Beneficial Effects of Omega-3 Fatty Acids on Obesity and Related Metabolic and Chronic Inflammatory Diseases
Abstract
1. Introduction
2. Results
2.1. Beneficial Effects of Omega-3 Fatty Acids on Obesity
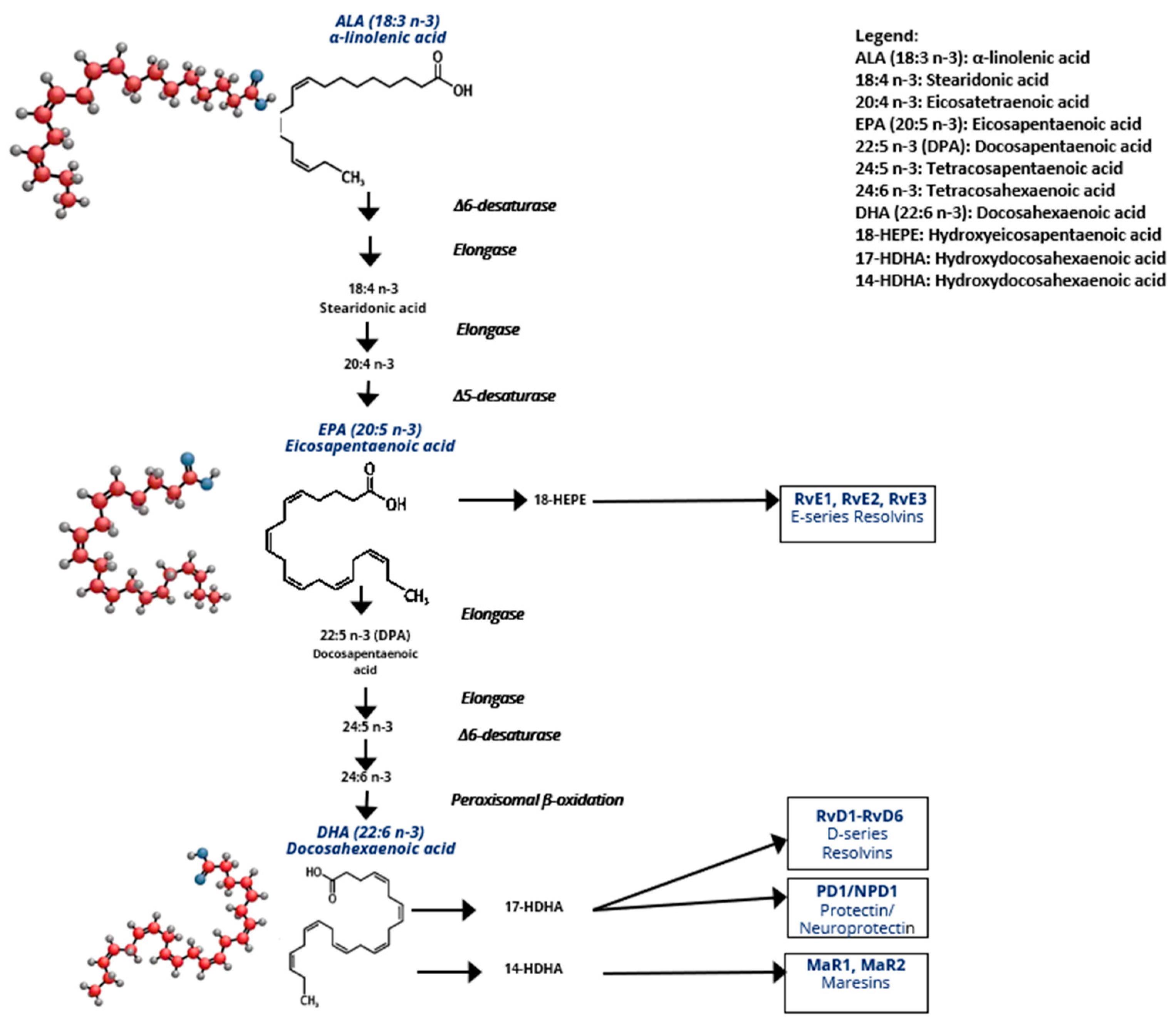
2.1.1. ω-3/ω-6 Eicosanoid Generation
2.1.2. Dietary Sources of n-3 PUFAs
2.1.3. Mechanisms of the Anti-Inflammatory Effects of n-3 PUFAs
2.1.4. Beneficial Effects of n-3 PUFAs on AT Inflammation and Adipose Metabolism in Obesity
2.2. Effects of n-3 PUFAs on Obesity-Related Metabolic Diseases
2.2.1. Cardiovascular Disease
2.2.2. Type 2 Diabetes (T2D)
2.2.3. Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD)
2.2.4. Chronic Kidney Disease (CKD)
2.3. n-3 PUFAs in Obesity Related-Chronic Inflammatory Immune Diseases
2.3.1. Mechanisms of AT Low-Grade Inflammation in Obesity
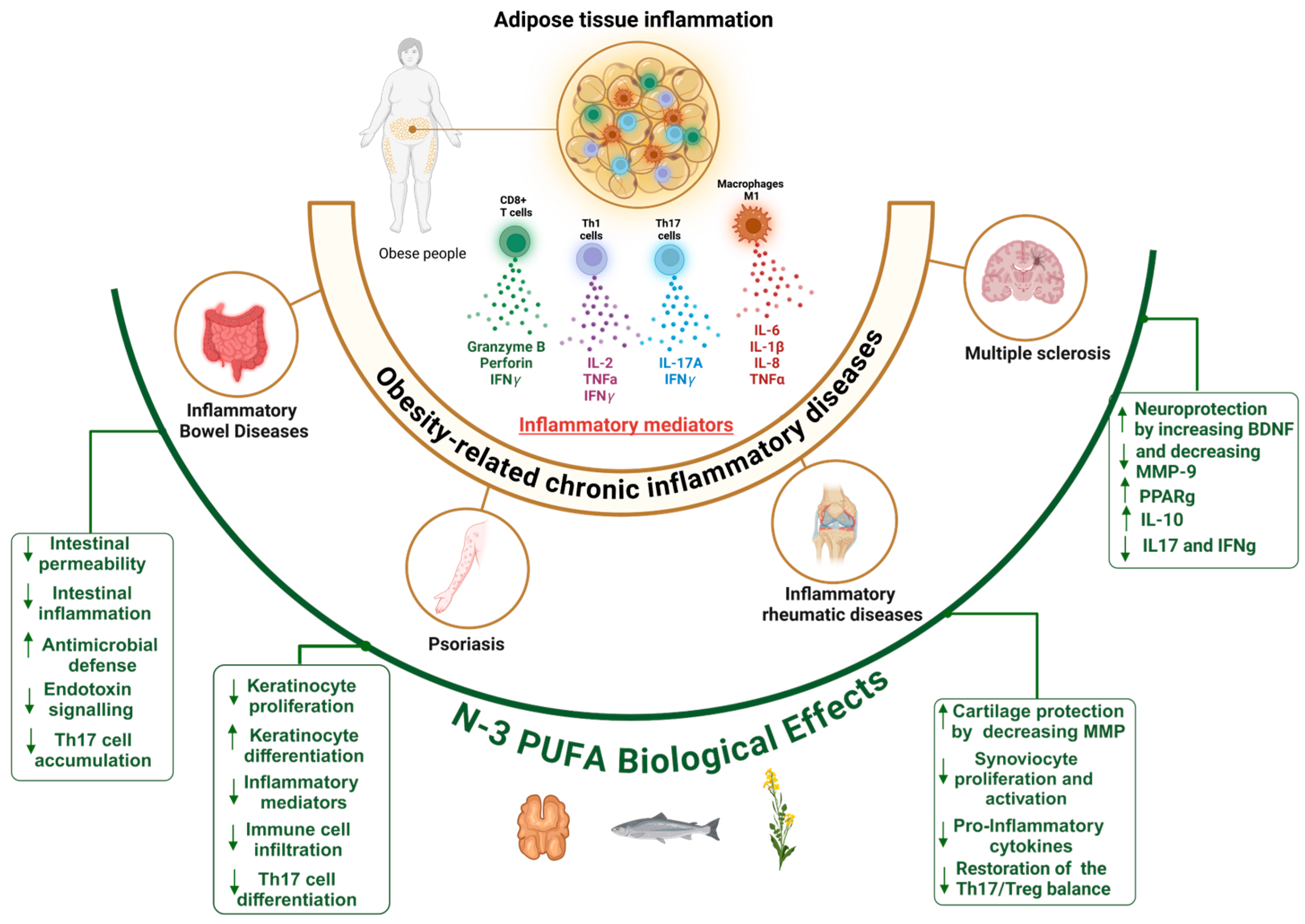
2.3.2. Shared Mechanisms of Inflammation in Obesity and Related Chronic Inflammatory Diseases
2.3.3. Inflammatory Bowel Diseases (IBDs)
2.3.4. Psoriasis
2.3.5. Inflammatory Rheumatic Diseases
Osteoarthritis
Rheumatoid Arthritis
2.3.6. Multiple Sclerosis
3. Conclusions
3.1. Limitations of Current Evidence
3.2. Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Arachidonic acid | MS | Multiple Sclerosis |
| ALA | Alpha-linolenic acid | MUFA | Monounsaturated Fatty Acid |
| ALT | Alanine amino-transferase | MyD88 | Myeloid Differentiation primary response 88 |
| AMPK | AMP-activated protein kinase | n-3 | Omega-3 |
| AP-1 | Activator Protein 1 | n-6 | Omega-6 |
| ASC | Adipose-derived stem cells | NF-κB | Nuclear factor kappa B |
| AST | Aspartate amino-transferase | NLRP3 | NOD-like receptor family pyrin domain containing 3 |
| AT | Adipose tissue | Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| BDNF | Brain-derived neurotrophic factor | OA | Osteoarthritis |
| BMI | Body mass index | ObOA | Obesity-associated osteoarthritis |
| CKD | Chronic kidney disease | PASI | Psoriasis Area Severity Index |
| COX | Cyclooxygenase | PD-L1 | Programmed Death-Ligand 1 |
| CRP | C-reactive protein | PDX | Protectin DX |
| CVD | Cardiovascular disease | PG | Prostaglandin |
| CYP | Cytochrome P450 | PLA2 | Phospholipase A2 |
| DHA | Docosahexaenoic acid | PPAR | Peroxisome proliferator-activated receptor |
| DGLA | Dihomo-gamma-linolenic acid | PPRE | Peroxisome proliferator-activated receptor response element |
| EDSS | Expanded Disability Status Scale | PUFA | Polyunsaturated fatty acid |
| EPA | Eicosapentaenoic acid | RA | Rheumatoid arthritis |
| ERK | Extracellular signal-regulated kinase | RAGE | Receptor for advanced glycation end-products |
| ESRD | End-ctage renal disease | RBC | Red blood cell |
| FA | Fatty acid | RORα | RAR-related Orphan Receptor alpha |
| FFAR4 | Free Fatty Acid Receptor 4 | ROS | Reactive Oxygen Species |
| GLP-1 | Glucagon-like peptide-1 | Rv | Resolvin |
| GLUT-4 | Glucose Transporter Type 4 | RXR | Retinoid X receptor |
| GM-CSF | Granulocyte-Macrophage Colony-Stimulating Factor | SFA | Saturated fatty acid |
| GPR120 | G protein-coupled receptor 120 | SIRT1 | Sirtuin 1 |
| GSDMD | Gasdermin D | SPM | Specialized pro-resolving mediator |
| HbA1c | Hemoglobin A1c | SPMs | Specialized pro-resolving mediators |
| HDL | High-density lipoprotein | SREBP-1 | Sterol Regulatory Element-Binding Protein-1 |
| HIF1α | Hypoxia-inducible factor 1-alpha | T2D | Type 2 diabetes |
| HMGB1 | High Mobility Group Box 1 | TAB1 | TAK1-binding protein 1 |
| IBD | Inflammatory bowel disease | TAK1 | Transforming growth factor beta-activated kinase 1 |
| ICAM-1 | Intercellular Adhesion Molecule 1 | TGFβ | Transforming growth factor beta |
| IFN | Interferon | Th1 | T helper 1 |
| IKK-β | Inhibitor of Nuclear Factor Kappa-B | Th17 | T helper 17 |
| IL- | Interleukin- | TLR | Toll-like receptor |
| JNK | c-Jun N-terminal kinase | Treg | Regulatory T cell |
| LA | Linoleic acid | TXA | Thromboxane A |
| LDL | Low-density lipoprotein | UC | Ulcerative colitis |
| LOX | Lipoxygenase | VEGF | Vascular Endothelial Growth Factor |
| LPS | Lipopolysaccharide | VLDL | Very low-density lipoproteins |
| LTB4 | Leukotriene B4 | ||
| LTB5 | Leukotriene B5 | ||
| MASLD | Metabolic dysfunction-associated steatotic liver disease | ||
| MCP-1 | Monocyte chemoattractant protein-1 | ||
| MMP | Matrix metalloproteinase | ||
| MRI | Magnetic resonance imaging | ||
References
- Deng, T.; Lyon, C.J.; Bergin, S.; Caligiuri, M.A.; Hsueh, W.A. Obesity, Inflammation, and Cancer. Annu. Rev. Pathol. 2016, 11, 421–449. [Google Scholar] [CrossRef] [PubMed]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in underweight and obesity from 1990 to 2022: A pooled analysis of 3663 population-representative studies with 222 million children, adolescents, and adults. Lancet 2024, 403, 1027–1050. [Google Scholar]
- Sakers, A.; De Siqueira, M.K.; Seale, P.; Villanueva, C.J. Adipose-tissue plasticity in health and disease. Cell 2022, 185, 419–446. [Google Scholar] [PubMed]
- Pestel, J.; Blangero, F.; Eljaafari, A. Pathogenic Role of Adipose Tissue-Derived Mesenchymal Stem Cells in Obesity and Obesity-Related Inflammatory Diseases. Cells 2023, 12, 348. [Google Scholar] [CrossRef] [PubMed]
- Chehimi, M.; Vidal, H.; Eljaafari, A. Pathogenic Role of IL-17-Producing Immune Cells in Obesity, and Related Inflammatory Diseases. J. Clin. Med. 2017, 6, 68. [Google Scholar] [CrossRef] [PubMed]
- Chakhtoura, M.; Haber, R.; Ghezzawi, M.; Rhayem, C.; Tcheroyan, R.; Mantzoros, C.S. Pharmacotherapy of obesity: An update on the available medications and drugs under investigation. eClinicalMedicine 2023, 58, 101882. [Google Scholar] [CrossRef]
- Cummings, D.E.; Cohen, R.V. Bariatric/Metabolic Surgery to Treat Type 2 Diabetes in Patients with a BMI < 35 kg/m2. Diabetes Care 2016, 39, 924–933. [Google Scholar]
- Negi, A.; Asokkumar, R.; Ravi, R.; Lopez-Nava, G.; Bautista-Castaño, I. Nutritional Management and Role of Multidisciplinary Follow-Up after Endoscopic Bariatric Treatment for Obesity. Nutrients 2022, 14, 3450. [Google Scholar] [CrossRef]
- Singh, M.; Thrimawithana, T.; Shukla, R.; Adhikari, B. Managing obesity through natural polyphenols: A review. Future Foods 2020, 1–2, 100002. [Google Scholar]
- Dong, L.; Qin, C.; Li, Y.; Wu, Z.; Liu, L. Oat phenolic compounds regulate metabolic syndrome in high fat diet-fed mice via gut microbiota. Food Biosci. 2022, 50, 101946. [Google Scholar]
- Li, Y.; Qin, C.; Dong, L.; Zhang, X.; Wu, Z.; Liu, L.; Yang, J.; Liu, L. Whole grain benefit: Synergistic effect of oat phenolic compounds and β-glucan on hyperlipidemia via gut microbiota in high-fat-diet mice. Food Funct. 2022, 13, 12686–12696. [Google Scholar] [CrossRef] [PubMed]
- Araujo, P.; Belghit, I.; Aarsæther, N.; Espe, M.; Lucena, E.; Holen, E. The Effect of Omega-3 and Omega-6 Polyunsaturated Fatty Acids on the Production of Cyclooxygenase and Lipoxygenase Metabolites by Human Umbilical Vein Endothelial Cells. Nutrients 2019, 11, 966. [Google Scholar] [CrossRef] [PubMed]
- Liddle, D.M.; Hutchinson, A.L.; Wellings, H.R.; Power, K.A.; Robinson, L.E.; Monk, J.M. Integrated Immunomodulatory Mechanisms through which Long-Chain n-3 Polyunsaturated Fatty Acids Attenuate Obese Adipose Tissue Dysfunction. Nutrients 2017, 9, 1289. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. n-3 PUFA and inflammation: From membrane to nucleus and from bench to bedside. Proc. Nutr. Soc. 2020, 79, 404–416. [Google Scholar] [CrossRef] [PubMed]
- Norris, P.C.; Gosselin, D.; Reichart, D.; Glass, C.K.; Dennis, E.A. Phospholipase A2 regulates eicosanoid class switching during inflammasome activation. Proc. Natl. Acad. Sci. USA 2014, 111, 12746–12751. [Google Scholar] [CrossRef] [PubMed]
- Spite, M.; Clària, J.; Serhan, C.N. Resolvins, specialized proresolving lipid mediators, and their potential roles in metabolic diseases. Cell Metab. 2014, 19, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Yang, R.; Martinod, K.; Kasuga, K.; Pillai, P.S.; Porter, T.F.; Oh, S.F.; Spite, M. Maresins: Novel macrophage mediators with potent antiinflammatory and proresolving actions. J. Exp. Med. 2009, 206, 15–23. [Google Scholar] [CrossRef]
- Abedi, E.; Sahari, M.A. Long-chain polyunsaturated fatty acid sources and evaluation of their nutritional and functional properties. Food Sci. Nutr. 2014, 2, 443–463. [Google Scholar] [CrossRef] [PubMed]
- Baker, E.J.; Miles, E.A.; Burdge, G.C.; Yaqoob, P.; Calder, P.C. Metabolism and functional effects of plant-derived omega-3 fatty acids in humans. Prog. Lipid Res. 2016, 64, 30–56. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The omega-6/omega-3 fatty acid ratio: Health implications. Oléagineux Corps Gras Lipides 2010, 17, 267–275. [Google Scholar] [CrossRef]
- Simopoulos, A.P. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef] [PubMed]
- Ben Othman, R.; Berriche, O.; Gamoudi, A.; Mizouri, R.; Jerab, D.; Ben Amor, N.; Mahjoub, F.; Jamoussi, H. Cross sectional study about nutritional risk factors of metabolically unhealthy obesity. Rom. J. Intern. Med. 2023, 61, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Jamoussi, H.; Chaabouni, S.; Gammoudi, A.; Mahjoub, F.; Ounaissa, K.; Berriche, O.; Amrouche, C.; Blouza, S. Apports spontanés en acides gras oméga 3 chez des diabétiques de type 2 tunisiens. Oléagineux Corps Gras Lipides 2014, 21, A501. [Google Scholar] [CrossRef]
- Snetselaar, L.G.; de Jesus, J.M.; DeSilva, D.M.; Stoody, E.E. Dietary Guidelines for Americans, 2020–2025: Understanding the Scientific Process, Guidelines, and Key Recommendations. Nutr. Today 2021, 56, 287–295. [Google Scholar] [CrossRef]
- Hara, T.; Kashihara, D.; Ichimura, A.; Kimura, I.; Tsujimoto, G.; Hirasawa, A. Role of free fatty acid receptors in the regulation of energy metabolism. Biochim. Biophys. Acta 2014, 1841, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Song, S.; Niu, Y.; Meng, M.; Wang, C. Eicosapentaenoic Acid (EPA) Induced Macrophages Activation through GPR120-Mediated Raf-ERK1/2-IKKβ-NF-κB p65 Signaling Pathways. Nutrients 2017, 9, 937. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.Y.; Talukdar, S.; Bae, E.J.; Imamura, T.; Morinaga, H.; Fan, W.; Li, P.; Lu, W.J.; Watkins, S.M.; Olefsky, J.M. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 2010, 142, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Jiang, W.; Spinetti, T.; Tardivel, A.; Castillo, R.; Bourquin, C.; Guarda, G.; Tian, Z.; Tschopp, J.; Zhou, R. Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation. Immunity 2013, 38, 1154–1163. [Google Scholar] [CrossRef]
- Li, A.C.; Glass, C.K. PPAR- and LXR-dependent pathways controlling lipid metabolism and the development of atherosclerosis. J. Lipid Res. 2004, 45, 2161–2173. [Google Scholar] [CrossRef]
- Varga, T.; Czimmerer, Z.; Nagy, L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2011, 1812, 1007–1022. [Google Scholar] [CrossRef]
- Li, H.; Ruan, X.Z.; Powis, S.H.; Fernando, R.; Mon, W.Y.; Wheeler, D.C.; Moorhead, J.F.; Varghese, Z. EPA and DHA reduce LPS-induced inflammation responses in HK-2 cells: Evidence for a PPAR-gamma-dependent mechanism. Kidney Int. 2005, 67, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Zapata-Gonzalez, F.; Rueda, F.; Petriz, J.; Domingo, P.; Villarroya, F.; Diaz-Delfin, J.; de Madariaga, M.A.; Domingo, J.C. Human dendritic cell activities are modulated by the omega-3 fatty acid, docosahexaenoic acid, mainly through PPAR(gamma):RXR heterodimers: Comparison with other polyunsaturated fatty acids. J. Leukoc. Biol. 2008, 84, 1172–1182. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Fernández, L.; Laiglesia, L.M.; Huerta, A.E.; Martínez, J.A.; Moreno-Aliaga, M.J. Omega-3 fatty acids and adipose tissue function in obesity and metabolic syndrome. Prostaglandins Other Lipid Mediat. 2015, 121, 24–41. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.Y.; McMurray, D.N.; Chapkin, R.S. Omega-3 fatty acids, lipid rafts, and T cell signaling. Eur. J. Pharmacol. 2016, 785, 2–9. [Google Scholar] [CrossRef]
- Hwang, D.H.; Kim, J.-A.; Lee, J.Y. Mechanisms for the activation of Toll-like receptor 2/4 by saturated fatty acids and inhibition by docosahexaenoic acid. Eur. J. Pharmacol. 2016, 785, 24–35. [Google Scholar] [CrossRef]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Bidu, C.; Escoula, Q.; Bellenger, S.; Spor, A.; Galan, M.; Geissler, A.; Bouchot, A.; Dardevet, D.; Morio, B.; Cani, P.D.; et al. The Transplantation of ω3 PUFA-Altered Gut Microbiota of fat-1 Mice to Wild-Type Littermates Prevents Obesity and Associated Metabolic Disorders. Diabetes 2018, 67, 1512–1523. [Google Scholar] [CrossRef] [PubMed]
- Flachs, P.; Mohamed-Ali, V.; Horakova, O.; Rossmeisl, M.; Hosseinzadeh-Attar, M.J.; Hensler, M.; Ruzickova, J.; Kopecky, J. Polyunsaturated fatty acids of marine origin induce adiponectin in mice fed a high-fat diet. Diabetologia 2006, 49, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Itoh, M.; Suganami, T.; Satoh, N.; Tanimoto-Koyama, K.; Yuan, X.; Tanaka, M.; Kawano, H.; Yano, T.; Aoe, S.; Takeya, M.; et al. Increased adiponectin secretion by highly purified eicosapentaenoic acid in rodent models of obesity and human obese subjects. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1918–1925. [Google Scholar] [CrossRef] [PubMed]
- da Cunha de Sá, R.D.C.; Simão, J.d.J.; Silva, V.S.d.; Farias, T.M.d.; Cruz, M.M.; Antraco, V.J.; Armelin-Correa, L.; Alonso-Vale, M.I. Fish Oil Enriched in EPA, but Not in DHA, Reverses the Metabolic Syndrome and Adipocyte Dysfunction Induced by a High-Fat Diet. Nutrients 2021, 13, 754. [Google Scholar] [CrossRef] [PubMed]
- Figueras, M.; Olivan, M.; Busquets, S.; López-Soriano, F.J.; Argilés, J.M. Effects of eicosapentaenoic acid (EPA) treatment on insulin sensitivity in an animal model of diabetes: Improvement of the inflammatory status. Obesity 2011, 19, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Liu, X.; Shen, W.; Deckelbaum, R.J.; Qi, K. The Regulation of Leptin, Leptin Receptor and Pro-opiomelanocortin Expression by N-3 PUFAs in Diet-Induced Obese Mice Is Not Related to the Methylation of Their Promoters. Nutr. Metab. 2011, 8, 31. [Google Scholar] [CrossRef]
- Yang, X.; Li, X.; Hu, M.; Huang, J.; Yu, S.; Zeng, H.; Mao, L. EPA and DHA differentially improve insulin resistance by reducing adipose tissue inflammation-targeting GPR120/PPARγ pathway. J. Nutr. Biochem. 2024, 130, 109648. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.U.; Ohmori, K.; Konishi, K.; Igarashi, J.; Hashimoto, T.; Kamitori, K.; Yamaguchi, F.; Tsukamoto, I.; Uyama, T.; Ishihara, Y.; et al. Eicosapentaenoic acid upregulates VEGF-A through both GPR120 and PPARγ mediated pathways in 3T3-L1 adipocytes. Mol. Cell. Endocrinol. 2015, 406, 10–18. [Google Scholar] [CrossRef]
- Pinel, A.; Pitois, E.; Rigaudiere, J.-P.; Jouve, C.; De Saint-Vincent, S.; Laillet, B.; Montaurier, C.; Huertas, A.; Morio, B.; Capel, F. EPA prevents fat mass expansion and metabolic disturbances in mice fed with a Western diet. J. Lipid Res. 2016, 57, 1382–1397. [Google Scholar] [CrossRef] [PubMed]
- Ruzickova, J.; Rossmeisl, M.; Prazak, T.; Flachs, P.; Sponarova, J.; Veck, M.; Tvrzicka, E.; Bryhn, M.; Kopecky, J. Omega-3 PUFA of marine origin limit diet-induced obesity in mice by reducing cellularity of adipose tissue. Lipids 2004, 39, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Jin, R.; Hao, J.; Zeng, J.; Yin, D.; Yi, Y.; Zhu, M.; Mandal, A.; Hua, Y.; Ng, C.K.; et al. Consumption of the Fish Oil High-Fat Diet Uncouples Obesity and Mammary Tumor Growth through Induction of Reactive Oxygen Species in Protumor Macrophages. Cancer Res. 2020, 80, 2564–2574. [Google Scholar] [CrossRef] [PubMed]
- Pinel, A.; Rigaudière, J.P.; Jouve, C.; Montaurier, C.; Jousse, C.; LHomme, M.; Morio, B.; Capel, F. Transgenerational supplementation with eicosapentaenoic acid reduced the metabolic consequences on the whole body and skeletal muscle in mice receiving an obesogenic diet. Eur. J. Nutr. 2021, 60, 3143–3157. [Google Scholar] [CrossRef]
- Pinel, A.; Rigaudière, J.-P.; Morio, B.; Capel, F. Adipose Tissue Dysfunctions in Response to an Obesogenic Diet Are Reduced in Mice after Transgenerational Supplementation with Omega 3 Fatty Acids. Metabolites 2021, 11, 838. [Google Scholar] [CrossRef] [PubMed]
- Delpino, F.M.; Figueiredo, L.M.; da Silva, B.G.C. Effects of omega-3 supplementation on body weight and body fat mass: A systematic review. Clin. Nutr. ESPEN 2021, 44, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Huerta, O.D.; Aguilera, C.M.; Mesa, M.D.; Gil, A. Omega-3 long-chain polyunsaturated fatty acids supplementation on inflammatory biomakers: A systematic review of randomised clinical trials. Br. J. Nutr. 2012, 107 (Suppl. S2), S159–S170. [Google Scholar] [CrossRef] [PubMed]
- Billingsley, H.; Carbone, S.; Canada, J.; Buckley, L.; Dixon, D.; Kadariya, D.; Wohlford, G.; Trankle, C.; Tassell, B.V.; Abbate, A. Omega-3 Red Blood Cell Content Is Associated with Fat Mass Index and Leptin in Subjects with Obesity and Heart Failure with Preserved Ejection Fraction (P21-001-19). Curr. Dev. Nutr. 2019, 3, nzz041.P21-001-19. [Google Scholar] [CrossRef]
- Skulas-Ray, A.C.; Flock, M.R.; Richter, C.K.; Harris, W.S.; West, S.G.; Kris-Etherton, P.M. Red Blood Cell Docosapentaenoic Acid (DPA n-3) is Inversely Associated with Triglycerides and C-reactive Protein (CRP) in Healthy Adults and Dose-Dependently Increases Following n-3 Fatty Acid Supplementation. Nutrients 2015, 7, 6390–6404. [Google Scholar] [CrossRef] [PubMed]
- Fontes, J.a.D.; Rahman, F.; Lacey, S.; Larson, M.G.; Vasan, R.S.; Benjamin, E.J.; Harris, W.S.; Robins, S.J. Red blood cell fatty acids and biomarkers of inflammation: A cross-sectional study in a community-based cohort. Atherosclerosis 2015, 240, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Banaszak, M.; Dobrzyńska, M.; Kawka, A.; Górna, I.; Woźniak, D.; Przysławski, J.; Drzymała-Czyż, S. Role of Omega-3 fatty acids eicosapentaenoic (EPA) and docosahexaenoic (DHA) as modulatory and anti-inflammatory agents in noncommunicable diet-related diseases—Reports from the last 10 years. Clin. Nutr. ESPEN 2024, 63, 240–258. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, R.; Odjidja, E.N.; Scott, D.; Shivappa, N.; Hébert, J.R.; Hodge, A.; de Courten, B. The dietary inflammatory index, obesity, type 2 diabetes, and cardiovascular risk factors and diseases. Obes. Rev. 2022, 23, e13349. [Google Scholar] [CrossRef]
- Wei, S.-G.; Yu, Y.; Zhang, Z.-H.; Felder, R.B. Proinflammatory cytokines upregulate sympathoexcitatory mechanisms in the subfornical organ of the rat. Hypertension 2015, 65, 1126–1133. [Google Scholar] [CrossRef]
- Barr, J.L.; Lindenau, K.L.; Brailoiu, E.; Brailoiu, G.C. Direct evidence of bradycardic effect of omega-3 fatty acids acting on nucleus ambiguus. Neurosci. Lett. 2020, 735, 135196. [Google Scholar]
- Abdelhamid, A.S.; Brown, T.J.; Brainard, J.S.; Biswas, P.; Thorpe, G.C.; Moore, H.J.; Deane, K.H.; Summerbell, C.D.; Worthington, H.V.; Song, F.; et al. Omega-3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2020, 3, CD003177. [Google Scholar] [CrossRef]
- Djuricic, I.; Calder, P.C. Beneficial Outcomes of Omega-6 and Omega-3 Polyunsaturated Fatty Acids on Human Health: An Update for 2021. Nutrients 2021, 13, 2421. [Google Scholar] [CrossRef]
- Manson, J.E.; Cook, N.R.; Lee, I.-M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Albert, C.M.; Gordon, D.; Copeland, T.; et al. Marine n−3 Fatty Acids and Prevention of Cardiovascular Disease and Cancer. N. Engl. J. Med. 2019, 380, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Siscovick, D.S.; Barringer, T.A.; Fretts, A.M.; Wu, J.H.Y.; Lichtenstein, A.H.; Costello, R.B.; Kris-Etherton, P.M.; Jacobson, T.A.; Engler, M.B.; Alger, H.M.; et al. Omega-3 Polyunsaturated Fatty Acid (Fish Oil) Supplementation and the Prevention of Clinical Cardiovascular Disease. Circulation 2017, 135, e867–e884. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle, R.T.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N. Engl. J. Med. 2019, 380, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Budoff, M.J.; Muhlestein, J.B.; Bhatt, D.L.; Le Pa, V.T.; May, H.T.; Shaikh, K.; Shekar, C.; Kinninger, A.; Lakshmanan, S.; Roy, S.K.; et al. Effect of icosapent ethyl on progression of coronary atherosclerosis in patients with elevated triglycerides on statin therapy: A prospective, placebo-controlled randomized trial (EVAPORATE): Interim results. Cardiovasc. Res. 2021, 117, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.J.; Lincoff, A.M.; Garcia, M.; Bash, D.; Ballantyne, C.M.; Barter, P.J.; Davidson, M.H.; Kastelein, J.J.P.; Koenig, W.; McGuire, D.K.; et al. Effect of High-Dose Omega-3 Fatty Acids vs Corn Oil on Major Adverse Cardiovascular Events in Patients at High Cardiovascular Risk: The STRENGTH Randomized Clinical Trial. JAMA 2020, 324, 2268–2280. [Google Scholar] [CrossRef] [PubMed]
- Gencer, B.; Djousse, L.; Al-Ramady, O.T.; Cook, N.R.; Manson, J.E.; Albert, C.M. Effect of Long-Term Marine ɷ-3 Fatty Acids Supplementation on the Risk of Atrial Fibrillation in Randomized Controlled Trials of Cardiovascular Outcomes: A Systematic Review and Meta-Analysis. Circulation 2021, 144, 1981–1990. [Google Scholar] [CrossRef] [PubMed]
- Tutor, A.; O’Keefe, E.L.; Lavie, C.J.; Elagizi, A.; Milani, R.; O’Keefe, J. Omega-3 fatty acids in primary and secondary prevention of cardiovascular diseases. Prog. Cardiovasc. Dis. 2024, 84, 19–26. [Google Scholar]
- Neuvonen, P.J. Drug interactions with HMG-CoA reductase inhibitors (statins): The importance of CYP enzymes, transporters and pharmacogenetics. Curr. Opin. Investig. Drugs 2010, 11, 323–332. [Google Scholar]
- de Lorgeril, M.; Salen, P.; Defaye, P.; Rabaeus, M. Recent findings on the health effects of omega-3 fatty acids and statins, and their interactions: Do statins inhibit omega-3? BMC Med. 2013, 11, 5. [Google Scholar] [CrossRef]
- Arnold, C.; Konkel, A.; Fischer, R.; Schunck, W.-H. Cytochrome P450-dependent metabolism of omega-6 and omega-3 long-chain polyunsaturated fatty acids. Pharmacol. Rep. 2010, 62, 536–547. [Google Scholar] [CrossRef]
- Bird, J.K.; Calder, P.C.; Eggersdorfer, M. The Role of n-3 Long Chain Polyunsaturated Fatty Acids in Cardiovascular Disease Prevention, and Interactions with Statins. Nutrients 2018, 10, 775. [Google Scholar] [CrossRef] [PubMed]
- O’Mahoney, L.L.; Matu, J.; Price, O.J.; Birch, K.M.; Ajjan, R.A.; Farrar, D.; Tapp, R.; West, D.J.; Deighton, K.; Campbell, M.D. Omega-3 polyunsaturated fatty acids favourably modulate cardiometabolic biomarkers in type 2 diabetes: A meta-analysis and meta-regression of randomized controlled trials. Cardiovasc. Diabetol. 2018, 17, 98. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Montaño, P.; García-González, V. Effects of Dietary Fatty Acids in Pancreatic Beta Cell Metabolism, Implications in Homeostasis. Nutrients 2018, 10, 393. [Google Scholar] [CrossRef] [PubMed]
- Lepretti, M.; Martucciello, S.; Burgos Aceves, M.A.; Putti, R.; Lionetti, L. Omega-3 Fatty Acids and Insulin Resistance: Focus on the Regulation of Mitochondria and Endoplasmic Reticulum Stress. Nutrients 2018, 10, 350. [Google Scholar] [CrossRef]
- Golpour, P.; Nourbakhsh, M.; Mazaherioun, M.; Janani, L.; Nourbakhsh, M.; Yaghmaei, P. Improvement of NRF2 gene expression and antioxidant status in patients with type 2 diabetes mellitus after supplementation with omega-3 polyunsaturated fatty acids: A double-blind randomised placebo-controlled clinical trial. Diabetes Res. Clin. Pract. 2020, 162, 108120. [Google Scholar] [CrossRef]
- Aas, V.; Rokling-Andersen, M.H.; Kase, E.T.; Thoresen, G.H.; Rustan, A.C. Eicosapentaenoic acid (20:5 n-3) increases fatty acid and glucose uptake in cultured human skeletal muscle cells. J. Lipid Res. 2006, 47, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Bhaswant, M.; Poudyal, H.; Brown, L. Mechanisms of enhanced insulin secretion and sensitivity with n-3 unsaturated fatty acids. J. Nutr. Biochem. 2015, 26, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Delpino, F.M.; Figueiredo, L.M.; da Silva, B.G.C.; da Silva, T.G.; Mintem, G.C.; Bielemann, R.M.; Gigante, D.P. Omega-3 supplementation and diabetes: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2022, 62, 4435–4448. [Google Scholar] [CrossRef] [PubMed]
- He, X.-X.; Wu, X.-L.; Chen, R.-P.; Chen, C.; Liu, X.-G.; Wu, B.-J.; Huang, Z.-M. Effectiveness of Omega-3 Polyunsaturated Fatty Acids in Non-Alcoholic Fatty Liver Disease: A Meta-Analysis of Randomized Controlled Trials. PLoS ONE 2016, 11, e0162368. [Google Scholar] [CrossRef]
- Jump, D.B.; Tripathy, S.; Depner, C.M. Fatty acid-regulated transcription factors in the liver. Annu. Rev. Nutr. 2013, 33, 249–269. [Google Scholar] [CrossRef] [PubMed]
- Herrera Vielma, F.; Valenzuela, R.; Videla, L.A.; Zúñiga-Hernández, J. N-3 Polyunsaturated Fatty Acids and Their Lipid Mediators as A Potential Immune-Nutritional Intervention: A Molecular and Clinical View in Hepatic Disease and Other Non-Communicable Illnesses. Nutrients 2021, 13, 3384. [Google Scholar] [CrossRef] [PubMed]
- Lytle, K.A.; Wong, C.P.; Jump, D.B. Docosahexaenoic acid blocks progression of western diet-induced nonalcoholic steatohepatitis in obese Ldlr-/- mice. PLoS ONE 2017, 12, e0173376. [Google Scholar] [CrossRef]
- Yang, W.; Tao, K.; Zhang, P.; Chen, X.; Sun, X.; Li, R. Maresin 1 protects against lipopolysaccharide/d-galactosamine-induced acute liver injury by inhibiting macrophage pyroptosis and inflammatory response. Biochem. Pharmacol. 2022, 195, 114863. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, D.; Zong, Y.; Yang, X. DHA Protects Hepatocytes from Oxidative Injury through GPR120/ERK-Mediated Mitophagy. Int. J. Mol. Sci. 2021, 22, 5675. [Google Scholar] [CrossRef] [PubMed]
- Depner, C.M.; Traber, M.G.; Bobe, G.; Kensicki, E.; Bohren, K.M.; Milne, G.; Jump, D.B. A metabolomic analysis of omega-3 fatty acid-mediated attenuation of western diet-induced nonalcoholic steatohepatitis in LDLR-/- mice. PLoS ONE 2013, 8, e83756. [Google Scholar] [CrossRef] [PubMed]
- Depner, C.M.; Philbrick, K.A.; Jump, D.B. Docosahexaenoic acid attenuates hepatic inflammation, oxidative stress, and fibrosis without decreasing hepatosteatosis in a Ldlr(-/-) mouse model of western diet-induced nonalcoholic steatohepatitis. J. Nutr. 2013, 143, 315–323. [Google Scholar] [CrossRef]
- Han, Y.-H.; Shin, K.-O.; Kim, J.-Y.; Khadka, D.B.; Kim, H.-J.; Lee, Y.-M.; Cho, W.-J.; Cha, J.-Y.; Lee, B.-J.; Lee, M.-O. A maresin 1/RORα/12-lipoxygenase autoregulatory circuit prevents inflammation and progression of nonalcoholic steatohepatitis. J. Clin. Investig. 2019, 129, 1684–1698. [Google Scholar] [CrossRef]
- Botolin, D.; Wang, Y.; Christian, B.; Jump, D.B. Docosahexaneoic acid (22:6,n-3) regulates rat hepatocyte SREBP-1 nuclear abundance by Erk- and 26S proteasome-dependent pathways. J. Lipid Res. 2006, 47, 181–192. [Google Scholar] [CrossRef]
- Kim, H.J.; Takahashi, M.; Ezaki, O. Fish oil feeding decreases mature sterol regulatory element-binding protein 1 (SREBP-1) by down-regulation of SREBP-1c mRNA in mouse liver. A possible mechanism for down-regulation of lipogenic enzyme mRNAs. J. Biol. Chem. 1999, 274, 25892–25898. [Google Scholar] [CrossRef]
- Sato, A.; Kawano, H.; Notsu, T.; Ohta, M.; Nakakuki, M.; Mizuguchi, K.; Itoh, M.; Suganami, T.; Ogawa, Y. Antiobesity effect of eicosapentaenoic acid in high-fat/high-sucrose diet-induced obesity: Importance of hepatic lipogenesis. Diabetes 2010, 59, 2495–2504. [Google Scholar] [CrossRef] [PubMed]
- Scorletti, E.; Bhatia, L.; McCormick, K.G.; Clough, G.F.; Nash, K.; Hodson, L.; Moyses, H.E.; Calder, P.C.; Byrne, C.D. WELCOME Study Effects of purified eicosapentaenoic and docosahexaenoic acids in nonalcoholic fatty liver disease: Results from the WELCOME* study. Hepatology 2014, 60, 1211–1221. [Google Scholar] [CrossRef] [PubMed]
- Vell, M.S.; Creasy, K.T.; Scorletti, E.; Seeling, K.S.; Hehl, L.; Rendel, M.D.; Schneider, K.M.; Schneider, C.V. Omega-3 intake is associated with liver disease protection. Front. Public Health 2023, 11, 1192099. [Google Scholar] [CrossRef] [PubMed]
- Musazadeh, V.; Karimi, A.; Malekahmadi, M.; Ahrabi, S.S.; Dehghan, P. Omega-3 polyunsaturated fatty acids in the treatment of non-alcoholic fatty liver disease: An umbrella systematic review and meta-analysis. Clin. Exp. Pharmacol. Physiol. 2023, 50, 327–334. [Google Scholar] [CrossRef]
- Aziz, T.; Niraj, M.K.; Kumar, S.; Kumar, R.; Parveen, H. Effectiveness of Omega-3 Polyunsaturated Fatty Acids in Non-alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Cureus 2024, 16, e68002. [Google Scholar] [CrossRef]
- Musa-Veloso, K.; Venditti, C.; Lee, H.Y.; Darch, M.; Floyd, S.; West, S.; Simon, R. Systematic review and meta-analysis of controlled intervention studies on the effectiveness of long-chain omega-3 fatty acids in patients with nonalcoholic fatty liver disease. Nutr. Rev. 2018, 76, 581–602. [Google Scholar] [CrossRef] [PubMed]
- Mas, E.; Barden, A.; Burke, V.; Beilin, L.J.; Watts, G.F.; Huang, R.-C.; Puddey, I.B.; Irish, A.B.; Mori, T.A. A randomized controlled trial of the effects of n-3 fatty acids on resolvins in chronic kidney disease. Clin. Nutr. 2016, 35, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Barden, A.E.; Burke, V.; Mas, E.; Beilin, L.J.; Puddey, I.B.; Watts, G.F.; Irish, A.B.; Mori, T.A. n-3 fatty acids reduce plasma 20-hydroxyeicosatetraenoic acid and blood pressure in patients with chronic kidney disease. J. Hypertens. 2015, 33, 1947–1953. [Google Scholar] [CrossRef] [PubMed]
- Taccone-Gallucci, M.; Manca-di-Villahermosa, S.; Battistini, L.; Stuffler, R.G.; Tedesco, M.; Maccarrone, M. N-3 PUFAs reduce oxidative stress in ESRD patients on maintenance HD by inhibiting 5-lipoxygenase activity. Kidney Int. 2006, 69, 1450–1454. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.R.; Juraschek, S.P.; Appel, L.J.; Madala, M.; Anderson, C.A.M.; Bleys, J.; Guallar, E. The effect of n-3 long-chain polyunsaturated fatty acid supplementation on urine protein excretion and kidney function: Meta-analysis of clinical trials. Am. J. Clin. Nutr. 2009, 89, 1937–1945. [Google Scholar] [CrossRef]
- Gharekhani, A.; Khatami, M.-R.; Dashti-Khavidaki, S.; Razeghi, E.; Abdollahi, A.; Hashemi-Nazari, S.-S.; Mansournia, M.-A. Effects of oral supplementation with omega-3 fatty acids on nutritional state and inflammatory markers in maintenance hemodialysis patients. J. Ren. Nutr. 2014, 24, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Svensson, M.; Schmidt, E.B.; Jørgensen, K.A.; Christensen, J.H.; OPACH Study Group. N-3 fatty acids as secondary prevention against cardiovascular events in patients who undergo chronic hemodialysis: A randomized, placebo-controlled intervention trial. Clin. J. Am. Soc. Nephrol. 2006, 1, 780–786. [Google Scholar] [PubMed]
- Zhu, W.; Dong, C.; Du, H.; Zhang, H.; Chen, J.; Hu, X.; Hu, F. Effects of fish oil on serum lipid profile in dialysis patients: A systematic review and meta-analysis of randomized controlled trials. Lipids Health Dis. 2014, 13, 127. [Google Scholar] [PubMed]
- Hosogai, N.; Fukuhara, A.; Oshima, K.; Miyata, Y.; Tanaka, S.; Segawa, K.; Furukawa, S.; Tochino, Y.; Komuro, R.; Matsuda, M.; et al. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes 2007, 56, 901–911. [Google Scholar] [PubMed]
- He, Q.; Gao, Z.; Yin, J.; Zhang, J.; Yun, Z.; Ye, J. Regulation of HIF-1{alpha} activity in adipose tissue by obesity-associated factors: Adipogenesis, insulin, and hypoxia. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E877–E885. [Google Scholar] [CrossRef]
- Lumeng, C.N.; Bodzin, J.L.; Saltiel, A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Investig. 2007, 117, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Zeng, Q.; Xie, L.; Zhang, H.; Hu, W.; Xiao, L.; Zhou, H.; Wang, F.; Xie, W.; Song, J.; et al. Adipose stem cells control obesity-induced T cell infiltration into adipose tissue. Cell Rep. 2024, 43, 113963. [Google Scholar] [CrossRef] [PubMed]
- Balcerczyk, A.; Eljaafari, A.; Pirola, L. Adipose stem cells drive T cell infiltration in obesity. Trends Endocrinol. Metab. 2024, 35, 931–933. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, S.; Manabe, I.; Nagasaki, M.; Eto, K.; Yamashita, H.; Ohsugi, M.; Otsu, M.; Hara, K.; Ueki, K.; Sugiura, S.; et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat. Med. 2009, 15, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Arner, P.; Caro, J.F.; Atkinson, R.L.; Spiegelman, B.M. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J. Clin. Investig. 1995, 95, 2409–2415. [Google Scholar] [CrossRef]
- Sabio, G.; Das, M.; Mora, A.; Zhang, Z.; Jun, J.Y.; Ko, H.J.; Barrett, T.; Kim, J.K.; Davis, R.J. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science 2008, 322, 1539–1543. [Google Scholar] [CrossRef] [PubMed]
- Eljaafari, A.; Robert, M.; Chehimi, M.; Chanon, S.; Durand, C.; Vial, G.; Bendridi, N.; Madec, A.-M.; Disse, E.; Laville, M.; et al. Adipose Tissue-Derived Stem Cells From Obese Subjects Contribute to Inflammation and Reduced Insulin Response in Adipocytes Through Differential Regulation of the Th1/Th17 Balance and Monocyte Activation. Diabetes 2015, 64, 2477–2488. [Google Scholar] [CrossRef] [PubMed]
- Chehimi, M.; Robert, M.; Bechwaty, M.E.; Vial, G.; Rieusset, J.; Vidal, H.; Pirola, L.; Eljaafari, A. Adipocytes, like their progenitors, contribute to inflammation of adipose tissues through promotion of Th-17 cells and activation of monocytes, in obese subjects. Adipocyte 2016, 5, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Winer, S.; Chan, Y.; Paltser, G.; Truong, D.; Tsui, H.; Bahrami, J.; Dorfman, R.; Wang, Y.; Zielenski, J.; Mastronardi, F.; et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat. Med. 2009, 15, 921–929. [Google Scholar] [CrossRef]
- Fabbrini, E.; Cella, M.; McCartney, S.A.; Fuchs, A.; Abumrad, N.A.; Pietka, T.A.; Chen, Z.; Finck, B.N.; Han, D.H.; Magkos, F.; et al. Association between specific adipose tissue CD4+ T-cell populations and insulin resistance in obese individuals. Gastroenterology 2013, 145, 366–374.e3. [Google Scholar] [CrossRef] [PubMed]
- Dalmas, E.; Venteclef, N.; Caer, C.; Poitou, C.; Cremer, I.; Aron-Wisnewsky, J.; Lacroix-Desmazes, S.; Bayry, J.; Kaveri, S.V.; Clément, K.; et al. T cell-derived IL-22 amplifies IL-1β-driven inflammation in human adipose tissue: Relevance to obesity and type 2 diabetes. Diabetes 2014, 63, 1966–1977. [Google Scholar] [CrossRef] [PubMed]
- Pestel, J.; Blangero, F.; Watson, J.; Pirola, L.; Eljaafari, A. Adipokines in obesity and metabolic-related-diseases. Biochimie 2023, 212, 48–59. [Google Scholar] [CrossRef]
- Artemniak-Wojtowicz, D.; Kucharska, A.M.; Pyrżak, B. Obesity and chronic inflammation crosslinking. Cent. Eur. J. Immunol. 2020, 45, 461–468. [Google Scholar] [CrossRef]
- Matarese, G. The link between obesity and autoimmunity. Science 2023, 379, 1298–1300. [Google Scholar] [CrossRef]
- Gremese, E.; Tolusso, B.; Gigante, M.R.; Ferraccioli, G. Obesity as a Risk and Severity Factor in Rheumatic Diseases (Autoimmune Chronic Inflammatory Diseases). Front. Immunol. 2014, 5, 576. [Google Scholar]
- Grzechnik, K.; Targońska-Stępniak, B. Metabolic Syndrome and Rheumatoid Arthritis Activity: An Analysis of Clinical, Laboratory, and Ultrasound Parameters. Nutrients 2023, 15, 4756. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, S.; Santos-Faria, D.; Leite Silva, J.; Ramos Rodrigues, J.; Sousa Neves, J.; Peixoto, D.; Tavares-Costa, J.; Alcino, S.; Afonso, C.; Teixeira, F. Obesity, metabolic syndrome and other comorbidities in rheumatoid arthritis and psoriatic arthritis: Influence on disease activity and quality of life. Acta Reumatol. Port. 2019, 44, 322–324. [Google Scholar] [PubMed]
- De Pergola, G.; Silvestris, F. Obesity as a major risk factor for cancer. J. Obes. 2013, 2013, 291546. [Google Scholar] [CrossRef] [PubMed]
- Blangero, F.; Robert, M.; Andraud, T.; Dumontet, C.; Vidal, H.; Eljaafari, A. Contribution of Mesenchymal Stem Cells from Obese Adipose Tissue to PD-L1 Over-Expression and Breast Cancer Progression through Pathogenic Th17 Cell Activation. Cancers 2023, 15, 2963. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Theiss, A.L.; Venuprasad, K. RORγt protein modifications and IL-17-mediated inflammation. Trends Immunol. 2021, 42, 1037–1050. [Google Scholar]
- Stockinger, B.; Omenetti, S. The dichotomous nature of T helper 17 cells. Nat. Rev. Immunol. 2017, 17, 535–544. [Google Scholar] [PubMed]
- Lee, Y.; Awasthi, A.; Yosef, N.; Quintana, F.J.; Xiao, S.; Peters, A.; Wu, C.; Kleinewietfeld, M.; Kunder, S.; Hafler, D.A.; et al. Induction and molecular signature of pathogenic TH17 cells. Nat. Immunol. 2012, 13, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Meyer Zu Horste, G.; Wu, C.; Wang, C.; Cong, L.; Pawlak, M.; Lee, Y.; Elyaman, W.; Xiao, S.; Regev, A.; Kuchroo, V.K. RBPJ Controls Development of Pathogenic Th17 Cells by Regulating IL-23 Receptor Expression. Cell Rep. 2016, 16, 392–404. [Google Scholar] [CrossRef] [PubMed]
- Ahern, P.P.; Izcue, A.; Maloy, K.J.; Powrie, F. The interleukin-23 axis in intestinal inflammation. Immunol. Rev. 2008, 226, 147–159. [Google Scholar] [CrossRef]
- Zhang, S.; Gang, X.; Yang, S.; Cui, M.; Sun, L.; Li, Z.; Wang, G. The Alterations in and the Role of the Th17/Treg Balance in Metabolic Diseases. Front. Immunol. 2021, 12, 678355. [Google Scholar]
- Chehimi, M.; Ward, R.; Pestel, J.; Robert, M.; Pesenti, S.; Bendridi, N.; Michalski, M.-C.; Laville, M.; Vidal, H.; Eljaafari, A. Omega-3 Polyunsaturated Fatty Acids Inhibit IL-17A Secretion through Decreased ICAM-1 Expression in T Cells Co-Cultured with Adipose-Derived Stem Cells Harvested from Adipose Tissues of Obese Subjects. Mol. Nutr. Food Res. 2019, 63, e1801148. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.J.; Fan, Y.-Y.; Monk, J.M.; Hou, T.Y.; Barhoumi, R.; McMurray, D.N.; Chapkin, R.S. n-3 PUFAs reduce T-helper 17 cell differentiation by decreasing responsiveness to interleukin-6 in isolated mouse splenic CD4+ T cells. J. Nutr. 2014, 144, 1306–1313. [Google Scholar] [CrossRef] [PubMed]
- Szilagyi, A. Relationship(s) between obesity and inflammatory bowel diseases: Possible intertwined pathogenic mechanisms. Clin. J. Gastroenterol. 2020, 13, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Harper, J.W.; Zisman, T.L. Interaction of obesity and inflammatory bowel disease. World J. Gastroenterol. 2016, 22, 7868–7881. [Google Scholar] [CrossRef] [PubMed]
- Bilski, J.; Mazur-Bialy, A.; Wojcik, D.; Surmiak, M.; Magierowski, M.; Sliwowski, Z.; Pajdo, R.; Kwiecien, S.; Danielak, A.; Ptak-Belowska, A.; et al. Role of Obesity, Mesenteric Adipose Tissue, and Adipokines in Inflammatory Bowel Diseases. Biomolecules 2019, 9, 780. [Google Scholar] [CrossRef]
- Scaioli, E.; Liverani, E.; Belluzzi, A. The Imbalance between n-6/n-3 Polyunsaturated Fatty Acids and Inflammatory Bowel Disease: A Comprehensive Review and Future Therapeutic Perspectives. Int. J. Mol. Sci. 2017, 18, 2619. [Google Scholar] [CrossRef]
- Monk, J.M.; Hou, T.Y.; Turk, H.F.; Weeks, B.; Wu, C.; McMurray, D.N.; Chapkin, R.S. Dietary n-3 polyunsaturated fatty acids (PUFA) decrease obesity-associated Th17 cell-mediated inflammation during colitis. PLoS ONE 2012, 7, e49739. [Google Scholar] [CrossRef]
- Monk, J.M.; Jia, Q.; Callaway, E.; Weeks, B.; Alaniz, R.C.; McMurray, D.N.; Chapkin, R.S. Th17 Cell Accumulation Is Decreased during Chronic Experimental Colitis by (n-3) PUFA in Fat-1 Mice1–23. J. Nutr. 2012, 142, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Willemsen, L.E.M.; Koetsier, M.A.; Balvers, M.; Beermann, C.; Stahl, B.; van Tol, E.A.F. Polyunsaturated fatty acids support epithelial barrier integrity and reduce IL-4 mediated permeability in vitro. Eur. J. Nutr. 2008, 47, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Beguin, P.; Errachid, A.; Larondelle, Y.; Schneider, Y.-J. Effect of polyunsaturated fatty acids on tight junctions in a model of the human intestinal epithelium under normal and inflammatory conditions. Food Funct. 2013, 4, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Usami, M.; Muraki, K.; Iwamoto, M.; Ohata, A.; Matsushita, E.; Miki, A. Effect of eicosapentaenoic acid (EPA) on tight junction permeability in intestinal monolayer cells. Clin. Nutr. 2001, 20, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Quiros, M.; Nusrat, A. Saving Problematic Mucosae: SPMs in Intestinal Mucosal Inflammation and Repair. Trends Mol. Med. 2019, 25, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.X.; Colgan, S.P. Special pro-resolving mediator (SPM) actions in regulating gastro-intestinal inflammation and gut mucosal immune responses. Mol. Aspects Med. 2017, 58, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.; Shah, P.S.; Steinhart, A.H.; Zlotkin, S.; Griffiths, A.M. Maintenance of remission in inflammatory bowel disease using omega-3 fatty acids (fish oil): A systematic review and meta-analyses. Inflamm. Bowel Dis. 2011, 17, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Radziszewska, M.; Smarkusz-Zarzecka, J.; Ostrowska, L.; Pogodziński, D. Nutrition and Supplementation in Ulcerative Colitis. Nutrients 2022, 14, 2469. [Google Scholar] [CrossRef] [PubMed]
- Hawthorne, A.B.; Daneshmend, T.K.; Hawkey, C.J.; Belluzzi, A.; Everitt, S.J.; Holmes, G.K.; Malkinson, C.; Shaheen, M.Z.; Willars, J.E. Treatment of ulcerative colitis with fish oil supplementation: A prospective 12 month randomised controlled trial. Gut 1992, 33, 922–928. [Google Scholar] [PubMed]
- Loeschke, K.; Ueberschaer, B.; Pietsch, A.; Gruber, E.; Ewe, K.; Wiebecke, B.; Heldwein, W.; Lorenz, R. n-3 fatty acids only delay early relapse of ulcerative colitis in remission. Dig. Dis. Sci. 1996, 41, 2087–2094. [Google Scholar] [CrossRef]
- Romano, C.; Cucchiara, S.; Barabino, A.; Annese, V.; Sferlazzas, C.; Diseases, S.I.S.G. of P.I.B. Usefulness of ω-3 fatty acid supplementation in addition to mesalazine in maintaining remission in pediatric Crohn’s disease: A double-blind, randomized, placebo-controlled study. World J. Gastroenterol. 2005, 11, 7118–7121. [Google Scholar]
- Chicco, F.; Magrì, S.; Cingolani, A.; Paduano, D.; Pesenti, M.; Zara, F.; Tumbarello, F.; Urru, E.; Melis, A.; Casula, L.; et al. Multidimensional Impact of Mediterranean Diet on IBD Patients. Inflamm. Bowel Dis. 2020, 27, 1–9. [Google Scholar]
- Fleming, P.; Kraft, J.; Gulliver, W.P.; Lynde, C. The Relationship of Obesity With the Severity of Psoriasis: A Systematic Review. J. Cutan. Med. Surg. 2015, 19, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Azfar, R.S.; Gelfand, J.M. Psoriasis and metabolic disease: Epidemiology and pathophysiology. Curr. Opin. Rheumatol. 2008, 20, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Liakou, A.I.; Zouboulis, C.C. Links and risks associated with psoriasis and metabolic syndrome. Psoriasis (Auckl.) 2015, 5, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Correia, B.; Torres, T. Obesity: A key component of psoriasis. Acta Biomed. 2015, 86, 121–129. [Google Scholar] [PubMed]
- Simard, M.; Rioux, G.; Morin, S.; Martin, C.; Guérin, S.L.; Flamand, N.; Julien, P.; Fradette, J.; Pouliot, R. Investigation of Omega-3 Polyunsaturated Fatty Acid Biological Activity in a Tissue-Engineered Skin Model Involving Psoriatic Cells. J. Investig. Dermatol. 2021, 141, 2391–2401.e13. [Google Scholar] [CrossRef] [PubMed]
- Morin, S.; Simard, M.; Flamand, N.; Pouliot, R. Biological action of docosahexaenoic acid in a 3D tissue-engineered psoriatic skin model: Focus on the PPAR signaling pathway. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 159032. [Google Scholar] [CrossRef]
- Honda, T.; Kabashima, K. Current understanding of the role of dietary lipids in the pathophysiology of psoriasis. J. Dermatol. Sci. 2019, 94, 314–320. [Google Scholar] [CrossRef]
- Morin, S.; Bélanger, S.; Cortez Ghio, S.; Pouliot, R. Eicosapentaenoic acid reduces the proportion of IL-17A-producing T cells in a 3D psoriatic skin model. J. Lipid Res. 2023, 64, 100428. [Google Scholar] [CrossRef]
- Son, S.-E.; Koh, J.-M.; Im, D.-S. Free Fatty Acid Receptor 4 (FFA4) Activation Ameliorates Imiquimod-Induced Psoriasis in Mice. Int. J. Mol. Sci. 2022, 23, 4482. [Google Scholar] [CrossRef] [PubMed]
- Arantes, E.L.; Dragano, N.; Ramalho, A.; Vitorino, D.; de-Souza, G.F.; Lima, M.H.M.; Velloso, L.A.; Araújo, E.P. Topical Docosahexaenoic Acid (DHA) Accelerates Skin Wound Healing in Rats and Activates GPR120. Biol. Res. Nurs. 2016, 18, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Sawada, Y.; Honda, T.; Nakamizo, S.; Otsuka, A.; Ogawa, N.; Kobayashi, Y.; Nakamura, M.; Kabashima, K. Resolvin E1 attenuates murine psoriatic dermatitis. Sci. Rep. 2018, 8, 11873. [Google Scholar] [CrossRef]
- Sorokin, A.V.; Norris, P.C.; English, J.T.; Dey, A.K.; Chaturvedi, A.; Baumer, Y.; Silverman, J.; Playford, M.P.; Serhan, C.N.; Mehta, N.N. Identification of proresolving and inflammatory lipid mediators in human psoriasis. J. Clin. Lipidol. 2018, 12, 1047–1060. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, A.V.; Arnardottir, H.; Svirydava, M.; Ng, Q.; Baumer, Y.; Berg, A.; Pantoja, C.J.; Florida, E.M.; Teague, H.L.; Yang, Z.-H.; et al. Comparison of the dietary omega-3 fatty acids impact on murine psoriasis-like skin inflammation and associated lipid dysfunction. J. Nutr. Biochem. 2023, 117, 109348. [Google Scholar] [CrossRef]
- Chen, X.; Hong, S.; Sun, X.; Xu, W.; Li, H.; Ma, T.; Zheng, Q.; Zhao, H.; Zhou, Y.; Qiang, Y.; et al. Efficacy of fish oil and its components in the management of psoriasis: A systematic review of 18 randomized controlled trials. Nutr. Rev. 2020, 78, 827–840. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.C.T.; Taghizadeh, M.; Nahavandi, M.; Jafarnejad, S. Efficacy of ω-3 supplementation in patients with psoriasis: A meta-analysis of randomized controlled trials. Clin. Rheumatol. 2019, 38, 977–988. [Google Scholar] [CrossRef] [PubMed]
- Visser, A.W.; de Mutsert, R.; le Cessie, S.; den Heijer, M.; Rosendaal, F.R.; Kloppenburg, M.; NEO Study Group. The relative contribution of mechanical stress and systemic processes in different types of osteoarthritis: The NEO study. Ann. Rheum. Dis. 2015, 74, 1842–1847. [Google Scholar] [CrossRef]
- Wang, X.; Hunter, D.; Xu, J.; Ding, C. Metabolic triggered inflammation in osteoarthritis. Osteoarthr. Cartil. 2015, 23, 22–30. [Google Scholar] [CrossRef]
- Thijssen, E.; van Caam, A.; van der Kraan, P.M. Obesity and osteoarthritis, more than just wear and tear: Pivotal roles for inflamed adipose tissue and dyslipidaemia in obesity-induced osteoarthritis. Rheumatology 2015, 54, 588–600. [Google Scholar] [CrossRef]
- Scotece, M.; Conde, J.; López, V.; Lago, F.; Pino, J.; Gómez-Reino, J.J.; Gualillo, O. Adiponectin and leptin: New targets in inflammation. Basic Clin. Pharmacol. Toxicol. 2014, 114, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Wann, A.K.T.; Mistry, J.; Blain, E.J.; Michael-Titus, A.T.; Knight, M.M. Eicosapentaenoic acid and docosahexaenoic acid reduce interleukin-1β-mediated cartilage degradation. Arthritis Res. Ther. 2010, 12, R207. [Google Scholar] [CrossRef] [PubMed]
- Caron, J.P.; Gandy, J.C.; Brown, J.L.; Sordillo, L.M. Omega-3 fatty acids and docosahexaenoic acid oxymetabolites modulate the inflammatory response of equine recombinant interleukin1β-stimulated equine synoviocytes. Prostaglandins Other Lipid Mediat. 2019, 142, 1–8. [Google Scholar] [PubMed]
- Jin, X.; Dong, X.; Sun, Y.; Liu, Z.; Liu, L.; Gu, H. Dietary Fatty Acid Regulation of the NLRP3 Inflammasome via the TLR4/NF-κB Signaling Pathway Affects Chondrocyte Pyroptosis. Oxid. Med. Cell. Longev. 2022, 2022, 3711371. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-L.; Jain, D.; McNeill, J.N.; Little, D.; Anderson, J.A.; Huebner, J.L.; Kraus, V.B.; Rodriguiz, R.M.; Wetsel, W.C.; Guilak, F. Dietary fatty acid content regulates wound repair and the pathogenesis of osteoarthritis following joint injury. Ann. Rheum. Dis. 2015, 74, 2076–2083. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Yi, Z.; Yin, E.; Lu, R.; You, H.; Yuan, X. Effect of omega-3 polyunsaturated fatty acids supplementation for patients with osteoarthritis: A meta-analysis. J. Orthop. Surg. Res. 2023, 18, 381. [Google Scholar] [CrossRef]
- Shi, W.; Xu, C.; Xu, Q.; Zhang, H.; Li, Z.; Li, H. Polyunsaturated fatty acids may not be helpful for people with osteoarthritis: A two-sample Mendelian randomization analysis. Sci. Rep. 2025, 15, 6065. [Google Scholar] [CrossRef]
- Dravid, A.A.; Dhanabalan, K.M.; Naskar, S.; Vashistha, A.; Agarwal, S.; Padhan, B.; Dewani, M.; Agarwal, R. Sustained release resolvin D1 liposomes are effective in the treatment of osteoarthritis in obese mice. J. Biomed. Mater. Res. A 2023, 111, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Sun, M. A meta-analysis of the relationship between body mass index and risk of rheumatoid arthritis. EXCLI J. 2018, 17, 1079–1089. [Google Scholar] [PubMed]
- Zheng, H.; Chen, C. Body mass index and risk of knee osteoarthritis: Systematic review and meta-analysis of prospective studies. BMJ Open 2015, 5, e007568. [Google Scholar] [CrossRef]
- Lievense, A.M. Influence of obesity on the development of osteoarthritis of the hip: A systematic review. Rheumatology 2002, 41, 1155–1162. [Google Scholar] [CrossRef]
- Dubovyk, V.; Vasileiadis, G.K.; Fatima, T.; Zhang, Y.; Kapetanovic, M.C.; Kastbom, A.; Rizk, M.; Söderbergh, A.; Zhao, S.S.; van Vollenhoven, R.F.; et al. Obesity is a risk factor for poor response to treatment in early rheumatoid arthritis: A NORD-STAR study. RMD Open 2024, 10, e004227. [Google Scholar] [CrossRef]
- George, M.D.; Baker, J.F. The Obesity Epidemic and Consequences for Rheumatoid Arthritis Care. Curr. Rheumatol. Rep. 2016, 18, 6. [Google Scholar] [CrossRef]
- van den Berg, W.B.; Miossec, P. IL-17 as a future therapeutic target for rheumatoid arthritis. Nat. Rev. Rheumatol. 2009, 5, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Eljaafari, A.; Tartelin, M.-L.; Aissaoui, H.; Chevrel, G.; Osta, B.; Lavocat, F.; Miossec, P. Bone marrow-derived and synovium-derived mesenchymal cells promote Th17 cell expansion and activation through caspase 1 activation: Contribution to the chronicity of rheumatoid arthritis. Arthritis Rheum. 2012, 64, 2147–2157. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lim, K.; Kim, K.H.; Kim, J.H.; Choi, J.S.; Shim, S.-C. N-3 polyunsaturated fatty acids restore Th17 and Treg balance in collagen antibody-induced arthritis. PLoS ONE 2018, 13, e0194331. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-E.; Lee, J.-H.; Koh, J.-M.; Im, D.-S. Free Fatty Acid 4 Receptor Activation Attenuates Collagen-Induced Arthritis by Rebalancing Th1/Th17 and Treg Cells. Int. J. Mol. Sci. 2024, 25, 5866. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim. Biophys. Acta 2015, 1851, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Greenwood, D.C.; Webster, J.; Uzokwe, C.; Tao, J.; Hardie, L.J.; Cade, J.E. Dose-Response Associations Between Diet and Risk of Rheumatoid Arthritis: A Meta-Analysis of Prospective Cohort Studies. Nutrients 2024, 16, 4050. [Google Scholar] [CrossRef]
- Adam, O.; Beringer, C.; Kless, T.; Lemmen, C.; Adam, A.; Wiseman, M.; Adam, P.; Klimmek, R.; Forth, W. Anti-inflammatory effects of a low arachidonic acid diet and fish oil in patients with rheumatoid arthritis. Rheumatol. Int. 2003, 23, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Gioxari, A.; Kaliora, A.C.; Marantidou, F.; Panagiotakos, D.P. Intake of ω-3 polyunsaturated fatty acids in patients with rheumatoid arthritis: A systematic review and meta-analysis. Nutrition 2018, 45, 114–124.e4. [Google Scholar] [CrossRef] [PubMed]
- Versini, M.; Jeandel, P.-Y.; Rosenthal, E.; Shoenfeld, Y. Obesity in autoimmune diseases: Not a passive bystander. Autoimmun. Rev. 2014, 13, 981–1000. [Google Scholar] [CrossRef] [PubMed]
- Ouellet, M.; Emond, V.; Chen, C.T.; Julien, C.; Bourasset, F.; Oddo, S.; LaFerla, F.; Bazinet, R.P.; Calon, F. Diffusion of docosahexaenoic and eicosapentaenoic acids through the blood-brain barrier: An in situ cerebral perfusion study. Neurochem. Int. 2009, 55, 476–482. [Google Scholar] [CrossRef]
- Dyall, S.C. Long-chain omega-3 fatty acids and the brain: A review of the independent and shared effects of EPA, DPA and DHA. Front. Aging Neurosci. 2015, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Shinto, L.; Marracci, G.; Baldauf-Wagner, S.; Strehlow, A.; Yadav, V.; Stuber, L.; Bourdette, D. Omega-3 fatty acid supplementation decreases matrix metalloproteinase-9 production in relapsing-remitting multiple sclerosis. Prostaglandins Leukot. Essent. Fatty Acids 2009, 80, 131–136. [Google Scholar] [PubMed]
- Ceccarini, M.R.; Ceccarelli, V.; Codini, M.; Fettucciari, K.; Calvitti, M.; Cataldi, S.; Albi, E.; Vecchini, A.; Beccari, T. The Polyunsaturated Fatty Acid EPA, but Not DHA, Enhances Neurotrophic Factor Expression through Epigenetic Mechanisms and Protects against Parkinsonian Neuronal Cell Death. Int. J. Mol. Sci. 2022, 23, 16176. [Google Scholar] [CrossRef] [PubMed]
- Majou, D.; Dermenghem, A.-L. DHA (omega-3 fatty acid) increases the action of brain-derived neurotrophic factor (BDNF). OCL 2024, 31, 1. [Google Scholar] [CrossRef]
- Serhan, C.N. Novel Pro-Resolving Lipid Mediators in Inflammation Are Leads for Resolution Physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Nylander, A.; Hafler, D.A. Multiple sclerosis. J. Clin. Investig. 2012, 122, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Langrish, C.L.; Chen, Y.; Blumenschein, W.M.; Mattson, J.; Basham, B.; Sedgwick, J.D.; McClanahan, T.; Kastelein, R.A.; Cua, D.J. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 2005, 201, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Unoda, K.; Doi, Y.; Nakajima, H.; Yamane, K.; Hosokawa, T.; Ishida, S.; Kimura, F.; Hanafusa, T. Eicosapentaenoic acid (EPA) induces peroxisome proliferator-activated receptors and ameliorates experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2013, 256, 7–12. [Google Scholar] [CrossRef]
- Nordvik, I.; Myhr, K.M.; Nyland, H.; Bjerve, K.S. Effect of dietary advice and n-3 supplementation in newly diagnosed MS patients. Acta Neurol. Scand. 2000, 102, 143–149. [Google Scholar] [CrossRef]
- AlAmmar, W.A.; Albeesh, F.H.; Ibrahim, L.M.; Algindan, Y.Y.; Yamani, L.Z.; Khattab, R.Y. Effect of omega-3 fatty acids and fish oil supplementation on multiple sclerosis: A systematic review. Nutr. Neurosci. 2021, 24, 569–579. [Google Scholar] [CrossRef]
- Ramirez-Ramirez, V.; Macias-Islas, M.A.; Ortiz, G.G.; Pacheco-Moises, F.; Torres-Sanchez, E.D.; Sorto-Gomez, T.E.; Cruz-Ramos, J.A.; Orozco-Aviña, G.; Celis de la Rosa, A.J. Efficacy of fish oil on serum of TNFα, IL-1β, and IL-6 oxidative stress markers in multiple sclerosis treated with interferon beta-1b. Oxid. Med. Cell. Longev. 2013, 2013, 709493. [Google Scholar] [CrossRef] [PubMed]
- Torkildsen, O.; Wergeland, S.; Bakke, S.; Beiske, A.G.; Bjerve, K.S.; Hovdal, H.; Midgard, R.; Lilleås, F.; Pedersen, T.; Bjørnarå, B.; et al. ω-3 fatty acid treatment in multiple sclerosis (OFAMS Study): A randomized, double-blind, placebo-controlled trial. Arch. Neurol. 2012, 69, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Farinotti, M.; Vacchi, L.; Simi, S.; Di Pietrantonj, C.; Brait, L.; Filippini, G. Dietary interventions for multiple sclerosis. Cochrane Database Syst. Rev. 2012, 12, CD004192. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Chen, H.; Li, Y.; Zhong, H.; Sun, W.; Wang, J.; Zhang, T.; Ma, J.; Yan, S.; Zhang, J.; et al. Maresin 1 improves the Treg/Th17 imbalance in rheumatoid arthritis through miR-21. Ann. Rheum. Dis. 2018, 77, 1644–1652. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.A.; Pinder, A.C. N-3 polyunsaturated fatty acids modulate the expression of functionally associated molecules on human monocytes and inhibit antigen-presentation in vitro. Clin. Exp. Immunol. 1997, 110, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Clària, J.; González-Périz, A.; López-Vicario, C.; Rius, B.; Titos, E. New insights into the role of macrophages in adipose tissue inflammation and Fatty liver disease: Modulation by endogenous omega-3 Fatty Acid-derived lipid mediators. Front. Immunol. 2011, 2, 49. [Google Scholar] [CrossRef]
- Peh, H.Y.; Chen, J. Pro-resolving lipid mediators and therapeutic innovations in resolution of inflammation. Pharmacol. Ther. 2025, 265, 108753. [Google Scholar] [CrossRef] [PubMed]
- Julliard, W.A.; Myo, Y.P.A.; Perelas, A.; Jackson, P.D.; Thatcher, T.H.; Sime, P.J. Specialized pro-resolving mediators as modulators of immune responses. Semin. Immunol. 2022, 59, 101605. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jerab, D.; Blangero, F.; da Costa, P.C.T.; de Brito Alves, J.L.; Kefi, R.; Jamoussi, H.; Morio, B.; Eljaafari, A. Beneficial Effects of Omega-3 Fatty Acids on Obesity and Related Metabolic and Chronic Inflammatory Diseases. Nutrients 2025, 17, 1253. https://doi.org/10.3390/nu17071253
Jerab D, Blangero F, da Costa PCT, de Brito Alves JL, Kefi R, Jamoussi H, Morio B, Eljaafari A. Beneficial Effects of Omega-3 Fatty Acids on Obesity and Related Metabolic and Chronic Inflammatory Diseases. Nutrients. 2025; 17(7):1253. https://doi.org/10.3390/nu17071253
Chicago/Turabian StyleJerab, Donia, Ferdinand Blangero, Paulo César Trindade da Costa, José Luiz de Brito Alves, Rym Kefi, Henda Jamoussi, Beatrice Morio, and Assia Eljaafari. 2025. "Beneficial Effects of Omega-3 Fatty Acids on Obesity and Related Metabolic and Chronic Inflammatory Diseases" Nutrients 17, no. 7: 1253. https://doi.org/10.3390/nu17071253
APA StyleJerab, D., Blangero, F., da Costa, P. C. T., de Brito Alves, J. L., Kefi, R., Jamoussi, H., Morio, B., & Eljaafari, A. (2025). Beneficial Effects of Omega-3 Fatty Acids on Obesity and Related Metabolic and Chronic Inflammatory Diseases. Nutrients, 17(7), 1253. https://doi.org/10.3390/nu17071253






