Tracking Pathways Linking Obesity with Heart Failure
Abstract
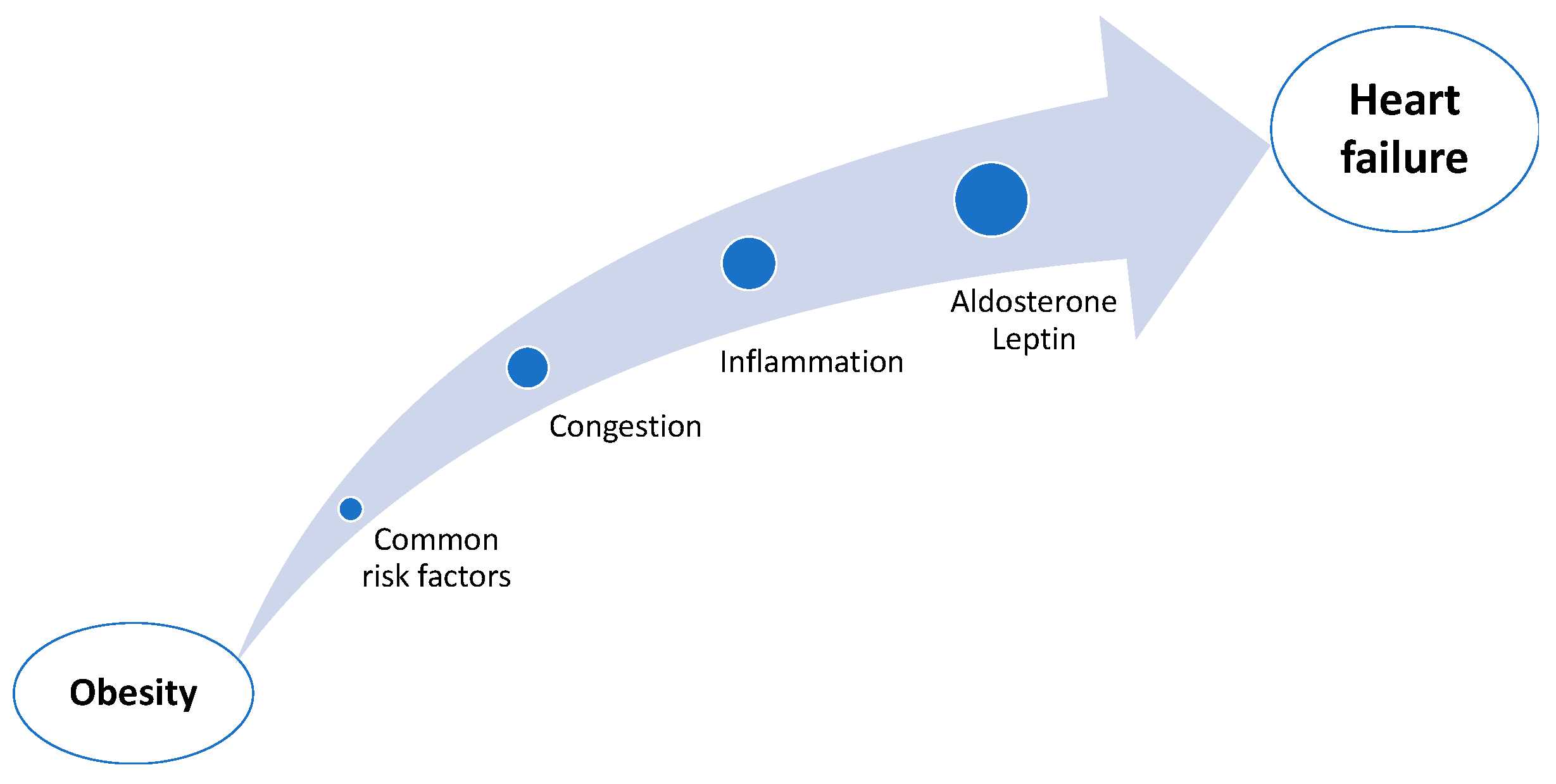
:1. Introduction
2. The Link Between Obesity and Heart Failure
2.1. Common Risk Factors
2.2. Congestion
2.3. Inflammation
2.4. Aldosterone, Leptin
3. Frailty, Sarcopenia, and Cachexia
4. The Obesity Paradox
5. Therapeutic Interventions
5.1. Lifestyle Modifications and Dietary Interventions
5.2. Pharmacological Interventions
5.3. Bariatric Surgery
5.4. Challenges and Considerations
6. Limitations
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BMI | Body Mass Index |
| WHO | World Health Organization |
| HF | Heart failure |
| LVEF | Left ventricular ejection fraction |
| HFrEF | Heart failure with reduced ejection fraction |
| HFmrEF | Heart failure with mildly reduced ejection fraction |
| HFpEF | Heart failure with preserved ejection fraction |
| DXA | Dual-energy X-ray Absorptiometry |
| BIA | Bioelectrical Impedance Analysis |
| DASH diet | Dietary Approaches to Stop Hypertension diet |
| GLP-1 receptor | Glucagon-like peptide-1 receptor |
| KCCQ-CSS | Kansas City Cardiomyopathy Questionnaire Clinical Summary Score |
| hs-CRP | High-sensitivity C-reactive protein |
| NT-proBNP | N-terminal pro-B-type natriuretic peptide |
| GIP receptor | glucose-dependent insulinotropic polypeptide receptor |
| IL-1 | Interleukin-1 |
References
- Obesity. Available online: https://www.who.int/health-topics/obesity (accessed on 28 August 2024).
- National Institutes of Health. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults—The Evidence Report. Obes. Res. 1998, 6 (Suppl. S2), 51S–209S.
- Ross, R.; Neeland, I.J.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; Cuevas, A.; Hu, F.B.; et al. Waist Circumference as a Vital Sign in Clinical Practice: A Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat. Rev. Endocrinol. 2020, 16, 177–189. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation; World Health Organization: Geneva, Switzerland, 2008.
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.-P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef] [PubMed]
- Piché, M.-E.; Poirier, P.; Lemieux, I.; Després, J.-P. Overview of Epidemiology and Contribution of Obesity and Body Fat Distribution to Cardiovascular Disease: An Update. Prog. Cardiovasc. Dis. 2018, 61, 103–113. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Savarese, G.; Becher, P.M.; Lund, L.H.; Seferovic, P.; Rosano, G.M.C.; Coats, A.J.S. Global Burden of Heart Failure: A Comprehensive and Updated Review of Epidemiology. Cardiovasc. Res. 2023, 118, 3272–3287. [Google Scholar] [CrossRef]
- Kenchaiah, S.; Evans, J.C.; Levy, D.; Wilson, P.W.F.; Benjamin, E.J.; Larson, M.G.; Kannel, W.B.; Vasan, R.S. Obesity and the Risk of Heart Failure. N. Engl. J. Med. 2002, 347, 305–313. [Google Scholar] [CrossRef]
- Pandey, A.; LaMonte, M.; Klein, L.; Ayers, C.; Psaty, B.M.; Eaton, C.B.; Allen, N.B.; De Lemos, J.A.; Carnethon, M.; Greenland, P.; et al. Relationship Between Physical Activity, Body Mass Index, and Risk of Heart Failure. J. Am. Coll. Cardiol. 2017, 69, 1129–1142. [Google Scholar] [CrossRef]
- Haass, M.; Kitzman, D.W.; Anand, I.S.; Miller, A.; Zile, M.R.; Massie, B.M.; Carson, P.E. Body Mass Index and Adverse Cardiovascular Outcomes in Heart Failure Patients with Preserved Ejection Fraction: Results from the Irbesartan in Heart Failure with Preserved Ejection Fraction (I-PRESERVE) Trial. Circ. Heart Fail. 2011, 4, 324–331. [Google Scholar] [CrossRef]
- Aune, D.; Sen, A.; Norat, T.; Janszky, I.; Romundstad, P.; Tonstad, S.; Vatten, L.J. Body Mass Index, Abdominal Fatness, and Heart Failure Incidence and Mortality: A Systematic Review and Dose–Response Meta-Analysis of Prospective Studies. Circulation 2016, 133, 639–649. [Google Scholar] [CrossRef]
- Del Gobbo, L.C.; Kalantarian, S.; Imamura, F.; Lemaitre, R.; Siscovick, D.S.; Psaty, B.M.; Mozaffarian, D. Contribution of Major Lifestyle Risk Factors for Incident Heart Failure in Older Adults. JACC Heart Fail. 2015, 3, 520–528. [Google Scholar] [CrossRef]
- Pandey, A.; Patel, K.V.; Vaduganathan, M.; Sarma, S.; Haykowsky, M.J.; Berry, J.D.; Lavie, C.J. Physical Activity, Fitness, and Obesity in Heart Failure with Preserved Ejection Fraction. JACC Heart Fail. 2018, 6, 975–982. [Google Scholar] [CrossRef]
- Welsh, A.; Hammad, M.; Piña, I.L.; Kulinski, J. Obesity and Cardiovascular Health. Eur. J. Prev. Cardiol. 2024, 31, 1026–1035. [Google Scholar] [CrossRef] [PubMed]
- Heianza, Y.; Qi, L. Impact of Genes and Environment on Obesity and Cardiovascular Disease. Endocrinology 2019, 160, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.L.; Borlaug, B.A. Impact of Obesity on Volume Status in Patients with Ambulatory Chronic Heart Failure. J. Card. Fail. 2020, 26, 112–117. [Google Scholar] [CrossRef]
- Borlaug, B.A.; Sharma, K.; Shah, S.J.; Ho, J.E. Heart Failure with Preserved Ejection Fraction. J. Am. Coll. Cardiol. 2023, 81, 1810–1834. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.; Dhingra, N.K.; Connelly, K.A. Obesity/Cardiometabolic Phenotype of Heart Failure with Preserved Ejection Fraction: Mechanisms to Recent Trials. Curr. Opin. Cardiol. 2024, 39, 92–97. [Google Scholar] [CrossRef]
- El Meouchy, P.; Wahoud, M.; Allam, S.; Chedid, R.; Karam, W.; Karam, S. Hypertension Related to Obesity: Pathogenesis, Characteristics and Factors for Control. IJMS-Int. J. Mol. Sci. 2022, 23, 12305. [Google Scholar] [CrossRef]
- Shariq, O.A.; McKenzie, T.J. Obesity-Related Hypertension: A Review of Pathophysiology, Management, and the Role of Metabolic Surgery. Gland. Surg. 2020, 9, 80–93. [Google Scholar] [CrossRef]
- Huby, A.-C.; Antonova, G.; Groenendyk, J.; Gomez-Sanchez, C.E.; Bollag, W.B.; Filosa, J.A.; De Chantemèle, E.J.B. Adipocyte-Derived Hormone Leptin Is a Direct Regulator of Aldosterone Secretion, Which Promotes Endothelial Dysfunction and Cardiac Fibrosis. Circulation 2015, 132, 2134–2145. [Google Scholar] [CrossRef]
- Vitale, C.; Jankowska, E.; Hill, L.; Piepoli, M.; Doehner, W.; Anker, S.D.; Lainscak, M.; Jaarsma, T.; Ponikowski, P.; Rosano, G.M.C.; et al. Heart Failure Association of the European Society of Cardiology Position Paper on Frailty in Patients with Heart Failure. Eur. J. Heart Fail. 2019, 21, 1299–1305. [Google Scholar] [CrossRef] [PubMed]
- Denfeld, Q.E.; Winters-Stone, K.; Mudd, J.O.; Gelow, J.M.; Kurdi, S.; Lee, C.S. The Prevalence of Frailty in Heart Failure: A Systematic Review and Meta-Analysis. Int. J. Cardiol. 2017, 236, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Camafort, M.; Kasiakogias, A.; Agabiti-Rosei, E.; Masi, S.; Iliakis, P.; Benetos, A.; Jeong, J.-O.; Lee, H.-Y.; Muiesan, M.L.; Sudano, I.; et al. Hypertensive Heart Disease in Older Patients: Considerations for Clinical Practice. Eur. J. Intern. Med. 2025, 134, 75–88. [Google Scholar] [CrossRef]
- Vidán, M.T.; Blaya-Novakova, V.; Sánchez, E.; Ortiz, J.; Serra-Rexach, J.A.; Bueno, H. Prevalence and Prognostic Impact of Frailty and Its Components in Non-dependent Elderly Patients with Heart Failure. Eur. J. Heart Fail. 2016, 18, 869–875. [Google Scholar] [CrossRef]
- Dent, E.; Morley, J.E.; Cruz-Jentoft, A.J.; Arai, H.; Kritchevsky, S.B.; Guralnik, J.; Bauer, J.M.; Pahor, M.; Clark, B.C.; Cesari, M.; et al. International Clinical Practice Guidelines for Sarcopenia (ICFSR): Screening, Diagnosis and Management. J. Nutr. Health Aging 2018, 22, 1148–1161. [Google Scholar] [CrossRef]
- Von Haehling, S.; Ebner, N.; Dos Santos, M.R.; Springer, J.; Anker, S.D. Muscle Wasting and Cachexia in Heart Failure: Mechanisms and Therapies. Nat. Rev. Cardiol. 2017, 14, 323–341. [Google Scholar] [CrossRef]
- Vest, A.R.; Chan, M.; Deswal, A.; Givertz, M.M.; Lekavich, C.; Lennie, T.; Litwin, S.E.; Parsly, L.; Rodgers, J.E.; Rich, M.W.; et al. Nutrition, Obesity, and Cachexia in Patients with Heart Failure: A Consensus Statement from the Heart Failure Society of America Scientific Statements Committee. J. Card. Fail. 2019, 25, 380–400. [Google Scholar] [CrossRef] [PubMed]
- Pelliccia, A.; Sharma, S.; Gati, S.; Bäck, M.; Börjesson, M.; Caselli, S.; Collet, J.-P.; Corrado, D.; Drezner, J.A.; Halle, M.; et al. 2020 ESC Guidelines on Sports Cardiology and Exercise in Patients with Cardiovascular Disease. Eur. Heart J. 2021, 42, 17–96. [Google Scholar] [CrossRef]
- Carbone, S.; Lavie, C.J.; Arena, R. Obesity and Heart Failure: Focus on the Obesity Paradox. Mayo Clin. Proc. 2017, 92, 266–279. [Google Scholar] [CrossRef]
- Padwal, R.; McAlister, F.A.; McMurray, J.J.V.; Cowie, M.R.; Rich, M.; Pocock, S.; Swedberg, K.; Maggioni, A.; Gamble, G.; Ariti, C.; et al. The Obesity Paradox in Heart Failure Patients with Preserved versus Reduced Ejection Fraction: A Meta-Analysis of Individual Patient Data. Int. J. Obes. 2014, 38, 1110–1114. [Google Scholar] [CrossRef]
- Keller, K.; Münzel, T.; Ostad, M.A. Sex-Specific Differences in Mortality and the Obesity Paradox of Patients with Myocardial Infarction Ages >70 y. Nutrition 2018, 46, 124–130. [Google Scholar] [CrossRef]
- Alpert, M.A.; Lavie, C.J.; Agrawal, H.; Kumar, A.; Kumar, S.A. Cardiac Effects of Obesity: Pathophysiologic, Clinical, and Prognostic Consequences—A Review. J. Cardiopulm. Rehabil. Prev. 2016, 36, 1–11. [Google Scholar] [CrossRef]
- Loprinzi, P.D. Physical Activity, Weight Status, and Mortality among Congestive Heart Failure Patients. Int. J. Cardiol. 2016, 214, 92–94. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.L.; Fonarow, G.C.; Horwich, T.B. Obesity and the Obesity Paradox in Heart Failure. Prog. Cardiovasc. Dis. 2014, 56, 409–414. [Google Scholar] [CrossRef]
- Benn, M.; Marott, S.C.W.; Tybjærg-Hansen, A.; Nordestgaard, B.G. Obesity Increases Heart Failure Incidence and Mortality: Observational and Mendelian Randomization Studies Totalling over 1 Million Individuals. Cardiovasc. Res. 2023, 118, 3576–3585. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Min, J.; Zhong, L.; Zhang, J.; Ye, L.; Chen, C. Life-Course Obesity and Heart Failure: A Two-Sample Mendelian Randomization Study. Intern. Emerg. Med. 2025, 20, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Bäck, M.; Rees, J.M.B.; Mason, A.M.; Burgess, S. Body Mass Index and Body Composition in Relation to 14 Cardiovascular Conditions in UK Biobank: A Mendelian Randomization Study. Eur. Heart J. 2020, 41, 221–226. [Google Scholar] [CrossRef]
- Fall, T.; Hägg, S.; Mägi, R.; Ploner, A.; Fischer, K.; Horikoshi, M.; Sarin, A.-P.; Thorleifsson, G.; Ladenvall, C.; Kals, M.; et al. The Role of Adiposity in Cardiometabolic Traits: A Mendelian Randomization Analysis. PLoS Med. 2013, 10, e1001474. [Google Scholar] [CrossRef]
- Ibrahim, R.; Pham, H.N.; Vest, A.R.; William, P. Managing Obesity in Heart Failure: Latest Evidence and Knowledge Gaps. Curr. Treat. Options Cardio. Med. 2024, 26, 355–367. [Google Scholar] [CrossRef]
- Soto, M.E.; Pérez-Torres, I.; Rubio-Ruiz, M.E.; Manzano-Pech, L.; Guarner-Lans, V. Interconnection between Cardiac Cachexia and Heart Failure—Protective Role of Cardiac Obesity. Cells 2022, 11, 1039. [Google Scholar] [CrossRef]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e177–e232. [Google Scholar] [CrossRef]
- Kim, J.Y. Optimal Diet Strategies for Weight Loss and Weight Loss Maintenance. J. Obes. Metab. Syndr. 2021, 30, 20–31. [Google Scholar] [CrossRef]
- Billingsley, H.E.; Carbone, S.; Driggin, E.; Kitzman, D.W.; Hummel, S.L. Dietary Interventions in Heart Failure with Preserved Ejection Fraction. JACC Adv. 2025, 4, 101465. [Google Scholar] [CrossRef]
- Wickman, B.E.; Enkhmaa, B.; Ridberg, R.; Romero, E.; Cadeiras, M.; Meyers, F.; Steinberg, F. Dietary Management of Heart Failure: DASH Diet and Precision Nutrition Perspectives. Nutrients 2021, 13, 4424. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A.; Gea, A.; Ruiz-Canela, M. The Mediterranean Diet and Cardiovascular Health: A Critical Review. Circ. Res. 2019, 124, 779–798. [Google Scholar] [CrossRef]
- Veronese, N.; Ragusa, F.S.; Maggi, S.; Witard, O.C.; Smith, L.; Dominguez, L.J.; Barbagallo, M.; Isanejad, M.; Prokopidis, K. Effect of the Mediterranean Diet on Incidence of Heart Failure in European Countries: A Systematic Review and Meta-Analysis of Cohort Studies. Eur. J. Clin. Nutr. 2025, 79, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrova, K.; Koelman, L.; Rodrigues, C.E. Dietary Patterns and Biomarkers of Oxidative Stress and Inflammation: A Systematic Review of Observational and Intervention Studies. Redox Biol. 2021, 42, 101869. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Beltrán-Velasco, A.I.; Redondo-Flórez, L.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Global Impacts of Western Diet and Its Effects on Metabolism and Health: A Narrative Review. Nutrients 2023, 15, 2749. [Google Scholar] [CrossRef]
- Cvetinovic, N.; Loncar, G.; Isakovic, A.M.; Von Haehling, S.; Doehner, W.; Lainscak, M.; Farkas, J. Micronutrient Depletion in Heart Failure: Common, Clinically Relevant and Treatable. IJMS-Int. J. Mol. Sci. 2019, 20, 5627. [Google Scholar] [CrossRef]
- Kukulska, A.; Garwacka-Czachor, E. Assessment of Adherence to Treatment Recommendations among Patients with Heart Failure: A Cross-Sectional Study. BMC Cardiovasc. Disord. 2024, 24, 337. [Google Scholar] [CrossRef]
- Driggin, E.; Cohen, L.P.; Gallagher, D.; Karmally, W.; Maddox, T.; Hummel, S.L.; Carbone, S.; Maurer, M.S. Nutrition Assessment and Dietary Interventions in Heart Failure: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2022, 79, 1623–1635. [Google Scholar] [CrossRef] [PubMed]
- White-Williams, C.; Rossi, L.P.; Bittner, V.A.; Driscoll, A.; Durant, R.W.; Granger, B.B.; Graven, L.J.; Kitko, L.; Newlin, K.; Shirey, M.; et al. Addressing Social Determinants of Health in the Care of Patients with Heart Failure: A Scientific Statement from the American Heart Association. Circulation 2020, 141, e841–e863. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, B.; Fonarow, G.C.; Goldberg, L.R.; Guglin, M.; Josephson, R.A.; Forman, D.E.; Lin, G.; Lindenfeld, J.; O’Connor, C.; Panjrath, G.; et al. Cardiac Rehabilitation for Patients with Heart Failure. J. Am. Coll. Cardiol. 2021, 77, 1454–1469. [Google Scholar] [CrossRef] [PubMed]
- Margulies, K.B.; Hernandez, A.F.; Redfield, M.M.; Givertz, M.M.; Oliveira, G.H.; Cole, R.; Mann, D.L.; Whellan, D.J.; Kiernan, M.S.; Felker, G.M.; et al. Effects of Liraglutide on Clinical Stability Among Patients with Advanced Heart Failure and Reduced Ejection Fraction: A Randomized Clinical Trial. JAMA 2016, 316, 500. [Google Scholar] [CrossRef]
- Jorsal, A.; Kistorp, C.; Holmager, P.; Tougaard, R.S.; Nielsen, R.; Hänselmann, A.; Nilsson, B.; Møller, J.E.; Hjort, J.; Rasmussen, J.; et al. Effect of Liraglutide, a Glucagon-like Peptide-1 Analogue, on Left Ventricular Function in Stable Chronic Heart Failure Patients with and without Diabetes (LIVE)—A Multicentre, Double-blind, Randomised, Placebo-controlled Trial. Eur. J. Heart Fail. 2017, 19, 69–77. [Google Scholar] [CrossRef]
- Verma, S.; Butler, J.; Borlaug, B.A.; Davies, M.; Kitzman, D.W.; Shah, S.J.; Petrie, M.C.; Barros, E.; Rönnbäck, C.; Vestergaard, L.S.; et al. Efficacy of Semaglutide by Sex in Obesity-Related Heart Failure with Preserved Ejection Fraction. J. Am. Coll. Cardiol. 2024, 84, 773–785. [Google Scholar] [CrossRef]
- Kosiborod, M.N.; Petrie, M.C.; Borlaug, B.A.; Butler, J.; Davies, M.J.; Hovingh, G.K.; Kitzman, D.W.; Møller, D.V.; Treppendahl, M.B.; Verma, S.; et al. Semaglutide in Patients with Obesity-Related Heart Failure and Type 2 Diabetes. N. Engl. J. Med. 2024, 390, 1394–1407. [Google Scholar] [CrossRef]
- Kouvari, M.; Panagiotakos, D.B.; Yannakoulia, M.; Georgousopoulou, E.; Critselis, E.; Chrysohoou, C.; Tousoulis, D.; Pitsavos, C. Transition from Metabolically Benign to Metabolically Unhealthy Obesity and 10-Year Cardiovascular Disease Incidence: The ATTICA Cohort Study. Metabolism 2019, 93, 18–24. [Google Scholar] [CrossRef]
- Waqas, S.A.; Sohail, M.U.; Saad, M.; Minhas, A.M.K.; Greene, S.J.; Fudim, M.; Fonarow, G.C.; Abramov, D.; Khan, M.S.; Ahmed, R. Efficacy of GLP-1 Receptor Agonists in Patients with Heart Failure and Mildly Reduced or Preserved Ejection Fraction: A Systematic Review and Meta-Analysis. J. Card. Fail. 2025; in press. [Google Scholar] [CrossRef]
- Karakasis, P.; Fragakis, N.; Patoulias, D.; Theofilis, P.; Sagris, M.; Koufakis, T.; Vlachakis, P.K.; Rangraze, I.R.; El Tanani, M.; Tsioufis, K.; et al. The Emerging Role of Glucagon-like Peptide-1 Receptor Agonists in the Management of Obesity-Related Heart Failure with Preserved Ejection Fraction: Benefits beyond What Scales Can Measure? Biomedicines 2024, 12, 2112. [Google Scholar] [CrossRef]
- Zheng, Z.; Zong, Y.; Ma, Y.; Tian, Y.; Pang, Y.; Zhang, C.; Gao, J. Glucagon-like Peptide-1 Receptor: Mechanisms and Advances in Therapy. Sig. Transduct. Target. Ther. 2024, 9, 234. [Google Scholar] [CrossRef]
- Shchendrygina, A.; Rakisheva, A.; Giverts, I.; Rustamova, Y.; Soloveva, A. Effects of Glucagon-like Peptide-1 Receptor Agonists on Cardiac Function, Exercise Capacity and Quality of Life. Card. Fail. Rev. 2024, 10, e10. [Google Scholar] [CrossRef] [PubMed]
- Abbate, A.; Toldo, S.; Marchetti, C.; Kron, J.; Van Tassell, B.W.; Dinarello, C.A. Interleukin-1 and the Inflammasome as Therapeutic Targets in Cardiovascular Disease. Circ. Res. 2020, 126, 1260–1280. [Google Scholar] [CrossRef]
- Lopatin, Y. Metabolic Therapy in Heart Failure. Card. Fail. Rev. 2015, 1, 112. [Google Scholar] [CrossRef] [PubMed]
- Ashrafian, H.; Le Roux, C.W.; Darzi, A.; Athanasiou, T. Effects of Bariatric Surgery on Cardiovascular Function. Circulation 2008, 118, 2091–2102. [Google Scholar] [CrossRef]
- Mentias, A.; Desai, M.Y.; Aminian, A.; Patel, K.V.; Keshvani, N.; Verma, S.; Cho, L.; Jacob, M.; Alvarez, P.; Lincoff, A.M.; et al. Trends and Outcomes Associated with Bariatric Surgery and Pharmacotherapies with Weight Loss Effects Among Patients with Heart Failure and Obesity. Circ. Heart Fail. 2024, 17, e010453. [Google Scholar] [CrossRef]
- Hamilton Health Sciences Corporation. Bariatric Surgery for the Reduction of Cardiovascular Events Feasibility Randomized Controlled Trial (BRAVE); Hamilton Health Sciences Corporation: Hamilton, ON, Canada, 2022. [Google Scholar]
- Washington, T.B.; Johnson, V.R.; Kendrick, K.; Ibrahim, A.A.; Tu, L.; Sun, K.; Stanford, F.C. Disparities in Access and Quality of Obesity Care. Gastroenterol. Clin. N. Am. 2023, 52, 429–441. [Google Scholar] [CrossRef]
- Masanam, M.K.; Grossman, D.A.; Neary, J.; Alimi, Y.R. Disparities in the Impact of Access to and Outcomes of Bariatric Surgery among Different Ethnoracial and Socioeconomic Populations: A Narrative Review of the Literature. Ann. Laparosc. Endosc. Surg. 2023, 8, 34. [Google Scholar] [CrossRef]
- Cascino, T.M.; Hummel, S.L. Nutrient Deficiencies in Heart Failure: A Micro Problem with Macro Effects? J. Am. Heart Assoc. 2018, 7, e010447. [Google Scholar] [CrossRef]
- Nelson, M.D.; Victor, R.G.; Szczepaniak, E.W.; Simha, V.; Garg, A.; Szczepaniak, L.S. Cardiac Steatosis and Left Ventricular Hypertrophy in Patients with Generalized Lipodystrophy as Determined by Magnetic Resonance Spectroscopy and Imaging. Am. J. Cardiol. 2013, 112, 1019–1024. [Google Scholar] [CrossRef]
| Intervention | HFrEF | HFpEF |
|---|---|---|
| Diet | SECRET
| |
| GLP-1 receptor agonists | FIGHT (liraglutide)
| STEP-HFpEF (semaglutide)
|
LIVE (liraglutide)
| ||
| Bariatric surgery | BRAVE N = 2000 patients ongoing | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manta, E.; Iliakis, P.; Fragoulis, C.; Leontsinis, I.; Stamoulopoulos, I.; Chrysohoou, C.; Tsioufis, K. Tracking Pathways Linking Obesity with Heart Failure. Nutrients 2025, 17, 1250. https://doi.org/10.3390/nu17071250
Manta E, Iliakis P, Fragoulis C, Leontsinis I, Stamoulopoulos I, Chrysohoou C, Tsioufis K. Tracking Pathways Linking Obesity with Heart Failure. Nutrients. 2025; 17(7):1250. https://doi.org/10.3390/nu17071250
Chicago/Turabian StyleManta, Eleni, Panagiotis Iliakis, Christos Fragoulis, Ioannis Leontsinis, Ioannis Stamoulopoulos, Christina Chrysohoou, and Konstantinos Tsioufis. 2025. "Tracking Pathways Linking Obesity with Heart Failure" Nutrients 17, no. 7: 1250. https://doi.org/10.3390/nu17071250
APA StyleManta, E., Iliakis, P., Fragoulis, C., Leontsinis, I., Stamoulopoulos, I., Chrysohoou, C., & Tsioufis, K. (2025). Tracking Pathways Linking Obesity with Heart Failure. Nutrients, 17(7), 1250. https://doi.org/10.3390/nu17071250







