8, 178. [Google Scholar]
- Wibmer, C.K.; Ayres, F.; Hermanus, T.; Madzivhandila, M.; Kgagudi, P.; Oosthuysen, B.; Lambson, B.E.; de Oliveira, T.; Vermeulen, M.; van der Berg, K.; et al. SARS-CoV-2 501Y.V2 escapes neutralization by South African COVID-19 donor plasma. Nat. Med. 2021, 27, 622–625. [Google Scholar] [CrossRef] [PubMed]
- Blumental, S.; Debré, P. Challenges and Issues of Anti-SARS-CoV-2 Vaccines. Front. Med. 2021, 8, 664179. [Google Scholar]
- Sunagar, R.; Singh, A.; Kumar, S. SARS-CoV-2: Immunity, Challenges with Current Vaccines, and a Novel Perspective on Mucosal Vaccines. Vaccines 2023, 11, 849. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.F.-W.; Yuan, S.; Chu, H.; Sridhar, S.; Yuen, K.-Y. COVID-19 drug discovery and treatment options. Nat. Rev. Microbiol. 2024, 22, 391–407. [Google Scholar] [CrossRef]
- Javanian, M.; Barary, M.; Ghebrehewet, S.; Koppolu, V.; Vasigala, V.; Ebrahimpour, S. A brief review of influenza virus infection. J. Med. Virol. 2021, 93, 4638–4646. [Google Scholar] [PubMed]
- Uyeki, T.M.; Hui, D.S.; Zambon, M.; Wentworth, D.E.; Monto, A.S. Influenza. Lancet 2022, 400, 693–706. [Google Scholar] [CrossRef]
- Comber, L.; O’Murchu, E.; Jordan, K.; Hawkshaw, S.; Marshall, L.; O’Neill, M.; Teljeur, C.; Ryan, M.; Carnahan, A.; Pérez Martín, J.J.; et al. Systematic review of the efficacy, effectiveness and safety of high-dose seasonal influenza vaccines for the prevention of laboratory-confirmed influenza in individuals ≥18 years of age. Rev. Med. Virol. 2023, 33, e2330. [Google Scholar]
- Chaachouay, N.; Zidane, L. Plant-Derived Natural Products: A Source for Drug Discovery and Development. Drugs Drug Candidates 2024, 3, 184–207. [Google Scholar] [CrossRef]
- Dias, D.A.; Urban, S.; Roessner, U. A historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Orhan, I.E.; Banach, M.; Rollinger, J.M.; Barreca, D.; Weckwerth, W.; Bauer, R.; Bayer, E.A.; et al. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, J.; Baker, C.; Cherry, L.; Dunne, E. Black elderberry (Sambucus nigra) supplementation effectively treats upper respiratory symptoms: A meta-analysis of randomized, controlled clinical trials. Complement. Ther. Med. 2019, 42, 361–365. [Google Scholar]
- Tiralongo, E.; Wee, S.S.; Lea, R.A. Elderberry Supplementation Reduces Cold Duration and Symptoms in Air-Travellers: A Randomized, Double-Blind Placebo-Controlled Clinical Trial. Nutrients 2016, 8, 182. [Google Scholar] [CrossRef] [PubMed]
- Zakay-Rones, Z.; Thom, E.; Wollan, T.; Wadstein, J. Randomized study of the efficacy and safety of oral elderberry extract in the treatment of influenza A and B virus infections. J. Int. Med. Res. 2004, 32, 132–140. [Google Scholar]
- Kinoshita, E.; Hayashi, K.; Katayama, H.; Hayashi, T.; Obata, A. Anti-influenza virus effects of elderberry juice and its fractions. Biosci. Biotechnol. Biochem. 2012, 76, 1633–1638. [Google Scholar]
- Setz, C.; Fröba, M.; Große, M.; Rauch, P.; Auth, J.; Steinkasserer, A.; Plattner, S.; Schubert, U. European Black Elderberry Fruit Extract Inhibits Replication of SARS-CoV-2 In Vitro. Nutraceuticals 2023, 3, 91–106. [Google Scholar] [CrossRef]
- Chen, C.; Zuckerman, D.M.; Brantley, S.; Sharpe, M.; Childress, K.; Hoiczyk, E.; Pendleton, A.R. Sambucus nigra extracts inhibit infectious bronchitis virus at an early point during replication. BMC Vet. Res. 2014, 10, 24. [Google Scholar]
- Swaminathan, K.; Dyason, J.C.; Maggioni, A.; von Itzstein, M.; Downard, K.M. Binding of a natural anthocyanin inhibitor to influenza neuraminidase by mass spectrometry. Anal. Bioanal. Chem. 2013, 405, 6563–6572. [Google Scholar]
- Roschek, B.J.; Fink, R.C.; McMichael, M.D.; Li, D.; Alberte, R.S. Elderberry flavonoids bind to and prevent H1N1 infection In Vitro. Phytochemistry 2009, 70, 1255–1261. [Google Scholar]
- Große, M.; Ruetalo, N.; Layer, M.; Hu, D.; Businger, R.; Rheber, S.; Setz, C.; Rauch, P.; Auth, J.; Fröba, M.; et al. Quinine Inhibits Infection of Human Cell Lines with SARS-CoV-2. Viruses 2021, 13, 647. [Google Scholar] [CrossRef]
- Latarissa, I.R.; Barliana, M.I.; Meiliana, A.; Lestari, K. Potential of Quinine Sulfate for COVID-19 Treatment and Its Safety Profile: Review. Clin. Pharmacol. Adv. Appl. 2021, 13, 225–234. [Google Scholar]
- Miller, L.H.; Rojas-Jaimes, J.; Low, L.M.; Corbellini, G. What Historical Records Teach Us about the Discovery of Quinine. Am. J. Trop. Med. Hyg. 2023, 108, 7–11. [Google Scholar] [PubMed]
- Achan, J.; Talisuna, A.O.; Erhart, A.; Yeka, A.; Tibenderana, J.K.; Baliraine, F.N.; Rosenthal, P.J.; D’Alessandro, U. Quinine, an old anti-malarial drug in a modern world: Role in the treatment of malaria. Malar. J. 2011, 10, 144. [Google Scholar]
- Khan, S.A.; Al-Balushi, K. Combating COVID-19: The role of drug repurposing and medicinal plants. J. Infect. Public. Health 2021, 14, 495–503. [Google Scholar]
- Malakar, S.; Sreelatha, L.; Dechtawewat, T.; Noisakran, S.; Yenchitsomanus, P.-t.; Chu, J.J.H.; Limjindaporn, T. Drug repurposing of quinine as antiviral against dengue virus infection. Virus Res. 2018, 255, 171–178. [Google Scholar]
- D’Alessandro, S.; Scaccabarozzi, D.; Signorini, L.; Perego, F.; Ilboudo, D.P.; Ferrante, P.; Delbue, S. The Use of Antimalarial Drugs against Viral Infection. Microorganisms 2020, 8, 85. [Google Scholar] [CrossRef]
- Baroni, A.; Paoletti, I.; Ruocco, E.; Ayala, F.; Corrado, F.; Wolf, R.; Tufano, M.A.; Donnarumma, G. Antiviral effects of quinine sulfate on HSV-1 HaCat cells infected: Analysis of the molecular mechanisms involved. J. Dermatol. Sci. 2007, 47, 253–255. [Google Scholar]
- Seeler, A.O.; Graessle, O.; Ott, W.H. Effect of Quinine on Influenza Virus Infections in Mice. J. Infect. Dis. 1946, 79, 156–158. [Google Scholar]
- Giusti, M.M.; Wrolstad, R.E. Characterization and Measurement of Anthocyanins by UV-Visible Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 00, F1.2.1–F1.2.13. [Google Scholar]
- de Wit, E.; Spronken, M.I.J.; Bestebroer, T.M.; Rimmelzwaan, G.F.; Osterhaus, A.D.M.E.; Fouchier, R.A.M. Efficient generation and growth of influenza virus A/PR/8/34 from eight cDNA fragments. Virus Res. 2004, 103, 155–161. [Google Scholar]
- Aguiar, J.A.; Tremblay, B.J.; Mansfield, M.J.; Woody, O.; Lobb, B.; Banerjee, A.; Chandiramohan, A.; Tiessen, N.; Cao, Q.; Dvorkin-Gheva, A.; et al. Gene expression and in situ protein profiling of candidate SARS-CoV-2 receptors in human airway epithelial cells and lung tissue. Eur. Respir. J. 2020, 56, 2001123. [Google Scholar] [CrossRef] [PubMed]
- Reed, L.J.; Muench, H. A Simple Method Of Estimating Fifty Per Cent Endpoints. Am. J. Epidemiol. 1936, 27, 493–497. [Google Scholar] [CrossRef]
- Gaush, C.R.; Hard, W.L.; Smith, T.F. Characterization of an established line of canine kidney cells (MDCK). Proceedings of the Society for Experimental Biology and Medicine. Soc. Exp. Biol. Med. 1966, 122, 931–935. [Google Scholar] [CrossRef]
- Corman, V.M.; Landt, O.; Kaiser, M.; Molenkamp, R.; Meijer, A.; Chu, D.K.; Bleicker, T.; Brünink, S.; Schneider, J.; Schmidt, M.L.; et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. Bull. Eur. Sur Les Mal. Transm. Eur. Commun. Dis. Bull. 2020, 25, 2000045. [Google Scholar] [CrossRef] [PubMed]
- Ianevski, A.; He, L.; Aittokallio, T.; Tang, J. SynergyFinder: A web application for analyzing drug combination dose-response matrix data. Bioinformatics 2017, 33, 2413–2415. [Google Scholar]
- Liu, Q.; Yin, X.; Languino, L.R.; Altieri, D.C. Evaluation of drug combination effect using a Bliss independence dose-response surface model. Stat. Biopharm. Res. 2018, 10, 112–122. [Google Scholar] [CrossRef]
- Owen, L.; Laird, K.; Shivkumar, M. Antiviral plant-derived natural products to combat RNA viruses: Targets throughout the viral life cycle. Lett. Appl. Microbiol. 2022, 75, 476–499. [Google Scholar] [CrossRef]
- Musarra-Pizzo, M.; Pennisi, R.; Ben-Amor, I.; Mandalari, G.; Sciortino, M.T. Antiviral Activity Exerted by Natural Products against Human Viruses. Viruses 2021, 13, 828. [Google Scholar] [CrossRef]
- Antonelli, G.; Turriziani, O. Antiviral therapy: Old and current issues. Int. J. Antimicrob. Agents 2012, 40, 95–102. [Google Scholar] [CrossRef]
- Özçelik, B.; Kartal, M.; Orhan, I. Cytotoxicity, antiviral and antimicrobial activities of alkaloids, flavonoids, and phenolic acids. Pharm. Biol. 2011, 49, 396–402. [Google Scholar] [CrossRef]
- Mohammadi Pour, P.; Fakhri, S.; Asgary, S.; Farzaei, M.H.; Echeverría, J. The Signaling Pathways, and Therapeutic Targets of Antiviral Agents: Focusing on the Antiviral Approaches and Clinical Perspectives of Anthocyanins in the Management of Viral Diseases. Front. Pharmacol. 2019, 10, 1207. [Google Scholar] [CrossRef] [PubMed]
- Grassauer, A.; Weinmuellner, R.; Meier, C.; Pretsch, A.; Prieschl-Grassauer, E.; Unger, H. Iota-Carrageenan is a potent inhibitor of rhinovirus infection. J. Virol. 2008, 5, 107. [Google Scholar] [CrossRef]
- Leibbrandt, A.; Meier, C.; König-Schuster, M.; Weinmüllner, R.; Kalthoff, D.; Pflugfelder, B.; Graf, P.; Frank-Gehrke, B.; Beer, M.; Fazekas, T.; et al. Iota-carrageenan is a potent inhibitor of influenza A virus infection. PLoS ONE 2010, 5, e14320. [Google Scholar] [CrossRef]
- Morokutti-Kurz, M.; Graf, C.; Prieschl-Grassauer, E. Amylmetacresol/2,4-dichlorobenzyl alcohol, hexylresorcinol, or carrageenan lozenges as active treatments for sore throat. Int. J. Gen. Med. 2017, 10, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Morokutti-Kurz, M.; Fröba, M.; Graf, P.; Große, M.; Grassauer, A.; Auth, J.; Schubert, U.; Prieschl-Grassauer, E. Iota-carrageenan neutralizes SARS-CoV-2 and inhibits viral replication in vitro. PLoS ONE 2021, 16, e0237480. [Google Scholar] [CrossRef]
- Schütz, D.; Conzelmann, C.; Fois, G.; Groß, R.; Weil, T.; Wettstein, L.; Stenger, S.; Zelikin, A.; Hoffmann, T.K.; Frick, M.; et al. Carrageenan-containing over-the-counter nasal and oral sprays inhibit SARS-CoV-2 infection of airway epithelial cultures. Am. J. Physiol. Lung Cell Mol. Physiol. 2021, 320, L750–L756. [Google Scholar] [CrossRef]
- Fröba, M.; Große, M.; Setz, C.; Rauch, P.; Auth, J.; Spanaus, L.; Münch, J.; Ruetalo, N.; Schindler, M.; Morokutti-Kurz, M.; et al. Iota-Carrageenan Inhibits Replication of SARS-CoV-2 and the Respective Variants of Concern Alpha, Beta, Gamma and Delta. Int. J. Mol. Sci. 2021, 22, 13202. [Google Scholar] [CrossRef]
- Figueroa, J.M.; Lombardo, M.E.; Dogliotti, A.; Flynn, L.P.; Giugliano, R.; Simonelli, G.; Valentini, R.; Ramos, A.; Romano, P.; Marcote, M.; et al. Efficacy of a Nasal Spray Containing Iota-Carrageenan in the Postexposure Prophylaxis of COVID-19 in Hospital Personnel Dedicated to Patients Care with COVID-19 Disease. Int. J. Gen. Med. 2021, 14, 6277–6286. [Google Scholar] [PubMed]
- Chahla, R.E.; Ruiz, L.M.; Ortega, E.S.; Morales, M.F.; Barreiro, F.; George, A.; Mancilla, C.; Amato, S.D.; Barrenechea, G.; Goroso, D.G.; et al. Intensive Treatment With Ivermectin and Iota-Carrageenan as Pre-exposure Prophylaxis for COVID-19 in Health Care Workers From Tucuman, Argentina. Am. J. Ther. 2021, 28, e601–e604. [Google Scholar] [CrossRef]
- Santhi, V.P.; Sriramavaratharajan, V.; Murugan, R.; Masilamani, P.; Gurav, S.S.; Sarasu, V.P.; Parthiban, S.; Ayyanar, M. Edible fruit extracts and fruit juices as potential source of antiviral agents: A review. J. Food Meas. Charact. 2021, 15, 5181–5190. [Google Scholar] [CrossRef]
- Ulbricht, C.; Basch, E.; Cheung, L.; Goldberg, H.; Hammerness, P.; Isaac, R.; Khalsa, K.P.; Romm, A.; Rychlik, I.; Varghese, M.; et al. An evidence-based systematic review of elderberry and elderflower (Sambucus nigra) by the Natural Standard Research Collaboration. J. Diet. Suppl. 2014, 11, 80–120. [Google Scholar] [PubMed]
- Zakay-Rones, Z.; Varsano, N.; Zlotnik, M.; Manor, O.; Regev, L.; Schlesinger, M.; Mumcuoglu, M. Inhibition of several strains of influenza virus in vitro and reduction of symptoms by an elderberry extract (Sambucus nigra L.) during an outbreak of influenza B Panama. J. Altern. Complement. Med. 1995, 1, 361–369. [Google Scholar] [PubMed]
- Krawitz, C.; Mraheil, M.A.; Stein, M.; Imirzalioglu, C.; Domann, E.; Pleschka, S.; Hain, T. Inhibitory activity of a standardized elderberry liquid extract against clinically-relevant human respiratory bacterial pathogens and influenza A and B viruses. BMC Complement. Altern. Med. 2011, 11, 16. [Google Scholar]
- Porter, R.S.; Bode, R.F. A Review of the Antiviral Properties of Black Elder (Sambucus nigra L.) Products. Phytother. Res. PTR 2017, 31, 533–554. [Google Scholar]
- Swaminathan, K.; Müller, P.; Downard, K.M. Substituent effects on the binding of natural product anthocyanidin inhibitors to influenza neuraminidase with mass spectrometry. Anal. Chim. Acta 2014, 828, 61–69. [Google Scholar] [CrossRef]
- Stich, L.; Plattner, S.; McDougall, G.; Austin, C.; Steinkasserer, A. Polysaccharides from European Black Elderberry Extract Enhance Dendritic Cell Mediated T Cell Immune Responses. Int. J. Mol. Sci. 2022, 23, 3949. [Google Scholar] [CrossRef]
- Ho, G.T.; Ahmed, A.; Zou, Y.F.; Aslaksen, T.; Wangensteen, H.; Barsett, H. Structure-activity relationship of immunomodulating pectins from elderberries. Carbohydr. Polym. 2015, 125, 314–322. [Google Scholar]
- Förster-Waldl, E.; Marchetti, M.; Schöll, I.; Focke, M.; Radauer, C.; Kinaciyan, T.; Nentwich, I.; Jäger, S.; Schmid, E.R.; Boltz-Nitulescu, G.; et al. Type I allergy to elderberry (Sambucus nigra) is elicited by a 33.2 kDa allergen with significant homology to ribosomal inactivating proteins. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2003, 33, 1703–1710. [Google Scholar]
- Reider, S.; Watschinger, C.; Längle, J.; Pachmann, U.; Przysiecki, N.; Pfister, A.; Zollner, A.; Tilg, H.; Plattner, S.; Moschen, A.R. Short- and Long-Term Effects of a Prebiotic Intervention with Polyphenols Extracted from European Black Elderberry-Sustained Expansion of Akkermansia spp. J. Pers. Med. 2022, 12, 1479. [Google Scholar] [CrossRef]
- Bray, P.G.; Mungthin, M.; Hastings, I.M.; Biagini, G.A.; Saidu, D.K.; Lakshmanan, V.; Johnson, D.J.; Hughes, R.H.; Stocks, P.A.; O’Neill, P.M.; et al. PfCRT and the trans-vacuolar proton electrochemical gradient: Regulating the access of chloroquine to ferriprotoporphyrin IX. Mol. Microbiol. 2006, 62, 238–251. [Google Scholar]
- Mauthe, M.; Orhon, I.; Rocchi, C.; Zhou, X.; Luhr, M.; Hijlkema, K.J.; Coppes, R.P.; Engedal, N.; Mari, M.; Reggiori, F. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy 2018, 14, 1435–1455. [Google Scholar] [CrossRef] [PubMed]
- Nugraha, R.V.; Ridwansyah, H.; Ghozali, M.; Khairani, A.F.; Atik, N. Traditional Herbal Medicine Candidates as Complementary Treatments for COVID-19: A Review of Their Mechanisms, Pros and Cons. Evid. Based Complement. Altern. Med. 2020, 2020, 2560645. [Google Scholar] [CrossRef] [PubMed]
- Savarino, A.; Boelaert, J.R.; Cassone, A.; Majori, G.; Cauda, R. Effects of chloroquine on viral infections: An old drug against today’s diseases? Lancet. Infect. Dis. 2003, 3, 722–727. [Google Scholar] [CrossRef]
- Eyal, S. The Fever Tree: From Malaria to Neurological Diseases. Toxins 2018, 10, 491. [Google Scholar] [CrossRef] [PubMed]
- Humphries, W. Just the tonic … gin becomes nation’s favourite spirit. The Times, 16 December 2017; p. 2017. [Google Scholar]
- Hall, A.P. The treatment of severe falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 1977, 71, 367–378. [Google Scholar] [CrossRef]
- Saguil, A.; Lauters, R. Quinine for Leg Cramps. Am. Fam. Physician 2016, 93, 177–178. [Google Scholar]
- Tange, R.A.; Dreschler, W.A.; Claessen, F.A.; Perenboom, R.M. Ototoxic reactions of quinine in healthy persons and patients with Plasmodium falciparum infection. Auris Nasus Larynx 1997, 24, 131–136. [Google Scholar] [CrossRef]
- Law, E.U. Regulation (EC) No 1334/2008 of the European Parliament and of the Council of 16 December 2008 on Flavourings and Certain Food Ingredients with Flavouring Properties for Use in and on Foods and Amending Council Regulation (EEC) No 1601/91, Regulations (EC) No 2232/96 and (EC) No 110/2008 and Directive 2000/13/EC. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:02008R1334-20190521 (accessed on 24 April 2024).
- Hall, A.P.; Czerwinski, A.W.; Madonia, E.C.; Evensen, K.L. Human plasma and urine quinine levels following tablets, capsules, and intravenous infusion. Clin. Pharmacol. Ther. 1973, 14, 580–585. [Google Scholar] [CrossRef]
- Soyinka, J.O.; Onyeji, C.O.; Omoruyi, S.I.; Owolabi, A.R.; Sarma, P.V.; Cook, J.M. Effects of concurrent administration of nevirapine on the disposition of quinine in healthy volunteers. J. Pharm. Pharmacol. 2009, 61, 439–443. [Google Scholar] [CrossRef]
- Shyr, Z.A.; Cheng, Y.-S.; Lo, D.C.; Zheng, W. Drug combination therapy for emerging viral diseases. Drug Discov. Today 2021, 26, 2367–2376. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, G.; Salam, A.; Horby, P.; Hayden, F.G.; Chen, C.; Pan, J.; Zheng, J.; Lu, B.; Guo, L.; et al. Comparative Effectiveness of Combined Favipiravir and Oseltamivir Therapy Versus Oseltamivir Monotherapy in Critically Ill Patients With Influenza Virus Infection. J. Infect. Dis. 2019, 221, 1688–1698. [Google Scholar]
- Boulon, R.; Mazeaud, C.; Farahani, M.D.; Broquière, M.; Iddir, M.; Charpentier, T.; Anton, A.; Ayotte, Y.; Woo, S.; Lamarre, A.; et al. Repurposing Drugs and Synergistic Combinations as Potential Therapies for Inhibiting SARS-CoV-2 and Coronavirus Replication. ACS Pharmacol. Transl. Sci. 2024, 7, 4043–4055. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.-K.; Lee, M.-M.; Ma, J.Y. Antiviral Effect of Isoquercitrin against Influenza A Viral Infection via Modulating Hemagglutinin and Neuraminidase. Int. J. Mol. Sci. 2022, 23, 13112. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, V.; Brognaro, H.; Prabhu, P.R.; de Souza, E.E.; Günther, S.; Reinke, P.Y.A.; Lane, T.J.; Ginn, H.; Han, H.; Ewert, W.; et al. Antiviral activity of natural phenolic compounds in complex at an allosteric site of SARS-CoV-2 papain-like protease. Commun. Biol. 2022, 5, 805. [Google Scholar] [CrossRef]
- Patras, A.; Brunton, N.P.; O’Donnell, C.; Tiwari, B.K. Effect of thermal processing on anthocyanin stability in foods; mechanisms and kinetics of degradation. Trends Food Sci. Technol. 2010, 21, 3–11. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Setz, C.; Rauch, P.; Setz, M.; Breitenberger, S.; Plattner, S.; Schubert, U. Synergistic Antiviral Activity of European Black Elderberry Fruit Extract and Quinine Against SARS-CoV-2 and Influenza A Virus. Nutrients 2025, 17, 1205. https://doi.org/10.3390/nu17071205
Setz C, Rauch P, Setz M, Breitenberger S, Plattner S, Schubert U. Synergistic Antiviral Activity of European Black Elderberry Fruit Extract and Quinine Against SARS-CoV-2 and Influenza A Virus. Nutrients. 2025; 17(7):1205. https://doi.org/10.3390/nu17071205
Chicago/Turabian StyleSetz, Christian, Pia Rauch, Melanie Setz, Stephan Breitenberger, Stephan Plattner, and Ulrich Schubert. 2025. "Synergistic Antiviral Activity of European Black Elderberry Fruit Extract and Quinine Against SARS-CoV-2 and Influenza A Virus" Nutrients 17, no. 7: 1205. https://doi.org/10.3390/nu17071205
APA StyleSetz, C., Rauch, P., Setz, M., Breitenberger, S., Plattner, S., & Schubert, U. (2025). Synergistic Antiviral Activity of European Black Elderberry Fruit Extract and Quinine Against SARS-CoV-2 and Influenza A Virus. Nutrients, 17(7), 1205. https://doi.org/10.3390/nu17071205





