Postprandial Energy Metabolism in the Regulation of Body Weight: Is there a Mechanistic Role for Dietary Calcium?
Abstract
:1. Introduction

2. Body Weight Regulation
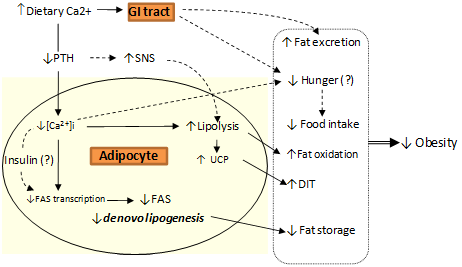
3. Calcium and Postprandial Energy Metabolism
3.1. Calcium, Fat Oxidation & Thermogenesis
| Authors | Study diets & duration | Significance between treatments | ||||
|---|---|---|---|---|---|---|
| ↑DIT | ↑FOR | ↓Genes | ↑Lipolysis | ↑Fecal fat | ||
| Cummings, James & Soares, 2006 [14] | CO (n = 8). 3 breakfast meals 176 mg and 532mg (D) and 575 ND each over 6 hr. | No | Yes | -- | Yes1 | -- |
| Soares et al., 2004 [18] | CO (n = 11). 2 breakfast meals 248 mg and 543 mg (D), followed by standard lunch 48 mg (D) over 8 h. | Yes | Yes | -- | Yes1 | -- |
| Boon et al., 2005 [11] | CO (n = 12). 3 diets of 1259 mg/d (high D), 1259 mg/d (low D, calcium carbonate) and 349 mg/d calcium, each for 1 week. | No | No2 | No | No | -- |
| Jacobsen et al., 2005 [16] | CO (n = 10). 3 dairy diets of 1,800 mg/d (15% protein), 1800 mg/d (23% protein) and 500 mg/d (15% protein), each for 1 week. | No | No | -- | -- | Yes3 |
| Melanson et al., 2005 [17] | CO (n = 19). Diets of 500 mg/d and 1,400 mg/d (D) each for 1 week and performed twice. Subjects studied on day 7 under energy deficit or in balance. | No | Yes4 | -- | -- | -- |
| St-Onge et al., 2007 [19] | CO (n = 14). Milk or fruit flavoured sugar drink supplemented over 1 week each. | Yes | Yes5 | -- | -- | -- |
| Boon et al., 2007 [10] | CO (n = 10). 4 diets of 2,500 mg/d, 1,200 mg/d and 400 mg/d from D and 1,200 mg/d from ND (calcium carbonate), each for 1 week. | -- | -- | Yes6 | -- | No7 |
| Bortolotti et al., 2008 [12] | CO (n = 10). D calcium (1,386 mg/d) or placebo (586 mg/d) over 5 weeks each. | No | No | No | No | -- |
| Buchowski et al., 2009 [13] | CO (n = 34). 500 mg/d and 1,500 mg/d calcium, each for 6 weeks weight loss, in lactose tolerant and intolerant subjects. | -- | -- | -- | -- | Yes |
| Teegarden et al., 2008 [20] | PT (n = 24). 3 diets of 500 mg/d, 1400 mg/d (ND) or 1400 mg/d (D) each for 12 weeks of weight loss. | No | Yes | -- | -- | -- |
| Gunther et al., 2005 [36] | PT (n = 26). 2 diets of low (<800 mg/d) or high D (1,000-1,400mg/d) for 1 year. | No | Yes | -- | -- | -- |
3.2. Calcium, Lipolysis and Lipogenesis
3.3. Calcium and Fat Excretion
4. Conclusions
Acknowledgements
Conflict of Interest
References
- Bessesen, D.H.; Bull, S.; Cornier, M.A. Trafficking of dietary fat and resistance to obesity. Phys. Behav. 2008, 94, 681–688. [Google Scholar]
- Hill, J.O. Understanding and addressing the epidemic of obesity: an energy balance perspective. Endocr. Rev. 2006, 27, 750–761. [Google Scholar]
- Martinez, J.A. Body-weight regulation: causes of obesity. Proc. Nutr. Soc. 2000, 59, 337–345. [Google Scholar]
- Zemel, M.B.; Sun, X.C. Calcitriol and energy metabolism. Nutr. Rev. 2008, 66, S139–S146. [Google Scholar]
- Zemel, M.B.; Shi, H.; Greer, B.; Dirienzo, D.B.; Zemel, P.C. Regulation of adiposity by dietary calcium. FASEB J. 2000, 14, 1132–1138. [Google Scholar]
- Tordoff, M.G. Calcium: Taste, intake, and appetite. Physiol. Rev. 2001, 81, 1567–1597. [Google Scholar] [PubMed]
- Astrup, A.; Chaput, J.P.; Gilbert, J.A.; Lorenzen, J.K. Dairy beverages and energy balance. Phys. Behav. 2010, 100, 67–75. [Google Scholar]
- McCarty, M.F.; Thomas, C.A. PTH excess may promote weight gain by impeding catecholamine-induced lipolysis-implications for the impact of calcium, vitamin D, and alcohol on body weight. Med. Hypotheses 2003, 61, 535–542. [Google Scholar]
- Flatt, J. Use and storage of carbohydrate and fat. Am. J. Clin. Nutr. 1995, 61, 952S–959S. [Google Scholar]
- Boon, N.; Hul, G.; Stegen, J.; Sluijsmans, W.; Valle, C.; Langin, D.; Viguerie, N.; Saris, W. An intervention study of the effects of calcium intake on faecal fat excretion, energy metabolism and adipose tissue mRNA expression of lipid-metabolism related proteins. Int. J. Obes. 2007, 31, 1704–1712. [Google Scholar]
- Boon, N.; Hul, G.B.; Viguerie, N.; Sicard, A.; Langin, D.; Saris, W.H. Effects of 3 diets with various calcium contents on 24-h energy expenditure, fat oxidation, and adipose tissue message RNA expression of lipid metabolism-related proteins. Am. J. Clin. Nutr. 2005, 82, 1244–1252. [Google Scholar] [PubMed]
- Bortolotti, M.; Rudelle, S.; Schneiter, P.; Vidal, H.; Loizon, E.; Tappy, L.; Acheson, K. Dairy calcium supplementation in overweight or obese persons: its effect on markers of fat metabolism. Amer. J. Clin. Nutr. 2008, 88, 877. [Google Scholar]
- Buchowski, M.S.; Aslam, M.; Dossett, C.; Dorminy, C.; Choi, L.; Acra, S. Effect of dairy and non-dairy calcium on fecal fat excretion in lactose digester and maldigester obese adults. Int. J. Obes. 2009, 34, 127–135. [Google Scholar]
- Cummings, N.; James, A.; Soares, M. The acute effects of different sources of dietary calcium on postprandial energy metabolism. Br. J. Nutr. 2006, 96, 138. [Google Scholar]
- Gunther, C.W.; Lyle, R.M.; Legowski, P.A.; James, J.M.; McCabe, L.D.; McCabe, G.P.; Peacock, M.; Teegarden, D. Fat oxidation and its relation to serum parathyroid hormone in young women enrolled in a 1-y dairy calcium intervention. Am. J. Clin. Nutr. 2005, 82, 1228–1234. [Google Scholar]
- Jacobsen, R.; Lorenzen, J.K.; Toubro, S.; Krog-Mikkelsen, I.; Astrup, A. Effect of short-term high dietary calcium intake on 24-h energy expenditure, fat oxidation, and fecal fat excretion. Int. J. Obes. 2005, 29, 292–301. [Google Scholar] [CrossRef]
- Melanson, E.L.; Donahoo, W.T.; Dong, F.; Ida, T.; Zemel, M.B. Effect of Low- and High-Calcium Dairy-Based Diets on Macronutrient Oxidation in Humans. Obesity 2005, 13, 2102–2112. [Google Scholar]
- Soares, M.J.; Chan She Ping-Delfos, W.; James, A.P.; Cummings, N.K. Dairy calcium and vitamin D stimulate postprandial thermogenesis: effect of sequential meals. Asia Pac. J. Clin. Nutr. 2004, 13, S56. [Google Scholar]
- St-Onge, M.P.; Claps, N.; Heshka, S.; Heymsfield, S.B.; Kosteli, A. Greater resting energy expenditure and lower respiratory quotient after 1 week of supplementation with milk relative to supplementation with a sugar-only beverage in children. Metabolism 2007, 56, 1699–1707. [Google Scholar]
- Teegarden, D.; White, K.M.; Lyle, R.M.; Zemel, M.B.; Van Loan, M.D.; Matkovic, V.; Craig, B.A.; Schoeller, D.A. Calcium and dairy product modulation of lipid utilization and energy expenditure. Obesity 2008, 16, 1566–1572. [Google Scholar]
- Wolever, T.; Jenkins, D.; Ocana, A.; Rao, V.; Collier, G. Second-meal effect: low-glycemic-index foods eaten at dinner improve subsequent breakfast glycemic response. Am. J. Clin. Nutr. 1988, 48, 1041–1047. [Google Scholar]
- Sawaya, A.L.; Fuss, P.J.; Dallal, G.E.; Tsay, R.; McCrory, M.A.; Young, V.; Robert, S.B. Meal palatability, substrate oxidation and blood glucose in young and older men. Phys. Behav. 2001, 72, 5–12. [Google Scholar]
- Allen, L.H. Calcium bioavailability and absorption: a review. Am J Clin Nutr 1982, 35, 783–808. [Google Scholar]
- Heaney, R.P.; Weaver, C.M.; Fitzsimmons, M.L. Influence of calcium load on absorption fraction. J. Bone Miner. Res. 1990, 5, 1135–1138. [Google Scholar]
- Heaney, R.P.; Dowell, S.M.; Hale, C.A.; Bendich, A. Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. J. Am. Coll. Nutr. 2003, 22, 142–146. [Google Scholar]
- Clark, C.A.; Gardiner, J.; McBurney, M.I.; Anderson, S.; Weatherspoon, L.J.; Henry, D.N.; Hord, N.G. Effects of breakfast meal composition on second meal metabolic responses in adults with type 2 diabetes mellitus. Eur. J. Clin. Nutr. 2006, 60, 1122–1129. [Google Scholar]
- Jenkins, D.J.; Wolever, T.M.; Taylor, R.H.; Griffiths, C.; Krzeminska, K.; Lawrie, J.A.; Bennett, C.M.; Goff, D.V.; Sarson, D.L.; Bloom, S.R. Slow release dietary carbohydrate improves second meal tolerance. Am. J. Clin. Nutr. 1982, 35, 1339–1349. [Google Scholar]
- Robertson, M.D.; Jackson, K.G.; Fielding, B.A.; Williams, C.M.; Frayn, K.N. Acute effects of meal fatty acid composition on insulin sensitivity in healthy post-menopausal women. Br. J. Nutr. 2002, 88, 635–640. [Google Scholar]
- Chan She Ping-Delfos, W.; Soares, M.J.; Cummings, N.K. Acute suppression of spontaneous food intake following dairy calcium and vitamin D. Asia Pac. J. Clin. Nutr. 2004, 13, S82. [Google Scholar]
- Soares, M.; Chan She Ping-Delfos, W. Second meal effects of dietary calcium and vitamin D. Eur. J. Clin. Nutr. 2008, 62, 872. [Google Scholar]
- Hunt, J.R.; Johnson, L.K.; Fariba Roughead, Z. Dietary protein and calcium interact to influence calcium retention: a controlled feeding study. Am. J. Clin. Nutr. 2009, 89, 1357–1365. [Google Scholar]
- Kerstetter, J.E.; O'Brien, K.O.; Caseria, D.M.; Wall, D.E.; Insogna, K.L. The impact of dietary protein on calcium absorption and kinetic measures of bone turnover in women. J. Clin. Endocrinol. Metab. 2005, 90, 26–31. [Google Scholar]
- Massey, L.K.; Whiting, S.J. Caffeine, urinary calcium, calcium metabolism and bone. J. Nutr. 1993, 123, 1611–1614. [Google Scholar] [PubMed]
- Pittas, A.G.; Lau, J.; Hu, F.B.; Dawson-Hughes, B. Review: The role of vitamin D and calcium in Type 2 Diabetes. A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2007, 92, 2017–2029. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Seino, Y.; Ishida, M.; Yamaoka, K.; Satomura, K.; Yabuuchi, H.; Seino, Y.; Imura, H. Effect of 1,25-Dihydroxyvitamin D3 on insulin secretion: Direct or mediated? Endocrinology 1986, 118, 1971–1976. [Google Scholar]
- Gunther, C.W.; Legowski, P.A.; Lyle, R.M.; McCabe, G.P.; Eagan, M.; Peacock, M. Dairy products do not lead to alterations in body weight or fat mass in young women in a 1-y intervention. Am. J. Clin. Nutr. 2005, 81, 751–756. [Google Scholar]
- Coppack, S.; Jensen, M.; Miles, J. In vivo regulation of lipolysis in humans. J. Lipid Res. 1994, 35, 177–193. [Google Scholar]
- Frayn, K.N. Non-esterified fatty acid metabolism and postprandial lipaemia. Atherosclerosis 1998, 141, S41–S46. [Google Scholar]
- Zemel, M.B.; Richards, J.; Mathis, S.; Milstead, A.; Gebhardt, L.; Silva, E. Dairy augmentation of total and central fat loss in obese subjects. Int. J. Obes. Relat. Metab. Disord. 2005, 29, 391–397. [Google Scholar]
- Zemel, M.B.; Richards, J.; Milstead, A.; Campbell, P. Effects of calcium and dairy on body composition and weight loss in African-American adults. Obesity 2005, 13, 1218–1225. [Google Scholar]
- Denke, M.A.; Fox, M.M.; Schulte, M.C. Short-Term dietary calcium fortification increases fecal saturated fat content and reduces serum lipids in men. J. Nutr. 1993, 123, 1047–1053. [Google Scholar]
- Govers, M.J.A.P.; Termont, D.S.M.L.; Lapré, J.A.; Kleibeuker, J.H.; Vonk, R.J.; Van der Meer, R. Calcium in milk products precipitates intestinal fatty acids and secondary bile acids and thus inhibits colonic Cytotoxicity in humans. Cancer Res. 1996, 56, 3270–3275. [Google Scholar]
- Heaney, R.P.; Recker, R.R. Estimation of true calcium absorption. Ann. Intern. Med. 1985, 103, 516–521. [Google Scholar]
- Christensen, R.; Lorenzen, J.K.; Svith, C.R.; Bartels, E.M.; Melanson, E.L.; Saris, W.H.; Tremblay, A.; Astrup, A. Effect of calcium from dairy and dietary supplements on faecal fat excretion: a meta-analysis of randomized controlled trials. Obesity Rev. 2009, 10, 475–486. [Google Scholar]
- Lorenzen, J.K.; Nielsen, S.; Holst, J.J.; Tetens, I.; Rehfeld, J.F.; Astrup, A. Effect of dairy calcium or supplementary calcium intake on postprandial fat metabolism, appetite, and subsequent energy intake. Am. J. Clin. Nutr. 2007, 85, 678–687. [Google Scholar] [PubMed]
- Major, G.C.; Chaput, J.P.; Ledoux, M.; St-Pierre, S.; Anderson, G.H.; Zemel, M.B.; Tremblay, A. Recent developments in calcium-related obesity research. Obesity Rev. 2008, 9, 428–445. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Soares, M.J.; She-Ping-Delfos, W.L.C. Postprandial Energy Metabolism in the Regulation of Body Weight: Is there a Mechanistic Role for Dietary Calcium? Nutrients 2010, 2, 586-598. https://doi.org/10.3390/nu2060586
Soares MJ, She-Ping-Delfos WLC. Postprandial Energy Metabolism in the Regulation of Body Weight: Is there a Mechanistic Role for Dietary Calcium? Nutrients. 2010; 2(6):586-598. https://doi.org/10.3390/nu2060586
Chicago/Turabian StyleSoares, Mario J., and Wendy L. Chan She-Ping-Delfos. 2010. "Postprandial Energy Metabolism in the Regulation of Body Weight: Is there a Mechanistic Role for Dietary Calcium?" Nutrients 2, no. 6: 586-598. https://doi.org/10.3390/nu2060586
APA StyleSoares, M. J., & She-Ping-Delfos, W. L. C. (2010). Postprandial Energy Metabolism in the Regulation of Body Weight: Is there a Mechanistic Role for Dietary Calcium? Nutrients, 2(6), 586-598. https://doi.org/10.3390/nu2060586