Effects of Long-Term Oral Administration of Arachidonic Acid and Docosahexaenoic Acid on the Immune Functions of Young Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals Used in the Experiments
| (mol%) | Control | ARA | DHA |
|---|---|---|---|
| PLA | 13.8 ± 0.01 | 6.95 ± 0.00 | 29.8 ± 0.03 |
| STA | 13.8 ± 0.01 | 5.91 ± 0.00 | 8.10 ± 0.04 |
| OLA | 42.5 ± 0.03 | 5.31 ± 0.00 | 16.3 ± 0.01 |
| LA | 20.0 ± 0.02 | 9.38 ± 0.01 | 1.96 ± 0.01 |
| ARA | ND | 45.1 ± 0.04 | 2.49 ± 0.02 |
| EPA | 0.13 ± 0.01 | 0.52 ± 0.00 | 6.61 ± 0.00 |
| DPA n-3 | ND | ND | 1.17 ± 0.01 |
| DHA | ND | ND | 32.6 ± 0.03 |
2.2. Cell Culture
2.3. Isolation of Rat Spleen Lymphocytes
2.4. Cytotoxic Activity of NK Cell Assay
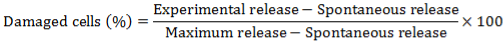
2.5. Sample Preparation for the Analysis of Fatty Acid Metabolites
2.6. LC-ESI-MS/MS-Based Analysis
| Compound | SRM Transition (m/z) | Compound | SRM Transition (m/z) | Compound | SRM Transition (m/z) |
|---|---|---|---|---|---|
| ARA | 303 > 259 | EPA | 301 > 257 | DHA | 327 > 283 |
| PGE2 | 351 > 271 | 5-HEPE | 317 > 115 | 7-HDoHE | 343 > 141 |
| PGD2 | 351 > 271 | 12-HEPE | 317 > 179 | 10-HDoHE | 343 > 153 |
| PGF2α | 353 > 193 | 15-HEPE | 317 > 219 | 14-HDoHE | 343 > 193 |
| 5-HETE | 319 > 115 | 18-HEPE | 317 > 259 | 17-HDoHE | 343 > 245 |
| 12-HETE | 319 > 179 | RvE2 | 333 > 115 | PD1 | 359 > 153 |
| 15-HETE | 319 > 219 | AA-d8 | 311 > 267 | PGE2-d4 | 355 > 275 |
| PGD2-d4 | 355 > 275 | PGF2α-d4 | 357 > 197 | 5-HETE-d8 | 327 > 116 |
2.7. Determination of Cytokine Levels
2.8. Analysis
3. Results
3.1. Measurement of Cytotoxic Activity of NK Cells

3.2. Analysis of Fatty Acid Metabolites
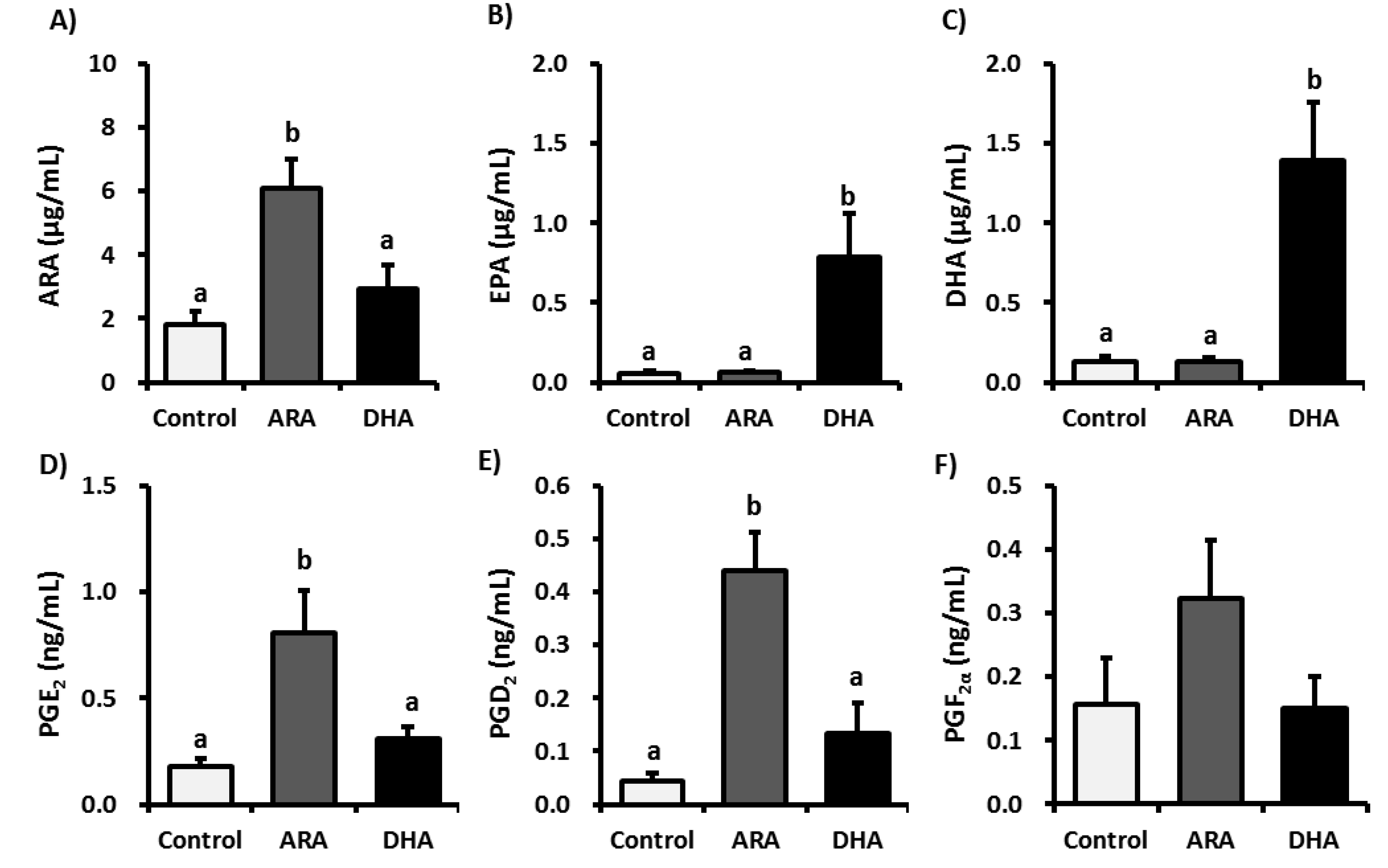

3.3. Analysis of Inflammatory Cytokines in Plasma
| (ng/mL) | Control | ARA | DHA |
|---|---|---|---|
| IL-1β | 107.4 ± 6.08 | 146.9 ± 31.1 | 91.26 ± 8.42 |
| IL-6 | 42.07 ± 11.6 | 27.87 ± 9.47 | 26.69 ± 8.74 |
| TNF-α | 20.84 ± 2.96 | 20.99 ± 2.17 | 21.65 ± 2.23 |
| IL-4 | 6.99 ± 0.60 | 6.99 ± 0.62 | 6.65 ± 0.79 |
| IL-10 | 129.4 ± 16.9 | 112.2 ± 8.25 | 112.8 ± 16.6 |
| IL-13 | 14.63 ± 0.80 | 17.54 ± 1.25 | 13.20 ± 2.02 |
4. Discussion
5. Conclusions
Acknowledgements
Conflict of Interest
References
- Chávez-Galán, L.; Arenas-Del Angel, M.C.; Zenteno, E.; Chávez, R.; Lascurain, R. Cell death mechanisms induced by cytotoxic lymphocytes. Cell. Mol. Immunol. 2009, 6, 15–25. [Google Scholar] [CrossRef]
- Seaman, W.E. Natural killer cells and natural killer T cells. Arthritis Rheum. 2000, 43, 1204–1217. [Google Scholar] [CrossRef]
- Hwang, Y.J.; Kim, J.; Park, D.S.; Hwang, K.A. Study on the immunomodulation effect of isodon japonicus extract via splenocyte function and NK anti-tumor activity. Int. J. Mol. Sci. 2012, 13, 4880–4888. [Google Scholar]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- O’Leary, J.G.; Goodarzi, M.; Drayton, D.L.; von Andrian, U.H. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat. Immunol. 2006, 7, 507–516. [Google Scholar] [CrossRef]
- Lu, L.M.; Zavitz, C.C.; Chen, B.; Kianpour, S.; Wan, Y.; Stämpfli, M.R. Cigarette smoke impairs NK cell-dependent tumor immune surveillance. J. Immunol. 2007, 178, 936–943. [Google Scholar]
- Cao, L.; Kulmburg, P.; Veelken, H.; Mackensen, A.; Mézes, B.; Lindemann, A.; Mertelsmann, R.; Rosenthal, F.M. Cytokine gene transfer in cancer therapy. Stem Cells 1998, 16, 251–260. [Google Scholar]
- Evans, C.; Dalgleish, A.G.; Kumar, D. Review article: Immune suppression and colorectal cancer. Aliment. Pharmacol. Ther. 2006, 24, 1163–1177. [Google Scholar]
- Budhu, A.; Wang, X.W. The role of cytokines in hepatocellular carcinoma. J. Leukoc. Biol. 2006, 80, 1197–1213. [Google Scholar] [CrossRef]
- Demaria, S.; Pikarsky, E.; Karin, M.; Coussens, L.M.; Chen, Y.C.; El-Omar, E.M.; Trinchieri, G.; Dubinett, S.M.; Mao, J.T.; Szabo, E.; et al. Cancer and inflammation: Promise for biologic therapy. J. Immunother. 2010, 33, 335–351. [Google Scholar] [CrossRef]
- Kundu, N.; Walser, T.C.; Ma, X.; Fulton, A.M. Cyclooxygenase inhibitors modulate NK activities that control metastatic disease. Cancer Immunol. Immunother. 2005, 54, 981–987. [Google Scholar] [CrossRef]
- Abrahao, A.C.; Castilho, R.M.; Squarize, C.H.; Molinolo, A.A.; dos Santos-Pinto, D., Jr.; Gutkind, J.S. A role for COX2-derived PGE2 and PGE2-receptor subtypes in head and neck squamous carcinoma cell proliferation. Oral Oncol. 2010, 46, 880–887. [Google Scholar] [CrossRef]
- Anwar-Mohamed, A.; El-Sherbeni, A.; Kim, S.H.; Elshenawy, O.H.; Althurwi, H.N.; Zordoky, B.N.; El-Kadi, A.O. Acute arsenic treatment alters cytochrome P450 expression and arachidonic acid metabolism in lung, liver and kidney of C57Bl/6 mice. Xenobiotica 2013, in press. [Google Scholar]
- Naraba, H.; Murakami, M.; Matsumoto, H.; Shimbara, S.; Ueno, A.; Kudo, I.; Oh-ishi, S. Segregated coupling of phospholipases A2, cyclooxygenases, and terminal prostanoid synthases in different phases of prostanoid biosynthesis in rat peritoneal macrophages. J. Immunol. 1998, 160, 2974–2982. [Google Scholar]
- Tilley, S.L.; Coffman, T.M.; Koller, B.H. Mixed messages: Modulation of inflammation and immune responses by prostaglandins and thromboxanes SourceDivision of Pulmonary and Critical Care Medicine. J. Clin. Invest. 2001, 108, 15–23. [Google Scholar]
- Vendramini-Costa, D.B.; Carvalho, J.E. Molecular link mechanisms between inflammation and cancer. Curr. Pharm. Des. 2012, 18, 3831–3852. [Google Scholar] [CrossRef]
- Murff, H.J.; Shu, X.O.; Li, H.; Yang, G.; Wu, X.; Cai, H.; Wen, W.; Gao, Y.T.; Zheng, W. Dietary polyunsaturated fatty acids and breast cancer risk in Chinese women: A prospective cohort study. Int. J. Cancer 2011, 128, 1434–1441. [Google Scholar] [CrossRef]
- Bougnoux, P.; Menanteau, J. Dietary fatty acids and experimental carcinogenesis. Bull. Cancer 2005, 92, 685–696. [Google Scholar]
- Greene, E.R.; Huang, S.; Serhan, C.N.; Panigrahy, D. Regulation of inflammation in cancer by eicosanoids. Prostaglandins Other Lipid Mediat. 2011, 96, 27–36. [Google Scholar]
- Giudetti, A.M.; Cagnazzo, R. Beneficial effects of n-3 PUFA on chronic airway inflammatory diseases. Prostaglandins Other Lipid Mediat. 2012, 99, 57–67. [Google Scholar] [CrossRef]
- Hata, A.N.; Breyer, R.M. Pharmacology and signaling of prostaglandin receptors: Multiple roles in inflammation and immune modulation. Pharmacol. Ther. 2004, 103, 147–166. [Google Scholar] [CrossRef]
- Fujino, H.; Salvi, S.; Regan, J.W. Differential regulation of phosphorylation of the cAMP response element-binding protein after activation of EP2 and EP4 prostanoid receptors by prostaglandin E2. Mol. Pharmacol. 2005, 68, 251–259. [Google Scholar]
- Joshi, P.C.; Zhou, X.; Cuchens, M.; Jones, Q. Prostaglandin E2 suppressed IL-15-mediated human NK cell function through down-regulation of common gamma-chain. J. Immunol. 2001, 166, 885–891. [Google Scholar]
- Simopoulos, A.P. Omega-3 fatty acids in inflammation and autoimmune diseases. J. Am. Coll. Nutr. 2002, 21, 495–505. [Google Scholar]
- Stewart, I.J.; Peel, S. Natural killer cytotoxicity and antibody-dependent cytotoxicity of cells of rat metrial glands. J. Reprod. Fertil. 1993, 98, 489–494. [Google Scholar] [CrossRef]
- Gardiner, C.M.; Meara, A.O.; Reen, D.J. Differential cytotoxicity of cord blood and bone marrow-derived natural killer cells. Blood 1998, 91, 207–213. [Google Scholar]
- Arita, M.; Iwamoto, R.; Isobe, Y. Lipidomics; Wiley-VCH: Weinheim, Germany, 2012; pp. 219–232. [Google Scholar]
- Ye, J.; Ortaldo, J.R.; Conlon, K.; Winkler-Pickett, R.; Young, H.A. Cellular and molecular mechanisms of IFN-gamma production induced by IL-2 and IL-12 in a human NK cell line. J. Leukoc. Biol. 1995, 58, 225–233. [Google Scholar]
- Zwirner, N.W.; Domaica, C.I. Cytokine regulation of natural killer cell effector functions. Biofactors 2010, 36, 274–288. [Google Scholar]
- Yu, Y.; Chadee, K. Prostaglandin E2 stimulates IL-8 gene expression in human colonic epithelial cells by a posttranscriptional mechanism. J. Immunol. 1998, 161, 3746–3752. [Google Scholar]
- Nakayama, T.; Mutsuga, N.; Yao, L.; Tosato, G. Prostaglandin E2 promotes degranulation-independent release of MCP-1 from mast cells. J. Leukoc. Biol. 2006, 79, 95–104. [Google Scholar] [CrossRef]
- Wang, X.S.; Lau, H.Y. Prostaglandin E potentiates the immunologically stimulated histamine release from human peripheral blood-derived mast cells through EP1/EP3 receptors. Allergy 2006, 61, 503–506. [Google Scholar] [CrossRef]
- Weller, C.L.; Collington, S.J.; Hartnell, A.; Conroy, D.M.; Kaise, T.; Barker, J.E.; Wilson, M.S.; Taylor, G.W.; Jose, P.J.; Williams, T.J. Chemotactic action of prostaglandin E2 on mouse mast cells acting via the PGE2 receptor 3. Proc. Natl. Acad. Sci. USA 2007, 104, 11712–11717. [Google Scholar]
- Kalinski, P. Regulation of immune responses by prostaglandin E2. J. Immunol. 2012, 188, 21–28. [Google Scholar] [CrossRef]
- Roper, R.L.; Ludlow, J.W.; Phipps, R.P. Prostaglandin E2 inhibits B lymphocyte activation by a cAMP-dependent mechanism: PGE-inducible regulatory proteins. Cell. Immunol. 1994, 154, 296–308. [Google Scholar] [CrossRef]
- Su, Y.; Huang, X.; Raskovalova, T.; Zacharia, L.; Lokshin, A.; Jackson, E.; Gorelik, E. Cooperation of adenosine and prostaglandin E2 (PGE2) in amplification of cAMP-PKA signaling and immunosuppression. Cancer Immunol. Immunother. 2008, 57, 1611–1623. [Google Scholar] [CrossRef]
- Holt, D.; Ma, X.; Kundu, N.; Fulton, A. Prostaglandin E (2) (PGE (2)) suppresses natural killer cell function primarily through the PGE (2) receptor EP4. Cancer Immunol. Immunother. 2011, 60, 1577–1586. [Google Scholar]
- Hirai, H.; Tanaka, K.; Yoshie, O.; Ogawa, K.; Kenmotsu, K.; Takamori, Y.; Ichimasa, M.; Sugamura, K.; Nakamura, M.; Takano, S.; Nagata, K. Prostaglandin D2 selectively induces chemotaxis in T helper type 2 cells, eosinophils, and basophils via seven-transmembrane receptor CRTH2. J. Exp. Med. 2001, 193, 255–261. [Google Scholar]
- Monneret, G.; Gravel, S.; Diamond, M.; Rokach, J.; Powell, W.S. Prostaglandin D2 is a potent chemoattractant for human eosinophils that acts via a novel DP receptor. Blood 2001, 98, 1942–1948. [Google Scholar]
- Gosset, P.; Bureau, F.; Angeli, V.; Pichavant, M.; Faveeuw, C.; Tonnel, A.B.; Trottein, F. Prostaglandin D2 affects the maturation of human monocyte-derived dendritic cells: Consequence on the polarization of naive Th cells. J. Immunol. 2003, 170, 4943–4952. [Google Scholar]
- Boie, Y.; Sawyer, N.; Slipetz, D.M.; Metters, K.M.; Abramovitz, M. Molecular cloning and characterization of the human prostanoid DP receptor. J. Biol. Chem. 1995, 270, 18910–18916. [Google Scholar]
- Faveeuw, C.; Gosset, P.; Bureau, F.; Angeli, V.; Hirai, H.; Maruyama, T.; Narumiya, S.; Capron, M.; Trottein, F. Prostaglandin D2 inhibits the production of interleukin-12 in murine dendritic cells through multiple signaling pathways. Eur. J. Immunol. 2003, 33, 889–898. [Google Scholar] [CrossRef]
- Chen, Y.; Perussia, B.; Campbell, K.S. Prostaglandin D2 suppresses human NK cell function via signaling through D prostanoid receptor. J. Immunol. 2007, 179, 2766–2773. [Google Scholar]
- Schwerbrock, N.M.; Karlsson, E.A.; Shi, Q.; Sheridan, P.A.; Beck, M.A. Fish oil-fed mice have impaired resistance to influenza infection. J. Nutr. 2009, 139, 1588–1594. [Google Scholar] [CrossRef]
- Yamashita, N.; Sugiyama, E.; Hamazaki, T.; Yano, S. Inhibition of natural killer cell activity by eicosapentaenoic acid in vivo and in vitro. Biochem. Biophys. Res. Commun. 1988, 150, 497–505. [Google Scholar] [CrossRef]
- Peterson, L.D.; Jeffery, N.M.; Thies, F.; Sanderson, P.; Newsholme, E.A.; Calder, P.C. Eicosapentaenoic and docosahexaenoic acids alter rat spleen leukocyte fatty acid composition and prostaglandin E2 production but have different effects on lymphocyte functions and cell-mediated immunity. Lipids 1998, 33, 171–180. [Google Scholar] [CrossRef]
- Chang, K.J.; Saito, H.; Tamura, Y.; Watanabe, K.; Yoshida, S. Effect of oral ingestion of eicosapentaenoic acid-ethyl ester on natural killer cell activity in rat spleen cells. Prostaglandins Leukot. Essent. Fatty Acid 1989, 37, 31–35. [Google Scholar] [CrossRef]
- Brouard, C.; Pascaud, M. Modulation of rat and human lymphocyte function by n-6 and n-3 polyunsaturated fatty acids and acetylsalicylic acid. Ann. Nutr. Metab. 1993, 37, 146–159. [Google Scholar] [CrossRef]
- Thies, F.; Nebe-von-Caron, G.; Powell, J.R.; Yaqoob, P.; Newsholme, E.A.; Calder, P.C. Dietary supplementation with eicosapentaenoic acid, but not with other long-chain n-3 or n-6 polyunsaturated fatty acids, decreases natural killer cell activity in healthy subjects aged > 55 y. Am. J. Clin. Nutr. 2001, 73, 539–548. [Google Scholar]
- Kakutani, S.; Ishikura, Y.; Tateishi, N.; Horikawa, C.; Tokuda, H.; Kontani, M.; Kawashima, H.; Sakakibara, Y.; Kiso, Y.; Shibata, H.; Morita, I. Supplementation of arachidonic acid-enriched oil increases arachidonic acid contents in plasma phospholipids, but does not increase their metabolites and clinical parameters in Japanese healthy elderly individuals: A randomized controlled study. Lipids Health Dis. 2011, 10, 241. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Juman, S.; Hashimoto, M.; Katakura, M.; Inoue, T.; Tanabe, Y.; Arita, M.; Miki, T.; Shido, O. Effects of Long-Term Oral Administration of Arachidonic Acid and Docosahexaenoic Acid on the Immune Functions of Young Rats. Nutrients 2013, 5, 1949-1961. https://doi.org/10.3390/nu5061949
Juman S, Hashimoto M, Katakura M, Inoue T, Tanabe Y, Arita M, Miki T, Shido O. Effects of Long-Term Oral Administration of Arachidonic Acid and Docosahexaenoic Acid on the Immune Functions of Young Rats. Nutrients. 2013; 5(6):1949-1961. https://doi.org/10.3390/nu5061949
Chicago/Turabian StyleJuman, Sachiko, Michio Hashimoto, Masanori Katakura, Takayuki Inoue, Yoko Tanabe, Makoto Arita, Tomohiro Miki, and Osamu Shido. 2013. "Effects of Long-Term Oral Administration of Arachidonic Acid and Docosahexaenoic Acid on the Immune Functions of Young Rats" Nutrients 5, no. 6: 1949-1961. https://doi.org/10.3390/nu5061949
APA StyleJuman, S., Hashimoto, M., Katakura, M., Inoue, T., Tanabe, Y., Arita, M., Miki, T., & Shido, O. (2013). Effects of Long-Term Oral Administration of Arachidonic Acid and Docosahexaenoic Acid on the Immune Functions of Young Rats. Nutrients, 5(6), 1949-1961. https://doi.org/10.3390/nu5061949




