The Role of Hypertriglyceridemia in the Development of Atherosclerosis and Endothelial Dysfunction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Drugs Used
2.2. Measurement of Plasma Lipid Concentration
2.3. Histological Examination
2.4. Measurement of Vascular Relaxing and Contracting Function

2.5. Statistics
3. Results
3.1. Effect of Hypertriglyceridemia on the Accumulation of Visceral Fat
| Weight of Tissues (g/Body Weight: kg) | Normal Rabbit # (n = 5) | PHT: 12-Month-Old (n = 5) | PHT: 33-Month-Old (n = 5) |
|---|---|---|---|
| Visceral Fat (Mesenteric Fat) | 39.73 ± 8.08 | 84.79 ± 3.59 * | 63.75 ± 10.99 * |
| Liver | 24.62 ± 1.45 | 39.97 ± 5.09 * | 27.37 ± 1.41 * |
| Kidney | 5.37 ± 0.16 | 7.44 ± 0.31 * | 7.09 ± 0.23 * |
3.2. Changes in the Concentration of Plasma Lipids in PHT Rabbits
3.3. Histological Examination of the Adipose Cells of Hypertriglyceridemic Rabbits



3.4. Histological Examination of the Liver


3.5. Histological Examination of the Aorta of Hypertriglyceridemic Rabbits


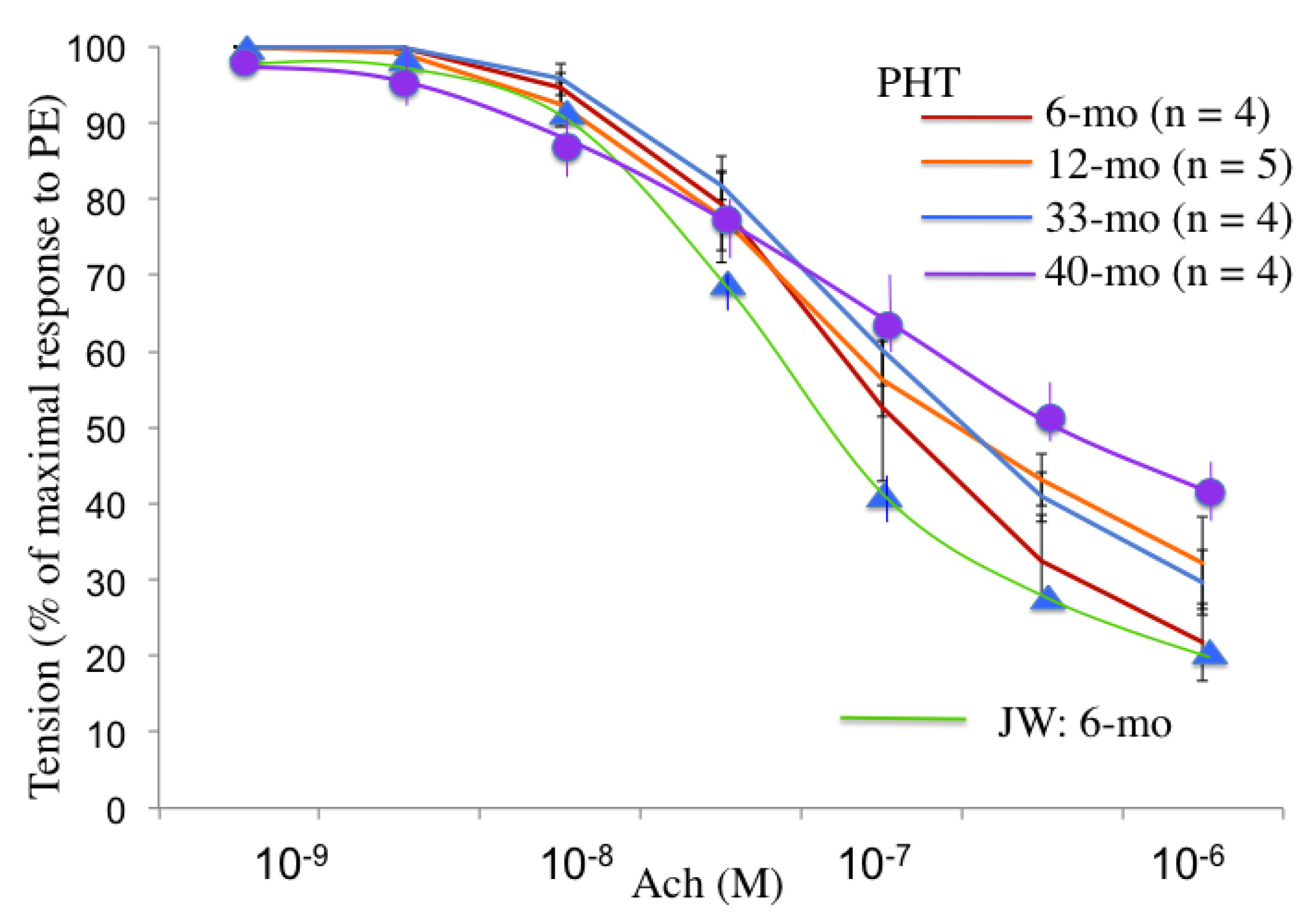
3.6. Endothelium-Dependent Relaxation in Hypertriglyceridemic Rabbits
4. Discussion
4.1. Adipose Tissue in PHT Rabbit
4.2. Endothelial Dysfunction and Atherosclerosis
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Carlson, L.A.; Boettiger, L.E. Risk factors for ischemic disease in men and women. Results of the 19-year follow-up of the Stockholm Prospective Study. Acta Med. Scand. 1985, 218, 207–211. [Google Scholar] [CrossRef]
- Susanna, B.; Giacomo, R.; Rong, T.; Johan, B. Alimentary lipemia, postprandial triglyceride-rich lipoproteins, and common carotid intima-media thickness in healthy, middle-aged men. Circulation 1999, 100, 723–728. [Google Scholar] [CrossRef]
- Koskinen, P.; Manttari, M.; Manninen, V.; Huttunen, J.K.; Heinonen, O.P.; Frick, M.H. Coronary heart disease incidence in NIDDM patients in the Helsinki Heart Study. Diabetes Care 1992, 15, 820–825. [Google Scholar]
- Patsch, J.R.; Miesenbock, G.; Hopferwieser, T.; Muhlberger, V.; Knapp, E.; Dunn, J.K.; Gotto, A.M., Jr.; Patsch, W. Relation of triglyceride metabolism and coronary artery disease: Studies in the postprandial state. Arterioscler. Thromb. 1992, 12, 1336–1345. [Google Scholar] [CrossRef]
- Iso, H.; Naito, Y.; Sato, S.; Kitamura, A.; Okamura, T.; Sankai, T. Serum triglycerides and risk of coronary heart disease among Japanese men and women. Am. J. Epidemiol. 2001, 153, 490–499. [Google Scholar] [CrossRef]
- Haffner, S.M.; D’Agostino, R., Jr.; Mykkanen, L.; Tracy, R.; Howard, B.; Rewers, M.; Selby, J.; Savage, P.J.; Saad, M.F. Insulin sensitivity in subjects with type 2 diabetes: Relationship to cardiovascular risk factors: The Insulin Resistance Atherosclerosis Study. Diabetes Care 1999, 22, 562–568. [Google Scholar] [CrossRef]
- Kannel, W.B.; Vasan, R.S. Triglycerides as vascular risk factors: New epidemiologic insights. Curr. Opin. Cardiol. 2009, 24, 345–350. [Google Scholar] [CrossRef]
- Nordestgaard, B.G.; Benn, M.; Schnohr, P.; Tybjaerg-Hansen, A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA 2007, 298, 299–308. [Google Scholar] [CrossRef]
- Miller, M.; Stone, N.J.; Ballantyne, C.; Bittner, V.; Criqui, M.H.; Ginsberg, H.N.; Goldberg, A.C.; Howard, W.J.; Jacobson, M.S.; Kris-Etherton, P.M.; et al. Triglycerides and Cardiovascular Disease. Circulation 2011, 123, 2292–2333. [Google Scholar] [CrossRef]
- Wang, Y.I.; Schulze, J.; Raymond, N.; Tomita, T.; Tam, K.; Simon, S.I.; Passerini, A.G. Endothelial inflammation correlates with subject triglycerides and waist size after a high-fat meal. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H784–H791. [Google Scholar] [CrossRef]
- Bae, J.H.; Bassenge, E.; Kim, K.B.; Kim, Y.N.; Kim, K.S.; Lee, H.J.; Moon, K.C.; Lee, M.S.; Park, K.Y.; Schwemmer, M. Postprandial hypertriglyceridemia impairs endothelial function by enhanced oxidant stress. Atherosclerosis 2001, 155, 517–523. [Google Scholar] [CrossRef]
- Vogel, R.A.; Corretti, M.C.; Plotnick, G.D. Effect of a single high-fat meal on endothelial function in healthy subjects. Am. J. Cardiol. 1997, 79, 350–354. [Google Scholar] [CrossRef]
- Mano, T.; Masuyama, T.; Yamamoto, K.; Naito, J.; Kondo, H.; Nagano, R.; Tanouchi, J.; Hori, M.; Inoue, M.; Kamada, T. Endothelial dysfunction in the early stage of atherosclerosis precedes appearance of intimal lesions assessable with intravascular ultrasound. Am. Heart J. 1996, 131, 231–238. [Google Scholar] [CrossRef]
- Fukuda, N.; Ito, T.; Ohwada, K.; Fujii, J. Upregulation of fatty acid synthesis and the suppression of hepatic triglyceride lipase as a direct cause of hereditary postprandial hypertriglyceridemia in rabbits. J. Clin. Biochem. Nutr. 2013, 53, 114–121. [Google Scholar] [CrossRef]
- Kawai, T.; Ito, T.; Ohwada, K.; Mera, Y.; Matsushita, M.; Tomoike, H. Hereditary postprandial hypertriglyceridemic rabbit exhibits insulin resistance and central obesity: A novel model of metabolic syndrome. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2752–2757. [Google Scholar] [CrossRef]
- Institute of Laboratory Animal Resources; Commission on Life Sciences; National Research Council. Guide for the Care and Use of Laboratory Animals; National Academy Press: Washington, DC, USA, 1996. Available online: http://www.nap.edu/readingroom/books/labrats/index.html (accessed on 18 March 2014).
- Hata, M.; Ito, T.; Ohwada, K. Kinetic analysis of apolipoproteins in postprandial hypertriglyceridaemia rabbits. Lab. Anim. 2009, 43, 174–181. [Google Scholar] [CrossRef]
- Ouchi, N.; Walsh, K. Adiponectin as an anti-inflammatory factor. Clin. Chem. Acta 2007, 380, 24–30. [Google Scholar] [CrossRef]
- Ito, T.; Ohwada, K.; Tomoike, H. A hereditary postprandial hypertriglyceridemic (PHT) rabbit model. Nihon Yakurigaku Zasshi 2005, 125, 301–306. (in Japanese). [Google Scholar] [CrossRef]
- Festi, D.; Colecchia, A.; Sacco, T.; Bondi, M.; Roda, E.; Marchesini, G. Hepatic steatosis in obese patients: Clinical aspects and prognostic significance. Obes. Rev. 2004, 5, 27–42. [Google Scholar] [CrossRef]
- Zilversmit, D.B. A proposal linking atherogenesis to the interaction of endothelial lipase with triglyceride-rich lipoprotein. Circ. Res. 1999, 17, 413–418. [Google Scholar]
- Alipour, A.; Elte, J.W.; van Zaanen, H.C.; Rietveld, A.O.; Cabezas, M.C. Postprandial inflammation and endothelial dysfunction. Biochem. Soc. Trans. 2007, 35, 466–469. [Google Scholar] [CrossRef]
- Tomkin, G.H.; Owens, D. The chylomicron: Relationship to atherosclerosis. Int. J. Vasc. Med. 2012, 2012. [Google Scholar] [CrossRef]
- Procter, S.D.; Mamo, J.C. Intimal retension of cholesterol derived from apolipoprotein B-100 and apolipoprotein B48-containing lipoproteins in carotid arteries of Watanabe hiritable hyperlipidemic rabbits. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1595–1600. [Google Scholar] [CrossRef]
- Nakano, T.; Nakajima, K.; Niimi, M.; Fujita, M.O.; Nakajima, Y.; Takeichi, S.; Kinoshita, M.; Matsusima, T.; Teramoto, T.; Tanaka, A. Detection of apolipoproteins B-48 and B-100 carrying particles in lipoprotein fractions extracted from human aortic atherosclerotic plaques in sudden cardiac death cases. Clin. Chim. Acta 2008, 390, 38–43. [Google Scholar] [CrossRef]
- Mero, N.; Malmström, R.; Steiner, G.; Taskinen, M.R.; Syvänne, M. Postprandial metabolism of apolipoprotein B-48- and B-100-containing particles in type 2 diabetes mellitus: Relations to angiographically verified severity of coronary artery disease. Atherosclerosis 2000, 150, 167–177. [Google Scholar] [CrossRef]
- Teno, S.; Uto, Y.; Nagashima, H.; Endoh, Y.; Iwamoto, Y.; Onmori, Y.; Takizawa, T. Association of postprandial hypertriglyceridemia and carotid intima-media thickness in patients with type 2 diabetes. Diabetes Care 2000, 23, 1401–1406. [Google Scholar] [CrossRef]
- Fukuda, N.; Ito, T.; Katahira, K.; Ohwada, K. Analysis of heart rate and blood pressure in PHT rabbit. Yamagata Med. J. 2010, 208, 69–72. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Matsumoto, S.; Gotoh, N.; Hishinuma, S.; Abe, Y.; Shimizu, Y.; Katano, Y.; Ishihata, A. The Role of Hypertriglyceridemia in the Development of Atherosclerosis and Endothelial Dysfunction. Nutrients 2014, 6, 1236-1250. https://doi.org/10.3390/nu6031236
Matsumoto S, Gotoh N, Hishinuma S, Abe Y, Shimizu Y, Katano Y, Ishihata A. The Role of Hypertriglyceridemia in the Development of Atherosclerosis and Endothelial Dysfunction. Nutrients. 2014; 6(3):1236-1250. https://doi.org/10.3390/nu6031236
Chicago/Turabian StyleMatsumoto, Saki, Nozomi Gotoh, Saori Hishinuma, Yohei Abe, Yoshimi Shimizu, Yumi Katano, and Akira Ishihata. 2014. "The Role of Hypertriglyceridemia in the Development of Atherosclerosis and Endothelial Dysfunction" Nutrients 6, no. 3: 1236-1250. https://doi.org/10.3390/nu6031236
APA StyleMatsumoto, S., Gotoh, N., Hishinuma, S., Abe, Y., Shimizu, Y., Katano, Y., & Ishihata, A. (2014). The Role of Hypertriglyceridemia in the Development of Atherosclerosis and Endothelial Dysfunction. Nutrients, 6(3), 1236-1250. https://doi.org/10.3390/nu6031236




