Investigating the Therapeutic Potential of the Ketogenic Diet in Modulating Neurodegenerative Pathophysiology: An Interdisciplinary Approach
Abstract
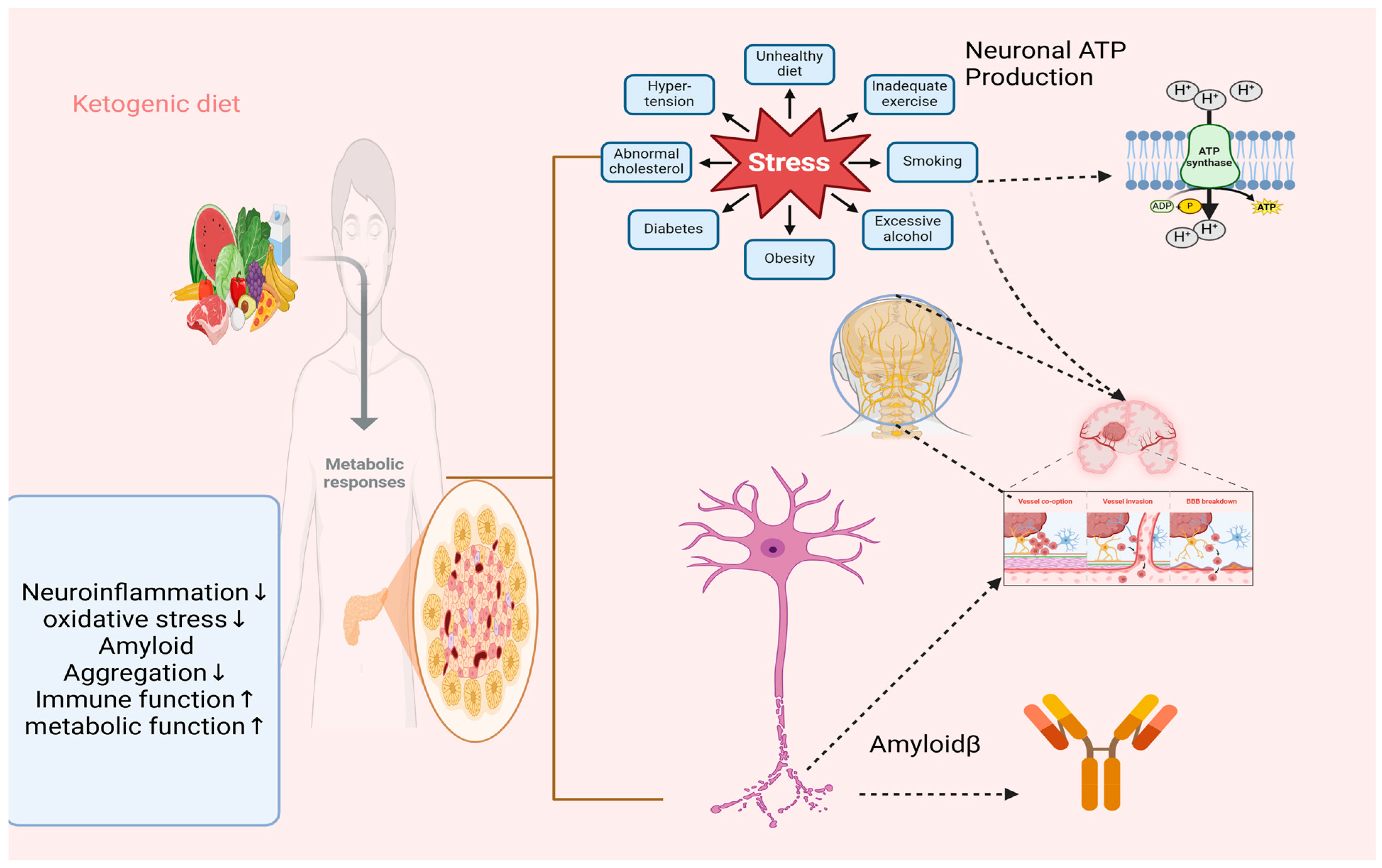
:1. Introduction
2. A Detailed Exploration of How Ketosis, Metabolic Shifts, and Hormonal Regulation Contribute to Therapeutic Effects
2.1. Induction of Ketosis and the Shift in Energy Sources: Mechanisms and Implications
2.2. The Composition and Mechanism of the Ketogenic Diet
2.3. Investigation of the Neuroprotective Effects of Ketone Bodies
2.4. The Historical Context and Mechanism of Action of the Subject
2.5. A Historical Overview of the Ketogenic Diet and Its Therapeutic Efficacy
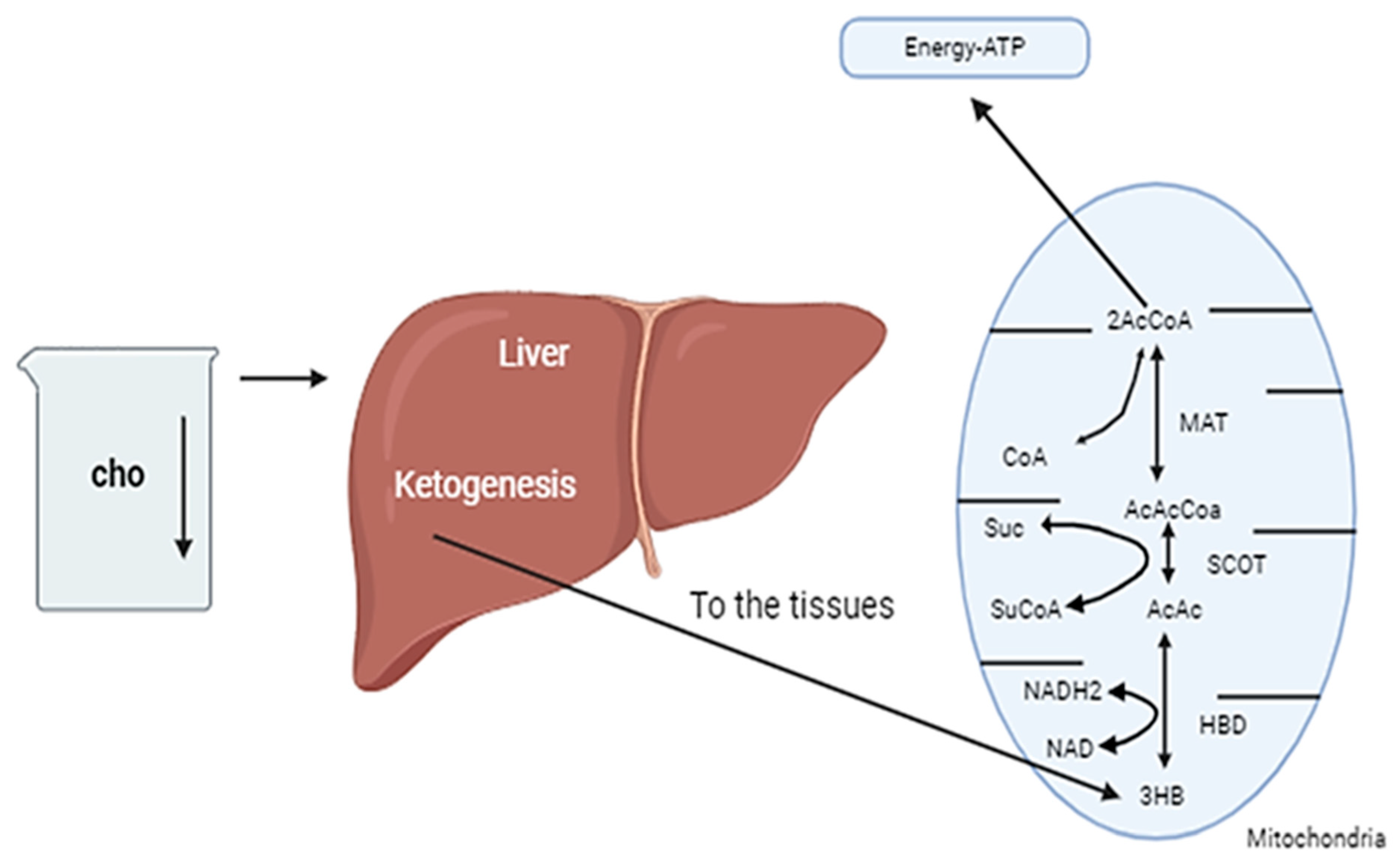
2.6. The Production of Ketone Bodies and Metabolic Adaptation
2.7. Ketone Bodies and Their Role in Energy Metabolism Within the Central Nervous System (CNS)
3. The Efficacy of the Ketogenic Diet in the Treatment of Neurodegenerative Diseases
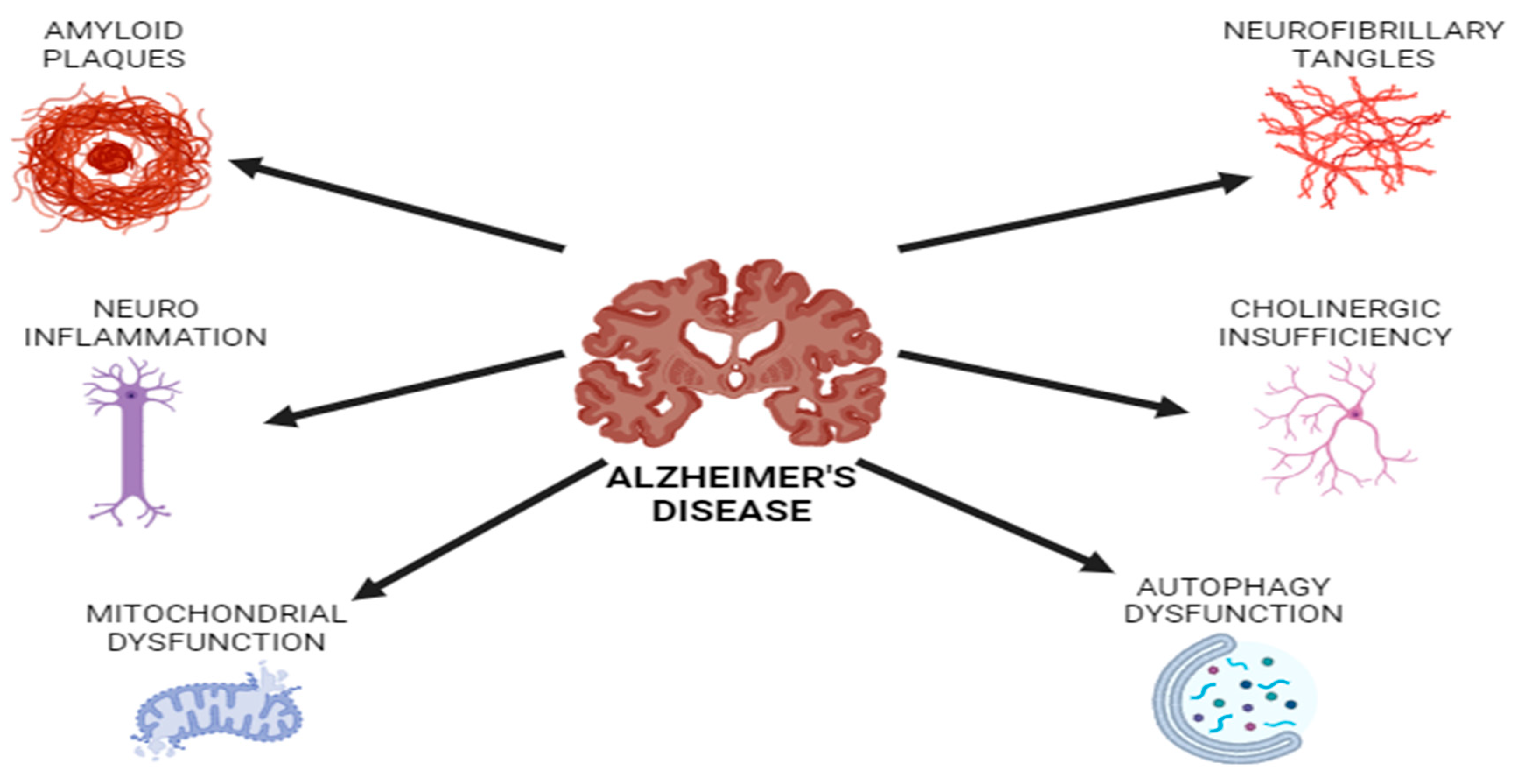
3.1. Alzheimer’s Disease
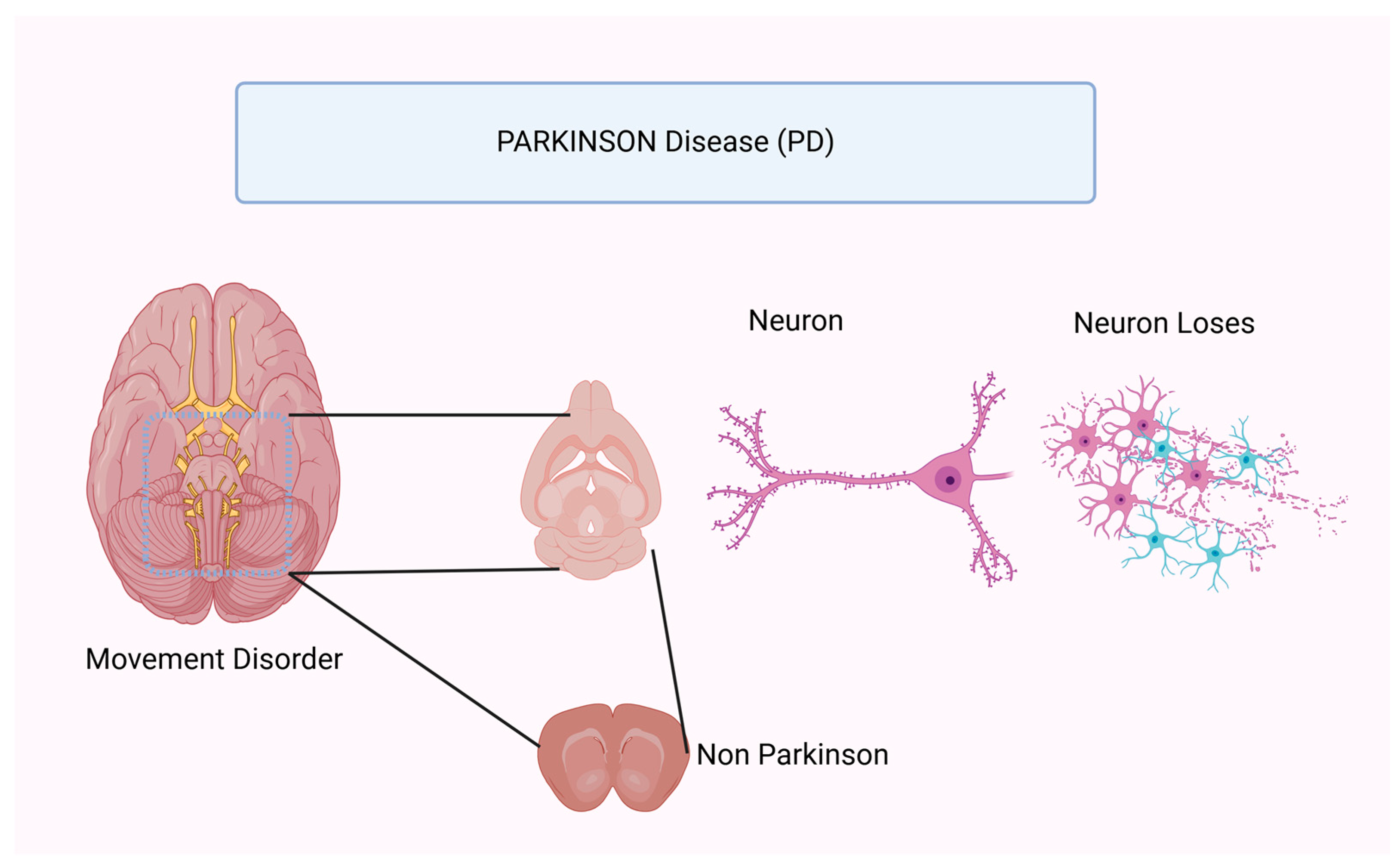
3.2. Parkinson’s Disease (PD)
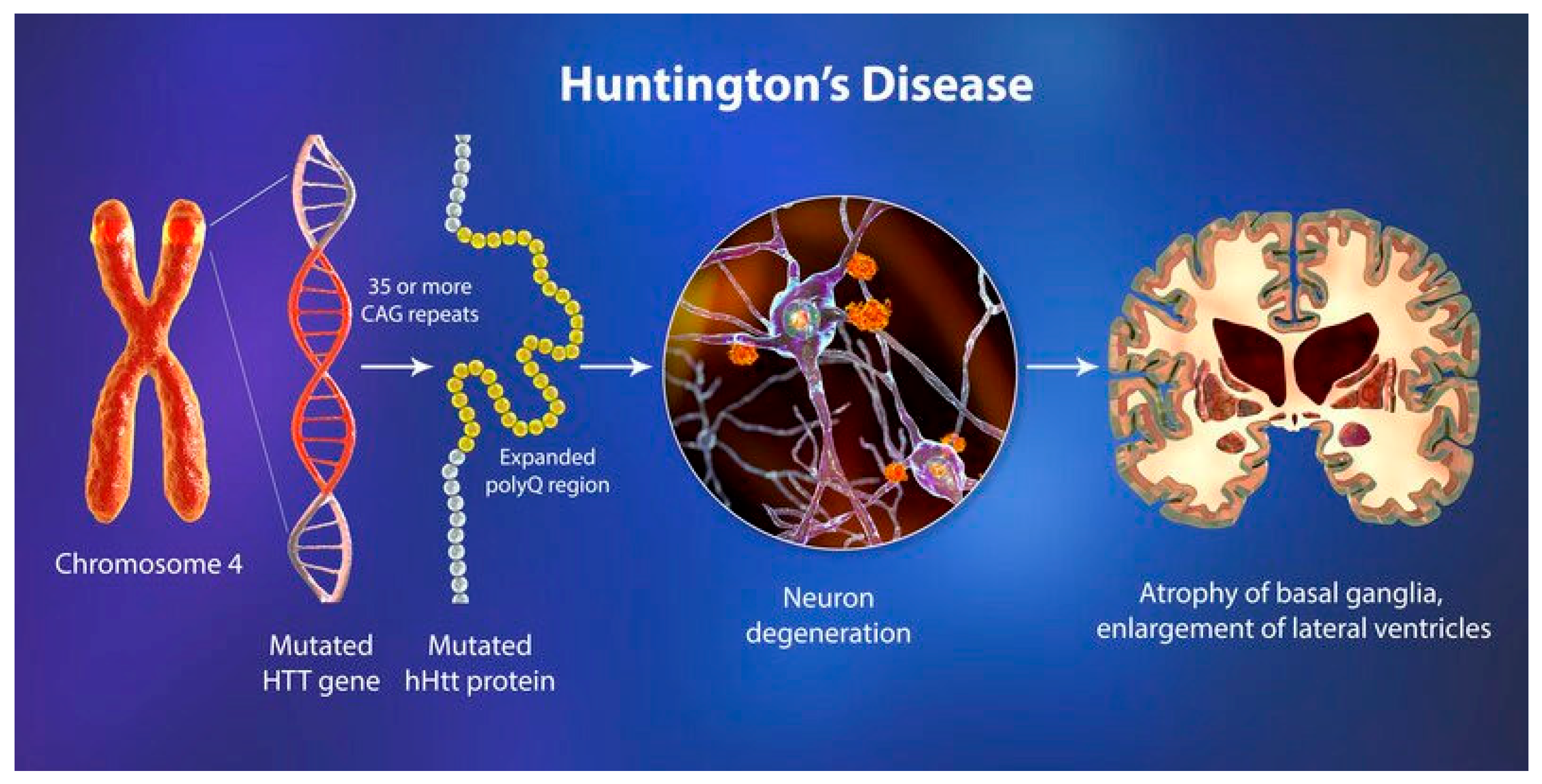
3.3. Huntington’s Disease
3.4. Multiple Sclerosis (MS)
3.5. Migraine
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, T.-L.; Wang, X.-H.; Jiang, W.; Zhang, S.-Y.; Nie, B.-B.; Zheng, Y.; Yan, F.; Lei, J.-F. Hypothermia selectively protects the anterior forebrain mesocircuit during global cerebral ischemia. Neural Regen. Res. 2022, 17, 1512–1517. [Google Scholar] [CrossRef]
- Sandrelli, F.; Bisaglia, M. Molecular and physiological determinants of amyotrophic lateral sclerosis: What the DJ-1 protein teaches us. Int. J. Mol. Sci. 2023, 24, 7674. [Google Scholar] [CrossRef]
- Koppel, S.J.; Pei, D.; Wilkins, H.M.; Weidling, I.W.; Wang, X.; Menta, B.W.; Perez-Ortiz, J.; Kalani, A.; Manley, S.; Novikova, L.; et al. A ketogenic diet differentially affects neuron and astrocyte transcription. J. Neurochem. 2021, 157, 1930–1945. [Google Scholar]
- Kawka, J.; Baranowska, A.; Baranowska, K.; Czyżewski, F.; Filipek, K.; Muciek, M.; Mrugała, S.; Mrugała, W.; Skierkowski, B.; Zalewska, N. The impact of eating disorders on glaucoma. J. Educ. Health Sport 2024, 66, 49959. [Google Scholar]
- Van der Auwera, I.; Wera, S.; Van Leuven, F.; Henderson, S.T. A ketogenic diet reduces amyloid beta 40 and 42 in a mouse model of Alzheimer’s disease. Nutr. Metab. 2005, 2, 28. [Google Scholar]
- Rawat, K.; Singh, N.; Kumari, P.; Saha, L. A review on preventive role of ketogenic diet (KD) in CNS disorders from the gut microbiota perspective. Rev. Neurosci. 2021, 32, 143–157. [Google Scholar] [CrossRef]
- Pinto, A.; Bonucci, A.; Maggi, E.; Corsi, M.; Businaro, R. Anti-oxidant and anti-inflammatory activity of ketogenic diet: New perspectives for neuroprotection in Alzheimer’s disease. Antioxidants 2018, 7, 63. [Google Scholar] [CrossRef]
- Lilamand, M.; Porte, B.; Cognat, E.; Hugon, J.; Mouton-Liger, F.; Paquet, C. Are ketogenic diets promising for Alzheimer’s disease? A translational review. Alzheimer’s Res. Ther. 2020, 12, 1–10. [Google Scholar] [CrossRef]
- Kovács, Z.; Brunner, B.; Ari, C. Beneficial effects of exogenous ketogenic supplements on aging processes and age-related neurodegenerative diseases. Nutrients 2021, 13, 2197. [Google Scholar] [CrossRef]
- Gough, S.M. Beneficial Effects of the Ketogenic Diet and Ketone Body Metabolism in Different Models of Neurodegeneration; University of Miami: Coral Gables, FL, USA, 2021. [Google Scholar]
- Wang, Y.; Zhang, J.; Zhang, Y.; Yao, J. Bibliometric analysis of global research profile on ketogenic diet therapies in neurological diseases: Beneficial diet therapies deserve more attention. Front. Endocrinol. 2023, 13, 1066785. [Google Scholar]
- Cunnane, S.C.; Trushina, E.; Morland, C.; Prigione, A.; Casadesus, G.; Andrews, Z.B.; Beal, M.F.; Bergersen, L.H.; Brinton, R.D.; de la Monte, S.; et al. Brain energy rescue: An emerging therapeutic concept for neurodegenerative disorders of ageing. Nat. Rev. Drug Discov. 2020, 19, 609–633. [Google Scholar]
- Kapogiannis, D.; Avgerinos, K.I. Brain glucose and ketone utilization in brain aging and neurodegenerative diseases. Int. Rev. Neurobiol. 2020, 154, 79–110. [Google Scholar]
- de Carvalho, T.S. Calorie restriction or dietary restriction: How far they can protect the brain against neurodegenerative diseases? Neural Regen. Res. 2022, 17, 1640–1644. [Google Scholar] [CrossRef]
- de la Rubia Ortí, J.E.; Fernández, D.; Platero, F.; García-Pardo, M.P. Can Ketogenic diet improve Alzheimer’s disease? Association with anxiety, depression, and glutamate system. Front. Nutr. 2021, 8, 744398. [Google Scholar]
- Taylor, M.K.; Swerdlow, R.H.; Sullivan, D.K. Dietary neuroketotherapeutics for Alzheimer’s disease: An evidence update and the potential role for diet quality. Nutrients 2019, 11, 1910. [Google Scholar] [CrossRef]
- Bianchi, V.E.; Herrera, P.F.; Laura, R. Effect of nutrition on neurodegenerative diseases. A systematic review. Nutr. Neurosci. 2021, 24, 810–834. [Google Scholar]
- Tidman, M.M.; White, D.; White, T. Effects of an low carbohydrate/healthy fat/ketogenic diet on biomarkers of health and symptoms, anxiety and depression in Parkinson’s disease: A pilot study. Neurodegener. Dis. Manag. 2022, 12, 57–66. [Google Scholar]
- Fontana, L.; Ghezzi, L.; Cross, A.H.; Piccio, L. Effects of dietary restriction on neuroinflammation in neurodegenerative diseases. J. Exp. Med. 2021, 218, e20190086. [Google Scholar]
- Lin, D.-T.; Kao, N.-J.; Cross, T.-W.L.; Lee, W.-J.; Lin, S.-H. Effects of ketogenic diet on cognitive functions of mice fed high-fat-high-cholesterol diet. J. Nutr. Biochem. 2022, 104, 108974. [Google Scholar]
- Jiang, Z.; Yin, X.; Wang, M.; Chen, T.; Wang, Y.; Gao, Z.; Wang, Z. Effects of ketogenic diet on neuroinflammation in neurodegenerative diseases. Aging Dis. 2022, 13, 1146. [Google Scholar] [CrossRef]
- Jensen, N.J.; Wodschow, H.Z.; Nilsson, M.; Rungby, J. Effects of ketone bodies on brain metabolism and function in neurodegenerative diseases. Int. J. Mol. Sci. 2020, 21, 8767. [Google Scholar] [CrossRef]
- Lilamand, M.; Mouton-Liger, F.; Di Valentin, E.; Ortiz, M.S.; Paquet, C. Efficacy and safety of ketone supplementation or ketogenic diets for Alzheimer’s disease: A mini review. Front. Nutr. 2022, 8, 807970. [Google Scholar] [CrossRef]
- Zhang, H.; Tao, Y.; Leng, S.X. Ketogenic diet: An effective treatment approach for neurodegenerative diseases. Curr. Neuropharmacol. 2022, 20, 2303–2319. [Google Scholar] [CrossRef]
- Zhu, H.; Bi, D.; Zhang, Y.; Kong, C.; Du, J.; Wu, X.; Wei, Q.; Qin, H. Ketogenic diet for human diseases: The underlying mechanisms and potential for clinical implementations. Signal Transduct. Target. Ther. 2022, 7, 11. [Google Scholar] [CrossRef]
- Davis, J.J.; Fournakis, N.; Ellison, J. Ketogenic diet for the treatment and prevention of dementia: A review. J. Geriatr. Psychiatry Neurol. 2021, 34, 3–10. [Google Scholar] [CrossRef]
- Oliveira, T.P.D.; Morais, A.L.B.; dos Reis, P.L.B.; Palotás, A.; Vieira, L.B. A potential role for the ketogenic diet in alzheimer’s disease treatment: Exploring pre-clinical and clinical evidence. Metabolites 2023, 14, 25. [Google Scholar] [CrossRef]
- Carlotta, B.; Serena, C.; Valentina, F.; Emanuele, C. Ketogenic diet and neurodegenerative diseases: A focus on Alzheimer’s disease, Parkinson’s disease and amyotrofic lateral sclerosis. PharmacologyOnLine 2020, 3, 17–23. [Google Scholar]
- Elamin, M.; Ruskin, D.N.; Masino, S.A.; Sacchetti, P. Ketogenic diet modulates NAD+-dependent enzymes and reduces DNA damage in hippocampus. Front. Cell. Neurosci. 2018, 12, 263. [Google Scholar]
- Azadian, M.; Tian, G.; Bazrafkan, A.; Maki, N.; Rafi, M.; Chetty, N.; Desai, M.; Otarola, I.; Aguirre, F.; Zaher, S.M.; et al. Overnight caloric restriction prior to cardiac arrest and resuscitation leads to improved survival and neurological outcome in a rodent model. Front. Neurosci. 2021, 14, 609670. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, X.; Xiao, A.; Han, J.; Wang, Z.; Wen, M. Ketogenic diet prevents chronic sleep deprivation-induced Alzheimer’s disease by inhibiting iron dyshomeostasis and promoting repair via Sirt1/Nrf2 pathway. Front. Aging Neurosci. 2022, 14, 998292. [Google Scholar]
- Morrill, S.J.; Gibas, K.J. Ketogenic diet rescues cognition in ApoE4+ patient with mild Alzheimer’s disease: A case study. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 1187–1191. [Google Scholar]
- Lim, J.-M.; Letchumanan, V.; Tan, L.T.-H.; Hong, K.-W.; Wong, S.-H.; Ab Mutalib, N.-S.; Lee, L.-H.; Law, J.W.-F. Ketogenic diet: A dietary intervention via gut microbiome modulation for the treatment of neurological and nutritional disorders (a narrative review). Nutrients 2022, 14, 3566. [Google Scholar] [CrossRef] [PubMed]
- Longo, R.; Peri, C.; Cricrì, D.; Coppi, L.; Caruso, D.; Mitro, N.; De Fabiani, E.; Crestani, M. Ketogenic diet: A new light shining on old but gold biochemistry. Nutrients 2019, 11, 2497. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, B.; Lee, M.-J. Ketogenic diet: A promising neuroprotective composition for managing Alzheimer’s diseases and its pathological mechanisms. Curr. Mol. Med. 2022, 22, 640–656. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, Z. Ketogenic diet: Delicacy or poison? Precis. Nutr. 2024, 3, e00063. [Google Scholar]
- Dabke, P.; Brogden, G.; Naim, H.Y.; Das, A.M. Ketogenic diet: Impact on cellular lipids in hippocampal murine neurons. Nutrients 2020, 12, 3870. [Google Scholar] [CrossRef]
- Lange, K.W.; Lange, K.M.; Makulska-Gertruda, E.; Nakamura, Y.; Reissmann, A.; Kanaya, S.; Hauser, J. Ketogenic diets and Alzheimer’s disease. Food Sci. Hum. Wellness 2017, 6, 1–9. [Google Scholar] [CrossRef]
- Saris, C.G.J.; Timmers, S. Ketogenic diets and Ketone suplementation: A strategy for therapeutic intervention. Front. Nutr. 2022, 9, 947567. [Google Scholar] [CrossRef]
- Li, R.; Liu, Y.; Liu, H.; Li, J. Ketogenic diets and protective mechanisms in epilepsy, metabolic disorders, cancer, neuronal loss, and muscle and nerve degeneration. J. Food Biochem. 2020, 44, e13140. [Google Scholar]
- McDonald, T.J.W.; Cervenka, M.C. Ketogenic diets for adult neurological disorders. Neurotherapeutics 2018, 15, 1018–1031. [Google Scholar]
- Morris, G.; Maes, M.; Berk, M.; Carvalho, A.F.; Puri, B.K. Nutritional ketosis as an intervention to relieve astrogliosis: Possible therapeutic applications in the treatment of neurodegenerative and neuroprogressive disorders. Eur. Psychiatry 2020, 63, e8. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.; Krishnan, V.; Praveen, S.; Hebbar, K. Dietary prospects of coconut oil for the prevention and treatment of Alzheimer’s disease (AD): A review of recent evidences. Trends Food Sci. Technol. 2021, 112, 201–211. [Google Scholar] [CrossRef]
- Qu, C.; Keijer, J.; Adjobo-Hermans, M.J.; van de Wal, M.; Schirris, T.; van Karnebeek, C.; Pan, Y.; Koopman, W.J. The ketogenic diet as a therapeutic intervention strategy in mitochondrial disease. Int. J. Biochem. Cell Biol. 2021, 138, 106050. [Google Scholar] [CrossRef] [PubMed]
- Alcover Galmes, F.P. Ketogenic diets: Neurodegenerative and rare diseases. Bachelor’s Thesis, University of Barcelona, Barcelona, Spain, 2020. [Google Scholar]
- Bohnen, J.L.; Albin, R.L.; Bohnen, N.I. Ketogenic interventions in mild cognitive impairment, Alzheimer’s disease, and Parkinson’s disease: A systematic review and critical appraisal. Front. Neurol. 2023, 14, 1123290. [Google Scholar] [CrossRef]
- Kraeuter, A.-K.; Phillips, R.; Sarnyai, Z. Ketogenic therapy in neurodegenerative and psychiatric disorders: From mice to men. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 101, 109913. [Google Scholar] [CrossRef]
- López-Ojeda, W.; Hurley, R.A. Ketone bodies and brain metabolism: New insights and perspectives for neurological diseases. J. Neuropsychiatry Clin. Neurosci. 2023, 35, 104–109. [Google Scholar] [CrossRef]
- Henderson, S.T. Ketone bodies as a therapeutic for Alzheimer’s disease. Neurotherapeutics 2008, 5, 470–480. [Google Scholar] [CrossRef]
- Yang, H.; Shan, W.; Zhu, F.; Wu, J.; Wang, Q. Ketone bodies in neurological diseases: Focus on neuroprotection and underlying mechanisms. Front. Neurol. 2019, 10, 585. [Google Scholar] [CrossRef]
- Waldman, H.S.; McAllister, M.J. Exogenous ketones as therapeutic signaling molecules in high-stress occupations: Implications for mitigating oxidative stress and mitochondrial dysfunction in future research. Nutr. Metab. Insights 2020, 13, 1178638820979029. [Google Scholar] [CrossRef]
- Norwitz, N.G.; Jaramillo, J.G.; Clarke, K.; Soto, A. Ketotherapeutics for neurodegenerative diseases. Int. Rev. Neurobiol. 2020, 155, 141–168. [Google Scholar]
- Dabke, P.; Das, A.M. Mechanism of action of ketogenic diet treatment: Impact of decanoic acid and beta—Hydroxybutyrate on sirtuins and energy metabolism in hippocampal murine neurons. Nutrients 2020, 12, 2379. [Google Scholar] [CrossRef] [PubMed]
- Augustin, K.; Khabbush, A.; Williams, S.; Eaton, S.; Orford, M.; Cross, J.H.; Heales, S.J.R.; Walker, M.C.; Williams, R.S.B. Mechanisms of action for the medium-chain triglyceride ketogenic diet in neurological and metabolic disorders. Lancet Neurol. 2018, 17, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Neth, B.J.; Mintz, A.; Whitlow, C.; Jung, Y.; Sai, K.S.; Register, T.C.; Kellar, D.; Lockhart, S.N.; Hoscheidt, S.; Maldjian, J.; et al. Modified ketogenic diet is associated with improved cerebrospinal fluid biomarker profile, cerebral perfusion, and cerebral ketone body uptake in older adults at risk for Alzheimer’s disease: A pilot study. Neurobiol. Aging 2020, 86, 54–63. [Google Scholar]
- Nagpal, R.; Neth, B.J.; Wang, S.; Craft, S.; Yadav, H. Modified Mediterranean-ketogenic diet modulates gut microbiome and short-chain fatty acids in association with Alzheimer’s disease markers in subjects with mild cognitive impairment. EBioMedicine 2019, 47, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, S.; Sangam, S.R.; Singh, S.; Joginapally, V.R. Modulatory Effects of Dietary Amino Acids on Neurodegenerative Diseases. Adv. Neurobiol. 2016, 12, 401–414. [Google Scholar]
- Jang, J.; Kim, S.R.; Lee, J.E.; Lee, S.; Son, H.J.; Choe, W.; Yoon, K.-S.; Kim, S.S.; Yeo, E.-J.; Kang, I. Molecular mechanisms of neuroprotection by ketone bodies and ketogenic diet in cerebral ischemia and neurodegenerative diseases. Int. J. Mol. Sci. 2023, 25, 124. [Google Scholar] [CrossRef]
- Choi, A.; Hallett, M.; Ehrlich, D. Nutritional ketosis in Parkinson’s disease—A review of remaining questions and insights. Neurotherapeutics 2021, 18, 1637–1649. [Google Scholar] [CrossRef]
- Munshi, M.I.; Yao, S.J.; Ben Mamoun, C. Redesigning therapies for pantothenate kinase–associated neurodegeneration. J. Biol. Chem. 2022, 298, 101577. [Google Scholar] [CrossRef]
- Dunn, E.; Zhang, B.; Sahota, V.K.; Augustin, H. Potential benefits of medium chain fatty acids in aging and neurodegenerative disease. Front. Aging Neurosci. 2023, 15, 1230467. [Google Scholar]
- Phillips, M.C.L.; Deprez, L.M.; Mortimer, G.M.N.; Murtagh, D.K.J.; McCoy, S.; Mylchreest, R.; Gilbertson, L.J.; Clark, K.M.; Simpson, P.V.; McManus, E.J.; et al. Randomized crossover trial of a modified ketogenic diet in Alzheimer’s disease. Alzheimer’s Res. Ther. 2021, 13, 1–12. [Google Scholar] [CrossRef]
- Kaviyarasan, S.; Sia, E.L.C.; Retinasamy, T.; Arulsamy, A.; Shaikh, M.F. Regulation of gut microbiome by ketogenic diet in neurodegenerative diseases: A molecular crosstalk. Front. Aging Neurosci. 2022, 14, 1015837. [Google Scholar]
- Heischmann, S.; Gano, L.B.; Quinn, K.; Liang, L.-P.; Klepacki, J.; Christians, U.; Reisdorph, N.; Patel, M. Regulation of kynurenine metabolism by a ketogenic diet. J. Lipid Res. 2018, 59, 958–966. [Google Scholar] [PubMed]
- Xiang, X. Research progress on the effects of ketogenic diet on central nervous system diseases. In Proceedings of the International Conference on Modern Medicine and Global Health (ICMMGH 2023), SPIE, Kuala Lumpur, Malaysia, 15 April 2023. [Google Scholar]
- Włodarek, D. Role of ketogenic diets in neurodegenerative diseases (Alzheimer’s disease and Parkinson’s disease). Nutrients 2019, 11, 169. [Google Scholar] [CrossRef] [PubMed]
- Höller, A.; Albrecht, U.; Sigl, S.B.; Zöggeler, T.; Ramoser, G.; Bernar, B.; Karall, D.; Scholl-Bürgi, S. Successful implementation of classical ketogenic dietary therapy in a patient with Niemann-Pick disease type C. Mol. Genet. Metab. Rep. 2021, 27, 100723. [Google Scholar]
- White, H.; Venkatesh, K.; Venkatesh, B. Systematic review of the use of ketones in the management of acute and chronic neurological disorders. J. Neurol. Neurosci. 2017, 8, 188. [Google Scholar]
- Alharbi, A.; Al-Sowayan, N.S. The effect of ketogenic-diet on health. Food Nutr. Sci. 2020, 11, 301–313. [Google Scholar] [CrossRef]
- Grochowska, K.; Przeliorz, A. The effect of the ketogenic diet on the therapy of neurodegenerative diseases and its impact on improving cognitive functions. Dement. Geriatr. Cogn. Disord. Extra 2022, 12, 100–106. [Google Scholar]
- Zarnowska, I.M. Therapeutic use of the ketogenic diet in refractory epilepsy: What we know and what still needs to be learned. Nutrients 2020, 12, 2616. [Google Scholar] [CrossRef]
- Mentzelou, M.; Dakanalis, A.; Vasios, G.K.; Gialeli, M.; Papadopoulou, S.K.; Giaginis, C. The relationship of ketogenic diet with neurodegenerative and psychiatric diseases: A scoping review from basic research to clinical practice. Nutrients 2023, 15, 2270. [Google Scholar] [CrossRef]
- Hersant, H.; Grossberg, G. The ketogenic diet and Alzheimer’s disease. J. Nutr. Health Aging 2022, 26, 606–614. [Google Scholar] [CrossRef]
- Polito, R.; La Torre, M.E.; Moscatelli, F.; Cibelli, G.; Valenzano, A.; Panaro, M.A.; Monda, M.; Messina, A.; Monda, V.; Pisanelli, D.; et al. The ketogenic diet and neuroinflammation: The action of beta-hydroxybutyrate in a microglial cell line. Int. J. Mol. Sci. 2023, 24, 3102. [Google Scholar] [CrossRef] [PubMed]
- Broom, G.M.; Shaw, I.C.; Rucklidge, J.J. The ketogenic diet as a potential treatment and prevention strategy for Alzheimer’s disease. Nutrition 2019, 60, 118–121. [Google Scholar] [PubMed]
- Sourbron, J.; Thevissen, K.; Lagae, L. The ketogenic diet revisited: Beyond ketones. Front. Neurol. 2021, 12, 720073. [Google Scholar]
- Ludwig, D.S. The ketogenic diet: Evidence for optimism but high-quality research needed. J. Nutr. 2020, 150, 1354–1359. [Google Scholar]
- Ramezani, M.; Fernando, M.; Eslick, S.; Asih, P.R.; Shadfar, S.; Bandara, E.M.S.; Hillebrandt, H.; Meghwar, S.; Shahriari, M.; Chatterjee, P.; et al. Ketone bodies mediate alterations in brain energy metabolism and biomarkers of Alzheimer’s disease. Front. Neurosci. 2023, 17, 1297984. [Google Scholar]
- Dyńka, D.; Kowalcze, K.; Paziewska, A. The role of ketogenic diet in the treatment of neurological diseases. Nutrients 2022, 14, 5003. [Google Scholar] [CrossRef]
- Dąbek, A.; Wojtala, M.; Pirola, L.; Balcerczyk, A. Modulation of cellular biochemistry, epigenetics and metabolomics by ketone bodies. Implications of the ketogenic diet in the physiology of the organism and pathological states. Nutrients 2020, 12, 788. [Google Scholar] [CrossRef]
- Kraeuter, A.-K.; Guest, P.C.; Sarnyai, Z. The therapeutic potential of ketogenic diet throughout life: Focus on metabolic, neurodevelopmental and neurodegenerative disorders. In Reviews on Biomarker Studies in Aging and Anti-Aging Research; Springer: Berlin/Heidelberg, Germany, 2019; pp. 77–101. [Google Scholar]
- Di Majo, D.; Cacciabaudo, F.; Accardi, G.; Gambino, G.; Giglia, G.; Ferraro, G.; Candore, G.; Sardo, P. Ketogenic and modified mediterranean diet as a tool to counteract neuroinflammation in multiple sclerosis: Nutritional suggestions. Nutrients 2022, 14, 2384. [Google Scholar] [CrossRef]
- Pietrzak, D.; Kasperek, K.; Rękawek, P.; Piątkowska-Chmiel, I. The therapeutic role of ketogenic diet in neurological disorders. Nutrients 2022, 14, 1952. [Google Scholar] [CrossRef]
- Caplliure-Llopis, J.; Peralta-Chamba, T.; Carrera-Juliá, S.; Cuerda-Ballester, M.; Drehmer-Rieger, E.; López-Rodriguez, M.M.; Ortí, J.E.d.l.R. Therapeutic alternative of the ketogenic Mediterranean diet to improve mitochondrial activity in Amyotrophic Lateral Sclerosis (ALS): A Comprehensive Review. Food Sci. Nutr. 2020, 8, 23–35. [Google Scholar]
- Camberos-Luna, L.; Massieu, L. Therapeutic strategies for ketosis induction and their potential efficacy for the treatment of acute brain injury and neurodegenerative diseases. Neurochem. Int. 2020, 133, 104614. [Google Scholar] [CrossRef] [PubMed]
- Grammatikopoulou, M.G.; Goulis, D.G.; Gkiouras, K.; Theodoridis, X.; Gkouskou, K.K.; Evangeliou, A.; Dardiotis, E.; Bogdanos, D.P. To keto or not to keto? A systematic review of randomized controlled trials assessing the effects of ketogenic therapy on Alzheimer disease. Adv. Nutr. 2020, 11, 1583–1602. [Google Scholar] [PubMed]
- Pluta, R.; Ulamek-Koziol, M. To treat or not to treat Alzheimer’s disease by the ketogenic diet? That is the question. Neural Regen. Res. 2020, 15, 857–858. [Google Scholar] [CrossRef] [PubMed]
- Powell, E. Welcome to Volume 13 of Neurodegenerative Disease Management; Taylor & Francis: Abingdon, UK, 2023; pp. 1–4. [Google Scholar]
- Habashy, K.J.; Ahmad, F.; Ibeh, S.; Mantash, S.; Kobeissy, F.; Issa, H.; Habis, R.; Tfaily, A.; Nabha, S.; Harati, H.; et al. Western and ketogenic diets in neurological disorders: Can you tell the difference? Nutr. Rev. 2022, 80, 1927–1941. [Google Scholar]
- Ryals, R.C.; Huang, S.J.; Wafai, D.; Bernert, C.; Steele, W.; Six, M.; Bonthala, S.; Titus, H.; Yang, P.; Gillingham, M.; et al. A ketogenic & low-protein diet slows retinal degeneration in rd10 mice. Translational Vis. Sci. Technol. 2020, 9, 18. [Google Scholar]
- Chinna-Meyyappan, A.; Gomes, F.A.; Koning, E.; Fabe, J.; Breda, V.; Brietzke, E. Effects of the ketogenic diet on cognition: A systematic review. Nutr. Neurosci. 2023, 26, 1258–1278. [Google Scholar]
| Blood Level | Normal Diet | Ketogenic Diet | Diabetic Ketoacidosis |
|---|---|---|---|
| Glucose (mg/dL) | 80–120 | 65–80 | >300 |
| Insulin (μU/L) | 6–23 | 6.6–9.4 | ≈0 |
| Ketone Bodies (mmol/L) | 0.1 | 7–8 | >25 |
| pH | 7.4 | 7.4 | <7.3 |
| Lactate (mmol/L) | 0.5–1.0 | 0.5–1.5 | >2.0 |
| Free Fatty Acids (μmol/L) | 400–500 | 800–1500 | >2000 |
| Triglycerides (mg/dL) | 50–150 | 50–200 | 150–500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shabbir, I.; Liu, K.; Riaz, B.; Rahim, M.F.; Zhong, S.; Aweya, J.J.; Cheong, K.-L. Investigating the Therapeutic Potential of the Ketogenic Diet in Modulating Neurodegenerative Pathophysiology: An Interdisciplinary Approach. Nutrients 2025, 17, 1268. https://doi.org/10.3390/nu17071268
Shabbir I, Liu K, Riaz B, Rahim MF, Zhong S, Aweya JJ, Cheong K-L. Investigating the Therapeutic Potential of the Ketogenic Diet in Modulating Neurodegenerative Pathophysiology: An Interdisciplinary Approach. Nutrients. 2025; 17(7):1268. https://doi.org/10.3390/nu17071268
Chicago/Turabian StyleShabbir, Iqra, Keying Liu, Bakhtawar Riaz, Muhammad Farhan Rahim, Saiyi Zhong, Jude Juventus Aweya, and Kit-Leong Cheong. 2025. "Investigating the Therapeutic Potential of the Ketogenic Diet in Modulating Neurodegenerative Pathophysiology: An Interdisciplinary Approach" Nutrients 17, no. 7: 1268. https://doi.org/10.3390/nu17071268
APA StyleShabbir, I., Liu, K., Riaz, B., Rahim, M. F., Zhong, S., Aweya, J. J., & Cheong, K.-L. (2025). Investigating the Therapeutic Potential of the Ketogenic Diet in Modulating Neurodegenerative Pathophysiology: An Interdisciplinary Approach. Nutrients, 17(7), 1268. https://doi.org/10.3390/nu17071268







