Caffeine Combined with Excitatory Neuromodulation Based on Transcranial Direct Current Stimulation (tDCS) Enhances Performance in a Time-Trial CrossFit® Workout: A Randomized, Placebo-Controlled, Double-Blind Study
Abstract
:1. Introduction
2. Methods
2.1. Experimental Approach
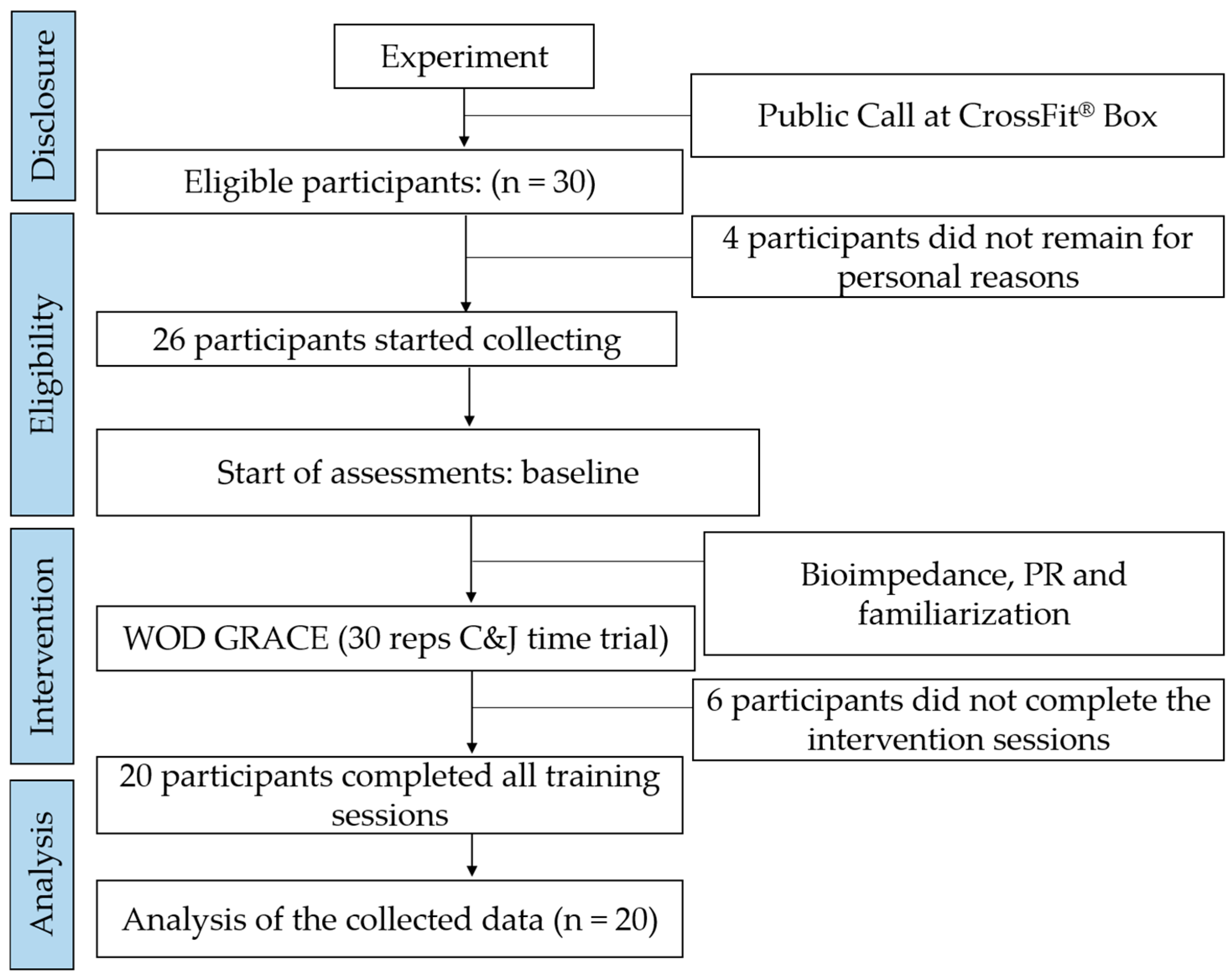
2.2. Participants
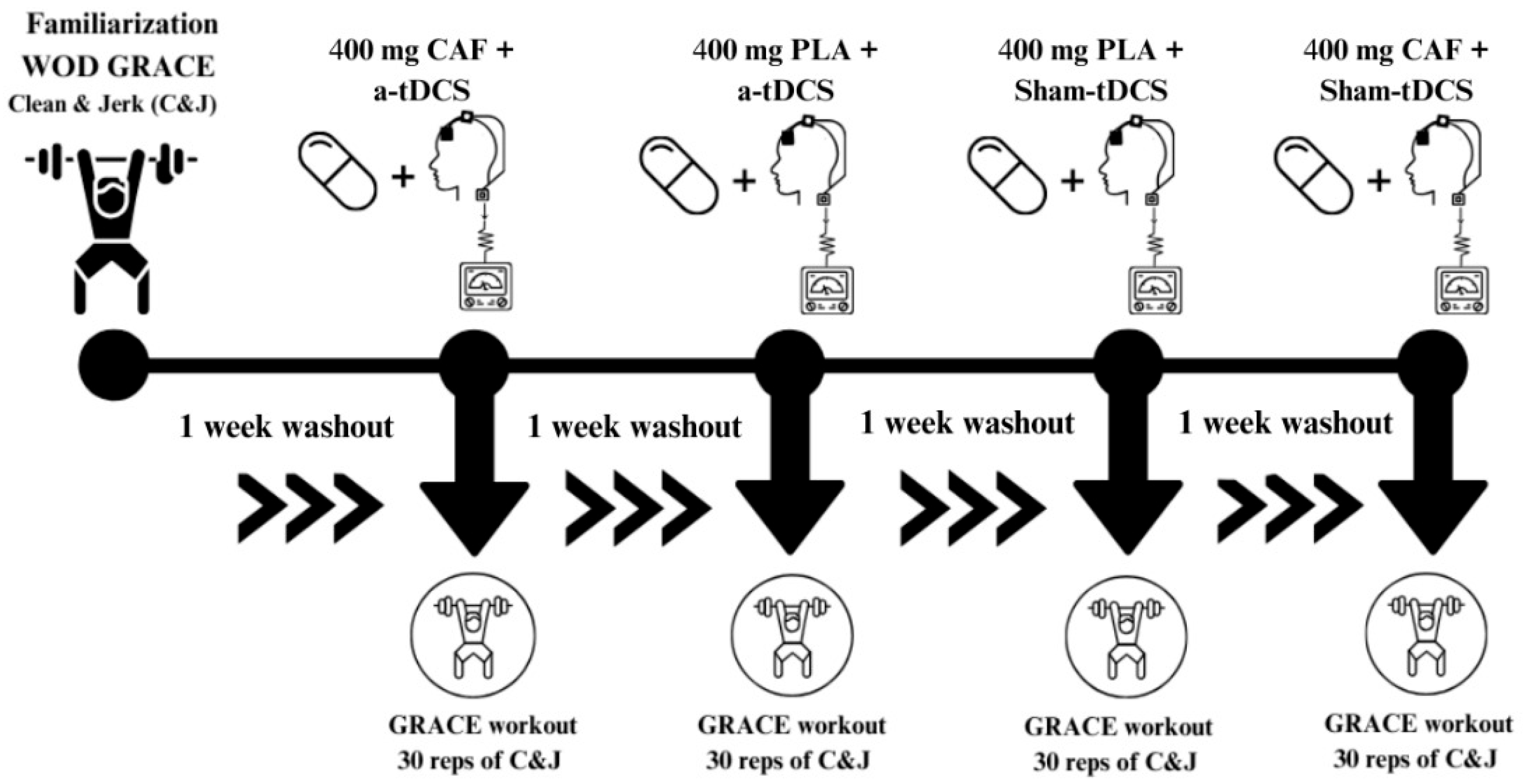
2.3. Study Design
2.4. Outcome Variables
2.5. Procedures
2.5.1. Bioimpedance
2.5.2. Personal Record
2.5.3. Participant Categorization—RX (as Prescribed)
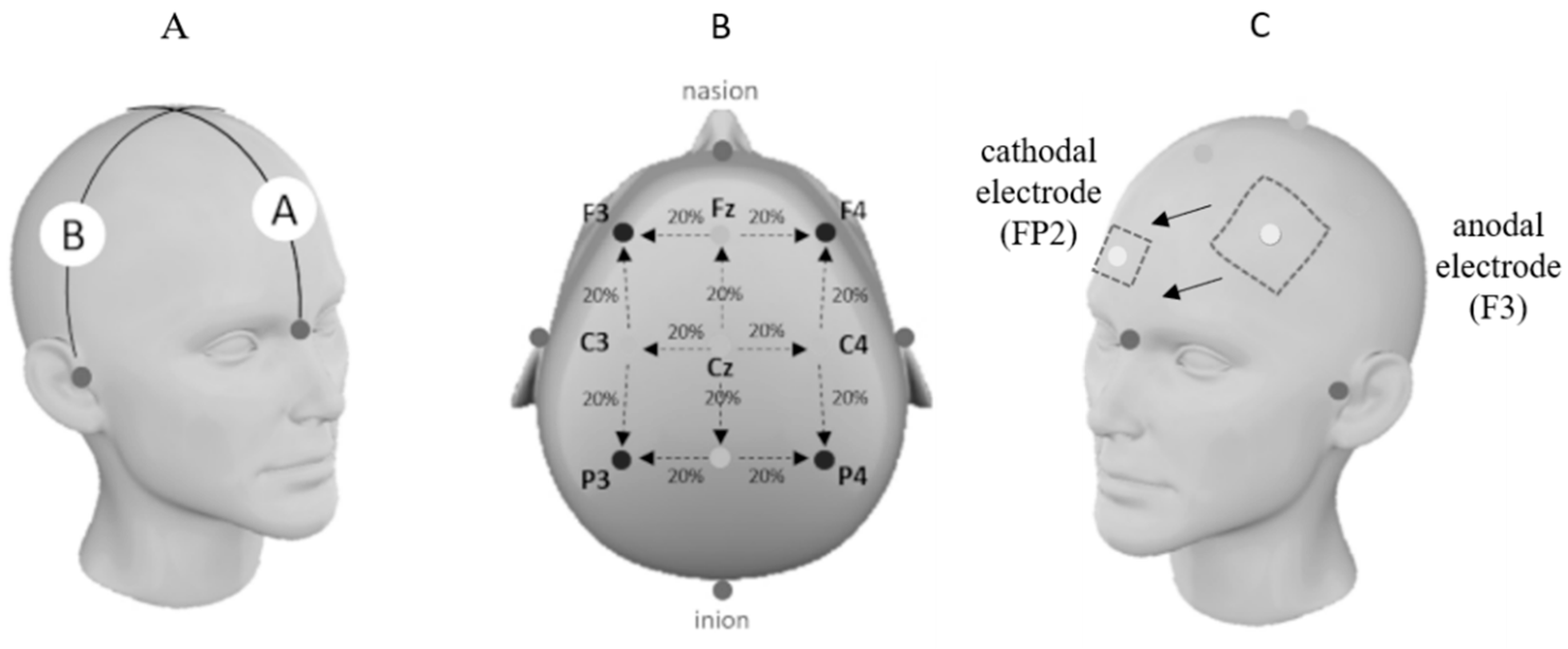
2.5.4. Transcranial Direct Current Stimulation (tDCS) Protocol
2.5.5. Caffeine (CAF) Supplementation
2.5.6. Experimental Protocol for C&J
2.5.7. Rating of Perceived Exertion (RPE) Scale
2.6. Allometry
2.7. Study Size
2.8. Randomization Process
2.9. Risk of Bias, Blinding, and Data Processing
2.10. Statistical Analysis
3. Results
3.1. General Information
3.2. Physical Performance
3.3. Participant Categorization and Allometry
3.4. Caffeine (CAF) Administration
3.5. Primary Outcome
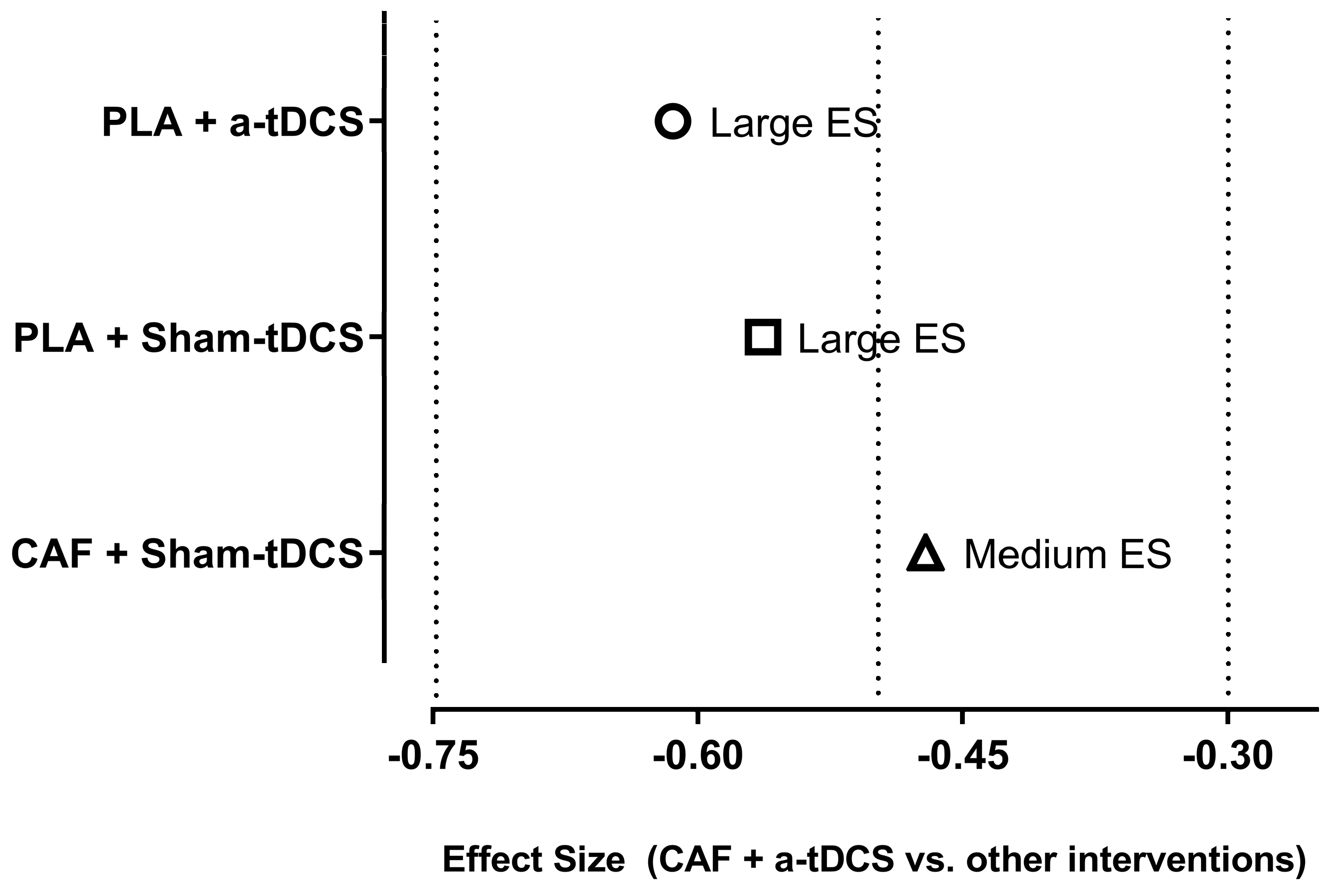
3.5.1. Comparison Between the Different Interventions
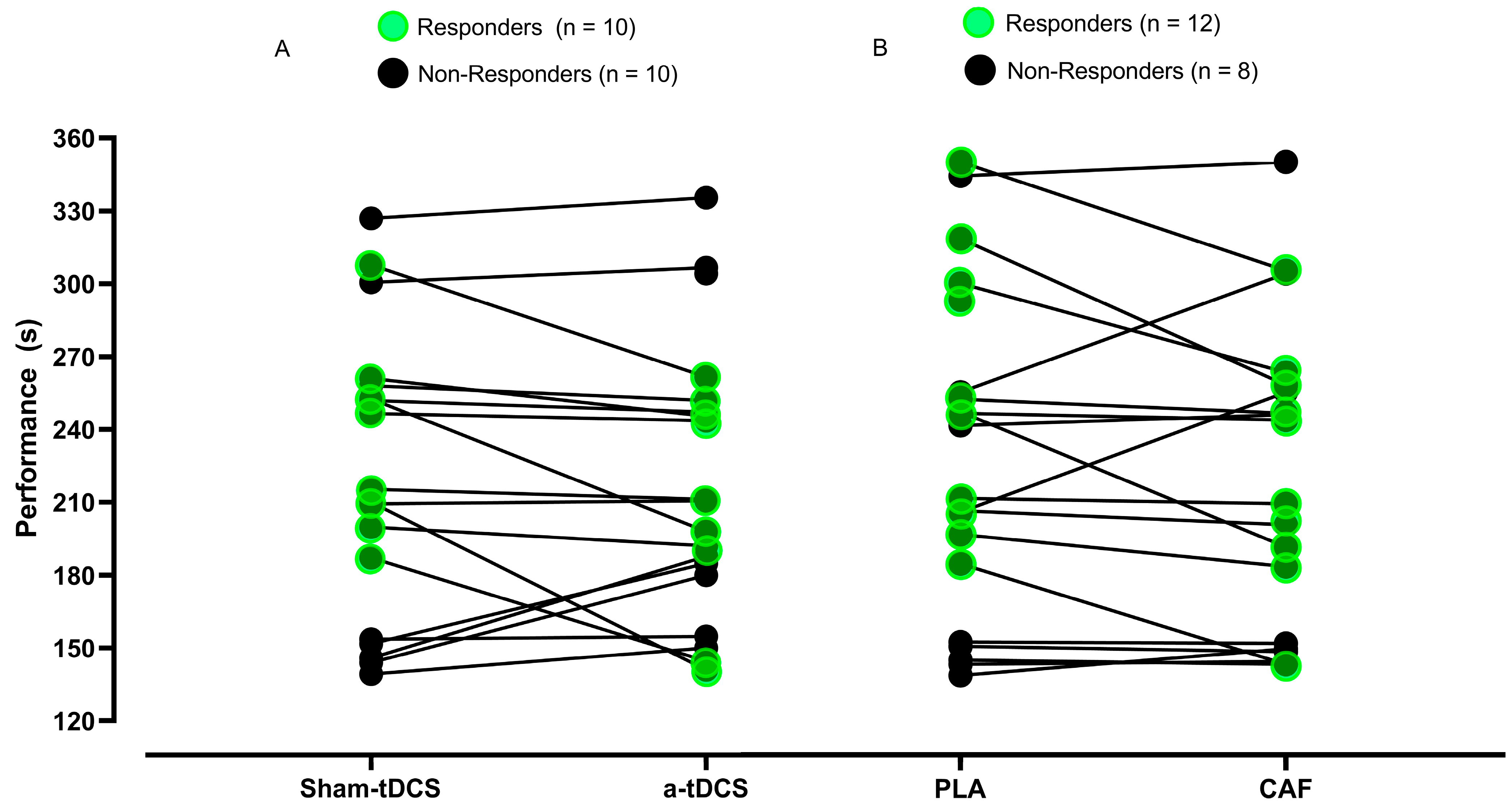
3.5.2. Responders and Non-Responders to the tDCS and CAF Interventions
3.6. Secondary Outcome
3.7. Relationship Between the Administered Caffeine (CAF) Dose and Performance
3.8. Perceptions and Adverse Effects
4. Discussion
4.1. Primary Outcome
4.2. Secondary Outcome
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CAF | Caffeine |
| tDCS | Transcranial Direct Current Stimulation |
| PLA | Placebo |
| PR | Personal Record |
| RPE | Rating of Perceived Exertion |
| RX | Prescribed Exercise Standard in CrossFit™ |
| DLPFC | Dorsolateral Prefrontal Cortex |
| VO2max | Maximal Oxygen Consumption |
| M1 | Primary Motor Cortex |
| BIA | Bioelectrical Impedance Analysis |
| BM | Body Mass |
| BMI | Body Mass Index |
| CAD | Coronary Artery Disease |
| NaHCO3 | Sodium Bicarbonate |
| ES | Effect Size |
| CI95% | 95% Confidence Interval |
| ACSM | American College of Sports Medicine |
| SPSS | Statistical Package for the Social Sciences |
| G*Power | Statistical Power Analysis Software |
| ICMJE | International Committee of Medical Journal Editors |
| STROBE | Strengthening the Reporting of Observational Studies in Epidemiology |
References
- Angius, L.; Hopker, J.; Mauger, A.R. The Ergogenic Effects of Transcranial Direct Current Stimulation on Exercise Performance. Front. Physiol. 2017, 8, 90. [Google Scholar] [CrossRef] [PubMed]
- Doherty, M.; Smith, P.M. Effects of caffeine ingestion on rating of perceived exertion during and after exercise: A meta-analysis. Scand. J. Med. Sci. Sports 2005, 15, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Grgic, J. Effects of Caffeine on Resistance Exercise: A Review of Recent Research. Sports Med. 2021, 51, 2281–2298. [Google Scholar] [CrossRef] [PubMed]
- Lattari, E.; Vieira, L.A.F.; Oliveira, B.R.R.; Unal, G.; Bikson, M.; de Mello Pedreiro, R.C.; Marques Neto, S.R.; Machado, S.; Maranhao-Neto, G.A. Effects of Transcranial Direct Current Stimulation With Caffeine Intake on Muscular Strength and Perceived Exertion. J. Strength Cond. Res. 2019, 33, 1237–1243. [Google Scholar] [CrossRef]
- Inacio, P.A.; Siqueira, J.P.; Sales, M.M.; Silva, W.A.; Leonardo, P.S.; Portugal, E.M.; Aprigliano, V.; Lopes-Martins, R.A.; Machado, S.; Sá Filho, A.S. Neuromodulación aplicada en la corteza prefrontal dorsolateral aumenta el rango de movimiento de la cadera: Un estudio controlado aleatorizado. Cuad. Psicol. Deporte 2025, 25, 242–256. [Google Scholar] [CrossRef]
- Lattari, E.; Andrade, M.L.; Filho, A.S.; Moura, A.M.; Neto, G.M.; Silva, J.G.; Rocha, N.B.; Yuan, T.F.; Arias-Carrion, O.; Machado, S. Can Transcranial Direct Current Stimulation Improve the Resistance Strength and Decrease the Rating Perceived Scale in Recreational Weight-Training Experience? J. Strength Cond. Res. 2016, 30, 3381–3387. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, X.; Nitsche, M.A.; Vicario, C.M.; Qi, F. Does a single session of transcranial direct current stimulation enhance both physical and psychological performance in national- or international-level athletes? A systematic review. Front. Physiol. 2024, 15, 1365530. [Google Scholar] [CrossRef]
- Vieira, L.A.F.; Lattari, E.; de Jesus Abreu, M.A.; Rodrigues, G.M.; Viana, B.; Machado, S.; Oliveira, B.R.R.; Maranhao Neto, G.A. Transcranial Direct Current Stimulation (tDCS) Improves Back-Squat Performance in Intermediate Resistance-Training Men. Res. Q. Exerc. Sport 2022, 93, 210–218. [Google Scholar] [CrossRef]
- Baharlouei, H.; Goosheh, M.; Moore, M.; Ramezani Ahmadi, A.H.; Yassin, M.; Jaberzadeh, S. The effect of transcranial direct current stimulation on rating of perceived exertion: A systematic review of the literature. Psychophysiology 2024, 61, e14520. [Google Scholar] [CrossRef]
- Machado, S.; Jansen, P.; Almeida, V.; Veldema, J. Is tDCS an Adjunct Ergogenic Resource for Improving Muscular Strength and Endurance Performance? A Systematic Review. Front. Psychol. 2019, 10, 1127. [Google Scholar] [CrossRef]
- Schlegel, P. CrossFit® Training Strategies from the Perspective of Concurrent Training: A Systematic Review. J. Sports Sci. Med. 2020, 19, 670–680. [Google Scholar] [PubMed]
- Rios, M.; Becker, K.M.; Cardoso, F.; Pyne, D.B.; Reis, V.M.; Moreira-Gonçalves, D.; Fernandes, R.J. Assessment of Cardiorespiratory and Metabolic Contributions in an Extreme Intensity CrossFit® Benchmark Workout. Sensors 2024, 24, 513. [Google Scholar] [CrossRef] [PubMed]
- Meier, N.; Rabel, S.; Schmidt, A. Determination of a CrossFit® Benchmark Performance Profile. Sports 2021, 9, 80. [Google Scholar] [CrossRef] [PubMed]
- De Souza, R.A.S.; da Silva, A.G.; de Souza, M.F.; Souza, L.K.F.; Roschel, H.; da Silva, S.F.; Saunders, B. A Systematic Review of CrossFit® Workouts and Dietary and Supplementation Interventions to Guide Nutritional Strategies and Future Research in CrossFit®. Int. J. Sport Nutr. Exerc. Metab. 2021, 31, 187–205. [Google Scholar] [CrossRef]
- Ziyaiyan, A.; Shabkhiz, F.; Hofmeister, M. Supplementation of caffeine and sodium bicarbonate together could not improve performance and performance-related factors in CrossFit participants: A randomized, double-blind, placebo-controlled study. J. Int. Soc. Sports Nutr. 2023, 20, 2206390. [Google Scholar] [CrossRef]
- Albernaz, T.; Sales, M.; Inacio, P.A.; Chiappa, G.; Barcelos, R.; Bezerra, M.; Oliveira-Silva, I.; Portugal, E.; Lopes-Martins, R.; Tavares, I.; et al. El “récord personal” estima el rendimiento de fuerza y resistencia en el entrenamiento Clean & Jerk: Un análisis del Crossfit® GRACE Benchmark. Cuad. Psicol. Deporte 2024, 24, 253–264. [Google Scholar]
- Bilondi, H.T.; Valipour, H.; Khoshro, S.; Jamilian, P.; Ostadrahimi, A.; Zarezadeh, M. The effect of caffeine supplementation on muscular strength and endurance: A meta-analysis of meta-analyses. Heliyon 2024, 10, e35025. [Google Scholar] [CrossRef]
- Berjisian, E.; Naderi, A.; Mojtahedi, S.; Grgic, J.; Ghahramani, M.H.; Karayigit, R.; Forbes, J.L.; Amaro-Gahete, F.J.; Forbes, S.C. Are Caffeine’s Effects on Resistance Exercise and Jumping Performance Moderated by Training Status? Nutrients 2022, 14, 4840. [Google Scholar] [CrossRef]
- Caetano, M.L.; Souza, M.L.R.; Loureiro, L.L.; Capistrano Junior, L.M. The Effects of Acute Caffeine Supplementation on Performance in Trained CrossFit Athletes: A Randomized, Double-Blind, Placebo-Controlled, and Crossover Trial. Sci. Sports 2023, 38, 701–707. [Google Scholar]
- McIntire, L.K.; McKinley, R.A.; Nelson, J.M.; Goodyear, C. Transcranial direct current stimulation versus caffeine as a fatigue countermeasure. Brain Stimul. 2017, 10, 1070–1078. [Google Scholar] [CrossRef]
- Drazen, J.M.; Van der Weyden, M.B.; Sahni, P.; Rosenberg, J.; Marusic, A.; Laine, C.; Kotzin, S.; Horton, R.; Hebert, P.C.; Haug, C.; et al. Uniform format for disclosure of competing interests in ICMJE journals. JAMA 2010, 303, 75–76. [Google Scholar] [CrossRef] [PubMed]
- Malta, M.; Cardoso, L.O.; Bastos, F.I.; Magnanini, M.M.; Silva, C.M. STROBE initiative: Guidelines on reporting observational studies. Rev. Saude Publica 2010, 44, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Santos Junior, E.R.; de Salles, B.F.; Dias, I.; Ribeiro, A.S.; Simão, R.; Willardson, J.M. Classification and Determination Model of Resistance Training Status. Strength Cond. J. 2021, 43, 77–86. [Google Scholar] [CrossRef]
- ACSM. ACSM’s Guidelines for Exercise Testing and Prescription; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; Volume 9 Ed. [Google Scholar]
- Ellis, K.J.; Bell, S.J.; Chertow, G.M.; Chumlea, W.C.; Knox, T.A.; Kotler, D.P.; Lukaski, H.C.; Schoeller, D.A. Bioelectrical impedance methods in clinical research: A follow-up to the NIH Technology Assessment Conference. Nutrition 1999, 15, 874–880. [Google Scholar] [CrossRef]
- Goldstein, E.R.; Ziegenfuss, T.; Kalman, D.; Kreider, R.; Campbell, B.; Wilborn, C.; Taylor, L.; Willoughby, D.; Stout, J.; Graves, B.S.; et al. International society of sports nutrition position stand: Caffeine and performance. J. Int. Soc. Sports Nutr. 2010, 7, 5. [Google Scholar] [CrossRef]
- Foster, C.; Florhaug, J.A.; Franklin, J.; Gottschall, L.; Hrovatin, L.A.; Parker, S.; Doleshal, P.; Dodge, C. A new approach to monitoring exercise training. J. Strength Cond. Res. 2001, 15, 109–115. [Google Scholar]
- Chamsi-Pasha, M.; Albar, M.A.; Chamsi-Pasha, H. Minimizing nocebo effect: Pragmatic approach. Avicenna J. Med. 2017, 7, 139–143. [Google Scholar] [CrossRef]
- Bartels, D.J.P.; van Laarhoven, A.I.M.; Stroo, M.; Hijne, K.; Peerdeman, K.J.; Donders, R.T.; van de Kerkhof, P.C.M.; Evers, A.W.M. Minimizing nocebo effects by conditioning with verbal suggestion: A randomized clinical trial in healthy humans. PLoS ONE 2017, 12, e0182959. [Google Scholar] [CrossRef]
- Claudino, J.G.; Gabbett, T.J.; Bourgeois, F.; Souza, H.S.; Miranda, R.C.; Mezencio, B.; Soncin, R.; Cardoso Filho, C.A.; Bottaro, M.; Hernandez, A.J.; et al. CrossFit Overview: Systematic Review and Meta-analysis. Sports Med. Open 2018, 4, 11. [Google Scholar] [CrossRef]
- Feito, Y.; Heinrich, K.M.; Butcher, S.J.; Poston, W.S.C. High-Intensity Functional Training (HIFT): Definition and Research Implications for Improved Fitness. Sports 2018, 6, 76. [Google Scholar] [CrossRef]
- Kalmar, J.M.; Cafarelli, E. Caffeine: A valuable tool to study central fatigue in humans? Exerc. Sport Sci. Rev. 2004, 32, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Cristina-Souza, G.; Santos, P.S.; Santos-Mariano, A.C.; Coelho, D.B.; Rodacki, A.; De-Oliveira, F.R.; Bishop, D.J.; Bertuzzi, R.; Lima-Silva, A.E. Caffeine Increases Endurance Performance via Changes in Neural and Muscular Determinants of Performance Fatigability. Med. Sci. Sports Exerc. 2022, 54, 1591–1603. [Google Scholar] [CrossRef] [PubMed]
- Monturil, I.L.C.; Sales, M.M.; Inacio, P.A.; Aprigliano, V.; Leonardo, P.S.; Oliveira-Silva, I.; Cunha, R.M.; Chiappa, G.R.; Fajemiroye, J.O.; Vieira, R.P.; et al. Not All Forms of Exercise Lead to Positive Affect: A Comparative Monitoring Between an Imposed and Self-Adjusted Prescription in Recreational Runners—A Cross-Sectional Randomized Controlled Study. Appl. Sci. 2025, 15, 1549. [Google Scholar] [CrossRef]
- Fogaca, L.J.; Santos, S.L.; Soares, R.C.; Gentil, P.; Naves, J.P.; Dos Santos, W.D.; Pimentel, G.D.; Bottaro, M.; Mota, J.F. Effect of caffeine supplementation on exercise performance, power, markers of muscle damage, and perceived exertion in trained CrossFit men: A randomized, double-blind, placebo-controlled crossover trial. J. Sports Med. Phys. Fit. 2020, 60, 181–188. [Google Scholar] [CrossRef]
- Teymoori, H.; Amiri, E.; Tahmasebi, W.; Hoseini, R.; Grospretre, S.; Machado, D. Effect of tDCS targeting the M1 or left DLPFC on physical performance, psychophysiological responses, and cognitive function in repeated all-out cycling: A randomized controlled trial. J. Neuroeng. Rehabil. 2023, 20, 97. [Google Scholar] [CrossRef]
- Angius, L.; Santarnecchi, E.; Pascual-Leone, A.; Marcora, S.M. Transcranial Direct Current Stimulation over the Left Dorsolateral Prefrontal Cortex Improves Inhibitory Control and Endurance Performance in Healthy Individuals. Neuroscience 2019, 419, 34–45. [Google Scholar] [CrossRef]
- Lattari, E.; Rosa Filho, B.J.; Fonseca Junior, S.J.; Murillo-Rodriguez, E.; Rocha, N.; Machado, S.; Maranhao Neto, G.A. Effects on Volume Load and Ratings of Perceived Exertion in Individuals’ Advanced Weight Training After Transcranial Direct Current Stimulation. J. Strength Cond. Res. 2020, 34, 89–96. [Google Scholar] [CrossRef]
- Kleemeyer, M.M.; Polk, T.A.; Schaefer, S.; Bodammer, N.C.; Brechtel, L.; Lindenberger, U. Exercise-Induced Fitness Changes Correlate with Changes in Neural Specificity in Older Adults. Front. Hum. Neurosci. 2017, 11, 123. [Google Scholar] [CrossRef]
- Warren, G.L.; Park, N.D.; Maresca, R.D.; McKibans, K.I.; Millard-Stafford, M.L. Effect of caffeine ingestion on muscular strength and endurance: A meta-analysis. Med. Sci. Sports Exerc. 2010, 42, 1375–1387. [Google Scholar] [CrossRef]
- Beedie, C.J.; Stuart, E.M.; Coleman, D.A.; Foad, A.J. Placebo effects of caffeine on cycling performance. Med. Sci. Sports Exerc. 2006, 38, 2159–2164. [Google Scholar] [CrossRef]
- Okano, A.H.; Fontes, E.B.; Montenegro, R.A.; Farinatti, P.d.T.V.; Cyrino, E.S.; Li, L.M.; Bikson, M.; Noakes, T.D. Brain stimulation modulates the autonomic nervous system, rating of perceived exertion and performance during maximal exercise. Br. J. Sports Med. 2015, 49, 1213–1218. [Google Scholar] [CrossRef] [PubMed]
- Lattari, E.; de Oliveira, B.S.; Oliveira, B.R.R.; de Mello Pedreiro, R.C.; Machado, S.; Neto, G.A.M. Effects of transcranial direct current stimulation on time limit and ratings of perceived exertion in physically active women. Neurosci. Lett. 2018, 662, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Alix-Fages, C.; Romero-Arenas, S.; Calderon-Nadal, G.; Jerez-Martinez, A.; Pareja-Blanco, F.; Colomer-Poveda, D.; Marquez, G.; Garcia-Ramos, A. Transcranial direct current stimulation and repeated sprint ability: No effect on sprint performance or ratings of perceived exertion. Eur. J. Sport Sci. 2022, 22, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Codella, R.; Alongi, R.; Filipas, L.; Luzi, L. Ergogenic Effects of Bihemispheric Transcranial Direct Current Stimulation on Fitness: A Randomized Cross-over Trial. Int. J. Sports Med. 2021, 42, 66–73. [Google Scholar] [CrossRef]
- Chen, C.H.; Chen, Y.C.; Jiang, R.S.; Lo, L.Y.; Wang, I.L.; Chiu, C.H. Transcranial Direct Current Stimulation Decreases the Decline of Speed during Repeated Sprinting in Basketball Athletes. Int. J. Environ. Res. Public Health 2021, 18, 6967. [Google Scholar] [CrossRef]
- Gallo, G.; Geda, E.; Codella, R.; Faelli, E.; Panasci, M.; Ranieri, L.E.; Pollastri, L.; Brighenti, S.; Molino, L.; Riba, U.; et al. Effects of Bilateral Dorsolateral Prefrontal Cortex High-Definition Transcranial Direct-Current Stimulation on Physiological and Performance Responses at Severe-Intensity Exercise Domain in Elite Road Cyclists. Int. J. Sports Physiol. Perform. 2022, 17, 1085–1093. [Google Scholar] [CrossRef]
- Garner, C.T.; Dykstra, R.M.; Hanson, N.J.; Miller, M.G. Transcranial Direct Current Stimulation with the Halo Sport Does Not Improve Performance on a Three-Minute, High Intensity Cycling Test. Int. J. Exerc. Sci. 2021, 14, 962–970. [Google Scholar] [CrossRef]
- Moreira, A.; Moscaleski, L.; Machado, D.G.d.S.; Bikson, M.; Unal, G.; Bradley, P.S.; Cevada, T.; da Silva, F.T.G.; Baptista, A.F.; Morya, E.; et al. Transcranial direct current stimulation during a prolonged cognitive task: The effect on cognitive and shooting performances in professional female basketball players. Ergonomics 2023, 66, 492–505. [Google Scholar] [CrossRef]
- Mesquita, P.H.C.; Lage, G.M.; Franchini, E.; Romano-Silva, M.A.; Albuquerque, M.R. Bi-hemispheric anodal transcranial direct current stimulation worsens taekwondo-related performance. Hum. Mov. Sci. 2019, 66, 578–586. [Google Scholar] [CrossRef]
- de Sousa Fortes, L.; Mazini-Filho, M.; Lima-Júnior, D.; Machado, D.G.; Albuquerque, M.R.; Fonseca, F.S.; Ferreira, M.E. Transcranial Stimulation Improves Volume and Perceived Exertion but does not Change Power. Int. J. Sports Med. 2021, 42, 630–637. [Google Scholar]
- Delleli, S.; Ouergui, I.; Messaoudi, H.; Trabelsi, K.; Ammar, A.; Glenn, J.M.; Chtourou, H. Acute Effects of Caffeine Supplementation on Physical Performance, Physiological Responses, Perceived Exertion, and Technical-Tactical Skills in Combat Sports: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 2996. [Google Scholar] [CrossRef] [PubMed]
- Butcher, S.J.; Neyedly, T.J.; Horvey, K.J.; Benko, C.R. Do physiological measures predict selected CrossFit® benchmark performance? Open Access J. Sports Med. 2015, 6, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Meier, N.; Schlie, J.; Schmidt, A. CrossFit®: ‘Unknowable’ or Predictable?-A Systematic Review on Predictors of CrossFit® Performance. Sports 2023, 11, 112. [Google Scholar] [CrossRef] [PubMed]



| Age | Exp | Body Mass | Height | BMI | Fat | Lean Mass | Total Water | |
|---|---|---|---|---|---|---|---|---|
| (Year) | (Months) | (kg) | (cm) | kg/m2 | (%) | (kg) | (L) | |
| Mean | 31.1 | 49.0 | 77.3 | 1.73 | 26.0 | 17.0 | 36.1 | 46.2 |
| SD | 5.0 | 30.0 | 12.3 | 0.08 | 2.9 | 8.3 | 5.7 | 7.0 |
| CI95% | 28.5–33.7 | 33.6–64.4 | 70.9–83.6 | 1.68–1.77 | 24.4–27.4 | 13.5–22.1 | 33.1–39.0 | 42.6–49.8 |
| Parameters | Percentage (%) |
|---|---|
| Itching | 70.0% |
| Pain | 5.5% |
| Burning Sensation | 50.0% |
| Warmth | 5.56% |
| Tingling | 100.0% |
| Fatigue | 0.0% |
| Other | 0.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sá Filho, A.S.; Albernaz-Silva, T.; Inacio, P.A.; Aprigliano, V.; Oliveira-Silva, I.; Chiappa, G.R.; Vieira, R.P.; de Aguiar, A.S.N.; Cunha, R.M.; Fajemiroye, J.O.; et al. Caffeine Combined with Excitatory Neuromodulation Based on Transcranial Direct Current Stimulation (tDCS) Enhances Performance in a Time-Trial CrossFit® Workout: A Randomized, Placebo-Controlled, Double-Blind Study. Nutrients 2025, 17, 1261. https://doi.org/10.3390/nu17071261
Sá Filho AS, Albernaz-Silva T, Inacio PA, Aprigliano V, Oliveira-Silva I, Chiappa GR, Vieira RP, de Aguiar ASN, Cunha RM, Fajemiroye JO, et al. Caffeine Combined with Excitatory Neuromodulation Based on Transcranial Direct Current Stimulation (tDCS) Enhances Performance in a Time-Trial CrossFit® Workout: A Randomized, Placebo-Controlled, Double-Blind Study. Nutrients. 2025; 17(7):1261. https://doi.org/10.3390/nu17071261
Chicago/Turabian StyleSá Filho, Alberto Souza, Thiago Albernaz-Silva, Pedro Augusto Inacio, Vicente Aprigliano, Iransé Oliveira-Silva, Gaspar R. Chiappa, Rodolfo P. Vieira, Antônio Sérgio Nakao de Aguiar, Raphael Martins Cunha, James Oluwagbamigbe Fajemiroye, and et al. 2025. "Caffeine Combined with Excitatory Neuromodulation Based on Transcranial Direct Current Stimulation (tDCS) Enhances Performance in a Time-Trial CrossFit® Workout: A Randomized, Placebo-Controlled, Double-Blind Study" Nutrients 17, no. 7: 1261. https://doi.org/10.3390/nu17071261
APA StyleSá Filho, A. S., Albernaz-Silva, T., Inacio, P. A., Aprigliano, V., Oliveira-Silva, I., Chiappa, G. R., Vieira, R. P., de Aguiar, A. S. N., Cunha, R. M., Fajemiroye, J. O., & Sales, M. M. (2025). Caffeine Combined with Excitatory Neuromodulation Based on Transcranial Direct Current Stimulation (tDCS) Enhances Performance in a Time-Trial CrossFit® Workout: A Randomized, Placebo-Controlled, Double-Blind Study. Nutrients, 17(7), 1261. https://doi.org/10.3390/nu17071261










