Branched-Chain Amino Acid Supplementation Enhances Substrate Metabolism, Exercise Efficiency and Reduces Post-Exercise Fatigue in Active Young Males
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Body Composition and Maximal Aerobic Capacity Assessment
2.4. Measurements During Experiments
2.5. Diet Record and Supplementation Procedures
2.6. Indirect Calorimetry Measurement
2.7. Perceptual Scales Assessment
2.8. Blood Samples Collection and Measurements
2.9. Statistical Analysis
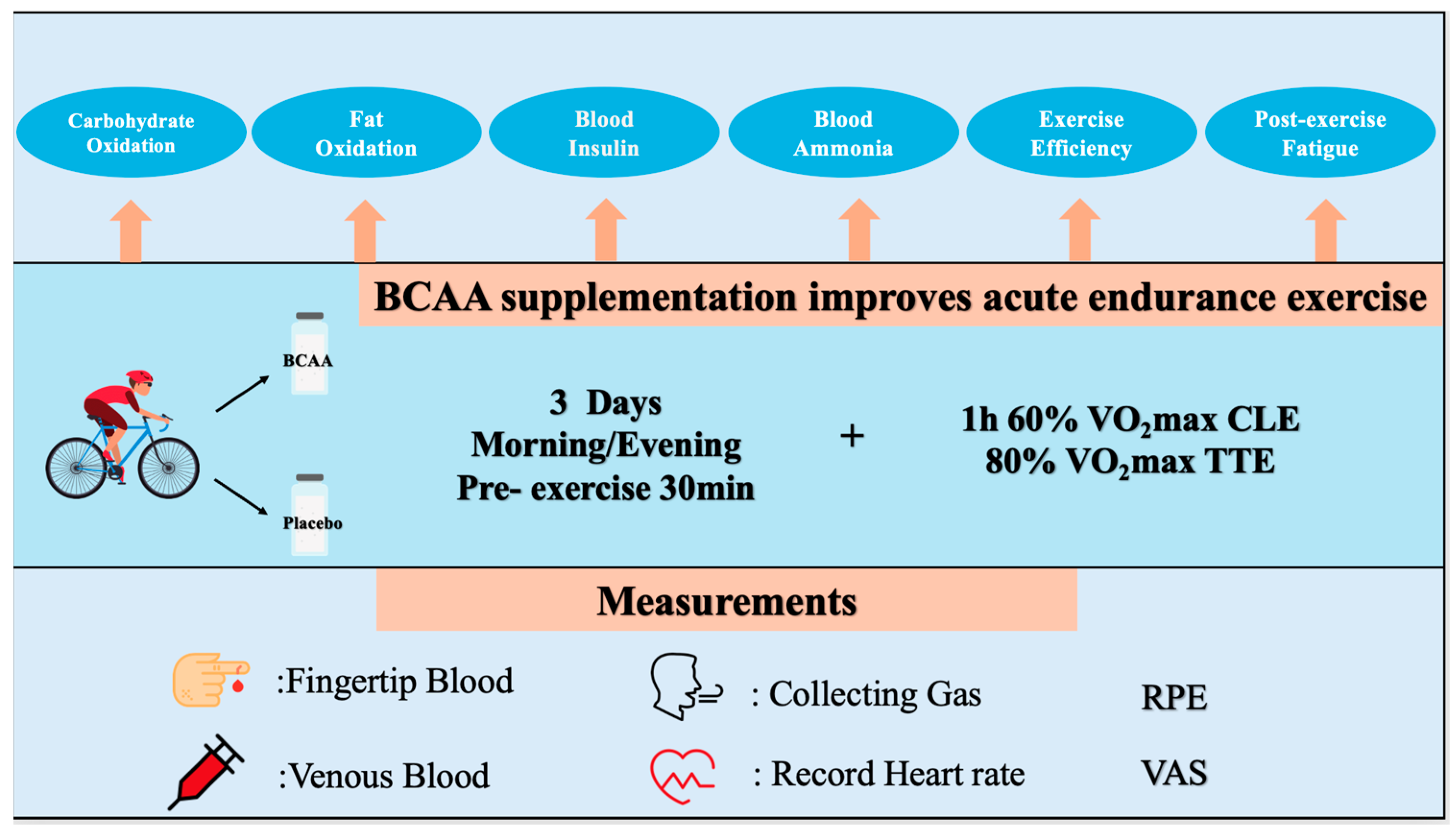
3. Results
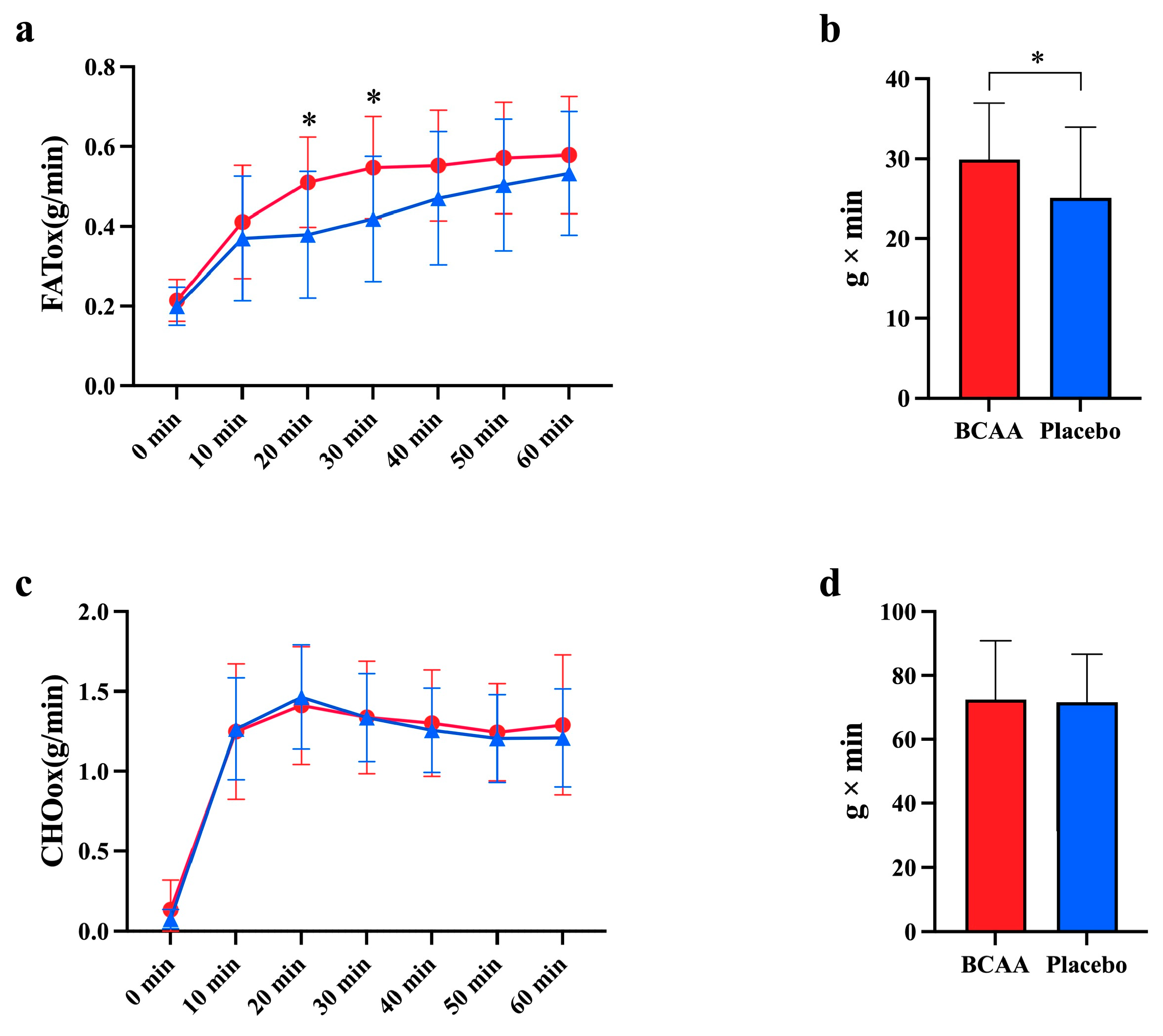
3.1. BCAA Supplementation Promotes Fat Oxidation During Endurance Exercise
3.2. BCAA Supplementation Enhances CHO Oxidation During TTE
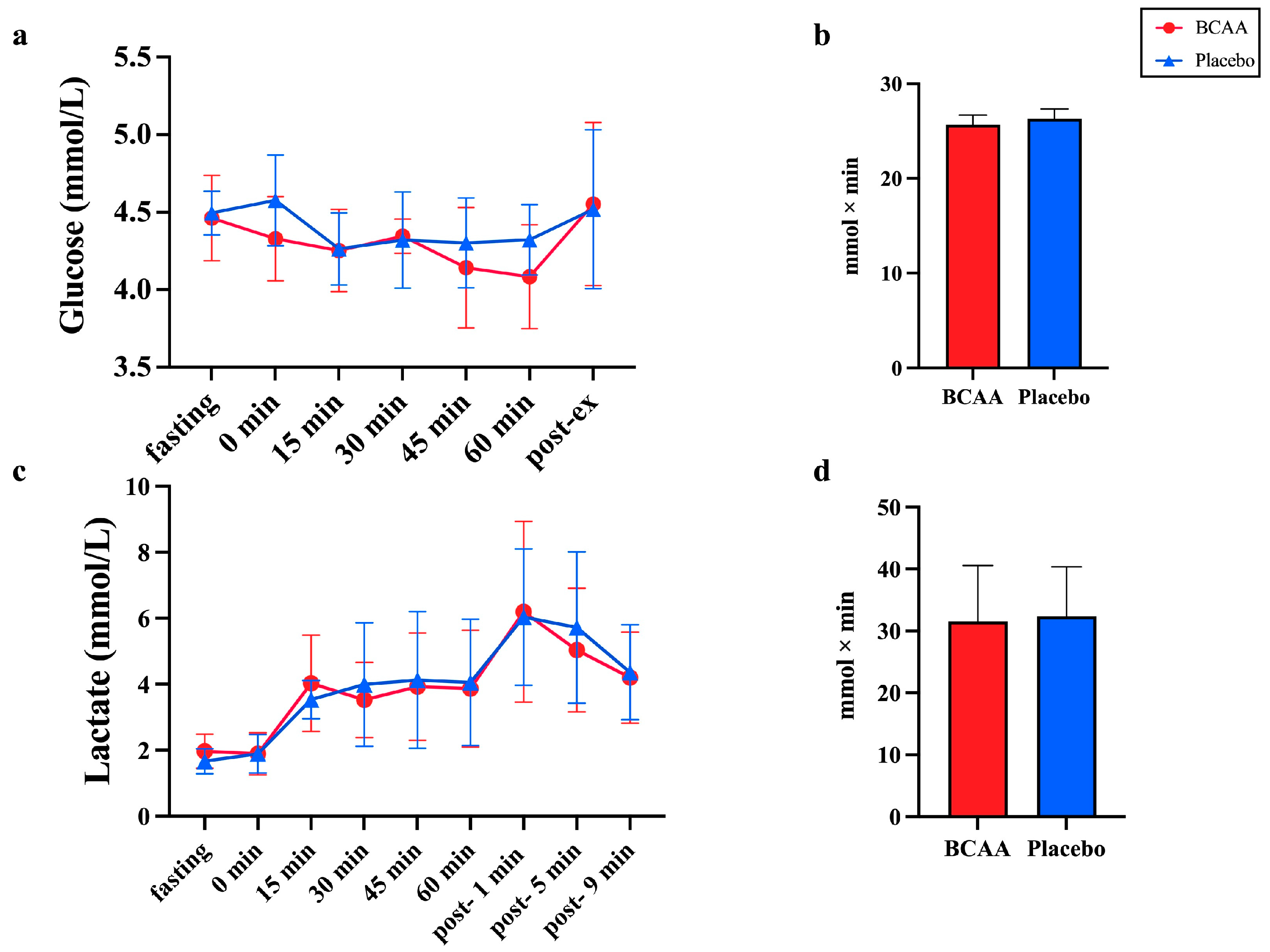
3.3. Blood Glucose and Lactate Are Not Affected by the BCAA Supplements
3.4. NEFA Levels Were Not Affected by BCAA Supplements
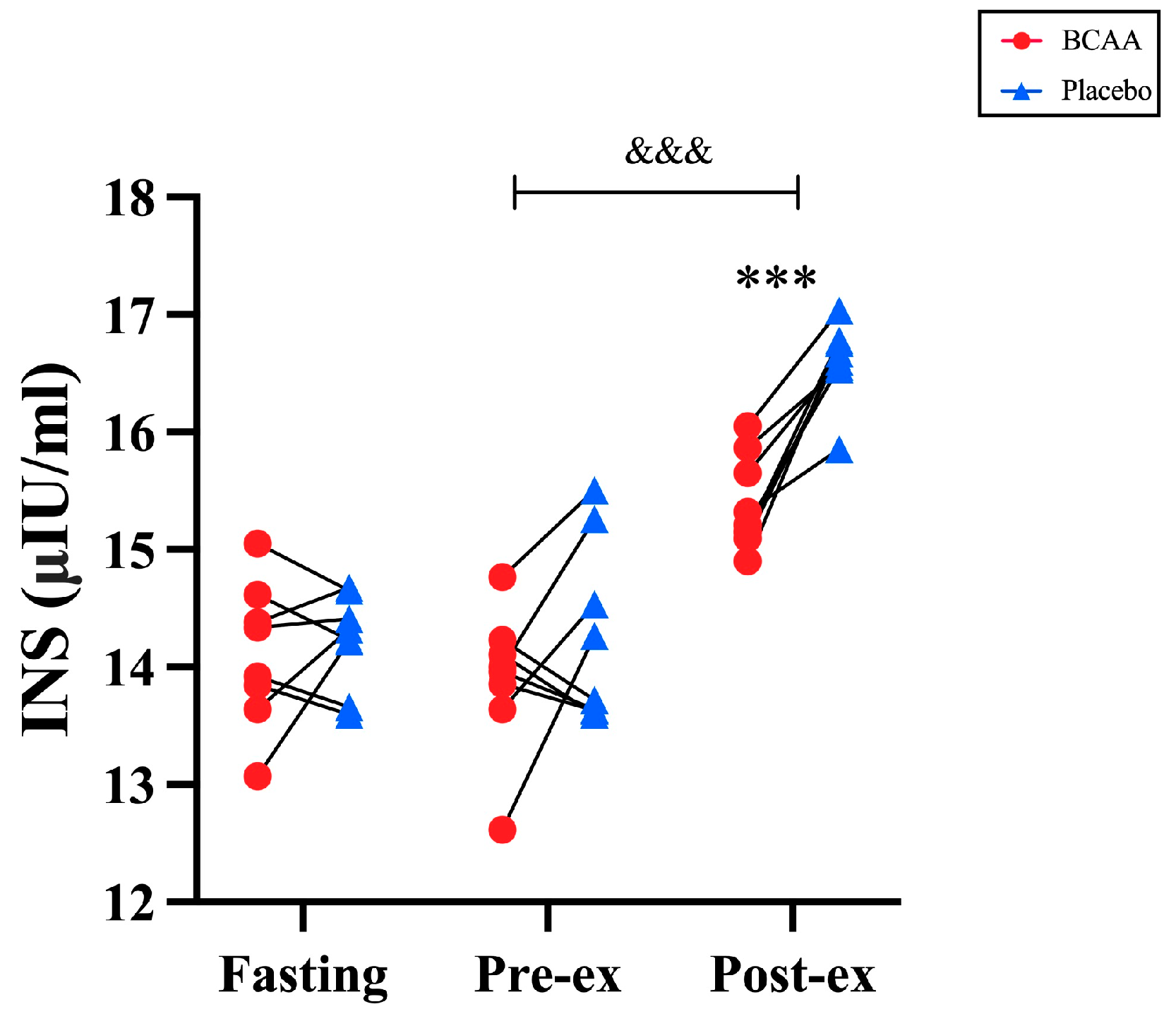
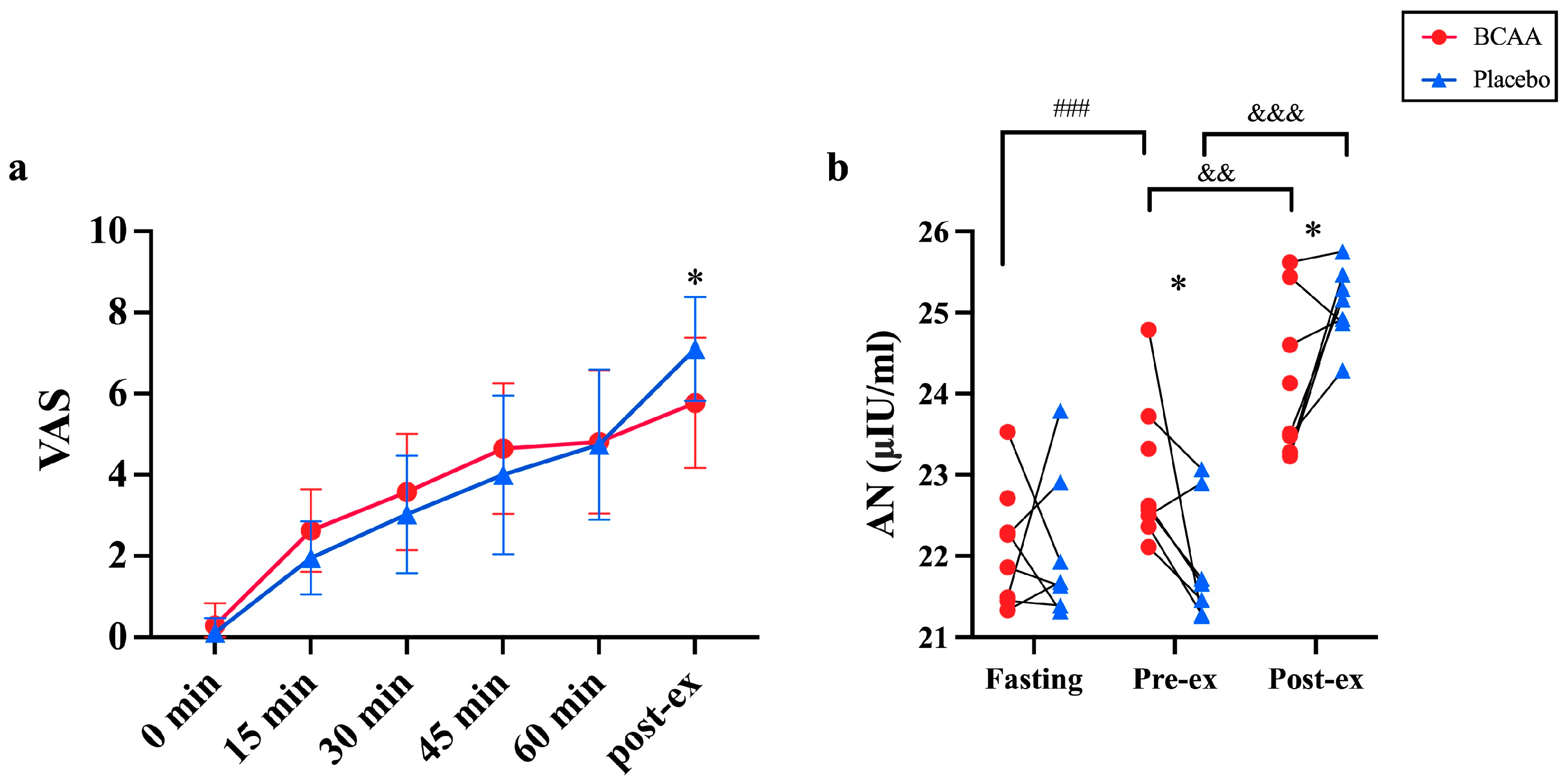
3.5. Blood Insulin Was Elevated by BCAA Supplementation
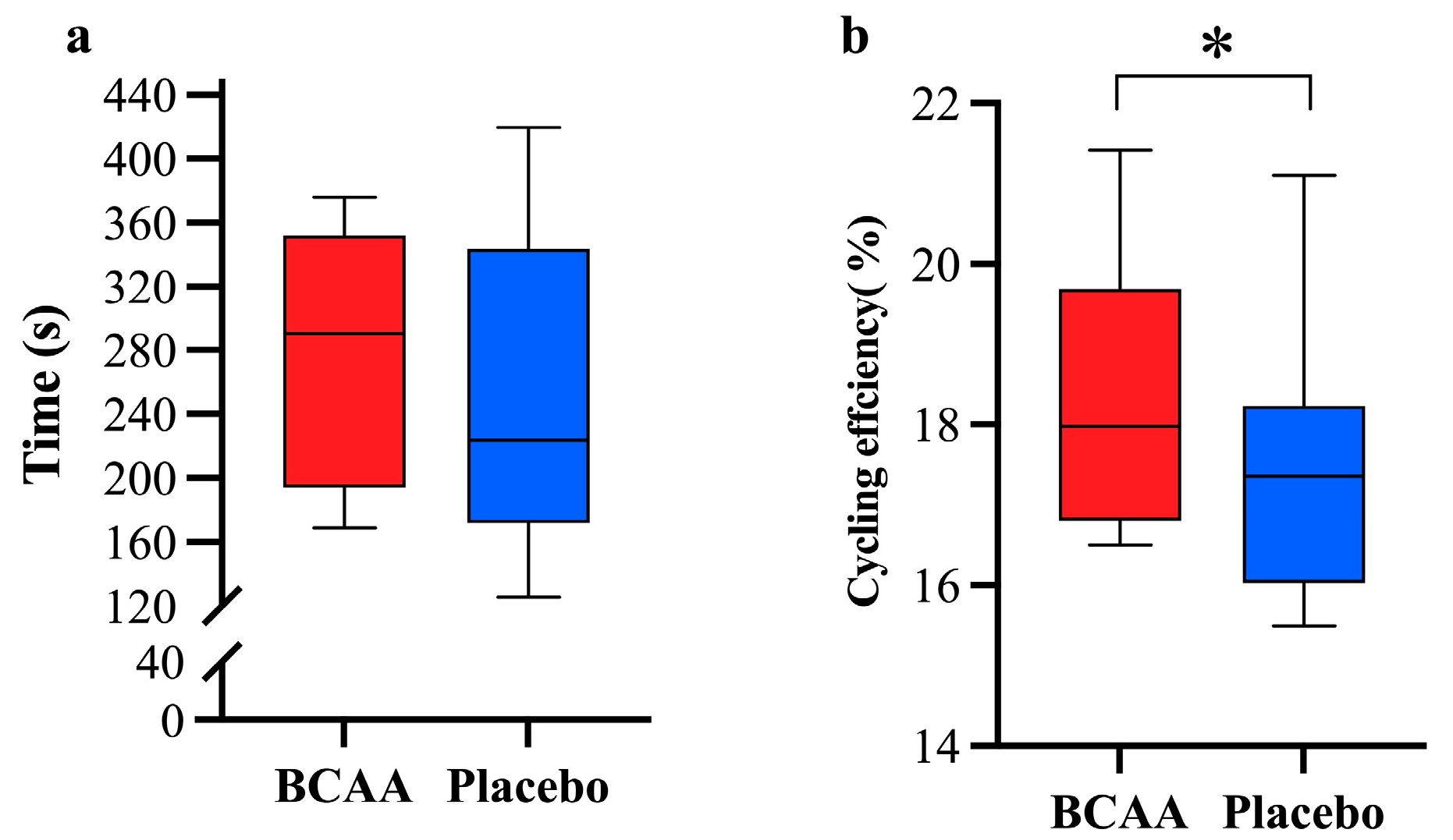
3.6. BCAAs Supplements Enhance Exercise Efficiency
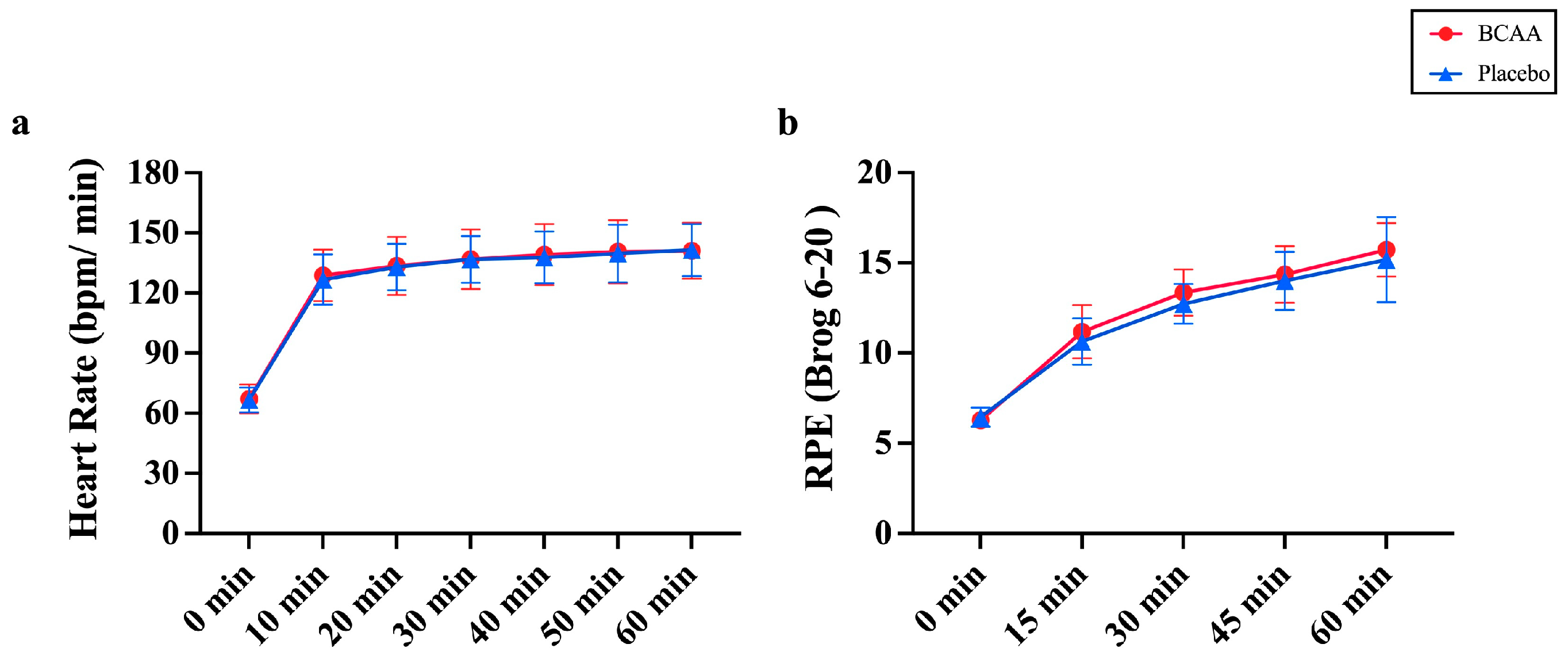
3.7. BCAA Supplements Do Not Alter Physiological Responses During Endurance Exercise
3.8. BCAA Supplements Reduce Acute Exercise-Caused Fatigue
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alghannam, A.F.; Ghaith, M.M.; Alhussain, M.H. Regulation of Energy Substrate Metabolism in Endurance Exercise. Int. J. Environ. Res. Public Health 2021, 18, 4963. [Google Scholar] [CrossRef] [PubMed]
- Romijn, J.A.; Coyle, E.F.; Sidossis, L.S.; Gastaldelli, A.; Horowitz, J.F.; Endert, E.; Wolfe, R.R. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am. J. Physiol. 1993, 265, E380–E391. [Google Scholar] [CrossRef] [PubMed]
- Hawley, J.A. Effect of increased fat availability on metabolism and exercise capacity. Med. Sci. Sports Exerc. 2002, 34, 1485–1491. [Google Scholar] [CrossRef] [PubMed]
- Hawley, J.A.; Leckey, J.J. Carbohydrate Dependence During Prolonged, Intense Endurance Exercise. Sports Med. 2015, 45 (Suppl. S1), S5–S12. [Google Scholar] [CrossRef]
- Burke, L.M.; Whitfield, J.; Heikura, I.A.; Ross, M.L.R.; Tee, N.; Forbes, S.F.; Hall, R.; McKay, A.K.A.; Wallett, A.M.; Sharma, A.P. Adaptation to a low carbohydrate high fat diet is rapid but impairs endurance exercise metabolism and performance despite enhanced glycogen availability. J. Physiol. 2021, 599, 771–790. [Google Scholar] [CrossRef]
- Neinast, M.; Murashige, D.; Arany, Z. Branched Chain Amino Acids. Annu. Rev. Physiol. 2019, 81, 139–164. [Google Scholar] [CrossRef]
- Shimomura, Y.; Murakami, T.; Nakai, N.; Nagasaki, M.; Harris, R.A. Exercise promotes BCAA catabolism: Effects of BCAA supplementation on skeletal muscle during exercise. J. Nutr. 2004, 134, 1583S–1587S. [Google Scholar] [CrossRef]
- Goto, M.; Shinno, H.; Ichihara, A. Isozyme patterns of branched-chain amino acid transaminase in human tissues and tumors. Gan 1977, 68, 663–667. [Google Scholar]
- Adeva-Andany, M.M.; Lopez-Maside, L.; Donapetry-Garcia, C.; Fernandez-Fernandez, C.; Sixto-Leal, C. Enzymes involved in branched-chain amino acid metabolism in humans. Amino Acids 2017, 49, 1005–1028. [Google Scholar] [CrossRef]
- Li, T.; Zhang, Z.; Kolwicz, S.C., Jr.; Abell, L.; Roe, N.D.; Kim, M.; Zhou, B.; Cao, Y.; Ritterhoff, J.; Gu, H.; et al. Defective Branched-Chain Amino Acid Catabolism Disrupts Glucose Metabolism and Sensitizes the Heart to Ischemia-Reperfusion Injury. Cell Metab. 2017, 25, 374–385. [Google Scholar] [CrossRef]
- Shimomura, Y.; Murakami, T.; Nakai, N.; Nagasaki, M.; Obayashi, M.; Li, Z.; Xu, M.; Sato, Y.; Kato, T.; Shimomura, N.; et al. Suppression of glycogen consumption during acute exercise by dietary branched-chain amino acids in rats. J. Nutr. Sci. Vitaminol. 2000, 46, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Kainulainen, H.; Hulmi, J.J.; Kujala, U.M. Potential role of branched-chain amino acid catabolism in regulating fat oxidation. Exerc. Sport. Sci. Rev. 2013, 41, 194–200. [Google Scholar] [CrossRef]
- Zhang, F.; Zhao, S.; Yan, W.; Xia, Y.; Chen, X.; Wang, W.; Zhang, J.; Gao, C.; Peng, C.; Yan, F.; et al. Branched Chain Amino Acids Cause Liver Injury in Obese/Diabetic Mice by Promoting Adipocyte Lipolysis and Inhibiting Hepatic Autophagy. EBioMedicine 2016, 13, 157–167. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, S.H.; Jeong, W.S.; Lee, H.Y. Effect of BCAA intake during endurance exercises on fatigue substances, muscle damage substances, and energy metabolism substances. J. Exerc. Nutr. Biochem. 2013, 17, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, M.; Huonker, M.; Dimeo, F.; Heinz, N.; Gastmann, U.; Treis, N.; Steinacker, J.M.; Keul, J.; Kajewski, R.; Haussinger, D. Serum amino acid concentrations in nine athletes before and after the 1993 Colmar ultra triathlon. Int. J. Sports Med. 1995, 16, 155–159. [Google Scholar] [CrossRef]
- van Hall, G.; MacLean, D.A.; Saltin, B.; Wagenmakers, A.J. Mechanisms of activation of muscle branched-chain alpha-keto acid dehydrogenase during exercise in man. J. Physiol. 1996, 494 Pt 3, 899–905. [Google Scholar] [CrossRef]
- Plotkin, D.L.; Delcastillo, K.; Van Every, D.W.; Tipton, K.D.; Aragon, A.A.; Schoenfeld, B.J. Isolated Leucine and Branched-Chain Amino Acid Supplementation for Enhancing Muscular Strength and Hypertrophy: A Narrative Review. Int. J. Sport. Nutr. Exerc. Metab. 2021, 31, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Bassit, R.A.; Sawada, L.A.; Bacurau, R.F.; Navarro, F.; Costa Rosa, L.F. The effect of BCAA supplementation upon the immune response of triathletes. Med. Sci. Sports Exerc. 2000, 32, 1214–1219. [Google Scholar] [CrossRef]
- Salem, A.; Ben Maaoui, K.; Jahrami, H.; AlMarzooqi, M.A.; Boukhris, O.; Messai, B.; Clark, C.C.T.; Glenn, J.M.; Ghazzaoui, H.A.; Bragazzi, N.L.; et al. Attenuating Muscle Damage Biomarkers and Muscle Soreness After an Exercise-Induced Muscle Damage with Branched-Chain Amino Acid (BCAA) Supplementation: A Systematic Review and Meta-analysis with Meta-regression. Sports Med. Open 2024, 10, 42. [Google Scholar] [CrossRef]
- Wilkinson, D.J.; Smeeton, N.J.; Watt, P.W. Ammonia metabolism, the brain and fatigue; revisiting the link. Prog. Neurobiol. 2010, 91, 200–219. [Google Scholar] [CrossRef]
- Cordeiro, L.M.S.; Rabelo, P.C.R.; Moraes, M.M.; Teixeira-Coelho, F.; Coimbra, C.C.; Wanner, S.P.; Soares, D.D. Physical exercise-induced fatigue: The role of serotonergic and dopaminergic systems. Braz. J. Med. Biol. Res. 2017, 50, e6432. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, E.A.; Blomstrand, E. Tryptophan, 5-hydroxytryptamine and a possible explanation for central fatigue. Adv. Exp. Med. Biol. 1995, 384, 315–320. [Google Scholar] [CrossRef]
- Blomstrand, E.; Hassmen, P.; Ek, S.; Ekblom, B.; Newsholme, E.A. Influence of ingesting a solution of branched-chain amino acids on perceived exertion during exercise. Acta Physiol. Scand. 1997, 159, 41–49. [Google Scholar] [CrossRef]
- Manaf, F.A.; Peiffer, J.J.; Maker, G.L.; Fairchild, T.J. Branched-chain amino acid supplementation improves cycling performance in untrained cyclists. J. Sci. Med. Sport. 2021, 24, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Gervasi, M.; Sisti, D.; Amatori, S.; Donati Zeppa, S.; Annibalini, G.; Piccoli, G.; Vallorani, L.; Benelli, P.; Rocchi, M.B.L.; Barbieri, E.; et al. Effects of a commercially available branched-chain amino acid-alanine-carbohydrate-based sports supplement on perceived exertion and performance in high intensity endurance cycling tests. J. Int. Soc. Sports Nutr. 2020, 17, 6. [Google Scholar] [CrossRef] [PubMed]
- De Palo, E.F.; Gatti, R.; Cappellin, E.; Schiraldi, C.; De Palo, C.B.; Spinella, P. Plasma lactate, GH and GH-binding protein levels in exercise following BCAA supplementation in athletes. Amino Acids 2001, 20, 1–11. [Google Scholar] [CrossRef]
- Watson, P.; Shirreffs, S.M.; Maughan, R.J. The effect of acute branched-chain amino acid supplementation on prolonged exercise capacity in a warm environment. Eur. J. Appl. Physiol. 2004, 93, 306–314. [Google Scholar] [CrossRef]
- Cheuvront, S.N.; Carter, R., 3rd; Kolka, M.A.; Lieberman, H.R.; Kellogg, M.D.; Sawka, M.N. Branched-chain amino acid supplementation and human performance when hypohydrated in the heat. J. Appl. Physiol. (1985) 2004, 97, 1275–1282. [Google Scholar] [CrossRef]
- Thomas, S.; Reading, J.; Shephard, R.J. Revision of the Physical Activity Readiness Questionnaire (PAR-Q). Can. J. Sport. Sci. 1992, 17, 338–345. [Google Scholar]
- Gualano, A.B.; Bozza, T.; Lopes De Campos, P.; Roschel, H.; Dos Santos Costa, A.; Luiz Marquezi, M.; Benatti, F.; Herbert Lancha Junior, A. Branched-chain amino acids supplementation enhances exercise capacity and lipid oxidation during endurance exercise after muscle glycogen depletion. J. Sports Med. Phys. Fitness 2011, 51, 82–88. [Google Scholar]
- Jackman, S.R.; Wallis, G.A.; Yu, J.; Philp, A.; Baar, K.; Tipton, K.D.; Witard, O.C. Co-Ingestion of Branched-Chain Amino Acids and Carbohydrate Stimulates Myofibrillar Protein Synthesis Following Resistance Exercise in Trained Young Men. Int. J. Sport. Nutr. Exerc. Metab. 2023, 33, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Cheng, I.S.; Wang, Y.W.; Chen, I.F.; Hsu, G.S.; Hsueh, C.F.; Chang, C.K. The Supplementation of Branched-Chain Amino Acids, Arginine, and Citrulline Improves Endurance Exercise Performance in Two Consecutive Days. J. Sports Sci. Med. 2016, 15, 509–515. [Google Scholar]
- Greer, B.K.; White, J.P.; Arguello, E.M.; Haymes, E.M. Branched-chain amino acid supplementation lowers perceived exertion but does not affect performance in untrained males. J. Strength. Cond. Res. 2011, 25, 539–544. [Google Scholar] [CrossRef]
- Jeukendrup, A.E.; Wallis, G.A. Measurement of substrate oxidation during exercise by means of gas exchange measurements. Int. J. Sports Med. 2005, 26 (Suppl. S1), S28–S37. [Google Scholar] [CrossRef]
- Moseley, L.; Jeukendrup, A.E. The reliability of cycling efficiency. Med. Sci. Sports Exerc. 2001, 33, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Koba, T.; Hamada, K.; Tsujimoto, H.; Mitsuzono, R. Branched-chain amino acid supplementation increases the lactate threshold during an incremental exercise test in trained individuals. J. Nutr. Sci. Vitaminol. 2009, 55, 52–58. [Google Scholar] [CrossRef]
- Dyck, D.J.; Peters, S.J.; Wendling, P.S.; Chesley, A.; Hultman, E.; Spriet, L.L. Regulation of muscle glycogen phosphorylase activity during intense aerobic cycling with elevated FFA. Am. J. Physiol. 1996, 270, E116–E125. [Google Scholar] [CrossRef] [PubMed]
- Norton, L.; Shannon, C.; Gastaldelli, A.; DeFronzo, R.A. Insulin: The master regulator of glucose metabolism. Metabolism 2022, 129, 155142. [Google Scholar] [CrossRef]
- Cox, D.J.; Palmer, T.N. Carcass glycogen repletion on carbohydrate re-feeding after starvation. Biochem. J. 1987, 245, 903–905. [Google Scholar] [CrossRef]
- Li, C.; Najafi, H.; Daikhin, Y.; Nissim, I.B.; Collins, H.W.; Yudkoff, M.; Matschinsky, F.M.; Stanley, C.A. Regulation of leucine-stimulated insulin secretion and glutamine metabolism in isolated rat islets. J. Biol. Chem. 2003, 278, 2853–2858. [Google Scholar] [CrossRef]
- Wojtaszewski, J.F.; Nielsen, J.N.; Richter, E.A. Invited review: Effect of acute exercise on insulin signaling and action in humans. J. Appl. Physiol. (1985) 2002, 93, 384–392. [Google Scholar] [CrossRef]
- Almeida, F.N.; Proenca, A.R.; Chimin, P.; Marcal, A.C.; Bessa-Lima, F.; Carvalho, C.R. Physical exercise and pancreatic islets: Acute and chronic actions on insulin secretion. Islets 2012, 4, 296–301. [Google Scholar] [CrossRef]
- Mikines, K.J.; Sonne, B.; Tronier, B.; Galbo, H. Effects of training and detraining on dose-response relationship between glucose and insulin secretion. Am. J. Physiol. 1989, 256, E588–E596. [Google Scholar] [CrossRef] [PubMed]
- Jackman, S.R.; Witard, O.C.; Jeukendrup, A.E.; Tipton, K.D. Branched-chain amino acid ingestion can ameliorate soreness from eccentric exercise. Med. Sci. Sports Exerc. 2010, 42, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Lewis, P.B.; Ruby, D.; Bush-Joseph, C.A. Muscle soreness and delayed-onset muscle soreness. Clin. Sports Med. 2012, 31, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Nicastro, H.; da Luz, C.R.; Chaves, D.F.; Bechara, L.R.; Voltarelli, V.A.; Rogero, M.M.; Lancha, A.H., Jr. Does Branched-Chain Amino Acids Supplementation Modulate Skeletal Muscle Remodeling through Inflammation Modulation? Possible Mechanisms of Action. J. Nutr. Metab. 2012, 2012, 136937. [Google Scholar] [CrossRef]
- Azevedo, R.A.; Silva-Cavalcante, M.D.; Lima-Silva, A.E.; Bertuzzi, R. Fatigue development and perceived response during self-paced endurance exercise: State-of-the-art review. Eur. J. Appl. Physiol. 2021, 121, 687–696. [Google Scholar] [CrossRef]
- Chaouloff, F.; Laude, D.; Elghozi, J.L. PHysical exercise: Evidence for differential consequences of tryptophan on 5-HT synthesis and metabolism in central serotonergic cell bodies and terminals. J. Neural Transm. 1989, 78, 121–130. [Google Scholar] [CrossRef]
- Pardridge, W.M. Kinetics of competitive inhibition of neutral amino acid transport across the blood-brain barrier. J. Neurochem. 1977, 28, 103–108. [Google Scholar] [CrossRef]
- Davis, J.M.; Bailey, S.P. Possible mechanisms of central nervous system fatigue during exercise. Med. Sci. Sports Exerc. 1997, 29, 45–57. [Google Scholar] [CrossRef]


| Characteristics | Mean ± SD |
|---|---|
| Age (year) | 21 ± 1 |
| Height (m) | 1.79 ± 0.08 |
| Body mass (kg) | 74.8 ± 10.3 |
| Body fat (%) | 16.7 ± 5.4 |
| BMI (kg/m2) | 23.5 ± 1.9 |
| VO2max (mL/(kg·min)) | 49 ± 9 |
| power VO2max (w) | 228 ± 28 |
| power at 60% VO2max (w) | 117 ± 21 |
| power at 80% VO2max (w) | 170 ± 25 |
| BCAA | Placebo | |
|---|---|---|
| Energy (kcal) | 2062 ± 338 | 2076 ± 348 |
| Carbohydrate (g) | 246 ± 50 | 246 ± 47 |
| Fat (g) | 64 ± 18 | 67 ± 17 |
| Protein (g) | 115 ± 11 | 116 ± 15 |
| CLE | TTE | |
|---|---|---|
| FATox (g/min) | ||
| BCAA | 0.51 ± 0.14 | 0.18 ± 0.18 |
| Placebo | 0.48 ± 0.16 | 0.19 ± 0.23 |
| CHOox (g/min) | ||
| BCAA | 1.33 ± 0.30 | 3.20 ± 0.87 * |
| Placebo | 1.35 ± 0.22 | 2.79 ± 1.04 |
| RER | ||
| BCAA | 0.85 ± 0.03 | 0.98 ± 0.05 |
| Placebo | 0.86 ± 0.03 | 0.98 ± 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luan, C.; Wang, Y.; Li, J.; Zhou, N.; Song, G.; Ni, Z.; Xu, C.; Tang, C.; Fu, P.; Wang, X.; et al. Branched-Chain Amino Acid Supplementation Enhances Substrate Metabolism, Exercise Efficiency and Reduces Post-Exercise Fatigue in Active Young Males. Nutrients 2025, 17, 1290. https://doi.org/10.3390/nu17071290
Luan C, Wang Y, Li J, Zhou N, Song G, Ni Z, Xu C, Tang C, Fu P, Wang X, et al. Branched-Chain Amino Acid Supplementation Enhances Substrate Metabolism, Exercise Efficiency and Reduces Post-Exercise Fatigue in Active Young Males. Nutrients. 2025; 17(7):1290. https://doi.org/10.3390/nu17071290
Chicago/Turabian StyleLuan, Chenglin, Yizhang Wang, Junxi Li, Nihong Zhou, Guilin Song, Zhen Ni, Chunyan Xu, Chunxue Tang, Pengyu Fu, Xintang Wang, and et al. 2025. "Branched-Chain Amino Acid Supplementation Enhances Substrate Metabolism, Exercise Efficiency and Reduces Post-Exercise Fatigue in Active Young Males" Nutrients 17, no. 7: 1290. https://doi.org/10.3390/nu17071290
APA StyleLuan, C., Wang, Y., Li, J., Zhou, N., Song, G., Ni, Z., Xu, C., Tang, C., Fu, P., Wang, X., Gong, L., & Zhang, E. (2025). Branched-Chain Amino Acid Supplementation Enhances Substrate Metabolism, Exercise Efficiency and Reduces Post-Exercise Fatigue in Active Young Males. Nutrients, 17(7), 1290. https://doi.org/10.3390/nu17071290








