Genetic Sensitivity to the Bitter Taste of 6-n-Propylthiouracil (PROP) and Its Association with Physiological Mechanisms Controlling Body Mass Index (BMI)
Abstract
:1. Introduction
2. Physiological Overview of Taste Sensitivity
3. Genetic Factors Contributing to PROP Sensitivity
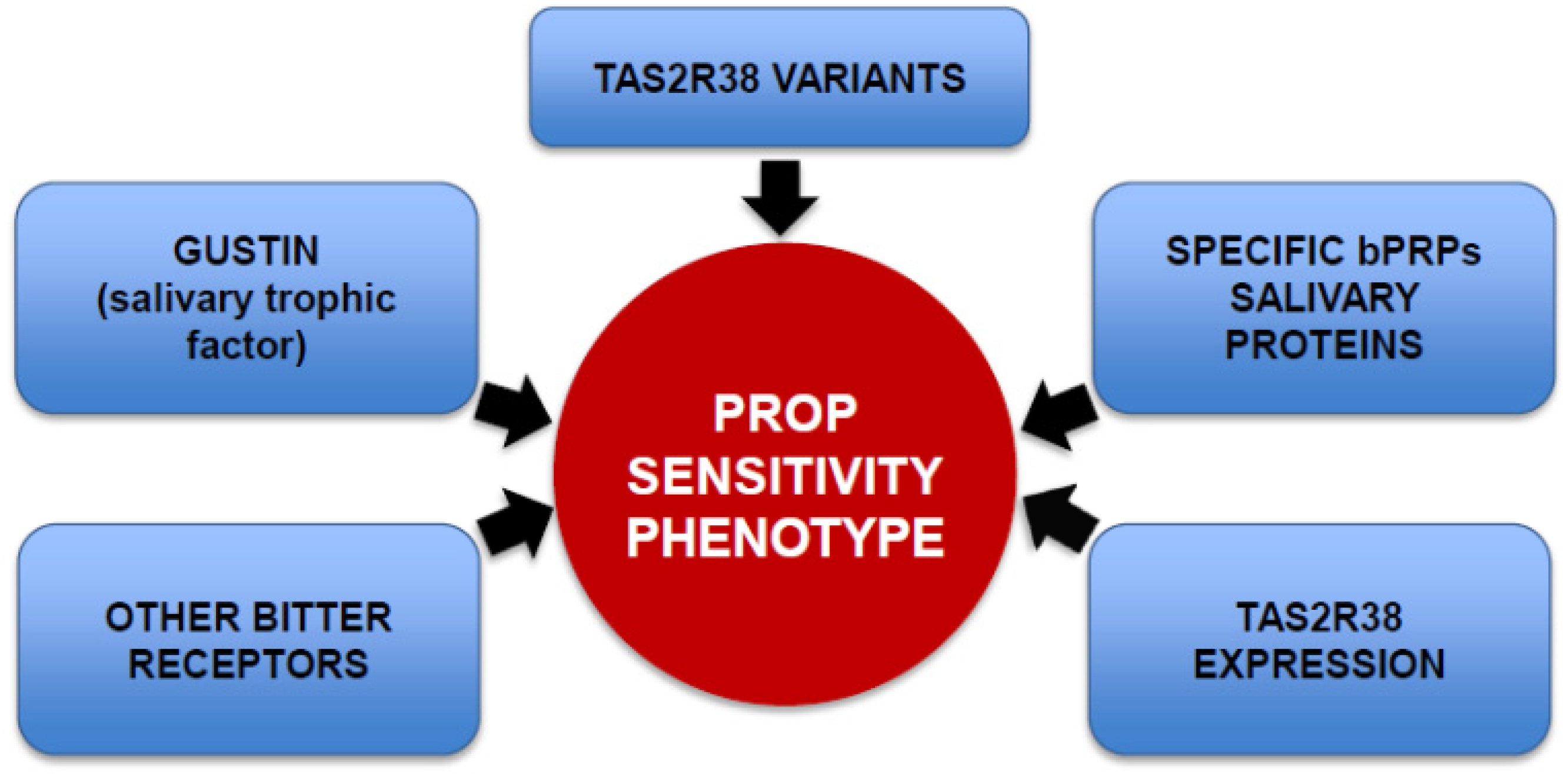
4. Nutritional Implications of PROP Bitter Taste Sensitivity
5. Variables that May Influence the Relationship between PROP Sensitivity and BMI

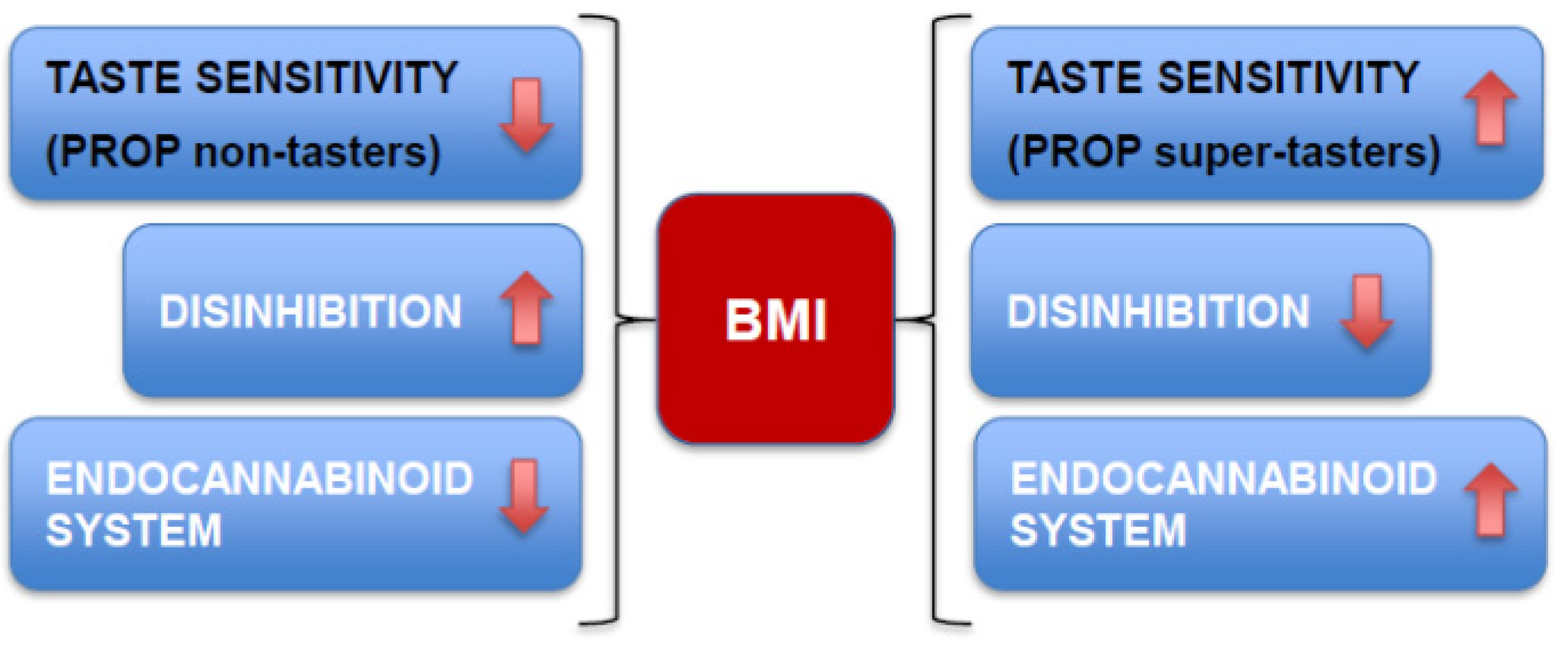
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Scott, K. Taste recognition: Food for thought. Neuron 2005, 48, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, N.; Roper, S.D. The cell biology of taste. J. Cell Biol. 2010, 190, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Mattes, R.D. Fat taste in humans: Is it a primary? In Fat Detection: Taste, Texture, and Post Ingestive Effects; Montmayeur, J.P., le Coutre, J., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 167–196. [Google Scholar]
- Ebba, S.; Abarintos, R.A.; Kim, D.G.; Tiyouh, M.; Stull, J.C.; Movalia, A.; Smutzer, G. The examination of fatty acid taste with edible strips. Physiol. Behav. 2012, 106, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Mattes, R.D. Oral fatty acid signaling and intestinal lipid processing: Support and supposition. Physiol. Behav. 2011, 105, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Pepino, M.Y.; Love-Gregory, L.; Klein, S.; Abumrad, N.A. The fatty acid translocase gene CD36 and lingual lipase influence oral sensitivity to fat in obese subjects. J. Lipid Res. 2012, 53, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Chevrot, M.; Poirier, H.; Passilly-Degrace, P.; Niot, I.; Besnard, P. CD36 as a lipid sensor. Physiol. Behav. 2011, 105, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Laugerette, F.; Passilly-Degrace, P.; Patris, B.; Niot, I.; Febbraio, M.; Montmayeur, J.P.; Besnard, P. CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J. Clin. Invest. 2005, 115, 3177–3184. [Google Scholar] [CrossRef] [PubMed]
- Sclafani, A.; Ackroff, K.; Abumrad, N.A. CD36 gene deletion reduces fat preference and intake but not post-oral fat conditioning in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R1823–R1832. [Google Scholar] [CrossRef] [PubMed]
- Tepper, B.J. Nutritional implications of genetic taste variation: The role of PROP sensitivity and other taste phenotypes. Annu. Rev. Nutr. 2008, 28, 367–388. [Google Scholar] [CrossRef] [PubMed]
- Soranzo, N.; Bufe, B.; Sabeti, P.C.; Wilson, J.F.; Weale, M.E.; Marguerie, R.; Meyerhof, W.; Goldstein, D.B. Positive selection on a high-sensitivity allele of the human bitter-taste receptor TAS2R16. Curr. Biol. 2005, 15, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Wooding, S.; Kim, U.K.; Bamshad, M.J.; Larsen, J.; Jorde, L.B.; Drayna, D. Natural selection and molecular evolution in PTC, a bitter-taste receptor gene. Am. J. Hum. Genet. 2004, 74, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Drewnowski, A.; Gomez-Carneros, C. Bitter taste, phytonutrients, and the consumer: A review. Am. J. Clin. Nutr. 2000, 72, 1424–1435. [Google Scholar] [PubMed]
- D’Archivio, M.; Filesi, C.; di Benedetto, R.; Gargiulo, R.; Giovannini, C.; Masella, R. Polyphenols, dietary sources and bioavailability. Ann. Ist. Super. Sanita 2007, 43, 348–361. [Google Scholar]
- Mandel, A.L.; Breslin, P.A.S. High endogenous salivary amylase activity is associated with improved glycemic homeostasis following starch ingestion in adults. J. Nutr. 2012, 142, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Vandenbeuch, A.; Clapp, T.R.; Kinnamon, S.C. Amiloride-sensitive channels in type I fungiform taste cells in mouse. BMC Neurosci. 2008, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Nelson, G.; Hoon, M.A.; Chandrashekar, J.; Zhang, Y.; Ryba, N.J.; Zuker, C.S. Mammalian sweet taste receptors. Cell 2001, 106, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Yarmolinsky, D.A.; Zuker, C.S.; Ryba, N.J. Common sense about taste: From mammals to insects. Cell 2009, 139, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, J.; Hoon, M.A.; Ryba, N.J.; Zuker, C.S. The receptors and cells for mammalian taste. Nature 2006, 444, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Ji, Q.; Liu, Z.; Snyder, L.A.; Benard, L.M.; Margolskee, R.F.; Max, M. The cysteine-rich region of T1R3 determines responses to intensely sweet proteins. J. Biol. Chem. 2004, 279, 45068–45075. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Staszewski, L.; Tang, H.; Adler, E.; Zoller, M.; Li, X. Different functional roles of T1R subunits in the heteromeric taste receptors. Proc. Natl. Acad. Sci. USA 2004, 101, 14258–14263. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Staszewski, L.; Xu, H.; Durick, K.; Zoller, M.; Adler, E. Human receptors for sweet and umami taste. Proc. Natl. Acad. Sci. USA 2002, 99, 4692–4696. [Google Scholar] [CrossRef] [PubMed]
- Nelson, G.; Chandrashekar, J.; Hoon, M.A.; Feng, L.; Zhao, G.; Ryba, N.J.; Zuker, C.S. An amino-acid taste receptor. Nature 2002, 416, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Damak, S.; Rong, M.; Yasumatsu, K.; Kokrashvili, Z.; Varadarajan, V.; Zou, S.; Jiang, P.; Ninomiya, Y.; Margolskee, R.F. Detection of sweet and umami taste in the absence of taste receptor T1R3. Science 2003, 301, 850–853. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, Y.; Pereira, E.; Margolskee, R.F.; Chaudhari, N.; Roper, S.D. Umami responses in mouse taste cells indicate more than one receptor. J. Neurosci. 2006, 26, 2227–2234. [Google Scholar] [CrossRef] [PubMed]
- Yasumatsu, K.; Horio, N.; Murata, Y.; Shirosaki, S.; Ohkuri, T.; Yoshida, R.; Ninomiya, Y. Multiple receptors underlie glutamate taste responses in mice. Am. J. Clin. Nutr. 2009, 90, 747S–752S. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Zhang, J.; Yang, H.; Zhang, Y.P. Adaptive diversification of bitter taste receptor genes in mammalian evolution. Mol. Biol. Evol. 2003, 20, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, J.; Mueller, K.L.; Hoon, M.A.; Adler, E.; Feng, L.; Guo, W.; Zuker, C.S.; Ryba, N.J. T2Rs function as bitter taste receptors. Cell 2000, 100, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Roper, S.D. Signal transduction and information processing in mammalian taste buds. Pflugers Arch. 2007, 454, 759–776. [Google Scholar] [CrossRef] [PubMed]
- Behrens, M.; Reichling, C.; Batram, C.; Brockhoff, A.; Meyerhof, W. Bitter taste receptors and their cells. Ann. N. Y. Acad. Sci. 2009, 1170, 111–115. [Google Scholar] [CrossRef]
- Mueller, K.L.; Hoon, M.A.; Erlenbach, I.; Chandrashekar, J.; Zuker, C.S.; Ryba, N.J. The receptors and coding logic for bitter taste. Nature 2005, 434, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Meyerhof, W.; Batram, C.; Kuhn, C.; Brockhoff, A.; Chudoba, E.; Bufe, B.; Appendino, G.; Behrens, M. The molecular receptive ranges of human TAS2R bitter taste receptors. Chem. Senses 2010, 35, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Murray, R.G. Cellular relations in mouse circumvallate taste buds. Microsc. Res. Tech. 1993, 26, 209–224. [Google Scholar] [CrossRef] [PubMed]
- Murray, R.G.; Murray, A.; Fujimoto, S. Fine structure of gustatory cells in rabbit taste buds. J. Ultrastruct. Res. 1969, 27, 444–461. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Crowley, H.H.; Rock, M.E.; Kinnamon, J.C. Taste cells with synapses in rat circumvallate papillae display snap-25-like immunoreactivity. J. Comp. Neurol. 2000, 424, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Heck, G.L.; DeSimone, J.A. The anion paradox in sodium taste reception: Resolution by voltage-clamp studies. Science 1991, 254, 724–726. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, J.; Yarmolinsky, D.; von Buchholtz, L.; Oka, Y.; Sly, W.; Ryba, N.J.; Zuker, C.S. The taste of carbonation. Science 2009, 326, 443–445. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.L.; Chen, X.; Hoon, M.A.; Chandrashekar, J.; Guo, W.; Trankner, D.; Ryba, N.J.; Zuker, C.S. The cells and logic for mammalian sour taste detection. Nature 2006, 442, 934–938. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.A.; Maruyama, Y.; Stimac, R.; Roper, S.D. Presynaptic (type III) cells in mouse taste buds sense sour (acid) taste. J. Physiol. 2008, 586, 2903–2912. [Google Scholar] [CrossRef] [PubMed]
- Tomchik, S.M.; Berg, S.; Kim, J.W.; Chaudhari, N.; Roper, S.D. Breadth of tuning and taste coding in mammalian taste buds. J. Neurosci. 2007, 27, 10840–10848. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, P.M.; Chen, J.Y.; Victor, J.D. Quality time: Representation of a multidimensional sensory domain through temporal coding. J. Neurosci. 2009, 29, 9227–9238. [Google Scholar]
- Miller, P.; Katz, D.B. Stochastic transitions between neural states in taste processing and decision-making. J. Neurosci. 2010, 30, 2559–2570. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.M.; Fontanini, A.; Katz, D.B. Gustatory processing: A dynamic systems approach. Curr. Opin. Neurobiol. 2006, 16, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Fox, A.L. The relationship between chemical constitution and taste. Proc. Natl. Acad. Sci. USA 1932, 18, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Bartoshuk, L.M. Comparing sensory experiences across individuals: Recent psychophysical advances illuminate genetic variation in taste perception. Chem. Senses. 2000, 25, 447–460. [Google Scholar] [CrossRef] [PubMed]
- Bartoshuk, L.M. The biological basis of food perception and acceptance. Food Qual. Pref. 1993, 4, 21–32. [Google Scholar] [CrossRef]
- Bartoshuk, L.M.; Duffy, V.B.; Miller, I.J. PTC/PROP tasting: Anatomy, psychophysics, and sex effects. Physiol. Behav. 1994, 56, 1165–1171. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.W.; Reed, D.R. The genetics of phenylthiocarbamide perception. Ann. Hum. Biol. 2001, 28, 111–142. [Google Scholar] [CrossRef] [PubMed]
- Bajec, M.R.; Pickering, G.J. Thermal taste, PROP responsiveness, and perception of oral sensations. Physiol. Behav. 2008, 95, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Yeomans, M.R.; Tepper, B.J.; Rietzschel, J.; Prescott, J. Human hedonic responses to sweetness: Role of taste genetics and anatomy. Physiol. Behav. 2007, 91, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Essick, G.K.; Chopra, A.; Guest, S.; McGlone, F. Lingual tactile acuity, taste perception, and the density and diameter of fungiform papillae in female subjects. Physiol. Behav. 2003, 80, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Shahbake, M.; Hutchinson, I.; Laing, D.G.; Jinks, A.L. Rapid quantitative assessment of fungiform papillae density in the human tongue. Brain Res. 2005, 1052, 196–201. [Google Scholar] [CrossRef]
- Melis, M.; Atzori, E.; Cabras, S.; Zonza, A.; Calo, C.; Muroni, P.; Nieddu, M.; Padiglia, A.; Sogos, V.; Tepper, B.J.; et al. The gustin (CA6) gene polymorphism, rs2274333 (A/G), as a mechanistic link between PROP tasting and fungiform taste papilla density and maintenance. PLoS One 2013, 8, e74151. [Google Scholar] [CrossRef] [PubMed]
- Tepper, B.J.; Nurse, R.J. Fat perception is related to PROP taster status. Physiol. Behav. 1997, 61, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Kalmus, H. Improvements in the classification of the taster genotypes. Ann. Hum. Genet. 1958, 22, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Bufe, B.; Breslin, P.A.; Kuhn, C.; Reed, D.R.; Tharp, C.D.; Slack, J.P.; Kim, U.K.; Drayna, D.; Meyerhof, W. The molecular basis of individual differences in phenylthiocarbamide and propylthiouracil bitterness perception. Curr. Biol. 2005, 15, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Kim, U.K.; Jorgenson, E.; Coon, H.; Leppert, M.; Risch, N.; Drayna, D. Positional cloning of the human quantitative trait locus underlying taste sensitivity to phenylthiocarbamide. Science 2003, 299, 1221–1225. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.E.; Bartoshuk, L.M.; Kidd, J.R.; Duffy, V.B. Supertasting and PROP bitterness depends on more than the TAS2R38 gene. Chem. Senses. 2008, 33, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Lipchock, S.V.; Mennella, J.A.; Spielman, A.I.; Reed, D.R. Human bitter perception correlates with bitter receptor messenger RNA expression in taste cells. Am. J. Clin. Nutr. 2013, 98, 1136–1143. [Google Scholar] [CrossRef] [PubMed]
- Olson, J.M.; Boehnke, M.; Neiswanger, K.; Roche, A.F.; Siervogel, R.M. Alternative genetic models for the inheritance of the phenylthiocarbamide taste deficiency. Genet. Epidemiol. 1989, 6, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Drayna, D.; Coon, H.; Kim, U.K.; Elsner, T.; Cromer, K.; Otterud, B.; Baird, L.; Peiffer, A.P.; Leppert, M.; Utah Genetic Reference Project. Genetic analysis of a complex trait in the Utah Genetic Reference Project: A major locus for ptc taste ability on chromosome 7q and a secondary locus on chromosome 16p. Hum. Genet. 2003, 112, 567–572. [Google Scholar]
- Reed, D.R.; Nanthakumar, E.; North, M.; Bell, C.; Bartoshuk, L.M.; Price, R.A. Localization of a gene for bitter-taste perception to human chromosome 5p15. Am. J. Hum. Genet. 1999, 64, 1478–1480. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, R. Role of saliva in the maintenance of taste sensitivity. Crit. Rev. Oral. Biol. Med. 2000, 11, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Bennick, A. Interaction of plant polyphenols with salivary proteins. Crit. Rev. Oral. Biol. Med. 2002, 13, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Dinnella, C.; Recchia, A.; Fia, G.; Bertuccioli, M.; Monteleone, E. Saliva characteristics and individual sensitivity to phenolic astringent stimuli. Chem. Senses 2009, 34, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Dinnella, C.; Recchia, A.; Tuorila, H.; Monteleone, E. Individual astringency responsiveness affects the acceptance of phenol-rich foods. Appetite 2011, 56, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Dinnella, C.; Recchia, A.; Vincenzi, S.; Tuorila, H.; Monteleone, E. Temporary modification of salivary protein profile and individual responses to repeated phenolic astringent stimuli. Chem. Senses 2010, 35, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Cabras, T.; Melis, M.; Castagnola, M.; Padiglia, A.; Tepper, B.J.; Messana, I.; Tomassini Barbarossa, I. Responsiveness to 6-n-propylthiouracil (PROP) is associated with salivary levels of two specific basic proline-rich proteins in humans. PLoS One 2012, 7, e30962. [Google Scholar] [CrossRef] [PubMed]
- Azen, E.A.; Latreille, P.; Niece, R.L. PRBI gene variants coding for length and null polymorphisms among human salivary Ps, PmF, PmS, and Pe proline-rich proteins (PRPs). Am. J. Hum. Genet. 1993, 53, 264–278. [Google Scholar] [PubMed]
- Melis, M.; Aragoni, M.C.; Arca, M.; Cabras, T.; Caltagirone, C.; Castagnola, M.; Crnjar, R.; Messana, I.; Tepper, B.J.; Barbarossa, I.T. Marked increase in PROP taste responsiveness following oral supplementation with selected salivary proteins or their related free amino acids. PLoS One 2013, 8, e59810. [Google Scholar] [CrossRef] [PubMed]
- Padiglia, A.; Zonza, A.; Atzori, E.; Chillotti, C.; Calo, C.; Tepper, B.J.; Barbarossa, I.T. Sensitivity to 6-n-propylthiouracil is associated with gustin (carbonic anhydrase VI) gene polymorphism, salivary zinc, and body mass index in humans. Am. J. Clin. Nutr. 2010, 92, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Henkin, R.I.; Martin, B.M.; Agarwal, R.P. Efficacy of exogenous oral zinc in treatment of patients with carbonic anhydrase VI deficiency. Am. J. Med. Sci. 1999, 318, 392–405. [Google Scholar] [CrossRef] [PubMed]
- Calo, C.; Padiglia, A.; Zonza, A.; Corrias, L.; Contu, P.; Tepper, B.J.; Barbarossa, I.T. Polymorphisms in TAS2R38 and the taste bud trophic factor, gustin gene co-operate in modulating PROP taste phenotype. Physiol. Behav. 2011, 104, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Genick, U.K.; Kutalik, Z.; Ledda, M.; Destito, M.C.S.; Souza, M.M.; Cirillo, C.A.; Godinot, N.; Martin, N.; Morya, E.; Sameshima, K.; et al. Sensitivity of genome-wide-association signals to phenotyping strategy: The PROP-TAS2R38 taste association as a benchmark. Plos One 2011, 6, e27745. [Google Scholar] [CrossRef] [PubMed]
- Feeney, E.L.; Hayes, J.E. Exploring associations between taste perception, oral anatomy and polymorphisms in the carbonic anhydrase (gustin) gene CA6. Physiol. Behav. 2014, 128, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, G.L.; Daun, H.; Tepper, B.J. Influence of PROP taster status and maternal variables on energy intake and body weight of pre-adolescents. Physiol. Behav. 2007, 90, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Whissell-Buechy, D.; Wills, C. Male and female correlations for taster (P.T.C.) phenotypes and rate of adolescent development. Ann. Hum. Biol. 1989, 16, 131–146. [Google Scholar]
- Zuniga, J.R.; Davis, S.H.; Englehardt, R.A.; Miller, I.J.; Schiffrman, S.S.; Phillips, C. Taste performance on the anterior human tongue varles with fungiform taste bud density. Chem. Senses 1993, 18, 449–460. [Google Scholar] [CrossRef]
- Correa, M.; Hutchinson, I.; Laing, D.G.; Jinks, A.L. Changes in fungiform papillae density during development in humans. Chem. Senses 2013, 38, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Gayathri Devi, A.; Henderson, S.A.; Drewnowski, A. Sensory acceptance of Japanese green tea and soy products is linked to genetic sensitivity to 6-n-propylthiouracil. Nutr. Cancer 1997, 29, 146–151. [Google Scholar]
- Bartoshuk, L.M.; Duffy, V.B.; Lucchina, L.A.; Prutkin, J.; Fast, K. PROP (6-n-propylthiouracil) supertasters and the saltiness of NaCl. Ann. N. Y. Acad. Sci. 1998, 855, 793–796. [Google Scholar] [CrossRef] [PubMed]
- Bartoshuk, L.; Fast, K.; Karrer, T.; Marino, S.; Price, R.; Reed, D. PROP supertasters and the perception of sweetness and bitterness. Chem. Senses 1992, 17, 594. [Google Scholar]
- Bartoshuk, L.M. Bitter taste of saccharin related to the genetic ability to taste the bitter substance 6-n-propylthiouracil. Science 1979, 205, 934–935. [Google Scholar] [CrossRef] [PubMed]
- Bartoshuk, L.M.; Rifkin, B.; Marks, L.E.; Bars, P. Taste and aging. J. Gerontol. 1986, 41, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Bartoshuk, L.M.; Rifkin, B.; Marks, L.E.; Hooper, J.E. Bitterness of KCl and benzoate: Related to genetic status for sensitivity to PTC/PROP. Chem. Senses 1988, 13, 517–528. [Google Scholar] [CrossRef]
- Gent, J.; Bartoshuk, L. Sweetness of sucrose, neohesperidin dihydrochalcone, and saccharin is related to genetic ability to taste the bitter substance 6-n-propylthiouracil. Chem. Senses 1983, 7, 265–272. [Google Scholar] [CrossRef]
- Prescott, J.; Swain-Campbell, N. Responses to repeated oral irritation by capsaicin, cinnamaldehyde and ethanol in PROP tasters and non-tasters. Chem. Senses 2000, 25, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Ly, A.; Drewnowski, A. PROP (6-n-propylthiouracil) tasting and sensory responses to caffeine, sucrose, neohesperidin dihydrochalcone and chocolate. Chem. Senses 2001, 26, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Duffy, V.B.; Davidson, A.C.; Kidd, J.R.; Kidd, K.K.; Speed, W.C.; Pakstis, A.J.; Reed, D.R.; Snyder, D.J.; Bartoshuk, L.M. Bitter receptor gene (TAS2R38), 6-n-propylthiouracil (PROP) bitterness and alcohol intake. Alcohol. Clin. Exp. Res. 2004, 28, 1629–1637. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.E.; Duffy, V.B. Revisiting sugar-fat mixtures: Sweetness and creaminess vary with phenotypic markers of oral sensation. Chem. Senses 2007, 32, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Duffy, V.B.; Bartoshuk, L.M.; Lucchina, L.A.; Snyder, D.J.; Tym, A. Supertasters of PROP (6-n-propylthiouracil) rate the highest creaminess to high-fat milk products. Chem. Senses 1996, 21, 598. [Google Scholar]
- Tepper, B.J.; Nurse, R.J. PROP taster status is related to fat perception and preference. Ann. N. Y. Acad. Sci. 1998, 855, 802–804. [Google Scholar] [CrossRef] [PubMed]
- Keller, K.L.; Steinmann, L.; Nurse, R.J.; Tepper, B.J. Genetic taste sensitivity to 6-n-propylthiouracil influences food preference and reported intake in preschool children. Appetite 2002, 38, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Dinehart, M.E.; Hayes, J.E.; Bartoshuk, L.M.; Lanier, S.L.; Duffy, V.B. Bitter taste markers explain variability in vegetable sweetness, bitterness, and intake. Physiol. Behav. 2006, 87, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, M.; Nakamura, K.; Tamai, Y.; Wada, K.; Sahashi, Y.; Watanabe, K.; Ohtsuchi, S.; Ando, K.; Nagata, C. Relationship of intake of plant-based foods with 6-n-propylthiouracil sensitivity and food neophobia in Japanese preschool children. Eur. J. Clin. Nutr. 2012, 66, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Robino, A.; Mezzavilla, M.; Pirastu, N.; Dognini, M.; Tepper, B.J.; Gasparini, P. A population-based approach to study the impact of PROP perception on food liking in populations along the silk road. PLoS One 2014, 9, e91716. [Google Scholar] [CrossRef] [PubMed]
- Duffy, V.; Lucchina, L.; Bartoshuk, L. Genetic variation in taste: Potential biomarker for cardiovascular disease risk? In Genetic Variations in Taste Sensitivity; Prescott, J., Tepper, B.J., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2004; pp. 195–228. [Google Scholar]
- Prescott, J.; Bartoshuk, L.M.; Prutkin, J. 6-n-Propylthiouracil tasting and the perception of nontaste oral sensations. In Genetic Variation in Taste Sensitivity; Prescott, J., Tepper, B.J., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2004; pp. 89–104. [Google Scholar]
- Hayes, J.E.; Duffy, V.B. Oral sensory phenotype identifies level of sugar and fat required for maximal liking. Physiol. Behav. 2008, 95, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Kirkmeyer, S.V.; Tepper, B.J. Understanding creaminess perception of dairy products using free-choice profiling and genetic responsivity to 6-n-propylthiouracil. Chem. Senses 2003, 28, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Drewnowski, A.; Henderson, S.A.; Barratt-Fornell, A. Genetic sensitivity to 6-n-propylthiouracil and sensory responses to sugar and fat mixtures. Physiol. Behav. 1998, 63, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Drewnowski, A.; Henderson, S.A.; Cockroft, J.E. Genetic sensitivity to 6-n-propylthiouracil has no influence on dietary patterns, body mass indexes, or plasma lipid profiles of women. J. Am. Diet. Assoc. 2007, 107, 1340–1348. [Google Scholar] [CrossRef] [PubMed]
- Duffy, V.B.; Bartoshuk, L.M. Food acceptance and genetic variation in taste. J. Am. Diet. Assoc. 2000, 100, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Forrai, G.; Bankovi, G. Taste perception for phenylthiocarbamide and food choice—A Hungarian twin study. Acta Physiol. Hung. 1984, 64, 33–40. [Google Scholar] [PubMed]
- Tepper, B.J.; Neilland, M.; Ullrich, N.V.; Koelliker, Y.; Belzer, L.M. Greater energy intake from a buffet meal in lean, young women is associated with the 6-n-propylthiouracil (PROP) non-taster phenotype. Appetite 2011, 56, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Tepper, B.J.; Ullrich, N.V. Influence of genetic taste sensitivity to 6-n-propylthiouracil (PROP), dietary restraint and disinhibition on body mass index in middle-aged women. Physiol. Behav. 2002, 75, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Tepper, B.J.; Koelliker, Y.; Zhao, L.; Ullrich, N.V.; Lanzara, C.; D’Adamo, P.; Ferrara, A.; Ulivi, S.; Esposito, L.; Gasparini, P. Variation in the bitter-taste receptor gene TAS2R38, and adiposity in a genetically isolated population in southern italy. Obesity 2008, 16, 2289–2295. [Google Scholar] [CrossRef] [PubMed]
- Shafaie, Y.; Koelliker, Y.; Hoffman, D.J.; Tepper, B.J. Energy intake and diet selection during buffet consumption in women classified by the 6-n-propylthiouracil bitter taste phenotype. Am. J. Clin. Nutr. 2013, 98, 1583–1591. [Google Scholar] [CrossRef] [PubMed]
- Gorovic, N.; Afzal, S.; Tjonneland, A.; Overvad, K.; Vogel, U.; Albrechtsen, C.; Poulsen, H.E. Genetic variation in the HTAS2R38 taste receptor and Brassica vegetable intake. Scand. J. Clin. Lab. Invest. 2011, 71, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Feeney, E.; O’Brien, S.; Scannell, A.; Markey, A.; Gibney, E.R. Genetic variation in taste perception: Does it have a role in healthy eating? Proc. Nutr. Soc. 2011, 70, 135–143. [Google Scholar] [CrossRef]
- Baranowski, T.; Baranowski, J.C.; Watson, K.B.; Jago, R.; Islam, N.; Beltran, A.; Martin, S.J.; Nguyen, N.; Tepper, B.J. 6-n-Propylthiouracil taster status not related to reported cruciferous vegetable intake among ethnically diverse children. Nutr. Res. 2011, 31, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Mennella, J.A.; Pepino, M.Y.; Reed, D.R. Genetic and environmental determinants of bitter perception and sweet preferences. Pediatrics 2005, 115, e216–e222. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, S.A.; Feeney, E.L.; Scannell, A.G.; Markey, A.; Gibney, E.R. Bitter taste perception and dietary intake patterns in irish children. J. Nutrigenet. Nutrigenomics 2013, 6, 43–58. [Google Scholar]
- Tepper, B.J.; Christensen, C.M.; Cao, J. Development of brief methods to classify individuals by PROP taster status. Physiol. Behav. 2001, 73, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Kirkmeyer, S.V.; Tepper, B.J. A paper screening test to assess genetic taste sensitivity to 6-n-propylthiouracil. Physiol. Behav. 2003, 78, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Mennella, J.; Pepino, M.Y.; Duke, F.; Reed, D. Age modifies the genotype-phenotype relationship for the bitter receptor TAS2R38. BMC Genet. 2010, 11, 60. [Google Scholar] [CrossRef] [PubMed]
- Timpson, N.; Heron, J.; Day, I.; Ring, S.; Bartoshuk, L.; Horwood, J.; Emmett, P.; Davey-Smith, G. Refining associations between TAS2R38 diplotypes and the 6-n-propylthiouracil (PROP) taste test: Findings from the AVON Longitudinal Study of Parents and Children. BMC Genet. 2007, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Running, C.A.; Mattes, R.D.; Tucker, R.M. Fat taste in humans: Sources of within- and between-subject variability. Prog. Lipid Res. 2013, 52, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Than, T.T.; Delay, E.R.; Maier, M.E. Sucrose threshold variation during the menstrual cycle. Physiol. Behav. 1994, 56, 237–239. [Google Scholar] [CrossRef] [PubMed]
- Alberti-Fidanza, A.; Fruttini, D.; Servili, M. Gustatory and food habit changes during the menstrual cycle. Int. J. Vitam. Nutr. Res. 1998, 68, 149–153. [Google Scholar]
- Glanville, E.V.; Kaplan, A.R. Taste perception and the menstrual cycle. Nature 1965, 205, 930–931. [Google Scholar] [CrossRef]
- Kuga, M.; Ikeda, M.; Suzuki, K. Gustatory changes associated with the menstrual cycle. Physiol. Behav. 1999, 66, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Whissell-Buechy, D. Effects of age and sex on taste sensitivity to phenylthiocarbamide (PTC) in the berkeley guidance sample. Chem. Senses 1990, 15, 39–57. [Google Scholar]
- Glanville, E.V.; Kaplan, A.R.; Fischer, R. Age, sex, and taste sensitivity. J. Gerontol. 1964, 19, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Mojet, J.; Christ-Hazelhof, E.; Heidema, J. Taste perception with age: Generic or specific losses in threshold sensitivity to the five basic tastes? Chem. Senses 2001, 26, 845–860. [Google Scholar] [CrossRef]
- Stunkard, A.J.; Messick, S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J. Psychosom. Res. 1985, 29, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Laessle, R.G.; Tuschl, R.J.; Kotthaus, B.C.; Pirke, K.M. Behavioral and biological correlates of dietary restraint in normal life. Appetite 1989, 12, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Tepper, B.J.; Trail, A.C.; Shaffer, S.E. Diet and physical activity in restrained eaters. Appetite 1996, 27, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.M.; Tepper, B.J. Use of reduced-calorie/reduced-fat foods by young adults: Influence of gender and restraint. Appetite 1995, 25, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Westenhoefer, J. Dietary restraint and disinhibition: Is restraint a homogeneous construct? Appetite 1991, 16, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Lawson, O.J.; Williamson, D.A.; Champagne, C.M.; DeLany, J.P.; Brooks, E.R.; Howat, P.M.; Wozniak, P.J.; Bray, G.A.; Ryan, D.H. The association of body weight, dietary intake, and energy expenditure with dietary restraint and disinhibition. Obes. Res. 1995, 3, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Bryant, E.J.; King, N.A.; Blundell, J.E. Disinhibition: Its effects on appetite and weight regulation. Obes. Rev. 2008, 9, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Tomassini Barbarossa, I.; Carta, G.; Murru, E.; Melis, M.; Zonza, A.; Vacca, C.; Muroni, P.; di Marzo, V.; Banni, S. Taste sensitivity to 6-n-propylthiouracil is associated with endocannabinoid plasma levels in normal-weight individuals. Nutrition 2013, 29, 531–536. [Google Scholar]
- Silvestri, C.; di Marzo, V. The endocannabinoid system in energy homeostasis and the etiopathology of metabolic disorders. Cell Metab. 2013, 17, 475–490. [Google Scholar] [CrossRef] [PubMed]
- Banni, S.; di Marzo, V. Effect of dietary fat on endocannabinoids and related mediators: Consequences on energy homeostasis, inflammation and mood. Mol. Nutr. Food Res. 2010, 54, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Piscitelli, F.; Carta, G.; Bisogno, T.; Murru, E.; Cordeddu, L.; Berge, K.; Tandy, S.; Cohn, J.S.; Griinari, M.; Banni, S.; et al. Effect of dietary krill oil supplementation on the endocannabinoidome of metabolically relevant tissues from high-fat-fed mice. Nutr. Metab. 2011, 8, 51. [Google Scholar] [CrossRef]
- DiPatrizio, N.V.; Astarita, G.; Schwartz, G.; Li, X.; Piomelli, D. Endocannabinoid signal in the gut controls dietary fat intake. Proc. Natl. Acad. Sci. USA 2011, 108, 12904–12908. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, P.; Piscitelli, F.; Scognamiglio, P.; Monteleone, A.M.; Canestrelli, B.; di Marzo, V.; Maj, M. Hedonic eating is associated with increased peripheral levels of ghrelin and the endocannabinoid 2-arachidonoyl-glycerol in healthy humans: A pilot study. J. Clin. Endocrinol. Metab. 2012, 97, E917–E924. [Google Scholar] [CrossRef] [PubMed]
- Mattes, R.D. Spices and energy balance. Physiol. Behav. 2012, 107, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Mattes, R.D. Fat taste and lipid metabolism in humans. Physiol. Behav. 2005, 86, 691–697. [Google Scholar] [CrossRef] [PubMed]
- Astrup, A.; Andersen, T.; Christensen, N.J.; Bulow, J.; Madsen, J.; Breum, L.; Quaade, F. Impaired glucose-induced thermogenesis and arterial norepinephrine response persist after weight reduction in obese humans. Am. J. Clin. Nutr. 1990, 51, 331–337. [Google Scholar] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tepper, B.J.; Banni, S.; Melis, M.; Crnjar, R.; Tomassini Barbarossa, I. Genetic Sensitivity to the Bitter Taste of 6-n-Propylthiouracil (PROP) and Its Association with Physiological Mechanisms Controlling Body Mass Index (BMI). Nutrients 2014, 6, 3363-3381. https://doi.org/10.3390/nu6093363
Tepper BJ, Banni S, Melis M, Crnjar R, Tomassini Barbarossa I. Genetic Sensitivity to the Bitter Taste of 6-n-Propylthiouracil (PROP) and Its Association with Physiological Mechanisms Controlling Body Mass Index (BMI). Nutrients. 2014; 6(9):3363-3381. https://doi.org/10.3390/nu6093363
Chicago/Turabian StyleTepper, Beverly J., Sebastiano Banni, Melania Melis, Roberto Crnjar, and Iole Tomassini Barbarossa. 2014. "Genetic Sensitivity to the Bitter Taste of 6-n-Propylthiouracil (PROP) and Its Association with Physiological Mechanisms Controlling Body Mass Index (BMI)" Nutrients 6, no. 9: 3363-3381. https://doi.org/10.3390/nu6093363
APA StyleTepper, B. J., Banni, S., Melis, M., Crnjar, R., & Tomassini Barbarossa, I. (2014). Genetic Sensitivity to the Bitter Taste of 6-n-Propylthiouracil (PROP) and Its Association with Physiological Mechanisms Controlling Body Mass Index (BMI). Nutrients, 6(9), 3363-3381. https://doi.org/10.3390/nu6093363







