Antioxidant, Polyphenol, Physical, and Sensory Changes in Myofibrillar Protein Gels Supplemented with Polyphenol-Rich Plant-Based Additives
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
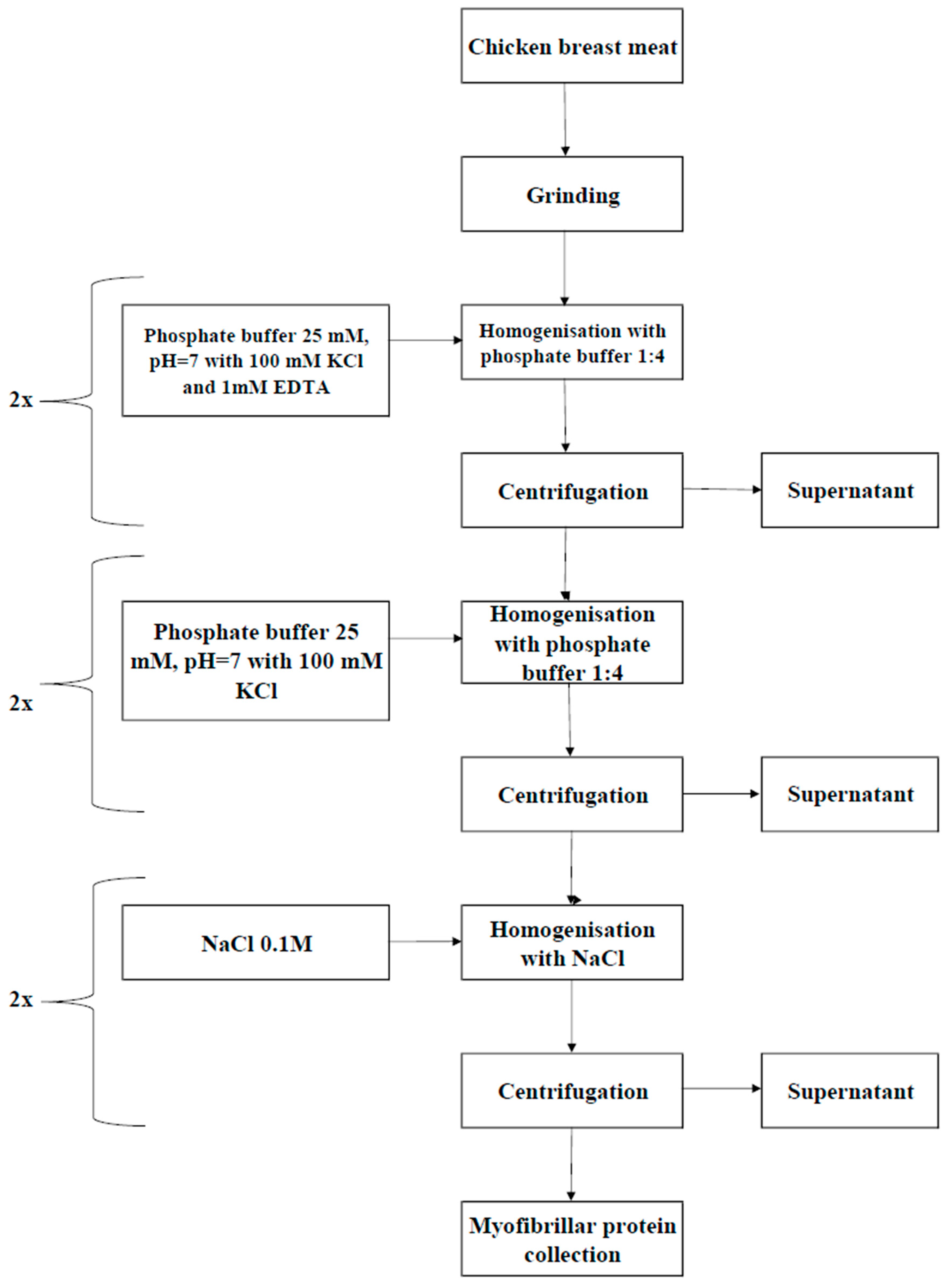
2.2. Isolation of Myofibrillar Protein
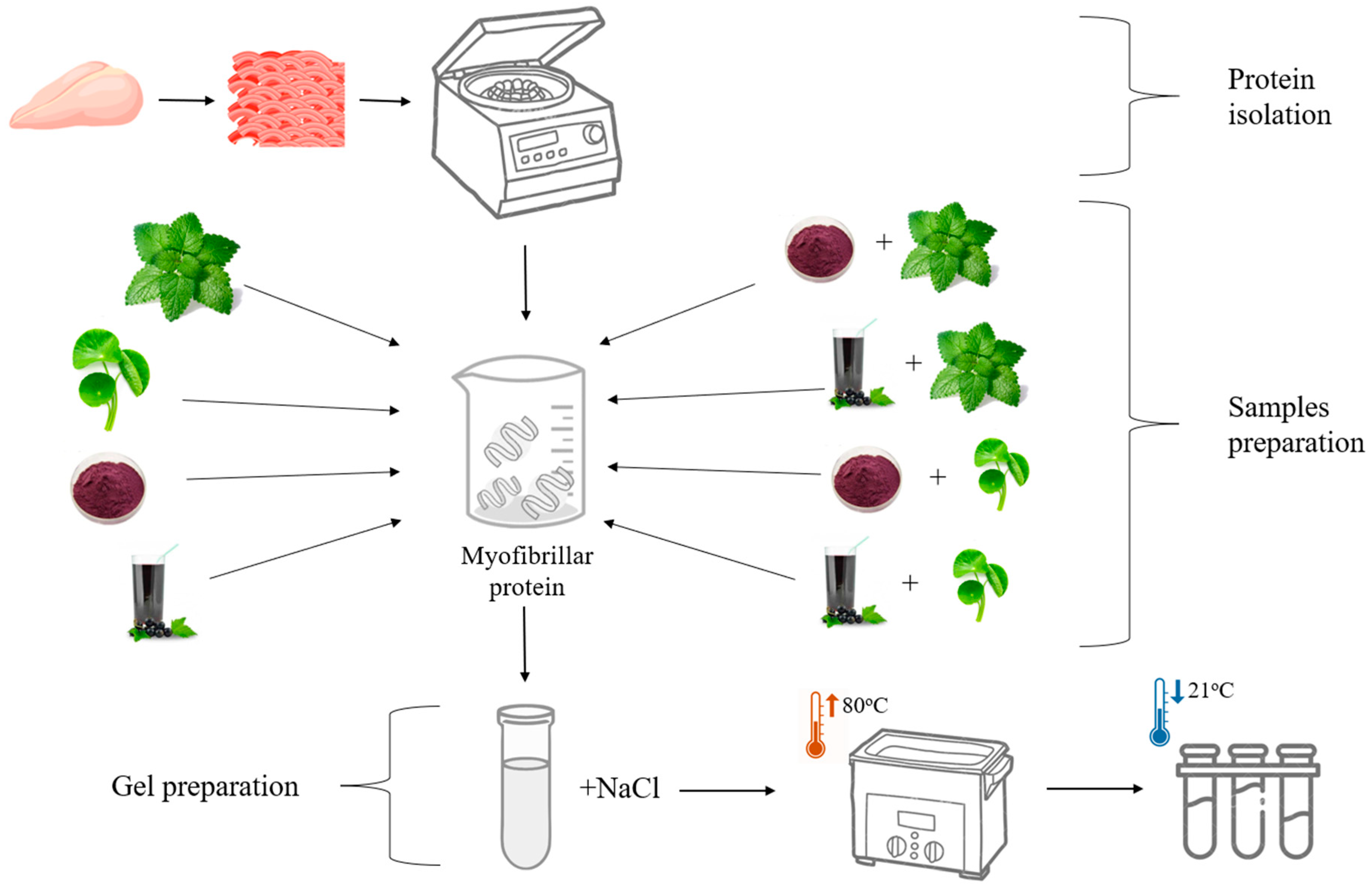
2.3. Preparation of Myofibrillar Protein Gels Supplemented with Plant-Based Additives
2.4. Analytical Methods
2.4.1. Antioxidant Attributes
Determination of 2,2′-Azinobis(3-ethylbenzothiazoline-6-sulfonate) (ABTS+) Radical Scavenging Activity
Determination of 1,1-Diphenyl-2-picrylhydrazyl (DPPH) Radical Scavenging Activity
Determination of Ferric-Reducing Antioxidant Power (FRAP) Assay
2.4.2. Determination of Polyphenolic Compounds by the LC-MS-PDA-Q/TOF and UPLC-PDA Methods
2.4.3. Physical Attributes
Determination of pH
Determination of CIE L*a*b* Color
Determination of Hardness and Cohesiveness
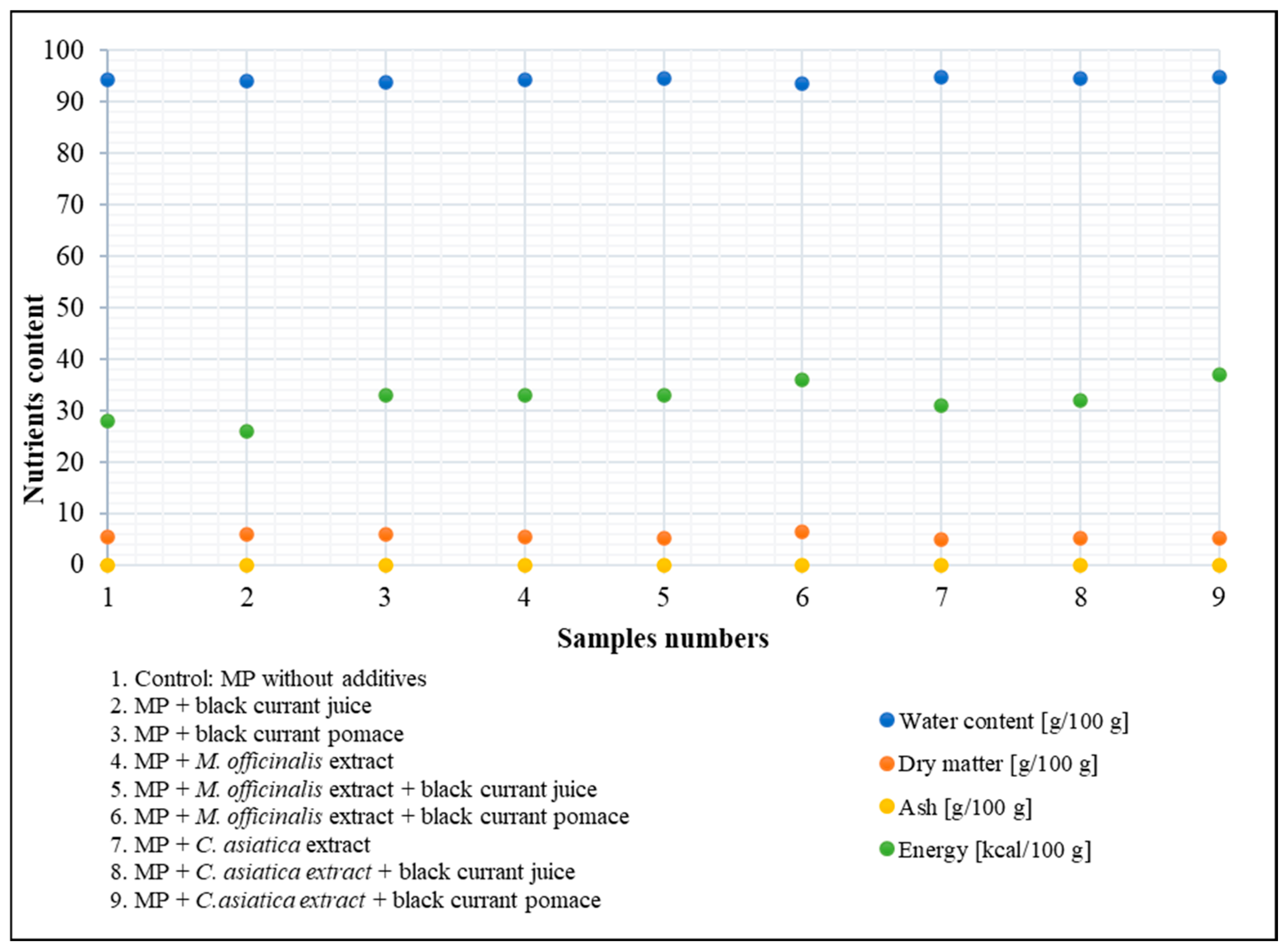
Determination of Calorific (Energy) Value
Determination of Dry Matter
Determination of Ash
2.4.4. Sensory Attributes
2.5. Statistical Analysis
3. Results and Discussion
3.1. Antioxidant Changes
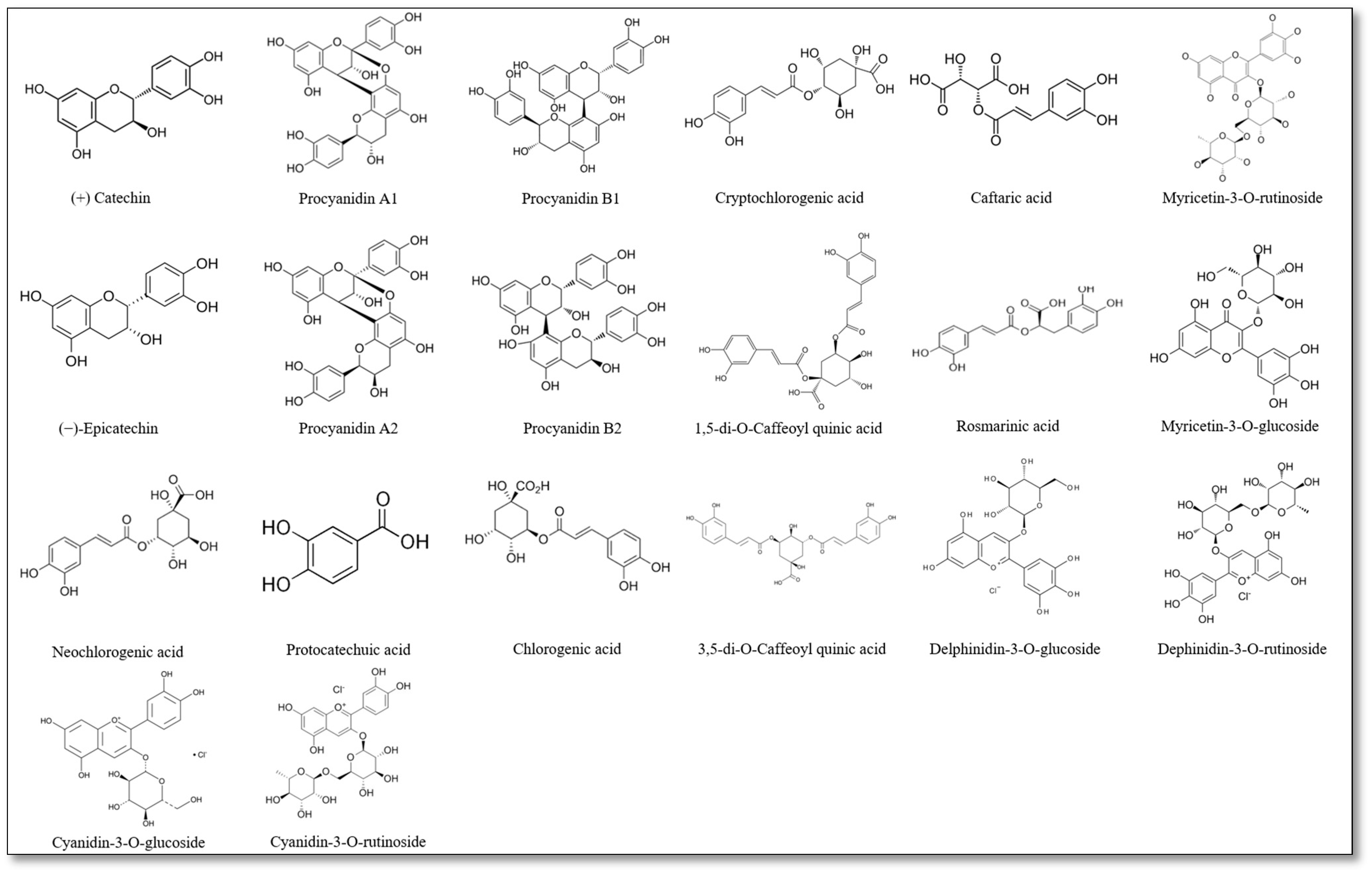
3.2. Polyphenolic Changes
3.3. Physical Changes
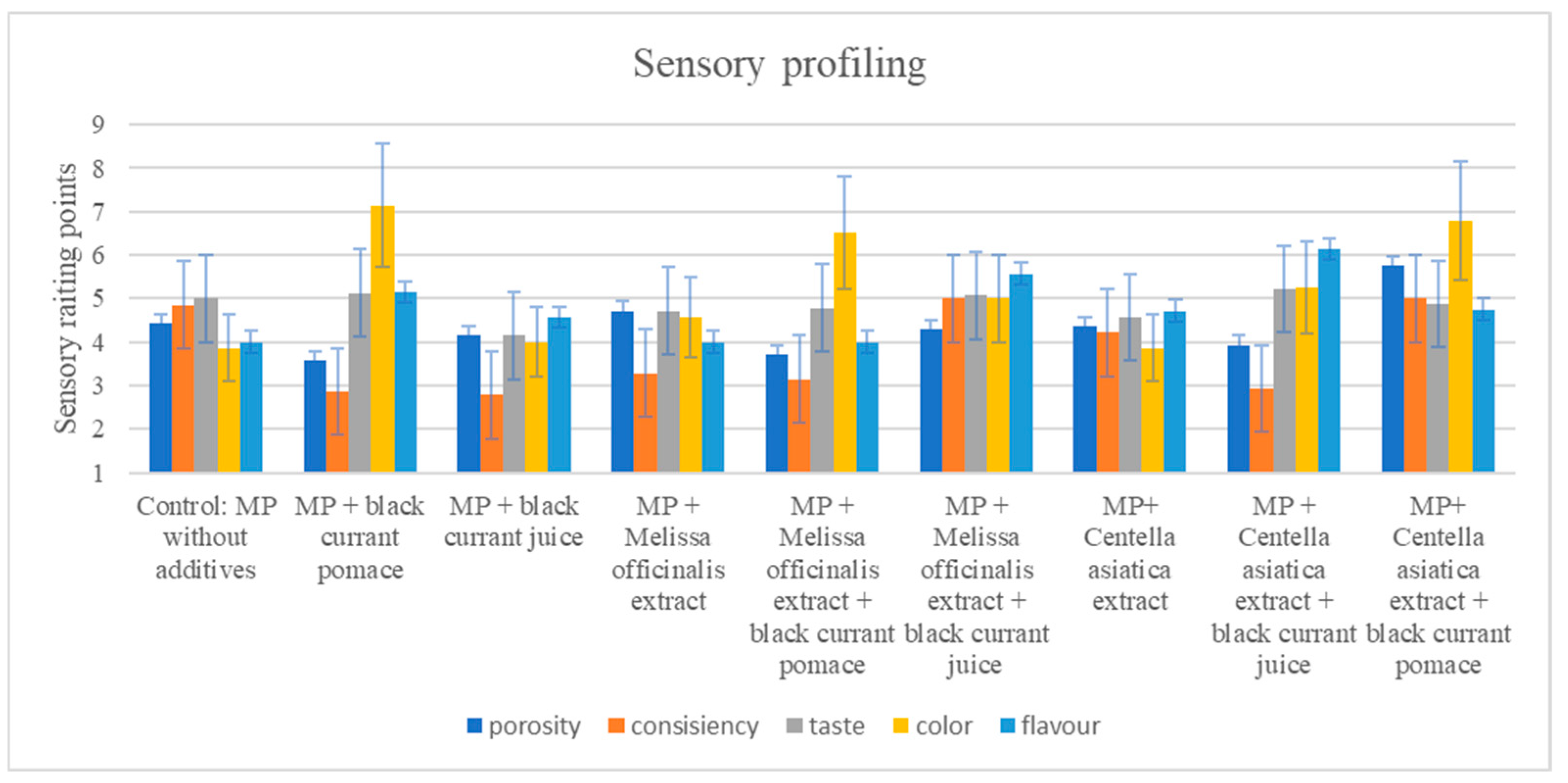
3.4. Sensory Changes
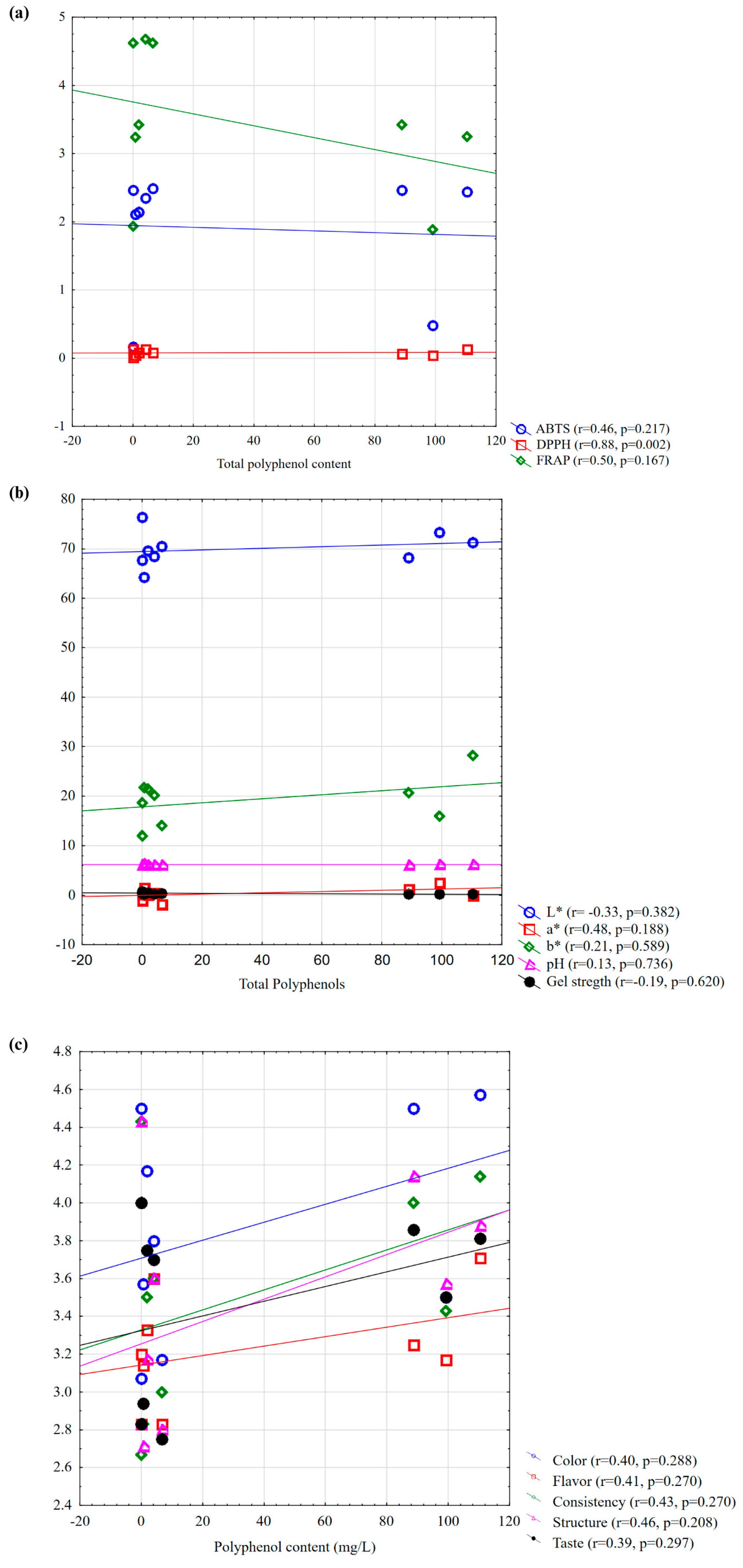
3.5. Relationships Between Antioxidant, Polyphenolic, Physicochemical, and Sensory Attributes
4. Limitations of the Current Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Topolska, K.; Florkiewicz, A.; Filipiak-Florkiewicz, A. Functional food—Consumer motivations and expectations. Int. J. Environ. Res. Public Health 2021, 18, 5327. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, D.; Li, B.; Lund, M.N.; Xing, Y.; Wang, Y.; Li, L. Engineering polyphenols with biological functions via polyphenol-protein interactions as additives for functional foods. Trends Food Sci. Technol. 2021, 110, 470–482. [Google Scholar]
- Iqbal, I.; Wilairatana, P.; Saqib, F.; Nasir, B.; Wahid, M.; Latif, M.F.; Mubarak, M.S. Plant polyphenols and their potential benefits on cardiovascular health: A review. Molecules 2023, 28, 6403. [Google Scholar] [CrossRef]
- Jin, Q.; Liu, T.; Qiao, Y.; Liu, D.; Yang, L.; Mao, H.; Zhan, Y. Oxidative stress and inflammation in diabetic nephropathy: Role of polyphenols. Front. Immunol. 2023, 14, 1185317. [Google Scholar]
- Kostenko, V.; Akimov, O.; Gutnik, O.; Kostenko, H.; Kostenko, V.; Romantseva, T.; Morhun, Y.; Nazarenko, S.; Taran, O. Modulation of redox-sensitive transcription factors with polyphenols as pathogenetically grounded approach in therapy of systemic inflammatory response. Heliyon 2023, 9, e16054. [Google Scholar]
- Li, W.; Chen, H.; Xu, B.; Wang, Y.; Zhang, C.; Cao, Y.; Xing, X. Research progress on classification, sources, and functions of dietary polyphenols for prevention and treatment of chronic diseases. J. Future Foods 2023, 3, 289–305. [Google Scholar]
- Cruz-Jentoft, A.J.; Hughes, B.D.; Scott, D.; Sanders, K.M.; Rizzoli, R. Nutritional strategies for maintaining muscle mass and strength from middle age to later life: A narrative review. Maturitas 2020, 132, 57–64. [Google Scholar]
- Małecki, J.; Muszyński, S.; Sołowiej, B.G. Proteins in food systems—Bionanomaterials, conventional and unconventional sources, functional properties, and development opportunities. Polymers 2021, 13, 2506. [Google Scholar] [CrossRef]
- Cheng, J.; Zhu, M.; Liu, X. Insight into the conformational and functional properties of myofibrillar protein modified by mulberry polyphenols. Food Chem. 2020, 308, 125592. [Google Scholar]
- Xu, Y.; Xu, X. Modification of myofibrillar protein functional properties prepared by various strategies: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 458–500. [Google Scholar]
- Li, K.; Fu, L.; Zhao, Y.Y.; Xue, S.W.; Wang, P.; Xu, X.L.; Bai, Y.H. Use of high-intensity ultrasound to improve emulsifying properties of chicken myofibrillar protein and enhance the rheological properties and stability of the emulsion. Food Hydrocoll. 2020, 98, 105275. [Google Scholar] [CrossRef]
- Liu, Y.; Mubango, E.; Dou, P.; Bao, Y.; Tan, Y.; Luo, Y.; Hong, H. Insight into the protein oxidation impact on the surface properties of myofibrillar proteins from bighead carp. Food Chem. 2023, 411, 135515. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, N.S.; Sintang, M.D.; Huda, N.; Mohd Zaini, H.; Akanda, M.J.H.; Pindi, W. Enhancing meat product quality: Exploring the effects of additives on myofibrillar protein functionality. Food Bioprocess Technol. 2024, 17, 1043–1060. [Google Scholar]
- Leicht, K.; Okpala, C.O.R.; Korzeniowska, M. Connecting polyphenols and myofibrillar proteins with their bioactive potentials: A terse review. Ann. Anim. Sci. 2024; in press. [Google Scholar]
- Abd El-Hack, M.E.; de Oliveira, M.C.; Attia, Y.A.; Kamal, M.; Almohmadi, N.H.; Youssef, I.M.; Khalifa, N.E.; Moustafa, M.; Al-Shehri, M.; Taha, A.E. The efficacy of polyphenols as an antioxidant agent: An updated review. Int. J. Biol. Macromol. 2023, 250, 126525. [Google Scholar] [CrossRef] [PubMed]
- Lang, Y.; Gao, N.; Zang, Z.; Meng, X.; Lin, Y.; Yang, S.; Li, B. Classification and antioxidant assays of polyphenols: A review. J. Future Foods 2024, 4, 193–204. [Google Scholar] [CrossRef]
- Grabska-Kobyłecka, I.; Szpakowski, P.; Król, A.; Książek-Winiarek, D.; Kobyłecki, A.; Głąbiński, A.; Nowak, D. Polyphenols and their impact on the prevention of neurodegenerative diseases and development. Nutrients 2023, 15, 3454. [Google Scholar] [CrossRef]
- Meremäe, K.; Raudsepp, P.; Rusalepp, L.; Anton, D.; Bleive, U.; Roasto, M. In vitro antibacterial and antioxidative activity and polyphenolic profile of the extracts of chokeberry, blackcurrant, and rowan berries and their pomaces. Foods 2024, 13, 421. [Google Scholar] [CrossRef]
- Untea, A.E.; Oancea, A.G.; Vlaicu, P.A.; Varzaru, I.; Saracila, M. Blackcurrant (fruits, pomace, and leaves) phenolic characterization before and after In Vitro digestion, free radical scavenger capacity, and antioxidant effects on iron-mediated lipid peroxidation. Foods 2024, 13, 1514. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Zhang, L.; Ren, J.; Bai, W. A review of poultry waste-to-wealth: Technological progress, modeling and simulation studies, and economic-environmental and social sustainability. Sustainability 2023, 15, 5620. [Google Scholar] [CrossRef]
- Peng, L.; Ma, L.; Dai, H.J.; Yu, Y.; Wang, H.X.; Zhu, H.K.; Zhang, Y.H. Recent progress in understanding the interaction mechanism between polyphenols and myofibrillar protein and its effects on protein properties. Food Chem. 2020, 342, 128268. [Google Scholar]
- Zhang, K.; Huang, J.; Wang, D.; Wan, X.; Wang, Y. Covalent polyphenols-proteins interactions in food processing: Formation mechanisms, quantification methods, bioactive effects, and applications. Front. Nutr. 2024, 11, 1371401. [Google Scholar]
- Cui, C.; Zhou, X.; Zhao, M.; Yang, B. Effect of thermal treatment on the enzymatic hydrolysis of chicken proteins. Innov. Food Sci. Emerg. Technol. 2009, 10, 37–41. [Google Scholar]
- Ryan, J.T.; Ross, R.P.; Bolton, D.; Fitzgerald, G.F.; Stanton, C. Bioactive Peptides from Muscle Sources: Meat and Fish. Nutrients 2011, 3, 765–791. [Google Scholar] [CrossRef]
- Nusairat, B.; Tellez-Isaias, G.; Qudsieh, R. An overview of poultry meat quality and myopathies. In Broiler Industry; IntechOpen: London, UK, 2022. [Google Scholar]
- Olson, D.G.; Stromer, M.H. Myofibril fragmentation and shear resistance of three bovine muscles during postmortem storage. J. Food Sci. 1976, 41, 1036–1041. [Google Scholar]
- AOAC International. Official Methods of Analysis of AOAC International, 21st ed.; AOAC International: Rockville, MD, USA, 2019. [Google Scholar]
- Camou, J.P.; Sebranek, J.G.; Olson, D.G. Effect of Heating Rate and Protein Concentration on Gel Strength and Water Loss of Muscle Protein Gels. J. Food Sci. 1989, 54, 850–854. [Google Scholar]
- Jeong, J.W.; Lee, S.Y.; Kim, J.H.; Yun, S.H.; Lee, J.; Mariano, E.; Hur, S.J. Analytical methods and effects of bioactive peptides derived from animal products: A mini-review. Food Sci. Anim. Resour. 2024, 44, 533. [Google Scholar]
- Gulcin, İ.; Alwasel, S.H. DPPH radical scavenging assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Kalpana, S.; Vijai, D.; Premalatha, S. Antioxidant activity of different solvent extracts of Barleria longiflora Linn. Int. J. Curr. Res. Biol. Med. 2016, 1, 1–8. [Google Scholar]
- Wojdyło, A.; Nowicka, P.; Turkiewicz, I.P.; Tkacz, K.; Hernandez, F. Comparison of bioactive compounds and health-promoting properties of fruits and leaves of apple, pear, and quince. Sci. Rep. 2021, 11, 20253. [Google Scholar]
- Hopper, Z.; Desbrow, B.; Roberts, S.; Irwin, C.G. Beverage sample preparation and procedures for bomb calorimetry: Establishing equivalency in methods. J. Food Compos. Anal. 2024, 128, 106033. [Google Scholar] [CrossRef]
- Civille, G.V.; Carr, B.T.; Osdoba, K.E. Sensory Evaluation Techniques; CRC Press: Boca Raton, FL, USA, 2024. [Google Scholar]
- Lawless, H.T.; Heymann, H. Sensory Evaluation of Food: Principles and Practices; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Shakeri, A.; Sahebkar, A.; Javadi, B. Melissa officinalis L.—A review of its traditional uses, phytochemistry, and pharmacology. J. Ethnopharmacol. 2016, 188, 204–228. [Google Scholar] [CrossRef]
- James, J.T.; Dubery, I.A. Pentacyclic triterpenoids from the medicinal herb, Centella asiatica (L.) Urban. Molecules 2009, 14, 3922–3941. [Google Scholar] [CrossRef]
- Dulf, F.V.; Vodnar, D.C.; Dulf, E.H.; Pintea, A. Phenolic compounds, antioxidant activity, and lipid oxidation inhibition in sunflower oil enriched with bilberry, blackberry, and blackcurrant extracts. Food Chem. 2016, 213, 441–448. [Google Scholar]
- Määttä-Riihinen, K.R.; Kamal-Eldin, A.; Mattila, P.H.; González-Paramás, A.M.; Törrönen, A.R. Distribution and contents of phenolic compounds in eighteen Scandinavian berry species. J. Agric. Food Chem. 2004, 52, 4477–4486. [Google Scholar] [CrossRef]
- Olszowy, M. What is responsible for antioxidant properties of polyphenolic compounds from plants? Plant Physiol. Biochem. 2019, 144, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Tarahi, M.; Gharagozlou, M.; Niakousari, M.; Hedayati, S. Protein–Chlorogenic Acid Interactions: Mechanisms, Characteristics, and Potential Food Applications. Antioxidants 2024, 13, 777. [Google Scholar] [CrossRef]
- Szydłowska, M.; Wojdyło, A.; Nowicka, P. Black and red currant pomaces as raw materials to create smoothies with In Vitro health-promoting potential. Foods 2024, 13, 2715. [Google Scholar] [CrossRef]
- Sun, X.; Sarteshnizi, R.A.; Udenigwe, C.C. Recent advances in protein–polyphenol interactions focusing on structural properties related to antioxidant activities. Curr. Opin. Food Sci. 2022, 45, 100840. [Google Scholar] [CrossRef]
- Gervasi, T.; Calderaro, A.; Barreca, D.; Tellone, E.; Trombetta, D.; Ficarra, S.; Smeriglio, A.; Mandalari, G.; Gattuso, G. Biotechnological applications and health-promoting properties of flavonols: An updated view. Int. J. Mol. Sci. 2022, 23, 1710. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, H.; Song, L.; Yang, Z.; Qiu, M.; Wang, J.; Shi, S. Anthocyanins: Promising natural products with diverse pharmacological activities. Molecules 2021, 26, 3807. [Google Scholar] [CrossRef] [PubMed]
- Pap, N.; Fidelis, M.; Azevedo, L.; do Carmo, M.A.V.; Wang, D.; Mocan, A.; Pereira, E.P.R.; Xavier-Santos, D.; Sant’Ana, A.S.; Yang, B.; et al. Berry polyphenols and human health: Evidence of antioxidant, anti-inflammatory, microbiota modulation, and cell-protecting effects. Curr. Opin. Food Sci. 2021, 42, 167–186. [Google Scholar]
- Kroll, J.; Rawel, H.M. Reactions of plant phenolics with food proteins and enzymes under special consideration of covalent bonds. Food Sci. Technol. Res. 2001, 7, 219–228. [Google Scholar]
- Rawel, H.M.; Rohn, S. Nature of hydroxycinnamate-protein interactions. Phytochem. Rev. 2020, 19, 1399–1422. [Google Scholar]
- Feng, Y.; Jin, C.; Lv, S.; Zhang, H.; Ren, F.; Wang, J. Molecular Mechanisms and Applications of Polyphenol-Protein Complexes with Antioxidant Properties: A Review. Antioxidants 2023, 12, 1577. [Google Scholar] [CrossRef]
- Jongberg, S.; Lund, M.N.; Skibsted, L.H. Protein oxidation in meat and meat products. Challenges for antioxidative protection. In Global Food Security and Wellness; Springer: New York, NY, USA, 2017; pp. 315–337. [Google Scholar]
- Zhang, Q.; Cheng, Z.; Wang, Y.; Fu, L. Dietary protein-phenolic interactions: Characterization, biochemical-physiological consequences, and potential food applications. Crit. Rev. Food Sci. Nutr. 2021, 61, 3589–3615. [Google Scholar]
- Muntaha, S.T.; Rakha, A.; Rasheed, H.; Fatima, I.; Butt, M.S.; Abdi, G.; Aadil, R.M. Polyphenol-protein particles: A nutraceutical breakthrough in nutrition and food science. J. Agric. Food Res. 2025, 19, 101641. [Google Scholar]
- Dai, S.; Liao, P.; Wang, Y.; Tian, T.; Tong, X.; Lyu, B.; Cheng, L.; Miao, L.; Qi, W.; Jiang, L.; et al. Soy protein isolate-catechin non-covalent and covalent complexes: Focus on structure, aggregation, stability and in vitro digestion characteristics. Food Hydrocoll. 2023, 135, 108108. [Google Scholar]
- Gao, Q.; Chen, J.; Zhou, G.; Xu, X. Different protein-anthocyanin complexes engineered by ultrasound and alkali treatment: Structural characterization and color stability. Food Chem. 2023, 427, 136693. [Google Scholar]
- Sadowska, U.; Armenta Villavicencio, R.; Dziadek, K.; Skoczylas, J.; Sadowski, S.K.; Kopeć, A. The identification of polyphenolic compounds and the determination of antioxidant activity in extracts and infusions of peppermint, lemon balm, and lavender. Appl. Sci. 2024, 14, 699. [Google Scholar] [CrossRef]
- Guo, A.; Xiong, Y.L. Myoprotein–phytophenol interaction: Implications for muscle food structure-forming properties. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2801–2824. [Google Scholar] [PubMed]
- Shahidi, F.; Yeo, J.D. Bioactivities of phenolics by focusing on suppression of oxidative stress and inflammation: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 524–544. [Google Scholar]
- Cao, H.; Saroglu, O.; Karadag, A.; Diaconeasa, Z.; Zoccatelli, G.; Conte-Junior, C.A.; Gonzalez-Aguilar, G.A.; Ou, J.; Bai, W.; Zamarioli, C.M.; et al. Available technologies on improving the stability of polyphenols in food processing. Food Front. 2021, 2, 109–139. [Google Scholar]
- Parisi, O.I.; Puoci, F.; Restuccia, D.; Farina, G.; Iemma, F.; Picci, N. Polyphenols and their formulations: Different strategies to overcome the drawbacks associated with their poor stability and bioavailability. In Polyphenols in Human Health and Disease; Academic Press: Cambridge, MA, USA, 2014; pp. 29–45. [Google Scholar]
- Zhang, L.; McClements, D.J.; Wei, Z.; Wang, G.; Liu, X.; Liu, F. Delivery of synergistic polyphenol combinations using biopolymer-based systems: Advances in physicochemical properties, stability and bioavailability. Crit. Rev. Food Sci. Nutr. 2020, 60, 2083–2097. [Google Scholar]
- Kaur, H.; Kaur, G. A critical appraisal of solubility enhancement techniques of polyphenols. J. Pharm. 2014, 2014, 180845. [Google Scholar]
- Azman, E.M.; Yusof, N.; Chatzifragkou, A.; Charalampopoulos, D. Stability enhancement of anthocyanins from blackcurrant (Ribes nigrum L.) pomace through intermolecular copigmentation. Molecules 2022, 27, 5489. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Andrade, C.; Morales, F.J. Unraveling the Contribution of Melanoidins to the Antioxidant Activity of Coffee Brews. J. Agric. Food Chem. 2005, 53, 1403–1407. [Google Scholar]
- Akharume, F.U.; Aluko, R.E.; Adedeji, A.A. Modification of plant proteins for improved functionality: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 198–224. [Google Scholar]
- Balasubramaniam, V.G.; Ramakrishnan, S.R.; Antony, U. Opportunities and Challenges of Plant Extracts in Food Industry. In Plant Extracts: Applications in the Food Industry; Academic Press: Cambridge, MA, USA, 2022; pp. 295–315. [Google Scholar]
- Boo, X.Y.; Chia, L.H. Formulation and in vitro evaluation of gels with Centella asiatica extract. J. Pharmacogn. Phytochem. 2024, 13, 109–117. [Google Scholar]
- Cîrstea, N.; Nour, V.; Corbu, A.R.; Codină, G.G. Blackcurrant pomace extract as a natural antioxidant in Vienna sausages reformulated by replacement of pork backfat with emulsion gels based on high oleic sunflower and flaxseed oils. Gels 2024, 10, 534. [Google Scholar] [CrossRef]
- Jongberg, S.; Terkelsen, L.d.S.; Miklos, R.; Lund, M.N. Green tea extract impairs meat emulsion properties by disturbing protein disulfide cross-linking. Meat Sci. 2015, 100, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, H.; Liu, Q.; Kong, B.; Diao, X. Effects of zein hydrolysates coupled with sage (Salvia officinalis) extract on the emulsifying and oxidative stability of myofibrillar protein prepared oil-in-water emulsions. Food Hydrocoll. 2019, 87, 149–157. [Google Scholar] [CrossRef]
- Munir, S.; Hu, Y.; Liu, Y.; Xiong, S. Enhanced properties of silver carp surimi-based edible films incorporated with pomegranate peel and grape seed extracts under acidic condition. Food Packag. Shelf Life 2019, 19, 114–120. [Google Scholar] [CrossRef]
- Cao, Y.; Xiong, Y.L. Chlorogenic acid-mediated gel formation of oxidatively stressed myofibrillar protein. Food Chem. 2015, 180, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Figura, L.O.; Teixeira, A.A. Food Physics: Physical Properties—Measurement and Applications, 2nd ed.; Springer: Cham, Switzerland, 2023. [Google Scholar]
- Harris, P.; Marshall, M.R. Ash Analysis. In Food Analysis, 4th ed.; Nollet, L.M.L., Toldrá, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 95–104. [Google Scholar]
- Gray, D.E.; McClements, D.J.; Decker, E.A. Influence of phytochemical extracts on the sensory properties of food products. Food Res. Int. 2010, 43, 1395–1403. [Google Scholar]
- Kapasakalidis, P.G.; Rastall, R.A.; Gordon, M.H. Extraction of polyphenols from processed black currant (Ribes nigrum L.) residues. J. Agric. Food Chem. 2006, 54, 4016–4021. [Google Scholar] [CrossRef]
- Miraj, S.; Kiani, S.; Keshavarz, M. Melissa officinalis L.: A review study with an antioxidant prospective. J. Evid.-Based Complement. Altern. Med. 2017, 22, 385–394. [Google Scholar] [CrossRef]
- Weber, F. Noncovalent Polyphenol–Macromolecule Interactions and Their Effects on the Sensory Properties of Foods. J. Agric. Food Chem. 2021, 70, 72–78. [Google Scholar] [CrossRef]
- Xu, D.; Yang, Z.; Chen, X.; Zhao, Y.; Liu, L. Stability of anthocyanins in food processing and storage: Influencing factors and countermeasures. Food Res. Int. 2021, 150, 110742. [Google Scholar]
- He, J.; Giusti, M.M. Anthocyanins: Natural Colorants with Health-Promoting Properties. Annu. Rev. Food Sci. Technol. 2010, 1, 163–187. [Google Scholar] [CrossRef]
- Kong, J.-M.; Chia, L.-S.; Goh, N.-K.; Chia, T.-F.; Brouillard, R. Analysis and biological activities of anthocyanins. Phytochemistry 2003, 64, 923–933. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Yang, X.; Li, J.; Fu, Q.; Zhou, J.; Zhao, J.; Zhang, N.; Liu, Q.; Wang, T.; Wang, H. Improvement of physicochemical and gel properties of chlorogenic acid-modified oxidized myofibrillar proteins by transglutaminase. LWT 2023, 178, 114582. [Google Scholar] [CrossRef]
- Castañeda-Ovando, A.; de Lourdes Pacheco-Hernández, M.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Lesschaeve, I.; Noble, A.C. Polyphenols: Factors influencing their sensory properties and their effects on food and beverage preferences. Am. J. Clin. Nutr. 2005, 81 (Suppl. S1), 330S–335S. [Google Scholar] [CrossRef]
- Pérez-Magariño, S.; Cano-Mozo, E.; Bueno-Herrera, M.; Canalejo, D.; Doco, T.; Ayestarán, B.; Guadalupe, Z. The Effects of Grape Polysaccharides Extracted from Grape By-Products on the Chemical Composition and Sensory Characteristics of White Wines. Molecules 2022, 27, 4815. [Google Scholar] [CrossRef]
- Velickovic, T.D.C.; Stanic-Vucinic, D.J. The Role of Dietary Phenolic Compounds in Protein Digestion and Processing Technologies to Improve Their Antinutritive Properties. Compr. Rev. Food Sci. Food Saf. 2018, 17, 82–103. [Google Scholar] [CrossRef]


| ABTS [mM Trolox] | DPPH [mM Trolox] | FRAP [mM/dm3] | |
|---|---|---|---|
| Control: Myofibrillar protein without additives | 0.17 a ± 0.01 | 0.01 a ± 0.04 | 1.93 a ± 0.05 |
| Myofibrillar protein + black currant juice | 0.48 b ± 0.02 | 0.04 b ± 0.02 | 1.89 a ± 0.14 |
| Myofibrillar protein + black currant pomace | 2.46 e ± 0.02 | 0.13 d ± 0.01 | 4.62 c ± 0.08 |
| Myofibrillar protein + M. officinalis extract | 2.49 e ± 0.05 | 0.08 c ± 0.04 | 4.62 c ± 0.08 |
| Myofibrillar protein + M. officinalis extract + black currant juice | 2.46 e ± 0.02 | 0.07 c ± 0.01 | 3.42 b ± 0.30 |
| Myofibrillar protein + M. officinalis extract + black currant pomace | 2.35 d ± 0.05 | 0.13 d ± 0.00 | 4.68 c ± 0.14 |
| Myofibrillar protein + C. asiatica extract | 2.14 c ± 0.02 | 0.08 c ± 0.01 | 3.42 b ± 0.31 |
| Myofibrillar protein + C. asiatica extract + black currant juice | 2.11 c ± 0.02 | 0.04 b ± 0.02 | 3.24 b ± 0.40 |
| Myofibrillar protein + C. asiatica extract + black currant pomace | 2.44 e ± 0.02 | 0.13 d ± 0.01 | 3.25 b ± 0.38 |
| p | 0.0000 | 0.0000 | 0.0000 |
| SEM | 0.208 | 0.0106 | 0.201 |
| Control: Myofibrillar protein without additives | Myofibrillar protein + M. officinalis extract | Myofibrillar protein + C. asiatica extract | Myofibrillar protein + black currant juice | Myofibrillar protein + black currant pomace | Myofibrillar protein + M.officinalis extract + black currant pomace | Myofibrillar protein + M. officinalis extract + black currant juice | Myofibrillar protein + C. asiatica extract + black currant juice | Myofibrillar protein + C. asiatica extract + black currant pomace | ||
|---|---|---|---|---|---|---|---|---|---|---|
| flavan-3-ols | Procyanidin B1 | − | − | − | − | + | + | − | − | + |
| (−)-Epigallocatechin | − | − | − | − | − | − | − | − | − | |
| (+)Catechin | − | + | − | − | + | + | − | − | + | |
| Procyanidin B2 | − | − | − | − | + | − | − | − | + | |
| (−)-Epicatechin | − | − | − | − | − | − | − | − | + | |
| Procyanidin C1 | − | − | − | − | − | − | − | − | − | |
| Procyanidin A1 | − | − | − | − | − | − | − | − | + | |
| Procyanidin A2 | − | − | − | − | + | − | − | − | − | |
| phenolic acids | Neochlorogenic acid | − | − | − | − | + | + | − | − | + |
| Protocatechuic acid | − | − | − | − | + | + | − | − | − | |
| Chlorogenic acid | − | − | + | − | + | + | − | + | + | |
| Caftaric acid | − | + | − | − | − | − | + | − | − | |
| Syringic acid | − | − | − | − | − | − | − | − | − | |
| Caffeic acid | − | − | − | − | − | − | − | − | − | |
| Cryptochlorogenic acid | − | − | − | − | + | + | − | − | + | |
| 4-Coumaric acid | − | − | − | − | − | − | − | − | − | |
| 3,5-di-O-Caffeoyl quinic acid | − | − | + | − | − | − | − | − | − | |
| 1,5-di-O-Caffeoyl quinic acid | − | − | + | − | − | − | − | − | − | |
| 3,4-di-O-Caffeoyl quinic acid | − | − | − | − | − | − | − | − | − | |
| Rosmarinic acid | − | + | − | − | − | + | + | − | − | |
| flavonols | Myricetin-3-O-rutinoside | − | − | − | − | + | + | − | − | + |
| Myricetin-3-O-glucoside | − | − | − | − | + | + | − | − | + | |
| Quercetin-3-O-rutinoside | − | − | − | − | − | − | − | − | − | |
| Quercetin-3-O-glucoside | − | − | − | − | − | − | − | − | − | |
| Quercetin-3,4′-di-O-glucoside | − | − | − | − | − | − | − | − | − | |
| Kaempferol-3-O-rutinoside | − | − | − | − | − | − | − | − | − | |
| anthocyanins | Delphinidin-3-O-glucoside | − | − | − | − | + | + | − | − | + |
| Dephinidin-3-O-rutinoside | − | − | − | − | + | + | − | − | + | |
| Cyanidin-3-O-glucoside | − | − | − | − | + | + | − | − | + | |
| Cyanidin-3-O-rutinoside | − | − | − | − | + | + | − | − | + |
| Color | pH | Gel Strength [N] | |||
|---|---|---|---|---|---|
| L | a | b | |||
| Control: Myofibrillar protein without additives | 76.36 c ± 1.82 | −0.92 ba ± 1.20 | 18.59 dc ± 2.06 | 6.17 b ± 0.01 | 0.87 c ± 0.28 |
| Myofibrillar protein + black currant pomace | 73.33 bc ± 2.49 | 2.59 c ± 1.28 | 15.99 bc ± 3.60 | 6.25 c ± 0.01 | 0.27 a ± 0.29 |
| Myofibrillar protein + black currant juice | 67.73 ba ± 4.50 | 0.23 bca ± 0.41 | 12.00 a ± 1.01 | 6.23 c ± 0.02 | 0.42 ab ± 0.15 |
| Myofibrillar protein + M. officinalis extract | 70.52 b ± 0.26 | −1.75 a ± 0.05 | 14.03 ab ± 1.16 | 6.13 a ± 0.01 | 0.36 ab ± 0.29 |
| Myofibrillar protein + M. officinalis extract + black currant pomace | 68.18 ba ± 2.81 | 1.23 bc ± 1.50 | 20.69 d ± 0.67 | 6.13 a ± 0.01 | 0.20 a ± 0.14 |
| Myofibrillar protein + M. officinalis extract + black currant juice | 68.53 ba ± 4.19 | 0.56 bca ± 0.84 | 20.15 d ± 1.04 | 6.13 a ± 0.00 | 0.28 a ± 0.12 |
| Myofibrillar protein + C. asiatica extract | 69.60 ba ± 3.94 | 0.16 bca ± 0.85 | 21.45 d ± 0.74 | 6.14 a ± 0.01 | 0.55 b ± 0.21 |
| Myofibrillar protein + C. asiatica extract + black currant pomace | 64.28 a ± 3.25 | 1.56 bc ± 3.39 | 21.73 d ± 2.33 | 6.37 d ± 0.02 | 0.33 ab ± 0.25 |
| Myofibrillar protein + C. asiatica extract + black currant juice | 71.31 bc ± 1.98 | 0.05 bca ± 1.37 | 28.20 e ± 3.99 | 6.26 c ± 0.04 | 0.21 a ± 0.17 |
| p | 0.008 | 0.007 | 0.000 | 0.000 | 0.000 |
| SEM | 0.811 | 0.339 | 0.955 | 0.015 | 0.036 |
| Color | Flavor | Consistency | Structure | Taste | |
|---|---|---|---|---|---|
| Control: Myofibrillar protein without additives | 4.50 bc ± 0.84 | 2.83 a ± 1.33 | 4.43 d ± 0.79 | 4.43 d ± 0.79 | 4.00 d ± 0.58 |
| Myofibrillar protein + black currant pomace | 3.50 ba ± 0.84 | 3.17 a ± 1.33 | 3.43 abcd ± 0.79 | 3.57 abcd ± 0.79 | 3.50 dcab ± 0.76 |
| Myofibrillar protein + black currant juice | 3.07 a ± 0.84 | 3.20 a ± 1.10 | 2.67 a ± 1.03 | 2.83 ab ± 0.98 | 2.83 ab ± 0.52 |
| Myofibrillar protein + M. officinalis extract | 3.17 a ± 0.98 | 2.83 a ± 0.98 | 3.00 abc ± 1.22 | 2.80 a ± 0.84 | 2.75 a ± 0.76 |
| Myofibrillar protein + M. officinalis extract + black currant pomace | 4.50 bc ± 0.84 | 3.25 a ± 1.41 | 4.00 bcd ± 1.00 | 4.14 cd ± 0.69 | 3.86 d ± 0.85 |
| Myofibrillar protein + M. officinalis extract + black currant juice | 3.80 bca ± 0.45 | 3.60 a ± 1.14 | 3.60 abcd ± 0.89 | 3.60 abcd ± 0.89 | 3.70 dcb ± 0.57 |
| Myofibrillar protein + C. asiatica extract | 4.17 bc ± 0.41 | 3.33 a ± 0.82 | 3.50 abcd ± 1.05 | 3.17 abc ± 0.75 | 3.75 dc ± 0.42 |
| Myofibrillar protein + C. asiatica extract + black currant juice | 4.57 c ± 0.79 | 3.71 a ± 0.76 | 4.14 cd ± 0.69 | 3.88 bcd ± 0.99 | 3.81 dc ± 0.92 |
| Myofibrillar protein + C. asiatica extract + black currant pomace | 3.57 bca ± 0.79 | 3.14 a ± 1.07 | 2.83 ab ± 0.75 | 2.71 a ± 0.76 | 2.94 cab ± 0.82 |
| p | 0.002 | 0.892 | 0.013 | 0.002 | 0.008 |
| SEM | 0.123 | 0.145 | 0.137 | 0.128 | 0.106 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leicht, K.; Okpala, C.O.R.; Nowicka, P.; Pérez-Alvarez, J.A.; Korzeniowska, M. Antioxidant, Polyphenol, Physical, and Sensory Changes in Myofibrillar Protein Gels Supplemented with Polyphenol-Rich Plant-Based Additives. Nutrients 2025, 17, 1232. https://doi.org/10.3390/nu17071232
Leicht K, Okpala COR, Nowicka P, Pérez-Alvarez JA, Korzeniowska M. Antioxidant, Polyphenol, Physical, and Sensory Changes in Myofibrillar Protein Gels Supplemented with Polyphenol-Rich Plant-Based Additives. Nutrients. 2025; 17(7):1232. https://doi.org/10.3390/nu17071232
Chicago/Turabian StyleLeicht, Katarzyna, Charles Odilichukwu R. Okpala, Paulina Nowicka, José Angel Pérez-Alvarez, and Małgorzata Korzeniowska. 2025. "Antioxidant, Polyphenol, Physical, and Sensory Changes in Myofibrillar Protein Gels Supplemented with Polyphenol-Rich Plant-Based Additives" Nutrients 17, no. 7: 1232. https://doi.org/10.3390/nu17071232
APA StyleLeicht, K., Okpala, C. O. R., Nowicka, P., Pérez-Alvarez, J. A., & Korzeniowska, M. (2025). Antioxidant, Polyphenol, Physical, and Sensory Changes in Myofibrillar Protein Gels Supplemented with Polyphenol-Rich Plant-Based Additives. Nutrients, 17(7), 1232. https://doi.org/10.3390/nu17071232








